Abstract
The use of Opuntia ficus-indica fruits in the agro-food sector is increasing for a multiplicity of players. This renewed interest is, in part, due to its organoleptic characteristics, nutritional value and health benefits. Furthermore, industries from different sectors intend to make use of its vast array of metabolites to be used in different fields. This trend represents an economic growth opportunity for several partners who could find new opportunities exploring non-conventional fruits, and such is the case for Opuntia ficus-indica. O. ficus-indica originates from Mexico, belongs to the Cactaceae family and is commonly known as opuntia, prickly pear or cactus pear. The species produces flowers, cladodes and fruits that are consumed either in raw or in processed products. Recent publications described that consumption of the fruit improves human health, exhibiting antioxidant activity and other relevant pharmacological activities through enzymatic and non-enzymatic mechanisms. Thus, we provide a systematic, scientific and rational review for researchers, consumers and other relevant stakeholders regarding the chemical composition and biological activities of O. ficus-indica fruits.
1. Introduction
Opuntia ficus-indica (L.) Mill, commonly known as prickly pear, Indian fig or nopal is a crassulacean acid metabolism (CAM) plant belonging to the Cactaceae family, Opuntia genus. Originating from Mexico, it is widespread through Central and South America, Australia, the Mediterranean basin and South Africa [1]. It is believed that O. ficus-indica was first introduced in Africa and Europe because it is the host of the cochineal insect Dactylopius coccus, a source of a commercial red dye [2]. The species can grow in arid and semiarid regions at high temperatures and low water availability [3].
Fresh cladodes, fruits and flowers are traditionally used for different purposes. Cladodes are rich in fibers such pectin, lignin, cellulose and hemicellulose [4] and can be used as animal feed, fodder or for human consumption [5]. Flowers are normally used as infusions for their diuretic activity [1].
O. ficus-indica fruits can present large color differences among cultivars, varying from green to white, yellow to orange and red to purple. These variations can be attributed to the betalain type pigments [6,7,8,9]. Fruits are highly flavored and a source of interesting and useful compounds, such as polyphenols [10,11,12,13,14,15,16], dietary fibers [17,18,19], carotenoids [9,20] vitamins [21], minerals [22] and amino acids [23,24]. O. ficus-indica fruit demand is increasing in domestic and international markets due to growing recognition of its nutritional and health value [25,26]. In addition, the species represents an opportunity for local growers to gain access to superior markets in which consumers place emphasis on exotic character and quality traits.
O. ficus-indica fruit or juice consumption may exert antioxidant activity through non-enzymatic mechanisms [27] or modifying SOD, CAT and GSH enzymatic levels [28]. Polyphenols present in syrup concentrates can display anti-cancer activity in tumorigenic lines of fibroblasts and neuroblastoma [11], and fermented juice could reduce UV-B damage induced in fibroblasts [29]. Besides its pharmacological importance, fruits have a considerable role contributing to the individual daily intake of minerals and other essential nutrients when are consumed fresh [22] or as food supplements [30].
The aim of this paper is to highlight the scientific literature and respective knowledge about O. ficus-indica fruit, concerning their biological activities and chemical composition relevant for food consumption, providing a systematic, scientific and rational review for consumers, stockholders and researchers.
2. Results and Discussion
2.1. Literature Search
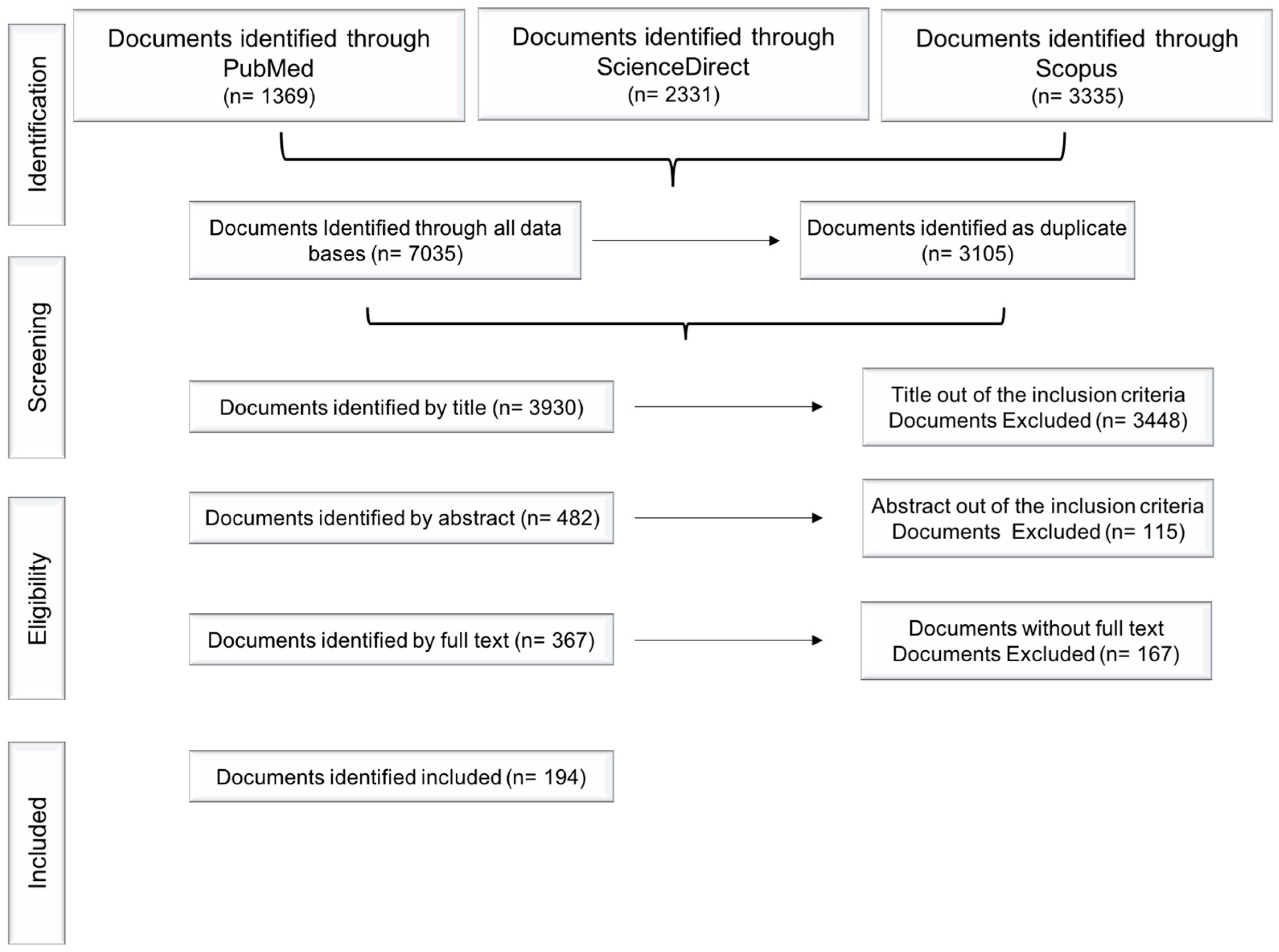
In this review, the literature search using “Opuntia. ficus-indica” and “prickly pear” keywords identified the following number of articles in the respective databases: PubMed® (n= 1369), ScienceDirect® (n = 2331) and Scopus® (n = 3335). After removing duplicates and excluding papers according to the methodology depicted in Figure 1, 482 articles were included by title, and from these, 367 articles were included by abstract and only 194 articles were selected by full text availability and were finally included in this study (Figure 1).
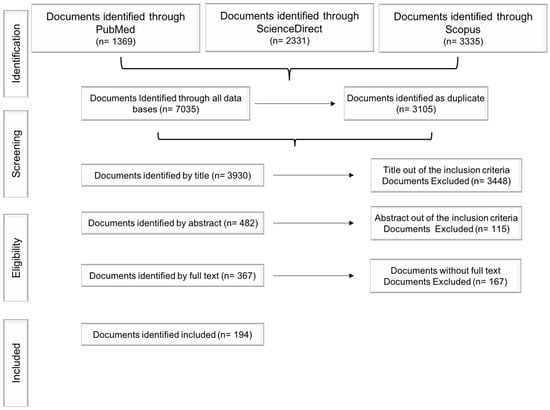

Figure 1.
Flow diagram: search strategy and analyzed data.
Since a single document sometimes delt with several topics, it is important to highlight that some of the analyzed papers were classified more than once into different categories. This resulted in a larger number of items, defined as “records”. In addition, once the total number of records assigned to the main categories was analyzed, it was found that some records could, in turn, be reclassified into subcategories, increasing again the number of items and were ultimately defined as “sub-records”. The search strategy is summarized in Figure 1.
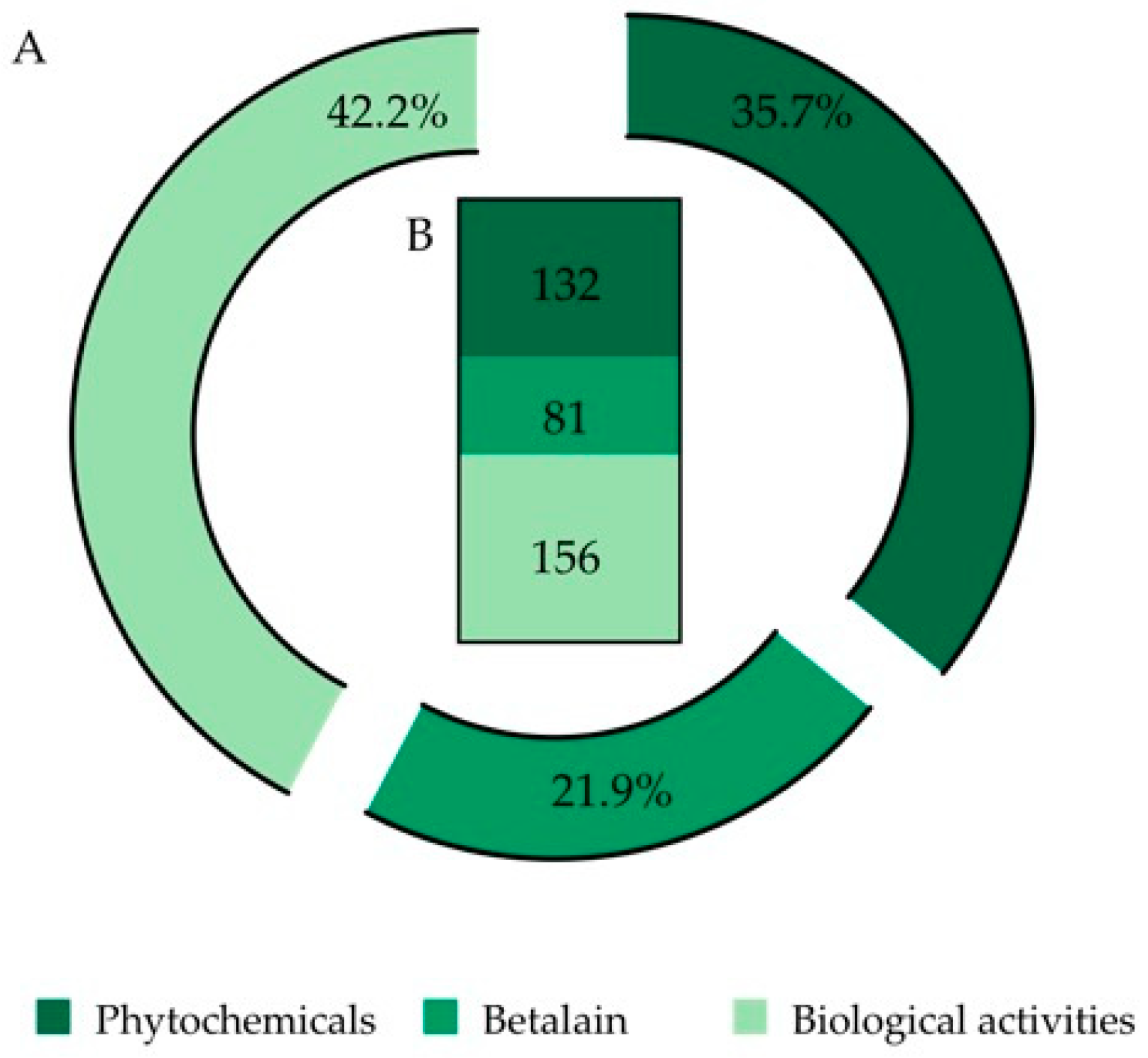
After the analysis of the 194 selected documents, a total number of 369 records was obtained, which were organized in three (3) categories: (i) phytochemicals, (ii) betalains and (iii) biological activities (Figure 2).
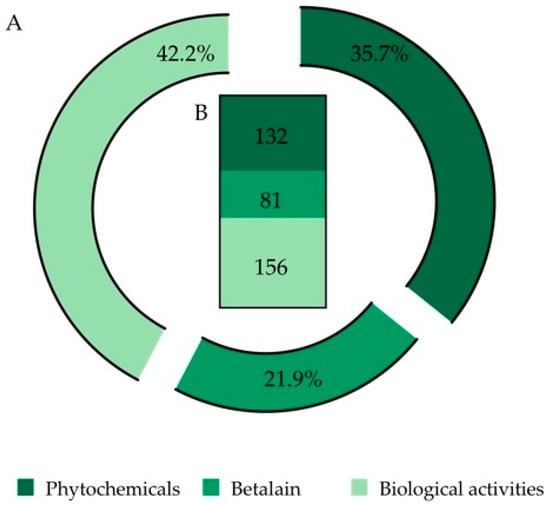
Figure 2.
Final output of 369 records (from 194 selected papers) organized into three categories: phytochemicals, betalains and biological activities. (A) Data expressed in percentage. (B) Data expressed as number of records.
In the first approach, the betalains were included in the phytochemicals category. However, due to their specific characteristics and due to the nature of the information output, e.g., extraction procedures, quantification, identification by analytical techniques, thermal stability and potential use in medicine and other industries, a single and unique category was created for these compounds.
2.1.1. Phytochemicals
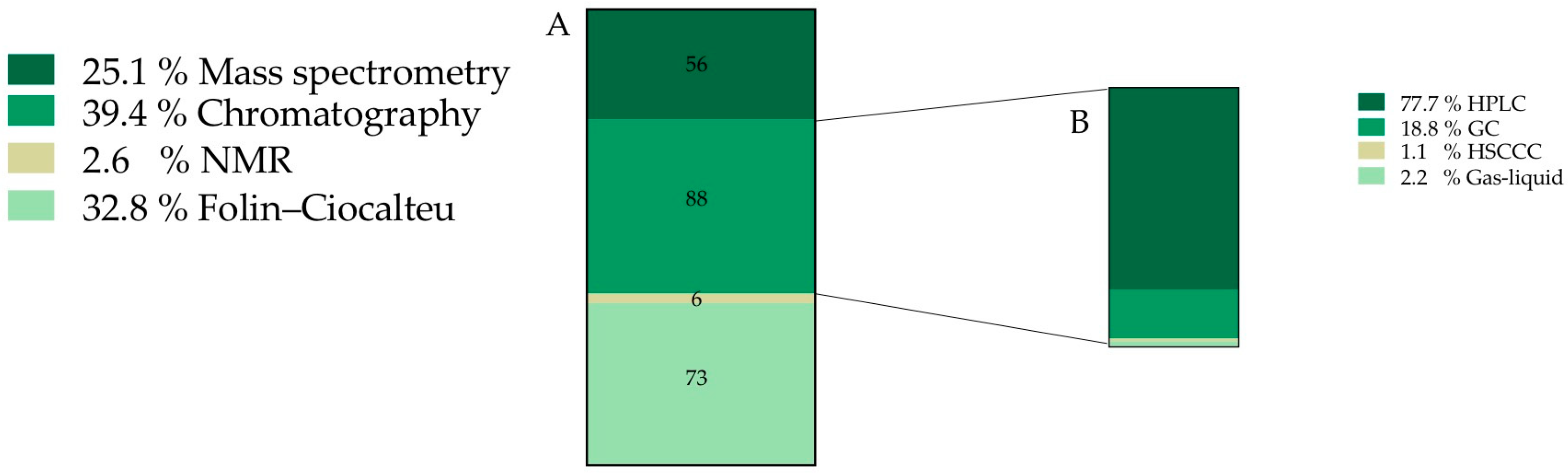
After screening the whole documents, it was found that 35.7% of the total information was related to the chemical characterization and composition of O. ficus-indica fruits (a total of 132 records). Once the 132 records assigned to chemical characterization were analyzed, new sub-categories were created, constituted by 223 final sub-records (Figure 3).
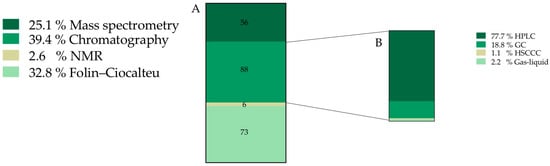
Figure 3.
Frequency of records classified into chemical characterization category (expressed as percentage) (A) Techniques used to characterize and quantify O. ficus-indica chemical composition. (B) Type of chromatographic technique. HPLC—High performance liquid chromatography, GC—Gas chromatography, HSCCC—High speed counter current chromatography.
Phenolic Content
Phenolic compounds have gained special attention due to their ability to scavenge free radicals or donate hydrogens to counterpoise reactive species. Furthermore, some polyphenols may be involved in several biological process such as nitric oxide regulation, anti-inflammatory processes or may possess antimicrobial activity [31].
O. ficus-indica fruits phenolic composition differs qualitatively and quantitatively depending on the variety [32,33]; part of the fruit, either peel, pulp or whole fruit [11,34]; sample preparation and extraction process [35,36,37]; geographic location [9,38]; season [39]; and storage conditions [27,39,40]. A full profile of identified phenolic compounds by different techniques is summarized in Appendix A.
In a recent study, the varieties “Colorada”, “Fresa”, “Blanco Buenavista” and “Blanco Fasnia” collected in the Canary Islands were subject to HPLC-ESI-QTOF. Among the findings, three phenolic acids and 14 flavonoids, mainly isorhamnetin glycoside derivatives, kaempferol glycosyl-rhamnoside and rutin were identified. Pulp fruit phenolic compounds ranged from 38 to 62 mg/100 g f.w for all the varieties. Peel phenolic content is significantly higher than pulp. The lowest content was reported for “Blanco Fasnia”, with 327 mg/100 g f.w, whereas the remaining varieties ranged from 4.3 to 4.5 g/100 g f.w [41]. Data about the range of total phenolic concentration (TP) based on O. ficus-indica fruits are presented in (Table 1).

Table 1.
Total phenolic content of O. ficus indica fruits and juices.
Processing techniques have a direct impact on TP yield [20,63]. Oven dried Morocco peel TP values ranged from 1.7 to 2.4 g GA/100 g d.w depending on the variety [51]. Mexican peel and pulp fruit subjected to high hydrostatic pressure (HHP) accounted for 11 and 4 g GA/g d.w [64]. Mexican green and red fruit residues mixed either with microcrystalline cellulose or lactose showed TP values ranging from 648 to 734 mg GA/100 g d.w [65]. Australian prickly pears treated with four different drying techniques (freeze dryer, microwave, oven dehydrator at 35 and 55 °C) showed that the freeze-drying technique reached the highest TP levels [35]. Fruits grown in drainage sediment had higher total phenolic compounds values than those grown in normal conditions [66], and wounded fruit pulp irradiated with UVB for 15, 90 and 180 min increased total phenolic accumulation by 52.5%, 101.8% and 38.8%, respectively, when compared with non-treated samples [67].
