Fusarium Wilt Invasion Results in a Strong Impact on Strawberry Microbiomes
Abstract
:1. Introduction
2. Results
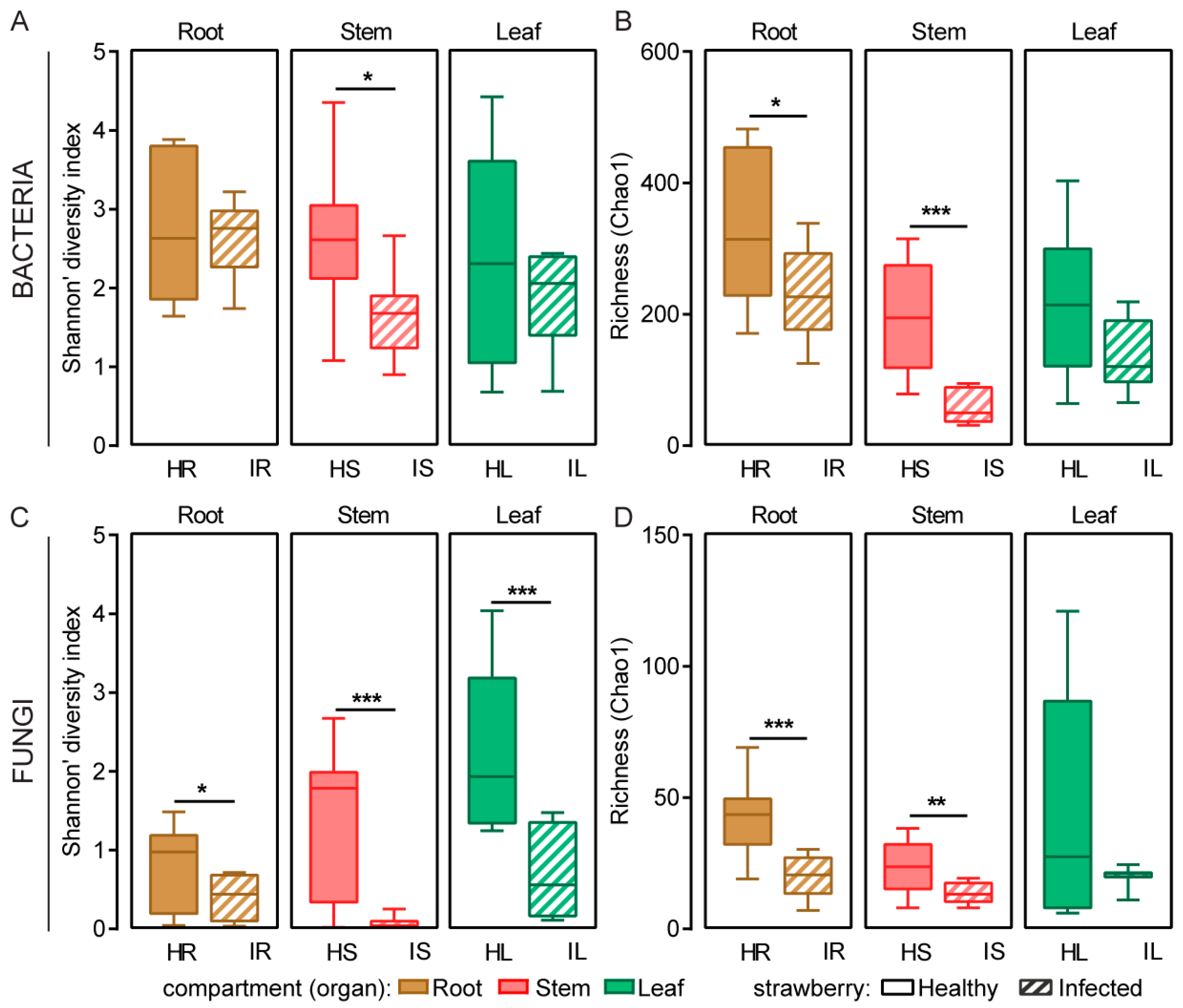
2.1. Microbial Community Diversity and Structure of Healthy and Infected Samples
2.2. Differences in Bacterial and Fungal Taxa in Healthy and Infected Samples
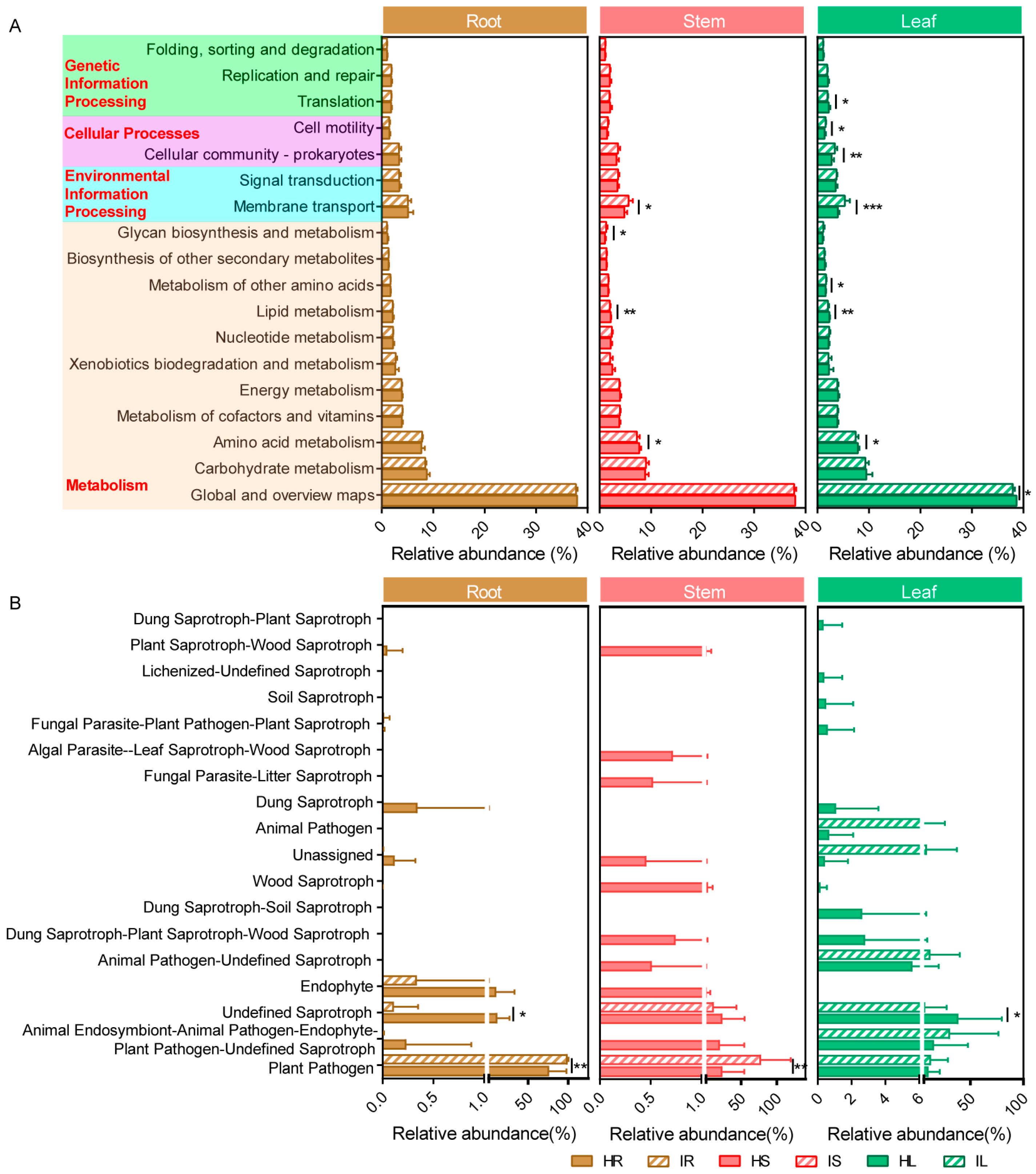
2.3. Potential Bacterial Metabolic Function and Fungal Functional Guilds of Healthy and Infected Samples
2.4. Differences in Fungal Isolation Taxa between Healthy and Infected Samples
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Sample Preparation and DNA Extraction
4.3. PCR Amplification and Illumina MiSeq Sequencing
4.4. Amplicon Sequence Processing and Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khare, E.; Mishra, J.; Arora, N.K. Multifaceted interactions between endophytes and plant: Developments and prospects. Front. Microbiol. 2018, 9, 2732. [Google Scholar] [CrossRef]
- Tian, Q.; Gong, Y.; Liu, S.; Ji, M.; Tang, R.; Kong, D.; Xue, Z.; Wang, L.; Hu, F.; Huang, L.; et al. Endophytic bacterial communities in wild rice (Oryza officinalis) and their plant growth-promoting effects on perennial rice. Front. Plant Sci. 2023, 14, 1184489. [Google Scholar] [CrossRef] [PubMed]
- Saati-Santamaria, Z.; Vicentefranqueira, R.; Kolarik, M.; Rivas, R.; Garcia-Fraile, P. Microbiome specificity and fluxes between two distant plant taxa in Iberian forests. Environ. Microbiome 2023, 18, 64. [Google Scholar] [CrossRef] [PubMed]
- Wulandari, A.P.; Triani, E.; Sari, K.; Prasetyani, M.; Nurzaman, M.; Purwati, R.D.; Ermawar, R.A.; Nuraini, A. Endophytic microbiome of Boehmeria nivea and their antagonism against latent fungal pathogens in plants. BMC Microbiol. 2022, 22, 320. [Google Scholar] [CrossRef] [PubMed]
- Omomowo, O.I.; Babalola, O.O. Bacterial and fungal endophytes: Tiny giants with immense beneficial potential for plant growth and sustainable agricultural productivity. Microorganisms 2019, 7, 481. [Google Scholar] [CrossRef] [PubMed]
- Chi, F.; Shen, S.H.; Cheng, H.P.; Jing, Y.X.; Yanni, Y.G.; Dazzo, F.B. Ascending migration of endophytic rhizobia, from roots to leaves, inside rice plants and assessment of benefits to rice growth physiology. Appl. Environ. Microbiol. 2005, 71, 7271–7278. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; Ye, W.W.; Yu, Z.; Shen, W.L.; Li, S.Z.; Wang, X.; Chen, J.J.; Wang, Y.C.; Zheng, X.B. Host niche, genotype, and field location shape the diversity and composition of the soybean microbiome. J. Integr. Agric. 2023, 22, 2412–2425. [Google Scholar] [CrossRef]
