Sweet Basil (Ocimum basilicum L.)―A Review of Its Botany, Phytochemistry, Pharmacological Activities, and Biotechnological Development
Abstract
1. Introduction
2. Methodology
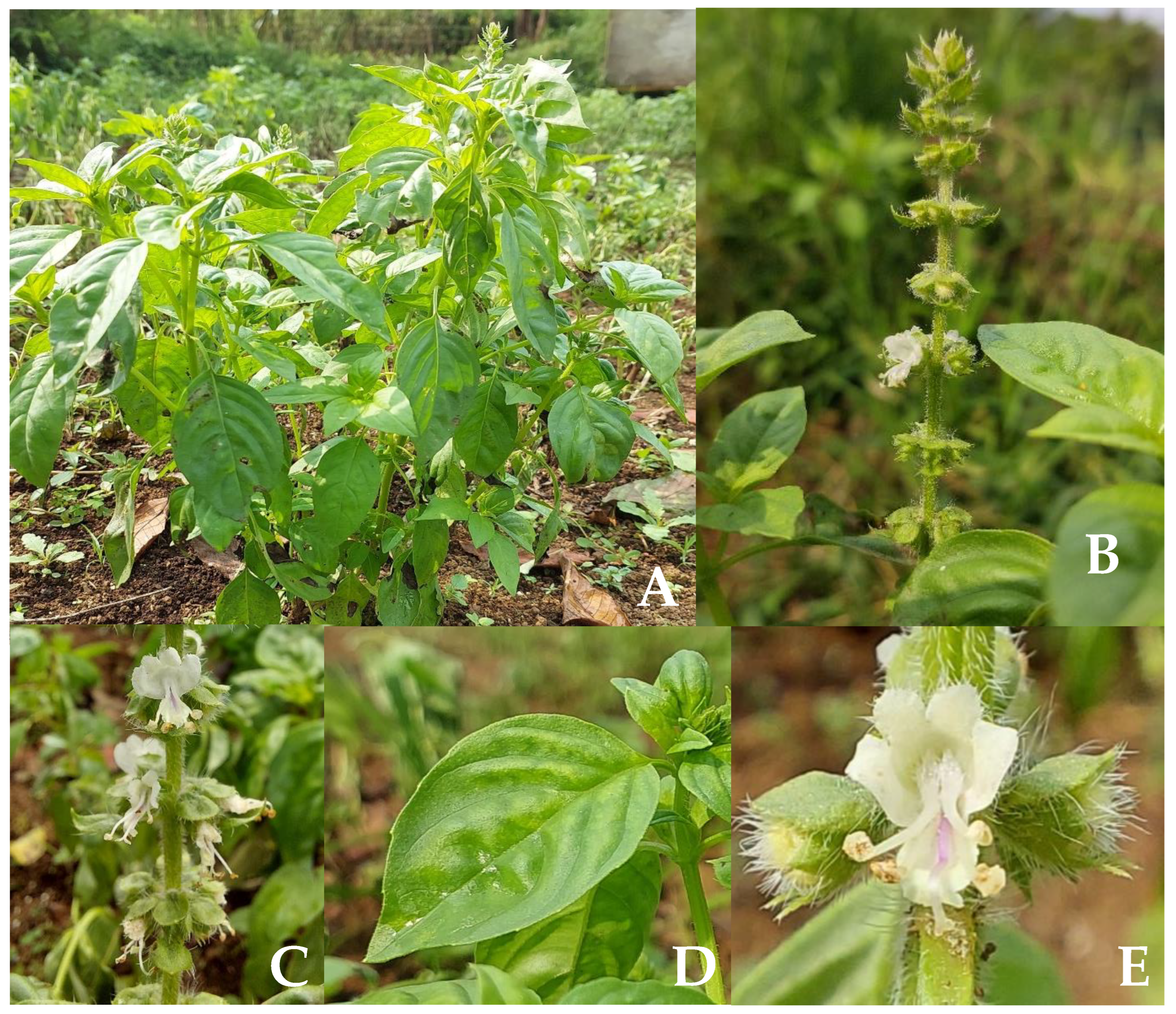
3. Ocimum basilicum L. Ecology and Morphology
4. Phytochemical Constituents
5. Ethnomedicinal Evidence for O. basilicum L.
6. Antimicrobial Activities and Biomedical Uses
6.1. Antiviral Activity
6.2. Antibacterial Activity
6.3. Antifungal Activity
6.4. Biomedical Activity
7. Biotechnological Development in O. basilicum L. Research
7.1. Green Nanotechnology Production in O. basilicum L. for Medical Application
7.2. Biotechnological Techniques for Improving the Metabolite Production of O. basilicum L.
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, S.I.; Sheikh, W.M.; Rather, M.A.; Venkatesalu, V.; Muzamil Bashir, S.; Nabi, S.U. Medicinal plants: Treasure for antiviral drug discovery. Phytother. Res. 2021, 35, 3447–3483. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.L.; Yu, H.; Luo, H.M.; Wu, Q.; Li, C.F.; Steinmetz, A. Conservation and sustainable use of medicinal plants: Problems, progress, and prospects. Chin. Med. 2016, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.; Stewart, L.E.; Darley, B.A.; Pham, A.M.; Esteban, I.; Panda, S.S. Plant-based natural products and extracts: Potential source to develop new antiviral drug candidates. Molecules 2021, 26, 6197. [Google Scholar] [CrossRef] [PubMed]
- Veeresham, C. Natural products derived from plants as a source of drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Süntar, I. Importance of ethnopharmacological studies in drug discovery: Role of medicinal plants. Phytochem. Rev. 2020, 19, 1199–1209. [Google Scholar] [CrossRef]
- Karpiński, T.M. Essential oils of lamiaceae family plants as antifungals. Biomolecules 2020, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Al Jaouni, S.; Saleh, A.M.; Wadaan, M.A.M.; Hozzein, W.N.; Selim, S.; AbdElgawad, H. Elevated CO2 induces a global metabolic change in basil (Ocimum basilicum L.) and peppermint (Mentha piperita L.) and improves their biological activity. J. Plant. Physiol. 2018, 224–225, 121–131. [Google Scholar] [CrossRef]
- Taek, M.M.; Bambang, P.E.W.; Agil, M. Plants used in traditional medicine for treatment of malaria by Tetun Ethnic people in West Timor Indonesia. Asian Pac. J. Trop. Med. 2018, 11, 630–637. [Google Scholar] [CrossRef]
- Silalahi, M.; Nisyawati; Walujo, E.B.; Supriatna, J.; Mangunwardoyo, W. The local knowledge of medicinal plants trader and diversity of medicinal plants in the Kabanjahe Traditional Market, North Sumatra, Indonesia. J. Ethnopharmacol. 2015, 175, 432–443. [Google Scholar] [CrossRef]
- Kasmawati, H.; Ihsan, S.; Munasari, D.; Ode Elafita, W. Ethnomedicine studies of traditional medicinal plants of the Muna Tribe in the Village of Bungi Southeast Sulawesi Province of Indonesia. Int. J. Sci. Res. 2019, 8, 1882–1887. [Google Scholar] [CrossRef]
- Aminian, A.R.; Mohebbati, R.; Boskabady, M.H. The effect of Ocimum basilicum L. and its main ingredients on respiratory disorders: An experimental, preclinical, and clinical review. Front. Pharmacol. 2022, 12, 805391. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Sharun, K.; Gugjoo, M.B.; Tiwari, R.; Alagawany, M.; Iqbal Yatoo, M.; Thakur, P.; Iqbal, H.M.N.; Chaicumpa, W.; Michalak, I.; et al. A comprehensive review on chemical profile and pharmacological activities of Ocimum basilicum. Food Rev. Int. 2023, 39, 119–147. [Google Scholar] [CrossRef]
- Abd El-Hamid, M.I.; El-Tarabili, R.M.; Bahnass, M.M.; Alshahrani, M.A.; Saif, A.; Alwutayd, K.M.; Safhi, F.A.; Mansour, A.T.; Alblwi, N.A.N.; Ghoneim, M.M.; et al. Partnering essential oils with antibiotics: Proven therapies against bovine Staphylococcus aureus mastitis. Front. Cell. Infect. Microbiol. 2023, 13, 1265027. [Google Scholar] [CrossRef] [PubMed]
- Barickman, T.C.; Olorunwa, O.J.; Sehgal, A.; Walne, C.H.; Reddy, K.R.; Gao, W. Interactive impacts of temperature and elevated CO2 on basil (Ocimum basilicum L.) root and shoot morphology and growth. Horticulturae 2021, 7, 112. [Google Scholar] [CrossRef]
- O’Leary, N. Taxonomic revision of Ocimum (lamiaceae) in Argentina. J. Torrey Bot. Soc. 2017, 144, 74–87. [Google Scholar] [CrossRef]
- Akbari, G.A.; Soltani, E.; Binesh, S.; Amini, F. Cold tolerance, productivity and phytochemical diversity in sweet basil (Ocimum basilicum L.) Accessions. Ind. Crops. Prod. 2018, 124, 677–684. [Google Scholar] [CrossRef]
- Li, Q.X.; Chang, C.L. Basil (Ocimum basilicum L.) oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 231–238. ISBN 9780124166417. [Google Scholar]
- Nazarian, H.; Amouzgar, D.; Sedghianzadeh, H. Effects of different concentrations of cadmium on growth and morphological changes in basil (Ocimum basilicum L.). Pak. J. Bot. 2016, 48, 945–952. [Google Scholar]
- Lal, R.K.; Gupta, P.; Chanotiya, C.S.; Sarkar, S. Traditional plant breeding in Ocimum. In The Ocimum Genome; Shasany, A.K., Kole, C., Eds.; Springer: Cham, Switzerland, 2018; pp. 89–98. [Google Scholar]
- Paton, A.; Harley, M.R.; Harley, M.M. Ocimum: An overview of classification and relationships. In Basil: The Genus Ocimum; Hiltunen, R., Holm, Y., Eds.; CRC Press: London, UK, 1999; pp. 1–38. ISBN 9781135296124. [Google Scholar]
- Kumar, A.; Shukla, A.K.; Shasany, A.K.; Sundaresan, V. Systematic position, phylogeny, and taxonomic revision of Indian Ocimum. In The Ocimum Genome; Shasany, A.K., Kole, C., Eds.; Springer: Cham, Switzerland, 2018; pp. 61–72. [Google Scholar]
- Varga, F.; Carović-Stanko, K.; Ristić, M.; Grdiša, M.; Liber, Z.; Šatović, Z. Morphological and biochemical intraspecific characterization of Ocimum basilicum L. Ind. Crops. Prod. 2017, 109, 611–618. [Google Scholar] [CrossRef]
- Padalia, R.C.; Verma, R.S.; Chauhan, A. Analyses of organ specific variations in essential oils of four Ocimum species. J. Essent. Oil Res. 2014, 26, 409–419. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S. Essential oils: Extraction, bioactivities, and their uses for food preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef]
- Majid, I.; Khan, S.; Aladel, A.; Dar, A.H.; Adnan, M.; Khan, M.I.; Mahgoub Awadelkareem, A.; Ashraf, S.A. Recent insights into green extraction techniques as efficient methods for the extraction of bioactive components and essential oils from foods. CYTA—J. Food 2023, 21, 101–114. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green extraction methods for polyphenols from plant matrices and their byproducts: A review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [PubMed]