Organic Acids
Organic acids are low weight molecular compounds directly produced from different organisms’ normal metabolism [68,69,70] and may be influenced by biotic and abiotic factors [27,71]. For instance, ascorbic acid is a water-soluble vitamin that is highly appreciated as a food additive for its antioxidant activity and is an important intermediate in redox reactions involved in bones, blood vessels and skin preservation [72]. Malic acid is normally used in candies, food and beverages as a pH control agent, flavoring agent, acidifier and preservative [73] and its consumption can improve skin conditions, boost immunity reduce risk of metal poisoning and promote oral health [74].
Organic acids accumulated in O. ficus-indica fruits are influenced by geographical location and processing techniques. For example, peel and pulp extracts of Portuguese varieties “Gialla”, “Rossa”, “Orange” and “Red” showed the presence of glutaric, malic, succinic and pyruvic acid [71], whereas malic, quinic, citric, piscidic acid and derivates, as well as caffeic and hydroxybenzoic acid, were identified by ESI-MS/MS from Portuguese fruit juice [75]. Sicilian “Rose”, “Gialla” and “Bianca” cultivars showed traces of succinic, lactic, pyruvic and isobutyric acid [76].
Ascorbic acid has been quantified in many O. ficus-indica fruits (Table 2). Its content seems to be higher than other organic acids [77] and also higher than the values reported for apples, grapes, bananas and pears [78].

Table 2.
Ascorbic acid concentration of O. ficus indica fruits and juices.
Carotenoids
Carotenoids are considered relevant in the prevention of certain oxidative stress-related diseases such as cancer. The most studied carotenoids are β-carotene, lycopene, lutein and zeaxanthin. The first two belong to the carotene class, featured by their carbon–hydrogen structure; the second group is called xanthophylls, which, besides their carbon–hydrogen structure, also present oxygen substituents [86].
Recently, carotenoid identification and quantification assisted by HPLC-DAD/MS was carried out in saponified and non-saponified extracts from Spanish O. ficus-indica fruits of “Sanguinos” and “Verdal” cultivars [9]. A summary of carotenoid concentrations is presented in (Table 3).
Lipid and Volatile Components
Fatty acids play pivotal roles in living organisms, being modulators of physiological functions and a source of energy [87]. Normally classified as saturated acids, monounsaturated acids (MUFAs), and polyunsaturated acids (PUFAs), their consumption has been reported to bring health benefits. For instance, linoleic and α-linolenic acid are two of the main representative compounds that cannot be synthesized by humans, known as dietary essential fatty acids, since they prevent deficiency symptoms. Additionally, PUFAs interact with nuclear receptor proteins that bind to certain regions of DNA and thereby can modulate the transcription of regulatory genes [88].

Table 3.
Carotenoid content of O. ficus indica fruits and juices.
Table 3.
Carotenoid content of O. ficus indica fruits and juices.
| Origin | Description | Total Carotenoids | Reference |
|---|---|---|---|
| Algeria | Orange fruit pulp | 503 μg/L | [79] |
| Algeria | Orange fruit pulp | 108.8 μg/100 g f.w | [42] |
| Italy | “Sulfarina” | 1.48 μg/100 g f.w | [89] |
| “Sanguinos” | 3.47 μg/100 g f.w | ||
| “Muscareda” | 1.45 μg/100 g f.w | ||
| Mexico | “Naranjona” | 85 mg /100 g f.w | [90] |
| “Blanca Cristalina” | 400 μg/100 g f.w | ||
| “Esmeralda” | 700 μg/100 g f.w | ||
| Morocco | Fruit juice | 20.8 μg/L | [82] |
| Morocco | “Akria” Fruit pulp | 110 μg/100 g f.w | [84] |
| “Drbana” Fruit pulp | 121.1 μg/100 g f.w | ||
| “Mlez” Fruit pulp | 150 μg/100 g f.w | ||
| Spain | “Sanguinos” Whole fruit | 478.1 μg/100 g f.w | [9] |
| “Sanguinos” Pulp | 255.9 μg/100 g f.w | ||
| “Sanguinos” Peel | 1.69 mg/100 g f.w | ||
| “Verdal” Whole Fruit | 444.9 μg/100 g f.w | ||
| “Verdal” Pulp | 379.4 μg/100 g f.w | ||
| Turkey | Whole fruit Fresh | 1.2 mg/100 g d.w | [91] |
| Whole Fruit Frozen | 1.2 mg/100 g d.w | ||
| Whole Fruit Sun-dried | 454 μg/100 g d.w | ||
| Whole Fruit Microwave-dried | 554 μg/100 g d.w | ||
| USA | Green Skinned | 290 μg/100 g f.w | [14] |
Fruits collected from different farms in Alicante (Spain) were analyzed for fatty acid composition. Pulp fruit showed approximately 16.9–34.8% monounsaturated fatty acids (MUFA) and 35.2–53.9% polyunsaturated fatty acids (PUFA), with oleic and linoleic acid as major compounds, whereas peel fruit showed 11.2–31% MUFA and 37–61.1% PUFA, with linoleic, oleic and palmitic acid found in higher amounts [92]. In addition, Egyptian peel fruit contained 41.2% PUFA, 30.1% MUFA and 28.7 SFA, and eicosadienoic acid was found as the major proportion, followed by oleic and palmitic acid [46]. On the other hand, peels from Tunisian fruits showed a high amount of PUFA, 60.2%, and FA, 23.4%, while pulp and seeds showed 25 and 22.4% PUFA. The general profile showed that linoleic acid was the most abundant, followed by oleic and palmitic acid [62].
Petroleum ether saponified extract of air-dried powdered of Egyptian fruit peels exhibited 15 fatty acids, amounting to 93.2% of the saponifiable matter, where ethyl linoleate was found as the major proportion, followed by ethyl oleate and methyl palmitate. The unsaponifiable extract was composed of 33 compounds amounting for 79.2%, in which two fatty alcohols were identified as major compounds, 1-tricosanol and 1-docosanol [93].
The total lipid content of O. ficus-indica peel fruit purchased on a German market may reach up to 36.8 g/kg d.w, where 63.3% of total lipids correspond to neutral lipids, 26.6% to glycolipids, and 8.75% to phospholipids. In addition, 14 fatty acids were identified ranging between C12 and C26, distributed across total lipids and neutral lipid fractions [21]. Insight suggests that recovered lipids could be suitable for commercial exploitation for food use or production of cosmetics.
The volatile composition may be affected by growing location, e.g., Italian and Greek juices of Opuntia fruits grown on different locations showed different volatile profiles, normally dominated by alcohols, aldehydes and, in minor proportion, terpenoids, which were the most abundant in all samples [94].
The extraction procedure has a direct effect on volatile profile, e.g., peel treated by ultrasonic-assisted solvent extraction showed different a profile than peels subjected to Soxhlet. This can be explained by the high temperature used in Soxhlet extraction that might be degraded in some compounds such as phytol, azulene and serverogenin acetate [36].
Headspace solid-phase microextraction (HS-SPME) and headspace GC-MS were used to analyze and summarize the volatile component of red, white and yellow Sicilian varieties. The most abundant compounds for all varieties were alcohols and esters, followed by terpenes and aldehydes [95]. The volatile profile of six Spanish varieties analyzed by HS-SPME followed by GC-MS resulted in the identification of 35 compounds, 28.5% were aldehydes, 20% terpenes, 20% esters, 17.1% alcohols, 5.7% terpenoids, 2.8% ketones and 2.8% linear hydrocarbons. The only common compounds in all varieties were ß-myrcene, p-cymene, D-limonene, (E)-ß-ocimene, y-terpinene, linalool, nonanal and 2,6-nonaadienal [96].
Sixteen volatile compounds were identified from yellow, red and white cultivars, using HS-SPME followed by GC-FID, and complemented by GC-MS when necessary. Only (E)-2-hexanal, (Z)-2-penten-1-ol and (E,Z)-2.6-nonadien-1-ol were found in all analyzed samples. The total range of volatile compounds was 8 mg/kg for red fruits, 11 mg/kg for yellow fruits and 10.8 mg/kg for white [97]. Furthermore, the n-hexane fraction of pinkish collected in Turkey resulted in fourteen compounds in which the main components were hexadecanoic acid (39.4%), followed by heptacosane (12.3%), methyl linoleate (6.8%), hexacosane (5.8%), tricosane (5.1%), methyl hexadecanoate (4.2%), camphor (2.8%), borneol (2.5%), verbenone (1.8%), pentacosane (1.7%) and α-terpineol (1.1%) [98].
Over 40 volatile compounds were identified as primary aldehydes and acids, however, results suggest that volatiles cannot be used as a fingerprint for sensory differentiation among cultivars [76].
2.1.2. Betalains
Betalains have developed growing interest as natural dyes due to their lack of toxicity, low cost, friendly extraction technology and easy application as food additives or nutraceuticals. Betalains are water-soluble pigments manly restricted to the Caryophyllales order and some fungi species from Basidiomycetes [99]. They are subdivided into two main types, red-purple betacyanin and yellow-withe betaxanthins [100], both considered to have antioxidant potential [20].
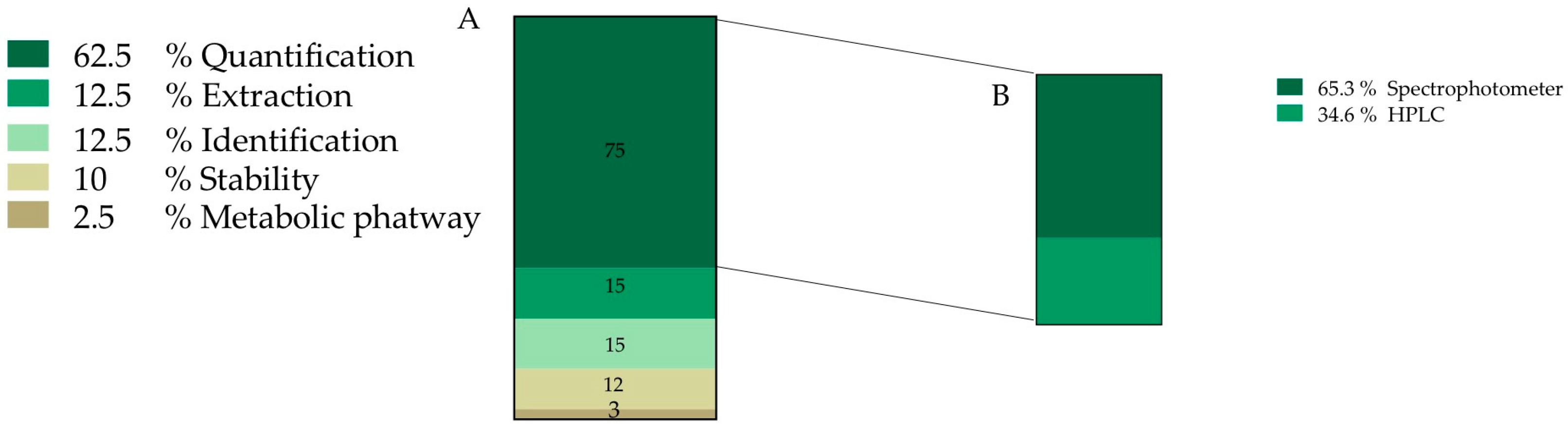
After document screening, 22% of the total information discussed is related to O. ficus-indica betalain (81 records) (Figure 2). This information was further divided into sub-categories and the final number of accounted new sub-records for this category was 120, where 62.5% of the information is related to pigment quantification. We also found that 65.3% of pigment quantification information is carried out by spectrophotometric techniques and the other 34.6% is usually performed assisted by chromatographic methodologies (Figure 4B).
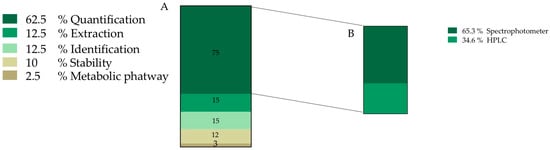
Figure 4.
Frequency of records classified into pigment category (A) Data obtained from 120 records. (B) Methodology used for pigment quantification; HPLC—High performance liquid chromatography.
Betalain identification is mainly performed by mass spectrometry and corresponds to 12.5% of collected information, followed by information about pigment stability, with 12.5%, and finally 2.5% was related to metabolic pathway information (Figure 4).
Identification of O. ficus-indica betalains have been performed several times [16,99,101]. Betalain profile of pulp and peels obtained from three different Portuguese fruits showed seven compounds, two isomers of indicaxanthin and five betacyanins (betanidin-5-O-β-sophoroside, etanidin-5-O-β-glucoside (betanin), isobetanin, gomphrenin I and betanidin) [70]. Cultivars “Naranjona” and “Roja pelota” subjected to HPLC-ESI-MS showed the presence of portulacaxanthin I, vulgaxanthin i, vulgaxanthin ii, vulgaxanthin iii, muscaaurin, indicaxanthin, betanin, iso-betanin and betanidin [101].
In another study, 18 betacyanins and 23 betaxanthins were identified from Mexican cultivars “Roja Lisa” red, “Selection 2-1-62” yellow-orange and “Cristalina” green [102]. Recently 14 betalains, 9 betaxanthins and 5 betacyanins were identified from the Canary Island varieties “Colorada”, “Fresa”, “Blanco Buenavista” and “Blanco Fasnia” [41].
No general consensus exists regarding which part of the fruit has better yield for betalain extraction. Instead, it seems that the cultivar plays a key role in betalain extraction, e.g., yellow fruits are the most suitable for indicaxanthin extraction, whereas reddish fruits for would be more appropriate for betanin [7]. A summary of O. ficus-indica betalains quantification is presented in Table 4.

Table 4.
Betalain quantification of O. ficus indica fruits and juices.
Since O. ficus-indica betalains are suitable for foodstuff coloring, several studies have been performed to evaluate the influence of pH, temperature, heating time, mass–solvent ratio for optimal pigment extraction [106] and clarification methods [107]. Yellow betaxanthin from Moroccan fruits showed thermal stability up to 70 °C for 30 min [100]. Betacyanin stability showed that temperatures above 70 °C reduced, in 75%, the stability of the pigments and betalains maintaining their integrity over a pH range from 3–7, with a maximum Chroma at 6.5 pH [99].
Moreover, pigment stability could be improved with the addition of 1% of citric acid [108] or ascorbic acid [109]. Storage and industrial processes include pasteurization, drying technique, fermentation and juice concentration may alter betalain stability, retention and yield. As an example, freeze-dried tissues showed a higher yield for pigment extraction than tissues treated with other drying techniques (oven, microwave and dehydrator) [35]. Juice stored at 10 °C and 95% of relative humidity resulted in betalain yield increment compared with room temperature storage [27] and pigment retention only decreased 5% when samples were stored for more than 28 days at 4 °C, protected from light [110].
Despite the presence of amino acids and reducing sugars, no Maillard browning was observed in concentrated juice suitable for coloring foodstuff, obtained at pilot-plant scale from typical operations for juice production [111,112] or storage [111]. Additionally, juice treated with high hydrostatic pressure HHP at 550 Mpa increased betalain concentrations compared with traditional heat treatment [113]. On the other hand, even though HPP increased pigment extraction yield, it may have reduced pigment stability by 25% [20]. Recently, a betanin extraction and purification process using a two-phase system based on tetrahydrofuran (THF) and sodium salts has been proposed, achieving 32.1 mg betanin /L [114].
2.1.3. Biological Activities
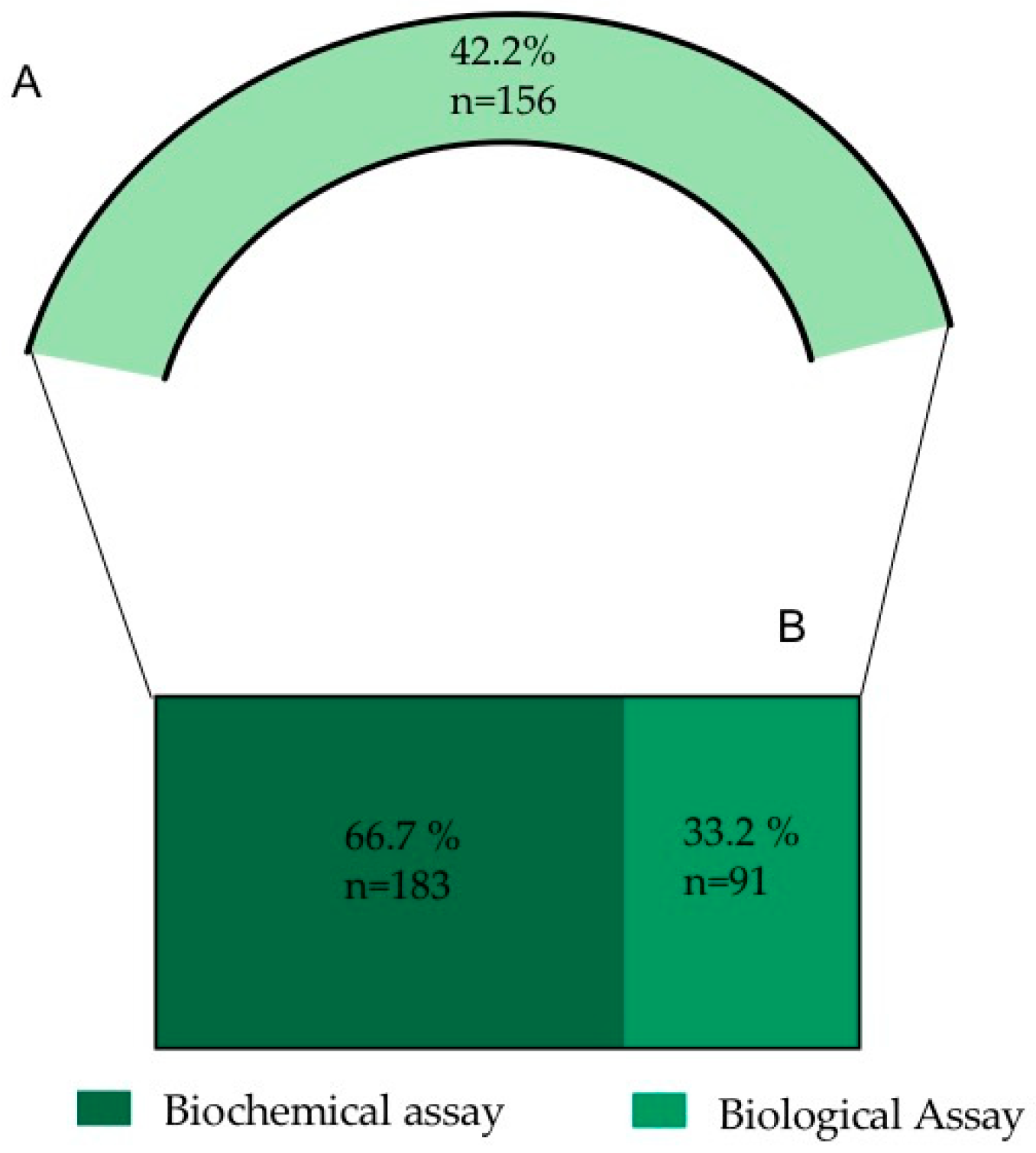
Following the screening of the selected references, 42.2% of the analyzed information is related to O. ficus-indica’s biological activities (156 records) (Figure 2). Due to the elevated number of records, the nature of the information and to simplify the analysis and discussion of the information, the data were subdivided into biochemical assays and biological assays (Figure 5).
