Physiological Responses of Agave maximiliana to Inoculation with Autochthonous and Allochthonous Arbuscular Mycorrhizal Fungi
Abstract
1. Introduction
2. Results and Discussion
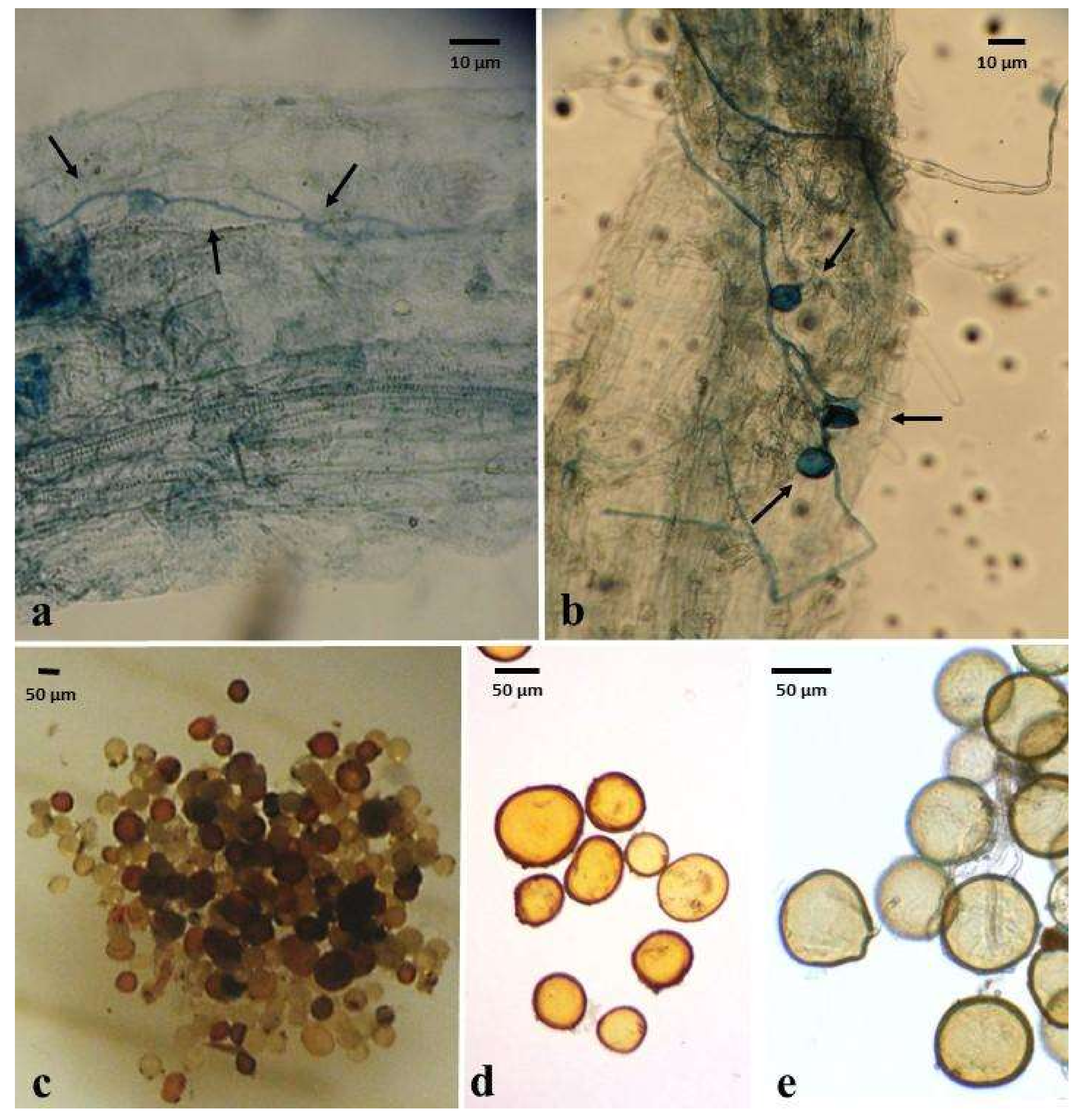
2.1. AMF Colonization
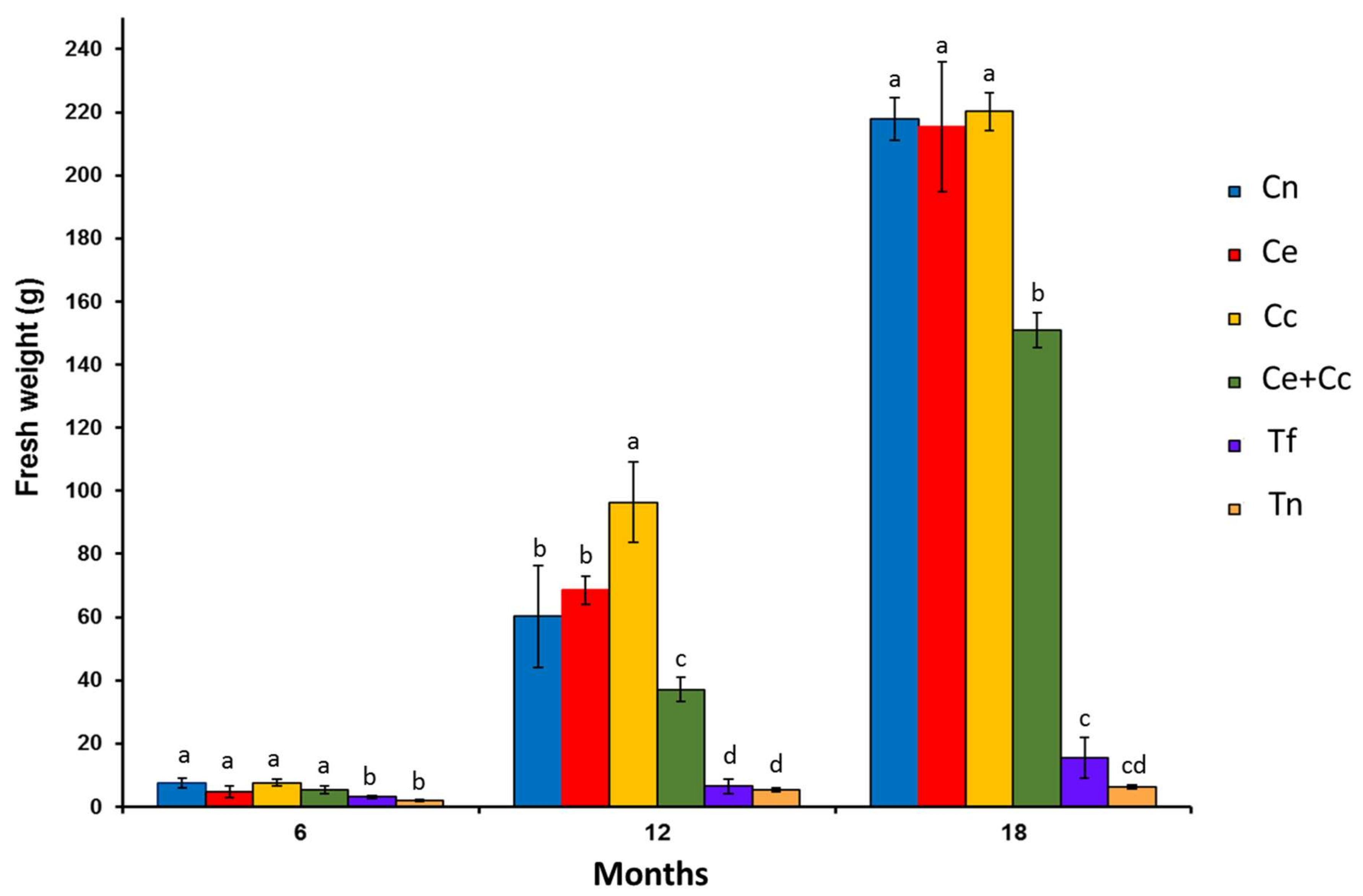
2.2. AMF Spore Density in Inoculated Treatments
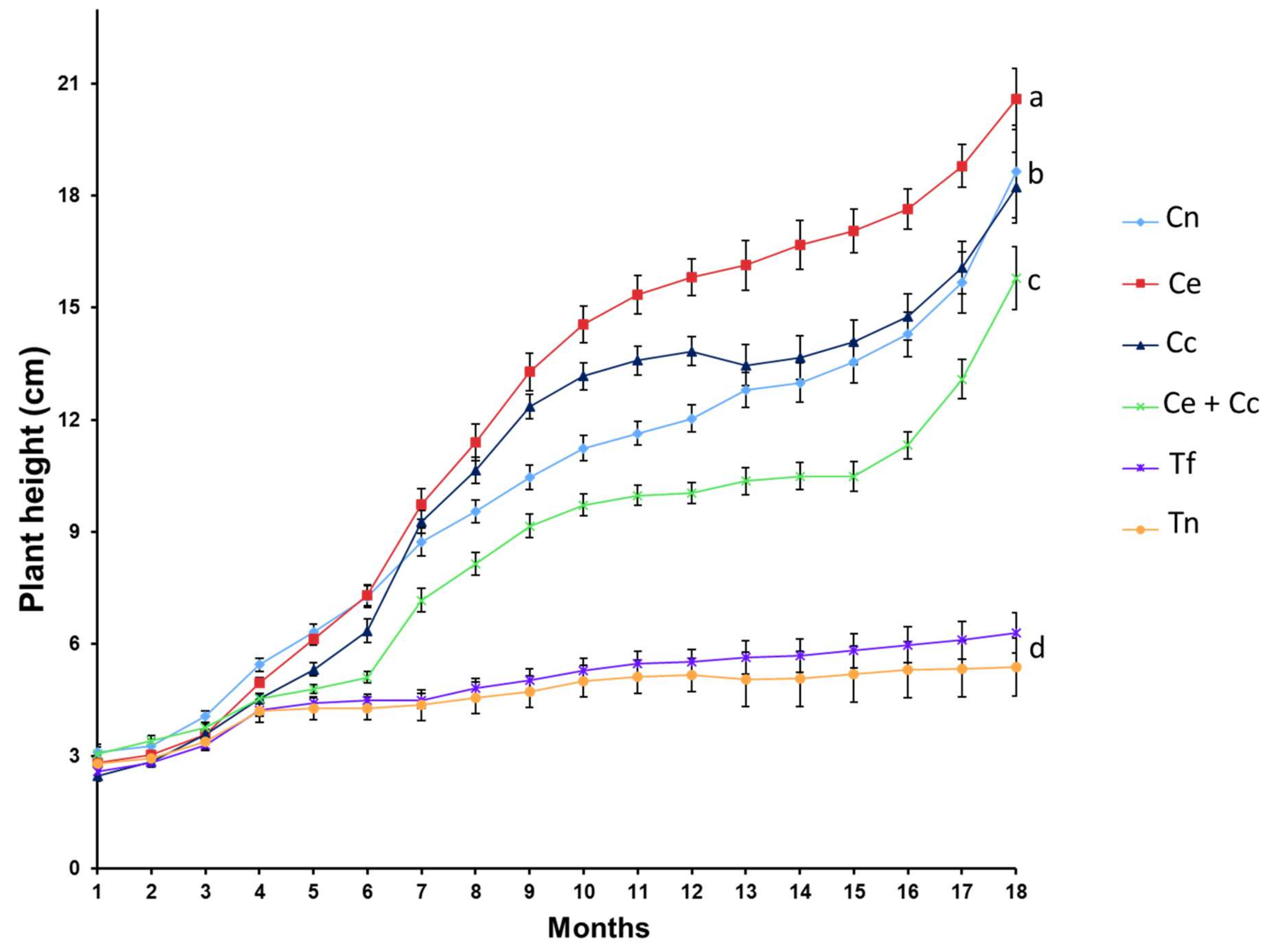
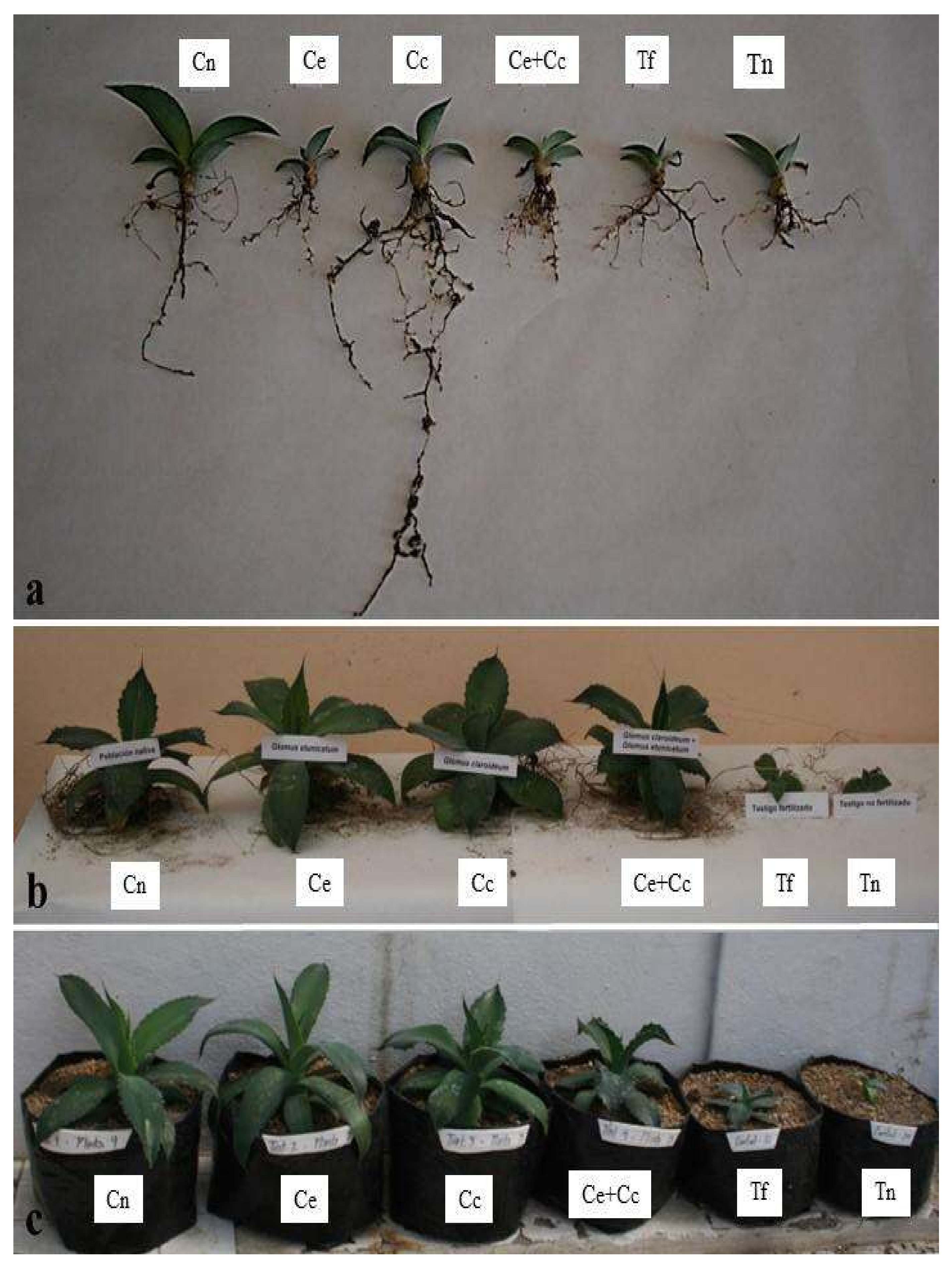
2.3. Plant Growth, Nutrition, and Metabolism
3. Materials and Methods
3.1. Plant Material
3.2. AMF Inoculums
3.3. Treatments
3.4. AMF Inoculation and Experiment
