Response of Cd, Zn Translocation and Distribution to Organic Acids Heterogeneity in Brassica juncea L.
Abstract
:1. Introduction
2. Results
2.1. Plant Biomass and Cd and Zn Contents
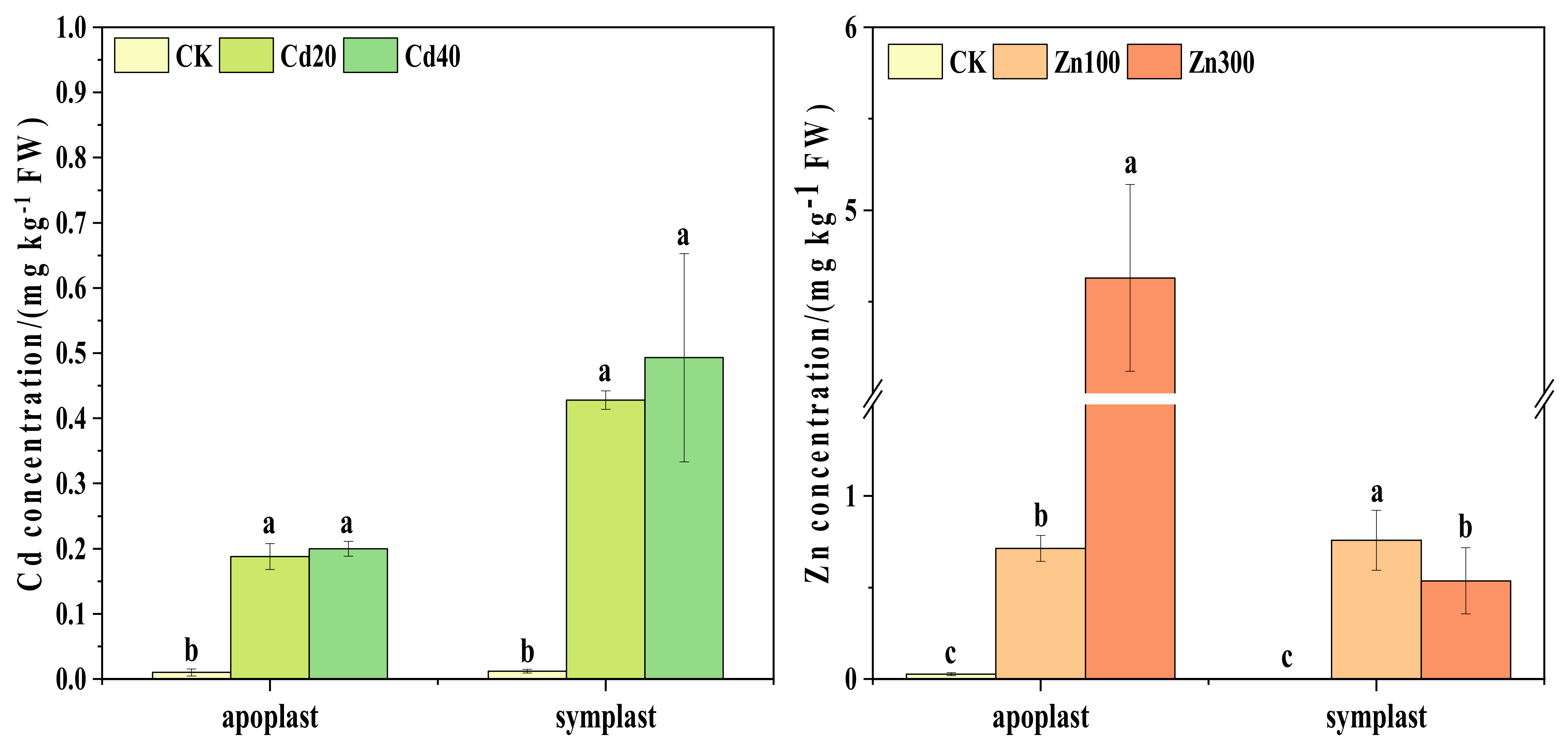
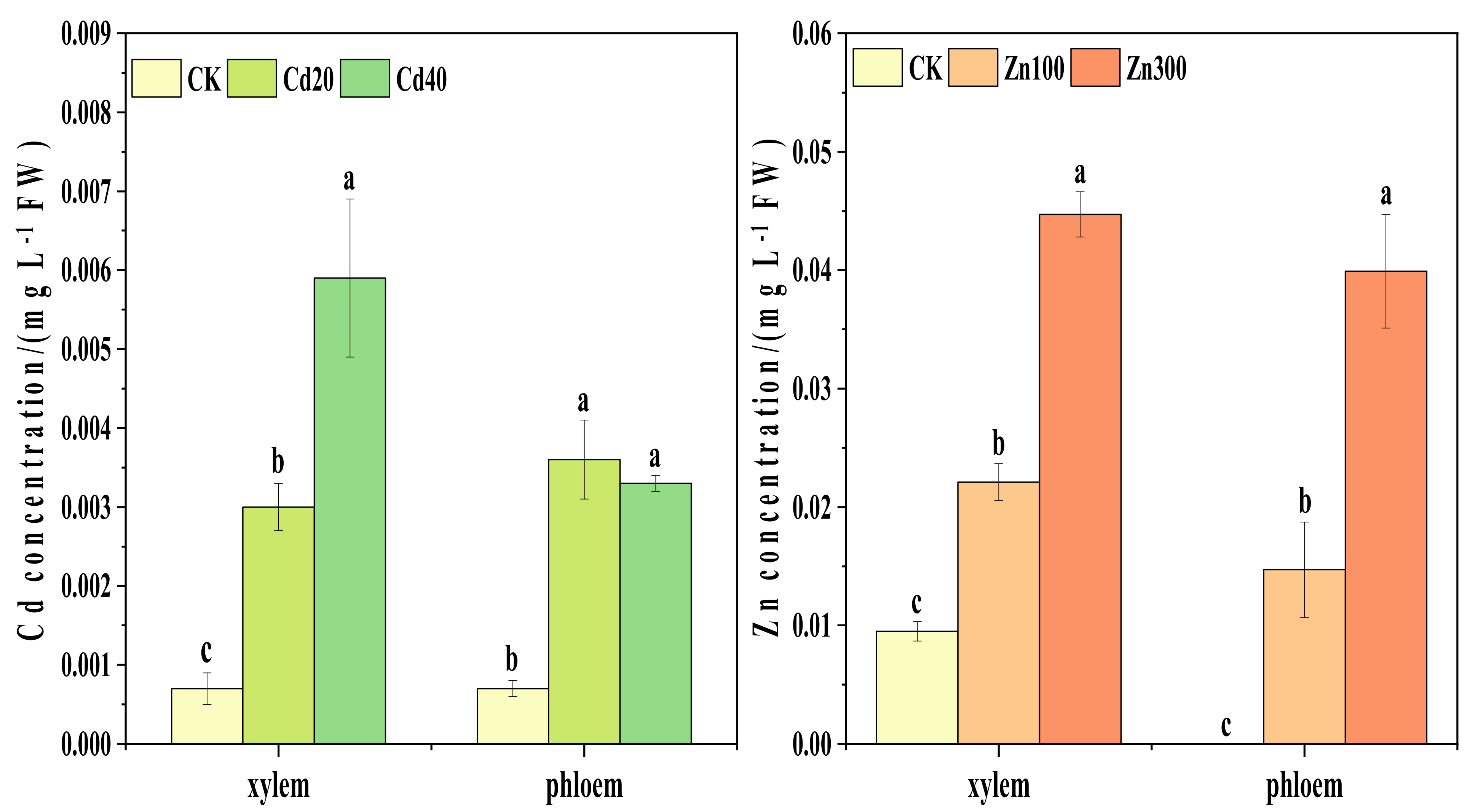
2.2. Contents of Cd and Zn in Sap Associated with Horizontal and Vertical Translocation Pathways of B. juncea
2.2.1. The Contents of Cd and Zn in the Sap Associated with the Horizontal Translocation Pathway of B. juncea
2.2.2. Contents of Cd and Zn in the Sap Associated with the Vertical Translocation Pathway of B. juncea
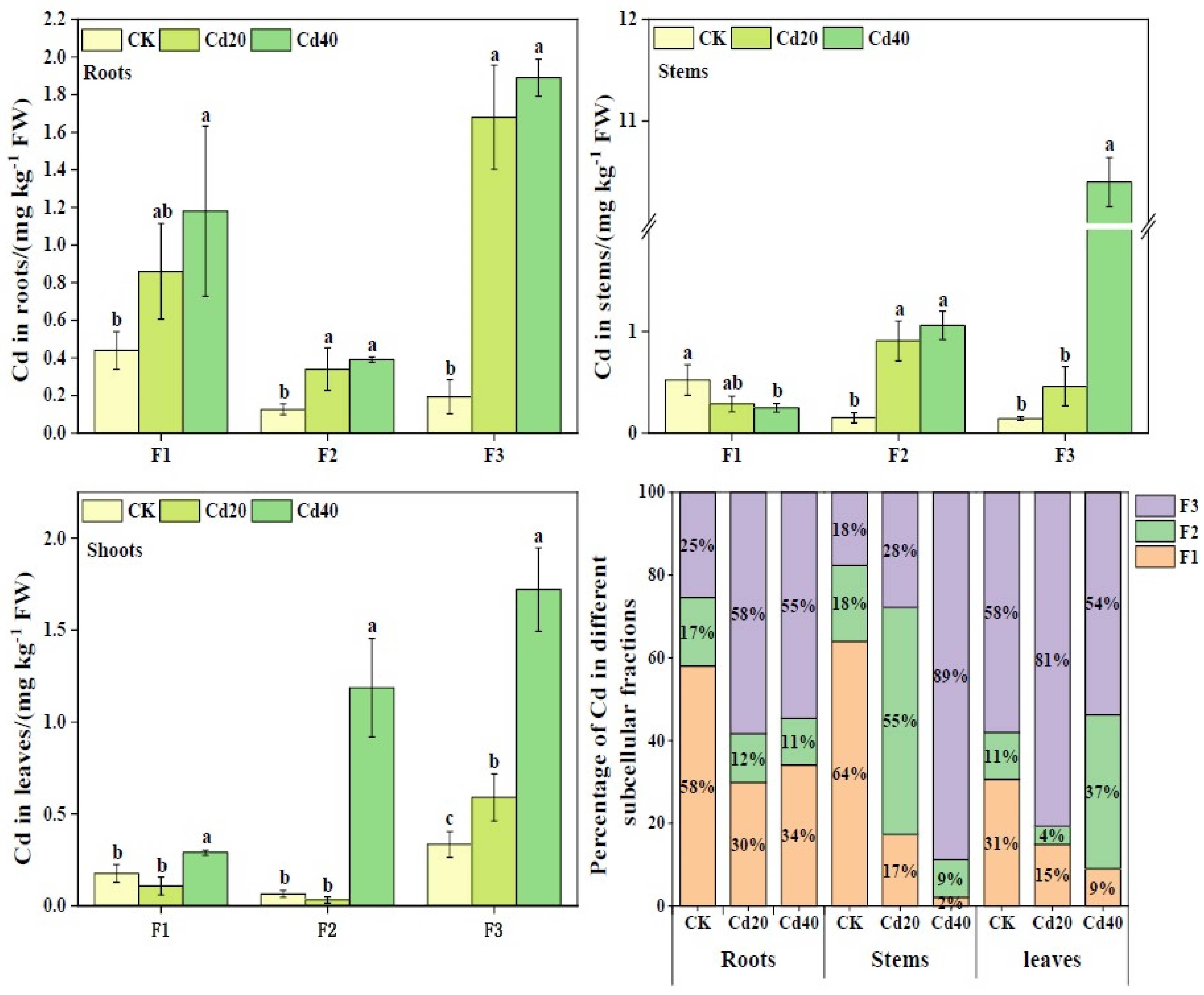
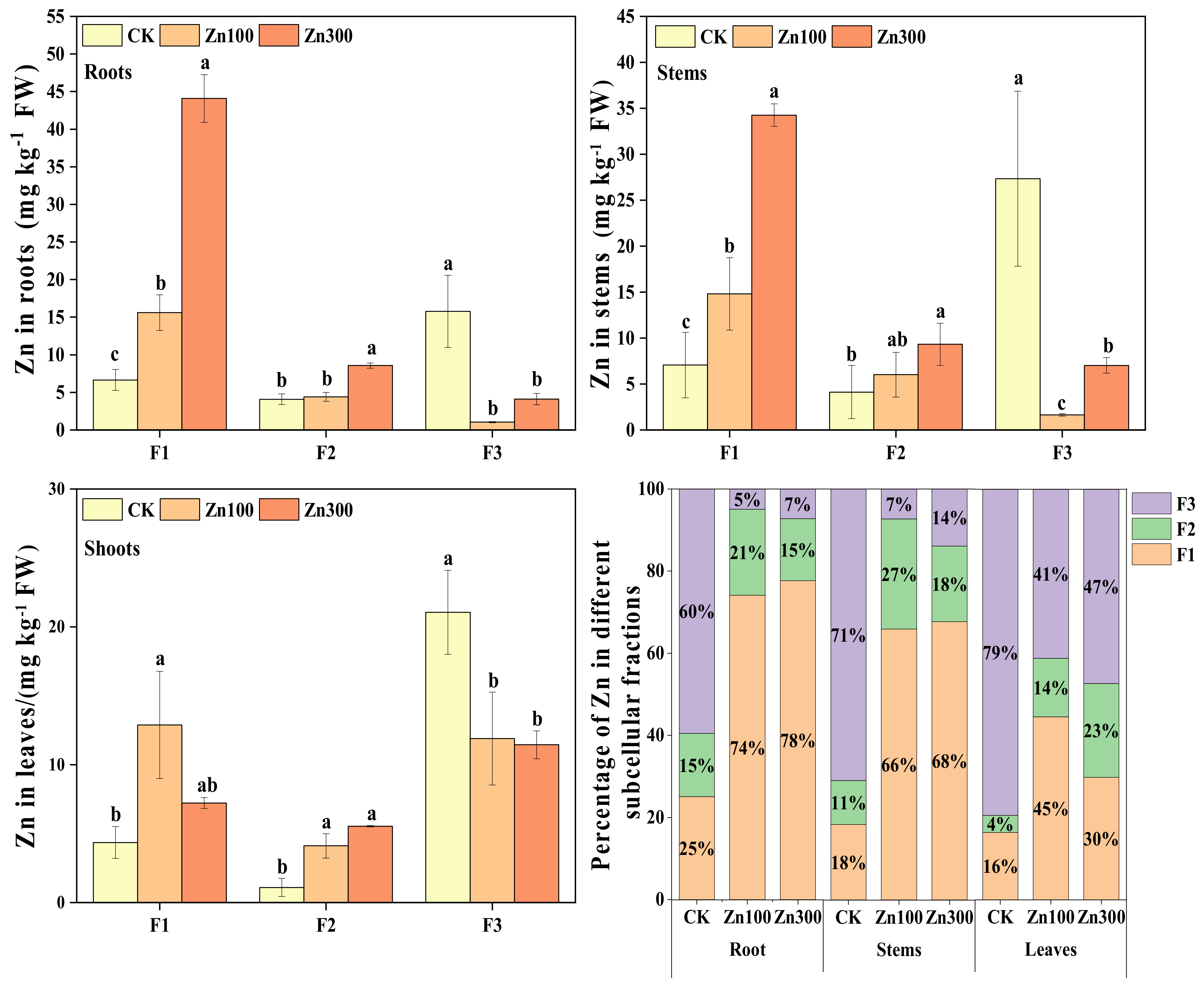
2.3. Subcellular Distribution of Cd and Zn in Roots, Stems and Leaves of B. juncea
2.3.1. Subcellular Distribution of Cd in Roots, Stems, and Leaves of B. juncea
2.3.2. Subcellular Distribution of Zn in Roots, Stems, and Leaves of B. juncea
2.4. Organic Acid Heterogeneity in B. juncea under Cd and Zn Treatments
2.4.1. Organic Acid Heterogeneity in B. juncea under Cd Treatments
2.4.2. Organic Acid Heterogeneity in B. juncea under Zn Treatments
2.5. Correlation of Organic Acid Contents with Cd and Zn Contents in Different Parts of B. juncea
3. Discussion
3.1. Heterogeneity of Cd and Zn Translocation and Distribution in B. juncea
3.2. Effect of Organic Acid Heterogeneity on the Translocation and Distribution of Cd and Zn in B. juncea
4. Materials and Methods
4.1. Plant Material and Soil
4.2. Experimental Design
4.3. Indicator Measurement Method
4.3.1. Plant Cd and Zn Contents
4.3.2. Extraction Method of Root Apoplast and Symplast Saps
4.3.3. Extraction Method of Xylem and Phloem Saps
4.3.4. Measurement of Subcellular Contents of Cd and Zn in Roots, Stems, and Leaves of B. juncea
4.3.5. Xylem Sap and Plant Organic Acid Contents of B. juncea
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, I.; Seleiman, M.F.; Chattha, M.U.; Jalal, R.S.; Mahmood, F.; Hassan, F.A.S.; Izzet, W.; Alhammad, B.A.; Ali, E.F.; Roy, R.; et al. Enhancing antioxidant defense system of mung bean with a salicylic acid exogenous application to mitigate cadmium toxicity. Not. Bot. Horti. Agrobo. 2021, 49, 12303. [Google Scholar] [CrossRef]