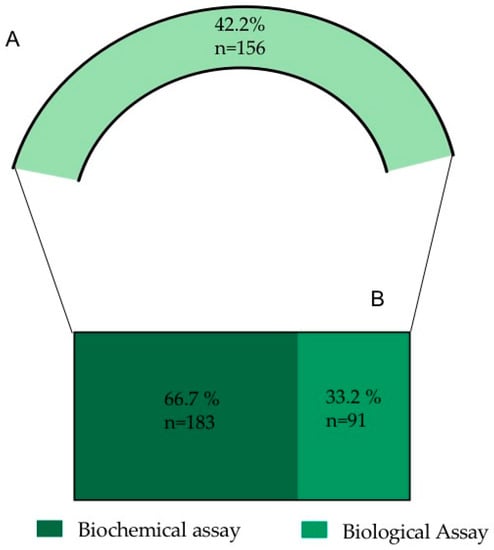
Figure 5.
(A) Percentage of records dealing with biological activities (from a total of 396 records used in the study). (B) Resulting sub-categories after the biological activities analysis.
As shown in previous sections, O. ficus-indica fruits contain a wide variety of chemical compounds, some of which are believed to be associated with different biological properties. For example, phenolic compounds present complex quantitative structure–activity relation (QSAR) properties [115], a situation that may explain the polyphenol’s ability to interact with electron oxidants, helping in the prevention of damaging free radical formation into biological systems [116]. In addition, phenolic compounds such as Isorhamnetin-3-O-glucoside, kaempferol 3-O-arabinoside and quercetin 3-O-rutinoside contain multiple polar functional groups that confer selective or unselective binding sites to biologically important molecules normally associated with health benefits [11,117].
Prickly pear is an important source of betalains, which, in addition to being of great interest as food colorings, could be a source of pharmacologically active compounds [118]. Compounds such as indicaxanthin and betanin could be important for maintaining a balance between free radicals and antioxidant defenses [80,119,120]. Additionally, there is evidence that betalains such as proline-betaxanthin, betanin, 17-hydroxy betanin and indicaxanthin can be used in the treatment of cancer [13,121,122].
It is important to highlight that most of the information on prickly pear fruit activities has been obtained from studies using plant extracts as raw material and less frequently from isolated or purified compounds. This gap makes it necessary to increase the number of studies related to the isolation and purification of target compounds in order to link the described effects to the responsible structures.
Biochemical Assays
From the biological activities records, 66.7% of the information corresponds to biochemical assays. This information was subdivided into different assay methods, taking into account the frequency of the mentioned methodologies (Figure 6).
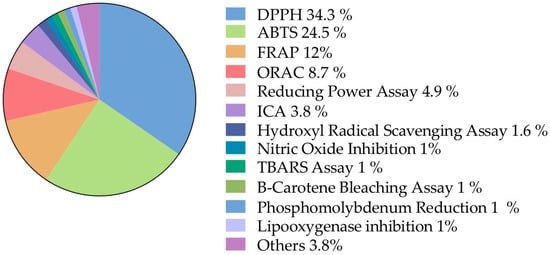
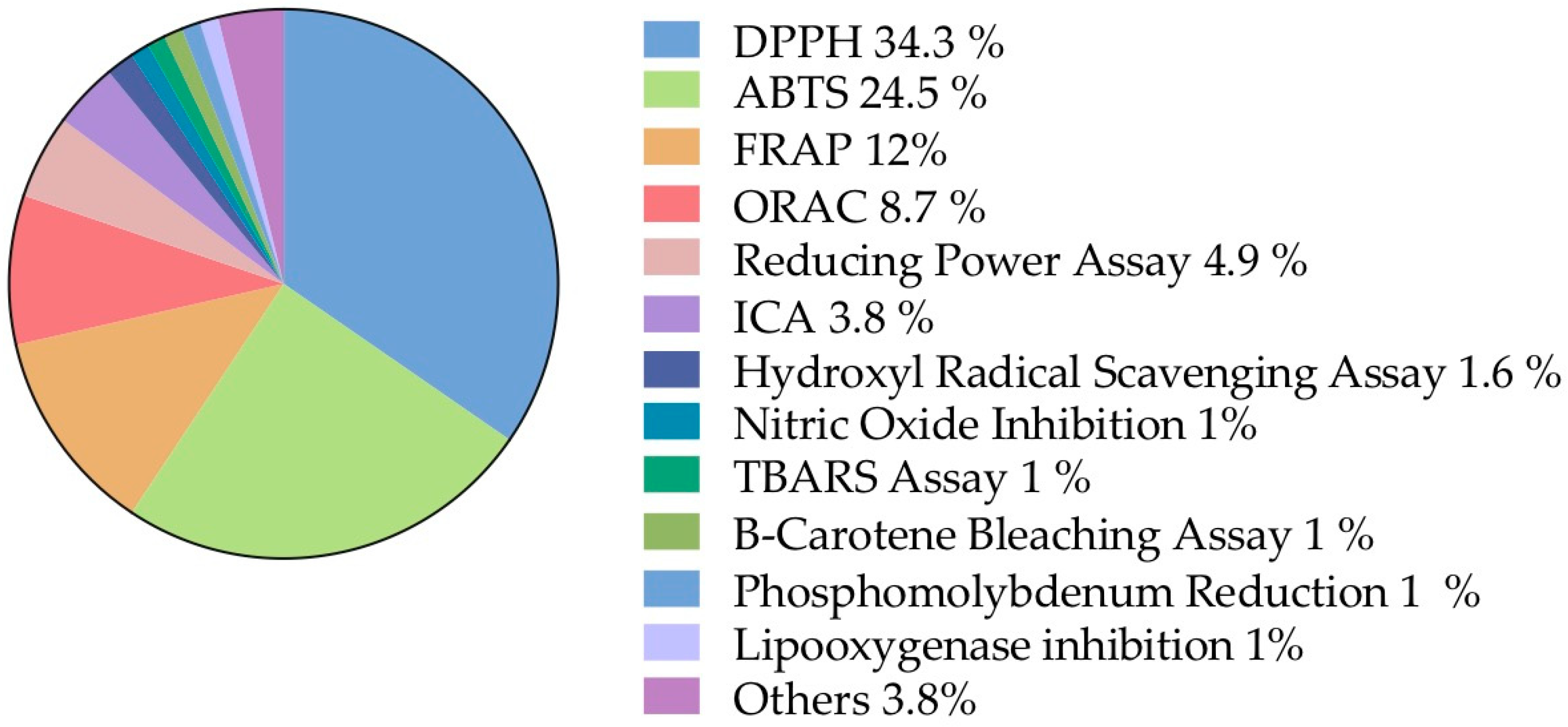
Figure 6.
Biochemical assays. Information classified, according to the frequency of methodologies mentioned, into the biological activities sub-category. DPPH: 2,2-diphenyl-1-picrylhydrazyl; ABTS: green–blue stable radical cationic chromophore, 2,2-azinobis-(3-ethylbenzothiazoline-6-sulfonate); FRAP: ferric reducing antioxidant power; ORAC: oxygen radical absorbance capacity; ICA: iron chelating activity; TBARS: Thiobarbituric acid reactive substances.
The biochemical interactions of O. ficus-indica fruit compounds have been determined through numerous spectrophotometric methods. Most of the methodologies correspond indirectly to the ratio of electrons/hydrogens that a certain mixture or compound can shift with the oxidant probe or substrate [123]. Nowadays, these principles are widely used across several methodologies and their importance is reflected in the high number of published documents attempting to give insight into the beneficial effects of different food matrixes, vegetables, fruits, spices and herbs [124]. Thereby, it is extremely important to understand the conceptual and technical limitations [125] to increase the quality of the obtained information. Factors such as blanks, reagent and probe concentration, light, kinetics, stoichiometry, pH, temperature and incubation time are commonly neglected and exert great influence upon the assays. This causes difficulties in the standardization of the assays [124,126]. The lack of standardization and the different ways of expressing the results (molar equivalent of Trolox, ascorbic acid, gallic acid and other oxidant equivalents, or in terms of EC50), makes it difficult to obtain an accurate idea of the results and also limits the direct comparison of the data extracted from different documents.
Overall, prickly pear fruits exhibit a high antioxidant capacity against free radical species, including superoxide radicals, hydrogen peroxide, hydroxyl radicals and singlet oxygen [80,89], which converts them as an important innovative and upcoming product for human health prevention against oxidative stress-related diseases. Detailed information is summarized in Appendix B.
The antioxidant potential is often related to the ascorbic acid, α-tocopherol, glutathione, carotenoids and phenolic compounds present in different tissues [127] and can be attributed to the structure–active relationship (SAR) and the interactions with the probe [128]. For example, glycosylation of molecules increases polarity, turning the molecule more hydrophilic [129] and the DPPH radical is spontaneously formed in organic solutions. This organic environment obstructs hydrophilic molecules by donating a hydrogen or an electron, thus reducing DPPH molecule [130].
On the other hand, the ABTS+· radical proved to be ambivalent and it can be solubilized in aqueous and in organic media [131]. Therefore, an aqueous environment promotes the hydrogen or electron donation of glycosylated molecules, corroborated by an increase in the antioxidant potential of O. ficus-indica fruit.
Biological Assays
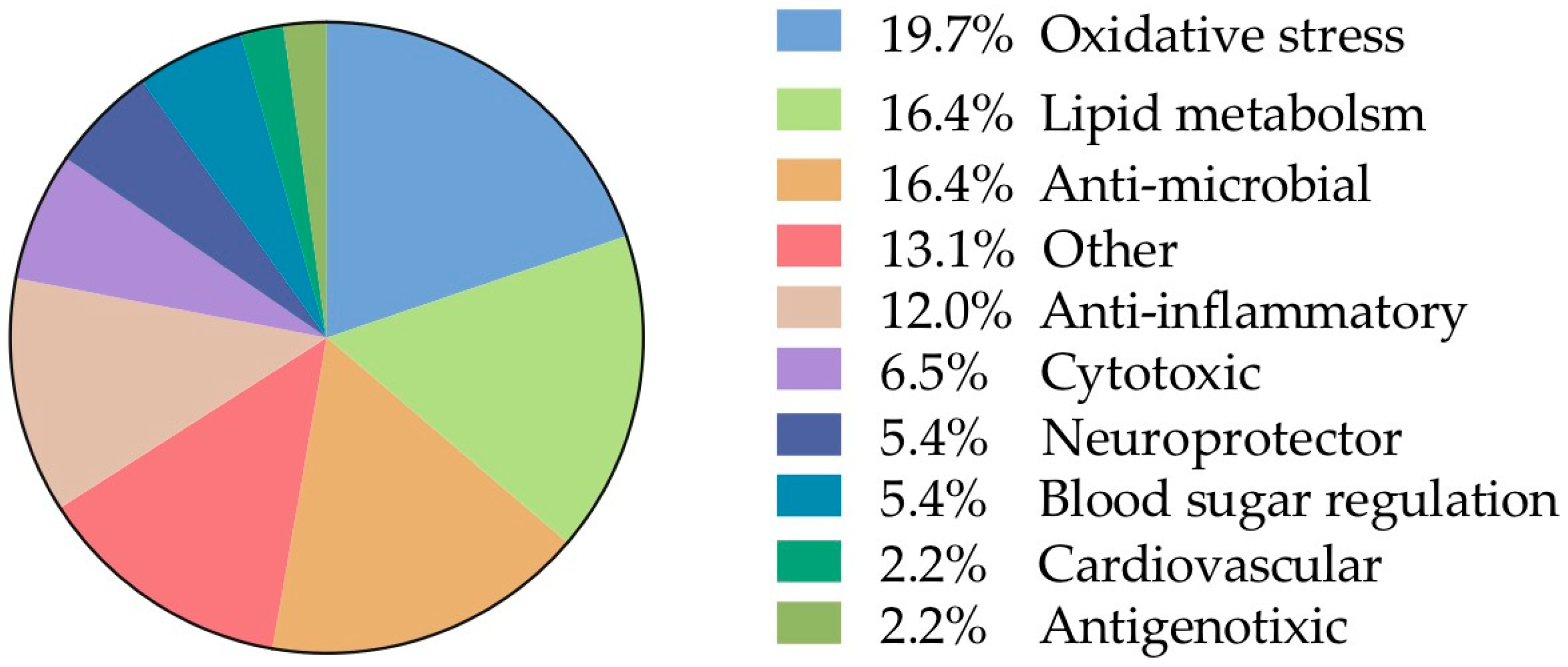
The remaining analyzed data allocated in the category “biological activities” was further subdivided taking into account the frequencies of the biological assays reported (Figure 7). From this, 19.7% was allocated into oxidative stress and 16.4% lipid metabolism, 16.4% antimicrobial, anti-inflammatory 12% were the other main categories of biological activity. Finally, individual records (n = 12) were found and to facilitate their analysis, they were grouped into a single category called “other” and represent 13.1% of the total information.
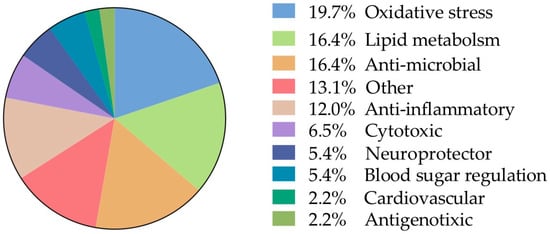
Figure 7.
Frequency of sub-records classified according to the biological interaction (91 in total).
Oxidative Stress
Wistar rats submitted to ethanol-induced injury and with a juice intake of 40 mL/kg b.w of Tunisian purple-skinned fruit resulted in 3.9-rold reduced malondialdehyde (MDA), 4.33-fold reduced protein carbonyl (PC), 2.23-fold increased GSH levels and 2.57-fold increased plasma scavenging activity in erythrocytes [28]. It also increased the activity of hepatic antioxidant defenses such as superoxide dismutase (SOD), catalase (CAT) and glutathione (GSH) by 1.3-fold, 2.68-fold and 3.44-fold, respectively, 2.42-fold reduced alanine aminotransferase (ALT), 2-fold reduced aspartate transferase (AST), and 3.22-fold reduced gamma-glutamyl transferase (GGT) produced by ethanol-induced injury in Wistar rats [132]. Radiation-induced colitis in female Wistar rats was significantly ameliorated by pretreatment of 1 g/kg body weight of peel extract for 10 days. MDA levels decreased 1-fold and SOD increased 2.7-fold compared to irradiated rats [93].
Enzymatic levels (SOD, GPx, GRx) of high fat-fed rats after O. ficus-indica vinegar administration of 7 mL/kg for seven days were slightly modified compared with the control [133]. A commercial product designed by Cactinea®, composed of spray-dried juice extract, tested in rats at a daily intake of 240 mg/kg for seven days, increased, by 1.03-fold, blood globular levels of glutathione peroxidase [134]. Daily intake of 100 mg/kg b.w peel or fruit extract increased GSH, SOD and CAT and decreased MDA levels in adult male Sprague-Dawley rats with aluminum chloride-induced neurotoxicity [45].
The aqueous extract of combined pulp and seed recovered endoplasmic reticulum homeostasis via the unfolded protein response pathway in Drosophila melanogaster [135].
PC-12 neuronal cell line viability increased up to 73%, after H2O2 insult, at 100 µg/mL of ethyl acetate extract of Korean “Saboten” fruits [136]. Portuguese wild fruits juice extracts showed an anti-proliferative effect on the human colon cancer cell line HT29, an effect that may be attributed to an increment of 12 to 16% of ROS compared to the control [57]. Extracts from yellow, red and orange fruits at 50, 75 and 100 μg/mL decreased lymphocyte mortality treated with 100 μM of t-BOOH, by approximately 4.0 to 55%, respectively, for red cactus pulp and peels, and 5.0 to 31% for orange and yellow pulp and peel extracts compared with non-treated cells [104].
In addition, some tests have been performed in humans. When compared with a 75 mg/day vitamin C intake, the intake of 250 g/day of O. ficus-indica fruit decreased, by 4.2 and 1.74-fold, MDA and LDL hydro-peroxide, respectively, and increased, by 1.48-fold, the GSH:GSSG ratio in healthy humans [137]. Additionally, MDA, total cholesterol (TC), creatine kinase (CK), LH and HDL levels decreased after 150 mL/day juice supplementation during the yo-yo intermittent recovery test (YYIRT) [138]. In a separate study, the consumption of juice reduced hydroperoxides and increased the ability to restore redox balance caused by high intensity exercise in healthy active women [139].
Lipid Metabolism
Collected information suggests that fruit consumption may have a direct influence on liver metabolism, exerting hepatic protection through several metabolic modifications. For example, a juice intake of 40 mL/kg b.w of Tunisian purple-skinned fruit increased SOD 1.38-fold, CAT 1.4-fold, and ethanol-induced damage in rat erythrocytes 1.27-fold [132], whereas of ethanol-induced erythrocytes damage was reversed in 149.9% [28]. The same dosage increased the hepatic activity of SOD, CAT and GSH 1.32-fold, 2.68-fold and 3.44-fold, respectively, decreased total cholesterol (TC) 1.33-fold, triglycerides (TG) 1.81-fold and reduced lipid peroxidation in ethanol-induced injury in rats [140]. Moreover, fruit vinegar seems to reduce histopathological lesions, decrease liver damage and regulate lipid metabolism caused by oxidative stress in high fat-fed rats [141].
Glutamine pyruvic transaminase (GPT) and glutamic oxaloacetic transaminase decreased after 24, 48 and 72 h of 3 mL/day juice intake in the CCL4 liver degenerative process of rats [142]. Jordanian fruit juice at 2 mL/kg bd administration reduced liver damage induced by Cyclophosphamide (CP) in mice [143]. N-butanol fractioned flavonoids at 10 and 100 µg/mL protected Sprague-Dawley rat hepatocytes against alcoholic oxidative stress, increasing cell viability up to 40% [144].
Administration of 0.3% peel extract of the “Verde Villanueva” variety with a hypercholesterolemic diet was able to reduce hepatic cholesterol levels by 35% in hyperlipidemic hamsters [13]. Fruit ingestion of 250 g/day significantly increased 111In–LDL and 111In–HDL binding by human platelets. Specific binding sites on platelets can be upregulated by prickly pear consumption [145].
O. ficus-indica vinegar consumption of 7 mL/kg/d reduced plasma TC in 31%, TG in 53.7%, LDL in 82.14% and increased HDL in 20.5% on high fat-fed rats [133]. Methanolic extracts of Sicilian pulp fruit at variable concentrations (1–5 mg) inhibited lipid oxidation in red blood cells from healthy patients in a dose-dependent manner [80]. Fruit ingestion of 250 g/day significantly increased 111In–LDL and 111In–HDL binding by human platelets, reducing hypercholesterolemia [145].