- Rezzoug, M.; Bakchiche, B.; Gherib, A.; Roberta, A.; Guido, F.; Kilinçarslan, Ö.; Mammadov, R.; Bardaweel, S.K. Chemical composition and bioactivity of essential oils and ethanolic extracts of Ocimum basilicum L. and Thymus algeriensis Boiss. & Reut. from the Algerian Saharan Atlas. BMC Complement. Altern. Med. 2019, 19, 146. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Hussain Sherazi, S.T.; Przybylski, R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008, 108, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Blajet, M.; Feder-Kubis, J. Monoterpenes and their derivatives—Recent development in biological and medical applications. Int. J. Mol. Sci. 2020, 21, 7078. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; El-Ansary, D.O.; Al-Mana, F.A.; Mahmoud, E.A. Saudi Rosmarinus officinalis and Ocimum basilicum L. polyphenols and biological activities. Processes 2020, 8, 446. [Google Scholar] [CrossRef]
- Ge, L.; Li, S.P.; Lisak, G. Advanced sensing technologies of phenolic compounds for pharmaceutical and biomedical analysis. J. Pharm. Biomed. Anal. 2020, 179, 112913. [Google Scholar] [CrossRef]
- Qamar, F.; Sana, A.; Naveed, S.; Faizi, S. Phytochemical characterization, antioxidant activity and antihypertensive evaluation of Ocimum basilicum L. in L-NAME induced hypertensive rats and its correlation analysis. Heliyon 2023, 9, e14644. [Google Scholar] [CrossRef]
- Joshi, R.K.; Agarwal, S.; Patil, P.; Alagarasu, K.; Panda, K.; Cherian, S.; Parashar, D.; Roy, S. Anti-dengue activity of lipophilic fraction of Ocimum basilicum L. Stem. Molecules 2023, 28, 1446. [Google Scholar] [CrossRef]
- Falowo, A.B.; Mukumbo, F.E.; Idamokoro, E.M.; Afolayan, A.J.; Muchenje, V. Phytochemical constituents and antioxidant activity of sweet basil (Ocimum basilicum L.) essential oil on ground beef from Boran and Nguni cattle. Int. J. Food. Sci. 2019, 2019, 2628747. [Google Scholar] [CrossRef]
- Rabbani, M.; Rabbani, M.; Ebrahim Sajjadi, S.; Vaezi, A. Evaluation of anxiolytic and sedative effect of essential oil and hydroalcoholic extract of Ocimum basilicum L. and chemical composition of its essential oil. Res. Pharm. Sci. 2015, 10, 535–543. [Google Scholar] [PubMed]
- Saaban, K.F.; Ang, C.H.; Khor, S.M.; Chuah, C.H. Chemical constituents and antioxidant capacity of Ocimum basilicum and Ocimum sanctum. Iran J. Chem. Chem. Eng. 2019, 38, 139–152. [Google Scholar] [CrossRef]
- Chenni, M.; Abed, D.E.; Rakotomanomana, N.; Fernandez, X.; Chemat, F. Comparative study of essential oils extracted from Egyptian basil leaves (Ocimum basilicum L.) using hydro-distillation and solvent-free microwave extraction. Molecules 2016, 21, 113. [Google Scholar] [CrossRef] [PubMed]
- Hapsari, I.P.; Feroniasanti, Y.M.L. Phytochemical screening and in vitro antibacterial activity of sweet basil leaves (Ocimum basilicum L.) essential oil against Cutibacterium acnes ATCC 11827. In AIP Conference Proceedings; AIP Publishing: New York, NY, USA, 2019; Volume 2099. [Google Scholar]
- Sharafati-Chaleshtori, R.; Rokni, N.; Rafieian-Kopaei, M.; Drees, F.; Salehi, E. Antioxidant and antibacterial activity of basil (Ocimum basilicum L.) Essential Oil in Beef Burger. J. Agric. Sci. Technol. 2015, 17, 817–826. [Google Scholar]
- El-Soud, N.H.A.; Deabes, M.; El-Kassem, L.A.; Khalil, M. Chemical composition and antifungal activity of Ocimum basilicum L. essential oil. Maced. J. Med. Sci. 2015, 3, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, H.R.; Akhtar, S.; Ismail, T.; Qamar, M.; Sestili, P.; Saeed, W.; Azeem, M.; Esatbeyoglu, T. Antioxidant effect of Ocimum basilicum essential oil and its effect on cooking qualities of supplemented chicken nuggets. Antioxidants 2022, 11, 1882. [Google Scholar] [CrossRef] [PubMed]
- Khadka, D.; Dhamala, M.K.; Li, F.; Aryal, P.C.; Magar, P.R.; Bhatta, S.; Thakur, M.S.; Basnet, A.; Cui, D.; Shi, S. The use of medicinal plants to prevent COVID-19 in Nepal. J. Ethnobiol. Ethnomed. 2021, 17, 26. [Google Scholar] [CrossRef] [PubMed]
- Camou-Guerrero, A.; Casas, A.; Moreno-Calles, A.I.; Aguilera-Lara, J.; Garrido-Rojas, D.; Rangel-Landa, S.; Torres, I.; Pérez-Negrón, E.; Solís, L.; Blancas, J.; et al. Ethnobotany in Mexico: History, development, and perspectives. In Ethnobotany of Mexico; Lira, R., Casas, A., Blancas, J., Eds.; Springer: New York, NY, USA, 2016; pp. 21–39. [Google Scholar]
- Abbasi, A.M.; Shah, M.H.; Khan, M.A. Ethnobotany and ethnomedicine. In Wild Edible Vegetables of Lesser Himalayas; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 19–29. [Google Scholar]
- Rodríguez-Calderón, Á.; Muñoz, J.A.; Moreno, D.; Celis, M. Describing and diff using the ethnobotanical knowledge of Bogotá D.C. (Colombia) through an online tool focused on common names of plants. Acta Bot. Brasilica 2019, 33, 303–314. [Google Scholar] [CrossRef]
- Mechaala, S.; Bouatrous, Y.; Adouane, S. Traditional knowledge and diversity of wild medicinal plants in El Kantara’s Area (Algerian Sahara Gate): An ethnobotany survey. Acta Ecol. Sin. 2022, 42, 33–45. [Google Scholar] [CrossRef]
- Anand, U.; Tudu, C.K.; Nandy, S.; Sunita, K.; Tripathi, V.; Loake, G.J.; Dey, A.; Proćków, J. Ethnodermatological use of medicinal plants in India: From Ayurvedic formulations to clinical perspectives—A review. J. Ethnopharmacol. 2022, 284, 114744. [Google Scholar] [CrossRef]
- Ojha, S.N.; Tiwari, D.; Anand, A.; Sundriyal, R.C. Ethnomedicinal knowledge of a Marginal Hill community of Central Himalaya: Diversity, usage pattern, and conservation concerns. J. Ethnobiol. Ethnomed. 2020, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.Z.; Arefin, M.K.; Alam, M.F.; Kibria, M.G.; Podder, S.L.; Hassan, M.A. Knowledge of ethnomedical plants and informant consensus in and around Lawachara National Park. J. Asiat. Soc. Bangladesh Sci. 2017, 43, 101–123. [Google Scholar] [CrossRef]
- Sohel, M.D.D.; Kawsar, M.D.H.; Sumon, M.D.H.U.; Sultana, T. Ethnomedicinal studies of Lalmohan Thana in Bhola District, Bangladesh. Altern. Integr. Med. 2016, 5, 317–327. [Google Scholar] [CrossRef]
- Hegde, H.V.; Hegde, G.R.; Kholkute, S.D. Herbal care for reproductive health: Ethno medicobotany from Uttara Kannada District in Karnataka, India. Complement. Ther. Clin. Pract. 2007, 13, 38–45. [Google Scholar] [CrossRef]