- Beckers, B.; Op De Beeck, M.; Weyens, N.; Boerjan, W.; Vangronsveld, J. Structural variability and niche differentiation in the rhizosphere and endosphere bacterial microbiome of field-grown poplar trees. Microbiome 2017, 5, 25. [Google Scholar] [CrossRef]
- Cregger, M.A.; Veach, A.M.; Yang, Z.K.; Crouch, M.J.; Vilgalys, R.; Tuskan, G.A.; Schadt, C.W. The Populus holobiont: Dissecting the effects of plant niches and genotype on the microbiome. Microbiome 2018, 6, 31. [Google Scholar] [CrossRef]
- Hu, Q.; Tan, L.; Gu, S.; Xiao, Y.; Xiong, X.; Zeng, W.A.; Feng, K.; Wei, Z.; Deng, Y. Network analysis infers the wilt pathogen invasion associated with non-detrimental bacteria. NPJ Biofilms Microbiomes 2020, 6, 8. [Google Scholar] [CrossRef]
- Saad, M.M.; Eida, A.A.; Hirt, H. Tailoring plant-associated microbial inoculants in agriculture: A roadmap for successful application. J. Exp. Bot. 2020, 71, 3878–3901. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, B.S.; Babalola, O.O. The endosphere microbial communities, a great promise in agriculture. Int. Microbiol. 2021, 24, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, L.; Qiu, J.; Qian, Y.; Wang, M. Comparative metabolomic analysis of the nutritional aspects from ten cultivars of the strawberry fruit. Foods 2023, 12, 1153. [Google Scholar] [CrossRef] [PubMed]
- Denoyes, B.; Prohaska, A.; Petit, J.; Rothan, C.; Lunn, J. Deciphering the genetic architecture of fruit color in strawberry. J. Exp. Bot. 2023, 74, 6306–6320. [Google Scholar] [CrossRef] [PubMed]
- Cho, G.; Kwak, Y.-S. Genetic variation of strawberry Fusarium wilt pathogen population in Korea. Mycobiology 2022, 50, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Khan, M.H.; Yuan, Z.; Hussain, S.; Cao, H.; Liu, Y. Response of soil microbiome structure and its network profiles to four soil amendments in monocropping strawberry greenhouse. PLoS ONE 2021, 16, e0245180. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Calabria, J.; Chen, H.; Somssich, M. The Arabidopsis thaliana–Fusarium oxysporum strain 5176 pathosystem: An overview. J. Exp. Bot. 2022, 73, 6052–6067. [Google Scholar] [CrossRef]
