Edible Halophytes and Halo-Tolerant Species in Apulia Region (Southeastern Italy): Biogeography, Traditional Food Use and Potential Sustainable Crops
Abstract
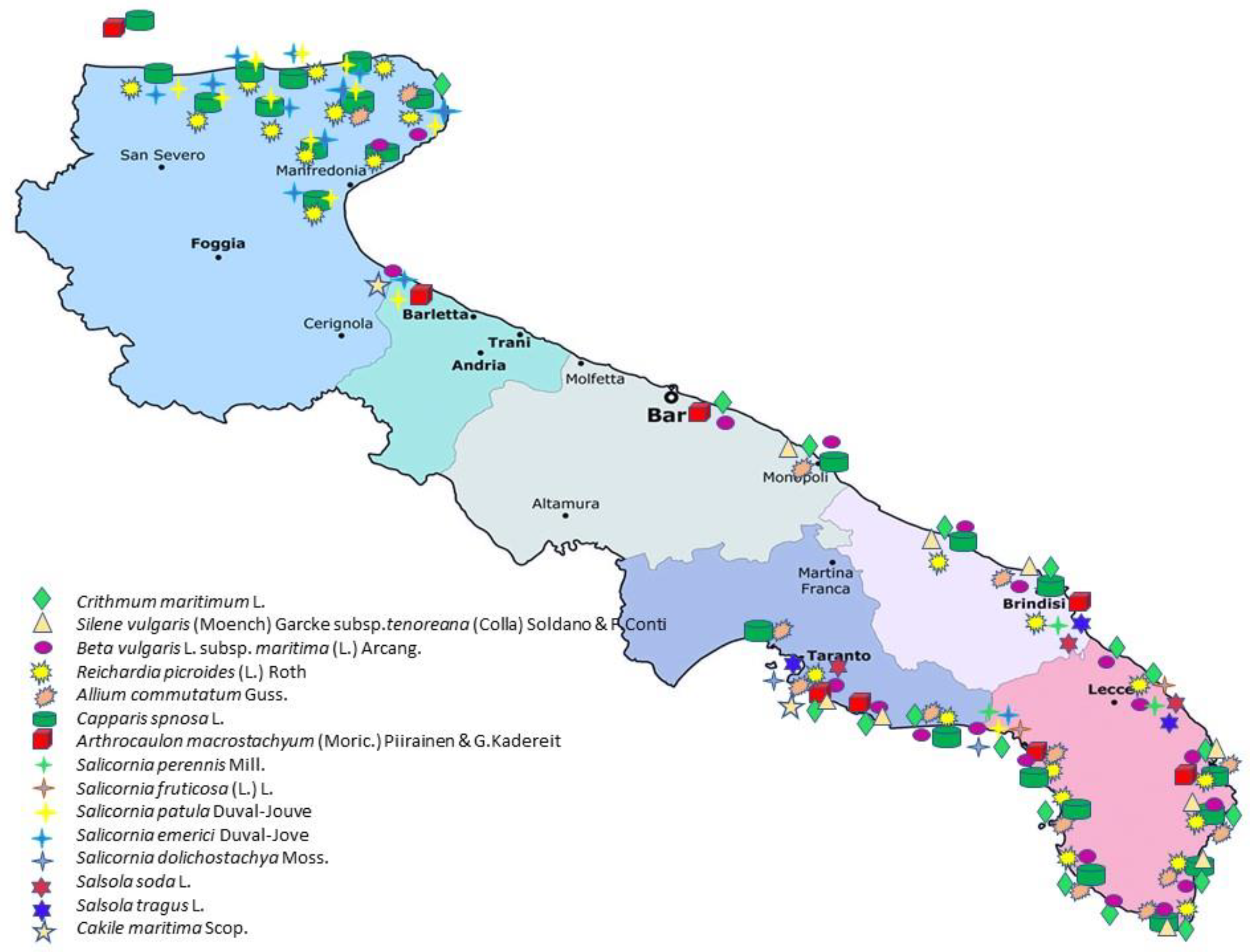
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Bibliographic Review; New Data Collection in the Field
2.3. Taxonomical Notes
3. Results
3.1. Salicornia sp. pl. (Annual)
3.1.1. Brief Description, Distribution and Ecology
3.1.2. Food Use
3.1.3. Domestication/Cultivation
3.1.4. Other Uses/Properties
3.2. Salicornia sp. pl. (Perennial) and Arthrocaulon macrostachyum (Moric.) Piirainen & G. Kadereit
3.2.1. Brief Description, Distribution and Ecology
3.2.2. Food Use
3.2.3. Domestication/Cultivation
3.2.4. Other Uses/Properties
3.3. Soda inermis Fourr. (=Salsola soda L.)
3.3.1. Brief Description, Distribution and Ecology
3.3.2. Food Use
3.3.3. Domestication/Cultivation
3.3.4. Other Uses/Properties
3.4. Cakile maritima Scop.
3.4.1. Brief Description, Distribution and Ecology
3.4.2. Food Use
3.4.3. Domestication/Cultivation
3.4.4. Other Uses/Properties
3.5. Crithmum maritimum L.
3.5.1. Brief Description, Distribution and Ecology
3.5.2. Food Use
3.5.3. Domestication/Cultivation
3.5.4. Other Uses/Properties
3.6. Reichardia picroides (L.) Roth
3.6.1. Brief Description, Distribution and Ecology
3.6.2. Food Use
3.6.3. Domestication/Cultivation
3.6.4. Other Uses/Properties
3.7. Silene vulgaris (Moench) Garcke subsp. tenoreana (Colla) Soldano & F. Conti
3.7.1. Brief Description, Distribution and Ecology
3.7.2. Food Use
3.7.3. Domestication/Cultivation
3.7.4. Other Uses/Properties
3.8. Allium commutatum Guss
3.8.1. Brief Description, Distribution and Ecology
3.8.2. Food Use
3.8.3. Domestication/Cultivation
3.8.4. Other Uses/Properties
3.9. Beta vulgaris L. subsp. maritima (L.) Arcang
3.9.1. Brief Description, Distribution and Ecology
3.9.2. Food Use
3.9.3. Domestication/Cultivation
3.9.4. Other Uses/Properties
3.10. Capparis spinosa L.
3.10.1. Brief Description, Distribution and Ecology
3.10.2. Food Use
3.10.3. Domestication/Cultivation
3.10.4. Other Uses/Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| (a) | |||||||||||||
| loc name 1 | loc name 2 | loc name 3 | loc name 4 | loc name 5 | loc name 6 | loc name 7 | loc name 8 | loc name 9 | loc name 10 | loc name 11 | loc name 12 | loc name 13 | |
| Isole Tremiti | Lesina | Apricena | Sannicandro Garganico | Cagnano | Ischitella | Vico del Gargano | Vieste | Peschici | S. Giovanni Rotondo | Manfredonia | Mattinata | Margherita di Savoia | |
| Salicornia patula | up; cu; hs | up; cu | up; cu; hs | up; cu; hs | up; cu; | up; cu; hs | up | up; cu; hs | up; cu; | up | up; cu | ||
| Salicornia emerici | up; cu; hs | up; cu; hs | up; cu; hs | up; cu; hs | up; cu; | up; cu; hs | up | up; cu; hs | up; cu; | up | |||
| Salicornia dolichostachya | |||||||||||||
| Salicornia perennis | up; cu | ||||||||||||
| Salicornia fruticosa | |||||||||||||
| Arthrocaulon macrostachyum | up; cu; hs | up; cu | |||||||||||
| Soda inermis | |||||||||||||
| Cakile maritima | up | ||||||||||||
| Crithmum maritimum | up; cu | ||||||||||||
| Reichardia picroides | up; cu | up; cu | up; cu | up; cu | up; cu | up; cu | up; cu | up; cu | up; cu | up; cu | up; cu | ||
| Silene vulgaris subsp. tenoreana | |||||||||||||
| Allium commutatum | up; cu | up; cu | up; cu | ||||||||||
| Beta vulgaris subsp. maritima | up; cu | up; cu | up; cu | up; cu | |||||||||
| Capparis spinosa | up; cu; hs | up; cu; hs | up; cu; hs | up; cu; hs | up; cu; hs | up; cu; hs | up; cu; hs | up; cu; hs | up; cu; hs | up; cu; hs; cul | up; cu; hs | up; cu; hs; cul | |
| (b) | |||||||||||||
| loc name 14 | loc name 15 | loc name 16 | loc name 17 | loc name 18 | loc name 19 | loc name 20 | loc name 21 | loc name 22 | loc name 23 | loc name 24 | |||
| Mola di Bari | Monopoli | Carovigno | Brindisi | Casalabate | San Cataldo | Torre dell'Orso | Otranto | Santa Cesarea | Tricase | S. Maria di Leuca | |||
| Salicornia patula | |||||||||||||
| Salicornia emerici | |||||||||||||
| Salicornia dolichostachya | up | up | |||||||||||
| Salicornia perennis | up; cu | up; cu | |||||||||||
| Salicornia fruticosa | up; cu | ||||||||||||
| Arthrocaulon macrostachyum | up; cu; hs | up; cu; hs | up; cu | ||||||||||
| Soda inermis | up | up | |||||||||||
| Cakile maritima | |||||||||||||
| Crithmum maritimum | up; cu | up; cu | up; cu | up; cu | up; cu | up; cu | up; cu | up; cu | up; cu | up; cu | up; cu | ||
| Reichardia picroides | up; cu | up; cu | up; cu | up; cu | up; cu | up; cu | up; cu | up; cu | |||||
| Silene vulgaris subsp. tenoreana | up | up; cu | up; cu | up; cu | |||||||||
| Allium commutatum | up; cu | up; cu | up; cu | up; cu | up; cu | up; cu | up; cu | up; cu | |||||
| Beta vulgaris subsp. maritima | up; cu; hs | up; cu; hs | up; cu | up; cu | up; cu | up; cu | up; cu | up; cu | up; cu | up; cu | up; cu | ||
| Capparis spinosa | up; cu; hs | up; cu | up; cu | up; cu | up; cu | up; cu | up; cu | up; cu | |||||
| (c) | |||||||||||||
| loc name 25 | loc name 26 | loc name 27 | loc name 28 | loc name 29 | loc name 30 | loc name 31 | loc name 32 | loc name 33 | loc name 34 | ||||
| Pescoluse | Torre Suda | Gallipoli | S. Maria al Bagno | S. Isidoro | Porto Cesareo | Salina di Monaci | San Pietro in Bevagna | S.giorgio jonico | Taranto (la Vela) | ||||
| Salicornia patula | up | ||||||||||||
| Salicornia emerici | up | up; cu | |||||||||||
| Salicornia dolichostachya | up | up | up; cu | ||||||||||
| Salicornia perennis | up | up; cu | up; cu | ||||||||||
| Salicornia fruticosa | up | ||||||||||||
| Arthrocaulon macrostachyum | up | up | up; cu | ||||||||||
| Soda inermis | up | ||||||||||||
| Cakile maritima | up | ||||||||||||
| Crithmum maritimum | up; cu | up; cu | up; cu | up; cu | up; cu | up; cu | up; cu | up; cu | |||||
| Reichardia picroides | up; cu | up; cu | up; cu | up; cu | up; cu | ||||||||
| Silene vulgaris subsp. tenoreana | up | up | |||||||||||
| Allium commutatum | up; cu | up; cu | up; cu | up; cu | up; cu | up; cu | |||||||
| Beta vulgaris subsp. maritima | up; cu | up; cu | up; cu | up; cu | up; cu | up; cu | up; cu | up; cu | |||||
| Capparis spinosa | up; cu; hs | up; cu | up; cu | up; cu | up; cu | ||||||||
Appendix B
References
- Shahid, S.A.; Zama, M.; Heng, L. Soil Salinity: Historical Perspectives and a World Overview of the Problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Zaman, M., Shahid, S.A., Heng, L., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 43–53. [Google Scholar] [CrossRef]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savouré, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Panta, S.; Flowers, T.; Lane, P.; Doyle, R.; Haros, G.; Shabala, S. Halophyte agriculture: Success stories. Environ. Exp. Bot. 2014, 107, 71–83. [Google Scholar] [CrossRef]
