A Critical Gap in Seagrass Protection: Impact of Anthropogenic Off-Shore Nutrient Discharges on Deep Posidonia oceanica Meadows
Abstract
:1. Introduction
2. Results
2.1. Univariate Analyses
2.1.1. Variations in Abiotic Factors
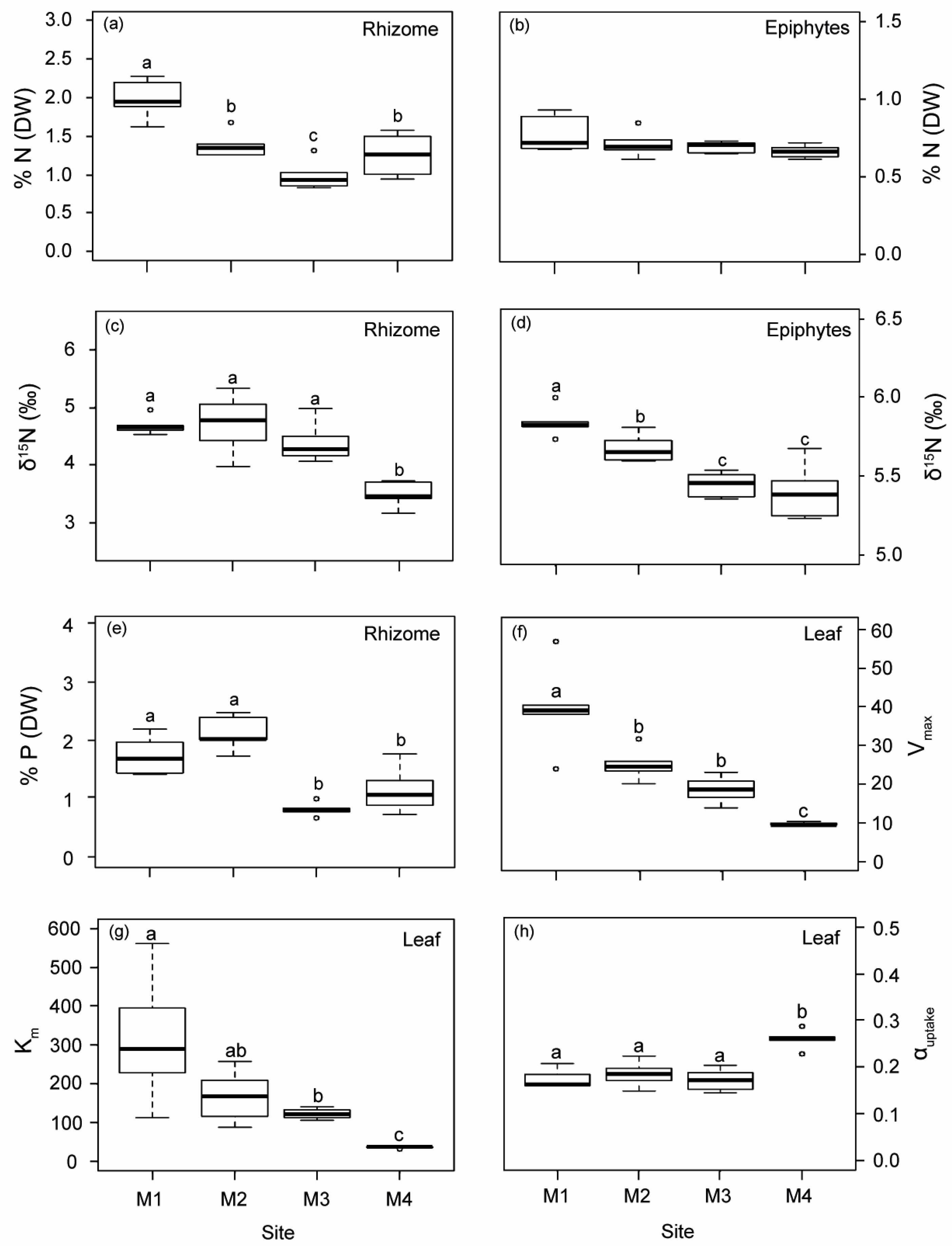
2.1.2. Variations of Biological Indicators of Anthropogenic Pressure
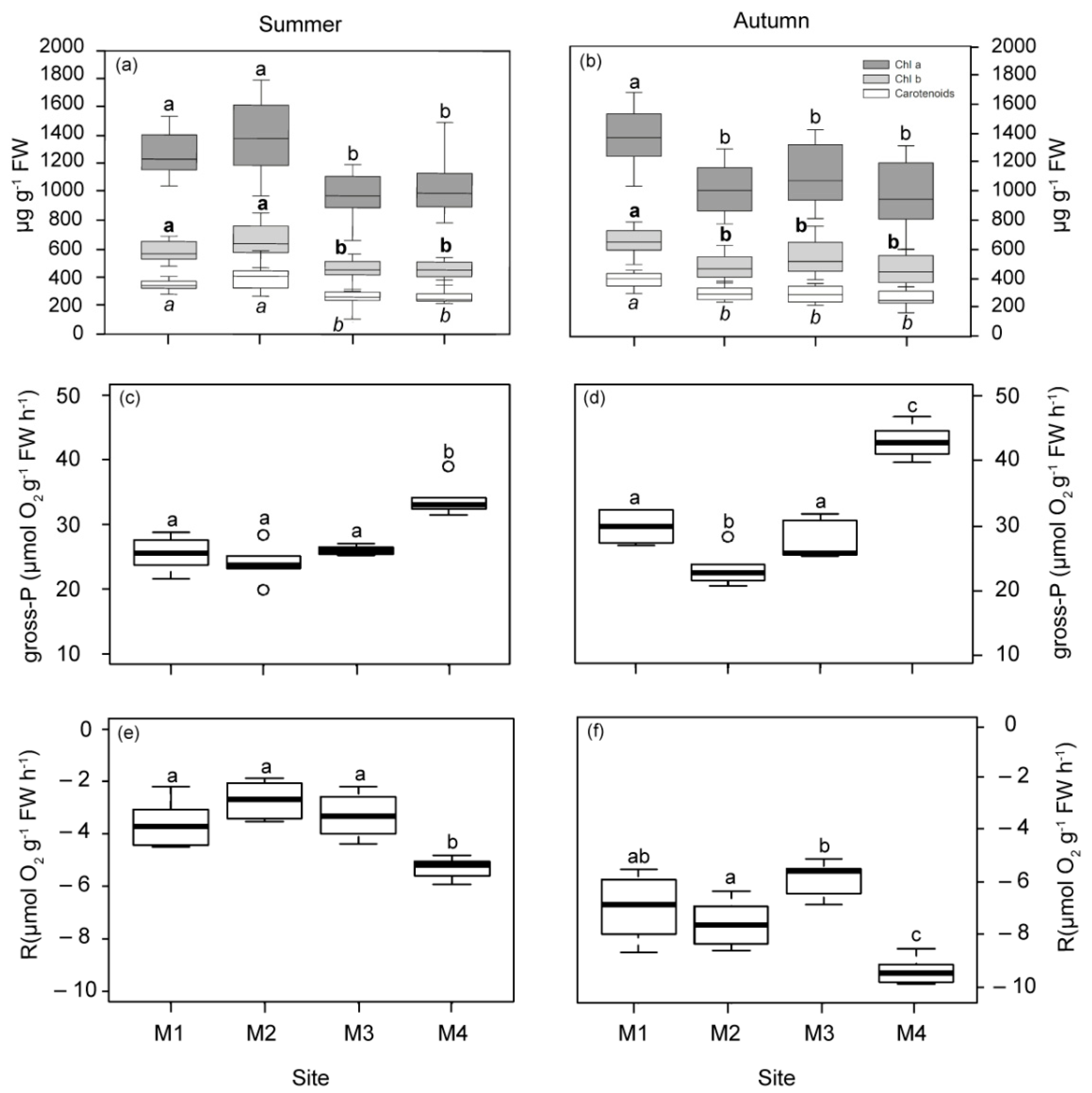
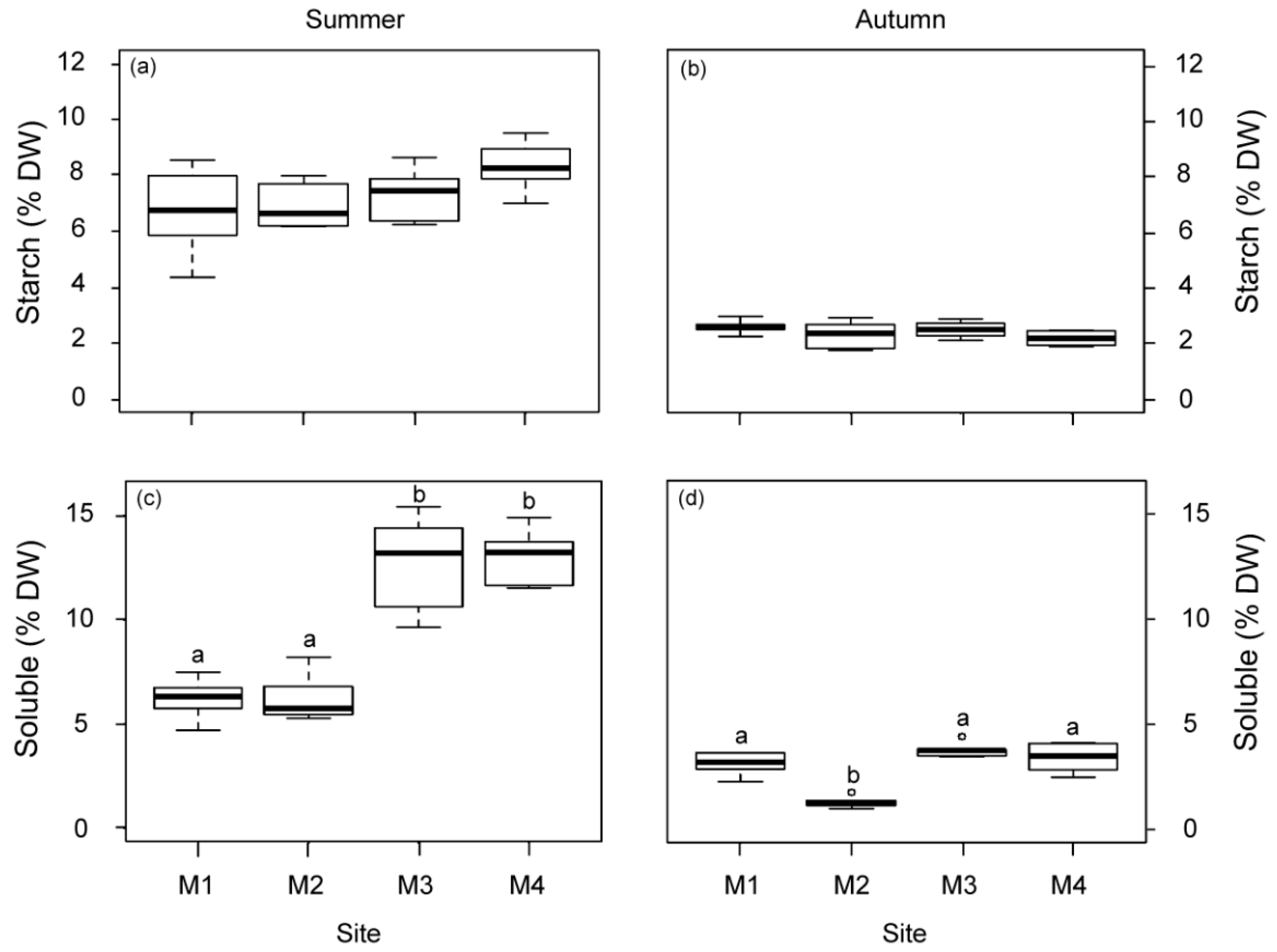
2.1.3. Physiological Responses of P. oceanica Plants
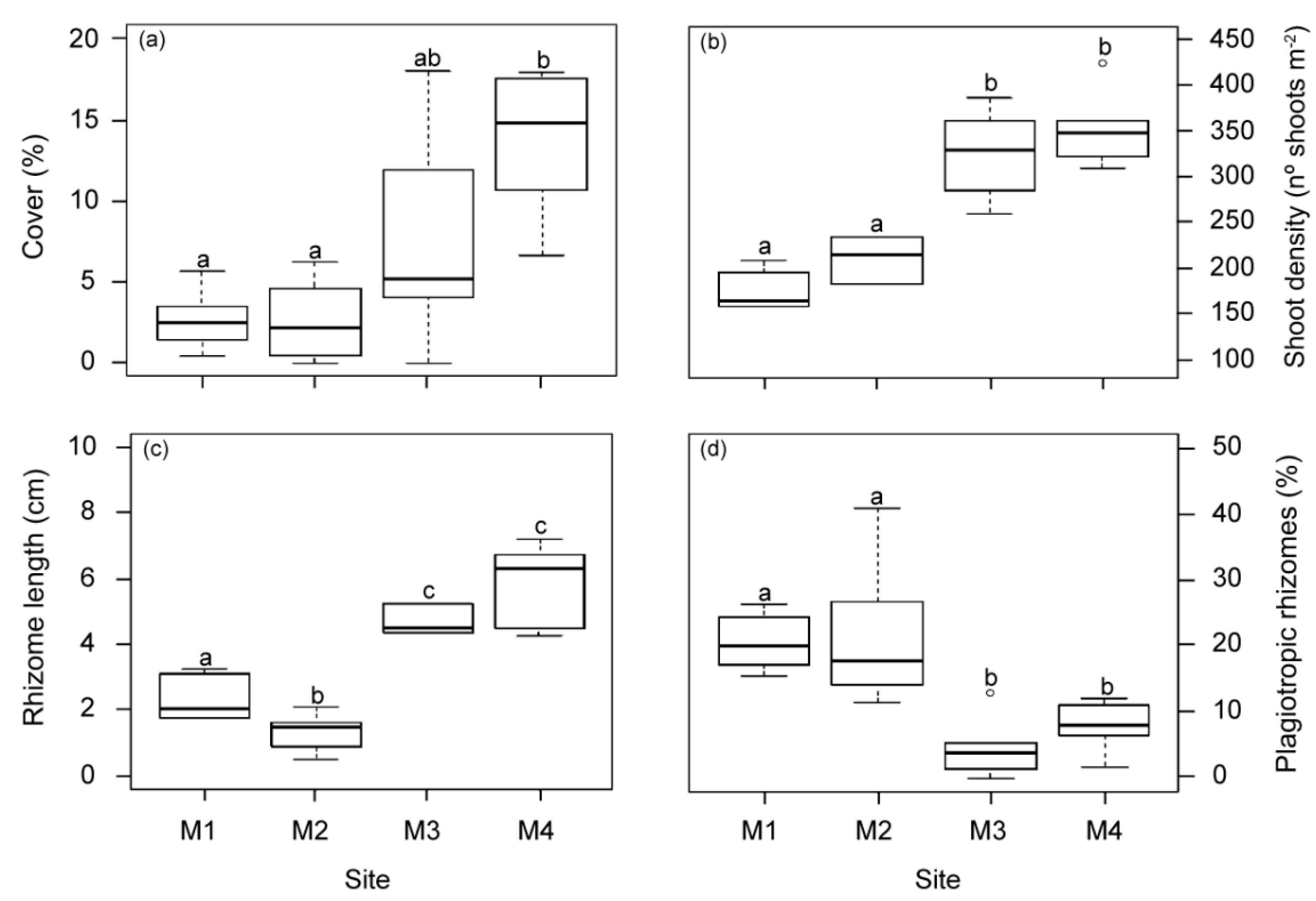
2.1.4. Changes at the Structural Meadow Level
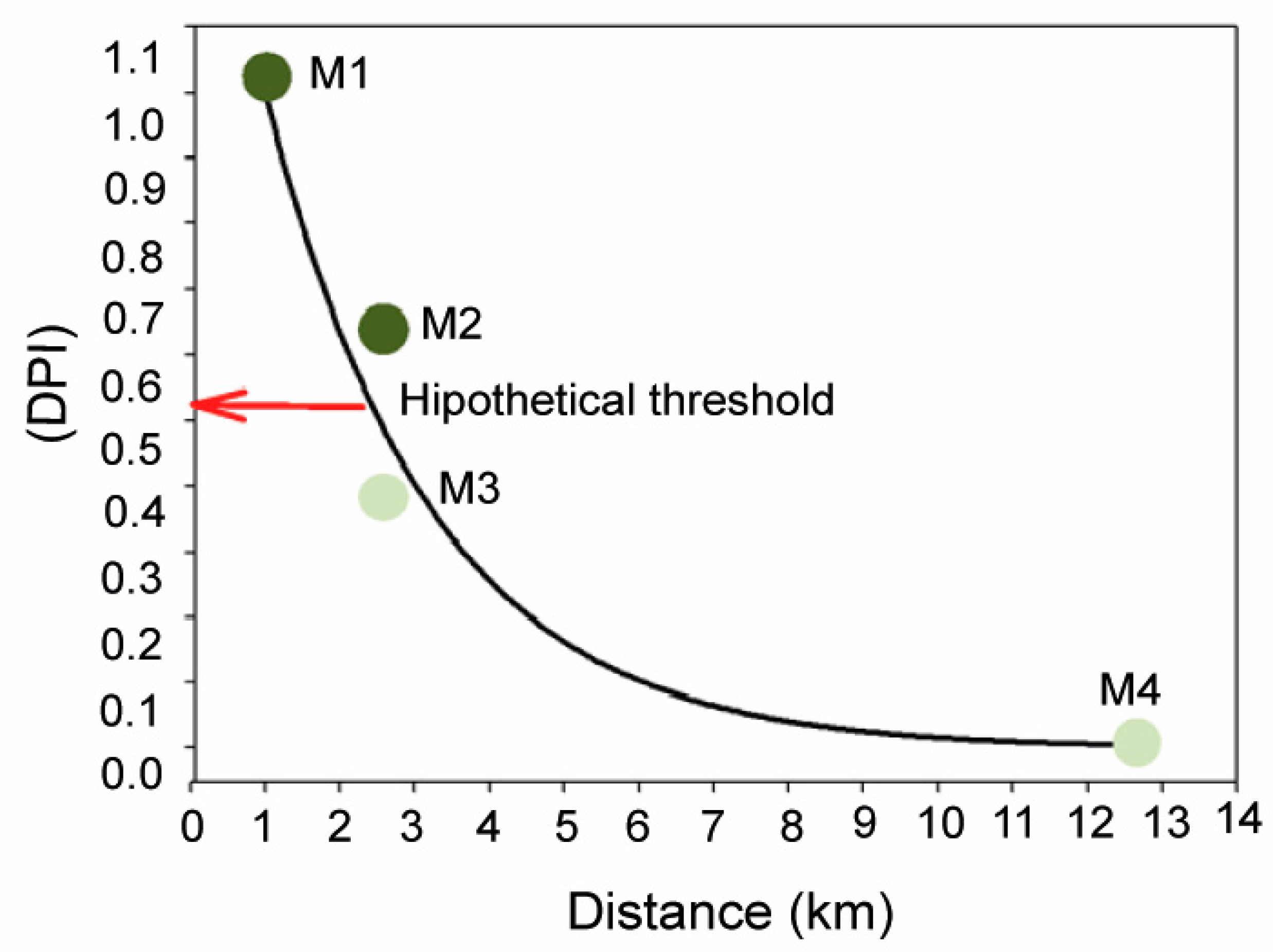
2.1.5. Synthesis of Variable Responses
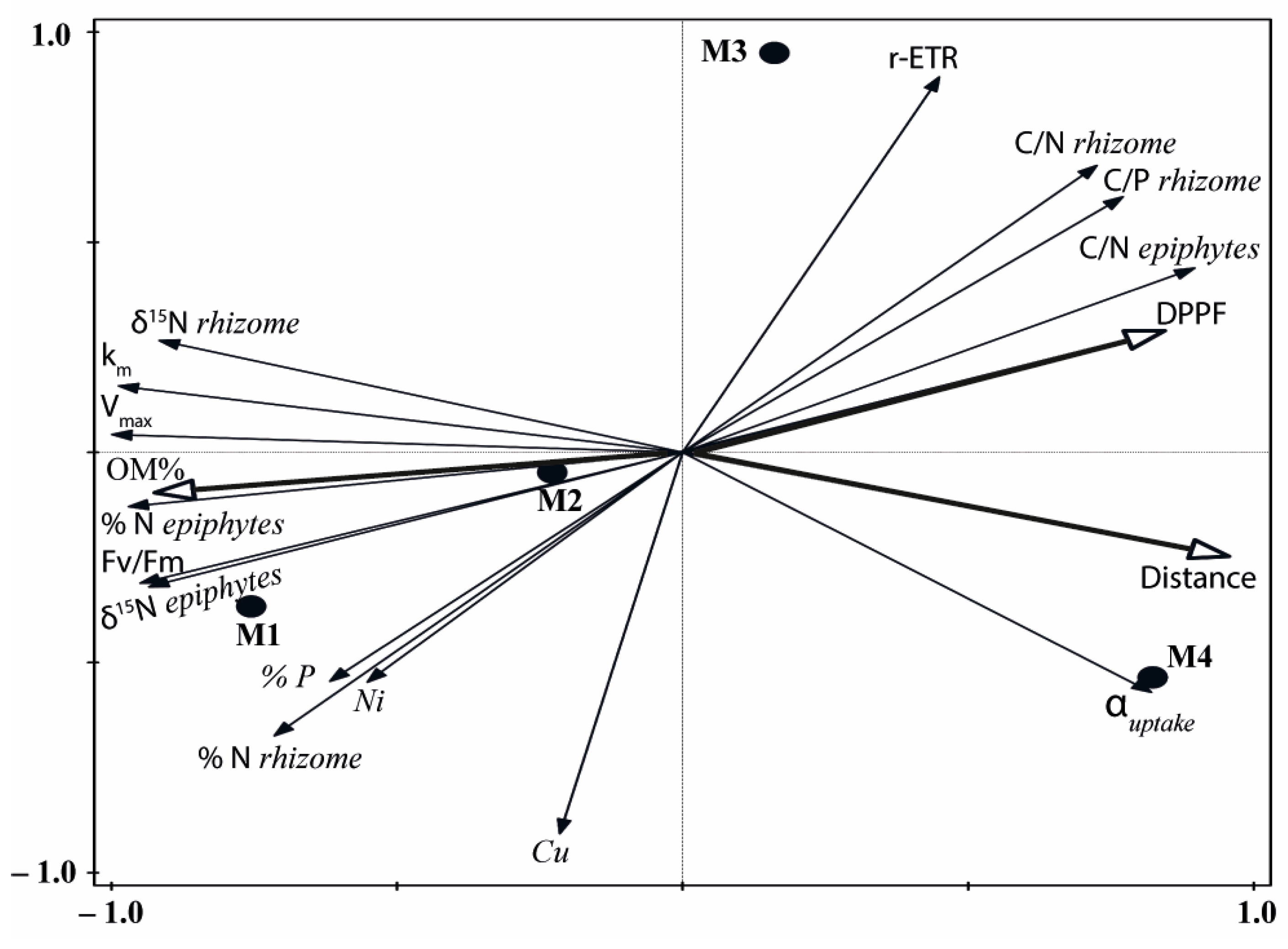
2.2. Multivariate Analysis
3. Discussion
4. Materials and Methods
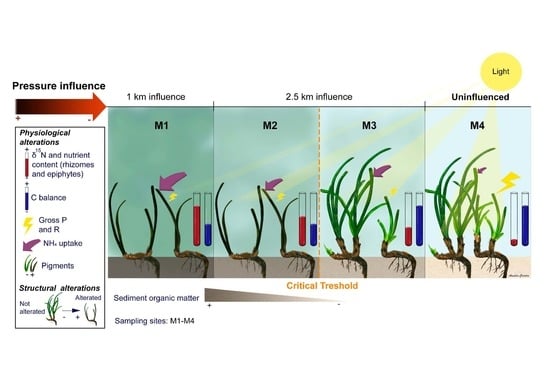
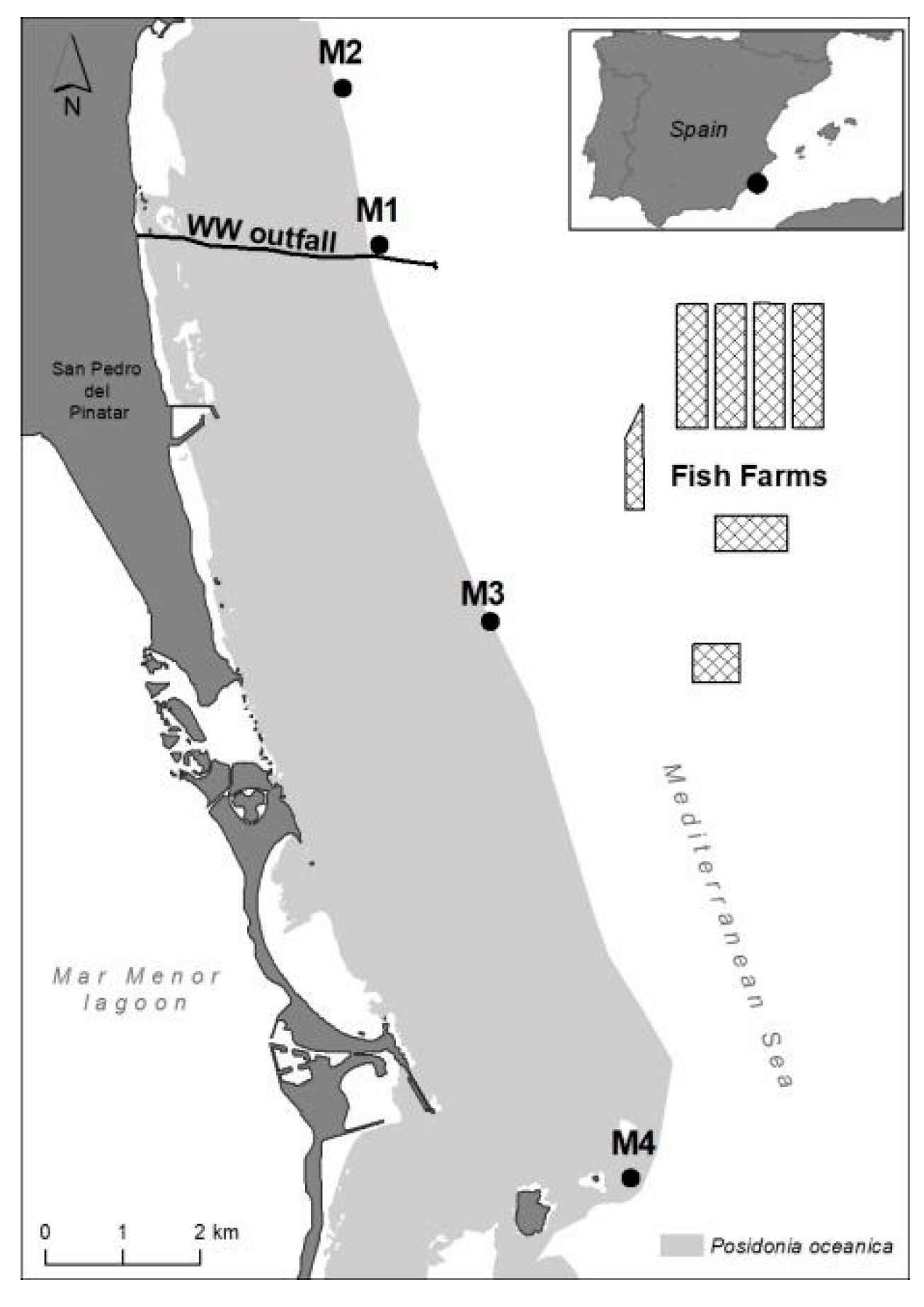
4.1. Study Site and Sampling Design
4.2. Abiotic Factors
4.3. Seagrass Bioindicators of Anthropogenic Pressure
4.4. P. oceanica Physiological Features
4.5. Meadow Structure Descriptors
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Small, C.; Nicholls, R.J. A Global Analysis of Human Settlement in Coastal Zones. J. Coast. Res. 2003, 19, 584–599. [Google Scholar]
- McGranahan, G.; Balk, D.; Anderson, B. The rising tide: Assessing the risks of climate change and human settlements in low elevation coastal zones. Environ. Urban. 2007, 19, 17–37. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Aber, J.D.; Howarth, R.W.; Likens, G.E.; Matson, P.A.; Schindler, D.W.; Schlesinger, W.H.; Tilman, D.G. Human alteration of the global nitrogen cycle: Sources and consequences. Ecol. Appl. 1997, 7, 737–750. [Google Scholar] [CrossRef] [Green Version]
- McGlathery, K.J.; Sundbäck, K.; Anderson, I.C. Eutrophication in shallow coastal bays and lagoons: The role of plants in the coastal filter. Mar. Ecol. Prog. Ser. 2007, 348, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Nixon, S.W.; Fulweiler, R.W. Nutrient Pollution, Eutrophication, and the Degradation of Coastal Marine Ecosystems. Glob. Loss Coast. Habitats: Rates Causes Conseq. 2009, 87–120. [Google Scholar]
- Tomascik, T.; Sander, F. Effects of eutrophication on reef-building corals. Mar. Biol. 1987, 94, 53–75. [Google Scholar] [CrossRef]
- Hemminga, M.; Duarte, C. Seagrass Ecology-Marten A. Hemminga, Carlos M. Duarte; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Short, F.T.; Wyllie-Echeverria, S. Natural and human-induced disturbance of seagrasses. Environ. Conserv. 1996, 23, 17–27. [Google Scholar] [CrossRef]
- Cambridge, M.L.; McComb, A.J. The loss of seagrasses in Cockburn Sound, Western Australia. I. The time course and magnitude of seagrass decline in relation to industrial development. Aquat. Bot. 1984, 20, 229–243. [Google Scholar] [CrossRef]
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.B.; Orth, R.J.; Dennison, W.C.; Olyarnik, S.; Calladine, A.; Fourqurean, J.W.; Heck, K.L., Jr.; Hughes, A.R.; et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12377–12381. [Google Scholar] [CrossRef] [Green Version]
- Boudouresque, C.F.; Bernard, G.; Pergent, G.; Shili, A.; Verlaque, M. Regression of Mediterranean seagrasses caused by natural processes and anthropogenic disturbances and stress: A critical review. Bot. Mar. 2009, 52, 395–418. [Google Scholar] [CrossRef]
- Cancemi, G.; De Falco, G.; Pergent, G. Effects of organic matter input from a fish farming facility on a Posidonia oceanica meadow. Estuarine Coast. Shelf Sci. 2003, 56, 961–968. [Google Scholar] [CrossRef]
- Invers, O.; Kraemer, G.P.; Pérez, M.; Romero, J. Effects of nitrogen addition on nitrogen metabolism and carbon reserves in the temperate seagrass Posidonia oceanica. J. Exp. Mar. Biol. Ecol. 2004, 303, 97–114. [Google Scholar] [CrossRef]
