Characterization of Endophytic Bacteria Isolated from Typha latifolia and Their Effect in Plants Exposed to Either Pb or Cd
Abstract
:1. Introduction
2. Results
2.1. Pb and Cd Content in the Soil and Roots of T. latifolia
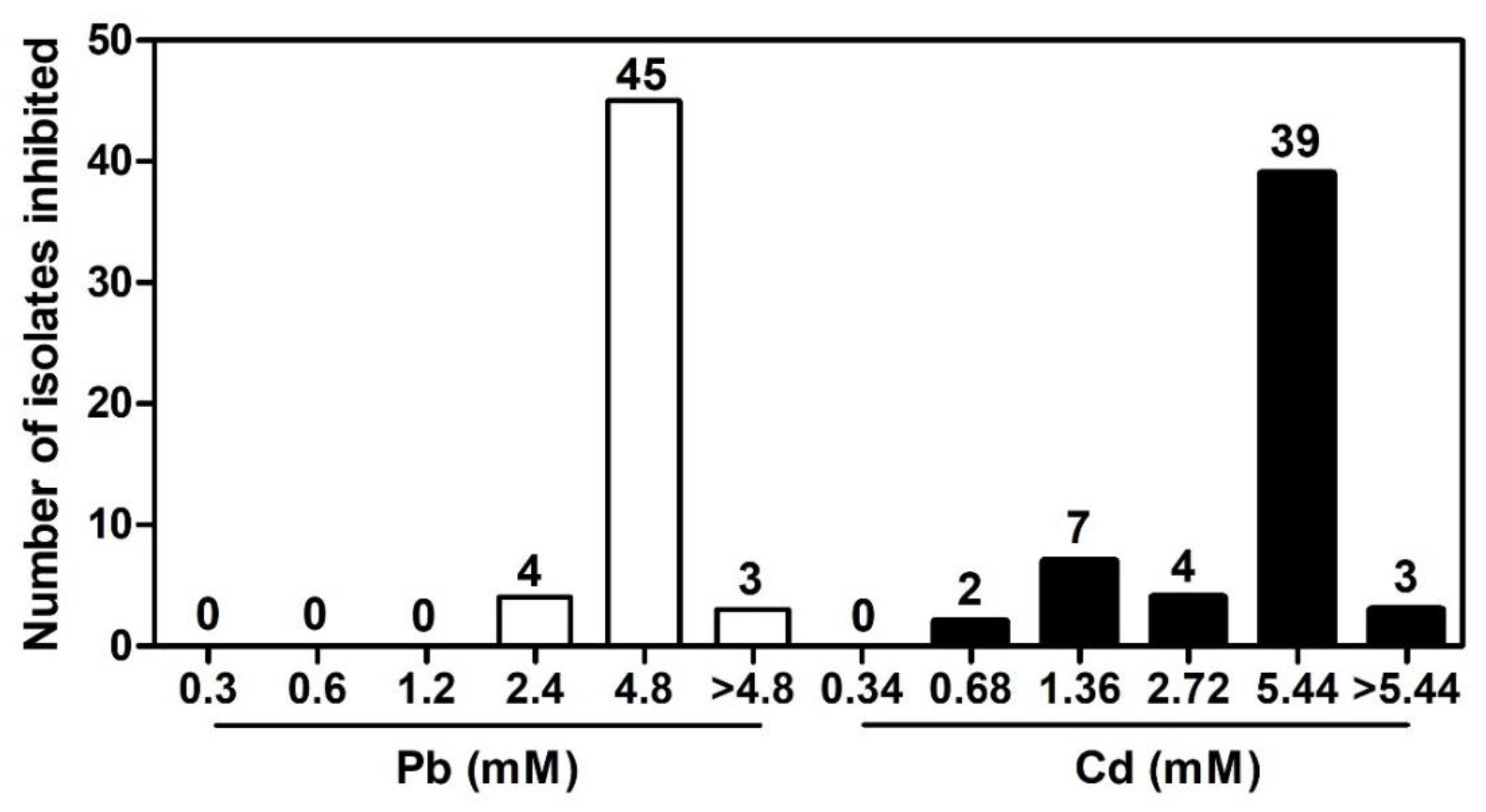
2.2. Isolation of Pb- and Cd-Tolerant Endophytic Bacteria from T. latifolia Roots
2.3. Identification of Bacterial Isolates
2.4. Antibiotic Susceptibility Test and Osmotic Tolerance
2.5. Screening for PGPR Abilities in Endophytic Bacterial Isolates
2.6. Effect of Bacterial Isolates in T. latifolia Seedlings
2.7. Effect of Bacterial Strains on T. latifolia Seedlings Exposed to Either Pb or Cd
3. Discussion
4. Materials and Methods
4.1. Study Site Description and Sample Collection
4.2. Heavy Metal Analysis
4.3. Isolation of Endophytic Bacteria from T. latifolia Roots
4.4. Pb and Cd Tolerance of Endophytic Bacteria
4.5. Osmotic Tolerance and Antibiotic Susceptibility Test
4.6. Molecular Identification of Endophytic Bacteria
4.7. Determination of PGPR Abilities in Bacteria
4.7.1. Phosphate Solubilization Activity
4.7.2. Siderophore Production
4.7.3. ACC Deaminase Detection
4.7.4. Bacterial Auxin Production
4.8. Plant–Bacteria Interaction Assays
4.8.1. Effect of Bacterial Strains in T. latifolia Seedlings
4.8.2. Effect of Bacterial Strains in T. latifolia Seedlings Exposed to Either Pb or Cd
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kumar, A.; Subrahmanyam, G.; Mondal, R.; Cabral-Pinto, M.M.S.; Shabnam, A.A.; Jigyasu, D.K.; Malyan, S.K.; Fagodiya, R.K.; Khan, S.A.; Kumar, A.; et al. Bio-remediation approaches for alleviation of cadmium contamination in natural resources. Chemosphere 2021, 268, 128855. [Google Scholar] [CrossRef] [PubMed]
- Małkowski, E.; Sitko, K.; Zieleźnik-Rusinowska, P.; Gieroń, Ż.; Szopiński, M. Heavy Metal Toxicity: Physiological Implications of Metal Toxicity in Plants. In Plant Metallomics and Functional Omics: A System-Wide Perspective; Sablok, G., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 253–301. [Google Scholar]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.; Cai, C.; Chi, H.; Reid, B.J.; Coulon, F.; Zhang, Y.; Hou, Y. Remediation of cadmium and lead polluted soil using thiol-modified biochar. J. Hazard. Mater. 2020, 388, 122037. [Google Scholar] [CrossRef]
- Salas-Moreno, M.; Marrugo-Negrete, J. Phytoremediation potential of Cd and Pb-contaminated soils by Paspalum fasciculatum Willd. ex Flüggé. Int. J. Phytoremediation 2020, 22, 87–97. [Google Scholar] [CrossRef]
- Alaboudi, K.A.; Ahmed, B.; Brodie, G. Phytoremediation of Pb and Cd contaminated soils by using sunflower (Helianthus annuus) plant. Ann. Agric. Sci. 2018, 63, 123–127. [Google Scholar] [CrossRef]
- Guo, Z.; Gao, Y.; Cao, X.; Jiang, W.; Liu, X.; Liu, Q.; Chen, Z.; Zhou, W.; Cui, J.; Wang, Q. Phytoremediation of Cd and Pb interactive polluted soils by switchgrass (Panicum virgatum L.). Int. J. Phytoremediation 2019, 21, 1486–1496. [Google Scholar] [CrossRef]