- Hussain, J.; Mehta, J.P.; Singh, A.; Bagri, A.S.; Singh, H.; Nautiyal, M.C.; Bussmann, R.W. Ethnomedicinal plants used in Khatling Valley of Western Himalaya, India. Ethnobot. Res. Appl. 2023, 25, 1–19. [Google Scholar] [CrossRef]
- Khajuria, A.K.; Manhas, R.K.; Kumar, H.; Bisht, N.S. Ethnobotanical study of traditionally used medicinal plants of Pauri District of Uttarakhand, India. J. Ethnopharmacol. 2021, 276, 114204. [Google Scholar] [CrossRef] [PubMed]
- Leelaveni, A.; Dash, S.; Behera, S.K.; Das, S.; Mandal, A.K. Ethno medicinal study in North West Ganjam, Odisha. Int. J. Innov. Sci. Res. 2018, 7, 1167–1174. [Google Scholar]
- Bora Ajit Kr Das, R. An inventory of ethnomedicinal plants among the Rabha Tribe residing nearby Chandubi Beel of Kamrup District (Assam). Int. J. Innov. Res. Sci. Technol. 2015, 1, 126–129. [Google Scholar]
- Tamang, S.; Singh, A.; Bussmann, R.W.; Shukla, V.; Nautiyal, M.C. Ethno-medicinal plants of tribal people: A case study in Pakyong subdivision of East Sikkim, India. Acta Ecol. Sin. 2023, 43, 34–46. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Morvin Yabesh, J.E.; Prabhu, S.; Manikandan, R.; Muralidharan, B. Quantitative ethnomedicinal study of plants used in the Nelliyampathy Hills of Kerala, India. J. Ethnopharmacol. 2015, 161, 238–254. [Google Scholar] [CrossRef]
- Magar, R.A.; Mallik, A.R.; Chaudhary, S.; Parajuli, S. Ethno-medicinal plants used by the people of Dharan, Eastern Nepal. Indian J. Tradit. Knowl. 2022, 21, 72–80. [Google Scholar]
- Ahmed, H.M. Ethnopharmacobotanical study on the medicinal plants used by herbalists in Sulaymaniyah Province, Kurdistan, Iraq. J. Ethnobiol. Ethnomed. 2016, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, S.; Khan, M.S.; Shah, M.; Shuaib, M.; Ahmad, M.; Zafar, M.; Begum, N.; Gul, S.; Ashfaq, S.; Mujahid, I.; et al. Traditional usage of medicinal plants among the local communities of Peshawar Valley, Pakistan. Acta Ecol. Sin. 2020, 40, 1–29. [Google Scholar] [CrossRef]
- Maneenoon, K.; Khuniad, C.; Teanuan, Y.; Saedan, N.; Prom-in, S.; Rukleng, N.; Kongpool, W.; Pinsook, P.; Wongwiwat, W. Ethnomedicinal plants used by traditional healers in Phatthalung Province, Peninsular Thailand. J. Ethnobiol. Ethnomed. 2015, 11, 43. [Google Scholar] [CrossRef] [PubMed]
- Montero, J.C.; Geducos, D.T. Ethnomedicinal plants used by the local folks in two selected villages of San Miguel, Surigao Del Sur, Mindanao, Philippines. Int. J. Agric. Technol. 2021, 17, 193–212. [Google Scholar]
- Hadanu, R.; Saparuddin, S.M.; Wahyuningrum, R.; Sartika, G.P. Ethnopharmacological survey of medicinal herbs in Tolaki-Mekongga Tribe of Kolaka Regency and East Kolaka Regency, Southeast Sulawesi, Indonesia. J. Med. Plants Stud. 2022, 10, 20–29. [Google Scholar] [CrossRef]
- Nisa, U.; Triyono, A.; Ardiyanto, D.; Novianto, F.; Fitriani, U.; Jannah, W.D.M.; Astana, P.R.W.; Zulkarnain, Z. Ethnopharmacological study of medicinal plants indigenous knowledge about low back pain therapy in Sumatra, Indonesia. J. Appl. Pharm. Sci. 2022, 12, 178–188. [Google Scholar] [CrossRef]
- Julung, H.; Supiandi, M.I.; Ege, B.; Zubaidah, S.; Mahanal, S. Ethnobotany of medicinal plants in the Dayak Linoh Tribe in Sintang District, Indonesia. Biodiversitas 2023, 24, 767–775. [Google Scholar] [CrossRef]
- Supiandi, M.I.; Julung, H.; Susanti, Y.; Zubaidah, S.; Mahanal, S. Potential of traditional medicinal plants in the Dayak Tamambaloh Tribe, West Kalimantan, Indonesia. Biodiversitas 2023, 24, 3384–3393. [Google Scholar] [CrossRef]
- De Santana, B.F.; Voeks, R.A.; Funch, L.S. Quilombola ethnomedicine: The role of age, gender, and culture change. Acta Bot. Bras. 2022, 36, e2020abb0500. [Google Scholar] [CrossRef]
- Nduche, M.U.; Omosun, G. The use of medicinal plants in the treatment of diarrhoea in Nigeria: Ethnomedical inventory of Abia State. Sch. J. Agric. Vet. Sci. 2016, 3, 270–274. [Google Scholar]
- Dubale, S.; Abdissa, N.; Kebebe, D.; Debella, A.; Zeynudin, A.; Suleman, S. Ethnomedicinal plants and associated indigenous knowledge for the treatment of different infectious diseases in Ethiopia. J. Herb. Med. 2023, 40, 100669. [Google Scholar] [CrossRef]
- Njoroge, G.N.; Bussmann, R.W. Traditional management of ear, nose and throat (ENT) diseases in Central Kenya. J. Ethnobiol. Ethnomed. 2006, 2, 54. [Google Scholar] [CrossRef] [PubMed]
- Abiodun, A.; Tunji, B. Medicinal weed diversity and ethno-medicinal weeds in Odigbo local government area, Ondo State, Nigeria. J. Med. Plants Stud. 2019, 7, 81–85. [Google Scholar]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Mnif, S.; Aifa, S. Cumin (Cuminum cyminum L.) from traditional uses to potential biomedical applications. Chem. Biodivers. 2015, 12, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Li, F.S.; Weng, J.K. Demystifying traditional herbal medicine with modern approaches. Nat. Plants 2017, 3, 17109. [Google Scholar] [CrossRef]
- Kurnia, D.; Putri, S.A.; Tumilaar, S.G.; Zainuddin, A.; Dharsono, H.D.A.; Amin, M.F. In silico study of antiviral activity of polyphenol compounds from Ocimum basilicum by molecular docking, ADMET, and drug-likeness analysis. Adv. Appl. Bioinf. Chem. 2023, 16, 37–47. [Google Scholar] [CrossRef]
- Hu, Q.; Xiong, Y.; Zhu, G.H.; Zhang, Y.N.; Zhang, Y.W.; Huang, P.; Ge, G.B. The SARS-CoV-2 main protease (Mpro): Structure, function, and emerging therapies for COVID-19. MedComm 2022, 3, e151. [Google Scholar] [CrossRef]
- Ćavar Zeljković, S.; Schadich, E.; Džubák, P.; Hajdúch, M.; Tarkowski, P. Antiviral activity of selected Lamiaceae essential oils and their monoterpenes against SARS-CoV-2. Front. Pharmacol. 2022, 13, 893634. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D.; Wu, X.; Xu, R.; Li, Y. Antiviral mechanism of carvacrol on HSV-2 infectivity through inhibition of RIP3-mediated programmed cell necrosis pathway and ubiquitin-proteasome system in BSC-1 cells. BMC Infect. Dis. 2020, 20, 283. [Google Scholar] [CrossRef] [PubMed]
- Arenas Buan, I.J.; Muncal Mendoza, D.V. Computational study of bioactive components of sweet basil (Ocimum basilicum Linn.), Luyang Dilaw (Curcuma longa Linn.) and Lagundi (Vitex negundo) as inhibitor against human immunodeficiency virus (HIV-1). Orient. J. Chem. 2020, 36, 767–772. [Google Scholar] [CrossRef]