- Xu, S.S.; Lin, S.Z.; Lai, Z.X. Cadmium impairs iron homeostasis in Arabidopsis thaliana by increasing the polysaccharide concentrations and the iron-binding capacity of root cell walls. Plant Soil 2015, 392, 71–85. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Dong, Y.W.; Zhu, N.; Jin, H.M. Foliar application of biosynthetic nano-selenium alleviates the toxicity of Cd, Pb, and Hg in Brassica chinensis by inhibiting heavy metal adsorption and improving antioxidant system in plant. Ecotoxicol. Environ. Saf. 2022, 240, 113681. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Ram, H.; Kumar, B. Mechanism of Zinc absorption in plants: Uptake, transport, translocation and accumulation. Rev. Environ. Sci. Biotechnol. 2016, 15, 89–109. [Google Scholar] [CrossRef]
- Angulo-Bejarano, P.I.; Puente-Rivera, J.; Cruz-Ortega, R. Metal and metalloid toxicity in plants: An overview on molecular aspects. Plants 2021, 10, 635. [Google Scholar] [CrossRef]
- Sofo, A.; Vitti, A.; Nuzzaci, M.; Tataranni, G.; Scopa, A.; Remans, T.; Falasca, G.; Altamura, M.M.; Degola, F.; Toppi, L.S. Correlation between hormonal homeostasis and morphogenic responses in Arabidopsis thaliana seedlings growing in a Cd/Cu/Zn multi-pollution context. Physiol. Plant. 2013, 149, 487–498. [Google Scholar] [CrossRef]
- Li, G.X.; Li, C.; Rengel, Z.; Liu, H.E.; Zhao, P. Excess Zn-induced changes in physiological parameters and expression levels of TaZips in two wheat genotypes. Environ. Exp. Bot. 2020, 177, 104133. [Google Scholar] [CrossRef]
- Salem, H.M.; Abdel-Salam, A.; Abdel-Salam, M.A.; Seleiman, M.F. Phytoremediation of Metal and Metalloids from Contaminated Soil. In Plants Under Metal and Metalloid Stress; Hasanuzzaman, M., Nahar, K., Fujita, M., Eds.; Springer: Singapore, 2018; pp. 249–262. [Google Scholar] [CrossRef]
- Ashraf, S.; Naveed, M.; Afzal, M.; Seleiman, M.F.; Al-Suhaibani, N.A.; Zahir, Z.A.; Mustafa, A.; Refay, Y.; Alhammad, B.A.; Ashraf, S.; et al. Unveiling the Potential of Novel Macrophytes for the Treatment of Tannery Effluent in Vertical Flow Pilot Constructed Wetlands. Water 2020, 12, 549. [Google Scholar] [CrossRef] [Green Version]
- Shahid, M.J.; Ali, S.; Shabir, G.; Siddique, M.; Rizwan, M.; Seleiman, M.F.; Afzal, M. Comparing the Performance of Four Macrophytes in Bacterial Assisted Floating Treatment Wetlands for the Removal of Trace Metals (Fe, Mn, Ni, Pb, and Cr) From Polluted River Water. Chemosphere 2019, 243, 125353. [Google Scholar] [CrossRef]