Anti-Microbial
Fruit extracts exhibited different levels of antimicrobial activity against several strains [11,58,146,147]. The antimicrobial activity normally depends on the nature of the biological sample and extraction method, for example, oven-dried fruits showed better activity compared to other samples [148]. A summary of the antimicrobial potential (both against bacteria and fungi) of O. ficus-indica fruits is presented in (Table 5).

Table 5.
Antimicrobial activity of O. ficus indica fruits.
Anti-Inflammatory
Fruit extracts exhibited also anti-inflammatory effects both in animals and in humans. Intake of yellow fruit, 20 g f.w. fruit equivalent /kg, significantly decreased the inflammatory response to carrageenin-induced rat pleurisy, inhibiting the exudate level, number of migrated leukocytes and decreasing the release of pro-inflammatory mediators, such as prostaglandin E2 (PGE2), NADPH oxidases (Nox), interleukin 1-β (IL1-β) and tumor necrosis factor-α (TNF-α) in Wistar rats [153]. Obese rats fed with vinegar (7 mL/kg daily) reduced obesity-induced heart injury trough anti-inflammatory effects, characterized by decreased pro-inflammatory biomarkers and anti-adiposity mechanisms [141].
Korean fruit extract administration on female Sprague-Dawley rats and male IRC mice showed a significant reduction by 1.52-fold in leukocyte migration, 20% β-glucuronidase and 63% swelling percentage after carrageenan-induced paw edema and gastric injuries [154]. Adult male Sprague-Dawley rats with aluminum chloride-induced neurotoxicity treated with 100 mg/kg b.w peel or pulp extract decreased TNF-α and nuclear factor kappa B (NF-κβ) and decreased IL1-β levels [45]. Nitric oxide accumulation decreased approximately 5-fold in female Wistar rats’ radiation-induced colitis [93].
Consumption of 200 g of ”Surfarina” fruits twice a day in a dietary regimen decreased pro-inflammatory cytokines in healthy humans [155]. Daily ingestion of juice drastically decreased recalcitrant cutaneous sarcoidosis in a 50-year-old Caucasian female patient [156]. “Sanguigna” fruit juice fermentation with L. mesenteroides modified anti- and pro-inflammatory cytokines in Caco-2/TC7 cells [152]. Purple-skinned “Pelota” fruits treated with high hydrostatic pressure decreased hyaluronidase inhibition by approximately 20% compared with non-treated samples [64].
Cytotoxic
The cytotoxic activity of O. ficus-indica fruit has been tested in several cancer cellular lines. For example, Mexican fruit juice of the “Pelon” variety at 0.5% final concentration, during 48 h of incubation, decreased the cell viability of mammary (MCF-7), prostate (PC-3) and hepatic (HepG2) cancer cell lines, in 7.6, 5.1, and 12.6%, respectively [13]. Juice of fruits collected in different regions of Portugal showed an anti-proliferative effect at all tested concentrations (2–20%) in the human colon cancer cellular line HT29 after 96 h of incubation [57]. Aqueous extract from yellow fruits showed a 50% cell viability reduction at 400 mg fresh pulp equivalent/mL in human colon cancer cells [122]. Increasing concentrations of pulp and peel ethanolic extract from Egyptian fruits decreased cellular viability of liver (HepG2), colon (Caco-2) and mammary (MCF-7) cancer cell lines [157].
Neuroprotector
The acetone fraction of fruit peel extract at 400 µg/mL alleviated neurodegenerative effects in flies, exerting anti-amyloidogenic potential and mitigating the disruption of lipid membranes [158].
Korean juice extract at 50, 100 and 200 mg/kg b.w reduced ethanol-induced psychomotor alterations in Sprague-Dawley rats [159]. Lyophilized peel and fruit methanolic extract did not show acute oral toxicity at 2 g/kg b.w adult male Sprague-Dawley rats. Moreover, daily intake of peel or pulp extract at 100 mg/kg reduced the acetylcholinesterase (AChE) in 55.9% and 31.9%, respectively, compared to AlCl3 values [45].
Methanolic extracts of Korean fruits at 1 mg/mL reduced LDH release induced by N-methyl-d-aspartate (NMDA 25 µM), kainate (KA 30 µM) and oxygen–glucose deprivation (OGD 50 min) induced neuronal injury in cultured mouse cortical cells in approximately 59%, 37% and 75%, respectively [159]. Cell viability of neuronal PC-12 lines pre-incubated for 24 h with 100 µg/mL of ethyl acetate extract fraction was increased up to 73% after H2O2 µM insult [136].
Blood Sugar Regulation
Animal experiments showed the potential of fruit extracts in blood sugar regulation. Oral intake of 5.88 mg/kg of blended pad and fruit extract (75:25) increased plasma insulin levels and lowered blood glucose in Wistar rats [160]. Acetone peel fruit extract of 2 g/kg efficiently reduced blood glucose levels in healthy “Balb-C mice [39].
Additionally, a commercial formulation under Opundia™ designation reduced blood glucose levels by 7% and increased serum insulin concentration by 28% in healthy athletes [161]. Opundia™ capsule intake increased serum insulin and may facilitate blood glucose disposal after exercise in healthy men [162]. Additionally, the same formulation reduced blood glucose concentration in obese pre-diabetic adults [163].
Cardiovascular
A commercial formulation under patent no. W098/39019 corresponding to 100 g of fruit was tested on male athletes, which improved heart rate variability and autonomic nervous system activity, improving performance during exercise and reducing fatigue after exercise [164]. On the other hand, daily administration of 150 mL of juice improved diastolic and systolic blood pressure and maximal heart rate after yo-yo intermittent recovery test [165]. Ingestion of 150 mL of fruit juice reduced autonomic cardiac regulation in healthy men feed with high-fat formulations [166].
Antigenotoxic
DNA damage of HT29 cells induced by 100 µM of H2O2 and treated with 10% v/v Sicilian yellow fruit aqueous extract decreased by approximately 10% after 24 h [167]. Still, increasing concentrations of Korean fruits’ methanolic extract (12.5–100 µg/mL) reduced human peripheral lymphocytes by 200 µM of H2O2-induced damage in a dose-dependent manner [168].
Others
Daily oral intake of 3 mL/rat of Sicilian fruit juice revealed protective action against ethanol-induced ulcers in rats, avoiding ROS excessive accumulation and maintaining the mucosal stability [81]. The ingestion of commercial capsules containing fruit extract resulted in a significant reduction in nausea, anorexia and dry mouth in healthy subjects with alcohol hangover symptoms [169].
Lyophilized powder juice extracted from Korean fruits and maltodextrin at 800–1600 mg/kg significantly reduced stress-induced acute gastric lesions in rats, reducing gastric mucosal TNF-α and myeloperoxidase [170]. Ethyl acetate fruit extract administered intraperitoneally at 100 mg/kg in BALB/c mice increased the sleeping time after thiopental exposure 60 mg/kg compared to non-administered mice, inhibiting the effect on the CNS associated with the GABAergic system, thus, suggesting a sedative-hypnotic effect [171]. Freeze-dried fruit powder may be effective and a safe alternative active ingredient to hormone replacement treatment for the management of postmenopausal symptoms [172]. Ovariectomized rats treated with 500 mg/kg/d had modified hepatic gene expression of cytochrome P450 (CYP450) and glucuronosyltransferase UGT isoforms, suggesting that OFI consumption may be used to mitigate postmenopausal symptoms in women [173].
Additionally, 240 mg/mL methanolic extract from yellow “Surfarina” Sicilian fruits resuspended in PBS inhibit 80% of the spontaneous contractions of mouse ileum longitudinal muscle by interfering with pathways of intracellular Ca2+ release in the smooth muscle cells [174]. Administration of 5 mL/100 g b.w of 15% infusion of Sicilian fruit aqueous extract reduced plasma uric acid levels in male Wistar rats [81]. Increasing doses of green-white juice fruit and aqueous seed extract showed a laxative effect on gastrointestinal motility of constipated-after-loperamide-treatment and healthy rats. Furthermore, fruit ripening stage modified small intestinal mobility, laxative effect and gastric emptying [175,176].
Extracts of yellow and red Spanish fruits showed a significant increase in the average lifespan of Caenorhabditis elegans from 12.8 to 13.7 days for 0.1% w/v of red extract and 14.2 days for 0.5% w/v of yellow extract [158,177]. Sicilian pulp and peel extracts from yellow, red and orange fruits showed a clear angiogenesis effect in fertilized Gallus gallus eggs at 50 µg of extract/egg, orange peel extract being the more active extract, with 90.73% vessel growth inhibition and yellow pulp extract being less active, with 54.35% vessel growth inhibition [97].
Incubation of human skin fibroblast with 1 and 10 µg/mL of fermented juice for 72 h white Aureobasidium pullulans KCCM 12017 and Pichia jadinii KFCC 11487P may reduce photoaging by UV- damage, increasing transforming growth factor-beta (TGF-β) [26]. Crude methanolic extract of fruit peel showed a tyrosinase inhibitory effect of 72% and successfully inhibited 88% of lipoxygenase effects. In addition, 1% of peel extract incorporated into an o/w emulsion was shown to protect the formulation after in vitro irradiation of UVA [57].
3. Conclusions
Fruit consumption is no longer a personal choice based on taste; instead, it has become a health and life quality issue because of fruits’ nutritional content, amount of minerals, fibers, vitamins and bioactive compounds. The present review aimed to examine and summarize the scientific literature concerning antioxidant properties, biological activities, chemical composition and pigment information on the published data of O. ficus-indica fruits.
As an emerging fruit, inclusion of O. ficus-indica in the daily diet is a challenge; however, reported in vitro and biological activities data suggest that periodic fruit consumption reduces the risk of developing chronic diseases. Furthermore, its protective effects are related to organic acids, phenolic compounds, lipid content and the synergistic interaction between them. In addition, fruits represent a promising source of natural red and yellow pigments (betaine and betaxanthin), especially peels that have high levels of pigments and may represent up to 45% of the fruit.
Betalains are neither allergenic nor toxic, and aside from its high molar extinction coefficients, which makes them a good candidate to replace synthetic colorants in the food industry, they may have antioxidant properties, increasing the beneficial properties of the final product. Apart from the medicinal and food industry potential, O. ficus-indica, due to its CAM metabolism, can be used as an agricultural alternative for farmers in arid or semiarid regions and those facing the effects of climate change, providing not only fruits but succulent flowers and pads (cladodes), increasing the commercial value of the species.
Author Contributions
Conceptualization and methodology, L.G.-S. and A.C.P.D.; validation, A.C.P.D. and E.R.; formal analysis and writing—original draft preparation, L.G.-S. and B.F.; writing—review and editing, L.G.-S., B.F., E.R. and A.C.P.D.; supervision, E.R. and A.C.P.D.; project administration, A.C.P.D.; funding acquisition, A.C.P.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the “Contrato-Programa” Financiamento Plurianual de Unidades de I&D, through the FCT I.P., UIDB/04050/2020 and UIDB/04033/2020 and CCDR-N (Norte Portugal Regional Coordination and Development Commission) through the project AgriFoodXXI (NORTE-01-0145-FEDER-000041).
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the financial support provided by a grant BD/AGRICHAINS/UTAD/2016_SET from the Programa Doutoral–Agrichains; to the European Social Funds and the Regional Operational Programme Norte 2020 (operation NORTE-08-5369-FSE-000054).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A

Table A1.
Summary of O. ficus-indica phenolic compound composition.
Table A1.
Summary of O. ficus-indica phenolic compound composition.
| Phenolic Compounds from O. ficus-indica Fruit | ||
|---|---|---|
| Compounds | Reference | |
| 1 | 1-O-feruloyl-β-D-glucopyranoside | [178] |
| 2 | 2-Feruloyl piscidic acid | [75] |
| 3 | Androsin, Aromadendrin | [172] |
| 4 | Benzoic acid | [157] |
| 5 | Benzyl-O-β-D-glucopyranoside | [178] |
| 6 | Betanin, Betaxanthin | [75,112] |
| 7 | Caffeic acid | [11,75,157] |
| 8 | Caffeic acid 4-O-glucuronide | [179] |
| 9 | Caffeine, Catechol, Chlorogenic acid, Cinnamic acid | [157] |
| 10 | Dihydroferulic acid 4-O-glucuronide | [179] |
| 11 | Ellagic acid | [157] |
| 12 | Eucomic acid | [45,150,179] |
| 13 | Ferulic acid | [157] |
| 14 | Ferulic acid glucoside | [75] |
| 15 | Gallic acid | [132,157] |
| 16 | Hydroxybenzoic acid, Isobetanin | [75] |
| 17 | Isorhamentin-O-(deoxyhexosyl-hexoside), Isorhamentin-O- (deoxyhexosyl-pentosyl-hexoside), Isorhamentin-O-(pentosyl- hexoside) | [150,171] |
| 18 | Isorhametin-3-O-glucoside | [11,180] |
| 19 | Isorhamnetin | [75,178,181] |
| 20 | Isorhamnetin 3-O-β-D-glucopyranoside | [178] |
| 21 | Isorhamnetin 3-O-galactoside 7-O-rhamnoside, Isorhamnetin 3-O- glucoside 7-O-rhamnoside | [148,179] |
| 22 | Isorhamnetin glucoside, Isorhamnetin glucosyl-di-rhamnoside, Isorhamnetin pentosyl-glucoside, Isorhamnetin pentosyl- rhamnoside, Isorhamnetin pentosyl-rutinoside | [75] |
| 23 | Isorhamnetin rutinoside | [75,180] |
| 24 | Isorhamnetin-O-(di-deoxyhexosyl-hexoside) | [150] |
| 25 | Kaempferol | [157,178] |
| 26 | Piscidic acid | [181] |
Appendix B

Table A2.
Summary of O. ficus-indica biochemical assays and major outcomes.
Table A2.
Summary of O. ficus-indica biochemical assays and major outcomes.