- Singh, P.; Chakraborty, P.; He, D.H.; Mergia, A. Extract prepared from the leaves of Ocimum basilicum inhibits the entry of Zika Virus. Acta Virol. 2019, 63, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Al-Amri, S.A.; Odisho, S.M.; Ibrahem, O.M. In vitro antiviral potential of Ocimum basilicum and Olea europaea leaves extract against Newcastle Disease Virus of poultry. Iraqi J. Vet. Med. 2015, 39, 94–99. [Google Scholar] [CrossRef]
- Kubiça, T.F.; Alves, S.H.; Weiblen, R.; Lovato, L.T. In vitro inhibition of the Bovine Viral Diarrhoea Virus by the essential oil of Ocimum basilicum (Basil) and Monoterpenes. Braz. J. Microbiol. 2014, 45, 209–214. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saied, A.; El-Ghoneimy, A.; Seddek, A.; Abdel-Ghafar, S.; Morad, S. Therapeutic effectiveness of Ocimum basilicum extract on bovine cutaneous papillomatosis. SVU-Int. J. Vet. Sci. 2020, 3, 60–77. [Google Scholar] [CrossRef]
- Chiang, L.-C.; Ng, L.-T.; Cheng, P.-W.; Chiang, W.; Lin, C.-C. Antiviral activities of extracts and selected pure constituents of Ocimum basilicum. Clin. Exp. Pharmacol. Physiol. 2005, 32, 811–816. [Google Scholar] [CrossRef]
- Yucharoen, R.; Anuchapreeda, S.; Tragoolpua, Y. Anti-herpes simplex virus activity of extracts from the culinary herbs Ocimum sanctum L., Ocimum basilicum L. and Ocimum americanum L. Afr. J. Biotechnol. 2011, 10, 860–866. [Google Scholar]
- Behbahani, M.; Mohabatkar, H.; Soltani, M. Anti-HIV-1 activities of aerial parts of Ocimum basilicum and its parasite Cuscuta campestris. J. Antivir. Antiretrovir. 2013, 5, 57–61. [Google Scholar] [CrossRef]
- Ben-Shabat, S.; Yarmolinsky, L.; Porat, D.; Dahan, A. Antiviral effect of phytochemicals from medicinal plants: Applications and drug delivery strategies. Drug. Deliv. Transl. Res. 2020, 10, 354–367. [Google Scholar] [CrossRef]
- Chandra, N.; Padiadpu, J. Network approaches to drug discovery. Expert Opin. Drug. Discov. 2013, 8, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ye, S. Research progress on antiviral constituents in traditional Chinese medicines and their mechanisms of action. Pharm. Biol. 2022, 60, 1063–1076. [Google Scholar] [CrossRef] [PubMed]
- Khare, T.; Anand, U.; Dey, A.; Assaraf, Y.G.; Chen, Z.S.; Liu, Z.; Kumar, V. Exploring phytochemicals for combating antibiotic resistance in microbial pathogens. Front. Pharmacol. 2021, 12, 720726. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Tang, J.; Khare, T.; Kumar, V. The alarming antimicrobial resistance in eskapee pathogens: Can essential oils come to the rescue? Fitoterapia 2020, 140, 104433. [Google Scholar] [CrossRef] [PubMed]
- Semeniuc, C.A.; Pop, C.R.; Rotar, A.M. Antibacterial activity and interactions of plant essential oil combinations against Gram-positive and Gram-negative bacteria. J. Food Drug Anal. 2017, 25, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Kabir, M.J.; Salehuddin, S.M.; Rahman, S.M.M.; Das, A.K.; Singha, S.K.; Alam, M.K.; Rahman, A. Antibacterial properties of essential oils and methanol extracts of sweet basil Ocimum basilicum occurring in Bangladesh. Pharm. Biol. 2010, 48, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Gaio, I.; Saggiorato, A.G.; Treichel, H.; Cichoski, A.J.; Astolfi, V.; Cardoso, R.I.; Toniazzo, G.; Valduga, E.; Paroul, N.; Cansian, R.L. Antibacterial activity of basil essential oil (Ocimum basilicum L.) in Italian-Type sausage. J. Consum. Prot. Food Saf. 2015, 10, 323–329. [Google Scholar] [CrossRef]
- Silva, V.A.; Da Sousa, J.P.; De Luna Freire Pessôa, H.; De Freitas, A.F.R.; Coutinho, H.D.M.; Alves, L.B.N.; Lima, E.O. Ocimum basilicum: Antibacterial activity and association study with antibiotics against bacteria of clinical importance. Pharm. Biol. 2016, 54, 863–867. [Google Scholar] [CrossRef]
- Al Abbasy, D.W.; Pathare, N.; Al-Sabahi, J.N.; Khan, S.A. Chemical composition and antibacterial activity of essential oil isolated from omani basil (Ocimum basilicum Linn.). Asian. Pac. J. Trop. Dis. 2015, 5, 645–649. [Google Scholar] [CrossRef]
- Khan, I.; Ahmad, K.; Khalil, A.T.; Khan, J.; Khan, Y.A.; Saqib, M.S.; Umar, M.N.; Ahmad, H. Evaluation of antileishmanial, antibacterial and brine shrimp cyto-toxic potential of crude methanolic extract of herb Ocimum basilicum (Lamiacea). J. Tradit. Chin. Med. 2015, 35, 316–322. [Google Scholar] [CrossRef]
- Ouibrahim, A.; Tlili-Ait-kaki, Y.; Bennadja, S.; Amrouni, S.; Djahoudi, A.G.; Djebar, M.R. Evaluation of antibacterial activity of Laurus nobilis L., Rosmarinus officinalis L. and Ocimum basilicum L. from Northeast of Algeria. Afr. J. Microbiol. Res. 2013, 7, 4968–4973. [Google Scholar] [CrossRef]
- Snoussi, M.; Dehmani, A.; Noumi, E.; Flamini, G.; Papetti, A. Chemical composition and antibiofilm activity of Petroselinum crispum and Ocimum basilicum essential oils against Vibrio ssp. strains. Microb. Pathog. 2016, 90, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.E.I.; Marei, G.I.; Rabea, E.I.; Taktak, N.E.M. Antimicrobial and antioxidant activities of hydrocarbon and oxygen-ated monoterpenes against some foodborne pathogens through in vitro and in silico studies. Pestic. Biochem. Physiol. 2019, 158, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant polyphenols as antioxidant and antibacterial agents for shelf-life extension of meat and meat products: Classification, structures, sources, and action mechanisms. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef] [PubMed]
- Anwar, F.; Alkharfy, K.M.; Mehmood, T.; Bakht, M.A.; Najeeb-ur-Rehman. Variation in chemical composition and effective antibacterial potential of Ocimum basilicum L. essential oil harvested from different regions of Saudi Arabia. Pharm. Chem. J. 2021, 55, 187–193. [Google Scholar] [CrossRef]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Assessment of factors influencing antimicrobial activity of carvacrol and cymene against Vibrio cholerae in Food. J. Biosci. Bioeng. 2010, 110, 614–619. [Google Scholar] [CrossRef] [PubMed]
- De Pascale, G.; Tumbarello, M. Fungal infections in the ICU: Advances in treatment and diagnosis. Curr. Opin. Crit. Care 2015, 21, 421–429. [Google Scholar] [CrossRef]
- Lee, Y.; Puumala, E.; Robbins, N.; Cowen, L.E. Antifungal drug resistance: Molecular mechanisms in Candida albicans and beyond. Chem. Rev. 2021, 121, 3390–3411. [Google Scholar] [CrossRef]