- Goswami, S.; Das, S. A study on cadmium phytoremediation potential of Indian Mustard, Brassica juncea. Int. J. Phytorem. 2015, 17, 583–588. [Google Scholar] [CrossRef]
- Mishra, R.; Datta, S.P.; Annapurna, K.; Meena, M.C.; Dwivedi, B.S.; Golui, D.; Bandyopadhyay, K. Enhancing the effectiveness of zinc, cadmium, and lead phytoextraction in polluted soils by using amendments and microorganisms. Environ. Sci. Pollut. Res. 2019, 26, 17224–17235. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.S.; Shekhawat, K.; Dass, A.; Kandpal, B.K.; Singh, V.K. Phytoremediation mechanism in Indian Mustard (Brassica juncea) and its enhancement through agronomic interventions. Proc. Natl. Acad. Sci. India Sect. B 2019, 89, 419–427. [Google Scholar] [CrossRef]
- Wu, Z.C.; Zhao, X.H.; Sun, X.C.; Tan, Q.L.; Tang, Y.F.; Nie, Z.J.; Hu, C.X. Xylem transport and gene expression play decisive roles in cadmium accumulation in shoots of two oilseed rape cultivars (Brassica napus). Chemosphere 2015, 119, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.C.; Wang, F.H.; Liu, S.; Du, Y.Q.; Li, F.R.; Du, R.Y.; Wen, D.; Zhao, J. Comparative responses to silicon and selenium in relation to cadmium uptake, compartmentation in roots, and xylem transport in flowering Chinese cabbage (Brassica campestris L. ssp. chinensis var. utilis) under cadmium stress. Environ. Exp. Bot. 2016, 131, 173–180. [Google Scholar] [CrossRef]
- Shen, Y.; Gu, R.; Sheng, Y.; Zeng, N.; Zhan, X. Acropetal translocation of phenanthrene in wheat seedlings: Xylem or phloem pathway? Environ. Pollut. 2020, 260, 114055. [Google Scholar] [CrossRef]
- Hazama, K.; Nagata, S.; Fujimori, T.; Yanagisawa, S.; Yoneyama, T. Concentrations of metals and potential metal-binding compounds and speciation of Cd, Zn and Cu in phloem and xylem saps from castor bean plants (Ricinus communis) treated with four levels of cadmium. Physiol. Plant. 2015, 154, 243–255. [Google Scholar] [CrossRef]
- Ye, W.L.; Guo, G.F.; Wu, F.; Fan, T.; Lu, H.J.; Chen, H.; Li, X.; Ma, Y. Absorption, translocation, and detoxification of Cd in two different castor bean (Ricinus communis L.) cultivars. Environ. Sci. Pollut. Res. 2018, 25, 28899–28906. [Google Scholar] [CrossRef]
- Xin, J.; Zhao, X.H.; Tan, Q.L.; Sun, X.C.; Hu, C.X. Comparison of cadmium absorption, translocation, subcellular distribution and chemical forms between two radish cultivars (Raphanus sativus L.). Ecotoxicol. Environ. Saf. 2017, 145, 258–265. [Google Scholar] [CrossRef]