- Edel-Hermann, V.; Lecomte, C. Current status of Fusarium oxysporum formae speciales and races. Phytopathology 2019, 109, 512–530. [Google Scholar] [CrossRef]
- Zuriegat, Q.; Zheng, Y.; Liu, H.; Wang, Z.; Yun, Y. Current progress on pathogenicity-related transcription factors in Fusarium oxysporum. Mol. Plant Pathol. 2021, 22, 882–895. [Google Scholar] [CrossRef]
- Henry, P.M.; Pincot, D.D.A.; Jenner, B.N.; Borrero, C.; Aviles, M.; Nam, M.H.; Epstein, L.; Knapp, S.J.; Gordon, T.R. Horizontal chromosome transfer and independent evolution drive diversification in Fusarium oxysporum f. sp. fragariae. New Phytol. 2021, 230, 327–340. [Google Scholar] [CrossRef]
- Henry, P.M.; Pincot, D.D.A.; Jenner, B.N.; Borrero, C.; Aviles, M.; Nam, M.H.; Epstein, L.; Knapp, S.J.; Gordon, T.R. The population of Fusarium oxysporum f. sp. fragariae, cause of Fusarium wilt of strawberry, in California. Plant Dis. 2017, 101, 550–556. [Google Scholar] [CrossRef]
- Tian, G.L.; Bi, Y.M.; Cheng, J.D.; Zhang, F.F.; Zhou, T.H.; Sun, Z.J.; Zhang, L.S. High concentration of ferulic acid in rhizosphere soil accounts for the occurrence of Fusarium wilt during the seedling stages of strawberry plants. Physiol. Mol. Plant Pathol. 2019, 108, 101435. [Google Scholar] [CrossRef]
- Ji, M.; Yao, K.; Li, G.; Wu, X.; Chen, H.; Zhuang, Y. Control effects of Bacillus subtilis DJ-6 and pyraclostrobin alone and in combination against Fusarium oxysporum. Agric. Sci. Technol. 2014, 15, 2020–2025. [Google Scholar]
- Tahat, M.M.; Aldakil, H.; Alananbeh, K.; Othman, Y.; Alsmairat, N. First report of strawberry wilt caused by Fusarium oxysporum Schltdl. in Jordan. Plant Dis. 2022, 107, 967. [Google Scholar] [CrossRef]
- Harsonowati, W.; Marian, M.; Surono; Narisawa, K. The effectiveness of a dark septate endophytic fungus, Cladophialophora chaetospira SK51, to mitigate strawberry Fusarium wilt disease and with growth promotion activities. Front. Microbiol. 2020, 11, 585. [Google Scholar] [CrossRef] [PubMed]
- Pastrana, A.M.; Watson, D.C.; Gordon, T.R. Transmission of Fusarium oxysporum f. sp. fragariae through stolons in strawberry plants. Plant Dis. 2019, 103, 1249–1251. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, X.; Yin, B.; Zhen, W.; Guo, J. Biochemical defenses induced by mycorrhizae fungi Glomus mosseae in controlling strawberry fusarium wilt. Open Biomed. Eng. J. 2015, 9, 301–304. [Google Scholar]