- Robbins, N.; Wright, G.D.; Cowen, L.E. Antifungal Drugs: The current armamentarium and development of new agents. Microbiol. Spectr. 2016, 4, FUNK-0002. [Google Scholar] [CrossRef]
- Kocić-Tanackov, S.; Dimić, G.; Lević, J.; Tanackov, I.; Tuco, D. Antifungal activities of basil (Ocimum basilicum L.) extract on Fusarium species. Afr. J. Biotechnol. 2011, 10, 10188–10195. [Google Scholar] [CrossRef]
- Stanojevic, L.P.; Marjanovic-Balaban, Z.R.; Kalaba, V.D.; Stanojevic, J.S.; Cvetkovic, D.J.; Cakic, M.D. Chemical composition, antioxidant and antimicrobial activity of basil (Ocimum basilicum L.) essential oil. J. Essent. Oil Bear. Plants 2017, 20, 1557–1569. [Google Scholar] [CrossRef]
- Beatović, D.; Krstić-Milošević, D.; Trifunović, S.; Šiljegović, J.; Glamočlija, J.; Ristić, M.; Jelačić, S. Chemical composition, antioxidant and antimicrobial activities of the essential oils of twelve Ocimum basilicum L. cultivars grown in Serbia. Nat. Prod. 2015, 9, 62–75. [Google Scholar]
- Ahmad, K.; talha Khalil, A.; Somayya aa Kafeel Ahmad, R.; Somayya, R. Antifungal, phytotoxic and hemagglutination activity of methanolic extracts of Ocimum basilicum. J. Tradit. Chin. Med. 2016, 36, 794–798. [Google Scholar] [CrossRef]
- Vlase, L.; Benedec, D.; Hanganu, D.; Damian, G.; Csillag, I.; Sevastre, B.; Mot, A.C.; Silaghi-Dumitrescu, R.; Tilea, I. Evaluation of antioxidant and antimicrobial activities and phenolic profile for Hyssopus officinalis, Ocimum basilicum and Teucrium chamaedrys. Molecules 2014, 19, 5490–5507. [Google Scholar] [CrossRef]
- Miron, D.; Battisti, F.; Silva, F.K.; Lana, A.D.; Pippi, B.; Casanova, B.; Gnoatto, S.; Fuentefria, A.; Mayorga, P. Antifungal activity and mechanism of action of monoterpenes against dermatophytes and yeasts. Rev. Bras. Farmacogn. 2014, 24, 660–667. [Google Scholar] [CrossRef]
- Khanzada, B.; Akhtar, N.; Okla, M.K.; Alamri, S.A.; Al-Hashimi, A.; Baig, M.W.; Rubnawaz, S.; AbdElgawad, H.; Hirad, A.H.; Haq, I.-U.; et al. Profiling of antifungal activities and in silico studies of natural polyphenols from some plants. Molecules 2021, 26, 7164. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; De Feo, V. Essential oils and antifungal activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef]
- Eid, A.M.; Jaradat, N.; Shraim, N.; Hawash, M.; Issa, L.; Shakhsher, M.; Nawahda, N.; Hanbali, A.; Barahmeh, N.; Taha, B.; et al. Assessment of anticancer, antimicrobial, antidiabetic, anti-obesity and antioxidant activity of Ocimum basilicum seeds essential oil from Palestine. BMC Complement. Med. Ther. 2023, 23, 221. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Attia, F.A.K.; Liu, Z.; Li, C.; Wei, J.; Kang, W. Antioxidant activity and total phenolic content of essential oils and extracts of sweet basil (Ocimum basilicum L.) plants. Food Sci. Hum. Wellness 2019, 8, 299–305. [Google Scholar] [CrossRef]
- Do, T.H.; Truong, H.B.; Nguyen, H.C. Optimization of extraction of phenolic compounds from Ocimum basilicum leaves and evaluation of their antioxidant activity. Pharm. Chem. J. 2020, 54, 162–169. [Google Scholar] [CrossRef]
- Osei Akoto, C.; Acheampong, A.; Boakye, Y.D.; Naazo, A.A.; Adomah, D.H. Anti-inflammatory, antioxidant, and anthelmintic activities of Ocimum basilicum (sweet basil) fruits. J. Chem. 2020, 2020, 2153534. [Google Scholar] [CrossRef]
- Aburjai, T.A.; Mansi, K.; Azzam, H.; Alqudah, D.A.; Alshaer, W.; Abuirjei, M. Chemical compositions and anticancer potential of essential oil from greenhouse-cultivated Ocimum basilicum leaves. Indian J. Pharm. Sci. 2020, 82, 179–184. [Google Scholar] [CrossRef]
- Behbahani, M. Evaluation of in vitro anticancer activity of Ocimum basilicum, Alhagi maurorum, Calendula officinalis and their parasite Cuscuta campestris. PLoS ONE 2014, 9, e116049. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, B.O.; Alsayadi, A.I.; Abutaha, N.; Al-Mekhlafi, F.A.; Wadaan, M.A. Evaluation of the anticancer potential of Morus nigra and Ocimum basilicum mixture against different cancer cell lines: An in vitro evaluation. Biomed. Res. Int. 2023, 2023, 9337763. [Google Scholar] [CrossRef] [PubMed]
- Kadan, S.; Saad, B.; Sasson, Y.; Zaid, H. In vitro evaluation of anti-diabetic activity and cytotoxicity of chemically analysed Ocimum basilicum extracts. Food Chem. 2016, 196, 1066–1074. [Google Scholar] [CrossRef]
- Zahra, K.; Khan, M.A.; Iqbal, F. Oral supplementation of Ocimum basilicum has the potential to improves the locomotory, exploratory, anxiolytic behavior and learning in adult male albino mice. Neurol. Sci. 2015, 36, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, S.; Hosseini, M.; Alipour, F.; Beheshti, F.; Rakhshandeh, H.; Mohammadipour, A.; Anaeigoudari, A.; Jalili-Nik, M.; Khazdair, M.R.; Jahani, A. Neuroprotective effects of the fractions of Ocimum basilicum in seizures induced by pentylenetetrazole in mice. Avicenna J. Phytomed. 2022, 12, 614–626. [Google Scholar] [CrossRef]
- Singh, V.; Kaur, K.; Kaur, S.; Shri, R.; Singh, T.G.; Singh, M. Trimethoxyflavones from Ocimum basilicum L. leaves improve long term memory in mice by modulating multiple pathways. J. Ethnopharmacol. 2022, 295, 115438. [Google Scholar] [CrossRef] [PubMed]
- Ayuob, N.N.; Balgoon, M.J.; Ali, S.; Alnoury, I.S.; ALmohaimeed, H.M.; AbdElfattah, A.A. Ocimum basilicum (basil) modulates apoptosis and neurogenesis in olfactory pulp of mice exposed to chronic unpredictable mild stress. Front. Psychiatry 2020, 11, 569711. [Google Scholar] [CrossRef]
- Rodrigues, L.B.; Oliveira Brito Pereira Bezerra Martins, A.; Cesário, F.R.; Ferreira, E.C.F.; de Albuquerque, T.R.; Martins Fernandes, M.N.; Fernandes da Silva, B.A.; Quintans Junior, L.J.; da Costa, J.G.; Melo Coutinho, H.D.; et al. Anti-inflammatory and antiedematogenic activity of the Ocimum basilicum essential oil and its main compound estragole: In vivo mouse models. Chem. Biol. Interact. 2016, 257, 14–25. [Google Scholar] [CrossRef]
- Złotek, U.; Szymanowska, U.; Karaś, M.; Świeca, M. Antioxidative and anti-inflammatory potential of phenolics from purple basil (Ocimum basilicum L.) leaves induced by jasmonic, arachidonic, and β-aminobutyric acid elicitation. Int. J. Food Sci. Technol. 2016, 51, 163–170. [Google Scholar] [CrossRef]
- Aye, A.; Jeon, Y.-D.; Lee, J.-H.; Bang, K.-S.; Jin, J.-S. Anti-inflammatory activity of ethanol extract of leaf and leaf callus of basil (Ocimum basilicum L.) on RAW 264.7 macrophage cells. Orient. Pharm. Exp. Med. 2019, 19, 217–226. [Google Scholar] [CrossRef]