- Perrino, E.V.; Valerio, F.; Jallali, S.; Trani, A.; Mezzapesa, G.N. Ecological and Biological Properties of Satureja cuneifolia Ten. and Thymus spinulosus Ten.: Two Wild Officinal Species of Conservation Concern in Apulia (Italy). A Preliminary Survey. Plants 2021, 10, 1952. [Google Scholar] [CrossRef] [PubMed]
- Valerio, F.; Mezzapesa, G.N.; Ghannouchi, A.; Mondelli, D.; Logrieco, A.F.; Perrino, E.V. Characterization and antimicrobial properties of essential oils from four wild taxa of Lamiaceae family growing in Apulia. Agronomy 2021, 11, 1431. [Google Scholar] [CrossRef]
- Casella, F.; Vurro, M.; Valerio, F.; Perrino, E.V.; Mezzapesa, G.; Boari, A. Phytotoxic effects of essential oils from six Lamiaceae species. Agronomy 2023, 13, 257. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phyt. 2008, 179, 954–963. [Google Scholar] [CrossRef]
- Grigore, M.N.; Toma, C. Definition and classification of halophytes. In Anatomical Adaptations of Halophytes; Springer: Cham, Switzerland, 2017; pp. 3–28. [Google Scholar]
- Ventura, Y.; Wuddineh, W.A.; Myrzabayeva, M.; Alikulov, Z.; Khozin-Goldberg, I.; Shpigel, M.; Samocha, T.M.; Sagi, M. Effect of seawater concentration on the productivity and nutritional value of annual Salicornia and perennial Sarcocornia halophytes as leafy vegetable crops. Sci. Hortic. 2011, 128, 189–196. [Google Scholar] [CrossRef]
- Loconsole, D.; Cristiano, G.; De Lucia, B. Glassworts: From wild salt marsh species to sustainable edible crops. Agriculture 2019, 9, 14. [Google Scholar] [CrossRef]
- Glenn, E.P.; Brown, J.J.; Blumwald, E. Salt Tolerance and Crop Potential of Halophytes. Crit. Rev. Plant Sci. 1999, 18, 227–255. [Google Scholar] [CrossRef]
- Ksouri, R.; Megdiche, W.; Falleh, H.; Trabelsi, N.; Boulaaba, M.; Smaoui, A.; Abdelly, C. Influence of biological, environmental and technical factors on phenolic content and antioxidant activities of Tunisian halophytes. Comptes Rendus Biol. 2008, 331, 865–873. [Google Scholar] [CrossRef]
- Benhammou, N.; Bekkara, F.A.; KadifkovaPanovska, T. Antioxidant activity of methanolic extracts and some bioactive compounds of Atriplex halimus. Comptes Rendus Chim. 2009, 12, 1259–1266. [Google Scholar] [CrossRef]
- Mishra, A.; Patel, M.K.; Jha, B. Non-targeted metabolomics and scavenging activity of reactive oxygen species reveal the potential of Salicornia brachiata as a functional food. J. Funct. Foods 2015, 13, 21–31. [Google Scholar] [CrossRef]
- Arya, S.S.; Devi, S.; Ram, K.; Kumar, S.; Kumar, N.; Mann, A.; Chand, G. Halophytes: The plants of therapeutic medicine. In Ecophysiology, Abiotic Stress Responses and Utilization of Halophytes; Hasanuzzaman, M., Nahar, K., Öztürk, M., Eds.; Springer: Singapore, 2019; pp. 271–287. [Google Scholar]
- del Rosario Marinoni, L.; Zabala, J.M.; Taleisnik, E.L.; Schrauf, G.E.; Richard, G.A.; Tomas, P.A.; Giavedoni, J.A.; Pensiero, J.F. Wild halophytic species as forage sources: Key aspects for plant breeding. Grass Forage Sci. 2019, 74, 321–344. [Google Scholar] [CrossRef]
- Castañeda-Loaiza, V.; Oliveira, M.; Santos, T.; Schüler, L.; Lima, A.R.; Gama, F. Wild vs cultivated halophytes: Nutritional and functional differences. Food Chem. 2020, 333, 127536. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Kanthaliya, B.; Arora, J. Halophytes: The nonconventional crops as source of biofuel production. In Handbook of halophytes: From Molecules to Ecosystems towards Biosaline Agriculture; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2020; pp. 1–28. [Google Scholar]
- Montesano, F.; Gattullo, C.; Parente, A.; Terzano, R.; Renna, M. Cultivation of potted sea fennel, an emerging mediterranean halophyte, using a renewable seaweed-based material as a peat substitute. Agriculture 2018, 8, 96. [Google Scholar] [CrossRef]
- Karakas, S.; Cullu, M.A.; Dikilitas, M. Comparison of two halophyte species (Salsola soda and Portulaca oleracea) for salt removal potential under different soil salinity conditions. Turk. J. Agric. For. 2017, 41, 183–190. [Google Scholar] [CrossRef]
- Ventura, Y.; Eshel, A.; Pasternak, D.; Sagi, M. The development of halophyte-based agriculture: Past and present. Ann. Bot. 2014, 115, 529–540. [Google Scholar] [CrossRef]
- Urbano, M.; Tomaselli, V.; Bisignano, V.; Veronico, G.; Hammer, K.; Laghetti, G. Salicornia patula Duval-Jouve: From gathering of wild plants to some attempts of cultivation in Apulia region (southern Italy). Genet. Resour. Crop Evol. 2017, 64, 1465–1472. [Google Scholar] [CrossRef]
- Bouchmaa, N.; Mrid, R.B.; Kabach, I.; Zouaoui, Z.; Karrouchi, K.; Chtibi, H.; Zyad, A.; Cacciola, F.; Nhiri, M. Beta vulgaris subsp. maritima: A Valuable Food with High Added Health Benefits. Appl. Sci. 2022, 12, 1866. [Google Scholar] [CrossRef]
- Maggini, R.; Benvenuti, S.; Leoni, F.; Pardossi, A. Terracrepolo (Reichardia picroides (L.) Roth.): Wild food or new horticultural crop? Sci. Hortic. 2018, 240, 224–231. [Google Scholar] [CrossRef]
- Rivera, D.; Inocencio, C.; Obón, C.; Alcarez, F. Review of Food and Medicinal Uses of Capparis L. subgenus Capparis (Capparidaceae). Econ. Bot. 2003, 57, 515–534. [Google Scholar] [CrossRef]
- Rivera, D.; Obón, C.; Heinrich, M.; Inocencio, C.; Verde, A.; Fajardo, J. Gathered Mediterranean food plants—Ethnobotanical investigations and historical development. Forum Nutr. 2006, 59, 18–74. [Google Scholar]
- Lavarra, P.; Angelini, P.; Augello, R.; Bianco, P.M.; Capogrossi, R.; Gennaio, R.; La Ghezza, V.; Marrese, M. Il Sistema Carta Della Natura Della Regione Puglia; Serie Rapporti: Ispra, Italy, 2014; p. 204. [Google Scholar]
- Accogli, R. Piante da Fibra e Piante Tintorie Spontanee Nell’economia del Salento (Puglia); Organo Ufficiale Associazione Geografi Italiani (AGEI)—Geotema Patron Editore: Bologna, Italy, 2009; Volume 35–36, pp. 99–102. [Google Scholar]
- Ditonno, N.; Lamusta, S.; Nardone, D. Sapori e Aromi da Piante e Frutti Spontanei della Puglia Peninsulare; Amici della “A. De Leo”: Brindisi, Italy, 1997; p. 794. [Google Scholar]
- Savo, V.; Salomone, F.; Mattoni, E.; Tofani, D.; Caneva, G. Traditional Salads and Soups with Wild Plants as a Source of Antioxidants: A Comparative Chemical Analysis of Five Species Growing in Central Italy. Evid.-Based Complement. Altern. Med. 2019, 5, 6782472. [Google Scholar] [CrossRef]
- Tava, A.; Biazzi, E.; Mella, M.; Doria, A.; Accogli, R.; Argentieri, M.P.; Avato, P. Triterpenic saponins from Medicago marina L. Phytochemistry 2019, 174, 112333. [Google Scholar] [CrossRef]
