Oxidative Stress Response and Metal Transport in Roots of Macleaya cordata Exposed to Lead and Zinc
Abstract
:1. Introduction
2. Results
2.1. Effects of Pb and Zn on Shoot Height and Root Length of M. cordata
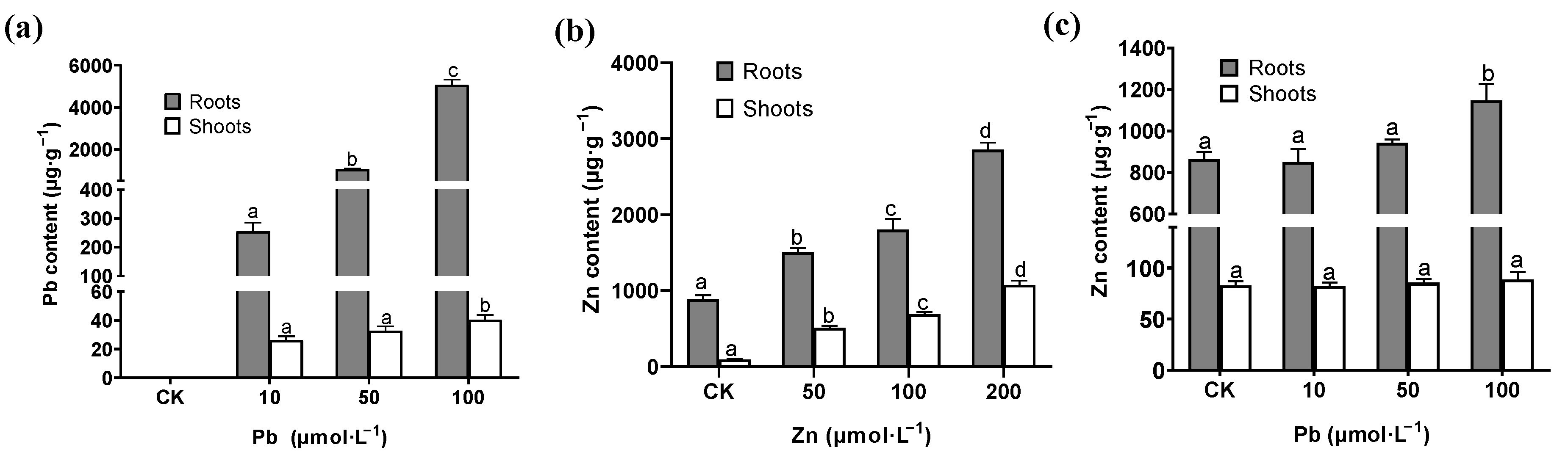
2.2. Content of Pb and Zn in Roots of M. cordata
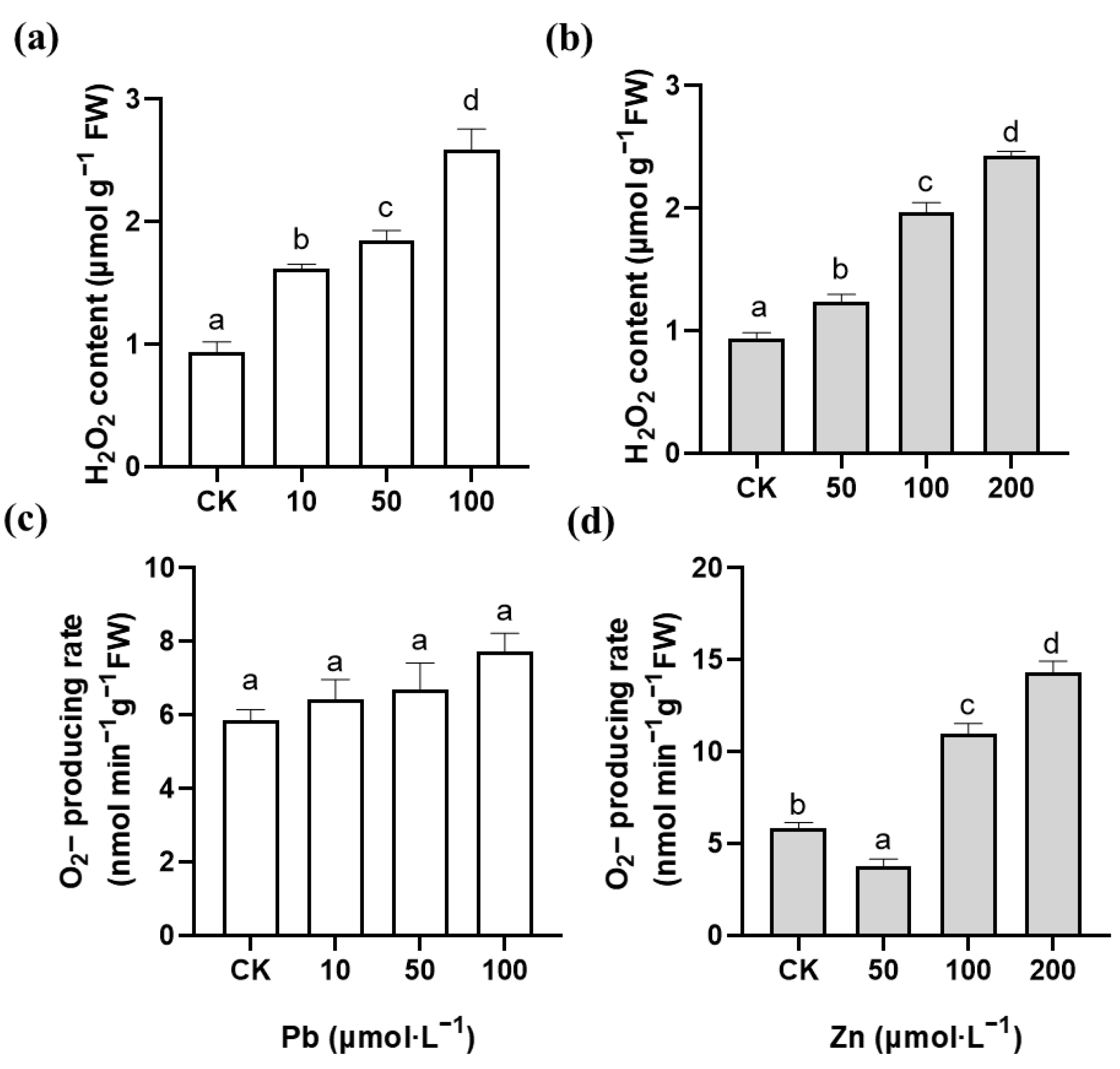
2.3. Effects of Pb and Zn on Production of ROS in Root Tips of M. cordata
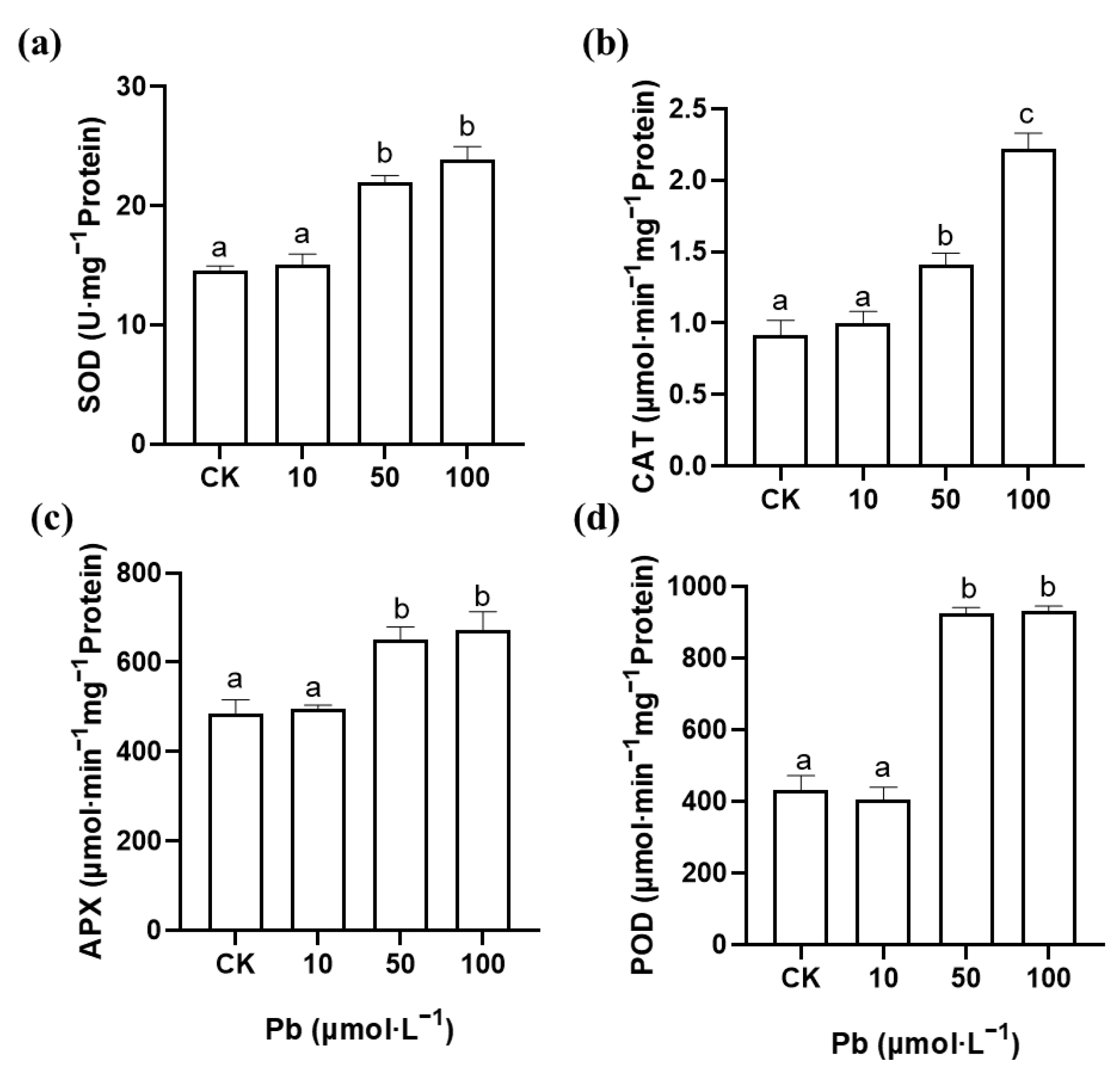
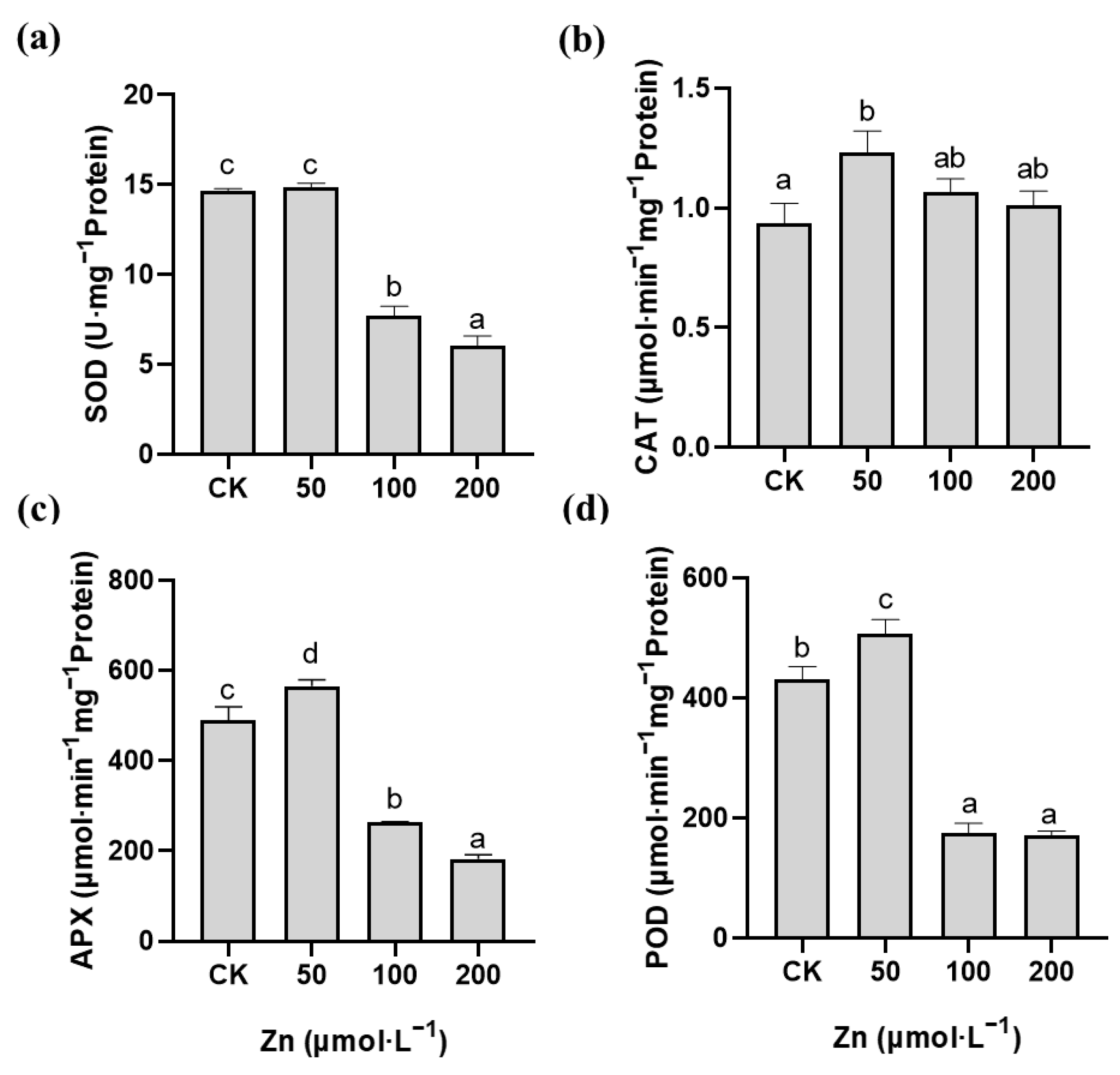
2.4. Effects of Pb and Zn on Antioxidant Enzyme Activity in Roots of M. cordata
3. Discussion
4. Materials and Methods
4.1. Hydroponic Culture
4.2. Metal Content Analysis
4.3. Histochemical Detection of Metals, H2O2 and O2− In Situ
4.4. Determination of H2O2, and O2− in the Root Extracts
4.5. Isolation of the Plasma Membrane and Determination of NADPH Oxidase Activity
4.6. Enzyme Activity Assay
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals-Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Lei, M.; Chen, T. Cost–benefit calculation of phytoremediation technology for heavy-metal-contaminated soil. Sci. Total Environ. 2016, 563–564, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.S.; Jin, H.P.; Robinson, B.; Naidu, R.; Huh, K.Y. Phytostabilization: A green approach to contaminant containment. Adv. Agron. 2011, 112, 145–204. [Google Scholar]
- Tang, Z.; Wang, H.; Chen, J.; Chang, J.; Zhao, F. Molecular mechanisms underlying the toxicity and detoxification of trace metals and metalloids in plants. J. Integr. Plant Biol. 2022. [Google Scholar] [CrossRef]
- Clemens, S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 2006, 88, 1707–1719. [Google Scholar] [CrossRef]
- Baker, A.J.M.; Reeves, R.D.; Hajar, A.S.M. Heavy metal accumulation and tolerance in British populations of the metallophyte Thlaspi caerulescens J & C Presl. (Brasicaceae). New Phytol. 1994, 127, 61–68. [Google Scholar] [PubMed]