- Ali Khan, B.; Ullah, S.; Khan, M.K.; Alshahrani, S.M.; Braga, V.A. Formulation and evaluation of Ocimum basilicum-based emulgel for wound healing using animal model. Saudi Pharm. J. 2020, 28, 1842–1850. [Google Scholar] [CrossRef]
- Antonescu (Mintas), I.A.; Antonescu, A.; Miere (Groza), F.; Fritea, L.; Teusdea, A.C.; Vicas, L.; Vicas, S.I.; Brihan, I.; Domuta, M.; Zdrinca, M.; et al. Evaluation of wound healing potential of novel hydrogel based on Ocimum basilicum and Trifolium pratense extracts. Processes 2021, 9, 2096. [Google Scholar] [CrossRef]
- Li, X.; Wu, J.; Xu, F.; Chu, C.; Li, X.; Shi, X.; Zheng, W.; Wang, Z.; Jia, Y.; Xiao, W. Use of ferulic acid in the management of diabetes mellitus and its complications. Molecules 2022, 27, 6010. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, A. Use of Biotechnology in Agriculture—Benefits and Risks; University of Hawaii: Honolulu, HI, USA, 2003. [Google Scholar]
- El Sheikha, A.F. Medicinal plants: Ethno-uses to biotechnology era. In Biotechnology and Production of Anti-Cancer Compounds; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–38. ISBN 9783319538808. [Google Scholar]
- Siahsar, B.; Rahimi, M.; Tavassoli, A.; Raissi, A. Application of biotechnology in production of medicinal plants. Am.-Euras. J. Agric. Environ. Sci. 2011, 11, 439–444. [Google Scholar]
- Stan, M.; Popa, A.; Toloman, D.; Silipas, T.D.; Vodnar, D.C. Antibacterial and antioxidant activities of ZnO nanoparticles synthesized using extracts of Allium sativum, Rosmarinus officinalis and Ocimum basilicum. Acta Metall. Sin. Engl. Lett. 2016, 29, 228–236. [Google Scholar] [CrossRef]
- Cai, M.; Wang, Y.; Wang, R.; Li, M.; Zhang, W.; Yu, J.; Hua, R. Antibacterial and antibiofilm activities of chitosan nanoparticles loaded with Ocimum basilicum L. essential oil. Int. J. Biol. Macromol. 2022, 202, 122–129. [Google Scholar] [CrossRef]
- Rahman, T.U.; Anwar, M.R.; Zeb, M.A.; Liaqat, W. Green synthesis, characterization, antibacterial activity of metal nanoparticles and composite oxides using leaves extract of Ocimum basilicum L. Microsc. Res. Tech. 2022, 85, 2857–2865. [Google Scholar] [CrossRef]
- Altikatoglu, M.; Attar, A.; Erci, F.; Cristache, C.M.; Isildak, I. Green synthesis of copper oxide nanoparticles using Ocimum basilicum extract and their antibacterial activity. Fresenius Environ. Bull. 2017, 26, 7832. [Google Scholar]
- Loza, K.; Heggen, M.; Epple, M. Synthesis, structure, properties, and applications of bimetallic nanoparticles of noble metals. Adv. Funct. Mater. 2020, 30, de1909260. [Google Scholar] [CrossRef]
- More, P.R.; Zannella, C.; Folliero, V.; Foglia, F.; Troisi, R.; Vergara, A.; Franci, G.; De Filippis, A.; Galdiero, M. Antimicrobial applications of green synthesized bimetallic nanoparticles from Ocimum basilicum. Pharmaceutics 2022, 14, 2457. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.R.; Sharif, S.; Shaheen, F.; Khalid, M.; Iqbal, Y.; Faisal, A.; Aziz, M.H.; Atif, M.; Ahmad, S.; Fakhar-e-Alam, M.; et al. Green synthesis of RGO-ZnO mediated Ocimum basilicum leaves extract nanocomposite for antioxidant, antibacterial, antidiabetic and photocatalytic activity. J. Saudi Chem. Soc. 2022, 26, 101438. [Google Scholar] [CrossRef]
- Malapermal, V.; Botha, I.; Krishna, S.B.N.; Mbatha, J.N. Enhancing antidiabetic and antimicrobial performance of Ocimum basilicum, and Ocimum sanctum (L.) using silver nanoparticles. Saudi J. Biol. Sci. 2017, 24, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Abdelsattar, A.S.; Farouk, W.M.; Mohamed Gouda, S.; Safwat, A.; Hakim, T.A.; El-Shibiny, A. Utilization of Ocimum basilicum extracts for zinc oxide nanoparticles synthesis and their antibacterial activity after a novel combination with phage. Mater. Lett. 2022, 309, 131344. [Google Scholar] [CrossRef]
- Abdelsattar, A.S.; Hakim, T.A.; Rezk, N.; Farouk, W.M.; Hassan, Y.Y.; Gouda, S.M.; El-Shibiny, A. Green synthesis of silver nanoparticles using Ocimum basilicum L. and Hibiscus sabdariffa L. extracts and their antibacterial activity in combination with phage zcse6 and sensing properties. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1951–1965. [Google Scholar] [CrossRef]
- Tantiwatcharothai, S.; Prachayawarakorn, J. Characterization of an antibacterial wound dressing from basil seed (Ocimum basilicum L.) mucilage-zno nanocomposite. Int. J. Biol. Macromol. 2019, 135, 133–140. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; El-Gendi, H.; Alotibi, F.O.; Al-Askar, A.A.; Elbeaino, T.; Behiry, S.I.; Abd-Elsalam, K.A.; Moawad, H. Ocimum basilicum-mediated synthesis of silver nanoparticles induces innate immune responses against Cucumber Mosaic Virus in squash. Plants 2022, 11, 2707. [Google Scholar] [CrossRef]
- Filip, S. Basil (Ocimum basilicum L.) a source of valuable phytonutrients. Int. J. Clin. Nutr. Diet. 2017, 3, 118. [Google Scholar] [CrossRef]
- Mith, H.; Yayi-Ladékan, E.; Sika Kpoviessi, S.D.; Yaou Bokossa, I.; Moudachirou, M.; Daube, G.; Clinquart, A. Chemical composition and antimicrobial activity of essential oils of Ocimum basilicum, Ocimum canum and Ocimum gratissimum in function of harvesting time. J. Essent. Oil Bear. Plants 2016, 19, 1413–1425. [Google Scholar] [CrossRef]
- Solouki, A.; Zare Mehrjerdi, M.; Azimi, R.; Aliniaeifard, S. Improving basil (Ocimum basilicum L.) essential oil yield following down-regulation of photosynthetic functionality by short-term application of abiotic elicitors. Biocatal. Agric. Biotechnol. 2023, 50, 102675. [Google Scholar] [CrossRef]
- Sipos, L.; Balázs, L.; Székely, G.; Jung, A.; Sárosi, S.; Radácsi, P.; Csambalik, L. Optimization of basil (Ocimum basilicum L.) production in led light environments—A review. Sci. Hortic. 2021, 289, 110486. [Google Scholar] [CrossRef]
- Carvalho, S.D.; Schwieterman, M.L.; Abrahan, C.E.; Colquhoun, T.A.; Folta, K.M. Light quality dependent changes in morphology, antioxidant capacity, and volatile production in sweet basil (Ocimum basilicum). Front. Plant Sci. 2016, 7, 1328. [Google Scholar] [CrossRef] [PubMed]
- Mandal, D.; Sarkar, T.; Chakraborty, R. Critical review on nutritional, bioactive, and medicinal potential of spices and herbs and their application in food fortification and nanotechnology. Appl. Biochem. Biotechnol. 2023, 195, 1319–1513. [Google Scholar] [CrossRef] [PubMed]