- Qiao, X.Q.; Zheng, Z.Z.; Zhang, L.F.; Wang, J.H.; Shi, G.X.; Xu, X.Y. Lead tolerance mechanism in sterilized seedlings of Potamogeton crispus L.: Subcellular distribution, polyamines and proline. Chemosphere 2015, 120, 179–187. [Google Scholar] [CrossRef]
- Huang, R.Z.; Jiang, Y.B.; Jia, C.H.; Jiang, S.M.; Yan, X.P. Subcellular distribution and chemical forms of cadmium in Morus alba L. Int. J. Phytorem. 2018, 20, 448–453. [Google Scholar] [CrossRef]
- Hart, J.J.; Welch, R.M.; Norcell, W.A.; Kochian, L.V. Zinc effects on cadmium accumulation and partitioning innearisogenic lines of durum wheat that differ in grain cadmium concentration. New Phytol. 2005, 167, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Ueno, D.; Iwashita, T.; Zhao, F.J.; Ma, J.F. Characterization of Cd translocation and identification of the Cd form in xylem sap of the Cd-hyperaccumulator Arabidopsis halleri. Plant Cell Physiol. 2008, 49, 540–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javed, M.T.; Akram, M.S.; Tanwir, K.; Chaudhary, H.J.; Ali, Q.; Stoltz, E.; Lindberg, S. Cadmium spiked soil modulates root organic acids exudation and ionic concentrations of two differentially Cd tolerant maize (Zea mays L.) cultivars. Ecotoxicol. Environ. Saf. 2020, 141, 216–225. [Google Scholar] [CrossRef]
- Li, M.T.; Luo, Z.X.; Yan, Y.M.; Wang, Z.H.; Chi, Q.Q.; Yan, C.Z.; Xing, B.S. Arsenate accumulation, distribution, and toxicity associated with titanium dioxide nanoparticles in Daphnia magna. Environ. Sci. Technol. 2016, 50, 9636–9643. [Google Scholar] [CrossRef]
- He, C.; Zhao, Y.; Wang, F.; Oh, K.; Zhao, Z.; Wu, C.; Zhang, X.; Chen, X.; Liu, X. Phytoremediation of soil heavy metals (Cd and Zn) by castor seedlings: Tolerance, accumulation and subcellular distribution. Chemosphere 2020, 252, 126471. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Nagata, S.; Yanagisawa, S.; Yoneyama, T. Copper in xylem and phloem saps from rice (Oryza sativa): The effect of moderate copper concentrations in the growth medium on the accumulation of five essential metals and a speciation analysis of copper-containing compounds. Funct. Plant Biol. 2013, 40, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Hanikenne, M.; Nouet, C. Metal hyperaccumulation and hypertolerance: A model for plant evolutionary genomics. Curr. Opin. Plant Biol. 2011, 14, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Jupa, R.; Luo, J.; Lux, A.; Kováč, J.; Wen, Y.; Zhou, Y.M.; Jan, J.; Liang, Y.C.; Li, T.Q. The apoplasmic pathway via the root apex and lateral roots contributes to Cd hyperaccumulation in the hyperaccumulator Sedum alfredii. J. Exp. Bot. 2016, 68, 739–751. [Google Scholar] [CrossRef] [Green Version]