3.5. Quantification of AMF Colonization
3.6. Recovery and Estimation of AMF Spore Density
3.7. Determination of Physiological Variables
3.8. Reducing Sugars
3.9. Determination of Foliar Nutrients
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García Mendoza, A.J.; Martínez, I.S.F.; Gutiérrez, D.S. Four new species of Agave (Asparagaceae, Agavoideae) from southern Mexico. Acta Bot. Mex. 2019, 126, e1461. [Google Scholar]
- García Mendoza, A.J. Los Agaves de México. Ciencias 2007, 87, 14–23. [Google Scholar]
- Álvarez-Ainza, M.; Arellano-Plaza, M.; de la Torre-González, F.J.; Gallardo-Valdez, J.; García-Barrón, S.E.; García-Galaz, A.; Gschaedler-Mathis, A.; Herrera-López, E.J.; López-Miranda, J.; Páez-Lerma, J.B.; et al. NOS-C y CPL-C. Bebidas destiladas de Agave. In Panorama del Aprovechamiento de los Agaves en México; Agared: Guadalajara, Mexico, 2017. [Google Scholar]
- IIEG. Radiografía Estadística de la Industria de la Raicilla 2022. Available online: https://iieg.gob.mx/ns/wp-content/uploads/2022/11/Radiograf%C3%ADa-Estad%C3%ADstica-de-la-Industria-de-la-Raicilla-2022.pdf (accessed on 2 March 2022).