- Lobiuc, A.; Vasilache, V.; Pintilie, O.; Stoleru, T.; Burducea, M.; Oroian, M.; Zamfirache, M.M. Blue and red LED illumination improves growth and bioactive compounds contents in acyanic and cyanic Ocimum basilicum L. microgreens. Molecules 2017, 22, 2111. [Google Scholar] [CrossRef] [PubMed]
- Açıkgöz, M.A. Establishment of cell suspension cultures of Ocimum basilicum L. and enhanced production of pharmaceutical active ingredients. Ind. Crops. Prod. 2020, 148, 112278. [Google Scholar] [CrossRef]
- Yilmaz, A.; Karik, Ü. AMF and PGPR enhance yield and secondary metabolite profile of basil (Ocimum basilicum L.). Ind. Crops. Prod. 2022, 176, 114327. [Google Scholar] [CrossRef]


| No. | Chemical Compound | Molecular Weight | Source | Extraction and Identification Method | Reference |
|---|---|---|---|---|---|
| Monoterpene Hydrocarbon | |||||
| 1. | α-pinene | 136.23 g/mol | Leaf | Hydrodistillation, GC-MS Maceration 24 h, GC-MS Hydrodistillation, solvent extraction, GC-MS | [34] [35] [36] |
| 2. | β-Myrcene | 136.23 g/mol | Leaf | Hydrodistillation, GC-MS | [34] |
| 3. | Citral | 153.23 g/mol | Leaf | Hydrodistillation, GC-MS | [34] |
| 4. | Camphene | 136.23 g/mol | Leaf | Maceration, GC-MS SFME, hydrodistillation 1 h, GC-MS | [32] [37] |
| 5. | Terpineol | 154.25 g/mol | Leaf | Maceration, GC-MS | [32] |
| 6. | Linalyl acetate | 196.29 g/mol | Leaf | Maceration, GC-MS | [32] |
| 7. | cis-Sabinene hydrate | 154.25 g/mol | Leaf | Maceration, GC-MS | [32] |
| 8. | (−)-trans-Pinocarvyl acetate | 194.27 g/mol | Leaf | Maceration, GC-MS | [32] |
| 9. | Limonene dioxide | 168.23 g/mol | Leaf | Maceration, GC-MS Hydrodistillation, GC-MS | [32] [38] |
| 10. | Geraniol | 154.25 g/mol | Leaf | Hydrodistillation, GC-MS | [38] |
| 11. | Carvone | 150.22 g/mol | Leaf | Hydrodistillation 3 h, GC-MS | [39] |
| 12. | Myrtenol | 152.23 g/mol | Leaf | Hydrodistillation, GC-MS | [40] |
| 13. | Fenchone | 152.23 g/mol | Aerial parts | Maceration 24 h, GC-MS Hydrodistillation 4 h, GC-MS | [35] [28] |
| Oxygenated Monoterpene | |||||
| 14. | l-Menthol | 156.26 g/mol | Leaf | Hydrodistillation, GC-MS | [34] |
| 15. | Linalool | 154.25 g/mol | Leaf | Maceration, GC-MS Hydrodistillation, GC-MS | [32] [38] |
| 16. | trans-linalool oxide | 170.25 g/mol | Leaf | Maceration, GC-MS | [32] |
| 17. | cis-Linalool-oxide | 213.27 g/mol | Leaf | Hydrodistillation, GC-MS Maceration, GC-MS | [34] [32] |
| 18. | Camphor | 152.23 g/mol | Leaf | Maceration, GC-MS Maceration 24 h, GC-MS SFME, hydrodistillation 1 h, GC-MS | [32] [35] [37] |
| 19. | Neral | 152.23 g/mol | Aerial parts | Maceration 24 h, GC-MS SFME, hydrodistillation 1 h, GC-MS | [35] [37] |
| 20. | 1,8-cineole (Eucalyptol) | 154.25 g/mol | Leaf | Hydrodistillation, GC-MS Maceration, GC-MS Hydrodistillation, solvent extraction, GC-MS | [34] [32] [36] |
| 21. | Estragole (Methyl chavicol) | 148.20 g/mol | Leaf, flower, inflorescence | Hydrodistillation, GC-MS Hydrodistillation 3 h, GC-MS Maceration 24 h, GC-MS Hydrodistillation, solvent extraction, GC-MS | [34] [39] [35] [36] |
| 22. | Eugenol | 164.20 g/mol | Leaf, aerial parts | Hydrodistillation, GC-MS Maceration 24 h, GC-MS SFME, hydrodistillation 1 h, GC-MS | [34] [35] [37] |
| 23. | Methyl eugenol | 173.23 g/mol | Leaf | Hydrodistillation 3 h, GC-MS Maceration 24 h, GC-MS | [39] [35] |
| 24. | Bornyl acetate | 196.29 g/mol | Aerial parts | Hydrodistillation 4 h, GC-MS | [28] |
| 25. | Methyl cinnamate | 162.18 g/mol | Leaf | Hydrodistillation, GC-MS | [40] |
| Sesquiterpene Hydrocarbon | |||||
| 26. | Copaene | 204.35 g/mol | Leaf | Hydrodistillation, GC-MS | [34] |
| 27. | Neoisolongifolene | 202.33 g/mol | Leaf | Hydrodistillation, GC-MS | [34] |
| 28. | α-Bergamotene | 204.35 g/mol | Leaf | SFME, hydrodistillation 1 h, GC-MS | [37] |
| 29. | trans-.alpha.-Bergamotene | 204.35 g/mol | Leaf | Hydrodistillation, GC-MS Hydrodistillation, solvent extraction, GC-MS Hydrodistillation, GC-MS | [34] [36] [38] |
| 30. | β-farnesene | 204.35 g/mol | Leaf | Hydrodistillation, GC-MS | [38] |
| 31. | Alloaromadendrene | 204.35 g/mol | Leaf | Hydrodistillation, GC-MS | [34] |
| 32. | γ-Cadinene | 204.35 g/mol | Leaf | SFME, hydrodistillation 1 h, GC-MS | [37] |
| 33. | Humulene | 204.35 g/mol | Leaf | Hydrodistillation, GC-MS | [34] |
| 34. | α-Humulene | 204.35 g/mol | Leaf | SFME, hydrodistillation 1 h, GC-MS | [37] |
| 35. | α-Copaene | 204.35 g/mol | Leaf | SFME, hydrodistillation 1 h, GC-MS | [37] |
| 36. | β-Copaene | 204.35 g/mol | Leaf | Hydrodistillation, GC-MS | [34] |
| 37. | β-Bisabolene | 204.35 g/mol | Leaf | Hydrodistillation, GC-MS | [34] |
| 38. | cis-muurola-3,5-diene | 204.35 g/mol | Leaf | Hydrodistillation, GC-MS | [34] |
| 39. | cis-.alpha.-Bisabolene | 204.35 g/mol | Leaf | Hydrodistillation, GC-MS | [34] |
| 40. | α-Cubebene | 204.35 g/mol | Leaf, aerial parts | Hydrodistillation, GC-MS Maceration 24 h, GC-MS Hydrodistillation, solvent extraction, GC-MS | [40] [35] [36] |
| 41. | Germacrene B | 204.35 g/mol | Leaf | Hydrodistillation, GC-MS | [40] |
| 42. | Germacrene D | 204.35 g/mol | Leaf | Hydrodistillation, GC-MS Hydrodistillation 4 h, GC-MS Maceration 24 h, GC-MS Hydrodistillation, GC-MS | [40] [28] [35] [38] |
| 43. | β-Elemene | 204.35 g/mol | Leaf | SFME, hydrodistillation 1 h, GC-MS | [37] |
| 44. | β-Cubebene | 204.35 g/mol | Aerial parts | Maceration 24 h, GC-MS Hydrodistillation, solvent extraction, GC-MS | [35] [36] |
| 45. | β-Caryophyllene | 204.35 g/mol | Aerial parts | Maceration 24 h, GC-MS Hydrodistillation, GC-MS SFME, hydrodistillation 1 h, GC-MS | [35] [38] [37] |
| Oxygenated Sesquiterpene | |||||
| 46. | α-Bisabolol | 222.37 g/mol | Aerial parts | Hydrodistillation 4 h, GC-MS | [28] |
| 47. | α-Cadinol | 222.37 g/mol | Aerial parts | Hydrodistillation 4 h, GC-MS | [28] |
| 48. | Nerolidol | 222.37 g/mol | Leaf | SFME, hydrodistillation 1 h, GC-MS | [37] |
| 49. | Caryophyllene oxide | 220.35 g/mol | Aerial parts | Maceration 24 h, GC-MS | [35] |
| Other Compounds | |||||
| 50. | trans-4-Methoxycinnamaldehyde | 162.18 g/mol | Leaf | Hydrodistillation, GC-MS | [34] |
| 51. | Mandelic Acid (Benzeneacetic acid, alpha.-hydroxy) | 152.15 g/mol | Leaf | Hydrodistillation, GC-MS | [34] |
| 52. | Phenylethanolamine | 137.18 g/mol | Leaf | Hydrodistillation, GC-MS | [34] |
| 53. | N-Benzyl-N-ethyl-p-isopropylbenzamide | 281.4 g/mol | Leaf | Hydrodistillation, GC-MS | [34] |