- Peuke, A.D. Correlations in concentrations, xylem and phloem flows, and partitioning of elements and ions in intact plants. A summary and statistical re-evaluation of modelling experiments in Ricinus communis. J. Exp. Bot. 2010, 61, 635–655. [Google Scholar] [CrossRef]
- Huang, X.; Duan, S.P.; Wu, Q.; Yu, M.; Shabala, S. Reducing cadmium accumulation in plants: Structure–function relations and tissue-specific operation of transporters in the spotlight. Plants 2020, 9, 223–240. [Google Scholar] [CrossRef] [Green Version]
- Fujimaki, S.; Suzui, N.; Ishioka, N.S.; Kawachi, N.; Ito, S.; Chino, M.; Shin-ichi, N. Tracing cadmium from culture to spikelet: Noninvasive imaging and quantitative characterization of absorption, transport, and accumulation of cadmium in an intact rice plant. Plant Physiol. 2010, 152, 1796–1806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Li, H.; Luo, J.; Ma, C.; Li, S.; Qu, L.; Gai, Y.; Jiang, X.; Janz, D.; Polle, A.; et al. A transcriptomic network underlies microstructural and physiological responses to cadmium in Populus x canescens (1 CW). Plant Physiol. 2013, 162, 424–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isaure, M.-P.; Huguet, S.; Meyer, C.-L.; Castillo-Michel, H.; Testemale, D.; Vantelon, D.; Saumitou-Laprade, P.; Verbruggen, N.; Sarret, G. Evidence of various mechanisms of Cd sequestration in the hyperaccumulator Arabidopsis halleri, the non-accumulator Arabidopsis lyrata, and their progenies by combined synchrotronbased techniques. J. Exp. Bot. 2015, 66, 3201–3214. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.T.; Zhu, Y.X.; Fan, T.; Peng, C.; Wang, J.R.; Sun, L.; Chen, C.Y. OsZIP7 functions in xylem loading in roots and inter-vascular transfer in nodes to deliver Zn/Cd to grain in rice. Biochem. Biophys. Res. Commun. 2019, 512, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Luo, N.; Li, Y.W.; Cai, Q.Y.; Li, H.Y.; Mo, C.H.; Wong, M.H. Cadmium in rice: Transport mechanisms, influencing factors, and minimizing measures. Environ. Pollut. 2017, 224, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Voiniciuc, C.; Pauly, M.; Usadel, B. Monitoring polysaccharide dynamics in the plant cell wall. Plant Physiol. 2018, 176, 2590–2600. [Google Scholar] [CrossRef] [Green Version]