- Hejna, M.; Moscatelli, A.; Stroppa, N.; Onelli, E.; Pilu, S.; Baldi, A.; Rossi, L. Bioaccumulation of heavy metals from wastewater through a Typha latifolia and Thelypteris palustris phytoremediation system. Chemosphere 2020, 241, 125018. [Google Scholar] [CrossRef]
- Bonanno, G.; Cirelli, G.L. Comparative analysis of element concentrations and translocation in three wetland congener plants: Typha domingensis, Typha latifolia and Typha angustifolia. Ecotoxicol. Environ. Saf. 2017, 143, 92–101. [Google Scholar] [CrossRef]
- Manoj, S.R.; Karthik, C.; Kadirvelu, K.; Arulselvi, P.I.; Shanmugasundaram, T.; Bruno, B.; Rajkumar, M. Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: A review. J. Environ. Manage. 2020, 254, 109779. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Páliz, K.; Caviedes, M.; Doukkali, B.; Mateos-Naranjo, E.; Rodríguez-Llorente, I.; Pajuelo, E. Screening beneficial rhizobacteria from Spartina maritima for phytoremediation of metal polluted salt marshes: Comparison of gram-positive and gram-negative strains. Environ. Sci. Pollut. Res. 2016, 23, 19825–19837. [Google Scholar] [CrossRef]
- Paredes-Páliz, K.I.; Mateos-Naranjo, E.; Doukkali, B.; Caviedes, M.A.; Redondo-Gómez, S.; Rodríguez-Llorente, I.D.; Pajuelo, E. Modulation of Spartina densiflora plant growth and metal accumulation upon selective inoculation treatments: A comparison of Gram negative and Gram positive rhizobacteria. Mar. Pollut. Bull. 2017, 125, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Chiboub, M.; Saadani, O.; Fatnassi, I.C.; Abdelkrim, S.; Abid, G.; Jebara, M.; Jebara, S.H. Characterization of efficient plant-growth-promoting bacteria isolated from Sulla coronaria resistant to cadmium and to other heavy metals. C. R. Biol. 2016, 339, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Liu, Q.F.; Liu, Y.; Zhu, J.N.; Zhang, Q. Endophytic bacterial diversity in roots of Typha angustifolia L. in the constructed Beijing Cuihu Wetland (China). Res. Microbiol. 2011, 162, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, U.D.; Saha, C.; Maiti, M.; Lahiri, S.; Ghosh, S.; Seal, A.; Mitra Ghosh, M. Root associated iron oxidizing bacteria increase phosphate nutrition and influence root to shoot partitioning of iron in tolerant plant Typha angustifolia. Plant Soil 2014, 381, 279–295. [Google Scholar] [CrossRef]
- Saha, C.; Mukherjee, G.; Agarwal-Banka, P.; Seal, A. A consortium of non-rhizobial endophytic microbes from Typha angustifolia functions as probiotic in rice and improves nitrogen metabolism. Plant Biol. J. 2016, 18, 938–946. [Google Scholar] [CrossRef]
- Shahid, M.J.; Ali, S.; Shabir, G.; Siddique, M.; Rizwan, M.; Seleiman, M.F.; Afzal, M. Comparing the performance of four macrophytes in bacterial assisted floating treatment wetlands for the removal of trace metals (Fe, Mn, Ni, Pb, and Cr) from polluted river water. Chemosphere 2020, 243, 125353. [Google Scholar] [CrossRef]
