Cultivation Using Coir Substrate and P or K Enriched Fertilizer Provides Higher Resistance to Drought in Ecologically Diverse Quercus Species
Abstract
:1. Introduction
2. Results
2.1. Water Stress Effects on Seedling Survival, Leaf Mortality, Plant Height, and Recovery after Irrigation
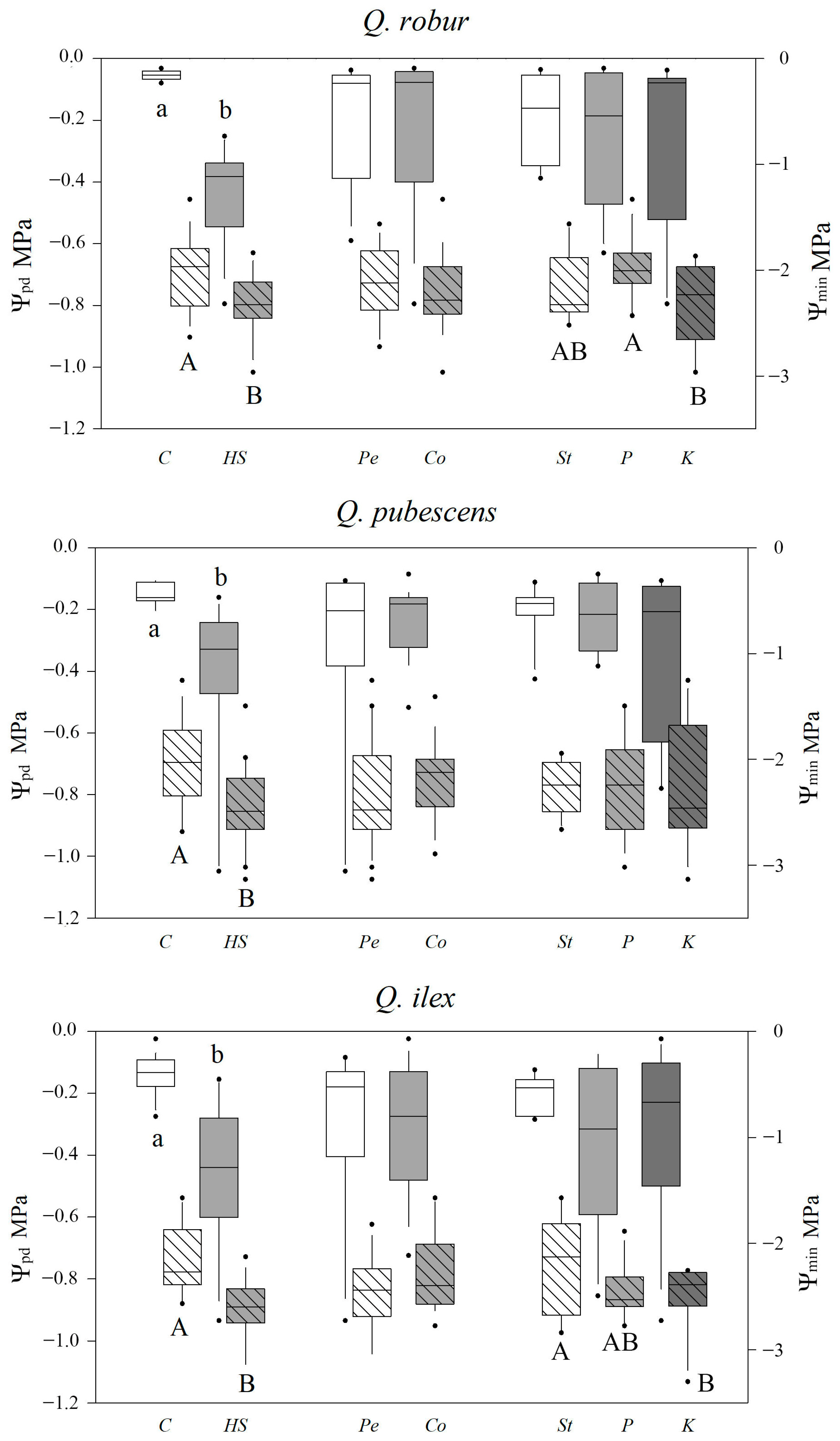
2.2. Leaf Water Potential
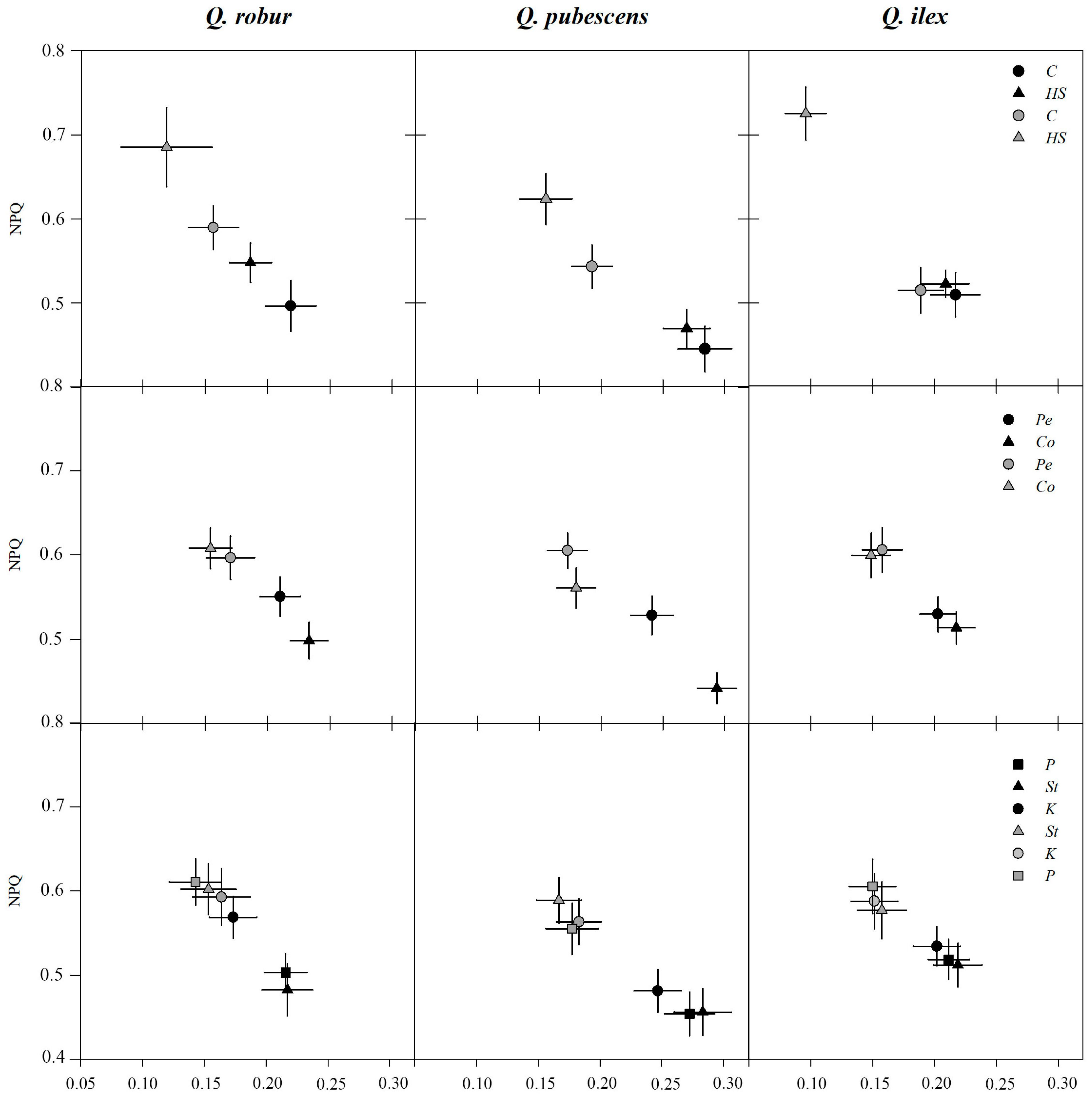
2.3. Chlorophyll Fluorescence
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Experimental Design
4.3. Seedling Survival, Leaf Mortality, Plant Height, and Recovery after Water Stress Treatments Application
4.4. Leaf Water Potential and Chlorophyll Fluorescence during Water Stress Experiment
4.5. Statistical Analysis
5. Conclusions
- Contrary to our hypothesis, seedlings grown in coir performed equally or better than in peat in water stress response; this could be due to the lower shoot-to-root ratio of seedlings grown in this substrate. This can be an advance in the way of looking for sustainable alternatives for peat.
- Nursery fertilization with high P promoted survival under water stress conditions in the trial, probably related to the physiological benefit of higher tissue P concentration after nursery cultivation.
- K-enriched fertilization at the nursery stage resulted in inconsistent findings under high water stress during the following growing season, with greater mortality in Q. robur, greater survival in Q. pubescens, and no effects in Q. ilex as compared to the standard fertilization, and no relevant effects on height growth. The negative effect on Q. robur could be due to the lack of accumulation of this element in the tissues after nursery cultivation in combination with the bigger size of the nursery seedlings that might have suffered the limited volume explorable by the roots in the pot.
- Q. pubescens—despite being described as a species with an intermediate stress resistance in Mediterranean environments—showed high resistance and acclimation to severe water stress, comparable, or better than Q. ilex.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef] [Green Version]
- Kovats, R.S.; Valentini, R.; Bouwer, L.M.; Georgopoulou, E.; Jacob, D.; Martin, E.; Rounsevell, M.; Soussana, J.-F. Europe. In Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part B: Regional Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Barros, V.R., Field, C.B., Dokken, D.J., Mastrandrea, M.D., Mach, K.J., Bilir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 1267–1326. [Google Scholar]
- Cook, B.I.; Mankin, J.S.; Anchukaitis, K.J. Climate Change and Drought: From Past to Future. Curr. Clim. Chang. Rep. 2018, 4, 164–179. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Lasanta, T.; Gracia, C. Aridification determines changes in forest growth in Pinus halepensis forests under semiarid Mediterranean climate conditions. Agric. For. Meteorol. 2010, 150, 614–628. [Google Scholar] [CrossRef]
- CRED. Centre for Research on the Epidemiology of Disasters 2021 Extreme Weather Events in Europe. CRED Crunch 64. Available online: https://www.emdat.be/cred-crunch-64-extreme-weather-events-europe (accessed on 27 December 2022).
- Tatarinov, F.; Rotenberg, E.; Maseyk, K.; Ogée, J.; Klein, T.; Yakir, D. Resilience to seasonal heat wave episodes in a Mediterranean pine forest. New Phytol. 2016, 210, 485–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Ramirez, N.; Santonja, M.; Baldy, V.; Ballini, C.; Montès, N. Shrub species richness decreases negative impacts of drought in a Mediterranean ecosystem. J. Veg. Sci. 2017, 28, 985–996. [Google Scholar] [CrossRef]
- Bussotti, F.; Pollastrini, M. Opportunities and threats of Mediterranean evergreen sclerophyllous woody species subjected to extreme drought events. Appl. Sci. 2020, 10, 8458. [Google Scholar] [CrossRef]
- Maestre, F.T.; Valladares, F.; Reynolds, J.F. Is the change of plant-plant interactions with abiotic stress predictable? A meta-analysis of field results in arid environments. J. Ecol. 2005, 937, 748–757. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Berry, J.A.; Smith, D.D.; Sperry, J.S.; Anderegg, L.D.L.; Field, C.B. The roles of hydraulic and carbon stress in a widespread climate-induced forest die-off. Proc. Natl. Acad. Sci. USA 2012, 109, 233–237. [Google Scholar] [CrossRef] [Green Version]
- Newbold, T.; Oppenheimer, P.; Etard, A.; Williams, J.J. Tropical and Mediterranean biodiversity is disproportionately sensitive to land-use and climate change. Nat. Ecol. Evol. 2020, 41, 1630–1638. [Google Scholar] [CrossRef]