- Tang, M.; Zhang, X.; Tan, X.R.; Liu, Y.; Wang, M.X. Accumulation, subcellular distribution, and chemical forms of zinc in three tree species. Chin. J. Appl. Ecol. 2021, 32, 4298–4306. [Google Scholar] [CrossRef]
- Kupper, H.; Andresen, E. Mechanisms of metal toxicity in plants. Metallomics 2016, 8, 269–285. [Google Scholar] [CrossRef]
- Claus, J.; Bohmann, A.; Chavarria-Krauser, A. Zinc uptake and radial transport in roots of Arabidopsis thaliana: A modelling approach to understand accumulation. Ann. Bot. 2013, 112, 369–380. [Google Scholar] [CrossRef]
- Kang, L.; Wu, Y.L.; Zhang, J.B.; An, Q.S.; Zhou, C.R.; Li, D.; Pan, C.P. Nano-selenium enhances the antioxidant capacity, organic acids and cucurbitacin B in melon (Cucumis melo L.) plants. Ecotoxicol. Environ. Saf. 2022, 241, 113777. [Google Scholar] [CrossRef]
- Hu, P.J.; Wang, Y.D.; Przybyłowicz, W.J.; Li, Z.; Barnabas, A.; Wu, L.H.; Luo, Y.M.; Mesjasz-Przybyłowicz, J. Elemental distribution by cryo-micro-PIXE in the zinc and cadmium hyperaccumulator Sedum plumbizincicola grown naturally. Plant Soil 2015, 388, 267–282. [Google Scholar] [CrossRef]
- Wang, W.J.; Zhang, M.Z.; Liu, J. Subcellular distribution and chemical forms of Cd in Bougainvillea spectabilis Willd.as an ornamental phytostabilizer: An integrated consideration. Int. J. Phytorem. 2018, 20, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.H.; Xue, B.H.; Song, G.L.; Shi, S.Q. Effects of citric acid on antioxidant system and carbon-nitrogen metabolism of Elymus dahuricus under Cd stress. Ecotoxicol. Environ. Saf. 2022, 233, 113321. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Marino, N.; De, I.T.F.; Matilla, A.J. Organic acids and soluble sugars in edible and nonedible parts of damson plum (Prunus domestica L. subsp. insititia cv. syriaca) fruits during development and ripening. Food Sci. Technol. Int. 2008, 14, 187–193. [Google Scholar] [CrossRef]
- Clemens, S.; Aarts, M.G.M.; Thomine, S.; Verbruggen, N. Plant science: The key to preventing slow cadmium poisoning. Trends Plant Sci. 2013, 18, 92–99. [Google Scholar] [CrossRef]