- Pergent-Martini, C.; Boudouresque, C.-F.; Pasqualini, V.; Pergent, G. Impact of fish farming facilities on Posidonia oceanica meadows: A review. Mar. Ecol. 2006, 27, 310–319. [Google Scholar] [CrossRef]
- Delgado, O.; Ruiz, J.; Pérez, M.; Romero, J.; Ballesteros, E. Effects of fish farming on seagrass (Posidonia oceanica) in a Mediterranean bay: Seagrass decline after organic loading cessation. Oceanol. Acta 1999, 22, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Orth, R.J.; Carruthers, T.J.B.; Dennison, W.C.; Duarte, C.M.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; Kendrick, G.A.; Kenworthy, W.J.; Olyarnik, S.; et al. A Global Crisis for Seagrass Ecosystems; Oxford Academic: Oxford, UK, 2006; Volume 56. [Google Scholar] [CrossRef] [Green Version]
- Burkholder, J.M.; Tomasko, D.A.; Touchette, B.W. Seagrasses and eutrophication. J. Exp. Mar. Biol. Ecol. 2007, 350, 46–72. [Google Scholar] [CrossRef]
- Boumaza, S.; Boudefoua, N.; Boumaza, R.; Semroud, R. Effects of urban effluents on spatial structure, morphology and total phenols of Posidonia oceanica: Comparison with a reference site. J. Exp. Mar. Biol. Ecol. 2014, 457, 113–119. [Google Scholar] [CrossRef]
- Blanco-Murillo, F.; Jimenez-Gutierrez, S.; Martínez-Vidal, J.; Guillén, J.E.; Sánchez-Lizaso, J.L. Spatiotemporal Trends Observed in 20 Years of Posidonia oceanica Monitoring along the Alicante Coast, Spain. Water 2022, 14, 274. [Google Scholar] [CrossRef]
- González-Correa, J.M.; Bayle Sempere, J.T.; Sánchez-Jerez, P.; Valle, C. Posidonia oceanica meadows are not declining globally. Analysis of population dynamics in marine protected areas of the Mediterranean Sea. Mar. Ecol. Prog. Ser. 2007, 336, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Holon, F.; Mouquet, N.; Boissery, P.; Bouchoucha, M.; Delaruelle, G.; Tribot, A.-S.; Deter, J. Fine-Scale Cartography of Human Impacts along French Mediterranean Coasts: A Relevant Map for the Management of Marine Ecosystems. PLoS ONE 2015, 10, e0135473. [Google Scholar] [CrossRef] [Green Version]
- Ruíz, J.M.; Pérez, M.; Romero, J.; Tomas, F. The importance of herbivory in the decline of a seagrass (Posidonia oceanica) meadow near a fish farm: An experimental approach. Bot. Mar. 2009, 52, 449–458. [Google Scholar] [CrossRef]
- Holon, F.; Boissery, P.; Guilbert, A.; Freschet, E.; Deter, J. The impact of 85 years of coastal development on shallow seagrass beds (Posidonia oceanica L. (Delile)) in South Eastern France: A slow but steady loss without recovery. Estuarine Coast. Shelf Sci. 2015, 165, 204–212. [Google Scholar] [CrossRef]
- Dennison, W.; Alberte, R. Role of daily light period in the depth distribution of Zostera marina (eelgrass). Mar. Ecol. Prog. Ser. 1985, 25, 51–61. [Google Scholar] [CrossRef]
- Tuya, F.; Ribeiro-Leite, L.; Arto-Cuesta, N.; Coca, J.; Haroun, R.; Espino, F. Decadal changes in the structure of Cymodocea nodosa seagrass meadows: Natural vs. human influences. Estuarine Coast. Shelf Sci. 2014, 137, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Ralph, P.J.; Durako, M.J.; Enríquez, S.; Collier, C.J.; Doblin, M.A. Impact of light limitation on seagrasses. J. Exp. Mar. Biol. Ecol. 2007, 350, 176–193. [Google Scholar] [CrossRef]
- Collier, C.J.; Lavery, P.S.; Ralph, P.J.; Masini, R.J. Shade-induced response and recovery of the seagrass Posidonia sinuosa. J. Exp. Mar. Biol. Ecol. 2009, 370, 89–103. [Google Scholar] [CrossRef]
- Basurco, B.; Larrazabal, G. Marine Fish Farming in Spain. Option Mediterr. Int. Cent. Adv. Mediterr. Agron. Stud. 2000, 30, 45–56. [Google Scholar]
- Ruiz, J.M.; Marco-Méndez, C.; Sánchez-Lizaso, J.L. Remote influence of off-shore fish farm waste on Mediterranean seagrass (Posidonia oceanica) meadows. Mar. Environ. Res. 2010, 69, 118–126. [Google Scholar] [CrossRef]