- Kamran, M.; Asif, S.; Nadeem, H.; Saddam, S.; Muhammad, R. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar]
- Fukao, Y.; Ferjani, A.; Tomioka, R.; Nagasaki, N.; Kurata, R.; Nishimori, Y.; Maeshima, F.M. iTRAQ analysis reveals mechanisms of growth defects due to excess zinc in Arabidopsis. Plant Physiol. 2011, 155, 1893–1907. [Google Scholar] [CrossRef] [Green Version]
- Reeves, R.D.; Baker, A.G.M. Metal accumulation plant. In Phytoremediation of Toxic Metals: Using Plant to Clean up the Environment; Terry, N., Banuelos, G.S., Eds.; John Wiley Sons Inc.: New York, NY, USA, 2000; pp. 193–224. [Google Scholar]
- Zhang, H.; Zhang, F.; Xia, Y.; Wang, G.; Shen, Z. Excess copper induces production of hydrogen peroxide in the leaf of Elsholtzia haichowensis through apoplastic and symplastic CuZn-superoxide dismutase. J. Hazard. Mater. 2010, 178, 834–843. [Google Scholar] [CrossRef]
- Deng, X.; Xia, Y.; Hu, W.; Zhang, H.; Shen, Z.G. Cadmium-induced oxidative damage and protective effects of N-acetyl-L-cysteine against cadmium toxicity in Solanum nigrum L. J. Hazard. Mater. 2010, 180, 722–729. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Lou, X.; Tang, M. Mycorrhizal and non-mycorrhizal Medicago truncatula roots exhibit differentially regulated NADPH oxidase and antioxidant response under Pb stress. Environ. Exp. Bot. 2019, 164, 10–19. [Google Scholar] [CrossRef]
- Jin, X.; Yang, X.; Islam, E.; Liu, D.; Mahmood, Q.; Li, H.; Li, J. Ultrastructural changes, zinc hyperaccumulation and its relation with antioxidants in two ecotypes of Sedum alfredii Hance. Plant Physiol. Biochem. 