- Hernández, L.J.; Medina, R.J.M. Agave maximiliana. Tecnoagave 2017, 47, 18–20. [Google Scholar]
- DOF. Declaración General de Protección de la Denominación de Origen “Raicilla.” Diario Oficial de la Federación. 2019. Available online: https://dof.gob.mx/nota_detalle.php?codigo=5564454&fecha=28/06/2019#gsc.tab=0 (accessed on 2 March 2022).
- López-Lozano, N.E.; Echeverría Molinar, A.; Ortiz Durán, E.A.; Hernández Rosales, M.; Souza, V. Bacterial diversity and interaction networks of Agave lechuguilla rhizosphere differ significantly from bulk soil in the oligotrophic basin of Cuatro Cienegas. Front. Plant Sci. 2020, 11, 1028. [Google Scholar] [CrossRef]
- Ruiz-Font, A. Biodiversidad del suelo, conservación de la naturaleza y sostenibilidad. Tecnol. Marcha 2008, 21, 184–190. [Google Scholar]
- Cui, M.; Nobel, P.S. Nutrient status, water uptake and gas exchange for three desert succulents infected with mycorrhizal fungi. New Phytol. 1992, 122, 643–649. [Google Scholar] [CrossRef]
- Robles-Martínez, M.L.; Robles, C.; Rivera-Becerril, F.; Ortega-Larrocea, M.P.; Pliego-Marín, L. Inoculation of native arbuscular mycorrhizal fungi consortia in Agave angustifolia Haw. Rev. Mex. Cienc. Agríc. 2013, 6, 1231–1240. [Google Scholar]
- Luna, C.L.A.; Monroy, A. Efecto de hongos micorrizógenos arbusculares (HMA) sobre el desarrollo de Agave salmiana y Opuntia streptacantha en condiciones de invernadero. In Plantas y Hongos—Micorrizas Arbusculares: Un Mutualismo Esencial en Zonas Semiáridas; Monroy-Ata, A., García-Sánchez, R., Eds.; Unidad de Investigación en Ecología Vegetal, FES-Zaragoza-UNAM: Mexico City, Mexico, 2009; pp. 25–36. [Google Scholar]
- Pimienta-Barrios, E.; Zañudo-Hernández, J.; López-Alcocer, E. Efecto de las micorrizas arbusculares en el crecimiento, fotosíntesis y anatomía foliar de plantas jóvenes de Agave tequilana. Acta Bot. Mex. 2009, 89, 63–78. [Google Scholar] [CrossRef]
- Ruiz, S.; Adriano, L.; Ovando, I.; Navarro, C.; Salvador, M. Biofertilization of micropropagated Agave tequilana: Effect on plant growth and production of hydrolytic enzymes. Afr. J. Biotechnol. 2011, 10, 9623–9630. [Google Scholar]
- Santiz-Gómez, J.A.; Rincón-Rosales, R.; Abud-Archila, M.; Ruíz-Valdiviezo, V.M.; Gutiérrez-Miceli, F.A.; Dendooven, L.; Mendez-Trujillo, V.; Rodríguez-Hernandez, L.; Gonzalez-Mendoza, D. Influence of mycorrhization on the growth and fructan production in micropropagated Agave grijalvensis (B. Ullrich) Plantlets. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 375–380. [Google Scholar] [CrossRef]
- Quiñones-Aguilar, E.E.; Montoya-Martínez, A.C.; Rincón-Enriquez, G.; Lobit, P.; López-Pérez, L. Effectiveness of native arbuscular mycorrhizal consortia on the growth of Agave inaequidens. J. Soil Sci. Plant Nutr. 2016, 16, 1052–1064. [Google Scholar] [CrossRef]
- Trinidad-Cruz, J.R.; Quiñones-Aguilar, E.E.; Rincón-Enríquez, G.; López-Pérez, L.; Hernández-Cuevas, L.V. Mycorrhization of Agave cupreata: Biocontrol of Fusarium oxysporum and plant growth promotion. Rev. Mex. Fitopatol. 2017, 35, 151–169. [Google Scholar]
- Martínez-López, J.R.; Vázquez-Alvarado, R.E.; Gutiérrez-Ornelas, E.; Peña del Río, M.A.; López-Cervantes, R.; Olivares-Sáenz, E.; Vidales-Contreras, J.; Valdez–Cepeda, R.D. Mycorrhiza efffect on nutritional quality and biomass production of Agave (Agave americana L.) and cactus pear (Opuntia lindheimeri Engelm). J. Prof. Assoc. Cactus Dev. 2009, 11, 69–77. [Google Scholar]
- Zacarías-Toledo, R.; González-Mendoza, D.; Rodriguez Mendiola, M.A.; Villalobos-Maldonado, J.J.; Gutiérrez-Oliva, V.F.; Dendooven, L.; Abud-Archila, M.; Arias-Castro, C.; Gutiérrez-Miceli, F.A. Plant growth and sugars content of Agave americana L. cultivated with vermicompost and rock phosphate and inoculated with Penicillium sp. and Glomus fasciculatum. Compost Sci. Util. 2016, 24, 259–265. [Google Scholar] [CrossRef]
- Montoya-Martinez, A.C.; Rincón-Enríquez, G.; Lobit, P.; López-Pérez, L.; Quiñones-Aguilar, E. Native arbuscular mycorrhizal fungi from the rhizosphere of Agave cupreata and their effect on Agave tequilana growth. Rev. Fitotec. Mex. 2019, 42, 429–438. [Google Scholar] [CrossRef]
- Rodríguez, H.G.; Morales, D.F.; Gutiérrez, C.R.; Aguilar, E.S.; Pérez, M.E. Generación de raíces transformadas de Agave salmiana Otto y su colonización por Glomus intraradices. Fitotec. Mex. 2007, 30, 215–222. [Google Scholar] [CrossRef]
- Ochoa-Meza, A.; Esqueda, M.; Fernández-Valle, R.; Herrera-Peraza, R. Variación estacional de hongos micorrízicos arbusculares asociados con Agave angustifolia Haw. en la Sierra Sonorense, Mexico. Rev. Fitotec. Mex. 2009, 32, 189–199. [Google Scholar] [CrossRef]