- Blondel, J.; Aronson, J.; Bodiou, J.Y.; Boeuf, G. The Mediterranean Region: Biological Diversity in Space and Time; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Merlin, M.; Perot, T.; Perret, S.; Korboulewsky, N.; Vallet, P. Effects of stand composition and tree size on resistance and resilience to drought in sessile oak and Scots pine. For. Ecol. Manag. 2015, 339, 22–33. [Google Scholar] [CrossRef]
- Pröll, G.; Hietz, P.; Delaney, C.M.; Katzensteiner, K. Substrate influences ecophysiological performance of tree seedlings. Tree Physiol. 2015, 36, 39–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, A.S.; Pinto, J.R. The scientific basis of the target plant concept: An overview. Forests 2021, 12, 1293. [Google Scholar] [CrossRef]
- Close, D.C.; Beadle, C.L.; Brown, P.H. The physiological basis of containerised tree seedling ‘transplant shock’: A review. Aust. For. 2005, 68, 112–120. [Google Scholar] [CrossRef]
- Cuesta, B.; Villar-Salvador, P.; Puértolas, J.; Jacobs, D.F.; Rey Benayas, J.M. Why do large, nitrogen rich seedlings better resist stressful transplanting conditions? A physiological analysis in two functionally contrasting Mediterranean forest species. For. Ecol. Manag. 2010, 260, 71–78. [Google Scholar] [CrossRef]
- Stanturf, J.A.; Schoenholtz, S.H.; Schweitzer, C.J.; Shepard, J.P. Achieving restoration success: Myths in bottomland hardwood forests. Restor. Ecol. 2001, 9, 189–200. [Google Scholar] [CrossRef]
- Vallejo, V.R.; Smanis, A.; Chirino, E.; Fuentes, D.; Valdecantos, A.; Vilagrosa, A. Perspectives in dryland restoration: Approaches for climate change adaptation. New For. 2012, 43, 561–579. [Google Scholar] [CrossRef] [Green Version]
- Beikircher, B.; Florineth, F.; Mayr, S. Restoration of rocky slopes based on planted gabions and use of drought-preconditioned woody species. Ecol. Eng. 2010, 36, 421–426. [Google Scholar] [CrossRef]
- Grossnickle, S.C. Why seedlings survive: Influence of plant attributes. New For. 2012, 43, 711–738. [Google Scholar] [CrossRef]
- Villar-Salvador, P.; Peñuelas, J.L.; Nicolás-Peragón, J.L.; Benito, L.F.; Domínguez-Lerena, S. Is nitrogen fertilization in the nursery a suitable tool for enhancing the performance of Mediterranean oak plantations? New For. 2013, 44, 733–751. [Google Scholar] [CrossRef]
- Toca, A.; Moler, E.; Nelson, A.; Jacobs, D.F. Environmental conditions in the nursery regulate root system development and architecture of forest tree seedlings: A systematic review. New For. 2022, 53, 1113–1143. [Google Scholar] [CrossRef]
- Wilson, B.C.; Jacobs, D.F. Quality Assessment of Temperate Zone Deciduous Hardwood Seedlings. New For. 2006, 31, 417–433. [Google Scholar] [CrossRef]
- Pinto, J.R.; Dumroese, R.K.; Davis, A.S.; Landis, T.D. Conducting seedling stocktype trials: A new approach to an old question. J. For. 2011, 109, 293–299. [Google Scholar]
- Dumroese, R.K.; Landis, T.D.; Pinto, J.R.; Haase, L.; Wilkinson, K.W.; Davis, A.S. Meeting forest restoration challenges: Using the Target Plant Concept. Reforesta 2016, 1, 37–52. [Google Scholar] [CrossRef] [Green Version]
- Haase, D.L.; Davis, A.S. Developing and supporting quality nursery facilities and staff are necessary to meet global forest and landscape restoration needs. Reforesta 2017, 4, 69–93. [Google Scholar] [CrossRef] [Green Version]
- van der Driessche, R. Changes in drought resistance and root growth capacity of container seedlings in response to nursery drought, nitrogen, and potassium treatments. Can. J. For. Res. 1992, 22, 740–749. [Google Scholar] [CrossRef]
- Valliere, J.M.; Zhang, J.; Sharifi, M.R.; Rundel, P.W. Can we condition native plants to increase drought tolerance and improve restoration success? Ecol. Appl. 2019, 29, e01863. [Google Scholar] [CrossRef]
- Villar-Salvador, P.; Planelles, R.; Oliet, J.; Peñuelas-Rubira, J.L.; Jacobs, D.F.; González, M. Drought tolerance and transplanting performance of holm oak (Quercus ilex) seedlings after drought hardening in the nursery. Tree Physiol. 2004, 24, 1147–1155. [Google Scholar] [CrossRef] [Green Version]
- Nardini, A.; Petruzzellis, F.; Marusig, D.; Tomasella, M.; Natale, S.; Altobelli, A.; Calligaris, C.; Floriddia, G.; Cucchi, F.; Forte, E.; et al. Water ‘on the rocks’: A summer drink for thirsty trees? New Phytol. 2021, 229, 199–212. [Google Scholar] [CrossRef]
- Cortina, J.; Vilagrosa, A.; Trubat, R. The role of nutrients for improving seedling quality in drylands. New For. 2013, 44, 719–732. [Google Scholar] [CrossRef]