- Shelef, O.; Stavi, I.; Zdruli, P.; Rachmilevitch, S. Land use change, a case study from southern Italy: General implications for agricultural subsidy policies. Land Degrad. Dev. 2015, 27, 868–870. [Google Scholar] [CrossRef]
- Perrino, E.V.; Ladisa, G.; Calabrese, G. Flora and plant genetic resources of ancient olive groves of Apulia (southern Italy). Genet. Resour. Crop Evol. 2014, 61, 23–53. [Google Scholar] [CrossRef]
- Wagensommer, R.P.; Fröhlich, T.; Fröhlich, M. First record of the southeast European species Cerinthe retorta Sibth. & Sm. (Boraginaceae) in Italy and considerations on its distribution and conservation status. Acta Bot. Gall. Bot. Lett. 2014, 161, 111–115. [Google Scholar] [CrossRef]
- Di Pietro, R.; Wagensommer, R.P. A new Sesleria juncifolia association from south-eastern Italy and its position in the amphi-Adriatic biogeographical context. Acta Bot. Croat. 2014, 73, 171–207. [Google Scholar] [CrossRef]
- Wagensommer, R.P.; Medagli, P.; Albano, A.; Peruzzi, L.; Bartolucci, F.; Villani, M.; Conti, F.; Passalacqua, N.G.; Alessandrini, A.; Barberis, G.; et al. Loci classici of the Italian endemic vascular plants described for Apulia. Inf. Bot. Ital. 2014, 46, 323–369. [Google Scholar]
- Perrino, E.V.; Signorile, G.; Marvulli, M. A first checklist of the vascular flora of the “Polignano a Mare” coast (Apulia, southern Italy). Nat. Croat. 2013, 22, 295–318. [Google Scholar]
- Corbetta, F. Lineamenti della vegetazione macrofitica dei Laghi di Lesina e di Varano [Outlines of the macrophytic vegetation of the Lesina and Varano lakes]. Giorn. Bot. Ital. 1970, 104, 165–191. [Google Scholar] [CrossRef]
- Géhu, J.-M.; Costa, M.; Scoppola, A.; Biondi, E.; Marchiori, S.; Peris, J.B.; Frank, J.; Caniglia, G.; Veri, L. Essai synsystématique et synchorologique sur les végétations littorals italiennes dans un but conservatoire. I. Dunes et vasessalees [Synsystematic and synchorological analysis on Italian coastal vegetation for a conservatory purpose. I. Dunes and salt marshes]. Doc. Phytosoc. 1984, 8, 394–474. [Google Scholar]
- Corbetta, F.; Gratani, L.; Moriconi, M.; Pirone, G. Lineamenti vegetazionali e caratterizzazione ecologica delle spiagge dell’arco jonico da Taranto alla foce del Sinni [Vegetation features and ecological characterization of the Ionian beaches from Taranto to the Sinni mouth]. Coll. Phytosoc. 1989, 19, 55–81. [Google Scholar]
- Bartolo, G.; Brullo, S.; Signorello, P. La classe Crithmo-Limonietea nella penisola Italiana. Colloq. phytosoc. 1992, 19, 55–81. [Google Scholar]
- Brullo, S.; De Marco, G. Antyllidion barbae-jovis new alliance of Crithmo-Limonietea. Arch. Bot. Ital. 1989, 65, 109–120. [Google Scholar]
- Biondi, E.; Casavecchia, S.; Guerra, V. Analysis of vegetation diversity in relation to the geomorphological characteristics in the Salento coasts (Apulia-Italy). Fitos 2006, 43, 25–38. [Google Scholar]
- Tomaselli, V.; Perrino, E.V.; Cimmarusti, G. Paludi Sfinale e Gusmay, due aree umide di rilevante interesse naturalistico nel Parco Nazionale del Gargano [Sfinale and Gusmay, two wetlands of significant naturalistic interest in the Gargano National Park]. Inf. Bot. Ital. 2008, 40, 183–192. [Google Scholar]
- Biondi, E.; Casavecchia, S. The halophilous retro-dunegrasslands of the Italian Adriatic coastline. Braun-Blanquetia 2010, 46, 11–127. [Google Scholar]
- Tomaselli, V.; Di Pietro, R.; Sciandrello, S. Plant communities structure and composition in three coastal wetlands in southern Apulia (Italy). Biologia 2011, 66, 1027–1043. [Google Scholar] [CrossRef]
- Sciandrello, S.; Tomaselli, V. Coastal salt marshes plant communities of the Salicornietea fruticosae class in Apulia (Italy). Biologia 2014, 69, 53–69. [Google Scholar] [CrossRef]
- Tomaselli, V.; Sciandrello, S. Contribution to the knowledge of the coastal vegetation of the Zone Umide dellaCapitanata (Apulia, Italy). Plant Biosyst. 2017, 151, 673–694. [Google Scholar] [CrossRef]
- Veronico, G.; Sciandrello, S.; Medagli, P.; Tomaselli, V. Vegetation survey and plant landscape mapping of the SCI IT9140002 “LitoraleBrindisino” (Puglia, Southern Italy). Plant Sociol. 2017, 54, 89–106. [Google Scholar]
- Tomaselli, V.; Terzi, M. Rocky coastal vegetation of the class Crithmo-Staticetea in the south-east of Italy. Acta Bot. Croat. 2019, 78, 46–56. [Google Scholar] [CrossRef]
- Tomaselli, V.; Veronico, G.; Sciandrello, S.; Forte, L. Therophytic halophilous vegetation classification in South-Eastern Italy. Phytocoenologia 2020, 50, 187–209. [Google Scholar] [CrossRef]
- Margiotta, B.; Colaprico, G.; Urbano, M.; Veronico, G.; Tommasi, F.; Tomaselli, V. Halophile wheatgrass Thinopyrum elongatum (Host) D.R. Dewey (Poaceae) in three Apulian coastal wetlands: Vegetation survey and genetic diversity. Plant Biosyst. 2020, 156, 1–15. [Google Scholar] [CrossRef]
- Brullo, S.; Giusso Del Galdo, G.; Siracusa, G.; Spampinato, G. Considerazioni fitogeografiche sulla vegetazione psammofila dei litorali italiani. Biogeographia 2001, 22, 93–137. [Google Scholar]
- Perrino, E.V.; Wagensommer, R.P. Aggiornamenti floristici per il Gargano (Puglia) con riferimento agli habitat della Direttiva 92/43/EEC. Inform. Bot. Ital. 2012, 44, 163–170. [Google Scholar]
- Perrino, E.V.; Tomaselli, V.; Costa, R.; Pavone, P. Conservation status of habitats (Directive 92/43 EEC) of coastal and low hill belts in a Mediterranean biodiversity hot spot (Gargano-Italy). Plant Biosyst. 2013, 147, 1006–1028. [Google Scholar] [CrossRef]
- Perrino, E.V.; Wagensommer, R.P.; Silletti, G.N.; Signorile, G.; Angiulli, F. Nuovi dati distributivi e relazione con la Direttiva 92/43/CEE di taxa critici pugliesi dalla Provincia di Bari. Inform. Bot. Ital. 2013, 45, 53–62. [Google Scholar]
- Tomaselli, V.; Tenerelli, P.; Sciandrello, S. Mapping and quantifying habitat fragmentation in small coastal areas: A case study of three protected wetlands in Apulia (Italy). Environ. Monit. Assess. 2012, 184, 693–713. [Google Scholar] [CrossRef]
- Adamo, M.; Tarantino, C.; Tomaselli, V.; Veronico, G.; Nagendra, H.; Blonda, P. Habitat mapping of coastal wetlands using expert knowledge and Earth observation data. J. Appl. Ecol. 2016, 53, 1521–1532. [Google Scholar] [CrossRef]
- Gavish, Y.; O’Connell, J.; Marsh, C.J.; Tarantino, C.; Blonda, P.; Tomaselli, V.; Kunin, W.E. Comparing the performance of flat and hierarchical habitat/land-cover classification models in a NATURA 2000 site. ISPRS J. Photogramm. Remote Sens. 2018, 136, 1–12. [Google Scholar] [CrossRef]
- Palombi, D. Erbario Salentino; Edizioni del Grifo: Lecce, Italy, 2005; p. 527. [Google Scholar]