| 54. | cis-2-(2-pentenyl) furan | 136.19 g/mol | Leaf | Maceration, GC-MS | [32] |
| Bacterial Species | Essential Oil/Extract | MIC Value | Reference |
|---|---|---|---|
| Gram Positive | |||
| Bacillus cereus (ATCC 11778) | Essential oil Essential oil and methanolic extract | 10.80 µL/mL 62.5 µg/mL | [92] [93] |
| Bacillus subtilis | Essential oil and methanolic extract | 125 µg/mL | [93] |
| Bacillus megaterium | Methanolic extract | 62.5 µg/mL | [93] |
| Enterococcus faecalis (ATCC 19433) | Essential oil | 0.75 mg/mL | [94] |
| Listeria monocytogenes | Essential oil and methanolic extract | 125 µg/mL | [93] |
| Micrococcus luteus (ATCC 10240) | Essential oil | 0.50 mg/mL | [94] |
| Sarcina sp. | Essential oil | 0.75 mg/mL | [94] |
| Staphylococcus aureus (ATCC 6538P) | Essential oil Essential oil Essential oil Essential oil and methanolic extract | 2.45 µL/mL 32 µg/mL 1 mg/mL 62.5 µg/mL | [92] [95] [94] [93] |
| Staphylococcus epidermidis | Essential oil | 0.75 mg/mL | [94] |
| Streptococcus mutans | Essential oil | 0.75 mg/mL | [94] |
| Gram Negative | |||
| Acinetobacter sp. | Essential oil | 0.75 mg/mL | [94] |
| Aeromonas sp. | Essential oil | 1 mg/mL | [94] |
| Citrobacter freundii (ATCC 8090) | Essential oil | 1 mg/mL | [94] |
| Escherichia coli (ATCC 25922) | Essential oil Methanolic extract | 10.80 µL/mL 125 µg/mL | [92] [93] |
| Klebsiella pneumoniae (ATCC 13833) | Essential oil | 0.75 mg/mL | [94] |
| Proteus mirabilis (ATCC 25933) | Essential oil | 1 mg/mL | [94] |
| P. vulgaris (ATCC 13315) | Essential oil | 0.75 mg/mL | [94] |
| Pseudomonas aeruginosa (ATCC 27853) P. aeruginosa (ATCC 25853) P. aeruginosa (1662339) | Essential oil Essential oil Essential oil | 22.68 µL/mL 256 µg/mL 32 µg/mL | [92] [95] [95] |
| Salmonella choleraesuis (ATCC 10708) | Essential oil | 0.5 mg/mL | [94] |
| Salmonella typhimurium (ATCC 14028) | Essential oil | 22.68 µL/mL | [92] |
| Serratia marcescens (ATCC 13880) | Essential oil | 0.25 mg/mL | [94] |
| Shigella boydii | Essential oil | 250 µg/mL | [93] |
| Shigella dysenteriae | Essential oil and methanolic extract | 250 µg/mL | [93] |
| Shigella flexneri (ATCC 12022) | Essential oil | 0.75 mg/mL | [94] |
| Vibrio parahaemolyticus | Essential oil | 250 µg/mL | [93] |
| Vibrio mimicus | Essential oil | 250 µg/mL | [93] |
| Yersinia enterocolitica (ATCC 10460) | Essential oil | 0.25 mg/mL | [94] |
| Bacterial Species | Essential Oil/Extract | Diameter of Zone Inhibition | Reference |
|---|---|---|---|
| Gram Positive | |||
| Bacillus cereus | Essential oil Ethyl acetate fraction | 25 mm 21.1 mm | [96] [93] |
| Bacillus subtilis | Ethyl acetate fraction Methanolic extract | 19.3 mm 31.86 mm | [93] [97] |
| Bacillus megaterium | Ethyl acetate fraction | 18.2 mm | [93] |
| Clostridium perfringens type C | Methanolic extract | 31.13 mm | [97] |
| Cutibacterium acnes (ATCC 11827) | Essential oil | 18.13 mm | [38] |
| Enterococcus sp. | Methanolic extract | 30.73 mm | [97] |
| Enterococcus faecalis (ATCC 19433) | Essential oil Essential oil | 10.3 mm 11.2 mm | [94] [98] |
| Listeria monocytogenes | Essential oil | 17.1 mm | [93] |
| Micrococcus luteus (ATCC 10240) | Essential oil | 13.5 mm | [94] |
| Sarcina sp. | Essential oil | 14.6 mm | [94] |
| Staphylococcus aureus (ATCC 6538) S. aureus (ATCC 6538) S. aureus (ATCC 25923) S. aureus | Essential oil Ethyl acetate fraction Essential oil Methanolic extract | 9 mm 17.1 mm 9.7 mm 30.66 mm | [96] [93] [98] [97] |
| Staphylococcus epidermidis (ATCC 12228) | Essential oil | 13.3 mm | [98] |
| Staphylococcus mutans (ATCC 25175) | Essential oil | 11 mm | [94] |
| Gram Negative | |||
| Acinetobacter sp. | Essential oil | 15 mm | [94] |
| Aeromonas sp. | Essential oil | 10.6 mm | [94] |
| Citrobacter freundii (ATCC 8090) | Essential oil | 11.6 mm | [94] |
| Escherichia coli E. coli E. coli E. coli E. coli (ATCC 25922) | Essential oil Essential oil Ethyl acetate fraction Methanolic extract Essential oil | 11 mm 10.3 mm 14.2 mm 28.30 mm 13.5 mm | [96] [94] [93] [97] [98] |
| Klebsiella pneumoniae | Essential oil Essential oil Methanolic extract | 12.2 mm 17.2 mm 26.66 mm | [94] [98] [97] |
| Proteus mirabilis (ATCC 25933) | Essential oil Essential oil | 11.3 mm 13.1 mm | [94] [98] |
| Proteus vulgaris (ATCC 13315) | Essential oil | 18 mm | [94] |
| Pseudomonas aeruginosa | Methanolic extract | 28.83 mm | [97] |
| Salmonella choleraesuis (ATCC 10708) | Essential oil | 10 mm | [94] |
| Salmonella typhymurium | Essential oil Methanolic extract | 10 mm 15.30 mm | [96] [97] |
| Serratia marcescens (ATCC 13880) | Essential oil Essential oil | 16.6 mm 10.4 mm | [94] [98] |
| Shigella boydii | Essential oil | 13.3 mm | [93] |
| Shigella dysenteriae | Ethyl acetate fraction | 15.2 mm | [93] |
| Shigella flexneri (ATCC 12022) | Essential oil | 17.1 mm | [94] |
| Vibrio parahaemolyticus | Ethyl acetate fraction | 16.2 mm | [93] |
| Vibrio mimicus | Methanolic extract | 51.2 mm | [93] |
| Xanthomonas sp. | Methanolic extract | 14.36 mm | [97] |
| Yersinia enterocolitica (ATCC 10460) | Essential oil | 12.6 mm | [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azizah, N.S.; Irawan, B.; Kusmoro, J.; Safriansyah, W.; Farabi, K.; Oktavia, D.; Doni, F.; Miranti, M. Sweet Basil (Ocimum basilicum L.)―A Review of Its Botany, Phytochemistry, Pharmacological Activities, and Biotechnological Development. Plants 2023, 12, 4148. https://doi.org/10.3390/plants12244148
Azizah NS, Irawan B, Kusmoro J, Safriansyah W, Farabi K, Oktavia D, Doni F, Miranti M. Sweet Basil (Ocimum basilicum L.)―A Review of Its Botany, Phytochemistry, Pharmacological Activities, and Biotechnological Development. Plants. 2023; 12(24):4148. https://doi.org/10.3390/plants12244148
Chicago/Turabian StyleAzizah, Nabilah Sekar, Budi Irawan, Joko Kusmoro, Wahyu Safriansyah, Kindi Farabi, Dina Oktavia, Febri Doni, and Mia Miranti. 2023. "Sweet Basil (Ocimum basilicum L.)―A Review of Its Botany, Phytochemistry, Pharmacological Activities, and Biotechnological Development" Plants 12, no. 24: 4148. https://doi.org/10.3390/plants12244148
APA StyleAzizah, N. S., Irawan, B., Kusmoro, J., Safriansyah, W., Farabi, K., Oktavia, D., Doni, F., & Miranti, M. (2023). Sweet Basil (Ocimum basilicum L.)―A Review of Its Botany, Phytochemistry, Pharmacological Activities, and Biotechnological Development. Plants, 12(24), 4148. https://doi.org/10.3390/plants12244148