- Poorter, H.; Hendrik, A.; Nagel, O. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: A quantitative review. Aust. J. Plant Physiol. 2000, 27, 591–607. [Google Scholar]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Moler, E.R.V.; Toca, A.; Jacobs, D.F.; Nelson, A.S. Root system adaptations represent untapped opportunities for forest tree seedling improvement. New For. 2022, 53, 1069–1091. [Google Scholar] [CrossRef]
- Luis, V.C.; Llorca, M.; Chirino, E.; Hernández, E.I.; Vilagrosa, A. Differences in morphology, gas exchange and root hydraulic conductance before planting in Pinus canariensis seedlings growing under different fertilization and light regimes. Trees Struct. Funct. 2010, 24, 1143–1150. [Google Scholar] [CrossRef]
- Domínguez, M.T.; Aponte, C.; Pérez-Ramos, I.M.; García, L.V.; Villar, R.; Marañón, T. Relationships between leaf morphological traits, nutrient concentrations and isotopic signatures for Mediterranean woody plant species and communities. Plant Soil 2012, 357, 407–424. [Google Scholar] [CrossRef] [Green Version]
- Razaq, M.; Zhang, P.; Shen, H.L. Influence of nitrogen and phosphorous on the growth and root morphology of Acer mono. PLoS ONE 2017, 12, e0171321. [Google Scholar] [CrossRef] [Green Version]
- Pemán, J.; Chirino, E.; Espelta, J.M.; Jacobs, D.F.; Martín-GómezRafael, P.; Navarro-Cerrillo, R.; Oliet, J.A.; Vilagrosa, A.; Villar-Salvador, P.; Gil-Pelegrín, E. Physiological keys for natural and artificial regeneration of oaks. In Oaks Physiological Ecology. Exploring the Functional Diversity of Genus Quercus L.; Springer: Cham, Switzerland, 2017; pp. 453–511. [Google Scholar]
- Sigala, J.A.; Uscola, M.; Oliet, J.A.; Jacobs, D.F. Drought tolerance and acclimation in Pinus ponderosa seedlings: The influence of nitrogen form. Tree Physiol. 2020, 40, 1165–1177. [Google Scholar] [CrossRef]
- Mondragón-Valero, A.; Lopéz-Cortés, I.; Salazar, D.M.; de Córdova, P.F. Physical mechanisms produced in the development of nursery almond trees (Prunus dulcis Miller) as a response to the plant adaptation to different substrates. Rhizosphere 2017, 3, 44–49. [Google Scholar] [CrossRef]
- Villar-Salvador, P.; Valladares, F.; Domínguez-Lerena, S.; Ruiz-Díez, B.; Fernández-Pascual, M.; Delgado, A.; Peñuelas, J.L. Functional traits related to seedling performance in the Mediterranean leguminous shrub Retama sphaerocarpa: Insights from a provenance, fertilization, and rhizobial inoculation study. Environ. Exp. Bot. 2008, 64, 145–164. [Google Scholar] [CrossRef]
- Grossnickle, S.C.; MacDonald, J.E. Seedling quality: History, application, and plant attributes. Forests 2018, 9, 283. [Google Scholar] [CrossRef] [Green Version]
- Oliet, J.A.; Puértolas, J.; Planelles, R.; Jacobs, D.F. Nutrient loading of forest tree seedlings to promote stress resistance and field performance: A Mediterranean perspective. New For. 2013, 44, 649–669. [Google Scholar] [CrossRef]
- Oliet, J.A.; Planelles, R.; Artero, F.; Jacobs, D.F. Nursery fertilization and tree shelters affect long-term field response of Acacia salicina Lindl. planted in Mediterranean semiarid conditions. For. Ecol. Manag. 2005, 215, 339–351. [Google Scholar] [CrossRef]
- Ovalle, J.F.; Arellano, E.; Oliet, J.; Becerra, P.; Ginocchio, R. Linking nursery nutritional status and water availability post-planting under intense summer drought: The case of a South American Mediterranean tree species. iForest—Biogeosci. For. 2016, 9, 758. [Google Scholar] [CrossRef]
- Oliet, J.A.; Salazar, J.M.; Villar, R.; Robredo, E.; Valladares, F. Fall fertilization of Holm oak affects N and P dynamics, root growth potential, and post-planting phenology and growth. Ann. For. Sci. 2011, 68, 647–656. [Google Scholar] [CrossRef] [Green Version]
- Heredia-Guerrero, N.; Oliet, J.A.; Villar-Salvador, P.; Benito, L.F.; Peñuelas, J.L. Fertilization regime interacts with fall temperature in the nursery to determine the frost and drought tolerance of the Mediterranean oak Quercus ilex subsp. ballota. For. Ecol. Manag. 2014, 331, 50–59. [Google Scholar] [CrossRef]
- Villar-Salvador, P.; Puértolas, J.; Cuesta, B.; Peñuelas, J.L.; Uscola, M.; Heredia-Guerrero, N.; Rey Benayas, J.M. Increase in size and nitrogen concentration enhances seedling survival in Mediterranean plantations. Insights from an ecophysiological conceptual model of plant survival. New For. 2012, 43, 755–770. [Google Scholar] [CrossRef]
- Ellsworth, D.S.; Crous, K.Y.; Lambers, H.; Cooke, J. Phosphorus recycling in photorespiration maintains high photosynthetic capacity in woody species. Plant Cell Environ. 2015, 38, 1142–1156. [Google Scholar] [CrossRef] [Green Version]