- Ferreira, J.G.; Andersen, J.H.; Borja, Á.; Bricker, S.B.; Camp, J.; Cardoso da Silva, M.; Garcés, E.; Heiskanen, A.S.; Humborg, C.; Ignatiades, L.; et al. Marine Strategy Framework Directive: A Tool for Eutrophication Assessment and Management in Marine Waters. EUR 2010, 24338, 49. [Google Scholar] [CrossRef]
- Telesca, L.; Belluscio, A.; Criscoli, A.; Ardizzone, G.; Apostolaki, E.T.; Fraschetti, S.; Gristina, M.; Knittweis, L.; Martin, C.S.; Pergent, G.; et al. Seagrass meadows (Posidonia oceanica) distribution and trajectories of change. Sci. Rep. 2015, 5, 12505. [Google Scholar] [CrossRef] [Green Version]
- Boudouresque, C.-F.; Blanfuné, A.; Pergent, G.; Pergent-Martini, C.; Perret-Boudouresque, M.; Thibaut, T. Impacts of Marine and Lagoon Aquaculture on Macrophytes in Mediterranean Benthic Ecosystems. Front. Mar. Sci. 2020, 7, 218. [Google Scholar] [CrossRef]
- Holon, F.; Marre, G.; Parravicini, V.; Mouquet, N.; Bockel, T.; Descamp, P.; Tribot, A.-S.; Boissery, P.; Deter, J. A predictive model based on multiple coastal anthropogenic pressures explains the degradation status of a marine ecosystem: Implications for management and conservation. Biol. Conserv. 2018, 222, 125–135. [Google Scholar] [CrossRef] [Green Version]
- Gilarranz, L.J.; Narwani, A.; Odermatt, D.; Siber, R.; Dakos, V. Regime shifts, trends, and variability of lake productivity at a global scale. Proc. Natl. Acad. Sci. USA 2022, 119, e2116413119. [Google Scholar] [CrossRef] [PubMed]
- Dattolo, E.; Marín-Guirao, L.; Ruiz, J.M.; Procaccini, G. Long-Term Acclimation to Reciprocal Light Conditions Suggests Depth-Related Selection in the Marine Foundation Species Posidonia Oceanica. Ecol. Evol. 2017, 7, 1148–1164. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, M.; Carpenter, S.; Foley, J.A.; Folke, C.; Walker, B. Catastrophic shifts in ecosystems. Nature 2001, 413, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P. Catastrophes, Phase Shifts, and Large-Scale Degradation of a Caribbean Coral Reef. Science 1994, 265, 1547–1551. [Google Scholar] [CrossRef] [Green Version]
- Scheffer, M.; Carpenter, S.R. Catastrophic regime shifts in ecosystems: Linking theory to observation. Trends Ecol. Evol. 2003, 18, 648–656. [Google Scholar] [CrossRef]
- Montefalcone, M.; Parravicini, V.; Bianchi, C.N. Quantification of Coastal Ecosystem Resilience. Treatise Estuar. Coast. Sci. 2012, 10, 49–70. [Google Scholar] [CrossRef]
- Gera, A.; Pagès, J.F.; Arthur, R.; Farina, S.; Roca, G.; Romero, J.; Alcoverro, T. The effect of a centenary storm on the long-lived seagrass Posidonia Oceanica. Limnol. Oceanogr. 2014, 59, 1910–1918. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, J.M.; Pérez, M.; Romero, J. Effects of Fish Farm Loadings on Seagrass (Posidonia oceanica) Distribution, Growth and Photosynthesis. Mar. Pollut. Bull. 2001, 42, 749–760. [Google Scholar] [CrossRef]
- Cabaço, S.; Machás, R.; Vieira, V.; Santos, R. Impacts of urban wastewater discharge on seagrass meadows (Zostera noltii). Estuarine Coast. Shelf Sci. 2008, 78, 1–13. [Google Scholar] [CrossRef]
- Holmer, M.; Argyrou, M.; Dalsgaard, T.; Danovaro, R.; Diaz-Almela, E.; Duarte, C.M.; Frederiksen, M.; Grau, A.; Karakassis, I.; Marbà, N.; et al. Effects of fish farm waste on Posidonia oceanica meadows: Synthesis and provision of monitoring and management tools. Mar. Pollut. Bull. 2008, 56, 1618–1629. [Google Scholar] [CrossRef] [Green Version]
- García-Sanz, T.; Ruiz, J.M.; Pérez, M.; Ruiz, M. Assessment of dissolved nutrients dispersal derived from offshore fish-farm using nitrogen stable isotope ratios (Δ15N) in macroalgal bioassays. Estuar. Coast. Shelf Sci. 2011, 91, 361–370. [Google Scholar] [CrossRef]
- Thomsen, E.; Herbeck, L.S.; Jennerjahn, T.C. The End of Resilience: Surpassed Nitrogen Thresholds in Coastal Waters Led to Severe Seagrass Loss after Decades of Exposure to Aquaculture Effluents: Long-Term Aquaculture Effluents Exposure Causes Seagrass Loss. Mar. Environ. Res. 2020, 160, 104986. [Google Scholar] [CrossRef]