- Accogli, R.; Mele, C.; Minonne, F.; Medagli, P.; Marchiori, S. Utilizzo delle piante spontanee mangerecce nel Salento. 2° Convegno Nazionale “Piante Mediterranee”. Atti Convegno 2007, I, 520–528. [Google Scholar]
- Leporatti, M.L.; Guarrera, P.M. Ethnobotanical remarks in Capitanata and Salento areas (Puglia Southern Italy). Etnobiología 2005, 5, 51–64. [Google Scholar]
- Accogli, R.; Marchiori, S. Ricerche etnobotaniche nell’agro di Fasano (Brindisi Puglia). In Proceedings of the IV Convegno Nazionale “Piante Mediterranee. Le Potenzialità del Territorio e dell’Ambiente”, Marina di Nova Siri (MT), Italy, 7–10 October 2009; p. 165. [Google Scholar]
- Accogli, R.; Medagli, P.; Marchiori, S. Conservazione della biodiversità vegetale: Indagini e reperimento di ancestori spontanei. Ital. J. Agric. 2009, 4, 225–230. [Google Scholar]
- Accogli, R.; Medagli, P. Erbe Spontanee Salentine. Guida al Riconoscimento e all’uso Delle Piante Alimentari Tradizionali; Edizioni Grifo: Lecce, Italy, 2014; p. 198. ISBN 9788898175642. [Google Scholar]
- Biscotti, N.; Pieroni, A. The hidden Mediterranean diet: Wild vegetables traditionally gathered and consumed in the Gargano area, Apulia, SE Italy. Acta Soc. Bot. Pol. 2015, 84, 327–338. [Google Scholar] [CrossRef]
- Biscotti, N.; Bonsanto, D.; Del Viscio, G. The traditional food use of wild vegetables in Apulia (Italy) in the light of Italian ethnobotanical literature. Ital. Bot. 2018, 5, 1–24. [Google Scholar] [CrossRef]
- Nardone, D.; Ditonno, N.; Lamusta, S. Fave e Favelle. Piante della Puglia Peninsulare Nelle voci Dialettali in Uso e di Tradizione. Aforismi. Modi di Dire. Farmaci. Cosmetici; Centro Studi Salentini: Lecce, Italy, 2012; p. 587. [Google Scholar]
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Albano, A.; Alessandrini, A.; Ardenghi, N.; Astuti, G.; Bacchetta, G.; Ballelli, S.; Banfi, E.; et al. An updated checklist of the vascular flora native to Italy. Plant Bios. 2018, 152, 179–303. [Google Scholar] [CrossRef]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora D’italia 2017–2019, 2nd ed.; Edagricole–Edizioni Agricole di NewBusiness Media: Bologna, Italy, 2019; Volume 1–4. [Google Scholar]
- The Angiosperm Phylogeny Group; Chase, M.W.; Christenhusz, M.J.M.; Fay, M.F.; Byng, J.W.; Judd, W.S.; Soltis, D.E.; Mabberley, D.J.; Sennikov, A.N.; Soltis, P.S.; et al. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- Kadereit, G.; Borsch, T.; Weising, K.; Freitag, H. Phylogeny of Amaranthaceae and Chenopodiaceae and the evolution of C4 photosynthesis. Intern. J. Plant Sci. 2003, 164, 959–986. [Google Scholar] [CrossRef]
- Rufo, L.; Fuente, V.; Sánchez-Mata, D. Sarcocornia plant communities of the Iberian Peninsula and the Balearic Islands. Phytocoenologia 2016, 46, 383–396. [Google Scholar] [CrossRef]
- Piirainen, M.; Liebisch, O.; Kadereit, G. Phylogeny, biogeography, systematics and taxonomy of Salicornioideae (Amaranthaceae/Chenopodiaceae)—A cosmopolitan, highlyspecializedhygrohalophyte lineage dating back to the Oligocene. Taxon 2017, 66, 109–132. [Google Scholar] [CrossRef]
- Kadereit, G.; Piirainen, M.; Lambinon, J.; Vanderpoorten, A. Cryptic taxa should have names: Reflections in the glasswort genus Salicornia (Amaranthaceae). Taxon 2012, 61, 1227–1239. [Google Scholar] [CrossRef]
- De La Fuente, V.; Rufo, L.; Rodríguez, N.; Sánchez-Mata, D.; Franco, A.; Amils, R. A study of Sarcocornia A.J. Scott (Chenopodiaceae) from Western Mediterranean Europe. Plant Biosyst. 2016, 150, 343–356. [Google Scholar] [CrossRef]
- Patel, S. Salicornia: Evaluating the halophytic extremophile as a food and a pharmaceutical candidate. Biotech 2016, 6, 104. [Google Scholar] [CrossRef]
- Barreira, L.; Resek, E.; Rodrigues, M.J.; Rocha, M.I.; Pereira, H.; Bandarra, N.; Moreira da Silva, M.; Varela, J.; Custódio, L. Halophytes: Gourmet food with nutritional health benefits? J. Food Comp. Anal. 2017, 59, 35–42. [Google Scholar] [CrossRef]
- Chevalier, A. Les Salicornes et leuremploi dans l’alimentation: Etude historique, botanique, economique. Jour. D’agric. Trad. Bot. Appl. 1922, 2, 697–785. [Google Scholar]
- Kim, S.M. Quality Characteristics of Low-Salt Kimchi with Salt Replaced by Salicornia herbacea L. Powder. J. Korean Soc. Food Cult. 2013, 28, 674–683. [Google Scholar] [CrossRef]
- Ventura, Y.; Sagi, M. Halophyte crop cultivation: The case for Salicornia and Sarcocornia. Environ. Exp. Bot. 2013, 92, 144–153. [Google Scholar] [CrossRef]
- Gunning, D. Cultivating Salicornia europaea (Marsh Samphire); Daithi O’ Murchu Marine Research Station & University College Cork: Dublin, Ireland, 2016; pp. 1–50. [Google Scholar]
- Limongelli, F.; Crupi, P.; Clodoveo, M.L.; Corbo, F.; Muraglia, M. Overview of the polyphenols in Salicornia: From recovery to health-promoting effect. Molecules 2022, 27, 7954. [Google Scholar] [CrossRef]
- Sánchez-Gavilán, I.; Ramírez, E.; de la Fuente, V. Bioactive Compounds in Salicornia patula Duval-Jouve: A Mediterranean Edible Euhalophyte. Foods 2021, 10, 410. [Google Scholar] [CrossRef] [PubMed]
- Tomaselli, V.; Adamo, M.; Veronico, G.; Sciandrello, S.; Tarantino, C.; Dimopoulos, P.; Medagli, P.; Nagendra, H.; Blonda, P. Definition and application of expert knowledge on vegetation pattern, phenology and seasonality for habitat mapping, as exemplified in a Mediterranean coastal site. Plant Biosyst. 2017, 151, 887–899. [Google Scholar] [CrossRef]
- Nisar, F.; Gul, B.; Khan, M.A.; Hamed, A. Heteromorhic seed of coastal halophytes Arthrocnemum macrostachyum and indicum display differential patterns of hydrogen peroxide accumulation, lipid peroxidation and antioxidat activities under increasing salinity. Plant PhysiolBiochem 2019, 144, 58–63. [Google Scholar] [CrossRef]
- Antunes, M.D.; Gago, C.; Guerreiro, A.; Sousa, A.R.; Julião, M.; Miguel, M.G.; Faleiro, M.L.; Panagopoulos, T. Nutritional Characterization and Storage Ability of Salicornia ramosissima and Sarcocornia perennis for Fresh Vegetable Salads. Horticulturae 2021, 7, 6. [Google Scholar] [CrossRef]
- Castagna, A.; Mariottini, G.; Gabriele, M.; Longo, V.; Souid, A.; Dauvergne, X.; Foggi, G.; Conte, G.; Santin, M.; Ranieri, A. Nutritional composition and bioactivity of Salicornia europaea L. plants grown in monocolture or intercropped with tomato plants in salt-affected soils. Horticulturae 2022, 8, 828. [Google Scholar] [CrossRef]
- Navarro-Torre, S.; Mateos-Naranjo, E.; Caviedes, M.A.; Pajuelo, E.; Rodríguez-Llorente, I.D. Isolation of plant-growth-promoting and metal-resistant cultivable bacteria from Arthrocnemum macrostachyum in the Odiel marshes with potential use in phytoremediation. Mar. Pollut. Bull. 2016, 110, 133–142. [Google Scholar] [CrossRef]