- Karthika, K.S.; Rashmi, I.; Parvathi, M.S. Biological functions, uptake and transport of essential nutrients in relation to plant growth. In Plant Nutrients and Abiotic Stress Tolerance; Springer: Singapore, 2018; pp. 1–49. [Google Scholar]
- Trubat, R.; Cortina, J.; Vilagrosa, A. Plant morphology and root hydraulics are altered by nutrient deficiency in Pistacia lentiscus (L.). Trees—Struct. Funct. 2006, 20, 334–339. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Morad-Talab, N.; Abd-Allah, E.F.; Ahmad, P.; Hajiboland, R. Plant growth under drought stress: Significance of mineral nutrients. In Water Stress and Crop Plants: A Sustainable Approach; Ahmad, P., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016; Volume 2, pp. 649–668. [Google Scholar]
- Tariq, A.; Pan, K.; Olatunji, O.A.; Graciano, C.; Li, Z.; Sun, F.; Zhang, L.; Wu, X.; Chen, W.; Song, D.; et al. Phosphorous fertilization alleviates drought effects on Alnus cremastogyne by regulating its antioxidant and osmotic potential. Sci. Rep. 2018, 8, 5644. [Google Scholar] [CrossRef]
- Ragel, P.; Raddatz, N.; Leidi, E.O.; Quintero, F.J.; Pardo, J.M. Regulation of K + nutrition in plants. Front. Plant Sci. 2019, 10, 281. [Google Scholar] [CrossRef] [Green Version]
- White, P.J.; Karley, A.J. Potassium. In Cell Biology of Metals and Nutrients; Springer: Berlin/Heidelberg, Germany, 2010; pp. 199–224. [Google Scholar]
- Sustr, M.; Soukup, A.; Tylova, E. Potassium in root growth and development. Plants 2019, 8, 435. [Google Scholar] [CrossRef] [Green Version]
- Zörb, C.; Senbayram, M.; Peiter, E. Potassium in agriculture—Status and perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, A.D.; Hermoso, J.; Flors, J.; Lidón, A.; Navarro-Cerrillo, R.M. Nursery location and potassium enrichment in Aleppo pine stock 2. Performance under real and hydrogel-mediated drought conditions. Forestry 2011, 84, 235–245. [Google Scholar] [CrossRef]
- Andivia, E.; Fernández, M.; Vázquez-Piqué, J. Autumn fertilization of Quercus ilex ssp. ballota (Desf.) Samp. nursery seedlings: Effects on morpho-physiology and field performance. Ann. For. Sci. 2011, 68, 543–563. [Google Scholar] [CrossRef] [Green Version]
- Schmilewski, G. Growing medium constituents used in the EU. In Proceedings of the International Symposium on Growing Media 2007, Nottingham, UK, 2–9 September 2007; pp. 33–46. [Google Scholar]
- Barrett, G.E.; Alexander, P.D.; Robinson, J.S.; Bragg, N.C. Achieving environmentally sustainable growing media for soilless plant cultivation systems—A review. Sci. Hortic. 2016, 212, 220–234. [Google Scholar] [CrossRef] [Green Version]
- Schmilewski, G. Socio-Economic Impact of the Peat and Growing Media Industry on Horticulture in the EU; European Peat and Growing Media Association (EPAGMA): Saterland-Sedelsberg, Germany, 2019. [Google Scholar]
- Caron, J.; Pepin, S.; Périard, Y. Physics of growing media in a green future. Acta Hortic. 2013, 1034, 309–317. [Google Scholar] [CrossRef]
- Di Lonardo, S.; Massa, D.; Orsenigo, S.; Zubani, L.; Rossi, G.; Fascella, G.; Cacini, S. Substitution of peat in the cultivation of two shrub species used for ecological restoration and recovery of degraded green areas. In Proceedings of the III Interna-tional Symposium on Growing Media, Composting and Substrate Analysis, Milan, Italy, 24–28 June 2019; Volume 1305, pp. 97–102. [Google Scholar]
- Tsakaldimi, M.; Ganatsas, P. A synthesis of results on wastes as potting media substitutes for the production of native plant species. Reforesta 2016, 1, 147–163. [Google Scholar] [CrossRef] [Green Version]
- Ugolini, F.; Mariotti, B.; Maltoni, A.; Tani, A.; Salbitano, F.; Izquierdo, C.G.; Macci, C.; Masciandaro, G.; Tognetti, R. A tree from waste: Decontaminated dredged sediments for growing forest tree seedlings. J. Environ. Manag. 2018, 211, 269–277. [Google Scholar] [CrossRef]
- Aung, A.; Youn, W.B.; Seo, J.M.; Dao, H.T.T.; Han, S.H.; Cho, M.S.; Park, B.B. Effects of three biomaterials mixed with growing media on seedling quality of Prunus sargentii. Forest Sci. Technol. 2019, 15, 13–18. [Google Scholar] [CrossRef] [Green Version]
- Mariotti, B.; Martini, S.; Raddi, S.; Tani, A.; Jacobs, D.F.; Oliet, J.A.; Maltoni, A. Coconut coir as a sustainable nursery growing media for seedling production of the ecologically diverse Quercus species. Forests 2020, 11, 522. [Google Scholar] [CrossRef]
- Picard, O. Final Report: Evaluation of the Community Aid Scheme for Forestry Measures in Agriculture of Regulation No 2080/92; Institute for Forestry Development: Auzeville, France, 2001; Available online: http://ec.europa.eu/agriculture/eval/reports/forest/text_en.pdf (accessed on 27 December 2022).