- Vítková, M.; Komarek, M.; Tejnecky, V.; Šillerova, H. Interactions of nanooxides with low-molecular-weight organic acids in a contaminated soil. J. Hazard. Mater. 2015, 293, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.P.; Cui, J.L.; Chan, T.S.; Dong, J.C.; Chen, D.L.; Li, X.D. Role of chelant on Cu distribution and speciation in Lolium multiflorum by synchrotron techniques. Sci. Total Environ. 2018, 621, 772–778. [Google Scholar] [CrossRef]
- Wei, Z.G.; Wong, J.W.; Hong, F.S.; Zhao, H.Y.; Li, H.X.; Hu, F. Determination of inorganic and organic anions in xylem saps of two contrasting oilseed rape (Brassica juncea L.) varieties: Roles of anions in long-distance transport of cadmium. Microchem. J. 2007, 86, 53–59. [Google Scholar] [CrossRef]
- Cornu, J.Y.; Bussière, S.; Coriou, C.; Robert, T.; Maucourt, M.; Deborde, C.; Moing, A.; Nguyen, C. Changes in plant growth, Cd partitioning and xylem sap composition in two sunflower cultivars exposed to low Cd concentrations in hydroponics. Ecotoxicol. Environ. Saf. 2020, 205, 111145. [Google Scholar] [CrossRef]
- Li, Q.; Guo, J.Y.; Zhang, X.Z.; Yu, H.Y.; Huang, F.; Zhang, L.; Zhang, M.; Li, T.X. Changes of non-protein thiols in root and organic acids in xylem sap involved in cadmium translocation of cadmium-safe rice line (Oryza sative L.). Plant Soil 2019, 439, 475–486. [Google Scholar] [CrossRef]
- Ge, T.D.; Tang, D.M.; Lu, B.; Xia, H.Y.; Song, S.W.; Huang, D.F. Influence of organic and inorganic nitrogen supply on the composition of tomato seedling root exudates, xylem and phloem sap grown in hydroponic culture. Acta Hortic. Sin. 2008, 35, 39–46. [Google Scholar] [CrossRef]
- Yang, L.P.; Zhu, J.; Wang, P.; Zeng, J.; Tan, R.; Yang, Y.Z.; Liu, Z.M. Effect of Cd on growth, physiological response, Cd subcellular distribution and chemical forms of Koelreuteria paniculata. Ecotoxicol. Environ. Saf. 2018, 160, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Yang, J.; Shang, H.; Song, J. Changes of characteristic sugar, fatty acid, organic acid and amino acid in jujubes at different dry mature stages. J. Food Compos. Anal. 2021, 104, 104104. [Google Scholar] [CrossRef]


| Heavy Metal | Treatment Concentrations/mg kg−1 | Shoots | Roots | TF | ||
|---|---|---|---|---|---|---|
| Biomass/g plant−1 | Contents/mg kg−1 | Biomass/g plant−1 | Contents/mg kg−1 | |||
| Cd | 0 | 4.50 ± 0.17 a | 0.49 ± 0.21 b | 1.68 ± 0.08 b | 0.66 ± 0.15 c | 0.90 |