| O. ficus-indica Fruit Antioxidant Power | |||||
|---|---|---|---|---|---|
| Origin | Variety | Sample Type | Outcome | Reference | |
| 1 | Saudi Arabia | Red/Purple and Orange/Yellow | Juice | Red peel and pulp presented, in general, higher antioxidant activity than orange juices, DPPH70.17 and 81.90 mg AA equivalent/100 mL red juice, respectively, while 234.96 and 391.90 mg AA equivalent/100 mL orange juice, phosphomolybdenum method 760.61 and 735.35 mg AA equivalent/100 mL red juice and 228.57 and 204.31 mg AA equivalent/100 mL orange juice and reducing power assay 149.49 and 123.23 mg AA equivalent/100 mL red juice and 83.12 and 81.38 mg AA equivalent/100 mL orange juice | [10] |
| 2 | Egypt | Not specified | Aqueous isopropyl (80%) | Peel extract showed EC50 values 15 µg/mL for DDPH assay and 22.5 µg/mL for hydroxyl radical scavenging assay | [46] |
| 3 | Italy | Red/Purple and Orange/Yellow | Methanol: formic acid: water (50:5:45) | TEAC values of red cultivar were higher than orange ones (0.61 vs. 0.37 mmol/100 g f.w), while ORAC assays have similar values between red and orange extracts (1.28 vs. 0.98 mM/100 g f.w) | [32] |
| 4 | Tunisia | Purple | Juice | After 30 min of incubation, juice samples were able to scavenge 10.3% of DPPH solution | [165] |
| 5 | Spain | Not specified | Methanol:water (80:20) 1% HCL | Mean EC50 values of pulp and peel for DPPH 59.35 and 56.58, ABTS 21.1 and 32.4 and FRAP 22 and 58.3 mM Trolox/kg d.w | [34] |
| 6 | Mexico | Red/Purple | Edible films with aqueous peel extracts 2% and 4% | Samples exert reducing power of 196.8 and 382.1 mg AA equivalents and DPPH values of 46.8 and 52.5 mg GA equivalents | [182] |
| 7 | South Africa | Not specified | Ethanol, methanol, water, hexane | DPHH and FRAP activity were higher following the solvent sequence, hexane > acidified methanol > ethanol > water. Additionally, oven dried samples were more active than freeze dried samples | [148] |
| 8 | Mexico, Chile & Brazil | Selenium tolerant | Not specified | Selenium tolerant samples, Red and Orange had, on average, 2,8-fold higher ABTS activity than cultivar growth in normal conditions | [66] |
| 9 | Turkey | Not specified | Methanol | DPPH scavenging % of fruits collected on different locations ranged from 52.21 to 53.31 | [38] |
| 10 | Mexico | Pelon Red and Alfajacuyan Green | Methanol:water (50:50) and acetone:water (70:30) | By-product (spines, glochids and epidermis) Pelon extract showed 47.35 and 65.76 for µM Trolox/g d.w for FRAP and ABTS assays, meanwhile Alfajacuyan extract showed 40.39 and 66.33 Trolox/g d.w respectively | [17] |
| 11 | Tunisia | Inermis | Seed cake protein | 0.2 mg/mL of protein showed 60% of scavenging activity for DPPH assay, the ability to reduce iron of the protein increased with increasing concentrations (1–5 mg/mL), additionally, increasing concentration (1–5 mg/mL) increased the ability of chelate iron with a maximum of 80%. | [183] |
| 12 | Tunisia | Orange | Peel fluor | DPPH scavenge activity was 274.7 mmol/g equiv Trolox | [60] |
| 13 | Algerian | Not specified | Vinegar | Vinegar reduces the hepatic and serum levels of T-BARS, AST, ALT and Alk-p in acute liver injury caused by free radical reactions | [133] |
| 14 | Italy | Red/Purple, Orange/Yellow and Withe | Methanol | Antioxidant potential of pulp extracts showed 5.31 (yellow), 4.36 (withe) and 4.20 (Red) µM Trolox/g of edible pulp for ABTS assay | [80] |
| 15 | Spain | Sanguinos Red, Verdal Orange | Methanol and Water | DPPH activity of Sanguinos and Verdal of analyzed tissued did not show differences, mean values were 129.78 (whole fruit), 115.66 (pulp) and 141.7 (peel) µM Trolox/100 g f.w. On the other hand, only peel tissue showed differences among verities for ORAC assay, mean values were 37.37 (whole fruit), 29.40 (pulp), 88.31 (Sanginos peel) and 48.35 (Verdal peel) µM Trolox /100 g f.w | [9] |
| 16 | Italy | Yellow | Depeptinazed Juice | Total antioxidant activity in depeptinazed juice was 5.21 mM Trolox | [184] |
| 17 | Mexico | Pelon Red | Juice | Pelon juice showed 21.0 mM Trolox/ juice liter for the ORAC assay | [13] |
| 18 | Algerian | Orange/Yellow | Methanol:water (70:30) | Peel extracts showed mean EC50 values of 1.03 mg/mL and 77.81 mg/mL for reducing power activity and DPPH | [43] |
| 19 | Argentina | Dark/Purple, Purple and white | Methanol | DPPH scavenging potential of fruits were expressed as vitamin C equivalent antioxidant capacity (VCEAC), purple cultivar showed higher values than dark purple and white; 0.57 mg/g, 0.53 mg/g and 0.48 mg/g VCEAC respectively | [185] |
| 20 | Mexico | Roja Lisa | Juice | Storage at 10 °C greatly increased 2.8-fold ABTS, 2.0-fold DPPH, 2.42 FRAP and 1.22-fold ORAC activity than fresh juice | [27] |
| 21 | Mexico | Pelon Red and Reyna Green | Blended Juice Red and Green (6:4) | Processing and storage juice parameters affect the antioxidant potential of juices, ABTS, DPPH and ICA values ranged from 110 to 341 µM Trolox/juice L, 1.93 to 2.27 µM Trolox/juice and 58 to 66% chelating activity | [186,187] |
| 22 | Tunisia | Not specified | Syrup | Concentrated syrup (74° Brix) exhibited high DPPH scavenging 3.63 µM Trolox/g f.w and 4.70 µM Trolox/g f.w for ABTS | [11] |
| 23 | Italy | Sanguino | Fruit puree | Fruit puree fermented with Leuconostoc mesenteroides showed a DPPH scavenging potential of 45–50%, considerably higher than raw juice, with 32.5%. | [152] |
| 24 | Mexico | Not specified | Methanol:water (1:2) | Peel flour extract showed 69% of ABTS scavenging corresponding to 2.9 µM Trolox | [188] |
| 25 | South Africa | Orange Ofer and Gymnocarpo, Red-pink Meyers and Sicilian Red- purple Nudosa | Aqueous | Evaluated cultivars showed average DPPH scavenging and iron chelating activity values of 70.78% and 93.86%, respectively | [189] |
| 26 | South Africa | Gymnocarpo, Meyers, Nepgen | Aqueous | Aqueous extracts of fresh and dried fruit, juice, preserves and chutney showed average values of 87,33% for iron chelating activity and 87.86% DPPH scavenge | [190] |
| 27 | Egypt | Not specified | Ethanolic and ethyl acetate | EC50 values of ethanolic pulp and peel extracts were 25.75 and 20.45 µg/mL, respectively, on the other hand, ethyl acetate extracts showed values of 39.77 and 35.77 µg/mL, respectively | [157] |
| 28 | Spain | Not specified | Methanol | Fruit extracts showed 6.70 µM Trolox/g f.w for DPPH assay and 5.22 µM Trolox/g f.w for ABTS assay | [12] |
| 29 | Spain | Yellow | Juice | Yellow juice showed 4.8 µM Trolox/g for DPPH assay and 5.9 µM Trolox/g for ABTS assay | [110] |
| 30 | Italy | Yellow 95% + Red 5% blend | Juice and aqueous extract | Fruit juice and aqueous extract showed EC50 values for DPPH scavenging of 6.75 µL/mL and 7.68 µL/mL, respectively | [81] |
| 31 | Morocco | Not specified | Juice | Fruit juice showed EC50 values for DPPH scavenging of 13.20 µL/mL | [191] |
| 32 | Mexico & Spain | Morada, Sanguino and Verdal | Methanol:water (1:1) | Peel extracts showed the highest ORAC activity, around 67.8 mM Trolox/L, Sanguinos, Verdal and Pelota whole fruit extracts presented high antioxidant activity of 42.2, 41.4, 45.0 mM Trolox/L | [192] |
| 33 | Mexico | Pelota and Sanguino | Methanol:water (1:1) | Samples treated with high hydrostatic pressure (HHP) showed ORAC values of 297.6 (peel) and 81.4 (pulp) for Pelota; 192.3 (peel) and 56.9 (pulp) µM Trolox/g d.w | [193] |
| 34 | Australia | Purple, Orange and White | Methanol:water (7:3) | Results indicated that there are differences in antioxidant activity not only among varieties, but sample preparation and type of tissue also have a great impact on total antioxidant capacity | [35] |
| 35 | Portugal | Orange, Green, Gialla and Rossa | Not specified | Peel and pulp extract average values were 3.34 and 2.93 µM Trolox/g f.w for ABTS, on the other hand, they showed 0.60 and 0.30 µM Trolox/g f.w for DPPG activity | [194] |
| 36 | Pakistan | Not specified | Methanol:water (1:1) | All fruit extracts showed mean EC50 values of 2.32 mg/mL, 2.68 mg/mL and 2.88 mg/mL for DPPH, reducing power activity and iron chelating activity | [195] |
| 37 | San Martin & Cristal | Mexican Cultivar | Juice | High hydrostatic pressure (HHP) increased antioxidant activity for ORAC of juices between 30–75% depending on the cultivar | [113] |
| 38 | Italy | Yellow-green and Red-Orange | Juice | Yellow-green Messina juices showed a maximum of 75.63% and red-orange Lakonia 69.90% DPPH scavenging activity | [196] |
| 39 | Tunisia | Not specified | Juice | DPPH scavenging activity of juice after 30 min of incubation was 10.3% | [165] |
| 40 | Italy | Yellow, Orange and Red | Aqueous | Red variety showed the highest antioxidant activity followed by orange and yellow varieties for DPPH, ABTS and FRAP assays | [40] |
| 41 | USA | Green | Not specified | Fruit extract showed 26.3 µM Trolox equivalent for ORAC, with 0.78 correlation coefficients between ORAC and flavonoid | [14] |
| 41 | Italy | Bastardoni and Agostani Red, Yellow and white | Methanol | Altitude has a great influence in antioxidant activity, Agostani varieties showed a maximum value of 17 µM Trolox/g at 480 mt, whereas Bastardoni varieties were not influenced by altitude, with mean values around 25 µM Trolox/g for ORAC assay | [150] |
| 43 | Italy | Gialla Orange-Red and Sanguigna | Ethanol:water (8:2) | Gialla and Sanguigna” skin extract showed EC50 values of 4.6 and 4.1 mg/mL DPPH scavenging, 2.65 and 2.08 mg/mL reducing power activity and 3.87 and 6.49 mg/mL β-carotene, respectively | [150] |
| 44 | Egypt | Red and Yellow- Orange | Methanol:water (7:3) | Peel extract showed average values almost two-fold higher than pulp extracts for ABTS 8.3 µM Trolox/100 mg), the same pattern was observed for the Fremy’s salt assay | [33] |
| 45 | Mexico | Huatusco, Copena, Jade, Solferino and Roja Villaneuva | Methanol | DPPH EC50 average values of peel extracts ranged from 20 to 160 µg d.w/mL and 30 and 100 µg d.w /mL | [197] |
| 46 | Portugal | White, Yellow, Orange & Red | Juice | Juice showed different EC50 values for DPPH, ranging from 0,65 g/L white to 1.12 g/L orange, antioxidant activity is influenced by the location where samples were collected | [198] |
| 47 | Iran | Not specified | Aqueous | Antioxidant activity of gum extract is dependent on concentration, showing a maximum activity of 80% at 400 µg/mL | [199] |
| 48 | India | Not specified | Methanol | Summer season extracts showed barely higher activities than winter season extracts for DPPH, ICA, NO, SOD, OH¯ scavenging, FRAP, H2O2 Scavenging and ABTS | [151] |
| 49 | Portugal | Not specified | Juice | Tramagal and Beja juice antioxidant activity was 33.4 and 27.8 mM Trolox for ORAC, 13.9 and 11.5 mM Trolox for HORAC, 14.9 and 11.4 mM Trolox for HOSC assay | [57] |
| 50 | South Korea | Not specified | Methanol:acetic acid (8:2) | Fruit extracts at maximum tested concentration 0.5 mg/mL showed 71.6% of DPPH, 63.6% of OH¯ and 78.8% alkyl radical scavenging activity | [168] |
| 50 | Italy | Orange, Red and Yellow | Methanol:water (9:1) | Pulp and peel extracts of orange cultivar showed approximately two-fold more activity for DPPH, ABTS, FRAP, ORAC and β-carotene than the yellow cultivar and 0.5-fold than the red cultivar | [104] |
| 52 | South Korea | Saboten | Ethanol:water (7:3) | Antioxidant activity of fractioned extracts measured by DPPH and ABTS were higher following the solvent sequence ethyl acetate > chloroform > n-butanol > water > n-hexane | [136] |
| 53 | USA | Red, purple, green and orange | Juice | Antioxidant activity of juice and pulp extracts of tested cultivars were: green 3.31 mM/L, 2.24 mM/kg ABTS and 5.45 mM/L, 3.68 mM/kg ORAC; orange 3.1 mM/L, 2.32 mM/kg ABTS and 5.83 mM/L, 4.36 mM/kg ORAC; red 3.71 mM/L, 2.60 mM/kg ABTS and 6.35 mM/L, 4.44 mM/kg ORAC; Purple 4.99 mM/L, 3.6 e mM/kg ABTS and 11.2 mM/L 8.16 mM/kg ORAC | [112] |
| 54 | South Korea | Not specified | Different solvent | After enzymatic hydrolysis, autoclaved and water extracts showed approximately 1-fold lower antioxidant activity for DPPH than ethanolic extracts. On the other hand, ethanolic extract was approximately 1.2-fold higher than autoclaved and water extract | [200] |
| 55 | Tunisia | Spiny (green-yellow and yellow) and Thornless (green and purple) | Methanol:acetic acid (9.0:0.1) | Peel and pulp extracts showed similar EC50 values for DPPH scavenging of 0.54 and 0.51 mg/mL and spiny and thornless of 0.57 and 0.56 mg/mL, respectively | [180] |
| 56 | Mexico | Purple | Juice | Antioxidant activity after sonication treatments at 60% and 80% amplitude levels for 15 min were similar to the control value of 26.3 mg VCEAC/100 mL juice, on the other hand, juice subjected to sonication treatments had an antioxidant activity similar to control 4.368 μM Trolox/L. The highest antioxidant activity was observed in juice treated at 80% amplitude level for 15 min 4.88 μM Trolox/L | [201] |
| 57 | Algeria | Not specified | Methanol:water (7:3) | Ec50 values of juice extracts were 3.52 mg/mL for DPPH, 0.80 mg/mL for ABTS, 8.04 for FRAP and 1043 μM Trolox/g d.w | [63] |
| 58 | Mexico | Pelon | Juice | Juice showed total antioxidant activity of 4.38 μM Trolox/g d.w and 4.91 μM FeSO4 Trolox/g d.w | [202] |
| 59 | Egypt | Not specified | Methanolic extract | DPPH IC50 values of peel and pulp extracts were 16.5 and 10.6 µg/mL | [45] |
| 60 | Portugal | Yellow | Ethanolic extract | Ohmic, high pressure extraction and combination of both techniques increased the antioxidant activity by 63%, 18% and 41%, respectively | [58] |
| 61 | Spain | Yellow | Methanol:water (8:2) | Hydrophilic total antioxidant activity increased during storage conditions. Meanwhile lipophilic total antioxidant activity remained constant during storage conditions | [40] |
| 62 | Saudi Arabia | Not specified | Different solvent | Solvent polarity and extraction procedure exert influence on the antioxidant activity. In a general way, samples with soxhlet extraction were more active than maceration ones. Additionally, for the DPPH assay, the acetone solvent was the most active, whereas the methanolic extract showed better activity for the reducing power and carotene assays | [59] |
| 63 | Tunisia | Yellow and red | Different solvent | Acetone fraction from yellow peel fruit with an EC50 of 784 μg/mL for the DPPH assay. Broadly. peel extracts were more active than pulp extract for the ABTS assay regardless of the solvent; all extracts showed ec50 values lower than 40 μg/mL. From all the tested samples, the yellow peel acetone extract was the most active with the highest reducing power of 1.23 mg/mL | [61] |
References
- De Leo, M.; De Abreu, M.B.; Pawlowska, A.; Cioni, P.; Braca, A. Profiling the chemical content of Opuntia ficus-indica flowers by HPLC–PDA-ESI-MS and GC/EIMS analyses. Phytochem. Lett. 2010, 3, 48–52. [Google Scholar] [CrossRef]
- Abascal, K.; Yarnell, E. A Review of Prickly Pear the Upscale Medicinal Food. Altern. Complement. Ther. 2000, 6, 265–271. [Google Scholar] [CrossRef]
- Kluge, M.; Ting, I.P. Crassulacean Acid Metabolism: Analysis of an Ecological Adaptation; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 30. [Google Scholar]
- Ayadi, M.; Abdelmaksoud, W.; Ennouri, M.; Attia, H. Cladodes from Opuntia ficus indica as a source of dietary fiber: Effect on dough characteristics and cake making. Ind. Crop. Prod. 2009, 30, 40–47. [Google Scholar] [CrossRef]
- Rocchetti, G.; Pellizzoni, M.; Montesano, D.; Lucini, L. Italian Opuntia ficus-indica Cladodes as Rich Source of Bioactive Compounds with Health-Promoting Properties. Foods 2018, 7, 24. [Google Scholar] [CrossRef]
- Salem, N.; Lamine, M.; Damergi, B.; Ezahra, F.; Feres, N.; Jallouli, S.; Hammami, M.; Khammassi, S.; Selmi, S.; Elkahoui, S.; et al. Natural colourants analysis and biological activities. Association to molecular markers to explore the biodiversity of Opuntia species. Phytochem. Anal. 2020, 31, 892–904. [Google Scholar] [CrossRef]
- Cejudo-Bastante, M.J.; Chaalal, M.; Louaileche, H.; Parrado, J.; Heredia, F.J. Betalain Profile, Phenolic Content, and Color Characterization of Different Parts and Varieties of Opuntia ficus-indica. J. Agric. Food Chem. 2014, 62, 8491–8499. [Google Scholar] [CrossRef]
- Khatabi, O.; Hanine, H.; Elothmani, D.; Hasib, A. Extraction and determination of polyphenols and betalain pigments in the Moroccan Prickly pear fruits (Opuntia ficus indica). Arab. J. Chem. 2016, 9, S278–S281. [Google Scholar] [CrossRef]
- Cano, M.; Gómez-Maqueo, A.; García-Cayuela, T.; Welti-Chanes, J. Characterization of carotenoid profile of Spanish Sanguinos and Verdal prickly pear (Opuntia ficus-indica, spp.) tissues. Food Chem. 2017, 237, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hameed, E.-S.S.; Nagaty, M.A.; Salman, M.S.; Bazaid, S.A. Phytochemicals, nutritionals and antioxidant properties of two prickly pear cactus cultivars (Opuntia ficus indica Mill.) growing in Taif, KSA. Food Chem. 2014, 160, 31–38. [Google Scholar] [CrossRef]