2008, 46, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Sai, C.; Li, D.; Xue, C.; Wang, K.; Hu, P.; Pei, Y.; Bai, J.; Jing, Y.; Li, Z.; Hua, H. Two pairs of enantiomeric alkaloid dimers from Macleaya cordata. Org. Lett. 2015, 17, 4102–4105. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, Y.; Huang, P.; Ma, Y.; Qing, Z.; Tang, Q.; Cao, H.; Cheng, P.; Zheng, Y.; Yuan, Z.; et al. The genome of medicinal plant Macleaya cordata provides new insights into benzylisoquinoline alkaloids metabolism. Mol. Plant 2017, 10, 975–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Hu, N.; Ding, D.; Hu, J.; Li, G.; Wang, Y. Phytoextraction of uranium from contaminated soil by Macleaya cordata before and after application of EDDS and CA. Environ. Sci. Pollut. Res. 2015, 22, 6155–6163. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; Li, J.; Zhang, H.; Xia, Y.; Chen, C.; Shen, Z.; Chen, Y. Several newly discovered Mo-enriched plants with a focus on Macleaya cordata. Environ. Sci. Pollut. Res. 2018, 25, 26493–26503. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Liu, Y.; Zeng, G.; Zheng, B.; Tan, X.; Liu, H.; Xie, J.; Gan, C.; Liu, W. Cadmium accumulation and tolerance of Macleaya cordata: A newly potential plant for sustainable phytoremediation in Cd-contaminated soil. Environ. Sci. Pollut. Res. 2016, 23, 10189–10199. [Google Scholar] [CrossRef]
- Cai, B.; Chen, Y.; Du, L.; Liu, Z.; He, L. Spent mushroom compost and calcium carbonate modification enhances phytoremediation potential of Macleaya cordata to lead-zinc mine tailings. J. Environ. Manag. 2021, 294, 113029. [Google Scholar] [CrossRef]
- Pan, G.; Zhang, H.; Liu, W.; Liu, P. Integrative study of subcellular distribution, chemical forms, and physiological responses for understanding manganese tolerance in the herb Macleaya cordata (papaveraceae). Ecotoxicol. Environ. Saf. 2019, 181, 455–462. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, W.; Chen, Y.; Xu, H.; Hou, D.; Lv, S.; Sun, X.; Wang, F.; Yang, L. The tolerance, absorption, and transport characteristics of Macleaya cordata in relation to lead, zinc, cadmium, and copper under hydroponic conditions. Appl. Sci. 2022, 12, 9598. [Google Scholar] [CrossRef]
- Zhang, H.; Xia, Y.; Wang, G.; Shen, Z. Excess copper induces accumulation of hydrogen peroxide and increases lipid peroxidation and total activity of copper-zinc superoxide dismutase in roots of Elsholtzia haichowensis. Planta 2008, 227, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Enot, M.M.; Weiland, F.; Mittal, P.; Hoffmann, P.; Sillero-Mahinay, M.; Pukala, T. Differential proteome analysis of the leaves of lead hyperaccumulator, Rhoeo discolor (L. Her.) Hance. J. Mass. Spectrom. 2021, 56, e4689. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.K.; Huang, H.G.; Yang, X.E.; Razafindrabe, B.; Inouhe, M. The detoxification of lead in Sedum alfredii H. is not related to phytochelatins but the glutathione. J. Hazard. Mater. 2010, 177, 437–444. [Google Scholar] [CrossRef]
- Meyer-Klaucke, W.; Kupper, H.; Mijovilovich, A.; Pmh, K. Tissue- and age-dependent differences in the complexation of cadmium and zinc in the cadmium/zinc hyperaccumulator Thlaspi caerulescens (Ganges ecotype) revealed by X-ray absorption spectroscopy. Plant Physiol. 2004, 134, 748–757. [Google Scholar]
- Vázquez, M.; Poschenrieder, C.; Barceló, J.; Baker, A.; And, P.H.; Cope, G.H. Compartmentation of zinc in roots and leaves of the zinc hyperaccumulator Thlaspi caerulescens J & C Presl. Bot. Acta 1994, 107, 243–250. [Google Scholar]