- El-Naker, N.A.; Yousef, A.F.; Yousef, L.F. A review of Arthrocnemum (Arthrocaulon) macrostachyum chemical content and bioactivity. Phytochem. Rev. 2020, 19, 1427–1448. [Google Scholar] [CrossRef]
- Hanif, Z.; Hafiz Haider, A.; Ghulam, R.; Asif, T.; Bhagirath Singh, C. Genus Salsola: Its Benefits, Uses, Environmental Perspectives and Future Aspects—A Review. J. Rangel. Sci. 2018, 8, 315–328. [Google Scholar]
- Renna, M.; Gonnella, M. Ethnobotany, Nutritional Traits, and Healthy Properties of Some Halophytes Used as Greens in the Mediterranean Basin. In Handbook of Halophytes; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Hammer, K. Chenopodiaceae. In J. Schultze-Motel, Rudolf MansfeldsVerzeichnislandwirtschaftlicher und gartnenscherKulturpflanzen (ohneZierpflanzen); Akademie-Verl.: Berlin, Germany, 1986; pp. 145–170. [Google Scholar]
- Hammer, K.; Pignone, D.; Cifarelli, S.; Perrino, P. Barilla (Salsola soda, Chenopodiaceae). Econ. Bot. 1990, 44, 410–412. Available online: http://www.jstor.org/stable/4255259 (accessed on 25 November 2022). [CrossRef]
- Tesi, R. Salsola. In Enciclopedia Agraria Italiana; REDA: Roma, Italy, 1980; p. 1008. [Google Scholar]
- Centofanti, T.; Bañuelos, G. Evaluation of the halophyte Salsola soda as an alternative crop for saline soils high in selenium and boron. J Environ. Manag. 2015, 157, 96–102. [Google Scholar] [CrossRef]
- Bisceglia, V. Sulle Piante Utili; Marelli: Milano, Italy, 1807. [Google Scholar]
- Colla, G.; Rouphael, Y.; Fallovo, C.; Cardarelli, M.; Graifenberg, A. Use of Salsola soda as a companion plant to improve greenhouse pepper (Capsicum annuum) under saline conditions. N. Zeal. J. Crop hortic. Sci. 2006, 34, 283–290. [Google Scholar] [CrossRef]
- Debez, A.; Braun, H.P.; Pich, A.; Taamalli, W.; Koyro, H.W.; Abdelly, C.; Huchzermeyer, B. Proteomic and physiological response of the halophyte Cakile maritima to moderate salinity at the germinative and vegetative stages. J. Protemics 2012, 75, 5667–5694. [Google Scholar] [CrossRef]
- Augedelo, A.; Carvajal, M.; Martinez-Ballesta, M.d.C. Halophytes of the Mediterranean Basin-Underutilized Species with the Potential to Be Nutritions Crops in the Scenario of the Climate Change. Foods 2021, 10, 119. [Google Scholar] [CrossRef]
- Gandour, M.; Tamaalli, W.; Trabelsi, N.; Hessini, K.; Sebei, K.; Debez, A.; Abdelly, C. How to optimize the seed and seed-oil production in the cash crop halophyte Cakile maritima? J. Med. Plants Res. 2011, 5, 5982–5987. [Google Scholar] [CrossRef]
- Arbelet-Bonnin, D.; Ben-Hamed-Louati, I.; Laurenti, P.; Abdelly, C.; Ben-Hame, K.; Bouteau, F. Cakile maritima, a promising model for halophyte studies and a putative cash crop for saline agriculture. Adv. Agric. 2019, 155, 45–78. [Google Scholar] [CrossRef]
- Farhat, N.; Hichri, S.T.M.; Hildebrandt, A.; Debez, A.; Braun, H.P. Composition and Stability of the Oxidative Phosphorylation System in the Halophile Plant Cakile maritima. Front. Plant Sci. 2019, 10, 1010. [Google Scholar] [CrossRef]
- Fuochi, V.; Barbagallo, I.I.; Distefano, A.; Puglisi, F.; Palmieri, R.; Di Rosa, M.; Giallongo, C.; Longhitano, L.; Fontana, P.; Sferrazzo, G.; et al. Biological properties of Cakile maritima Scop. (Brassicaceae) extracts. Eur. Rev. Med. L Pharm. Sci. 2019, 23, 2280–2292. [Google Scholar] [CrossRef]
- Ksouri, R.; Megdiche, W.; Debez, A.; Falleh, H.; Grignon, C.; Abdelly, C. Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol. Biochem. 2007, 45, 244–249. [Google Scholar] [CrossRef]
- Meot-Duros, L.; Le Floch, G.; Magné, C. Radical scavenging, antioxidant and antimicrobial activities of halophytic species. J. Ethnopharmacol. 2008, 116, 258–262. [Google Scholar] [CrossRef]
- Shiri, M.; Rabbi, M.; El Amrani, A.; Abdelly, A. The Halophyte Cakile maritima Reduces Phenanthrene Phytotoxicity. J. Phytorimediation 2015, 17, 925–928. [Google Scholar] [CrossRef]
- Atia, S.; Barhoumi, Z.; Mokded, R.; Abdelly, C.; Smaoui, A. Environmental eco-physiology and economical potential of the halophyte Crithmum maritimum L. (Apiaceae). J. Med. Plants Res. 2011, 5, 3564–3571. [Google Scholar] [CrossRef]
- Renna, M.; Gonnella, M.; Caretto, S.; Mita, G.; Serio, F. Sea fennel (Crithmum maritimum L.): From underutilized crop to new dried product for food use. Genet. Resour. Crop Evol. 2017, 64, 205–216. [Google Scholar] [CrossRef]
- Franke, W. Vitamin C in sea fennel (Crithmum maritimum), an edible wild plant. Econ. Bot. 1982, 36, 163–165. [Google Scholar] [CrossRef]
- Renna, M.; Gonnella, M. The use of the sea fennel as a new spice-colorant in culinary preparations. Int. J. Gastron. Food Sci. 2012, 1, 111–115. [Google Scholar] [CrossRef]
- Atia, A.; Ben, H.K.; Debez, A.; Abdelly, C. Salt and seawater effects on the germination of Crithmum maritimum. In Biosaline Agriculture and Salinity Tolerance in Plants; Öztürk, M., Waisel, Y., Khan, M.A., Görk, G., Eds.; Birkhäuser Verlag: Basel, Switzerland, 2006; pp. 29–33. [Google Scholar]
- Atia, A.; Debez, A.; Barhoumi, Z.; Smaoui, A.; Abdelly, C. Interactive effects of salinity, nitrate, light, and seed weight on the germination of the halophyte Crithmum maritimum. Acta Biol. Hung 2009, 60, 433–439. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Maloupa, E. Micropropagation and salt tolerance of in vitro grown Crithmum maritimum L. Plant Cell Tiss. Organ. Cult. 2008, 94, 209–217. [Google Scholar] [CrossRef]
- Martini, A.N.; Papafotiou, M.; Evangelopoulos, K. Effect of substrate type and depth on the establishment of the edible and medicinal native species Crithmum maritimum on an extensive urban Mediterranean green roof. Acta Hortic. 2017, 1189, 451–454. [Google Scholar] [CrossRef]
- Nektarios, P.A.; Nydrioti, E.; Kapsali, T.; Ntoulas, N. Crithmum maritimum growth in extensive green roof systems with different substrate type, depth and irrigation regime. Acta Hortic. 2016, 1108, 303–308. [Google Scholar] [CrossRef]
- Zenobi, S.; Fiorentini, M.; Zitti, S.; Aquilanti, L.; Foligni, R.; Mannozzi, C.; Mozzon, M.; Orsini, R. Crithmum maritimum L.: First results on phenological development and biomass production in Mediterranean areas. Agronomy 2021, 11, 773. [Google Scholar] [CrossRef]
- Pereira, C.G.; Barreira, L.; da Rosa Neng, N.; Nogueira, J.M.F.; Marques, C.; Santos, T.; Varela, J.; Custodio, L. Searching for new sources of innovative products for the food industry within halophyte aromatic plants: In vitro antioxidant activity and phenolic and mineral contents of infusions and decoctions of Crithmum maritimum L. Food Chem. Toxicol. 2017, 107, 581–589. [Google Scholar] [CrossRef]