| 20 | 4.18 ± 0.16 b | 3.27 ± 0.60 a | 1.65 ± 0.06 b | 11.55 ± 3.05 b | 0.28 | |
| 40 | 4.54 ± 0.09 a | 3.80 ± 0.62 a | 2.38 ± 0.09 a | 31.83 ± 6.13 a | 0.11 | |
| 0 | 4.50 ± 0.17 a | 21.85 ± 4.49 b | 1.68 ± 0.08 a | 30.30 ± 3.32 c | 0.72 | |
| Zn | 100 | 4.06 ± 0.13 b | 51.30 ± 9.51 a | 1.77 ± 0.10 a | 106.8 ± 12.65 b | 0.48 |
| 300 | 3.18 ± 0.13 c | 53.55 ± 8.98 a | 0.91 ± 0.08 b | 211.43 ± 10.56 a | 0.25 | |
| Parts | Heavy Metal | Organic Acid | ||||||
|---|---|---|---|---|---|---|---|---|
| Oxalic Acid | Tartaric Acid | Malic Acid | Malonic Acid | Acetic Acid | Citric Acid | Succinic Acid | ||
| roots | Cd | −0.758 | 0.536 | −0.204 | −0.243 | −0.820 * | −0.256 | −0.657 |
| Zn | −0.307 | 0.025 | −0.588 | −0.435 | 0.141 | −0.412 | −0.866 | |
| shoots | Cd | −0.531 | 0.011 | 0.210 | −0.524 | −0.624 | 0.324 | 0.134 |
| Zn | −0.262 | −0.796 * | −0.860 * | −0.430 | −0.742 | 0.797 * | −0.091 | |
| Parts | Heavy Metal | Organic Acid | ||||||
|---|---|---|---|---|---|---|---|---|
| Oxalic Acid | Tartaric Acid | Malic Acid | Malonic Acid | Acetic Acid | Citric Acid | Succinic Acid | ||
| Vacuole of roots | Cd | −0.968 ** | −0.032 | 0.369 | 0.237 | −0.749 | −0.653 | −0.918 ** |
| Zn | −0.442 | −0.455 | 0.840 * | 0.836 * | 0.474 | 0.673 | 0.828 * | |
| Vacuole of leaves | Cd | 0.020 | 0.511 | 0.707 | 0.003 | −0.232 | 0.342 | 0.638 |
| Zn | 0.088 | 0.861 ** | 0.383 | 0.584 | 0.889 ** | −0.810 ** | 0.568 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, Y.; Li, Z.; Yang, Z.; Wang, J.; Li, B.; Zu, Y. Response of Cd, Zn Translocation and Distribution to Organic Acids Heterogeneity in Brassica juncea L. Plants 2023, 12, 479. https://doi.org/10.3390/plants12030479
Liao Y, Li Z, Yang Z, Wang J, Li B, Zu Y. Response of Cd, Zn Translocation and Distribution to Organic Acids Heterogeneity in Brassica juncea L. Plants. 2023; 12(3):479. https://doi.org/10.3390/plants12030479
Chicago/Turabian StyleLiao, Yumeng, Zuran Li, Zhichen Yang, Jixiu Wang, Bo Li, and Yanqun Zu. 2023. "Response of Cd, Zn Translocation and Distribution to Organic Acids Heterogeneity in Brassica juncea L." Plants 12, no. 3: 479. https://doi.org/10.3390/plants12030479
APA StyleLiao, Y., Li, Z., Yang, Z., Wang, J., Li, B., & Zu, Y. (2023). Response of Cd, Zn Translocation and Distribution to Organic Acids Heterogeneity in Brassica juncea L. Plants, 12(3), 479. https://doi.org/10.3390/plants12030479