- Hart, M.M.; Reader, R.J. Taxonomic basis for variation in the colonization strategy of arbuscular mycorrhizal fungi. New Phytol. 2002, 153, 335–344. [Google Scholar] [CrossRef]
- Pouyu-Rojas, E.; Siqueira, J.O.; Donizetti, S.J.G. Compatibilidade simbiótica de fungos micorrízicos arbusculares com espécies arbóreas tropicais. Rev. Bras. Ciênc. Solo 2006, 30, 413–424. [Google Scholar] [CrossRef]
- Gavito, M.E.; Pérez-Castillo, D.; González-Monterrubio, C.F.; Vieyra-Hernández, T.; Martínez-Trujillo, M. High compatibility between arbuscular mycorrhizal fungal communities and seedlings of different land use types in a tropical dry ecosystem. Mycorrhiza 2008, 19, 47–60. [Google Scholar] [CrossRef]
- De Oliveira, J.R.G.; de Resende, G.M.; de Melo, N.F.; Yano-Melo, A.M. Symbiotic compatibility between arbuscular mycorrhizal fungi (autoctone or exotic) and three native species of the Caatinga in different phosphorus levels. Acta Sci. Biol. Sci. 2017, 39, 59–69. [Google Scholar] [CrossRef]
- An, G.-H.; Kobayashi, S.; Enoki, H.; Sonobe, K.; Muraki, M.; Karasawa, T.; Ezawa, T. How does arbuscular mycorrhizal colonization vary with host plant genotype? An example based on maize (Zea mays) germplasms. Plant Soil 2010, 327, 441–453. [Google Scholar] [CrossRef]
- Chagnon, B.L.; Bradley, R.L.; Maherali, H.; Klironomos, J.N. A trait-based framework to understand life history of mycorrhizal fungi. Trends Plant Sci. 2013, 18, 484–491. [Google Scholar] [CrossRef]
- López-García, A.; Azcón-Aguilar, C.; Barea, J.M. The interactions between plant life form and fungal traits of arbuscular mycorrhizal fungi determine the symbiotic community. Oecologia 2014, 176, 1075–1086. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M. Competition for infection between vesicular-arbuscular mycorrhizal fungi. New Phytol. 1984, 97, 427–435. [Google Scholar] [CrossRef]
- Engelmoer, D.J.P.; Behm, J.E.; Kiers, E.T. Intense competition between arbuscular mycorrhizal mutualism in an in vitro root microbiome negatively affects total fungal abundance. Mol. Ecol. 2014, 3, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Cano, C.; Bago, A. Competition and substrate colonization strategies of three polyxenically grown arbuscular mycorrhizal fungi. Mycologya 2005, 97, 1201–1214. [Google Scholar] [CrossRef]
- García-Martínez, L.I.; Sánchez-Mendoza, S.; Bautista-Cruz, A. Combination of mycorrhizal fungi and phosphorus fertilization in the growth of two wild agaves. Terra Latinoam. 2020, 38, 771–780. [Google Scholar]
- Ríos-Ramírez, S.C.; Enríquez del Valle, J.R.; Rodríguez-Ortiz, G.; Ruiz-Luna, J.; Velasco-Velasco, V.A. Crecimiento de Agave angustifolia Haw. con relación a la condición nutrimental. Rev. Mex. Cienc. Agríc. 2021, 12, 865–873. [Google Scholar] [CrossRef]
- Cruz, G.H.; Enríquez-del Valle, J.R.; Velasco, V.V.A.; Ruiz, L.J.; Campos, A.G.V.; Aquino, G.D.E. Nutrimentos y carbohidratos en plantas de Agave angustifolia Haw. y Agave karwinskii Zucc. Rev. Mex. Cienc. Agríc. 2013, 4, 1161–1173. [Google Scholar]
- Uvalle, B.J.X.; Vélez, G.C. Nutrición del agave tequilero (Agave tequilana Weber, var. Azul). In Conocimiento y Prácticas Agronómicas para la Producción de Agave tequilana Weber en la Zona de Denominación de Origen del Tequila; Pérez Domínguez, J.F., Del Real Laborde, J.I., Eds.; Libro Técnico 4; Centro de Investigación Regional del Pacífico Centro, Campo Experimental Centro-Altos de Jalisco: Tepatitlán, Mexico, 2007; pp. 69–88. [Google Scholar]
- Nikolaeva, V.B.; Niño de la Cruz, V. Deficiencia inducida de elementos nutritivos en el cultivo de agave. In Conocimiento y Prácticas Agronómicas para la Producción de Agave tequilana Weber en la Zona de Denominación de Origen del Tequila; Pérez Domínguez, J.F., Del Real Laborde, J.I., Eds.; Libro Técnico 4; Centro de Investigación Regional del Pacífico Centro, Campo Experimental Centro-Altos de Jalisco: Tepatitlán, Mexico, 2007; pp. 89–116. [Google Scholar]
- Quero, E.; Nobel, P.S. Predictions of field productivity for Agave lechuguilla. J. Appl. Ecol. 1987, 24, 1053–1062. [Google Scholar] [CrossRef]
- Bautista-Justo, M.; García-Oropeza, L.; Salcedo-Hernández, R.; Parra-Negrete, L.A. Azúcares en agaves (Agave tequilana Weber) cultivados en el Estado de Guanajuato. Acta Univ. 2001, 11, 33–38. [Google Scholar] [CrossRef]
- Vázquez-García, J.A.; Vargas-Rodríguez, Y.L.; Cházaro-Basáñez, M. Diversidad, endemismo, abundancia y riqueza de especies de Agave en Jalisco. In Agaves del Occidente de México; Vázquez-García, J.A., Cházaro-Basáñez, M., Hernández-Vera, G., Flores-Berrios, E., Vargas-Rodríguez, Y.L., Eds.; Serie Fronteras de Biodiversidad 3; Universidad de Guadalajara-Consejo Regulador del Tequila AC.-Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco A.C.-Comisión Nacional Forestal: Guadalajara, Mexico, 2007; pp. 23–31. [Google Scholar]