- Dhaouadi, K.; Raboudi, F.; Funez-Gomez, L.; Pamies, D.; Estevan, C.; Hamdaoui, M.; Fattouch, S. Polyphenolic Extract of Barbary-Fig (Opuntia ficus-indica) Syrup: RP–HPLC–ESI–MS Analysis and Determination of Antioxidant, Antimicrobial and Cancer-Cells Cytotoxic Potentials. Food Anal. Methods 2013, 6, 45–53. [Google Scholar] [CrossRef]
- Fernández-López, J.A.; Almela, L.; Obón, J.M.; Castellar, R. Determination of Antioxidant Constituents in Cactus Pear Fruits. Plant Foods Hum. Nutr. 2010, 65, 253–259. [Google Scholar] [CrossRef]
- Chavez-Santoscoy, R.A.; Gutierrez-Uribe, J.A.; Serna-Saldívar, S.O. Phenolic Composition, Antioxidant Capacity and In Vitro Cancer Cell Cytotoxicity of Nine Prickly Pear (Opuntia spp.) Juices. Plant Foods Hum. Nutr. 2009, 64, 146–152. [Google Scholar] [CrossRef]
- Kuti, J.O. Antioxidant compounds from four Opuntia cactus pear fruit varieties. Food Chem. 2004, 85, 527–533. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Fernández-López, J.A.; Castellar, R.; Obón, J.M.; Almela, L. Screening and mass-spectral confirmation of betalains in cactus pears. Chromatographia 2002, 56, 591–595. [Google Scholar] [CrossRef]
- Bensadón, S.; Hervert-Hernández, D.; Sáyago-Ayerdi, S.G.; Goñi, I. By-Products of Opuntia ficus-indica as a Source of Antioxidant Dietary Fiber. Plant Foods Hum. Nutr. 2010, 65, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gutiérrez, M.G.; Amaya-Guerra, C.A.; Quintero-Ramos, A.; de Jesús Ruiz-Anchondo, T.; Gutiérrez-Uribe, J.A.; Baez-González, J.G.; Lardizabal-Gutiérrez, D.; Campos-Venegas, K. Effect of soluble fiber on the physicochemical properties of cactus pear (Opuntia ficus indica) encapsulated using spray drying. Food Sci. Biotechnol. 2014, 23, 755–763. [Google Scholar] [CrossRef]
- Zenteno-Ramirez, G.; Juárez-Flores, B.I.; Aguirre-Rivera, J.R.; Ortiz-Pérez, M.D.; Zamora-Pedraza, C.; Rendón-Huerta, J.A. Evaluation of Sugars and Soluble Fiber in the Juice of Prickly Pear Varieties (Opuntia Spp.). Agrociencia 2015, 49, 141–152. [Google Scholar]
- Cano, M.P.; Gómez-Maqueo, A.; Fernández-López, R.; Welti-Chanes, J.; García-Cayuela, T. Impact of high hydrostatic pressure and thermal treatment on the stability and bioaccessibility of carotenoid and carotenoid esters in astringent persimmon (Diospyros kaki Thunb, var. Rojo Brillante). Food Res. Int. 2019, 123, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.F.; Mörsel, J.-T. Recovered lipids from prickly pear [Opuntia ficus-indica (L.) Mill] peel: A good source of polyunsaturated fatty acids, natural antioxidant vitamins and sterols. Food Chem. 2003, 83, 447–456. [Google Scholar] [CrossRef]
- Aregahegn, A.; Chandravanshi, B.; Atlabachew, M.; Ababa, A. Mineral contents of fruits of cactus pear (Opuntia ficus indica) grown in ethiopia. Acta Hortic. 2013, 979, 117–126. [Google Scholar] [CrossRef]
- Ali, H.S.M.; Al-Khalifa, A.S.; Brückner, H. Taurine is absent from amino components in fruits of Opuntia ficus-indica. Springerplus 2014, 3, 663. [Google Scholar] [CrossRef] [PubMed]
- Kugler, F.; Graneis, S.; Schreiter, P.P.-Y.; Stintzing, F.C.; Carle, R. Determination of Free Amino Compounds in Betalainic Fruits and Vegetables by Gas Chromatography with Flame Ionization and Mass Spectrometric Detection. J. Agric. Food Chem. 2006, 54, 4311–4318. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Morreale, V.; Pecoraino, M.; Pagliaro, M. Solar air drying for innovative Opuntia ficus-indica cladode dehydration. 4open 2019, 2, 1. [Google Scholar] [CrossRef]
- Valero-Galván, J.; González-Fernández, R.; Sigala-Hernández, A.; Núñez-Gastélum, J.A.; Ruiz-May, E.; Rodrigo-García, J.; Larqué-Saavedra, A.; del Rocio Martínez-Ruiz, N. Sensory attributes, physicochemical and antioxidant characteristics, and protein profile of wild prickly pear fruits (O. macrocentra Engelm., O. phaeacantha Engelm., and O. engelmannii Salm-Dyck ex Engelmann.) and commercial prickly pear fruits (O. ficus-indica (L.) Mill.). Food Res. Int. 2021, 140, 109909. [Google Scholar] [CrossRef]
- Cruz-Bravo, R.K.; Guzmán-Maldonado, S.H.; Araiza-Herrera, H.A.; Zegbe, J.A. Storage alters physicochemical characteristics, bioactive compounds and antioxidant capacity of cactus pear fruit. Postharvest Biol. Technol. 2019, 150, 105–111. [Google Scholar] [CrossRef]
- Alimi, H.; Hfaeidh, N.; Bouoni, Z.; Sakly, M.; Ben Rhouma, K. Protective effect of Opuntia ficus indica f. inermis prickly pear juice upon ethanol-induced damages in rat erythrocytes. Alcohol 2012, 46, 235–243. [Google Scholar] [CrossRef]
- Cho, D.-W.; Kim, D.-E.; Lee, D.-H.; Jung, K.-H.; Hurh, B.-S.; Kwon, O.W.; Kim, S.Y. Metabolite profiling of enzymatically hydrolyzed and fermented forms of Opuntia ficus-indica and their effect on UVB-induced skin photoaging. Arch. Pharmacal Res. 2014, 37, 1159–1168. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Wójtowicz, A.; Oniszczuk, T.; Matwijczuk, A.; Dib, A.; Markut-Miotła, E. Opuntia Fruits as Food Enriching Ingredient, the First Step towards New Functional Food Products. Molecules 2020, 25, 916. [Google Scholar] [CrossRef]
- Haida, Z.; Hakiman, M. A comprehensive review on the determination of enzymatic assay and nonenzymatic antioxidant activities. Food Sci. Nutr. 2019, 7, 1555–1563. [Google Scholar] [CrossRef]
- Albano, C.; Negro, C.; Tommasi, N.; Gerardi, C.; Mita, G.; Miceli, A.; De Bellis, L.; Blando, F. Betalains, Phenols and Antioxidant Capacity in Cactus Pear [Opuntia ficus-indica (L.) Mill.] Fruits from Apulia (South Italy) Genotypes. Antioxidants 2015, 4, 269–280. [Google Scholar] [CrossRef]
- Moussa-Ayoub, T.E.; El-Hady, E.-S.A.A.; Omran, H.T.; El-Samahy, S.K.; Kroh, L.W.; Rohn, S. Influence of cultivar and origin on the flavonol profile of fruits and cladodes from cactus Opuntia ficus-indica. Food Res. Int. 2014, 64, 864–872. [Google Scholar] [CrossRef]
- Andreu, L.; Nuncio-Jáuregui, N.; Carbonell-Barrachina, Á.A.; Legua, P.; Hernández, F. Antioxidant properties and chemical characterization of Spanish Opuntia ficus-indica Mill. cladodes and fruits. J. Sci. Food Agric. 2018, 98, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- Gouws, C.A.; D’Cunha, N.M.; Georgousopoulou, E.N.; Mellor, D.D.; Naumovski, N. The effect of different drying techniques on phytochemical content and in vitro antioxidant properties of Australian-grown prickly pears (Opuntia ficus indica). J. Food Process. Preserv. 2019, 43, e13900. [Google Scholar] [CrossRef]
- Karunanithi, A.; Venkatachalam, S. Ultrasonic-assisted solvent extraction of phenolic compounds from Opuntia ficus-indica peel: Phytochemical identification and comparison with soxhlet extraction. J. Food Process. Eng. 2019, 42, e13126. [Google Scholar] [CrossRef]
- Surano, B.; Leiva, G.; Marshall, G.; Maglietti, F.; Schebor, C. Pulsed electric fields using a multiple needle chamber to improve bioactive compounds extraction from unprocessed Opuntia ficus-indica fruits. J. Food Eng. 2022, 317, 110864. [Google Scholar] [CrossRef]
- Belviranlı, B.; Al-Juhaimi, F.; Özcan, M.M.; Ghafoor, K.; Babiker, E.E.; Alsawmahi, O.N. Effect of location on some physico-chemical properties of prickly pear (Opuntia ficus-indica L.) fruit and seeds. J. Food Process. Preserv. 2019, 43, e13896. [Google Scholar] [CrossRef]
- Herrera, M.D.; Zegbe, J.A.; Melero-Meraz, V.; Cruz-Bravo, R.K. Functional Properties of Prickly Pear Cactus Fruit Peels Undergoing Supplemental Irrigation and Fruit Storage Conditions. Plant Foods Hum. Nutr. 2021, 76, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Coll, L.; García-Pastor, M.; Valero, D.; Amorós, A.; Almansa, M.S.; Legua, P.; Hernández, F. Influence of Storage on Physiological Properties, Chemical Composition, and Bioactive Compounds on Cactus Pear Fruit (Opuntia ficus-indica (L.) Mill.). Agriculture 2021, 11, 62. [Google Scholar] [CrossRef]
- Gómez-Maqueo, A.; Antunes-Ricardo, M.; Welti-Chanes, J.; Cano, M.P. Digestive Stability and Bioaccessibility of Antioxidants in Prickly Pear Fruits from the Canary Islands: Healthy Foods and Ingredients. Antioxidants 2020, 9, 164. [Google Scholar] [CrossRef]
- Mazari, A.; Yahiaoui, K.; Fedjer, Z.; Mahdeb, A. Physical characteristics, phytochemical content and antioxidant activity of cactus pear fruits growing in northeast Algeria. J. Prof. Assoc. cactus Dev. 2018, 20, 178–188. [Google Scholar] [CrossRef]
- Chougui, N.; Djerroud, N.; Naraoui, F.; Hadjal, S.; Aliane, K.; Zeroual, B.; Larbat, R. Physicochemical properties and storage stability of margarine containing Opuntia ficus-indica peel extract as antioxidant. Food Chem. 2015, 173, 382–390. [Google Scholar] [CrossRef]
- Benattia, F.K.; Arrar, Z. Antioxidative and Antiradical Activities of Bioactive Compounds of Extracts from Algerian Prickly Pear (Opuntia ficus-indica. L.) Fruits. Curr. Nutr. Food Sci. 2018, 14, 211–217. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Sobeh, M.; Badr, W.K.; Abdelfattah, M.A.; Ali, Z.Y.; El-Tantawy, M.E.; Rabeh, M.A.; Wink, M. HPLC-PDA-MS/MS profiling of secondary metabolites from Opuntia ficus-indica cladode, peel and fruit pulp extracts and their antioxidant, neuroprotective effect in rats with aluminum chloride induced neurotoxicity. Saudi J. Biol. Sci. 2020, 27, 2829–2838. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Razek, A.G.; Shehata, M.G.; Badr, A.; Gromadzka, K.; Stępień, L. The Effect of Chemical Composition of Wild Opuntia ficus indica Byproducts on its Nutritional Quality, Antioxidant and Antifungal Efficacy. Egypt. J. Chem. 2019, 62, 47–61. [Google Scholar] [CrossRef]
- Karabagias, V.K.; Karabagias, I.K.; Prodromiti, M.; Gatzias, I.; Badeka, A. Bio-functional alcoholic beverage preparation using prickly pear juice and its pulp in combination with sugar and blossom honey. Food Biosci. 2020, 35, 100591. [Google Scholar] [CrossRef]
- Palmeri, R.; Parafati, L.; Arena, E.; Grassenio, E.; Restuccia, C.; Fallico, B. Antioxidant and Antimicrobial Properties of Semi-Processed Frozen Prickly Pear Juice as Affected by Cultivar and Harvest Time. Foods 2020, 9, 235. [Google Scholar] [CrossRef]
- Bouzoubaâ, Z.; Essoukrati, Y.; Tahrouch, S.; Hatimi, A.; Gharby, S.; Harhar, H. Phytochemical study of prickly pear from southern Morocco. J. Saudi Soc. Agric. Sci. 2016, 15, 155–161. [Google Scholar] [CrossRef]
- Ettalibi, F.; Elmahdaoui, H.; Amzil, J.; Gadhi, C.; Harrak, H. Drying impact on physicochemical and biochemical criteria of prickly pear fruit peels of three varieties of Opuntia spp. Mater. Today: Proc. 2020, 27, 3243–3248. [Google Scholar] [CrossRef]
- Bourhia, M.; Elmahdaoui, H.; Ullah, R.; Ibenmoussa, S.; Shahat, A. Physicochemical evaluation of the fruit pulp of Opuntia spp. growing in the Mediterranean area under hard climate conditions. Open Chem. 2020, 18, 565–575. [Google Scholar] [CrossRef]
- Monter-Arciniega, A.; Hernández-Falcón, T.A.; Cruz-Cansino, N.D.S.; Ramírez-Moreno, E.; Alanís-García, E.; Arias-Rico, J.; Ariza-Ortega, J.A. Functional Properties, Total Phenolic Content and Antioxidant Activity of Purple Cactus Pear (Opuntia ficus-indica) Waste: Comparison with Commercial Fibers. Waste Biomass Valorization 2019, 10, 2897–2906. [Google Scholar] [CrossRef]
- Gonzalez, F.P.H.; Saucedo, V.C.; Guerra, R.D.; Suarez, E.J.; Soto, H.R.M.; Lopez, J.A.; Garcia, C.E.; Hernández, R.G. Post-harvest quality and quantification of betalains, phenolic compounds and antioxidant activity in fruits of three cultivars of prickly pear (Opuntia ficus-indica L. Mill). J. Hortic. Sci. 2021, 16, 91–102. [Google Scholar] [CrossRef]
- Martínez, E.; Sandate-Flores, L.; Rodríguez-Rodríguez, J.; Rostro-Alanis, M.; Parra-Arroyo, L.; Antunes-Ricardo, M.; Serna-Saldívar, S.; Iqbal, H.; Parra-Saldívar, R. Underutilized Mexican Plants: Screening of Antioxidant and Antiproliferative Properties of Mexican Cactus Fruit Juices. Plants 2021, 10, 368. [Google Scholar] [CrossRef]
- Ordoñez, E.; Leon-Arevalo, A.; Rivera-Rojas, H.; Vargas, E. Cuantificación de Polifenoles Totales y Capacidad Antioxidante En Cáscara y Semilla de Cacao (Theobroma cacao L.), Tuna (Opuntia ficus indica Mill), Uva (Vitis vinífera) y Uvilla (Pourouma cecropiifolia). Sci. Agropecu. 2019, 10, 175–183. [Google Scholar] [CrossRef]
- Morales, N.X.C.; Gómez, K.Y.V.; Schweiggert, R.M.; Delgado, G.T.C. Stabilisation of betalains and phenolic compounds extracted from red cactus pear (Opuntia ficus-indica) by spray and freeze-drying using oca (Oxalis tuberosa) starch as drying aid. Food Sci. Technol. Int. 2021, 27, 456–469. [Google Scholar] [CrossRef]
- Serra, A.T.; Poejo, J.; Matias, A.A.; Bronze, M.R.; Duarte, C.M. Evaluation of Opuntia spp. derived products as antiproliferative agents in human colon cancer cell line (HT29). Food Res. Int. 2013, 54, 892–901. [Google Scholar] [CrossRef]
- Alexandre, E.M.C.; Coelho, M.C.; Ozcan, K.; Pinto, C.A.; Teixeira, J.A.; Saraiva, J.A.; Pintado, M. Emergent Technologies for the Extraction of Antioxidants from Prickly Pear Peel and Their Antimicrobial Activity. Foods 2021, 10, 570. [Google Scholar] [CrossRef]
- Atiya, A.; Majrashi, T.; Abdul Qadir, S.F.; Alqahtani, A.S.; Ali Alosman, A.S.; Alahmari, A.A.; Mesfer Al Aldabsh, A.N.; Alshahrani, A.T.; Alshahrani, R.R.M. Influence of solvent selection and extraction methods on the determination of polyphenols, antioxidant, lipoxygenase and tyrosinase inhibition activities of Opuntia ficus-indica fruits peel and pulp collected from the Kingdom of Saudi Arabia (KSA). Nat. Prod. Res. 2021, 1–8. [Google Scholar] [CrossRef]
- Bouazizi, S.; Montevecchi, G.; Antonelli, A.; Hamdi, M. Effects of prickly pear (Opuntia ficus-indica L.) peel flour as an innovative ingredient in biscuits formulation. Lwt 2020, 124, 109155. [Google Scholar] [CrossRef]
- El Mannoubi, I. Effect of extraction solvent on phenolic composition, antioxidant and antibacterial activities of skin and pulp of Tunisian red and yellow–orange Opuntia ficus indica fruits. J. Food Meas. Charact. 2021, 15, 643–651. [Google Scholar] [CrossRef]
- Albergamo, A.; Potortí, A.G.; Di Bella, G.; Ben Amor, N.; Vecchio, G.L.; Nava, V.; Rando, R.; Ben Mansour, H.; Turco, V.L. Chemical Characterization of Different Products from the Tunisian Opuntia ficus-indica (L.) Mill. Foods 2022, 11, 155. [Google Scholar] [CrossRef]
- Zeghad, N.; Ahmed, E.; Belkhiri, A.; Heyden, Y.V.; Demeyer, K.; Zeghad, N.; Ahmed, E.; Belkhiri, A.; Heyden, Y.V.; Demeyer, K. Antioxidant activity of Vitis vinifera, Punica granatum, Citrus aurantium and Opuntia ficus indica fruits cultivated in Algeria. Heliyon 2019, 5, e01575. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Maqueo, A.; Ortega-Hernández, É.; Serrano-Sandoval, S.N.; Jacobo-Velázquez, D.A.; García-Cayuela, T.; Cano, M.P.; Welti-Chanes, J. Addressing key features involved in bioactive extractability of vigor prickly pears submitted to high hydrostatic pressurization. J. Food Process. Eng. 2020, 43, e13202. [Google Scholar] [CrossRef]
- Manzur-Valdespino, S.; Ramírez-Moreno, E.; Arias-Rico, J.; Jaramillo-Morales, O.A.; Calderón-Ramos, Z.G.; Delgado-Olivares, L.; Córdoba-Díaz, M.; Córdoba-Díaz, D.; Cruz-Cansino, N.D.S. Opuntia ficus-indica L. Mill Residues—Properties and Application Possibilities in Food Supplements. Appl. Sci. 2020, 10, 3260. [Google Scholar] [CrossRef]
- Bañuelos, G.S.; Stushnoff, C.; Walse, S.S.; Zuber, T.; Yang, S.I.; Pickering, I.; Freeman, J.L. Biofortified, selenium enriched, fruit and cladode from three Opuntia Cactus pear cultivars grown on agricultural drainage sediment for use in nutraceutical foods. Food Chem. 2012, 135, 9–16. [Google Scholar] [CrossRef]
- Ortega-Hernández, E.; Welti-Chanes, J.; Jacobo-Velázquez, D.A. Effects of UVB Light, Wounding Stress, and Storage Time on the Accumulation of Betalains, Phenolic Compounds, and Ascorbic Acid in Red Prickly Pear (Opuntia ficus-indica cv. Rojo Vigor). Food Bioprocess Technol. 2018, 11, 2265–2274. [Google Scholar] [CrossRef]
- Sauer, M.; Porro, D.; Mattanovich, D.; Branduardi, P. Microbial production of organic acids: Expanding the markets. Trends Biotechnol. 2008, 26, 100–108. [Google Scholar] [CrossRef]
- Tsao, G.T.; Cao, N.J.; Du, J.; Gong, C.S. Production of Multifunctional Organic Acids from Renewable Resources. In Recent Progress in Bioconversion of Lignocellulosics; Springer: Berlin/Heidelberg, Germany, 1999; Volume 65, pp. 243–280. [Google Scholar] [CrossRef]
- Goldberg, I.; Rokem, J.S.; Pines, O. Organic acids: Old metabolites, new themes. J. Chem. Technol. Biotechnol. Int. Res. Process. Environ. Clean Technol. 2006, 81, 1601–1611. [Google Scholar] [CrossRef]
- López-Bucio, J.; Nieto-Jacobo, M.F.; Ramírez-Rodríguez, V.; Herrera-Estrella, L. Organic acid metabolism in plants: From adaptive physiology to transgenic varieties for cultivation in extreme soils. Plant Sci. 2000, 160, 1–13. [Google Scholar] [CrossRef]