- Pereira, A.G.; Fraga-Corral, M.; García-Oliveira, P.; Jimenez-Lopez, C.; Lourenço-Lopes, C.; Carpena, M.; Otero, P.; Gullón, P.; Prieto, M.A.; Simal-Gandara, J. Culinary and nutritional value of edible wild plants from northern Spain rich in phenolic compounds with potential health benefits. Food Funct. 2020, 11, 8493. [Google Scholar] [CrossRef]
- Atia, A.; Debez, A.; Barhoumi, Z.; Abdelly, C.; Smaoui, A. Histochemical localization of essential oils and bioactive substances in the seed coat of the halophyte Crithmum maritimum L. (Apiaceae). J. Plant Biol. 2009, 52, 448–452. [Google Scholar] [CrossRef]
- Cornara, L.; D’Arrigo, C.; Pioli, F.; Borghesi, B.; Bottino, C.; Patrone, E.; Mariotti, M.G. Micromorphological investigation on the leaves of the rock samphire (Crithmum maritimum L.): Occurrence of hesperidin and diosmin crystals. Plant Biosyst. 2009, 143, 283–292. [Google Scholar] [CrossRef]
- Meot-Duros, L.; Magné, C. Antioxidant activity and phenol content of Crithmum maritimum L. leaves. Plant Physiol. Biochem. 2009, 47, 37–41. [Google Scholar] [CrossRef]
- Meot-Duros, L.; Cérantola, S.; Talarmin, H. New antibacterial and cytotoxic activities of falcarindiol isolated in Crithmum maritimum L. leaf extract. Food Chem. Toxicol. 2010, 48, 553–557. [Google Scholar] [CrossRef]
- Generalić Mekinić, I.; Blažević, I.; Mudnić, I.; Burčul, F.; Grga, M.; Skroza, D.; Jerčić, I.; Ljubenkov, I.; Boban, M.; Miloš, M.; et al. Sea fennel (Crithmum maritimum L.): Phytochemical profile, antioxidative, cholinesterase inhibitory and vasodilatory activity. J. Food Sci. Technol. 2016, 53, 3104–3112. [Google Scholar] [CrossRef]
- Gil, L.; Pinya, S.; Tejada, S.; Capó, X.; Sureda, A. Antioxidant Defenses in Wild Growing Halophyte Crithmum maritimum from Inland and Coastline Populations. Chem. Biodiv. 2019, 16, e1800448. [Google Scholar] [CrossRef]
- Jallali, I.; Zaouali, Y.; Missaoui, I.; Smeoui, A.; Abdelly, C.; Ksouri, R. Variability of antioxidant and antibacterial effects of essential oils and acetonic extracts of two edible halophytes: Crithmum maritimum L. and Inula crithmoïdes L. Food Chem. 2014, 145, 1031–1038. [Google Scholar] [CrossRef]
- Gnocchi, D.; Cesari, G.; Calabrese, G.J.; Capone, R.; Sabbà, C.; Mazzocca, A. Inhibition of Hepatocellular Carcinoma Growth by Ethyl Acetate Extracts of Apulian Brassica oleracea L. and Crithmum maritimum L. Plant Foods Hum. Nutr. 2020, 75, 33–40. [Google Scholar] [CrossRef]
- D’Agostino, G.; Giambra, B.; Palla, F.; Bruno, M.; Badalamenti, N. The Application of the Essential Oils of Thymus vulgaris L. and Crithmum maritimum L. as Biocidal on Two TholuBommalu Indian Leather Puppets. Plants 2021, 10, 1508. [Google Scholar] [CrossRef]
- Renna, M. Reviewing the Prospects of Sea Fennel (Crithmum maritimum L.) as Emerging Vegetable Crop. Plants 2018, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Azeñas, V.; Janner, I.; Medrano, H.; Gulías, J. Performance evaluation of five Mediterranean species to optimize ecosystem services of green roofs under water-limited conditions. J. Environ. Manag. 2018, 212, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, A.A.; Assimakopoulou, A.; Panagopoulos, P.; Bakea, M.; Vidalis, N.; Karapanos, I.C.; Petropoulos, S.A. Impact of Salinity on the Growth and Chemical Composition of Two Underutilized Wild Edible Greens: Taraxacum officinale and Reichardia picroides. Horticulturae 2021, 7, 160. [Google Scholar] [CrossRef]
- Guarrera, M.P.; Savo, V. Wild food plants used in traditional vegetable mixtures in Italy. J. Ethnopharmacol. 2016, 185, 202–234. [Google Scholar] [CrossRef] [PubMed]
- Ranfa, A.; Bodesmo, M. An Ethnobotanical investigation of traditional knowledge and uses of edible wild plants in the Umbria Region, Central Italy. J. Appl. Bot. Food Qual. 2017, 90, 246–258. [Google Scholar] [CrossRef]
- Tittel, C. Compositae. In Rudolf Mansfeld Verzeichnis landwirtschaftelicher und gartnerishcer Kulturpflanzed (ohne Zierpflanzen); Schultze-Motel, J., Ed.; Akademie-Verlag: Berlin, Germany, 1986; pp. 1261–1335. [Google Scholar]
- Maggini, R.; Benvenuti, S.; Leoni, F.; Incrocci, L.; Pardossi, A. Effects of NaCl on hydroponic cultivation of Reichardia picroides (L.) roth. Agronomy 2021, 11, 2352. [Google Scholar] [CrossRef]
- Pieroni, A. Medicinal plants and food medicines in the folk traditions of the upper Lucca 471 Province, Italy. J. Ethnopharmacol. 2000, 70, 235–273. [Google Scholar] [CrossRef]
- Loi, M.C.; Poli, F.; Sacchetti, G.; Selenu, M.B.; Ballero, M. Ethnopharmacology of Ogliastra (Villagrande Strisaili, Sardinia, Italy). Fitoterapia 2004, 75, 277–295. [Google Scholar] [CrossRef]
- Atzei, A.; Orioni, D.S.; Sotgiu, R. Contributo alla conoscenza degli usi etnobotanici nella 362 Gallura (Sardegna). Boll. Della Soc. Sarda Sci. Nat. 1991, 28, 137–177. [Google Scholar]
- Aouachria, S.; Boumerfeg, S.; Benslama, A.; Benbacha, F.; Guemmez, T.; Khennouf, S.; Arrar, L.; Baghiani, A. Acute, sub-acutetoxicity and antioxidant activities (in vitro and in vivo) of Reichardia picroides crude estract. J. Ethnopharmacol. 2017, 208, 105–116. [Google Scholar] [CrossRef]
- Fragopulou, E.; Detopoulou, P.; Nomikos, T.; Pliakis, E.; Panagiotakos, D.B.; Antonopoulou, S. Mediterranean wild plants reduce prostprandial platelet aggregation in patients with metabolic syndrome. Metabolism 2012, 61, 325–334. [Google Scholar] [CrossRef]
- Guarrera, P.M.; Salerno, G.; Caneva, G. Food, Flavouring and feed plant traditions in the Tyrrhenian sector of Basilicata, Italy. J Ethnobiol. Ethnomed. 2006, 2, 37. [Google Scholar] [CrossRef]
- Sebasky, M.; Keller, S.R.; Taylor, D.R. Investigating past range dynamics for a weed of cultivation Silene vulgaris. Ecol. Evol. 2016, 6, 4800–4811. [Google Scholar] [CrossRef]
- Taffetani, F. Rugni, Speragne e Crispigne. Piante Spontanee negli usi del Territorio maceratese. In Fondazione Cassa di Risparmio della Provincia di Macerata. Carime Arte srl; Pigini Group: Loreto, Italy, 2005. [Google Scholar]
- Arcidiacono, S.; Napoli, M.; Oddo, G.; Pavone, P. Piante selvatiche d’uso popolare nei territori di Alcara li Fusi e Militello Rosmarino (Messina, N-E Sicilia). Quad. Bot. Amb. 2007, 18, 105–146. [Google Scholar]
- Zampiva, F. Le erbe nella cucina popolare veneta. Erbor. Domani 1983, 4, 60–69. [Google Scholar]
- Laghetti, G.; Hammer, K.; Perrino, P. Collecting in north-west Italy. FAO/IBPGR Newsl. 1993, 91/92, 23. [Google Scholar]
- Laghetti, G.; Perrino, P.; Hammer, K. Utilization of Silene vulgaris (Moench) Garcke in Italy. Econ. Bot. 1994, 48, 337–339. [Google Scholar] [CrossRef]
- Hammer, K.; Kniipffer, H.; Laghetti, G.; Perrino, P. Seeds from the Past: A Catalogue of the Crop Germplasm of South Italy and Sicily; Germplasm Institute: Bari, Italy, 1992; p. 174.