- Cabrera-Toledo, D.; Vargas-Ponce, O.; Ascencio-Ramírez, S.; Valadez-Sandoval, L.M.; Pérez-Alquicira, J.; Morales-Saavedra, J.; Huerta-Galván, O.F. Morphological and genetic variation in monocultures, forestry systems and wild populations of Agave maximiliana of western Mexico: Implications for its conservation. Front. Plant Sci. 2020, 11, 817. [Google Scholar] [CrossRef] [PubMed]
- Torres-García, I.; Rendón-Sandoval, F.J.; Blancas, J.; Casas, A.; Moreno-Calles, A.I. The genus Agave in agroforestry systems of Mexico. Bot. Sci. 2019, 97, 263–290. [Google Scholar] [CrossRef]
- Sylvia, D.M.; Jarstfer, A.G. Sheared-root inocula of vesicular-arbuscular mycorrhizal fungi. Appl. Environ. Microbiol. 1992, 58, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Van Haef, J.M.N.; Berlijin, J.D. Horticultura: Manuales para Educación Agropecuaria—Producción Vegetal, 2nd ed.; SEP-Trillas: Mexico City, Mexico, 1990; p. 112. [Google Scholar]
- Augé, R.M. Arbuscular mycorrhizae and soil/plant water relations. Can. J. Soil Sci. 2004, 84, 373–381. [Google Scholar] [CrossRef]
- Blaszkowski, J. Taxonomy of Arbuscular Fungi: Species Descriptions and Illustrations. 2003. Available online: http://www.zor.zut.edu.pl/Glomeromycota/Taxonomy.html (accessed on 1 March 2022).
- INVAM. International Culture Collection of (Vesicular) Arbuscular Mycorrhizal Fungi. Available online: http://invam.ku.edu/ (accessed on 2 March 2022).
- Gerdemann, J.H.; Trappe, J.M. The Endogonaceae in the Pacific Northwest; Mycologia Memoir; New York Botanical Garden, in Collaboration with the Mycological Society of America: New York, NY, USA, 1974; Volume 5, pp. 1–74. [Google Scholar]
- Walker, C. Species in the Endogonaceae: A new species (Glomus occultum) and a new combination (Glomus geosporum). Mycotaxon 1982, 15, 49–61. [Google Scholar]
- Berch, S.M.; Fortin, J.A. A Lectotype for Glomus microcarpum (Endogonaceae, Zygomycetes). Mycologia 1984, 76, 190–193. [Google Scholar] [CrossRef]
- Schenck, N.C.; Spain, J.L.; Sieverding, E.; Howeler, R.H. Several new and unreported vesicular-arbuscular mycorrhizal fungi (Endogonaceae) from Colombia. Mycologia 1984, 76, 685–699. [Google Scholar] [CrossRef]
- Oehl, F.; Sýkorová, Z.; Redecker, D.; Wiemken, A.; Sieverding, E. Acaulospora alpina, a new arbuscular mycorrhizal fungal species characteristic for high mountainous and alpine regions of the Swiss Alps. Mycologia 2006, 98, 286–294. [Google Scholar] [CrossRef]
- Oehl, F.; Da Silva, G.A.; Goto, B.T.; Sieverding, E. Glomeromycota: Three new genera and glomoid species reorganized. Mycotaxon 2011, 116, 75–120. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular—Arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Gazey, C.; Abbott, L.K.; Robson, A.D. VA mycorrhizal spores from three species of Acaulospora: Germination, longevity and hyphal growth. Mycol. Res. 1993, 97, 785–790. [Google Scholar] [CrossRef]
- Giovanetti, M.L. Spore germination and pre-symbiotic mycelial growth. In Arbuscular Mycorrhizas: Structure and Function; Kapulnik, Y., Douds, D.D., Jr., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 47–68. [Google Scholar]
- Gerdemann, J.W.; Nicolson, T.H. Spores of mycorrhizal endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Negrulescu, A.; Patrulea, V.; Mincea, M.M.; Ionascu, C.; Vlad-Oros, B.A.; Ostafe, V. Adapting the reducing sugars method with dinitrosalicylic acid to microtiter plates and microwave heating. J. Braz. Chem. Soc. 2012, 23, 2176–2182. [Google Scholar] [CrossRef]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen—Total. In Methods of Soil Analysis, 2nd ed.; Page, A.L., Ed.; Agronomy Monographs; John Wiley & Sons: Hoboken, NJ, USA, 1983; pp. 595–624. [Google Scholar] [CrossRef]
- Alcántar-González, G.; Sandoval-Villa, M. Manual de Análisis Químico de Tejido Vegetal: Guía de Muestreo, Preparación, Análisis e Interpretación; Sociedad Mexicana de la Ciencia del Suelo, A.C.: Chapingo, Mexico, 1999; p. 155. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 3rd ed.; Prentice Hall: Hoboken, NJ, USA, 1996; p. 662. [Google Scholar]
- SAS Institute. JMP, version 4.0.4; SAS Institute Inc.: Cary, NC, USA, 2001. [Google Scholar]
- Zúñiga Estrada, L. Nutrición de Agave tequilana y Manejo de los Fertilizantes en un Sistema de Producción Intensiva (Riego por Goteo); Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias, Centro de Investigación Regional del Noreste, Campo Experimental Las Huastecas: Villa Cuauhtémoc, Mexico, 2013; p. 46.