- Su, D.; Chen, S.; Zhou, W.; Yang, J.; Luo, Z.; Zhang, Z.; Tian, Y.; Dong, Q.; Shen, X.; Wei, S.; et al. Comparative analysis of the microbial community structures between healthy and anthracnose-infected strawberry rhizosphere soils using Illumina sequencing technology in Yunnan province, southwest of China. Front. Microbiol. 2022, 13, 881450. [Google Scholar] [CrossRef]
- Yang, J.; Wei, S.; Su, D.; Zhang, Z.; Chen, S.; Luo, Z.; Shen, X.; Lai, Y.; Jamil, A.; Tong, J.; et al. Comparison of the rhizosphere soil microbial community structure and diversity between powdery mildew-infected and noninfected strawberry plants in a greenhouse by high-throughput sequencing technology. Curr. Microbiol. 2020, 77, 1724–1736. [Google Scholar] [CrossRef]
- Zhou, D.; Jing, T.; Chen, Y.; Wang, F.; Qi, D.; Feng, R.; Xie, J.; Li, H. Deciphering microbial diversity associated with Fusarium wilt-diseased and disease-free banana rhizosphere soil. BMC Microbiol. 2019, 19, 161. [Google Scholar] [CrossRef]
- Xue, C.; Penton, C.; Shen, Z.; Zhang, R.; Huang, Q.; Li, R.; Ruan, Y.; Shen, Q. Manipulating the banana rhizosphere microbiome for biological control of Panama disease. Sci. Rep. 2015, 5, 11124. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Li, R.; Ren, Y.; Liu, C.; Zhao, Q.; Wu, H.; Jousset, A.; Shen, Q. Distinct roles for soil fungal and bacterial communities associated with the suppression of vanilla Fusarium wilt disease. Soil. Biol. Biochem. 2017, 107, 198–207. [Google Scholar] [CrossRef]
- Fernandes, L.B.; Ghag, S.B. Molecular insights into the jasmonate signaling and associated defense responses against wilt caused by Fusarium oxysporum. Plant Physiol. Biochem. 2022, 174, 22–34. [Google Scholar] [CrossRef]
- Dongzhen, F.; Xilin, L.; Xiaorong, C.; Wenwu, Y.; Yunlu, H.; Yi, C.; Jia, C.; Zhimin, L.; Litao, G.; Tuhong, W.; et al. Fusarium species and Fusarium oxysporum species complex genotypes associated with yam wilt in south-central China. Front. Microbiol. 2020, 11, 1964. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kaushik, N.; Sharma, A.; Marzouk, T.; Djébali, N. Exploring the potential of endophytes and their metabolites for bio-control activity. 3Biotech 2022, 12, 277. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhu, J.; Zhao, X.; Shi, J.; Jiang, C.; Shao, D. Beneficial effects of endophytic fungi colonization on plants. Appl. Microbiol. Biotechnol. 2019, 103, 3327–3340. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Jiang, H.; Chang, G.; Liang, S.; Ma, K.; Cai, Y.; Tian, B.; Shi, X. Effects of rhizosphere microbial communities on cucumber Fusarium wilt disease suppression. Microorganisms 2023, 11, 1576. [Google Scholar] [CrossRef] [PubMed]