- Johnston, C.S.; Steinberg, F.M.; Rucker, R.B. Ascorbic Acid. In Handbook of Vitamins; CRC Press: Boca Raton, FL, USA, 2007; Volume 4, pp. 489–520. [Google Scholar]
- Marques, C.; Sotiles, A.R.; Farias, F.O.; Oliveira, G.; Mitterer-Daltoé, M.L.; Masson, M.L. Full physicochemical characterization of malic acid: Emphasis in the potential as food ingredient and application in pectin gels. Arab. J. Chem. 2020, 13, 9118–9129. [Google Scholar] [CrossRef]
- Hossain, F.; Akhtar, S.; Anwar, M. Nutritional Value and Medicinal Benefits of Pineapple. Int. J. Nutr. Food Sci. 2015, 4, 84–88. [Google Scholar] [CrossRef]
- Mata, A.; Ferreira, J.; Semedo, C.; Serra, T.; Duarte, C.; Bronze, M. Contribution to the characterization of Opuntia spp. juices by LC–DAD–ESI-MS/MS. Food Chem. 2016, 210, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Maamoun, A.A.; Ehrlich, A.; Fahmy, S.; Wesjohann, L.A. Assessment of sensory metabolites distribution in 3 cactus Opuntia ficus-indica fruit cultivars using UV fingerprinting and GC/MS profiling techniques. LWT 2017, 80, 145–154. [Google Scholar] [CrossRef]
- Kaur, M.; Kaur, A.; Sharma, R. Pharmacological actions of Opuntia ficus indica: A Review. J. Appl. Pharm. Sci. 2012, 2, 15–18. [Google Scholar] [CrossRef]
- Kristbergsson, K.; Otles, S. Functional Properties of Traditional Foods; Springer: Cham, Switzerland, 2016; Volume 12, ISBN 1489976620. [Google Scholar]
- Lamia, I.; Zouhir, C.; Youcef, A. Characterization and transformation of the Opuntia ficus indica fruits. J. Food Meas. Charact. 2018, 12, 2349–2357. [Google Scholar] [CrossRef]
- Butera, D.; Tesoriere, L.; Di Gaudio, F.; Bongiorno, A.; Allegra, M.; Pintaudi, A.M.; Kohen, R.; Livrea, M.A. Antioxidant Activities of Sicilian Prickly Pear (Opuntia ficus indica) Fruit Extracts and Reducing Properties of Its Betalains: Betanin and Indicaxanthin. J. Agric. Food Chem. 2002, 50, 6895–6901. [Google Scholar] [CrossRef]
- Galati, E.M.; Mondello, M.R.; Giuffrida, D.; Dugo, G.; Miceli, N.; Pergolizzi, S.; Taviano, M.F. Chemical Characterization and Biological Effects of Sicilian Opuntia ficus indica (L.) Mill. Fruit Juice: Antioxidant and Antiulcerogenic Activity. J. Agric. Food Chem. 2003, 51, 4903–4908. [Google Scholar] [CrossRef]
- El Kharrassi, Y.; Mazri, M.A.; Benyahia, H.; Benaouda, H.; Nasser, B.; El Mzouri, E.H. Fruit and juice characteristics of 30 accessions of two cactus pear species (Opuntia ficus indica and Opuntia megacantha) from different regions of Morocco. LWT Food Sci. Technol. 2016, 65, 610–617. [Google Scholar] [CrossRef]
- Bourhia, M.; Elmahdaoui, H.; Ullah, R.; Bari, A.; Benbacer, L. Promising Physical, Physicochemical, and Biochemical Background Contained in Peels of Prickly Pear Fruit Growing under Hard Ecological Conditions in the Mediterranean Countries. BioMed Res. Int. 2019, 2019, 9873146. [Google Scholar] [CrossRef] [PubMed]
- Bourhia, M.; Elmahdaoui, H.; Moussa, S.I.; Ullah, R.; Bari, A. Potential Natural Dyes Food from the Powder of Prickly Pear Fruit Peels (Opuntia spp.) Growing in the Mediterranean Basin under Climate Stress. BioMed Res. Int. 2020, 2020, 7579430. [Google Scholar] [CrossRef]
- Gómez-Maqueo, A.; Soccio, M.; Cano, M.P. In Vitro Antioxidant Capacity of Opuntia spp. Fruits Measured by the LOX-FL Method and its High Sensitivity Towards Betalains. Plant Foods Hum. Nutr. 2021, 76, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Amaya, D.B. Carotenes and xanthophylls as antioxidants. In Handbook of Antioxidants for Food Preservation; Elsevier: Amsterdam, The Netherlands, 2015; pp. 17–50. [Google Scholar] [CrossRef]
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free Fatty Acid Receptors in Health and Disease. Physiol. Rev. 2020, 100, 171–210. [Google Scholar] [CrossRef] [PubMed]
- Benatti, P.; Peluso, G.; Nicolai, R.; Calvani, M. Polyunsaturated Fatty Acids: Biochemical, Nutritional and Epigenetic Properties. J. Am. Coll. Nutr. 2004, 23, 281–302. [Google Scholar] [CrossRef]
- Tesoriere, L.; Fazzari, M.; Allegra, M.; Livrea, M.A. Biothiols, Taurine, and Lipid-Soluble Antioxidants in the Edible Pulp of Sicilian Cactus Pear (Opuntia ficus-indica) Fruits and Changes of Bioactive Juice Components upon Industrial Processing. J. Agric. Food Chem. 2005, 53, 7851–7855. [Google Scholar] [CrossRef]
- Hernández-Pérez, T.; Carrillo-López, A.; Guevara-Lara, F.; Cruz-Hernández, A.; Paredes-López, O. Biochemical and Nutritional Characterization of Three Prickly Pear Species with Different Ripening Behavior. Plant Foods Hum. Nutr. 2005, 60, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Bakar, B.; Çakmak, M.; Ibrahim, M.S.; Özer, D.; Saydam, S.; Karatas, F. Investigation of Amounts of Vitamins, Lycopene, and Elements in the Fruits of Opuntia ficus-indica Subjected to Different Pretreatments. Biol. Trace Element Res. 2020, 198, 315–323. [Google Scholar] [CrossRef]
- Andreu-Coll, L.; Cano-Lamadrid, M.; Sendra, E.; Carbonell-Barrachina, A.; Legua, P.; Hernández, F. Fatty acid profile of fruits (pulp and peel) and cladodes (young and old) of prickly pear [Opuntia ficus-indica (L.) Mill.] from six Spanish cultivars. J. Food Compos. Anal. 2019, 84, 103294. [Google Scholar] [CrossRef]
- Elsawi, S.A.; Radwan, R.R.; Elbatanony, M.M.; El-Feky, A.M.; Sherif, N.H. Prophylactic Effect of Opuntia ficus indica Fruit Peel Extract against Irradiation-Induced Colon Injury in Rats. Planta Medica 2020, 86, 61–69. [Google Scholar] [CrossRef]
- Karabagias, V.K.; Karabagias, I.K.; Louppis, A.; Badeka, A.; Kontominas, M.G.; Papastephanou, C. Valorization of Prickly Pear Juice Geographical Origin Based on Mineral and Volatile Compound Contents Using LDA. Foods 2019, 8, 123. [Google Scholar] [CrossRef]
- Agozzino, P.; Avellone, G.; Ceraulo, L.; Ferrugia, M.; Filizzola, F. Volatile Profiles of Sicilian Prickly Pear (Opuntia ficus-indica) by SPME-GC/MS Analysis. Ital. J. food Sci. 2005, 17, 341–348. [Google Scholar]
- Andreu-Coll, L.; Noguera-Artiaga, L.; Carbonell-Barrachina, Á.A.; Legua, P.; Hernández, F. Volatile composition of prickly pear fruit pulp from six Spanish cultivars. J. Food Sci. 2020, 85, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Arena, E.; Campisi, S.; Fallico, B.; Lanza, M.C.; Maccarone, E. Aroma Value of Volatile Compounds of Prickly Pear (Opuntia ficus indica (L.) Mill., Cactaceae). Ital. J. Food Sci. 2001, 13, 311–319. [Google Scholar]
- Karadağ, A.E.; Demirci, B.; Polat, D.Ç.; Okur, M.E. Characterization of Opuntia ficus-indica (L.) Mill. Fruit Volatiles and Antibacterial Evaluation. Nat. Volatiles Essent. Oils 2018, 5, 35–38. [Google Scholar]
- Stintzing, F.C.; Schieber, A.; Carle, R. Evaluation of colour properties and chemical quality parameters of cactus juices. Eur. Food Res. Technol. 2003, 216, 303–311. [Google Scholar] [CrossRef]
- Gengatharan, A.; Dykes, G.A.; Choo, W.S. Betalains: Natural plant pigments with potential application in functional foods. LWT Food Sci. Technol. 2015, 64, 645–649. [Google Scholar] [CrossRef]
- Mondragon-Jacobo, C.; Yahia, E.M.; Castellanos, E. Identification and Quantification of Pigments in Prickly Pear Fruit. In VI International Postharvest Symposium 877; International Society for Horticultural Science: Leuven, Belgium, 2009; pp. 1129–1136. [Google Scholar] [CrossRef]
- Dantas, R.L.; Silva, S.M.; Santos, L.F.; Dantas, A.L.; Lima, R.P.; Soares, L.G. Betalains and Antioxidant Activity in Fruits of Cactaceae from Brazilian Semiarid. In VIII International Congress on Cactus Pear and Cochineal 1067; International Society for Horticultural Science: Leuven, Belgium, 2013; pp. 151–157. [Google Scholar] [CrossRef]
- Cova, A.M.; Crascì, L.; Panico, A.; Catalfo, A.; De Guidi, G. Antioxidant capability and phytochemicals content of Sicilian prickly fruits. Int. J. Food Sci. Nutr. 2015, 66, 881–886. [Google Scholar] [CrossRef]
- Smeriglio, A.; Bonasera, S.; Germanò, M.P.; D’Angelo, V.; Barreca, D.; Denaro, M.; Monforte, M.T.; Galati, E.M.; Trombetta, D. Opuntia ficus-indica (L.) Mill. fruit as source of betalains with antioxidant, cytoprotective, and anti-angiogenic properties. Phytother. Res. 2019, 33, 1526–1537. [Google Scholar] [CrossRef]
- Gómez-Maqueo, A.; Steurer, D.; Welti-Chanes, J.; Cano, M.P. Bioaccessibility of Antioxidants in Prickly Pear Fruits Treated with High Hydrostatic Pressure: An Application for Healthier Foods. Molecules 2021, 26, 5252. [Google Scholar] [CrossRef]
- Maran, J.P.; Manikandan, S. Response surface modeling and optimization of process parameters for aqueous extraction of pigments from prickly pear (Opuntia ficus-indica) fruit. Dye. Pigment. 2012, 95, 465–472. [Google Scholar] [CrossRef]
- Mejia, J.; Yáñez-Fernandez, J. Clarification Processes of Orange Prickly Pear Juice (Opuntia spp.) by Microfiltration. Membranes 2021, 11, 354. [Google Scholar] [CrossRef]
- Moßhammer, M.R.; Rohe, M.; Stintzing, F.C.; Carle, R. Stability of yellow-orange cactus pear (Opuntia ficus-indica [L.] Mill. cv. ‘Gialla’) betalains as affected by the juice matrix and selected food additives. Eur. Food Res. Technol. 2007, 225, 21–32. [Google Scholar] [CrossRef]
- El Gharras, H.; Hasib, A.; Jaouad, A.; Schoefs, B.; El-Bouadili, A. Stability of vacuolar betaxanthin pigments in juices from Moroccan yellow Opuntia ficus indica fruits. Int. J. Food Sci. Technol. 2008, 43, 351–356. [Google Scholar] [CrossRef]
- Fernández-López, J.A.; Roca, M.J.; Angosto, J.M.; Obón, J.M. Betaxanthin-Rich Extract from Cactus Pear Fruits as Yellow Water-Soluble Colorant with Potential Application in Foods. Plant Foods Hum. Nutr. 2018, 73, 146–153. [Google Scholar] [CrossRef]
- Moßhammer, M.R.; Stintzing, F.C.; Carle, R. Cactus Pear Fruits (Opuntia Spp.): A Review of Processing Technologies and Current Uses. J. Prof. Assoc. Cactus Dev. 2006, 8, 1–25. [Google Scholar]
- Stintzing, F.C.; Herbach, K.M.; Mosshammer, M.R.; Carle, R.; Yi, W.; Sellappan, S.; Akoh, C.C.; Bunch, R.; Felker, P. Color, Betalain Pattern, and Antioxidant Properties of Cactus Pear (Opuntia spp.) Clones. J. Agric. Food Chem. 2005, 53, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Aguilar, D.M.; Avellaneda, Z.J.E.; Martín-Belloso, O.; Gutiérrez-Uribe, J.; Valdez-Fragoso, A.; García, R.M.; Torres, J.A.; Welti-Chanes, J. Effect of High Hydrostatic Pressure on the Content of Phytochemical Compounds and Antioxidant Activity of Prickly Pears (Opuntia ficus-indica) Beverages. Food Eng. Rev. 2015, 7, 198–208. [Google Scholar] [CrossRef]
- Santos, R.P.; Souza, L.M.; Balieiro, A.L.; Soares, C.M.F.; Lima, Á.S.; Souza, R.L. Integrated process of extraction and purification of betanin from Opuntia ficus-indica using aqueous two-phase systems based on THF and sodium salts. Sep. Sci. Technol. 2018, 53, 734–744. [Google Scholar] [CrossRef]
- Amic, D.; Davidovic-Amic, D.; Beslo, D.; Rastija, V.; Lucic, B.; Trinajstic, N. SAR and QSAR of the Antioxidant Activity of Flavonoids. Curr. Med. Chem. 2007, 14, 827–845. [Google Scholar] [CrossRef]
- Abou-Elella, F.M.; Ali, R.F.M. Antioxidant and Anticancer Activities of Different Constituents Extracted from Egyptian Prickly Pear Cactus (Opuntia ficus-indica) Peel. Biochem. Anal. Biochem. 2014, 3, 1009–2161. [Google Scholar] [CrossRef]
- Handique, J.; Baruah, J. Polyphenolic compounds: An overview. React. Funct. Polym. 2002, 52, 163–188. [Google Scholar] [CrossRef]
- Azeredo, H.M. Betalains: Properties, sources, applications, and stability—A review. Int. J. Food Sci. Technol. 2009, 44, 2365–2376. [Google Scholar] [CrossRef]
- Khan, M.I. Plant Betalains: Safety, Antioxidant Activity, Clinical Efficacy, and Bioavailability. Compr. Rev. Food Sci. Food Saf. 2016, 15, 316–330. [Google Scholar] [CrossRef]
- Terzo, S.; Attanzio, A.; Calvi, P.; Mulè, F.; Tesoriere, L.; Allegra, M.; Amato, A. Indicaxanthin from Opuntia ficus-indica Fruit Ameliorates Glucose Dysmetabolism and Counteracts Insulin Resistance in High-Fat-Diet-Fed Mice. Antioxidants 2021, 11, 80. [Google Scholar] [CrossRef]
- Yin, Z.; Yang, Y.; Guo, T.; Veeraraghavan, V.P.; Wang, X. Potential chemotherapeutic effect of betalain against human non-small cell lung cancer through PI3K /Akt/ mTOR signaling pathway. Environ. Toxicol. 2021, 36, 1011–1020. [Google Scholar] [CrossRef]
- Naselli, F.; Tesoriere, L.; Caradonna, F.; Bellavia, D.; Attanzio, A.; Gentile, C.; Livrea, M.A. Anti-proliferative and pro-apoptotic activity of whole extract and isolated indicaxanthin from Opuntia ficus-indica associated with re-activation of the onco-suppressor p16INK4a gene in human colorectal carcinoma (Caco-2) cells. Biochem. Biophys. Res. Commun. 2014, 450, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Abramovič, H.; Grobin, B.; Ulrih, N.P.; Cigić, B. Relevance and Standardization of In Vitro Antioxidant Assays: ABTS, DPPH, and Folin–Ciocalteu. J. Chem. 2018, 2018, 4608405. [Google Scholar] [CrossRef]
- Schaich, K.; Tian, X.; Xie, J. Reprint of “Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays”. J. Funct. Foods 2015, 18, 782–796. [Google Scholar] [CrossRef]
- Apak, R.; Gorinstein, S.; Böhm, V.; Schaich, K.M.; Özyürek, M.; Güçlü, K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report). Pure Appl. Chem. 2013, 85, 957–998. [Google Scholar] [CrossRef]
- Goupy, P.; Dufour, C.; Loonis, M.; Dangles, O. Quantitative Kinetic Analysis of Hydrogen Transfer Reactions from Dietary Polyphenols to the DPPH Radical. J. Agric. Food Chem. 2003, 51, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Bendary, E.; Francis, R.R.; Ali, H.M.G.; Sarwat, M.I.; El Hady, S. Antioxidant and structure–activity relationships (SARs) of some phenolic and anilines compounds. Ann. Agric. Sci. 2013, 58, 173–181. [Google Scholar] [CrossRef]
- Wang, T.-Y.; Li, Q.; Bi, K.-S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B. Some methodological problems in the determination of antioxidant activity using chromogen radicals: A practical case. Trends Food Sci. Technol. 2000, 11, 419–421. [Google Scholar] [CrossRef]
- Cano, A.; Acosta, M.; Arnao, M.B. A method to measure antioxidant activity in organic media: Application to lipophilic vitamins. Redox Rep. 2000, 5, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Alimi, H.; Hfaeidh, N.; Bouoni, Z.; Sakly, M.; Ben Rhouma, K. Ameliorative effect of Opuntia ficus indica juice on ethanol-induced oxidative stress in rat erythrocytes. Exp. Toxicol. Pathol. 2013, 65, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Bouazza, A.; Bitam, A.; Amiali, M.; Bounihi, A.; Yargui, L.; Koceir, E.A. Effect of fruit vinegars on liver damage and oxidative stress in high-fat-fed rats. Pharm. Biol. 2016, 54, 260–265. [Google Scholar] [CrossRef]
- Bisson, J.-F.; Daubié, S.; Hidalgo, S.; Guillemet, D.; Linarés, E. Diuretic and antioxidant effects of Cacti-Nea®, a dehydrated water extract from prickly pear fruit, in rats. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2009, 24, 587–594. [Google Scholar] [CrossRef]
- Souid, S.; Brahmi, D.; Lepesant, J.-A.; Zourgui, L. Cactus (Opuntia ficus indica) extract improves endoplasmic reticulum stress in Drosophila melanogaster. Afr. J. Biotechnol. 2011, 10, 14699–14705. [Google Scholar] [CrossRef]
- Son, J.-E.; Lee, B.H.; Nam, T.G.; Im, S.; Chung, D.K.; Lee, J.M.; Chun, O.K.; Kim, D.-O. Flavonols from the Ripe Fruits of O puntia ficus-indica Var. saboten Protect Neuronal PC-12 Cells against Oxidative Stress. J. Food Biochem. 2014, 38, 518–526. [Google Scholar] [CrossRef]
- Tesoriere, L.; Butera, D.; Pintaudi, A.M.; Allegra, M.; Livrea, M.A. Supplementation with cactus pear (Opuntia ficus-indica) fruit decreases oxidative stress in healthy humans: A comparative study with vitamin C. Am. J. Clin. Nutr. 2004, 80, 391–395. [Google Scholar] [CrossRef]
- Khouloud, A.; Abedelmalek, S.; Chtourou, H.; Souissi, N. The effect of Opuntia ficus-indica juice supplementation on oxidative stress, cardiovascular parameters, and biochemical markers following yo-yo Intermittent recovery test. Food Sci. Nutr. 2018, 6, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Bellafiore, M.; Pintaudi, A.M.; Thomas, E.; Tesoriere, L.; Bianco, A.; Cataldo, A.; Cerasola, D.; Traina, M.; Livrea, M.A.; Palma, A. Redox and autonomic responses to acute exercise-post recovery following Opuntia ficus-indica juice intake in physically active women. J. Int. Soc. Sports Nutr. 2021, 18, 43. [Google Scholar] [CrossRef]
- Alimi, H.; Hfaeidh, N.; Mbarki, S.; Bouoni, Z.; Sakly, M.; Ben Rouma, K. Evaluation of Opuntia ficus indica f. inermis fruit juice hepatoprotective effect upon ethanol toxicity in rats. Gen. Physiol. Biophys. 2012, 31, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Bounihi, A.; Bitam, A.; Bouazza, A.; Yargui, L.; Koceir, E.A. Fruit vinegars attenuate cardiac injury via anti-inflammatory and anti-adiposity actions in high-fat diet-induced obese rats. Pharm. Biol. 2017, 55, 43–52. [Google Scholar] [CrossRef]
- Galati, E.M.; Mondello, M.R.; Lauriano, E.R.; Taviano, M.F.; Galluzzo, M.; Miceli, N. Opuntia ficus indica (L.) Mill. fruit juice protects liver from carbon tetrachloride-induced injury. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2005, 19, 796–800. [Google Scholar] [CrossRef]