- Carranza-Álvarez, C.; Alonso-Castro, A.J.; Alfaro-De La Torre, M.C.; García-De La Cruz, R.F. Accumulation and distribution of heavy metals in Scirpus americanus and Typha latifolia from an artificial lagoon in San Luis Potosí, México. Water Air Soil Pollut. 2008, 188, 297–309. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Carranza-Álvarez, C.; Alfaro-De la Torre, M.C.; Chávez-Guerrero, L.; García-De la Cruz, R.F. Removal and accumulation of cadmium and lead by Typha latifolia exposed to single and mixed metal solutions. Arch Environ. Contam. Toxicol. 2009, 57, 688–696. [Google Scholar] [CrossRef]
- Leura-Vicencio, A.; Alonso-Castro, A.J.; Carranza-Álvarez, C.; Loredo-Portales, R.; Alfaro-De La Torre, M.C.; García-De La Cruz, R.F. Removal and accumulation of As, Cd and Cr by Typha latifolia. Bull. Environ. Contam. Toxicol. 2013, 90, 650–653. [Google Scholar] [CrossRef]
- Rolón-Cárdenas, G.A.; Arvizu-Gómez, J.L.; Pacheco-Aguilar, J.R.; Vázquez-Martínez, J.; Hernández-Morales, A. Cadmium-tolerant endophytic Pseudomonas rhodesiae strains isolated from Typha latifolia modify the root architecture of Arabidopsis thaliana Col-0 in presence and absence of Cd. Braz. J. Microbiol. 2020, 52, 349–361. [Google Scholar] [CrossRef]
- Zapata-Morales, A.L.; Alfaro-De la Torre, M.C.; Hernández-Morales, A.; García-De la Cruz, R.F. Isolation of cultivable bacteria associated with the root of Typha latifolia in a constructed wetland for the removal of diclofenac or naproxen. Water Air Soil Pollut. 2020, 231, 423. [Google Scholar] [CrossRef]
- Kinuthia, G.K.; Ngure, V.; Beti, D.; Lugalia, R.; Wangila, A.; Kamau, L. Levels of heavy metals in wastewater and soil samples from open drainage channels in Nairobi, Kenya: Community health implication. Sci. Rep. 2020, 10, 8434. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Barajas, E.; Ramírez-Díaz, M.I.; Riveros-Rosas, H.; Cervantes, C. Heavy Metal Resistance in Pseudomonads. In Pseudomonas: Volume 6: Molecular Microbiology, Infection and Biodiversity; Ramos, J.L., Filloux, A., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 255–282. [Google Scholar]
- Chen, L.; Luo, S.; Chen, J.; Wan, Y.; Liu, C.; Liu, Y.; Pang, X.; Lai, C.; Zeng, G. Diversity of endophytic bacterial populations associated with Cd-hyperaccumulator plant Solanum nigrum L. grown in mine tailings. Appl. Soil Ecol. 2012, 62, 24–30. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Q.; Huang, X.; Guo, X. Endophytic bacterial diversity in roots of Typha and the relationship of water quality factors in reclaimed water replenishment constructed wetland. China Environ. Sci. 2016, 36, 875–886. [Google Scholar]
- Mazhar, S.H.; Herzberg, M.; Ben Fekih, I.; Zhang, C.; Bello, S.K.; Li, Y.P.; Su, J.; Xu, J.; Feng, R.; Zhou, S.; et al. Comparative insights into the complete genome sequence of highly metal resistant Cupriavidus metallidurans strain BS1 isolated from a Gold–Copper mine. Front. Microbiol. 2020, 11, 47. [Google Scholar] [CrossRef] [Green Version]