- Jones, B.L.; Cullen-Unsworth, L.C.; Unsworth, R.K.F. Tracking Nitrogen Source Using Δ15 N Reveals Human and Agricultural Drivers of Seagrass Degradation across the British Isles. Front. Plant Sci. 2018, 9, 133. [Google Scholar] [CrossRef] [Green Version]
- Schubert, P.R.; Karez, R.; Reusch, T.B.H.; Dierking, J. Isotopic signatures of eelgrass (Zostera marina L.) as bioindicator of anthropogenic nutrient input in the western Baltic Sea. Mar. Pollut. Bull. 2013, 72, 64–70. [Google Scholar] [CrossRef]
- Rattan, R.K.; Datta, S.P.; Chhonkar, P.K.; Suribabu, K.; Singh, A.K. Long-term impact of irrigation with sewage effluents on heavy metal content in soils, crops and groundwater—A case study. Agric. Ecosyst. Environ. 2005, 109, 310–322. [Google Scholar] [CrossRef]
- Dean, R.J.; Shimmield, T.M.; Black, K.D. Copper, zinc and cadmium in marine cage fish farm sediments: An extensive survey. Environ. Pollut. 2007, 145, 84–95. [Google Scholar] [CrossRef]
- Leoni, V.; Vela, A.; Pasqualini, V.; Pergent-Martini, C.; Pergent, G. Effects of experimental reduction of light and nutrient enrichments (N and P) on seagrasses: A review. Aquat. Conserv. Mar. Freshw. Ecosyst. 2008, 18, 202–220. [Google Scholar] [CrossRef]
- Pérez, M.; García, T.; Invers, O.; Ruiz, J.M. Physiological responses of the seagrass Posidonia oceanica as indicators of fish farm impact. Mar. Pollut. Bull. 2008, 56, 869–879. [Google Scholar] [CrossRef]
- Apostolaki, E.T.; Vizzini, S.; Karakassis, I. Leaf vs. epiphyte nitrogen uptake in a nutrient enriched Mediterranean seagrass (Posidonia oceanica) meadow. Aquat. Bot. 2012, 96, 58–62. [Google Scholar] [CrossRef]
- Udy, J.; Dennison, W. Physiological responses of seagrasses used to identify anthropogenic nutrient inputs. Mar. Freshw. Res. 1997, 48, 605–614. [Google Scholar] [CrossRef]
- Lapointe, B.E.; Barile, P.J.; Matzie, W.R. Anthropogenic nutrient enrichment of seagrass and coral reef communities in the Lower Florida Keys: Discrimination of local versus regional nitrogen sources. J. Exp. Mar. Biol. Ecol. 2004, 308, 23–58. [Google Scholar] [CrossRef]
- Bruland, G.L.; MacKenzie, R.A. Nitrogen Source Tracking with δ15 N Content of Coastal Wetland Plants in Hawaii. J. Environ. Qual. 2010, 39, 409–419. [Google Scholar] [CrossRef]
- Vieira, R.; Martin, A.; Engelen, A.H.; Thomsen, M.S.; Arenas, F. Interactive effects of co-occurring anthropogenic stressors on the seagrass, Zostera noltei. Ecol. Indic. 2019, 109, 105780. [Google Scholar] [CrossRef]
- Short, F.T.; Burdick, D.M.; Kaldy, J.E. Mesocosm experiments quantify the effects of eutrophication on eelgrass, Zostera marina. Limnol. Oceanogr. 1995, 40, 740–749. [Google Scholar] [CrossRef] [Green Version]
- Hauxwell, J.; Cebrián, J.; Valiela, I. Eelgrass Zostera marina loss in temperate estuaries: Relationship to land-derived nitrogen loads and effect of light limitation imposed by algae. Mar. Ecol. Prog. Ser. 2003, 247, 59–73. [Google Scholar] [CrossRef] [Green Version]
- Lapointe, B.E.; Herren, L.W.; Brewton, R.A.; Alderman, P.K. Nutrient over-enrichment and light limitation of seagrass communities in the Indian River Lagoon, an urbanized subtropical estuary. Sci. Total. Environ. 2020, 699, 134068. [Google Scholar] [CrossRef]
- Ralph, P.J.; Tomasko, D.; Moore, K.; Seddon, S.; Macinnis-Ng, C.M.O. Human Impacts on Seagrasses: Eutrophication, Sedimentation, and Contamination. In Seagrasses: Biology, Ecology and Conservation; Springer: Cham, The Netherlands, 2006; pp. 567–593. [Google Scholar] [CrossRef]