- Sarret, G.; Saumitou-Laprade, P.; Bert, V.; Proux, O.; Hazemann, J.L.; Traverse, A.; Marcus, M.; Manceau, A. Forms of zinc accumulated in the hyperaccumulator Arabidopsis halleri. Plant Physiol. 2003, 130, 1815–1826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinozaki, D.; Yoshimoto, K. Autophagy balances the zinc-iron seesaw caused by Zn-stress. Trends Plant Sci. 2021, 26, 882–884. [Google Scholar] [CrossRef]
- Romero-Puertas, M.; Rodriguez-Serrano, M.; Corpas, F.; Gomez, M.; Rio, L.; Sandalio, L. Cadmium-induced subcellular accumulation of O2·− and H2O2 in pea leaves. Plant Cell Environ. 2004, 27, 1122–1134. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J. Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol. 2001, 42, 1265–1273. [Google Scholar] [CrossRef] [Green Version]
- Quan, L.; Zhang, B.; Shi, W.; Li, H. Hydrogen peroxide in plants: A versatile molecule of reactive oxygen species network. J. Integr. Plant Biol. 2008, 50, 2–18. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, S.; Xu, H.; Hou, D.; Li, Y.; Wang, F. H2O2 is involved in the metallothionein-mediated rice tolerance to copper and cadmium toxicity. Int. J. Mol. Sci. 2017, 18, 2083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Xia, Y.; Chen, C.; Zhuang, K.; Song, Y.; Shen, Z. Analysis of copper-binding proteins in rice radicles exposed to excess copper and hydrogen peroxide stress. Front. Plant Sci. 2016, 7, 1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sgherri, C.; Quartacci, M.F.; Navari-Izzo, F. Early production of activated oxygen species in root apoplast of wheat following copper excess. J. Plant Physiol. 2007, 164, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, H.; Wang, G.; Xu, L.; Shen, Z. Cadmium-induced accumulation of hydrogen peroxide in the leaf apoplast of Phaseolus aureus and Vicia sativa and the roles of different antioxidant enzymes. J. Hazard. Mater. 2009, 168, 16–87. [Google Scholar] [CrossRef] [PubMed]