- Shuaib, M.; Azam, N.; Bahadur, S.; Romman, M.; Yu, Q.; Xuexiu, C. Variation and succession of microbial communities under the conditions of persistent heavy metal and their survival mechanism. Microbial. Pathogenesis 2021, 150, 104713. [Google Scholar] [CrossRef]
- Dahanayake, P.S.; Hossain, S.; Wickramanayake, M.V.K.S.; Heo, G.-J. Antibiotic and heavy metal resistance genes in Aeromonas spp. isolated from marketed Manila Clam (Ruditapes philippinarum) in Korea. J. Appl. Microbiol. 2019, 127, 941–952. [Google Scholar] [CrossRef]
- Dickinson, A.W.; Power, A.; Hansen, M.G.; Brandt, K.K.; Piliposian, G.; Appleby, P.; O’Neill, P.A.; Jones, R.T.; Sierocinski, P.; Koskella, B.; et al. Heavy metal pollution and co-selection for antibiotic resistance: A microbial palaeontology approach. Environ. Int. 2019, 132, 105117. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Cruz-Paredes, C.; Zhang, S.; Rousk, J. Can heavy metal pollution induce bacterial resistance to heavy metals and antibiotics in soils from an ancient land-mine? J. Hazard. Mater. 2021, 411, 124962. [Google Scholar] [CrossRef]
- Zhang, H.; Song, Y.; Tan, R. Biology and chemistry of endophytes. Nat. Prod. Rep. 2006, 23, 753–771. [Google Scholar] [CrossRef]
- Ullah, A.; Heng, S.; Munis, M.F.H.; Fahad, S.; Yang, X. Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: A review. Environ. Exp. Bot. 2015, 117, 28–40. [Google Scholar] [CrossRef]
- Rolón-Cárdenas, G.A.; Arvizu-Gómez, J.L.; Soria-Guerra, R.E.; Pacheco-Aguilar, J.R.; Alatorre-Cobos, F.; Hernández-Morales, A. The role of auxins and auxin-producing bacteria in the tolerance and accumulation of cadmium by plants. Environ. Geochem. Health 2022, 44, 3743–3764. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Rajkumar, M.; Moreno, A.; Zhang, C.; Freitas, H. Serpentine endophytic bacterium Pseudomonas azotoformans ASS1 accelerates phytoremediation of soil metals under drought stress. Chemosphere 2017, 185, 75–85. [Google Scholar] [CrossRef]
- Choińska-Pulit, A.; Sobolczyk-Bednarek, J.; Łaba, W. Optimization of copper, lead and cadmium biosorption onto newly isolated bacterium using a Box-Behnken design. Ecotoxicol. Environ. Saf. 2018, 149, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Wu, L.; Chen, G.; Feng, G. Complete genome sequence of Pseudomonas azotoformans S4, a potential biocontrol bacterium. J. Biotechnol. 2016, 227, 25–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sang, M.K.; Kim, E.N.; Han, G.D.; Kwack, M.S.; Jeun, Y.C.; Kim, K.D. Priming-mediated systemic resistance in cucumber induced by Pseudomonas azotoformans GC-B19 and Paenibacillus elgii MM-B22 against Colletotrichum orbiculare. Phytopathology 2014, 104, 834–842. [Google Scholar] [CrossRef] [Green Version]
- Heidari-Nonakaran, S.; Pazhouhandeh, M.; Keyvani, A.; Abdollahipour, F.Z.; Shirzad, A. Isolation and identification of Pseudomonas azotoformans for induced calcite precipitation. World J. Microbiol. Biotechnol. 2015, 31, 1993–2001. [Google Scholar] [CrossRef]