- Kopecky, J.; Samkova, Z.; Sarikhani, E.; Kyselkova, M.; Omelka, M.; Kristufek, V.; Divis, J.; Grundmann, G.G.; Moenne-Loccoz, Y.; Sagova-Mareckova, M. Bacterial, archaeal and micro-eukaryotic communities characterize a disease-suppressive or conducive soil and a cultivar resistant or susceptible to common scab. Sci. Rep. 2019, 9, 14883. [Google Scholar] [CrossRef]
- Lee, S.M.; Kong, H.G.; Song, G.C.; Ryu, C.M. Disruption of Firmicutes and Actinobacteria abundance in tomato rhizosphere causes the incidence of bacterial wilt disease. ISME J. 2021, 15, 330–347. [Google Scholar] [CrossRef]
- Munakata, Y.; Gavira, C.; Genestier, J.; Bourgaud, F.; Hehn, A.; Slezack-Deschaumes, S. Composition and functional comparison of vetiver root endophytic microbiota originating from different geographic locations that show antagonistic activity towards Fusarium graminearum. Microbiol. Res. 2021, 243, 126650. [Google Scholar] [CrossRef]
- Omotayo, O.P.; Babalola, O.O. Fusarium verticillioides of maize plant: Potentials of propitious phytomicrobiome as biocontrol agents. Front. Fungal Biol. 2023, 4, 1095765. [Google Scholar]
- Ballot, A.; Dore, J.; Rey, M.; Meiffren, G.; Langin, T.; Joly, P.; Dreux-Zigha, A.; Taibi, A.; Prigent-Combaret, C. Dimethylpolysulfides production as the major mechanism behind wheat fungal pathogen biocontrol-by Arthrobacter and Microbacterium actinomycetes. Microbiol. Spectr. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Palyzova, A.; Svobodova, K.; Sokolova, L.; Novak, J.; Novotny, C. Metabolic profiling of Fusarium oxysporum f. sp. conglutinans race 2 in dual cultures with biocontrol agents Bacillus amyloliquefaciens, Pseudomonas aeruginosa, and Trichoderma harzianum. Folia Microbiol. 2019, 64, 779–787. [Google Scholar] [CrossRef]
- Deepthi, B.V.; Poornachandra Rao, K.; Chennapa, G.; Naik, M.K.; Chandrashekara, K.T.; Sreenivasa, M.Y. Antifungal attributes of Lactobacillus plantarum MYS6 against fumonisin producing Fusarium proliferatum associated with poultry feeds. PLoS ONE 2016, 11, e0155122. [Google Scholar] [CrossRef] [PubMed]