- Pergent, G.; Mendez, S.; Pergent-Martini, C.; Pasqualini, V. Preliminary data on the impact of fish farming facilities on Posidonia oceanica meadows in the Mediterranean. Oceanol. Acta 1999, 22, 95–107. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, J.M.; Romero, J. Effects of in situ experimental shading on the Mediterranean seagrass Posidonia oceanica. Mar. Ecol. Prog. Ser. 2001, 215, 107–120. [Google Scholar] [CrossRef]
- Duarte, C.M. Seagrass depth limits. Aquat. Bot. 1991, 40, 363–377. [Google Scholar] [CrossRef]
- GEAM-IEO. Red de Seguimiento Científico de Las Praderas de Posidonia Oceanica y Cambio Climático En La Región de Murcia: Síntesis de Resultados; Informe científico-técnico de la anualidad 2018; Instituto Español de Oceanografía, Centro Oceanográfico de Murcia: Murcia, Spain, 2019; p. 148. [Google Scholar]
- Ruiz, J.M.; Romero, J. Effects of disturbances caused by coastal constructions on spatial structure, growth dynamics and photosynthesis of the seagrass Posidonia oceanica. Mar. Pollut. Bull. 2003, 46, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Beer, S.; Bjork, M.; Beardall, J. Photosynthesis in the Marine Environment; John Wiley & Sons: New York, NY, USA, 2014. [Google Scholar]
- Gera, A.; Alcoverro, T.; Mascaró, O.; Pérez, M.; Romero, J. Exploring the utility of Posidonia oceanica chlorophyll fluorescence as an indicator of water quality within the European Water Framework Directive. Environ. Monit. Assess. 2011, 184, 3675–3686. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A. Photosynthesis in Aquatic Plants. In Ecophysiology of Photosynthesis; Springer: Berlin/Heidelberg, Germany, 1995; pp. 299–318. [Google Scholar] [CrossRef]
- Dennison, W.C.; Alberte, R.S. Photosynthetic responses of Zostera marina L. (Eelgrass) to in situ manipulations of light intensity. Oecologia 1982, 55, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Ralph, P.J.; Burchett, M.D. Photosynthetic response of Halophila ovalis to heavy metal stress. Environ. Pollut. 1998, 103, 91–101. [Google Scholar] [CrossRef]
- Moustakas, M.; Malea, P.; Charitonidou, K.; Sperdouli, I. Copper bioaccumulation, photosystem II functioning, and oxidative stress in the seagrass Cymodocea nodosa exposed to copper oxide nanoparticles. Environ. Sci. Pollut. Res. 2017, 24, 16007–16018. [Google Scholar] [CrossRef]
- Sánchez-Lizaso, J.L.; De la Ossa-Carretero, J.A.; Del Pilar-Ruso, Y.; Fernández-Torquemada, Y.; Martínez-García, E.; Marco-Méndez, C. Programa de Vigilancia Ambiental Del Vertido de Las Desalinizadoras I y II Del Nuevo Canal de Cartagena En San Pedro Del Pinatar; Report n° 10; Department Of Marine Sciences and Applied Biology of the University of Alicante: Alicante, Spain, 2015. [Google Scholar]
- Alcoverro, T.; Manzanera, M.; Romero, J. Annual metabolic carbon balance of the seagrass Posidonia oceanica: The importance of carbohydrate reserves. Mar. Ecol. Prog. Ser. 2001, 211, 105–116. [Google Scholar] [CrossRef]
- Olesen, B.; Enríquez, S.; Duarte, C.M.; Sand-Jensen, K. Depth-acclimation of photosynthesis, morphology and demography of Posidonia oceanica and Cymodocea nodosa in the Spanish Mediterranean Sea. Mar. Ecol. Prog. Ser. 2002, 236, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Collier, C.J.; Lavery, P.S.; Masini, R.J.; Ralph, P.J. Morphological, growth and meadow characteristics of the seagrass Posidonia sinuosa along a depth-related gradient of light availability. Mar. Ecol. Prog. Ser. 2007, 337, 103–115. [Google Scholar] [CrossRef] [Green Version]
- Ruocco, M.; Entrambasaguas, L.; Dattolo, E.; Milito, A.; Marín-Guirao, L.; Procaccini, G. A king and vassals’ tale: Molecular signatures of clonal integration in Posidonia oceanica under chronic light shortage. J. Ecol. 2020, 109, 294–312. [Google Scholar] [CrossRef]
- Olivé, I.; García-Sánchez, M.P.; Brun, F.G.; Vergara, J.J.; Pérez-Lloréns, J.L. Interactions of light and organic matter under contrasting resource simulated environments: The importance of clonal traits in the seagrass Zostera noltii. Hydrobiologia 2009, 629, 199–208. [Google Scholar] [CrossRef]