- Saleem, M.; Asghar, H.N.; Zahir, Z.A.; Shahid, M. Impact of lead tolerant plant growth promoting rhizobacteria on growth, physiology, antioxidant activities, yield and lead content in sunflower in lead contaminated soil. Chemosphere 2018, 195, 606–614. [Google Scholar] [CrossRef]
- Soto, J.; Ortiz, J.; Herrera, H.; Fuentes, A.; Almonacid, L.; Charles, T.C.; Arriagada, C. Enhanced arsenic tolerance in Triticum aestivum inoculated with arsenic-resistant and plant growth promoter microorganisms from a heavy metal-polluted soil. Microorganisms 2019, 7, 348. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Wu, K.; Khan, A.; Jiang, Y.; Ling, Z.; Liu, P.; Chen, Y.; Tao, X.; Li, X. A novel Pseudomonas gessardii strain LZ-E simultaneously degrades naphthalene and reduces hexavalent chromium. Bioresour. Technol. 2016, 207, 370–378. [Google Scholar] [CrossRef] [Green Version]
- Begum, N.; Afzal, S.; Zhao, H.; Lou, L.; Cai, Q. Shoot endophytic plant growth-promoting bacteria reduce cadmium toxicity and enhance switchgrass (Panicum virgatum L.) biomass. Acta Physiologiae. Plantarum. 2018, 40, 170. [Google Scholar] [CrossRef]
- Begum, N.; Hu, Z.; Cai, Q.; Lou, L. Influence of PGPB Inoculation on HSP70 and HMA3 Gene Expression in Switchgrass under Cadmium Stress. Plants 2019, 8, 504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, M.L.; Gerbino, E.; Cavallero, G.J.; Casabuono, A.C.; Couto, A.S.; Gomez-Zavaglia, A.; Ramirez, S.A.M.; Vullo, D.L. Infrared spectroscopy with multivariate analysis to interrogate the interaction of whole cells and secreted soluble exopolimeric substances of Pseudomonas veronii 2E with Cd(II), Cu(II) and Zn(II). Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 228, 117820. [Google Scholar] [CrossRef] [PubMed]
- Garavaglia, L.; Cerdeira, S.B.; Vullo, D.L. Chromium (VI) biotransformation by β- and γ-Proteobacteria from natural polluted environments: A combined biological and chemical treatment for industrial wastes. J. Hazard. Mater. 2010, 175, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Lazzarini Behrmann, I.C.; Grattieri, M.; Minteer, S.D.; Ramirez, S.A.; Vullo, D.L. Online self-powered Cr(VI) monitoring with autochthonous Pseudomonas and a bio-inspired redox polymer. Anal. Bioanal. Chem. 2020, 412, 6449–6457. [Google Scholar] [CrossRef] [PubMed]
- Diazbarriga, F.; Santos, M.A.; Mejia, J.D.; Batres, L.; Yanez, L.; Carrizales, L.; Vera, E.; Delrazo, L.M.; Cebrian, M.E. Arsenic and cadmium exposure in children living near a smelter complex in San Luis Potosí, Mexico. Environ. Res. 1993, 62, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Rolón-Cárdenas, G.A.; Martínez-Martínez, J.G.; Arvizu-Gómez, J.L.; Soria-Guerra, R.E.; Alfaro-De la Torre, M.C.; Alatorre-Cobos, F.; Rubio-Santiago, J.; González-Balderas, R.d.M.; Carranza-Álvarez, C.; Macías-Pérez, J.R.; et al. Enhanced Cd-accumulation in Typha latifolia by Interaction with Pseudomonas rhodesiae GRC140 under axenic hydroponic conditions. Plants 2022, 11, 1447. [Google Scholar] [CrossRef]