- Pacwa-Płociniczak, M.; Płociniczak, T.; Yu, D.; Kurola, J.M.; Sinkkonen, A.; Piotrowska-Seget, Z.; Romantschuk, M. Effect of Silene vulgaris and Heavy Metal Pollution on Soil Microbial Diversity in Long-Term Contaminated Soil. Water Air Soil Pollut. 2018, 229, 13. [Google Scholar] [CrossRef]
- Antonidiadis, V.; Shaheen, S.M.; Stark, H.J.; Wennrich, R.; Levizou, E.; Merbach, I.; Rinklebe, J. Phytoremediation potential of twelve wild plants species for toxic elements in a contaminated soil. Environ Int. 2021, 146, 106233. [Google Scholar] [CrossRef]
- Tison, J.M.; Jauzein, P.; Michaud, H. Flore de France Méditerranéenne Continentale; Naturalia Publications: Turriers, Italy, 2014. [Google Scholar]
- GRIN. Germplasm Resources Information Network—(GRIN) [Online Database]. National Germplasm Resources Laboratory, Beltsville, Maryland. 2010. Available online: http://www.ars-rin.gov/cgibin/npgs/html/index.pl (accessed on 30 November 2022).
- Véla, E.; Kell, S.P. Allium commutatum. IUCN Red List Threat. Species 2018, e.T172281A19174707. [Google Scholar]
- Geraci, A.; Amato, F.; Di Noto, G.; Bazan, G.; Schicchi, R. The wild taxa utilized as vegetables in Sicily (Italy): A traditional component of the Mediterranean diet. J. Ethnob. Ethnomed. 2018, 14, 14. [Google Scholar] [CrossRef]
- Mathew, B. A review of Allium section Allium; Royal Botanic Gardens, Kew: Richmond, UK, 1996; ISBN 9780947643935. [Google Scholar]
- Vujević, M.; Pevalek-Kozlina, B.; Pavlica, M.; Šolić, M.E. Shoot and root regeneration from callus tissue of Allium commutatumGuss. Acta Bot. Croat. 1999, 58, 57–64. [Google Scholar]
- Loizzo, M.R.; Tundis, R.; Sut, S.; Dall’Acqua, S.; Ilardi, V.; Leporini, M.; Falco, T.; Sicari, V.; Bruno, M. High-Performance Liquid Chromatography/Electrospray Ionization Tandem Mass Spectrometry (HPLC-ESI-MSn) Analysis and Bioactivity Useful for Prevention of “Diabesity” of Allium commutatum Guss. Plant Foods Hum. Nutr. 2020, 75, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Demirtas, I.; Erenler, R.; Elmastas, M.; Goktasoglu, A. Studie on the antioxidant potential of flavones of Allium vineale isolated from its water-soluble fraction. Food Chem. 2013, 136, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Stajner, D.; Igic, R.; Popovic, B.M.; Malencic, D.j. Comparative study of antioxidant properties of wild growing and cultivated Allium species. Phytother. Res. 2007, 22, 113–117. [Google Scholar] [CrossRef]
- Leys, M.; Petit, E.J.; El-Bahloul, Y.; Liso, C.; Fournet, S.; Arnaud, J.F. Spatial genetic structure in Beta vulgaris subsp. maritima and Beta macrocarpa reveals the effect of contrasting mating system, influence of marine currents, and footprints of postglacial recolonization routes. Ecol. Evol. 2014, 4, 1828–1852. [Google Scholar] [CrossRef]
- Bianco, V.; Mariani, R.; Santamaria, P. Piante Spontanee Nella Cucina Tradizionale Molese; Levante Ed: Bari, Italy, 2009; pp. 84–89. [Google Scholar]
- Lentini, F.; Venza, F. Wild food plants of popular use in Sicily. J. Ethnob. Ethnomed. 2007, 3, 15. [Google Scholar] [CrossRef]
- Goldman, I.L.; Janick, J. Evolution of root morphology in table beet: Historical and iconographic. Front. Plant. Sci. 2021, 12, 689926. [Google Scholar] [CrossRef]
- Perrino, E.V.; Wagensommer, R.P. Crop Wild Relatives (CWR) Priority in Italy: Distribution, Ecology, In Situ and Ex Situ Conservation and Expected Actions. Sustainability 2021, 13, 1682. [Google Scholar] [CrossRef]
- Perrino, E.V.; Perrino, P. Crop wild relatives: Know how past and present to improve future research, conservation and utilization strategies, especially in Italy: A review. Genet. Resour. Crop Evol. 2020, 67, 1067–1105. [Google Scholar] [CrossRef]
- Yolcu, S.; Alavilli, H.; Ganesh, P.; Panigrahy, M.; Song, K. Salt and drought stress responses in cultivated beets (Beta vulgaris (L.) andwild beet (Beta maritima L.). Plants 2021, 10, 1843. [Google Scholar] [CrossRef]
- Zardi-Bergaoui, A.; Nejma, A.B.; Harzallah-Skhiri, F.; Flamini, G.; Ascrizzi, R.; Jannet, H.B. Chemical composition and biological studies of the essential oil from aerial parts of Beta vulgaris subsp. maritima (L.) Arcang. Growing in Tunisia. Chem Biodivers. 2017, 14, e1700234. [Google Scholar] [CrossRef]
- Inocencio, C.; Alcaraz, F.; Calderon, F.; Obon, C.; Rivera, D. The use of floral characters in Capparis sect. Capparis to determine the botanical and geographical origin of capers. Eur. Food Res. Technol. 2022, 214, 335–339. [Google Scholar] [CrossRef]
- Chedraoui, S.; Abi-Rizk, A.; EI-Beyrouthy, M.; Chalak, L.; Ouaini, N.; Rajjou, L. Capparis spinosa L. in a systematic review: A xerophilous species of multi values and promising potentialities. Front. Plant Sci. 2017, 8, 1845. [Google Scholar] [CrossRef]
- Ashraf, U.; Chaudhry, M.N.; Ahmad, S.R.; Ashraf, I.; Arslan, M.; Noor, H.; Jabbar, M. Impacts of climate change on Capparis spinosa L. based on ecological niche modeling. PeerJ 2018, 6, e5792. [Google Scholar] [CrossRef]
- Barbera, G.; Di Lorenzo, R. The caper culture in Italy. Acta Hortic. 1984, 144, 167–172. [Google Scholar] [CrossRef]
- Barbera, G.; Di Lorenzo, R.; Barone, E. Observations on Capparis populations cultivated in Sicily and on their vegetative and productive behavior. Agric. Mediterr. 1991, 121, 32–39. [Google Scholar]
- Sottile, F.; Caltagirone, C.; Peano, C.; Del Signore, M.B.; Barone, E. Can the Caper (Capparis spinosa L.) Still Be Considered a Difficult-to-Propagate Crop? Horticulturae 2021, 7, 316. [Google Scholar] [CrossRef]
- La Bella, S.; Rossini, F.; Licata, M.; Virga, G.; Ruggeri, R.; Iacuzzi, N.; Leto, C.; Tuttolomondo, T. Four-Year Study on the Bio-Agronomic Response of Biotypes of Capparis spinosa L. on the Island of Linosa (Italy). Agriculture 2021, 11, 327. [Google Scholar] [CrossRef]
- Laghetti, G.; Hammer, K.; Perrino, P. Plant genetic resources in Pantelleria and Pelagie archipelago, Italy: Collecting and conservation of local crop germplasm. FAO/IBPGR Plant Gen. Res. Newsl. 1996, 108, 17–25. [Google Scholar]
- Gan, L.; Zhang, C.; Yin, Y.; Lin, Z.; Huang, Y.; Xiang, J.; Fu, C.H.; Li, M.T. Anatomical adaptation of the xerophilus medicinal plant, Capparis spinosa, to drougth conditions. Hortic. Environ. Biotechnol. 2013, 54, 156–161. [Google Scholar] [CrossRef]
- Santo, A.; Dessì, L.; Ucchesu, M.; Bou Dagher Kharrat, M.; Charbel Sark, R.; Accogli, R.; Buhagiar, J.; Kyratsis, A.; Fournakaru, C.; Bacchetta, G. Seed germination ecology and salt stress response in eight Mediterranean populations of Sarcopoterium spinosum (L.) Spach. Plant Species Biol. 2019, 34, 110–121. [Google Scholar] [CrossRef]
- Annaz, H.; Sane, Y.; Bitchagno, G.T.M.; Ben Bakrim, W.; Drissi, B.; Mahdi, I.; El Bouhssini, M.; Sobeh, M. Caper (Capparis spinosa L.): An Update Review on Its Phytochemistry, Nutritional Value, Traditional Uses and Therapeutic Potential. Front. Pharmacol. 2022, 13, 878749. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Maggi, F.; Daglia, M.; Habetmariam, S.; Rastrelli, L.; Nabavi, S.M.P. Pharmacological effects of Capparis spinosa L. Phytother. Res. 2016, 30, 1733–1744. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Karkanis, A.; Martins, N.; Ferreira, C.F.R. Edible halophytes of the Mediterranean basin: Potential candidates for novel food products. Trends Food Sci. Technol. 2018, 74, 69–84. [Google Scholar] [CrossRef]