- Van Katwijk, M.; Vergeer, L.; Schmitz, G.; Roelofs, J. Ammonium toxicity in eelgrass Zostera marina. Mar. Ecol. Prog. Ser. 1997, 157, 159–173. [Google Scholar] [CrossRef]
- Touchette, B.W.; Burkholder, J.A.M. Review of nitrogen and phosphorus metabolism in seagrasses. J. Exp. Mar. Biol. Ecol. 2000, 250, 133–167. [Google Scholar] [CrossRef] [PubMed]
- Villazán, B.; Pedersen, M.F.; Brun, F.G.; Vergara, J.J. Elevated ammonium concentrations and low light form a dangerous synergy for eelgrass Zostera marina. Mar. Ecol. Prog. Ser. 2013, 493, 141–154. [Google Scholar] [CrossRef] [Green Version]
- Van Der Heide, T.; Smolders, A.J.P.; Rijkens, B.G.A.; Van Nes, E.H.; Van Katwijk, M.M.; Roelofs, J.G.M. Toxicity of reduced nitrogen in eelgrass (Zostera marina) is highly dependent on shoot density and pH. Oecologia 2008, 158, 411–419. [Google Scholar] [CrossRef] [Green Version]
- Sarà, G.; Scilipoti, D.; Mazzola, A.; Modica, A. Effects of fish farming waste to sedimentary and particulate organic matter in a southern Mediterranean area (Gulf of Castellammare, Sicily): A multiple stable isotope study (Δ13C and Δ15N). Aquaculture 2004, 234, 199–213. [Google Scholar] [CrossRef]
- McGlathery, K.J.; Reidenbach, M.A.; D’Odorico, P.; Fagherazzi, S.; Pace, M.L.; Porter, J.H. Nonlinear Dynamics and Alternative Stable States in Shallow Coastal Systems. Oceanography 2012, 25, 56–67. [Google Scholar] [CrossRef]
- Marín-Guirao, L.; Bernardeau-Esteller, J.; Belando, M.D.; García-Muñoz, R.; Ramos-Segura, A.; Alcoverro, T.; Minguito-Frutos, M.; Ruiz, J.M. Photo-acclimatory thresholds anticipate sudden shifts in seagrass ecosystem state under reduced light conditions. Mar. Environ. Res. 2022, 177, 105636. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Fernández, F. Análisis y Mejora de La Fluidodinámica En Instalaciones Depuradoras de Aguas Residuales; Universidad Politécnica de Cartagena: Cartagena, Spain, 2019. [Google Scholar]
- Ruiz, J.M.; Guillén, J.E.; Ramos-Segura, A.; Otero, M.M. Atlas de Las Praderas Marinas de España/IEO/IEL/UICN/Murcia-Alicante-Málaga; Instituto de Ecologia Litoral: El Campello, Spain, 2015. [Google Scholar]
- Marín-Guirao, L.; Ruiz, J.M.; Dattolo, E.; Garcia-Munoz, R.; Procaccini, G. Physiological and molecular evidence of differential short-term heat tolerance in Mediterranean seagrasses. Sci. Rep. 2016, 6, 28615. [Google Scholar] [CrossRef] [Green Version]
- Kramer, M.G. Delaware Geological Survey Laboratory Procedures Manual; Delaware Geological Survey: Newark, DE, USA, 1987. [Google Scholar]
- Holmer, M.; Georgiev, V.G.; Karamfilov, V.K. Effects of point source of untreated sewage waters on seagrass (Zostera marina and Z. noltii) beds in the South-Western Black Sea. Aquat. Bot. 2016, 133, 1–9. [Google Scholar] [CrossRef]
- Sandoval-Gil, J.M.; Camacho-Ibar, V.F.; del Carmen Ávila-López, M.; Hernández-López, J.; Zertuche-González, J.A.; Cabello-Pasini, A. Dissolved inorganic nitrogen uptake kinetics and Δ15N of Zostera marina L. (eelgrass) in a coastal lagoon with oyster aquaculture and upwelling influence. J. Exp. Mar. Biol. Ecol. 2015, 472, 1–13. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Marín-Guirao, L.; Sandoval-Gil, J.M.; Ruíz, J.M.; Sánchez-Lizaso, J.L. Photosynthesis, growth and survival of the Mediterranean seagrass Posidonia oceanica in response to simulated salinity increases in a laboratory mesocosm system. Estuarine Coast. Shelf Sci. 2011, 92, 286–296. [Google Scholar] [CrossRef]
- Marín-Guirao, L.; Sandoval-Gil, J.M.; Bernardeau-Esteller, J.; Ruíz, J.M.; Sánchez-Lizaso, J.L. Responses of the Mediterranean seagrass Posidonia oceanica to hypersaline stress duration and recovery. Mar. Environ. Res. 2013, 84, 60–75. [Google Scholar] [CrossRef]