- Tewari, S.; Sharma, S. Rhizobial-metabolite based biocontrol of fusarium wilt in pigeon pea. Microb. Pathog. 2020, 147, 104278. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhao, Y.; Fu, X.; Yu, C.; Gao, K.; Liu, H. Isolation and identification of Talaromyces sp. strain Q2 and its biocontrol mechanisms involved in the control of Fusarium wilt. Front. Microbiol. 2021, 12, 724842. [Google Scholar] [CrossRef] [PubMed]
- Vats, S.; Saxena, S. Endophytic Fusarium species, a unique bioresource for disaggregator of misfolded alpha-synuclein. Arch. Microbiol. 2023, 205, 224. [Google Scholar] [CrossRef]
- Khalmuratova, I.; Choi, D.H.; Kim, J.G.; Lee, I.S. Endophytic fungi of salt-tolerant plants: Diversity and ability to promote plant growth. J. Microbiol. Biotechnol. 2021, 31, 1526–1532. [Google Scholar] [CrossRef]
- Raza, W.; Ling, N.; Yang, L.; Huang, Q.; Shen, Q. Response of tomato wilt pathogen Ralstonia solanacearum to the volatile organic compounds produced by a biocontrol strain Bacillus amyloliquefaciens SQR-9. Sci. Rep. 2016, 6, 24856. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Y.; Wang, X.; Wang, W. Exogenous regulators enhance the yield and stress resistance of chlamydospores of the biocontrol agent Trichoderma harzianum T4. J. Fungi 2022, 8, 1017. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, X.; Zhang, Z.; Chen, Y.; Tian, Q.; Zeng, D.; Xu, M.; Wang, Y.; Dong, S.; Ma, Z.; et al. Fusarium-produced vitamin B6 promotes the evasion of soybean resistance by Phytophthora sojae. J. Integr. Plant Biol. 2023, 65, 2204–2217. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Q.; Wei, X.; Xue, Q.; Lai, H. Biocontrol effects of Penicillium griseofulvum against monkshood (Aconitum carmichaelii Debx.) root diseases caused by Sclerotium rolfsiii and Fusarium spp. J. Appl. Microbiol. 2019, 127, 1532–1545. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Wang, D.; Mao, Z.; Pan, L.; Liao, J.; Cai, Z. Infection of Plasmodiophora brassicae changes the fungal endophyte community of tumourous stem mustard roots as revealed by high-throughput sequencing and culture-dependent methods. PLoS ONE 2019, 14, e0214975. [Google Scholar] [CrossRef]
- Douanla-Meli, C.; Langer, E.; Talontsi Mouafo, F. Fungal endophyte diversity and community patterns in healthy and yellowing leaves of Citrus limon. Fungal Ecol. 2013, 6, 212–222. [Google Scholar] [CrossRef]