- Sagi, M.; Fluhr, R. Superoxide production by plant homologues of the gp91phox NADPH oxidase. Modulation of activity by calcium and by tobacco mosaic virus infection. Plant Physiol. 2001, 126, 1281–1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aebi, H. Catalase in vitro. Method. Enzymol. 1984, 105, 121–126. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in Spinach chloroplasts. Plant Cell Physiol. 1980, 22, 867–880. [Google Scholar]
- Zheng, X.; Huystee, R. Peroxidase-regulated elongation of segments from peanut hypocotyls. Plant Sci. 1992, 81, 47–56. [Google Scholar] [CrossRef]
- Giannopolitis, C.; Ries, S. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye-binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Sun, X.; Hwarari, D.; Du, X.; Wang, Y.; Xu, H.; Lv, S.; Wang, T.; Yang, L.; Hou, D. Oxidative Stress Response and Metal Transport in Roots of Macleaya cordata Exposed to Lead and Zinc. Plants 2023, 12, 516. https://doi.org/10.3390/plants12030516
Zhang H, Sun X, Hwarari D, Du X, Wang Y, Xu H, Lv S, Wang T, Yang L, Hou D. Oxidative Stress Response and Metal Transport in Roots of Macleaya cordata Exposed to Lead and Zinc. Plants. 2023; 12(3):516. https://doi.org/10.3390/plants12030516
Chicago/Turabian StyleZhang, Hongxiao, Xijing Sun, Delight Hwarari, Xinlong Du, Yinghao Wang, Huawei Xu, Shufang Lv, Ting Wang, Liming Yang, and Dianyun Hou. 2023. "Oxidative Stress Response and Metal Transport in Roots of Macleaya cordata Exposed to Lead and Zinc" Plants 12, no. 3: 516. https://doi.org/10.3390/plants12030516
APA StyleZhang, H., Sun, X., Hwarari, D., Du, X., Wang, Y., Xu, H., Lv, S., Wang, T., Yang, L., & Hou, D. (2023). Oxidative Stress Response and Metal Transport in Roots of Macleaya cordata Exposed to Lead and Zinc. Plants, 12(3), 516. https://doi.org/10.3390/plants12030516