- Rey Benayas, J.M.; Camacho-Cruz, A. Performance of Quercus ilex saplings planted in abandoned Mediterranean cropland after long-term interruption of their management. For. Ecol. Manag. 2004, 194, 223–233. [Google Scholar] [CrossRef]
- Pausas, J.G.; Bladé, C.; Valdecantos, A.; Seva, J.P.; Fuentes, D.; Alloza, J.A.; Vilagrosa, A.; Bautista, S.; Cortina, J.; Vallejo, R. Pines and oaks in the restoration of Mediterranean landscapes of Spain: New perspectives for an old practice—A review. Plant Ecol. 2004, 171, 209–220. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Valladares, F. Tolerance to shade, drought, and waterlogging of temperate northern hemisphere trees and shrubs. Ecol. Monogr. 2006, 76, 521–547. [Google Scholar] [CrossRef]
- Gil-Pelegrín, E.; Peguero-Pina, J.J.; Sancho-Knapik, D. Oaks Physiological Ecology. Exploring the Functional Diversity of Genus Quercus L.; Tree Physiology; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Sardans, J.; Rodà, F.; Peñuelas, J. Effects of a nutrient pulse supply on nutrient status of the Mediterranean trees Quercus ilex subsp. ballota and Pinus halepensis on different soils and under different competitive pressure. Trees Struct. Funct. 2006, 20, 619–632. [Google Scholar] [CrossRef]
- Carstensen, A.; Herdean, A.; Schmidt, S.B.; Sharma, A.; Spetea, C.; Pribil, M.; Husted, S. The impacts of phosphorus deficiency on the photosynthetic electron transport chain. Plant Physiol. 2018, 177, 271–284. [Google Scholar] [CrossRef] [Green Version]
- Haldimann, P.; Gallé, A.; Feller, U. Impact of an exceptionally hot dry summer on photosynthetic traits in oak (Quercus pubescens) leaves. Tree Physiol. 2008, 28, 785–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cakmak, I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J. Plant Nutr. Soil Sci. 2005, 168, 521–530. [Google Scholar] [CrossRef]
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef] [Green Version]
- Noguera, P.; Abad, M.; Puchades, R.; Noguera, V.; Maquieira, A.; Martinez, J. Physical and chemical proper-ties of coir waste and their relation to plant growth. Acta Hortic. 1997, 450, 365–374. [Google Scholar] [CrossRef]
- Lobo, A.; Torres-Ruiz, J.M.; Burlett, R.; Lemaire, C.; Parise, C.; Francioni, C.; Truffaut, L.; Tomášková, I.; Hansen, J.K.; Kjær, E.D.; et al. Assessing inter- and intraspecific variability of xylem vulnerability to embolism in oaks. For. Ecol. Manag. 2018, 424, 53–61. [Google Scholar] [CrossRef]
- Poorter, H.; Bühler, J.; Van Dusschoten, D.; Climent, J.; Postma, J.A. Pot size matters: A meta-analysis of the effects of rooting volume on plant growth. Funct. Plant Biol. 2012, 39, 839–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinoza, S.E.; Santelices, R.E.; Cabrera, A.M.; Magni, C.R. Interactive effects of water stress, container size and fertilizer on survival, gas exchange and morphological traits of Quillaja saponaria seedlings. Bosque 2017, 38, 409–414. [Google Scholar] [CrossRef]
- Figueiredo, F.A. Condutividade hidráulica de raiz e capacidade fotossintética de mudas clonais de eucalipto com indução de deformações radiculares. Ciência Florestal 2014, 24, 277–287. [Google Scholar] [CrossRef] [Green Version]
- Sousa-Santos, C.; Cerqueira, A.F.; Dalmolin, Â.C.; de Almeida, Á.A.; dos Santos, M.S.; Avelino, N.R.; dos Santos, R.B.; de Souza Júnior, J.O.; Mielke, M.S. Morphophysiological Changes in Genipa americana Seedlings in Response to Root Deformation and Substrate Attributes. J. Soil Sci. Plant Nutr. 2022, 22, 2755–2764. [Google Scholar] [CrossRef]
- Oliet, J.A.; Ortiz de Urbina, E.; Sánchez-Pinillos, M.; Tardío-Cerrillo, G. Matching seedling size to planting conditions: Interactive response with soil moisture. iForest—Biogeosci. For. 2019, 12, 220. [Google Scholar] [CrossRef] [Green Version]
- Andivia, E.; Villar-Salvador, P.; Oliet, J.A.; Puértolas, J.; Dumroese, R.K.; Ivetić, V.; Molina-Vengas, R.; Arellano, E.C.; Li, G.; Ovalle, J.F. Climate and species stress resistance modulate the higher survival of large seedlings in forest restorations worldwide. Ecol. Appl. 2021, 31, e02394. [Google Scholar] [CrossRef]
- Raddi, S.; Giannetti, F.; Martini, S.; Farinella, F.; Chirici, G.; Tani, A.; Maltoni, A.; Mariotti, B. Monitoring drought response and chlorophyll content in Quercus by consumer-grade, near-infrared (NIR) camera: A comparison with reflectance spectroscopy. New For. 2022, 53, 241–265. [Google Scholar] [CrossRef]