- Martínez, R.S.; Santos, A.T.; Robles, C.; Spinola, A.G.; Mendoza, T.M.H.; Rincón, J.A.S.; Bautista Sánchez, G.; Pedro Santos, E.C. Crecimiento y sólidos solubles de Agave potatorum Zucc. inducidos por riego y fertilización. Rev. Fitotec. Mex. 2012, 35, 61–68. [Google Scholar] [CrossRef]
- Ávila Núñez, R.; Hernández, R.; Rivas, B.; Chirinos, M. Contenido de azúcares totales, reductores y no reductores en Agave cocui Trelease. Multiciencias 2012, 12, 129–135. [Google Scholar]
| Mycorrhizal Colonization (%) * | Number of Spores/100 g of Soil ** | |||
|---|---|---|---|---|
| Age (Months) | Treatment | Median | Minimum and Maximum Values of Quartiles | |
| 6 | Cn | 23.60 a | 10.55–36.66 | 20.13 ± 2.82 a |
| Ce | 6.44 a | 1.68–11.2 | 32.85 ± 5.95 a | |
| Cc | 22.21 a | 11.66–32.77 | 16.22 ± 3.05 a | |
| Ce + Cc | 31.85 a | 29.55–34.16 | 26.67 ± 10.47 a | |
| 12 | Cn | 19.615 a | 18.33–20.90 | 17.07 ± 10.50 b |
| Ce | 38.74 a | 35–42.48 | 37.26 ± 11.00 b | |
| Cc | 37.57 a | 32.22–42.93 | 2922.68 ± 1600.50 a | |
| Ce + Cc | 22.05 a | 12.50–31.60 | 915.46 ± 314.00 a | |
| 18 | Cn | 55.27 a | 47.77–62.77 | 51.05 ± 8.66 b |
| Ce | 48.33 a | 43.33–53.33 | 188.16 ± 155.22 b | |
| Cc | 63.88 a | 62.77–65.00 | 831.27 ± 132.72 a | |
| Ce + Cc | 64.44 a | 61.66–67.22 | 87.81 ± 34.67 b | |
| Age (Months) | Treatment | Growth Variables | ||||
|---|---|---|---|---|---|---|
| Base diameter (mm) | Number of leaves + | Foliar length (cm) | Radicle length (cm) | Radicle biomass (g) | ||
| 6 * | Cn | 9.97 ± 0.13 a | 5.55 ± 0.14 ab | 7.25 ± 0.30 a | 19.4 ± 4.58 a | 0.39 ± 0.15 a |
| Ce | 10.11 ± 0.06 a | 5.55 ± 0.66 ab | 7.28 ± 0.28 a | 10.52 ± 1.69 b | 0.15 ± 0.04 a | |
| Cc | 10.06 ± 0.14 a | 6.27 ± 0.21 a | 6.33 ± 0.32 a | 12.42 ± 2.55 ab | 0.28 ± 0.07 a | |
| Ce + Cc | 9.63 ± 0.18 a | 5.16 ± 0.14 b | 5.01 ± 0.15 b | 9.7 ± 2.06 ab | 0.26 ± 0.07 a | |
| Tf | 8.40 ± 0.28 b | 4.22 ± 0.19 c | 4.48 ± 0.16 b | 8.6 ± 0.41 b | 0.17 ± 0.03 a | |
| Tn | 8.16 ± 0.52 b | 4.00 ± 0.28 c | 4.27 ± 0.30 b | 7.12 ± 0.44 b | 0.14 ± 0.02 a | |
| 12 ** | Cn | 30.05 ± 0.84 ab | 10.46 ± 0.29 a | 12.03 ± 0.36 c | 21.64 ± 4.94 b | 2.48 ± 0.78 a |
| Ce | 27.27 ± 0.60 bc | 10.61 ± 0.18 a | 15.82 ± 0.49 a | 23.4 ± 2.15 ab | 2.67 ± 0.37 a | |
| Cc | 31.94 ± 0.84 a | 11.15 ± 0.27 a | 13.83 ± 0.39 b | 41.9 ± 6.87 a | 3.88 ± 0.35 a | |
| Ce + Cc | 24.70 ± 0.62 c | 8.69 ± 0.20 b | 10.03 ± 0.28 d | 32.52 ± 5.73 ab | 2.20 ± 0.23 a | |
| Tf | 12.44 ± 0.77 d | 5.76 ± 0.25 c | 5.51 ± 0.33 e | 17.34 ± 3.67 b | 0.30 ± 0.11 b | |
| Tn | 11.01 ± 0.98 d | 4.84 ± 0.41 c | 5.16 ± 0.44 e | 15.7 ± 2.07 b | 0.32 ± 0.07 b | |
| 18 *** | Cn | 46.20 ± 0.69 a | 16.37 ± 0.70 ab | 18.65 ± 1.25 ab | 46.6 ± 3.72 a | 7.72 ± 0.84 a |
| Ce | 44.67 ± 1.52 ab | 16.12 ± 0.39 ab | 20.60 ± 0.82 a | 34.2 ± 4.70 b | 7.64 ± 1.03 a | |
| Cc | 48.48 ± 1.48 a | 17.25 ± 0.61 a | 18.22 ± 0.95 ab | 38.4 ± 2.65 ab | 10.80 ± 1.59 a | |
| Ce + Cc | 38.91 ± 1.39 b | 14.12 ± 0.66 b | 15.78 ± 0.84 b | 39.6 ± 1.53 ab | 8.00 ± 0.98 a | |