- Al-Kubaisy, K.N.; Al Essa, L.Y.; Shawagfeh, M.T. Stimulation of Hepatocytes Repair by Fruit Juice of Opuntia ficus indica in Anti Cancer Drug Cyclophosphamide (CP)-Induced Liver Toxicity in Mice. Annu. Res. Rev. Biol. 2016, 10, 1–8. [Google Scholar] [CrossRef]
- Sung, S.; Kim, J.; Kim, T.; Kim, H.; Park, S.; Kim, H. Hepatoprotective flavonoids in Opuntia ficus-indica fruits by reducing oxidative stress in primary rat hepatocytes. Pharmacogn. Mag. 2017, 13, 472–476. [Google Scholar] [CrossRef]
- Oguogho, A.; Efthimiou, Y.; Iliopoulos, J.; Stomatopoulos, J.; Ahmadzadehfar, H.; Schmid, P.; Steinbrenner, D.; Sinzinger, H. Prickly Pear Changes 111indium–LDL and 111indium–HDL Platelet Binding Correlating to Improvement of Platelet Function in Hypercholesterolemia. J. Prof. Assoc. Cactus Dev. 2010, 12, 67–79. [Google Scholar]
- Bargougui, A.; Tag, H.M.; Bouaziz, M.; Triki, S. Antimicrobial, Antioxidant, Total Phenols and Flavonoids Content of Four Cactus (Opuntia ficus-indica) Cultivars. Biomed. Pharmacol. J. 2019, 12, 1353–1368. [Google Scholar] [CrossRef]
- Trigo, J.P.; Alexandre, E.M.C.; Saraiva, J.A.; Pintado, M.E. High value-added compounds from fruit and vegetable by-products—Characterization, bioactivities, and application in the development of novel food products. Crit. Rev. Food Sci. Nutr. 2020, 60, 1388–1416. [Google Scholar] [CrossRef]
- Aruwa, C.; Amoo, S.; Kudanga, T. Extractable and macromolecular antioxidants of Opuntia ficus-indica cladodes: Phytochemical profiling, antioxidant and antibacterial activities. South Afr. J. Bot. 2019, 125, 402–410. [Google Scholar] [CrossRef]
- Bargougui, A.; Champy, P.; Triki, S.; Bories, C.; Le Pape, P.; Loiseau, P. Antileishmanial activity of Opuntia ficus-indica fractions. Biomed. Prev. Nutr. 2014, 4, 101–104. [Google Scholar] [CrossRef]
- Melgar, B.; Dias, M.I.; Ciric, A.; Sokovic, M.; Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Barros, L.; Ferreira, I. By-product recovery of Opuntia spp. peels: Betalainic and phenolic profiles and bioactive properties. Ind. Crop. Prod. 2017, 107, 353–359. [Google Scholar] [CrossRef]
- Saravanakumar, A.; Ganesh, M.; Peng, M.M.; Aziz, A.S.; Jang, H.T. Comparative antioxidant and antimycobacterial activities ofOpuntia ficus-indica fruit extracts from summer and rainy seasons. Front. Life Sci. 2015, 8, 182–191. [Google Scholar] [CrossRef]
- Di Cagno, R.; Filannino, P.; Vincentini, O.; Lanera, A.; Cavoski, I.; Gobbetti, M. Exploitation of Leuconostoc mesenteroides strains to improve shelf life, rheological, sensory and functional features of prickly pear (Opuntia ficus-indica L.) fruit puree. Food Microbiol. 2016, 59, 176–189. [Google Scholar] [CrossRef]
- Allegra, M.; Tesoriere, L.; Livrea, M.; Ianaro, A.; Panza, E. Cactus pear fruit extract exerts anti-inflammatory effects in carrageenin-induced rat pleurisy. In VIII International Congress on Cactus Pear and Cochineal 1067; International Society for Horticultural Science: Leuven, Belgium, 2013; pp. 19–25. [Google Scholar] [CrossRef]
- Park, E.-H.; Kahng, J.-H.; Paek, E.-A. Studies on the pharmacological action of cactus: Identification of its anti-inflammatory effect. Arch. Pharmacal Res. 1998, 21, 30–34. [Google Scholar] [CrossRef]
- Attanzio, A.; Tesoriere, L.; Vasto, S.; Pintaudi, A.M.; Livrea, M.A.; Allegra, M. Short-term cactus pear [Opuntia ficus-indica (L.) Mill] fruit supplementation ameliorates the inflammatory profile and is associated with improved antioxidant status among healthy humans. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef]
- Barrick, B.J.; Bruce, A.J.; Kalaaji, A.N.; Bauer, B.A. Clinical Improvement of Recalcitrant Cutaneous Sarcoidosis with Regular Nutritional Supplementation with Extract of the Prickly Pear Cactus. Explore 2013, 9, 377–378. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I.; Elmelegy, A.A.; Eldesoky, S.E.; Safwat, G. Phytochemical Screening, Antimicrobial, Antiaxidant, Anticancer Activities and Nutritional Values of Cactus (Opuntia ficus indicia) Pulp and Peel. Fresenius Environmental and Advances in Food Sciences. 2019, 28, 1545–16562. [Google Scholar]
- Briffa, M.; Ghio, S.; Neuner, J.; Gauci, A.J.; Cacciottolo, R.; Marchal, C.; Caruana, M.; Cullin, C.; Vassallo, N.; Cauchi, R.J. Extracts from two ubiquitous Mediterranean plants ameliorate cellular and animal models of neurodegenerative proteinopathies. Neurosci. Lett. 2017, 638, 12–20. [Google Scholar] [CrossRef]
- Peña, I.J.I.D.; Yoon, S.Y.; Kim, H.J.; Shim, H.; Kim, J.H.; Cheong, N.; Paek, S.H.; Seo, Y.K.; Park, S.J.; Moon, B.S.; et al. Alleviating effects of Opuntia ficus indica extracts on psychomotor alterations induced by ethanol in rats. Food Sci. Biotechnol. 2014, 23, 2063–2068. [Google Scholar] [CrossRef]
- Butterweck, V.; Semlin, L.; Feistel, B.; Pischel, I.; Bauer, K.; Verspohl, E.J. Comparative evaluation of two different Opuntia ficus-indica extracts for blood sugar lowering effects in rats. Phytother. Res. 2011, 25, 370–375. [Google Scholar] [CrossRef]
- Deldicque, L.; Van Proeyen, K.; Ramaekers, M.; Pischel, I.; Sievers, H.; Hespel, P. Additive insulinogenic action of Opuntia ficus-indica cladode and fruit skin extract and leucine after exercise in healthy males. J. Int. Soc. Sports Nutr. 2013, 10, 45. [Google Scholar] [CrossRef]
- Van Proeyen, K.; Ramaekers, M.; Pischel, I.; Hespel, P. Opuntia ficus-indica Ingestion Stimulates Peripheral Disposal of Oral Glucose Before and After Exercise in Healthy Men. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 284–291. [Google Scholar] [CrossRef]
- Godard, M.P.; Ewing, B.A.; Pischel, I.; Ziegler, A.; Benedek, B.; Feistel, B. Acute blood glucose lowering effects and long-term safety of OpunDia™ supplementation in pre-diabetic males and females. J. Ethnopharmacol. 2010, 130, 631–634. [Google Scholar] [CrossRef]
- Schmitt, L.; Fouillot, J.-P.; Nicolet, G.; Midol, A. Opuntia ficus indica Increases Heart-Rate Variability in High-Level Athletes. Int. J. Sport Nutr. Exerc. Metab. 2008, 18, 169–178. [Google Scholar] [CrossRef]
- Aloui, K.; Abedelmalek, S.; Boussetta, N.; Shimi, I.; Chtourou, H.; Souissi, N. Opuntia ficus-indica juice supplementation: What role it plays on diurnal variation of short-term maximal exercise? Biol. Rhythm. Res. 2017, 48, 315–330. [Google Scholar] [CrossRef]
- Gouws, C.A.; McKune, A.; Tee, N.; Somerset, S.; Mortazavi, R. Prickly pear juice consumption after fat intake affects postprandial heart rate variability but not traditional risk factors of cardiovascular disease in healthy men. Nutrition 2021, 96, 111555. [Google Scholar] [CrossRef]
- Koss-Mikołajczyk, I.; Kusznierewicz, B.; Wiczkowski, W.; Sawicki, T.; Bartoszek, A. The comparison of betalain composition and chosen biological activities for differently pigmented prickly pear (Opuntia ficus-indica) and beetroot (Beta vulgaris) varieties. Int. J. Food Sci. Nutr. 2019, 70, 442–452. [Google Scholar] [CrossRef]
- Siriwardhana, N.; Shahidi, F.; Jeon, Y.-J. Potential antioxidative effects of cactus pear fruit (Opuntia ficus-indica) extract on radical scavenging and dna damage reduction in human peripheral lymphocytes. J. Food Lipids 2006, 13, 445–458. [Google Scholar] [CrossRef]
- Wiese, J.; McPherson, S.; Odden, M.C.; Shlipak, M.G. Effect of Opuntia ficus indica on Symptoms of the Alcohol Hangover. Arch. Intern. Med. 2004, 164, 1334–1340. [Google Scholar] [CrossRef]
- Kim, S.H.; Jeon, B.J.; Kim, D.H.; Kim, T.I.; Lee, H.K.; Han, D.S.; Lee, J.-H.; Kim, J.W.; Sung, S.H. Prickly Pear Cactus (Opuntia ficus indica var. saboten) Protects Against Stress-Induced Acute Gastric Lesions in Rats. J. Med. Food 2012, 15, 968–973. [Google Scholar] [CrossRef]
- Akkol, E.K.; Ilhan, M.; Karpuz, B.; Genç, Y.; Sobarzo-Sánchez, E. Sedative and Anxiolytic Activities of Opuntia ficus indica (L.) Mill.: An Experimental Assessment in Mice. Molecules 2020, 25, 1844. [Google Scholar] [CrossRef]
- An, B.H.; Jeong, H.; Zhou, W.; Liu, X.; Kim, S.; Jang, C.Y.; Kim, H.-S.; Sohn, J.; Park, H.-J.; Sung, N.-H.; et al. Evaluation of the Biological Activity of Opuntia ficus indica as a Tissue- and Estrogen Receptor Subtype-Selective Modulator. Phytotherapy Res. 2016, 30, 971–980. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, S.; Kim, M.-Y.; Lee, J.; An, B.H.; Kim, H.-D.; Jeong, H.; Song, Y.S.; Chang, M. Inhibitory and Inductive Effects of Opuntia ficus indica Extract and Its Flavonoid Constituents on Cytochrome P450s and UDP-Glucuronosyltransferases. Int. J. Mol. Sci. 2018, 19, 3400. [Google Scholar] [CrossRef]
- Baldassano, S.; Tesoriere, L.; Rotondo, A.; Serio, R.; Livrea, M.A.; Mulè, F. Inhibition of the Mechanical Activity of Mouse Ileum by Cactus Pear (Opuntia ficus indica, L., Mill.) Fruit Extract and Its Pigment Indicaxanthin. J. Agric. Food Chem. 2010, 58, 7565–7571. [Google Scholar] [CrossRef]
- Rtibi, K.; Selmi, S.; Grami, D.; Amri, M.; Sebai, H.; Marzouki, L. Opposite Effect of Opuntia ficus-indica L. Juice Depending on Fruit Maturity Stage on Gastrointestinal Physiological Parameters in Rat. J. Med. Food 2018, 21, 617–624. [Google Scholar] [CrossRef]
- Rtibi, K.; Selmi, S.; Saidani, K.; Grami, D.; Amri, M.; Sebai, H.; Marzouki, L. Reverse Effect of Opuntia ficus-indica L. Juice and Seeds Aqueous Extract on Gastric Emptying and Small-Bowel Motility in Rat. J. Food Sci. 2018, 83, 205–211. [Google Scholar] [CrossRef]
- Guerrero-Rubio, M.A.; Hernández-García, S.; García-Carmona, F.; Gandía-Herrero, F. Extension of life-span using a RNAi model and in vivo antioxidant effect of Opuntia fruit extracts and pure betalains in Caenorhabditis elegans. Food Chem. 2019, 274, 840–847. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, T.B.; Yang, H.; Sung, S.H. Phenolic Compounds Isolated from Opuntia ficus-indica Fruits. Nat. Prod. Sci. 2016, 22, 117–121. [Google Scholar] [CrossRef]
- Aruwa, C.E.; Amoo, S.; Kudanga, T. Phenolic compound profile and biological activities of Southern African Opuntia ficus-indica fruit pulp and peels. LWT 2019, 111, 337–344. [Google Scholar] [CrossRef]
- Yeddes, N.; Chérif, J.K.; Guyot, S.; Sotin, H.; Ayadi, M.T. Comparative Study of Antioxidant Power, Polyphenols, Flavonoids and Betacyanins of the Peel and Pulp of Three Tunisian Opuntia Forms. Antioxidants 2013, 2, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Scarano, P.; Tartaglia, M.; Zuzolo, D.; Prigioniero, A.; Guarino, C.; Sciarrillo, R. Recovery and Valorization of Bioactive and Functional Compounds from the Discarded of Opuntia ficus-indica (L.) Mill. Fruit Peel. Agronomy 2022, 12, 388. [Google Scholar] [CrossRef]
- Aparicio-Fernández, X.; Vega-Ahuatzin, A.; Ochoa-Velasco, C.E.; Cid-Pérez, S.; Hernández-Carranza, P.; Ávila-Sosa, R. Physical and Antioxidant Characterization of Edible Films Added with Red Prickly Pear (Opuntia ficus-indica L.) cv. San Martín Peel and/or Its Aqueous Extracts. Food Bioprocess Technol. 2018, 11, 368–379. [Google Scholar] [CrossRef]
- Borchani, M.; Yaich, H.; Abbès, F.; Blecker, C.; Besbes, S.; Attia, H.; Masmoudi, M. Physicochemical, Functional and Antioxidant Properties of the Major Protein Fractions Extracted from Prickly Pear (Opuntia ficus indica L.) Seed Cake. Waste Biomass- Valorization 2021, 12, 1749–1760. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Drioli, E. Physico-chemical parameters of cactus pear (Opuntia ficus-indica) juice clarified by microfiltration and ultrafiltration processes. Desalination 2010, 250, 1101–1104. [Google Scholar] [CrossRef]
- Cayupán, Y.S.C.; Ochoa, M.J.; Nazareno, M.A. Health-promoting substances and antioxidant properties of Opuntia sp. fruits. Changes in bioactive-compound contents during ripening process. Food Chem. 2011, 126, 514–519. [Google Scholar] [CrossRef]
- Del Socorro Cruz-Cansino, N.; Ramírez-Moreno, E.; León-Rivera, J.E.; Delgado-Olivares, L.; Alanís-García, E.; Ariza-Ortega, J.A.; de Jesús Manríquez-Torres, J.; Jaramillo-Bustos, D.P. Shelf life, physicochemical, microbiological and antioxidant properties of purple cactus pear (Opuntia ficus indica) juice after thermoultrasound treatment. Ultrason. Sonochemistry 2015, 27, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Cansino, N.D.S.; Montiel-Columna, N.I.; Bautista-Velueta, P.G.; Pérez-Tinoco, M.R.; Alanís-García, E.; Ramírez-Moreno, E. Optimization of Thermoultrasound Conditions for the Processing of A Prickly Pear Juice Blend (Opuntia Ficus Indica) Using Response Surface Methodology. J. Food Qual. 2016, 39, 780–791. [Google Scholar] [CrossRef]
- Diaz-Vela, J.; Totosaus, A.; Cruz-Guerrero, A.E.; de Lourdes Pérez-Chabela, M. In vitroevaluation of the fermentation of added-value agroindustrial by-products: Cactus pear (Opuntia ficus-indica L.) peel and pineapple (Ananas comosus) peel as functional ingredients. Int. J. Food Sci. Technol. 2013, 48, 1460–1467. [Google Scholar] [CrossRef]
- Du Toit, A. Antioxidant Content and Potential of Fresh and Processed Cladodes and Fruit from Different Coloured Cactus Pear (Opuntia ficus-indica and Opuntia Robusta) Cultivars. Ph.D. Thesis, University of the Free State, Bloemfontein, South Africa, 2013. [Google Scholar]
- Du Toit, A.; de Wit, M.; Osthoff, G.; Hugo, A. Relationship and correlation between antioxidant content and capacity, processing method and fruit colour of cactus pear fruit. Food Bioprocess Technol. 2018, 11, 1527–1535. [Google Scholar] [CrossRef]
- Ghazi, Z.; Ramdani, M.; Tahri, M.; Rmili, R.; Elmsellem, H.; El Mahi, B.; Fauconnier, M.-L. Chemical Composition and Antioxidant Activity of Seeds Oils and Fruit Juice of Opuntia ficus indica and Opuntia dillenii from Morocco. J. Mater. Environ. Sci. 2015, 6, 2338–2345. [Google Scholar]
- García-Cayuela, T.; Gómez-Maqueo, A.; Guajardo-Flores, D.; Welti-Chanes, J.; Cano, M.P. Characterization and quantification of individual betalain and phenolic compounds in Mexican and Spanish prickly pear (Opuntia ficus-indica L. Mill) tissues: A comparative study. J. Food Compos. Anal. 2019, 76, 1–13. [Google Scholar] [CrossRef]
- Gómez-Maqueo, A.; García-Cayuela, T.; Welti-Chanes, J.; Cano, M.P. Enhancement of anti-inflammatory and antioxidant activities of prickly pear fruits by high hydrostatic pressure: A chemical and microstructural approach. Innov. Food Sci. Emerg. Technol. 2019, 54, 132–142. [Google Scholar] [CrossRef]
- Miguel, M.G.; Gago, C.; Valente, R.; Guerreiro, A.; Antunes, D.; Manhita, A.; Barrocas-Dias, C. Qualitative evaluation of fruits from different Opuntia ficus-indica ecotypes/cultivars harvested in South Portugal. J. Food Biochem. 2018, 42, e12652. [Google Scholar] [CrossRef]
- Ishtiaque, S.; Naz, S.; Siddiqi, R.; Abdullah, S.U.; Khan, K.; Ahmed, J.; Badaruddin, M. Antioxidant Activities and Total Phenolics Contents from Extracts of Terminalia catappa, Carrisa carandas, and Opuntia ficus indica Fruits. Recent Innov. Chem. Eng. Formerly Recent Patents Chem. Eng. 2014, 7, 106–112. [Google Scholar] [CrossRef]
- Karabagias, V.K.; Karabagias, I.K.; Gatzias, I.; Riganakos, K.A. Characterization of prickly pear juice by means of shelf life, sensory notes, physicochemical parameters and bio-functional properties. J. Food Sci. Technol. 2019, 56, 3646–3659. [Google Scholar] [CrossRef]
- Ramírez-Ramos, M.; Medina-Dzul, K.; García-Mateos, R.; Corrales-García, J.; Ybarra-Moncada, C.; Castillo-González, A.M. Nutraceutical Components, Antioxidant Activity, and Color of 11 Varieties of Prickly Pear (Opuntia Sp.). J. Appl. Bot. Food Qual 2018, 91, 211–218. [Google Scholar] [CrossRef]
- Reis, C.M.G.; Gouveia, C.; Vitorino, M.C.; Gazarini, L.; Ribeiro, M.M.A.; Peres, M.F. Bioactive Compounds and Morphology in Opuntia Spp. Fruits from Portuguese Ecotypes. Bulg. J. Agric. Sci. 2017, 23, 929–938. [Google Scholar]
- Salehi, E.; Emam-Djomeh, Z.; Askari, G.; Fathi, M. Opuntia ficus indica fruit gum: Extraction, characterization, antioxidant activity and functional properties. Carbohydr. Polym. 2019, 206, 565–572. [Google Scholar] [CrossRef]
- Suh, S.; Kim, Y.E.; Yang, H.-J.; Ko, S.; Hong, G.-P. Influence of autoclave treatment and enzymatic hydrolysis on the antioxidant activity of Opuntia ficus-indica fruit extract. Food Sci. Biotechnol. 2017, 26, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Zafra-Rojas, Q.Y.; Cruz-Cansino, N.; Ramírez-Moreno, E.; Delgado-Olivares, L.; Villanueva-Sánchez, J.; Alanís-García, E. Effects of ultrasound treatment in purple cactus pear (Opuntia ficus-indica) juice. Ultrason. Sonochemistry 2013, 20, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Zenteno-Ramírez, G.; Juárez-Flores, B.I.; Aguirre-Rivera, J.R.; Monreal-Montes, M.; García, J.M.; Serratosa, M.P.; Santos, M.Á.V.; Pérez, M.D.O.; Rendón-Huerta, J.A. Juices of prickly pear fruits (Opuntia spp.) as functional foods. Ital. J. Food Sci. 2018, 30, 614–627. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).