- Alcoverro, T.; Zimmerman, R.C.; Kohrs, D.G.; Alberte, R.S. Resource allocation and sucrose mobilization in light-limited eelgrass Zostera marina. Mar. Ecol. Prog. Ser. 1999, 187, 121–131. [Google Scholar] [CrossRef] [Green Version]
- Baly, E.C.C. The Kinetics of Photosynthesis. Proc. R. Soc. London. Ser. B Biol. Sci. 1935, 117, 218–239. [Google Scholar] [CrossRef]
- Marín-Guirao, L.; Bernardeau-Esteller, J.; Ruiz, J.M.; Sandoval-Gil, J.M. Resistance of Posidonia oceanica seagrass meadows to the spread of the introduced green alga Caulerpa cylindracea: Assessment of the role of light. Biol. Invasions 2015, 17, 1989–2009. [Google Scholar] [CrossRef]
- Matta, J.L.; Chapman, D.J. Photosynthetic responses and daily carbon balance ofColpomenia peregrina: Seasonal variations and differences between intertidal and subtidal populations. Mar. Biol. 1991, 108, 303–313. [Google Scholar] [CrossRef]
- Lopez y Royo, C.; Casazza, G.; Pergent-Martini, C.; Pergent, G. A biotic index using the seagrass Posidonia oceanica (BiPo), to evaluate ecological status of coastal waters. Ecol. Indic. 2010, 10, 380–389. [Google Scholar] [CrossRef]
- Fernández-Torquemada, Y.; Díaz-Valdés, M.; Colilla, F.; Luna, B.; Sánchez-Lizaso, J.L.; Ramos-Esplá, A.A. Descriptors from Posidonia oceanica (L.) Delile meadows in coastal waters of Valencia, Spain, in the context of the EU Water Framework Directive. ICES J. Mar. Sci. 2008, 65, 1492–1497. [Google Scholar] [CrossRef] [Green Version]
- Marbà, N.; Santiago, R.; Díaz-Almela, E.; Álvarez, E.; Duarte, C.M. Seagrass (Posidonia oceanica) vertical growth as an early indicator of fish farm-derived stress. Estuarine Coast. Shelf Sci. 2006, 67, 475–483. [Google Scholar] [CrossRef]
- Romero, J.; Martínez-Crego, B.; Alcoverro, T.; Pérez, M. A multivariate index based on the seagrass Posidonia oceanica (POMI) to assess ecological status of coastal waters under the water framework directive (WFD). Mar. Pollut. Bull. 2007, 55, 196–204. [Google Scholar] [CrossRef]
- Martínez-Crego, B.; Vergés, A.; Alcoverro, T.; Romero, J. Selection of multiple seagrass indicators for environmental biomonitoring. Mar. Ecol. Prog. Ser. 2008, 361, 93–109. [Google Scholar] [CrossRef]
- Underwood., A.J. Experiments in Ecology: Their Local Design and Interpretation Using Analysis of Variance; Cambridge Univ. Press: Cambridge, UK, 1997. [Google Scholar]
- Rutherford, A. Introducing Anova and Ancova: A GLM Approach; Thousand Oaks and New Delhi: London, UK, 2001. [Google Scholar]
- De Kock, W.; Hasler-Sheetal, H.; Holmer, M.; Tsapakis, M.; Apostolaki, E.T. Metabolomics and traditional indicators unveil stress of a seagrass (Cymodocea nodosa) meadow at intermediate distance from a fish farm. Ecol. Indic. 2020, 109, 105765. [Google Scholar] [CrossRef]
- Griffiths, L.L.; Connolly, R.M.; Brown, C.J. Critical gaps in seagrass protection reveal the need to address multiple pressures and cumulative impacts. Ocean Coast. Manag. 2020, 183, 104946. [Google Scholar] [CrossRef]
- Ontoria, Y.; Cuesta-Gracia, A.; Ruiz, J.M.; Romero, J.; Pérez, M. The negative effects of short-term extreme thermal events on the seagrass Posidonia oceanica are exacerbated by ammonium additions. PLoS ONE 2019, 14, e0222798. [Google Scholar] [CrossRef] [Green Version]
- Pazzaglia, J.; Santillán-Sarmiento, A.; Helber, S.B.; Ruocco, M.; Terlizzi, A.; Marín-Guirao, L.; Procaccini, G. Does Warming Enhance the Effects of Eutrophication in the Seagrass Posidonia Oceanica? Front. Mar. Sci. 2020, 7, 1067. [Google Scholar] [CrossRef]
- Boudouresque, C.-F.; Blanfuné, A.; Pergent, G.; Thibaut, T. Restoration of Seagrass Meadows in the Mediterranean Sea: A Critical Review of Effectiveness and Ethical Issues. Water 2021, 13, 1034. [Google Scholar] [CrossRef]