| Tf | 16.12 ± 1.63 c | 7.5 ± 0.42 c | 6.27 ± 0.54 c | 25 ± 4.81 bc | 0.56 ± 0.21 b | |
| Tn | 12.26 ± 1.84 c | 6.12 ± 0.89 c | 5.37 ± 0.77 c | 18.6 ± 1.80 c | 0.28 ± 0.05 b | |
| Treatment | Reducing Sugars | Minerals | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Free (μg/mL) | Total (μg/mL) |
N (%) |
K (%) |
Ca (%) |
Mg (%) |
P (ppm) |
Bo (ppm) | Cu (ppm) |
Fe (ppm) |
Mn (ppm) |
Zn (ppm) | |
| Cn | 230 ± 0.04 a | 310 ± 0.14 b | 1.44 ± 0.05 a | 4.25 ± 0.12 a | 2.44 ± 0.14 a | 1.31 ± 0.07 a | 2785.58 ± 488.46 b | 40.41 ± 1.95 a | <4.81 | 125.44 ± 6.90 b | 43.93 ± 3.36 b | 15.61 ± 3.21 b |
| Ce | 160 ± 0.02 b | 220 ± 0.01 b | 1.54 ± 0.08 a | 4.09 ± 0.16 a | 2.27 ± 0.08 a | 1.41 ± 0.02 a | 2391.85 ± 165.34 b | 41.56 ± 3.05 a | <4.81 | 160.44 ± 27.01 b | 61.91 ± 3.41 a | 17.34 ± 0.48 b |
| Cc | 180 ± 0.04 b | 260 ± 0.03 b | 1.77 ± 0.07 a | 4.39 ± 0.05 a | 2.24 ± 0.04 a | 1.37 ± 0.03 a | 3350.93 ± 203.47 a | 44.64 ± 0.83 a | <4.81 | 163.26 ± 59.51 b | 47.86 ± 6.38 b | 21.75 ± 1.96 a |
| Ce + Cc | 220 ± 0.06 a | 350 ± 0.07 b | 1.48 ± 0.06 a | 4.95 ± 0.28 a | 2.38 ± 0.27 a | 1.58 ± 0.04 a | 3210.83 ± 356.44 a | 43.74 ± 1.55 a | <4.81 | 137.79 ± 30.06 b | 33.20 ± 2.72 c | 23.94 ± 1.07 a |
| Tf | 140 ± 0.06 c | 780 ± 0.14 a | 1.49 ± 0.39 a | 2.77 ± 0.31 b | 2.15 ± 0.17 a | 0.99 ± 0.08 b | 620.29 ± 81.72 c | 43.36 ± 1.04 a | <4.81 | 197.24 ± 495.85 a | 34.76 ± 5.79 c | 16.47 ± 8.40 b |
| Tn | 120 ± 0.02 c | 620 ± 0.18 a | 1.47 ± 0.53 a | 1.93 ± 0.10 b | 1.67 ± 0.06 b | 0.73 ± 0.06 b | 401.70 ± 26.72 c | 43.97 ± 3.38 a | <4.81 | 169.22 ± 98.88 a | 28.76 ± 8.33 c | 9.13 ± 0.86 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Cuevas, L.V.; Salinas-Escobar, L.A.; Segura-Castruita, M.Á.; Palmeros-Suárez, P.A.; Gómez-Leyva, J.F. Physiological Responses of Agave maximiliana to Inoculation with Autochthonous and Allochthonous Arbuscular Mycorrhizal Fungi. Plants 2023, 12, 535. https://doi.org/10.3390/plants12030535
Hernández-Cuevas LV, Salinas-Escobar LA, Segura-Castruita MÁ, Palmeros-Suárez PA, Gómez-Leyva JF. Physiological Responses of Agave maximiliana to Inoculation with Autochthonous and Allochthonous Arbuscular Mycorrhizal Fungi. Plants. 2023; 12(3):535. https://doi.org/10.3390/plants12030535
Chicago/Turabian StyleHernández-Cuevas, Laura Verónica, Luis Alberto Salinas-Escobar, Miguel Ángel Segura-Castruita, Paola Andrea Palmeros-Suárez, and Juan Florencio Gómez-Leyva. 2023. "Physiological Responses of Agave maximiliana to Inoculation with Autochthonous and Allochthonous Arbuscular Mycorrhizal Fungi" Plants 12, no. 3: 535. https://doi.org/10.3390/plants12030535
APA StyleHernández-Cuevas, L. V., Salinas-Escobar, L. A., Segura-Castruita, M. Á., Palmeros-Suárez, P. A., & Gómez-Leyva, J. F. (2023). Physiological Responses of Agave maximiliana to Inoculation with Autochthonous and Allochthonous Arbuscular Mycorrhizal Fungi. Plants, 12(3), 535. https://doi.org/10.3390/plants12030535