- Ren, M.; Qin, Z.; Li, X.; Wang, L.; Wang, Y.; Zhang, J.; Huang, Y.; Yang, S. Selenite antagonizes the phytotoxicity of Cd in the cattail Typha angustifolia. Ecotoxicol. Environ. Saf. 2020, 189, 109959. [Google Scholar] [CrossRef]
- Liu, K.-J.; Xu, X.-D. First Report of Gray Leaf Spot of Maize Caused by Cercospora zeina in China. Plant Disease 2013, 97, 1656. [Google Scholar] [CrossRef]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef]
- Patel, N.P.; Shimpi, G.G.; Haldar, S. A comparative account of resistance and antagonistic activity of healthy and bleached coral-associated bacteria as an indicator of coral health status. Ecol. Indic. 2021, 120, 106886. [Google Scholar] [CrossRef]
- Chen, W.-P.; Kuo, T.-T. A simple and rapid method for the preparation of gram-negative bacterial genomic DNA. Nucleic Acids Res. 1993, 21, 2260. [Google Scholar] [CrossRef] [PubMed]
- Abdelmoteleb, A.; Gonzalez-Mendoza, D. Isolation and identification of phosphate solubilizing Bacillus spp. from Tamarix ramosissima rhizosphere and their effect on growth of Phaseolus vulgaris under salinity stress. Geomicrobiol. J. 2020, 37, 901–908. [Google Scholar] [CrossRef]
- Alexander, D.B.; Zuberer, D.A. Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol. Fertil. Soils 1991, 12, 39–45. [Google Scholar] [CrossRef]
- Penrose, D.M.; Glick, B.R. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol. Plant 2003, 118, 10–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dworkin, M.; Foster, J. Experiments with some microorganisms which utilize ethane and hydrogen. J. Bacteriol. 1958, 75, 592–601. [Google Scholar] [CrossRef] [Green Version]
- Ruiza, D.; Agaras, B.; de Werrab, P.; Wall, L.G.; Valverde, C. Characterization and screening of plant probiotic traits of bacteria isolated from rice seeds cultivated in Argentina. J. Microbiol. 2011, 49, 902–912. [Google Scholar] [CrossRef]



| Strain | Identity | Gene Bank Accession Number | ||
|---|---|---|---|---|
| 16S rRNA | gyrA | rpoD | ||
| JEP3 | Pseudomonas azotoformans | MT280006 | MW924893 | MW924899 |
| JEP8 | Pseudomonas florescens | MT280007 | MW924894 | MW924900 |
| JEP33 | Pseudomonas gessardii | MT280008 | MW924895 | MW924901 |
| JEC8 | Pseudomonas veronii. | MT280009 | MW924890 | MW924896 |
| JEC9 | Pseudomonas veronii. | MT280010 | MW924891 | MW924897 |
| JEC11 | Pseudomonas veronii | MT280011 | MW924892 | MW924898 |
| Class | Antibiotic | Content (µg) | Strain | |||||
|---|---|---|---|---|---|---|---|---|
| JEP3 | JEP8 | JEP33 | JEC8 | JEC9 | JEC11 | |||
| Penicillins | Penicillin (PEN) | 6 (10 IU) | S | S | S | S | S | S |
| Ampicillin (AMP) | 10 | S | S | S | S | S | S | |
| Carbenicillin (CAR) | 100 | S | S | S | S | S | S | |
| Dicloxacillin (DCX) | 1 | S | S | S | S | S | S | |
| Tetracyclines | Tetracycline (TET) | 30 | R | R | R | R | R | R |
| Cephalosporins | Cephalothin (CEF) | 30 | S | S | S | S | S | S |
| Ceftriaxone (CRO) | 30 | I | R | S | S | S | R | |
| Cefotaxime (CTX) | 30 | S | S | S | S | S | S | |