- Cameron, J.; Abouchar, J. The Precautionary Principle: A Fundamental Principle of Law and The Precautionary Principle: A Fundamental Principle of Law and Policy for the Protection of the Global Environment Policy for the Protection of the Global Environment. BC Int’l Comp. L. Rev. 1991, 14, 1–27. [Google Scholar]

| Sites | Summer | Autumn | |||||
|---|---|---|---|---|---|---|---|
| % E0 | DPPF ± SE | % DPPFred | % E0 | DPPF ± SE | % DPPFred | OM % ± SE | |
| M1 | 10.2 | 4.13 ± 0.20 a | −32.8% | 6.82 | 1.55 ± 0.13 a | −37.1% | 3.49 ± 0.21 a |
| M2 | 11.66 | 4.43 ± 0.21 a | −28.0% | 9.07 | 2.20 ± 0.17 b | −10.6% | 3.56 ± 0.04 a |
| M3 | 11.23 | 4.68 ± 0.21 a | −23.9% | 11.01 | 2.34 ± 0.15 b | −5.0% | 2.11 ± 0.08 b |
| M4 | 15.33 | 6.15 ± 0.31 b | 11.27 | 2.46 ± 0.14 b | 1.41 ± 0.09 c | ||
| Variable Type/Name | Site/Variable Response | ||
|---|---|---|---|
| Abiotic factors: | M1 | M2 | M3 |
| Daily Photosynthetic Photon Flux (DPPF) | – | – | – |
| % of surficial irradiance (% E0) | – | – | – |
| Sediment organic matter content (OM %) | + | + | + |
| Distance (km) | – | – | – |
| Biological indicators of anthropogenic influence: | |||
| Nitrogen stable isotope δ15N rhizomes | + | + | + |
| %N rhizomes | + | ||
| C/N rhizomes | – | ||
| % P rhizomes | + | + | |
| C/P rhizomes | – | – | |
| Nitrogen stable isotope δ15N epiphytes | + | + | |
| % N epiphytes | |||
| C/N epiphytes | – | – | |
| Ni rhizomes | + | ||
| Cu rhizomes | |||
| maximum N uptake rate (Vmax) | + | + | + |
| half saturation constant (km) | + | + | + |
| Efficiency of N uptake (α uptake) | – | – | – |
| P. oceanica physiological variables: | |||
| Chl a concentration | + | + | |
| Chl b concentration | + | + | |
| carotenoids concentration | + | + | |
| net photosynthetic rate (net-Pmax) | – | – | – |
| gross photosynthetic rate (gross-P) | – | – | – |
| respiration rate (R) | – | – | – |
| photosynthetic efficiency (α) | – | ||
| saturation irradiance (Ek) | – | – | – |
| compensation irradiance (Ec) | – | ||
| relative electro transport rate (r-ETR) | – | ||
| Maximum quantum yield (Fv/Fm) | + | + | |
| daily metabolic carbon balance (CB) | – | – | |
| daily saturation period (Hk) | – | – | – |
| daily compensation period (Hc) | |||
| Non-structural carbohydrates-soluble fraction | – | – | |
| Non-structural carbohydrates-starch fraction | |||
| Meadow structure descriptors | |||
| shoot density | – | – | |
| meadow cover | – | – | – |
| vertical rhizome length | – | – | |
| proportion of plagiotropic rhizomes | + | + | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Casero, J.; Belando, M.D.; Bernardeau-Esteller, J.; Marín-Guirao, L.; García-Muñoz, R.; Sánchez-Lizaso, J.L.; Ruiz, J.M. A Critical Gap in Seagrass Protection: Impact of Anthropogenic Off-Shore Nutrient Discharges on Deep Posidonia oceanica Meadows. Plants 2023, 12, 457. https://doi.org/10.3390/plants12030457
Jiménez-Casero J, Belando MD, Bernardeau-Esteller J, Marín-Guirao L, García-Muñoz R, Sánchez-Lizaso JL, Ruiz JM. A Critical Gap in Seagrass Protection: Impact of Anthropogenic Off-Shore Nutrient Discharges on Deep Posidonia oceanica Meadows. Plants. 2023; 12(3):457. https://doi.org/10.3390/plants12030457
Chicago/Turabian StyleJiménez-Casero, Judit, Maria Dolores Belando, Jaime Bernardeau-Esteller, Lazaro Marín-Guirao, Rocio García-Muñoz, José Luis Sánchez-Lizaso, and Juan Manuel Ruiz. 2023. "A Critical Gap in Seagrass Protection: Impact of Anthropogenic Off-Shore Nutrient Discharges on Deep Posidonia oceanica Meadows" Plants 12, no. 3: 457. https://doi.org/10.3390/plants12030457
APA StyleJiménez-Casero, J., Belando, M. D., Bernardeau-Esteller, J., Marín-Guirao, L., García-Muñoz, R., Sánchez-Lizaso, J. L., & Ruiz, J. M. (2023). A Critical Gap in Seagrass Protection: Impact of Anthropogenic Off-Shore Nutrient Discharges on Deep Posidonia oceanica Meadows. Plants, 12(3), 457. https://doi.org/10.3390/plants12030457