- Vahid, H.; Rakhandeh, H.; Ghorbani, A. Antidiabetic proprierties of Capparis spinosa L. and its components. Biomed Pharmacother. 2017, 92, 293–302. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Z.F. Phytochemical and Pharmacological Properties of Capparis spinosa as a Medicinal Plant. Nutrients 2018, 10, 116. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, Y.; Gao, W.; Jiang, B.; Shi, L. Capparis spinosa Alleviates DSS-Induced Ulcerative Colitis via Regulation of the Gut Microbiota and Oxidative Stress. Evid. Based Complement. Altern. Med. 2021, 15, 1227876. [Google Scholar] [CrossRef]
- Rahnavard, R.; Razavi, N. A review on the medical effects of Capparis spinosa L. Adv. Herb. Med. 2016, 2, 44–53. [Google Scholar]
- Saleem, H.; Khurshid, U.; Sarfraz, M.; Ahmad, I.; Alamri, A.; Anwar, S.; Alamri, A.S.; Locatelli, M.; Tartaglia, A.; Mahomoodally, M.F.; et al. Investigation into the biological properties, secondary metabolites composition, and toxicity of aerial and root parts of Capparis spinosa L.: An important medicinal food plant. Food Chem. Toxicol. 2021, 155, 112404. [Google Scholar] [CrossRef]
- Kdimy, A.; El Yadini, M.; Guaadaoui, A.; Bourais, I.; El Hajjaji, S.; Le, H.V. Phytochemistry, Biological Activities, Therapeutic Potential, and Socio-Economic Value of the Caper Bush (Capparis Spinosa L.). Chem. Biod. 2022, 19, e202200300. [Google Scholar] [CrossRef]
- Faran, M. Capparis spinosa-The Plant on the Wall. In Medicinal and Aromatic Plants of the Middle-East. Medicinal and Aromatic Plants of the World; Yaniv, Z., Dudai, N., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 2. [Google Scholar]
- Stinca, A.; Chianese, G.; D’Auria, G.; Del Guacchio, E.; Fascetti, S.; Perrino, E.V.; Rosati, L.; Salerno, G.; Santangelo, A. New alien vascular species for the flora of southern Italy. Webbia 2017, 72, 295–301. [Google Scholar] [CrossRef]
- Gibbs, J.P. Wetlands loss and biodiversity conservation. Cons. Biol. 2000, 14, 314–317. [Google Scholar] [CrossRef]
- van der Maarel, E. Some remarks on the functions of European coastal ecosystems. Phytocoenologia 2003, 33, 187–202. [Google Scholar] [CrossRef]
- Underwood, E.C.; Viers, J.H.; Klausmeyer, K.R.; Cox, R.L.; Shaw, M.R. Threats and biodiversity in the Mediterranean biome. Divers. Distrib. 2009, 15, 188–197. [Google Scholar] [CrossRef]
- Tomaselli, V.; Veronico, G.; Adamo, M. Monitoring and recording changes in natural landscapes: A case study from two coastal wetlands in SE Italy. Land 2021, 10, 50. [Google Scholar] [CrossRef]
- Maes, J.; Fabrega, N.; Zulian, G.; Barbosa, A.; Vizcaino, P.; Ivits, E.; Polce, C.; Vandecasteele, I.; Marí Rivero, I.; Guerra, C.; et al. Mapping and Assessment of Ecosystems and their Services. JRC Science and Policy Reports; European Commission: Brussels, Belgium, 2015.
- Liquete, C.; Piroddi, C.; Drakou, E.G.; Gurney, L.; Katsanevakis, S.; Charef, A.; Egoh, B. Current Status and Future Prospects for the Assessment of Marine and Coastal Ecosystem Services: A Systematic Review. PLoS ONE 2013, 8, e67737. [Google Scholar] [CrossRef]
- Perrino, E.V.; Wagensommer, R.P. Crop Wild Relatives (CWRs) Threatened and Endemic to Italy: Urgent Actions for Protection and Use. Biology 2022, 11, 193. [Google Scholar] [CrossRef]
- Pisani, D.; Pazienza, P.; Perrino, E.V.; Caporale, D.; De Lucia, C. The Economic Valuation of Ecosystem Services of Biodiversity Components in Protected Areas: A Review for a Framework of Analysis for the Gargano National Park. Sustainability 2021, 13, 11726. [Google Scholar] [CrossRef]
- Dimita, R.; Min Allah, S.; Luvisi, A.; Greco, D.; De Bellis, L.; Accogli, R.; Mininni, C.; Negro, C. Volatile Compounds and Total Phenolic Content of Perilla frutescens at Microgreens and Mature Stages. Horticulturae 2022, 8, 71. [Google Scholar] [CrossRef]
- Dronkers, J. Integrated Coastal Zone Management (ICZM). Available online: http://www.coastalwiki.org/wiki/Integrated_Coastal_Zone_Management_(ICZM) (accessed on 23 December 2022).
- Tomaselli, V.; Mantino, F.; Tarantino, C.; Albanese, G.; Adamo, M. Changing landscapes: Habitat monitoring and landtransformation in a long-time used Mediterranean coastal wetland. Wetl. Ecol Manag. 2022. [Google Scholar] [CrossRef]
- O’leary, J.W.; Glenn, E.P. Global distribution and potential for halophytes. In Halophytes as a Resource for Livestock and for Rehabilitation of Degraded Lands; Squires, V.R., Ayoub, A.T., Eds.; Tasks for Vegetation Science; Springer: Dordrecht, The Netherlands, 1994; Volume 32. [Google Scholar] [CrossRef]














| Taxon | Life Form | Geoelement | Protection | Use | Propagation |
|---|---|---|---|---|---|
| Salicornia patula | T scap | W-European | regional level | food/medical | generative |
| Salicornia emerici | T scap | Steno-Medit. | no | food/medical | generative |
| Salicornia dolichostachya | T scap | Steno-Medit. | no | food/medical | generative |
| Salicornia perennis | Ch succ | Euri-Medit. | regional level | food/medical | generative |
| Salicornia fruticosa | Ch succ | Euri-Medit. | no | food/medical | generative |
| Arthrocaulon macrostachyum | Ch succ/P succ | Medit. | no | food/medical | generative/vegetative |
| Soda inermis | T scap | Paleotemp. | no | food/medical/industrial | generative |
| Cakile maritima | T scap | Medit.-Atl. (Steno-) | no | food/medical | generative |
| Crithmum maritimum | Ch suffr | Euri-Medit./Steno-Medit. | regional level | food/medical | generative |
| Reichardia picroides | H scap | Steno-Medit. | no | food/medical | generative |
| Silene vulgaris subsp. tenoreana | H scap | Paleotemp./Subcosmop. | no | food/medical/phytoremediation | generative |
| Allium commutatum | G bulb | Steno-E-Medit. | LC (Least Concern) | food/medical | generative/vegetative |
| Beta vulgaris subsp. maritima | H scap | Euri-Medit. | no | food/medical | generative |
| Capparis spinosa | NP | Eurasiat. | regional level | food/medical | generative/vegetative |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Accogli, R.; Tomaselli, V.; Direnzo, P.; Perrino, E.V.; Albanese, G.; Urbano, M.; Laghetti, G. Edible Halophytes and Halo-Tolerant Species in Apulia Region (Southeastern Italy): Biogeography, Traditional Food Use and Potential Sustainable Crops. Plants 2023, 12, 549. https://doi.org/10.3390/plants12030549
Accogli R, Tomaselli V, Direnzo P, Perrino EV, Albanese G, Urbano M, Laghetti G. Edible Halophytes and Halo-Tolerant Species in Apulia Region (Southeastern Italy): Biogeography, Traditional Food Use and Potential Sustainable Crops. Plants. 2023; 12(3):549. https://doi.org/10.3390/plants12030549
Chicago/Turabian StyleAccogli, Rita, Valeria Tomaselli, Paolo Direnzo, Enrico Vito Perrino, Giuseppe Albanese, Marcella Urbano, and Gaetano Laghetti. 2023. "Edible Halophytes and Halo-Tolerant Species in Apulia Region (Southeastern Italy): Biogeography, Traditional Food Use and Potential Sustainable Crops" Plants 12, no. 3: 549. https://doi.org/10.3390/plants12030549
APA StyleAccogli, R., Tomaselli, V., Direnzo, P., Perrino, E. V., Albanese, G., Urbano, M., & Laghetti, G. (2023). Edible Halophytes and Halo-Tolerant Species in Apulia Region (Southeastern Italy): Biogeography, Traditional Food Use and Potential Sustainable Crops. Plants, 12(3), 549. https://doi.org/10.3390/plants12030549