- Niehaus, E.M.; Díaz-Sánchez, V.; von Bargen, K.W.; Kleigrewe, K.; Humpf, H.U.; Limón, M.C.; Tudzynski, B. Fusarins and fusaric acid in fusaria. In Biosynthesis and Molecular Genetics of Fungal Secondary Metabolites; Fungal Ecology Book Series; Springer: New York, NY, USA, 2014; pp. 239–262. [Google Scholar]
- Nag, P.; Paul, S.; Shriti, S.; Das, S. Defence response in plants and animals against a common fungal pathogen, Fusarium oxysporum. Curr. Res. Microb. Sci. 2022, 3, 100135. [Google Scholar] [CrossRef] [PubMed]
- Perincherry, L.; Lalak-Kańczugowska, J.; Stępień, Ł. Fusarium-produced mycotoxins in plant-pathogen Interactions. Toxins 2019, 11, 664. [Google Scholar] [CrossRef] [PubMed]
- Formela-Luboinska, M.; Remlein-Starosta, D.; Waskiewicz, A.; Karolewski, Z.; Bocianowski, J.; Stepien, L.; Labudda, M.; Jeandet, P.; Morkunas, I. The role of saccharides in the mechanisms of pathogenicity of Fusarium oxysporum f. sp. lupini in yellow lupine (Lupinus luteus L.). Int. J. Mol. Sci. 2020, 21, 7258. [Google Scholar] [CrossRef]
- Singh, V.K.; Singh, H.B.; Upadhyay, R.S. Role of fusaric acid in the development of ‘Fusarium wilt’ symptoms in tomato: Physiological, biochemical and proteomic perspectives. Plant Physiol. Biochem. 2017, 118, 320–332. [Google Scholar] [CrossRef]
- Yang, H.; Ye, W.; Ma, J.; Zeng, D.; Rong, Z.; Xu, M.; Wang, Y.; Zheng, X. Endophytic fungal communities associated with field-grown soybean roots and seeds in the Huang-Huai region of China. PeerJ 2018, 6, e4713. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sutton, D.A.; Rinaldi, M.G.; Sarver, B.A.J.; Balajee, S.A.; Schroers, H.-J.; Summerbell, R.C.; Robert, V.A.R.G.; Crous, P.W.; Zhang, N.; et al. Internet-accessible DNA sequence database for identifying fusaria from human and animal infections. J. Clin. Microbiol. 2010, 48, 3708–3718. [Google Scholar] [CrossRef]
- Druzhinina, I.; Kubicek, C.P. Species concepts and biodiversity in Trichoderma and Hypocrea: From aggregate species to species clusters? J. Zhejiang Univ.-Sci. B 2005, 6, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.K.; Wang, X.C.; Zhuang, W.Y.; Cheng, X.H.; Zhao, P. New species of Talaromyces (Fungi) isolated from soil in southwestern China. Biology 2021, 10, 745. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Rott, M.; Schlaeppi, K.; Ver Loren van Themaat, E.; Ahmadinejad, N.; Assenza, F.; Rauf, P.; Huettel, B.; Reinhardt, R.; Schmelzer, E.; et al. Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature 2012, 488, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Schlaeppi, K.; Dombrowski, N.; Oter, R.G.; Ver Loren van Themaat, E.; Schulze-Lefert, P. Quantitative divergence of the bacterial root microbiota in Arabidopsis thaliana relatives. Proc. Natl. Acad. Sci. USA 2014, 111, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Santos-Medellin, C.; Edwards, J.; Liechty, Z.; Nguyen, B.; Sundaresan, V. Drought stress results in a compartment-specific restructuring of the rice root-associated microbiomes. mBio 2017, 8, e00764-17. [Google Scholar] [CrossRef] [PubMed]
- Maarastawi, S.A.; Frindte, K.; Linnartz, M.; Knief, C. Crop rotation and straw application impact microbial communities in Italian and Philippine soils and the rhizosphere of Zea mays. Front. Microbiol. 2018, 9, 1295. [Google Scholar] [CrossRef]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Koljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Zhou, G.; Qiu, X.; Chen, L.; Zhang, C.; Ma, D.; Zhang, J. Succession of organics metabolic function of bacterial community in response to addition of earthworm casts and zeolite in maize straw composting. Bioresour. Technol. 2019, 280, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Toju, H.; Kishida, O.; Katayama, N.; Takagi, K. Networks depicting the fine-scale co-occurrences of fungi in soil horizons. PLoS ONE 2016, 11, 0165987. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Zhang, X.; Qiu, X.; Chen, J.; Wang, Y.; Zhang, G.; Jia, S.; Shen, X.; Ye, W.; Yan, Z. Fusarium Wilt Invasion Results in a Strong Impact on Strawberry Microbiomes. Plants 2023, 12, 4153. https://doi.org/10.3390/plants12244153
Yang H, Zhang X, Qiu X, Chen J, Wang Y, Zhang G, Jia S, Shen X, Ye W, Yan Z. Fusarium Wilt Invasion Results in a Strong Impact on Strawberry Microbiomes. Plants. 2023; 12(24):4153. https://doi.org/10.3390/plants12244153
Chicago/Turabian StyleYang, Hongjun, Xu Zhang, Xiaohong Qiu, Jiajia Chen, Yuanhua Wang, Geng Zhang, Sizhen Jia, Xiangqi Shen, Wenwu Ye, and Zhiming Yan. 2023. "Fusarium Wilt Invasion Results in a Strong Impact on Strawberry Microbiomes" Plants 12, no. 24: 4153. https://doi.org/10.3390/plants12244153
APA StyleYang, H., Zhang, X., Qiu, X., Chen, J., Wang, Y., Zhang, G., Jia, S., Shen, X., Ye, W., & Yan, Z. (2023). Fusarium Wilt Invasion Results in a Strong Impact on Strawberry Microbiomes. Plants, 12(24), 4153. https://doi.org/10.3390/plants12244153