- Picon, C.; Guehl, J.; Aussenac, G. Growth dynamics, transpiration and water-use efficiency in Quercus robur plants submitted to elevated CO2 and drought. Ann. Sci. For. 1996, 53, 431–446. [Google Scholar] [CrossRef] [Green Version]
- Neumann, P.M. Recent Advances in Understanding the Regulation of Whole-Plant Growth Inhibition by Salinity, Drought and Colloid Stress. Adv. Bot. Res. 2011, 57, 33–48. [Google Scholar]
- Delıgöz, A.; Bayar, E. Drought stress responses of seedlings of two oak species (Quercus cerris and Quercus robur). Turk. J. Agric. For. 2018, 42, 114–123. [Google Scholar] [CrossRef]
- Spieß, N.; Oufir, M.; Matušíková, I.; Stierschneider, M.; Kopecky, D.; Homolka, A.; Burg, K.; Fluch, S.; Hausman, J.F.; Wilhelm, E. Ecophysiological and transcriptomic responses of oak (Quercus robur) to long-term drought exposure and rewatering. Environ. Exp. Bot. 2012, 77, 117–126. [Google Scholar] [CrossRef]
- Fort, C.; Fauveau, M.L.; Muller, F.; Label, P.; Granier, A.; Dreyer, E. Stomatal conductance, growth and root signaling in young oak seedlings subjected to partial soil drying. Tree Physiol. 1997, 17, 281–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, R.; Haase, D.L. The Use of Coir as a Containerized Growing Medium for Douglas-fir Seedlings. Npj 2000, 1, 107–111. [Google Scholar] [CrossRef]
- Rippy, J.F.M.; Nelson, P.V. Cation exchange capacity and base saturation variation among Alberta, Canada, moss peats. HortScience 2007, 42, 349–352. [Google Scholar] [CrossRef] [Green Version]
- Schmilewski, G. The role of peat in assuring the quality of growing media. Mires Peat 2008, 3, 2. [Google Scholar]
- Abad, M.; Noguera, P.; Puchades, R.; Maquieira, A.; Noguera, V. Physico-chemical and chemical properties of some coconut coir dusts for use as a peat substitute for containerised ornamental plants. Bioresour. Technol. 2002, 82, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.R.; Konduru, S.; Stamps, R.H. Source variation in physical and chemical properties of coconut coir dust. HortScience 1996, 31, 965–967. [Google Scholar] [CrossRef] [Green Version]
- Munroe, R.; McGrath, D.; Henry, J. Increasing amounts of coir dust in substrates do not improve physical properties or growth of tree seedlings in a novel air-pruning propagation tray. J. Environ. Hortic. 2018, 36, 92–103. [Google Scholar] [CrossRef]
- Améglio, T.; Archer, P.; Cohen, M.; Valancogne, C.; Daudet, F.A.; Dayau, S.; Cruiziat, P. Significance and limits in the use of predawn leaf water potential for tree irrigation. Plant Soil 1999, 207, 155–167. [Google Scholar] [CrossRef]
- Damesin, C.; Rambal, S. Field study of leaf photosynthetic performance by a Mediterranean deciduous oak tree (Quercus pubescens) during a severe summer drought. New Phytol. 1995, 131, 159–167. [Google Scholar]
- Damesin, C.; Rambal, S.; Joffre, R. Co-occurrence of trees with different leaf habit: A functional approach on Mediterranean oaks. Acta Oecol. 1998, 19, 195–204. [Google Scholar] [CrossRef]
- Ripullone, F.; Rivelli, A.R.; Baraldi, R.; Guarini, R.; Guerrieri, R.; Magnani, F.; Peñuelas, J.; Raddi, S.; Borghetti, M. Effectiveness of the photochemical reflectance index to track photosynthetic activity over a range of forest tree species and plant water statuses. Funct. Plant Biol. 2011, 38, 177–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baquedano, F.J.; Castillo, F.J. Drought tolerance in the Mediterranean species Quercus coccifera, Quercus ilex, Pinus halepensis, and Juniperus phoenicea. Photosynthetica 2007, 45, 229–238. [Google Scholar] [CrossRef]
- Cochard, H.; Martin, R.; Gross, P.; Bogeat-Triboulot, M.B. Temperature effects on hydraulic conductance and water relations of Quercus robur L. J. Exp. Bot. 2000, 51, 1255–1259. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Xiong, L. General mechanisms of drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef]
- Adams, W.W., III; Muller, O.; Cohu, C.M.; Demmig-Adams, B. Photosystem II Efficiency and Non-Photochemical Fluorescence Quenching in the Context of Source-Sink Balance. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria; Demmig-Adams, B., Garab, G., Adams, W.W., III, Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 503–529. [Google Scholar]
- Ogaya, R.; Peñuelas, J. Comparative field study of Quercus ilex and Phillyrea latifolia: Photosynthetic response to experimental drought conditions. Environ. Exp. Bot. 2003, 50, 137–148. [Google Scholar] [CrossRef]