| Ceftazidime (CAZ) | 30 | S | S | S | S | S | S | |
| Cefuroxime (CXM) | 30 | S | S | S | S | S | S | |
| Quinolones | Pefloxacin (PEF) | 5 | R | R | R | R | R | R |
| Macrolides | Erythromycin (ERY) | 15 | S | S | S | S | S | S |
| Aminoglycoside | Amikacin (AMK) | 30 | R | R | R | R | R | R |
| Gentamicin (GEN) | 10 | R | R | R | R | R | R | |
| Netilmicin (NET) | 30 | R | R | R | R | R | R | |
| Phenicols | Chloramphenicol (CHL) | 30 | I | S | S | R | S | S |
| Nitrofurans | Nitrofurantoin (NIT) | 300 | S | S | S | S | S | S |
| Diaminopyrimidine/Sulfonamides | Trimethoprim/Sulfamethoxazole (SXT) | 25 | S | S | S | S | S | S |
| MAR index | 0.28 | 0.33 | 0.28 | 0.33 | 0.28 | 0.33 | ||
| Strain | Siderophore Production | ACC Activity | Solubilization Ca(PO3)2 | Indole Compounds Production (µg/mL) | |
|---|---|---|---|---|---|
| Salkowski | IAA | ||||
| JEP3 | + | + | + | 23.2 ± 1.3 ab | 0.079 ± 0.001 bc |
| JEP8 | + | − | + | 17.5 ± 1.5 b | 0.094 ± 0.009 b |
| JEP33 | + | + | + | 14.7± 0.5 bc | 0.069 ± 0.0004 c |
| JEC8 | + | + | + | 31.9 ± 7.1 a | 0.080 ± 0.002 bc |
| JEC9 | + | + | + | 6.3 ± 1.0 c | 0.115 ± 0.013 a |
| JEC11 | + | + | + | 8.4 ± 2.0 c | 0.080 ± 0.003 bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubio-Santiago, J.; Hernández-Morales, A.; Rolón-Cárdenas, G.A.; Arvizu-Gómez, J.L.; Soria-Guerra, R.E.; Carranza-Álvarez, C.; Rubio-Salazar, J.E.; Rosales-Loredo, S.; Pacheco-Aguilar, J.R.; Macías-Pérez, J.R.; et al. Characterization of Endophytic Bacteria Isolated from Typha latifolia and Their Effect in Plants Exposed to Either Pb or Cd. Plants 2023, 12, 498. https://doi.org/10.3390/plants12030498
Rubio-Santiago J, Hernández-Morales A, Rolón-Cárdenas GA, Arvizu-Gómez JL, Soria-Guerra RE, Carranza-Álvarez C, Rubio-Salazar JE, Rosales-Loredo S, Pacheco-Aguilar JR, Macías-Pérez JR, et al. Characterization of Endophytic Bacteria Isolated from Typha latifolia and Their Effect in Plants Exposed to Either Pb or Cd. Plants. 2023; 12(3):498. https://doi.org/10.3390/plants12030498
Chicago/Turabian StyleRubio-Santiago, Jesús, Alejandro Hernández-Morales, Gisela Adelina Rolón-Cárdenas, Jackeline Lizzeta Arvizu-Gómez, Ruth Elena Soria-Guerra, Candy Carranza-Álvarez, Jocabed Eunice Rubio-Salazar, Stephanie Rosales-Loredo, Juan Ramiro Pacheco-Aguilar, José Roberto Macías-Pérez, and et al. 2023. "Characterization of Endophytic Bacteria Isolated from Typha latifolia and Their Effect in Plants Exposed to Either Pb or Cd" Plants 12, no. 3: 498. https://doi.org/10.3390/plants12030498
APA StyleRubio-Santiago, J., Hernández-Morales, A., Rolón-Cárdenas, G. A., Arvizu-Gómez, J. L., Soria-Guerra, R. E., Carranza-Álvarez, C., Rubio-Salazar, J. E., Rosales-Loredo, S., Pacheco-Aguilar, J. R., Macías-Pérez, J. R., Aldaba-Muruato, L. R., & Vázquez-Martínez, J. (2023). Characterization of Endophytic Bacteria Isolated from Typha latifolia and Their Effect in Plants Exposed to Either Pb or Cd. Plants, 12(3), 498. https://doi.org/10.3390/plants12030498