- Živčák, M.; Brestič, M.; Olšovská, K.; Slámka, P. Performance index as a sensitive indicator of water stress in Triticum aestivum L. Plant Soil Environ. 2008, 54, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Cuervo-Alarcon, L.; Arend, M.; Müller, M.; Sperisen, C.; Finkeldey, R.; Krutovsky, K.V. A candidate gene association analysis identifies SNPs potentially involved in drought tolerance in European beech (Fagus sylvatica L.). Sci. Rep. 2021, 11, 2386. [Google Scholar] [CrossRef] [PubMed]
- Bantis, F.; Radoglou, K.; Brüggemann, W. Differential ecophysiological responses to seasonal drought of three co-existing oak species in northern Greece. Plant Biosyst. 2019, 153, 378–384. [Google Scholar] [CrossRef]
- Koller, S.; Holland, V.; Brüggemann, W. Effects of drought stress on the evergreen Quercus ilex L., the deciduous Q. robur L. and their hybrid Q. × turneri Willd. Photosynthetica 2013, 51, 574–582. [Google Scholar] [CrossRef]
- Umar, M.; Siddiqui, Z.S. Florescence assessment of sunflower genotypes against drought stress environment. Pak. J. Bot. 2020, 52, 1181–1188. [Google Scholar] [CrossRef]
- Leuschner, C.; Ellenberg, H. Ecology of Central European non-forest vegetation: Coastal to alpine, natural to man-made habitats. Ecology 2017, 2, 1093. [Google Scholar]
- Früchtenicht, E.; Neumann, L.; Klein, N.; Bonal, D.; Brüggemann, W. Response of Quercus robur and two potential climate change winners—Quercus pubescens and Quercus ilex—To two years summer drought in a semi-controlled competition study: I—Tree water status. Environ. Exp. Bot. 2018, 152, 107–117. [Google Scholar] [CrossRef]
- Friedrichs, D.A.; Bntgen, U.; Frank, D.C.; Esper, J.; Neuwirth, B.; Loffler, J. Complex climate controls on 20th century oak growth in Central-West Germany. Tree Physiol. 2009, 29, 39–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urli, M.; Lamy, J.B.; Sin, F.; Burlett, R.; Delzon, S.; Porté, A.J. The high vulnerability of Quercus robur to drought at its southern margin paves the way for Quercus ilex. Plant Ecol. 2015, 216, 117–187. [Google Scholar] [CrossRef]
- Früchtenicht, E.; Bock, J.; Feucht, V.; Brüggemann, W. Reactions of three European oak species (Q. robur, Q. petraea and Q. ilex) to repetitive summer drought in sandy soil. Trees For. People 2021, 5, 100093. [Google Scholar] [CrossRef]
- Terradas, J.; Savé, R. The influence of summer and winter stress and water relationships on the distribution of Quercus ilex L. In Quercus ilex L. Ecosystems: Function, Dynamics and Management; Romane, F., Terradas, J., Eds.; Springer: Dordrecht, The Netherlands, 1992; Volume 13, pp. 137–145. [Google Scholar]
- de la Torre, J.R.; Viñas, J.I.G.; Ruiz del Castillo y de Navascués, J.; Cardo, Ó.G. Flora Mayor; Organismo Autonomo de Parques Nacionales: Madrid, Spain, 2006; p. 1756. [Google Scholar]
- Weber, P.; Bugmann, H.; Rigling, A. Radial growth responses to drought of Pinus sylvestris and Quercus pubescens in an inner-Alpine dry valley. J. Veg. Sci. 2007, 18, 777–792. [Google Scholar] [CrossRef]
- Vodnik, D.; Gričar, J.; Lavrič, M.; Ferlan, M.; Hafner, P.; Eler, K. Anatomical and physiological adjustments of pubescent oak (Quercus pubescens Willd.) from two adjacent sub-Mediterranean ecosites. Environ. Exp. Bot. 2019, 165, 208–218. [Google Scholar] [CrossRef]
- Nardini, A.; Pitt, F. Drought resistance of Quercus pubescens as a function of root hydraulic conductance, xylem embolism and hydraulic architecture. New Phytol. 1999, 143, 485–493. [Google Scholar] [CrossRef]
- Gallé, A.; Haldimann, P.; Feller, U. Photosynthetic performance and water relations in young pubescent oak (Quercus pubescens) trees during drought stress and recovery. New Phytol. 2007, 174, 799–810. [Google Scholar] [CrossRef]
- Tognetti, R.; Longobucco, A.; Raschi, A. Vulnerability of xylem to embolism in relation to plant hydraulic resistance in Quercus pubescens and Quercus ilex co-occurring in a Mediterranean coppice stand in central Italy. New Phytol. 1998, 139, 437–447. [Google Scholar] [CrossRef]
- Fotelli, M.N.; Radoglou, K.M.; Constantinidou, H.-I. Water stress responses of seedlings of four Mediterranean oak species. Tree Physiol. 2000, 20, 1065–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peguero-Pina, J.J.; Mendoza-Herrer, Ó.; Gil-Pelegrín, E.; Sancho-Knapik, D. Cavitation limits the recovery of gas exchange after severe drought stress in Holm Oak (Quercus ilex L.). Forests 2018, 9, 443. [Google Scholar] [CrossRef] [Green Version]
- Seidel, H.; Menzel, A. Above-ground dimensions and acclimation explain variation in drought mortality of scots pine seedlings from various provenances. Front. Plant Sci. 2016, 7, 1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera, J.C.; Savi, T.; Mattocks, J.; De Berardinis, F.; Scheffknecht, S.; Hietz, P.; Rosner, S.; Forneck, A. Container volume affects drought experiments in grapevines: Insights on xylem anatomy and time of dehydration. Physiol. Plant. 2021, 173, 2181–2190. [Google Scholar] [CrossRef] [PubMed]
- Köhl, K.I.; Mulugeta Aneley, G.; Haas, M.; Peters, R. Confounding Factors in Container-Based Drought Tolerance Assessments in Solanum tuberosum. Agronomy 2021, 11, 865. [Google Scholar] [CrossRef]
- Campany, C.E.; Medlyn, B.E.; Duursma, R.A. Reduced growth due to belowground sink limitation is not fully explained by reduced photosynthesis. Tree Physiol. 2017, 37, 1042–1054. [Google Scholar] [CrossRef] [Green Version]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanism, Regulation & Adaptation; Taylor and Francis: London UK; New York, NY, USA, 2000; pp. 445–483. [Google Scholar]
- Anderson, D.R.; Burnham, K.P.; White, G.C. Comparison of Akaike information criterion and consistent Akaike information criterion for model selection and statistical inference from capture-recapture studies. J. Appl. Stat. 1998, 25, 263–282. [Google Scholar] [CrossRef]

| Live Seedlings at T1 | Seedlings Survival at Trec | |||||
| Q. robur | Q. pubescens | Q. ilex | Q. robur | Q. pubescens | Q. ilex | |
| Substrate | 0.937 | 0.205 | 0.398 | 0.756 | 0.077 | 0.822 |
| Fertilization | 0.036 | 0.000 | 0.057 | 0.020 | 0.000 | 0.117 |
| Sub × Fert | 0.799 | 0.715 | 0.241 | 0.583 | 0.211 | 0.077 |
| Live seedlings at T1 | Seedlings survival at Trec | ||
| Q. robur | Pe | 37.8 | 51.1 |
| Co | 35.6 | 55.6 | |
| St | 36.7 | 63.3 | |
| P | 53.3 | 63.3 | |
| K | 20.0 | 33.3 | |
| P > St and K * | P and St > K * | ||
| Q. pubescens | Pe | 42.2 | 77.8 |
| Co | 55.6 | 91.1 | |
| St | 20.0 | 66.7 | |
| P | 30.0 | 86.7 | |
| K | 96.7 | 100 | |
| K > P and St * | K and P > St * | ||
| Q. ilex | Pe | 48.9 | 66.7 |
| Co | 57.8 | 68.9 | |
| St | 36.7 | 53.3 | |
| P | 66.7 | 76.7 | |
| K | 56.7 | 73.3 |
| Q. robur | Q. pubescens | Q. ilex | |
| Co | 57.3 a | 33.2 a | 58.0 a |
| Pe | 72.1 b | 48.5 b | 66.2 b |
| p < 0.0001 | p < 0.0001 | p < 0.0001 | |
| K | 68.3 b | 45.9 b | 61.9 |
| P | 64.9 ab | 38.3 a | 60.5 |
| St | 61.0 a | 38.5 a | 64.0 |
| p = 0.029 | p = 0.001 | p = 0.208 | |
| Sub × Fert | p = 0.358 | p = 0.593 | p = 0.166 |
| T0 | T1 | ||||
| Q. robur | C | 3.61 ± 0.31 | 4.94 ± 0.27 | b | |
| HS | 4.73 ± 0.29 | 3.37 ± 0.29 | a | ||
| Pe | 3.98 ± 0.30 | 4.48 ± 0.27 | |||
| Co | 4.34 ± 0.31 | 3.86 ± 0.33 | |||
| St | 4.63 ± 0.40 | b | 4.60 ± 0.41 | ||
| P | 3.52 ± 0.29 | a | 3.85 ± 0.29 | ||
| K | 4.32 ± 0.40 | a | 4.08 ± 0.40 | ||
| Q. pubescens | C | 7.11 ± 0.57 | 9.06 ± 0.61 | ||
| HS | 6.93 ± 0.82 | 8.59 ± 0.64 | |||
| Pe | 5.67 ± 0.58 | a | 8.00 ± 0.61 | a | |
| Co | 8.06 ± 0.68 | b | 9.62 ± 0.64 | b | |
| St | 7.02 ± 0.95 | 8.17 ± 0.93 | |||
| P | 5.87 ± 0.66 | 9.16 ± 0.69 | |||
| K | 8.04 ± 0.83 | 8.96 ± 0.73 | |||
| Q. ilex | C | 10.08 ± 0.73 | 7.50 ± 0.45 | b | |
| HS | 9.54 ± 0.75 | 4.04 ± 0.41 | a | ||
| Pe | 9.09 ± 0.65 | 5.51 ± 0.45 | |||
| Co | 10.42 ± 0.79 | 6.13 ± 0.53 | |||
| St | 12.95 ± 0.91 | b | 5.58 ± 0.57 | ||
| P | 9.25 ± 0.90 | a | 6.15 ± 0.70 | ||
| K | 7.45 ± 0.62 | a | 5.74 ± 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariotti, B.; Martini, S.; Raddi, S.; Ugolini, F.; Oliet, J.A.; Jacobs, D.F.; Maltoni, A. Cultivation Using Coir Substrate and P or K Enriched Fertilizer Provides Higher Resistance to Drought in Ecologically Diverse Quercus Species. Plants 2023, 12, 525. https://doi.org/10.3390/plants12030525
Mariotti B, Martini S, Raddi S, Ugolini F, Oliet JA, Jacobs DF, Maltoni A. Cultivation Using Coir Substrate and P or K Enriched Fertilizer Provides Higher Resistance to Drought in Ecologically Diverse Quercus Species. Plants. 2023; 12(3):525. https://doi.org/10.3390/plants12030525
Chicago/Turabian StyleMariotti, Barbara, Sofia Martini, Sabrina Raddi, Francesca Ugolini, Juan A. Oliet, Douglass F. Jacobs, and Alberto Maltoni. 2023. "Cultivation Using Coir Substrate and P or K Enriched Fertilizer Provides Higher Resistance to Drought in Ecologically Diverse Quercus Species" Plants 12, no. 3: 525. https://doi.org/10.3390/plants12030525
APA StyleMariotti, B., Martini, S., Raddi, S., Ugolini, F., Oliet, J. A., Jacobs, D. F., & Maltoni, A. (2023). Cultivation Using Coir Substrate and P or K Enriched Fertilizer Provides Higher Resistance to Drought in Ecologically Diverse Quercus Species. Plants, 12(3), 525. https://doi.org/10.3390/plants12030525






