Toxicity and Anti-Inflammatory Activity of Phenolic-Rich Extract from Nopalea cochenillifera (Cactaceae): A Preclinical Study on the Prevention of Inflammatory Bowel Diseases
Abstract
:1. Introduction
2. Results
2.1. Physicochemical Analysis of Nopalea cochenillifera Extract
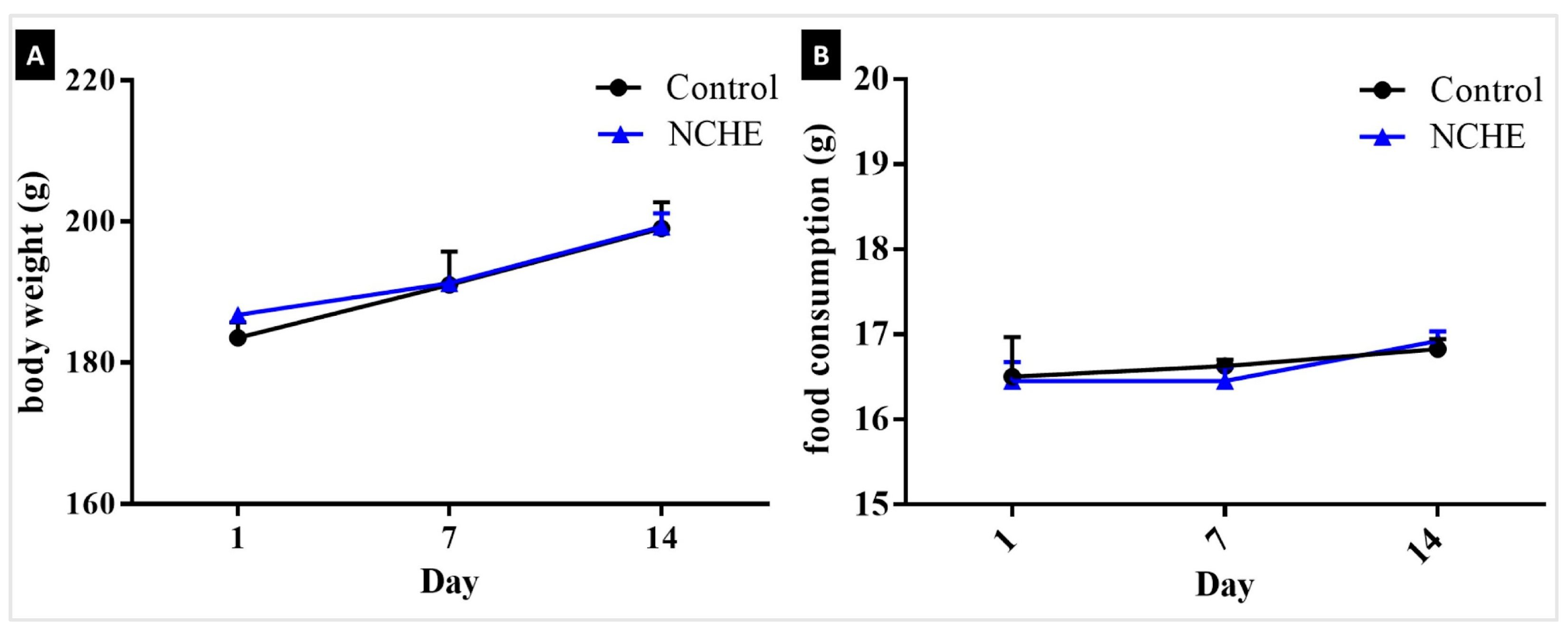
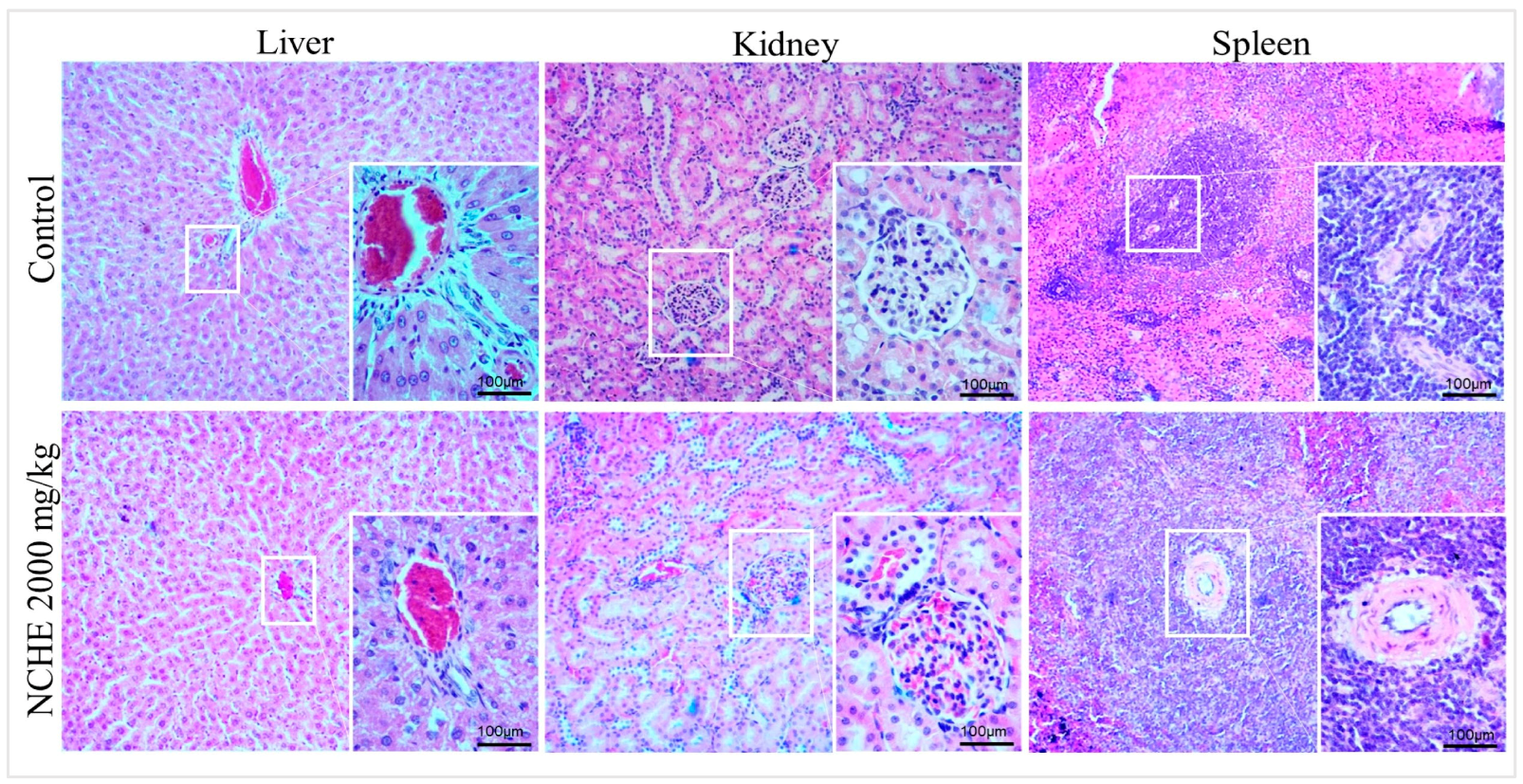
2.2. Acute Toxicity
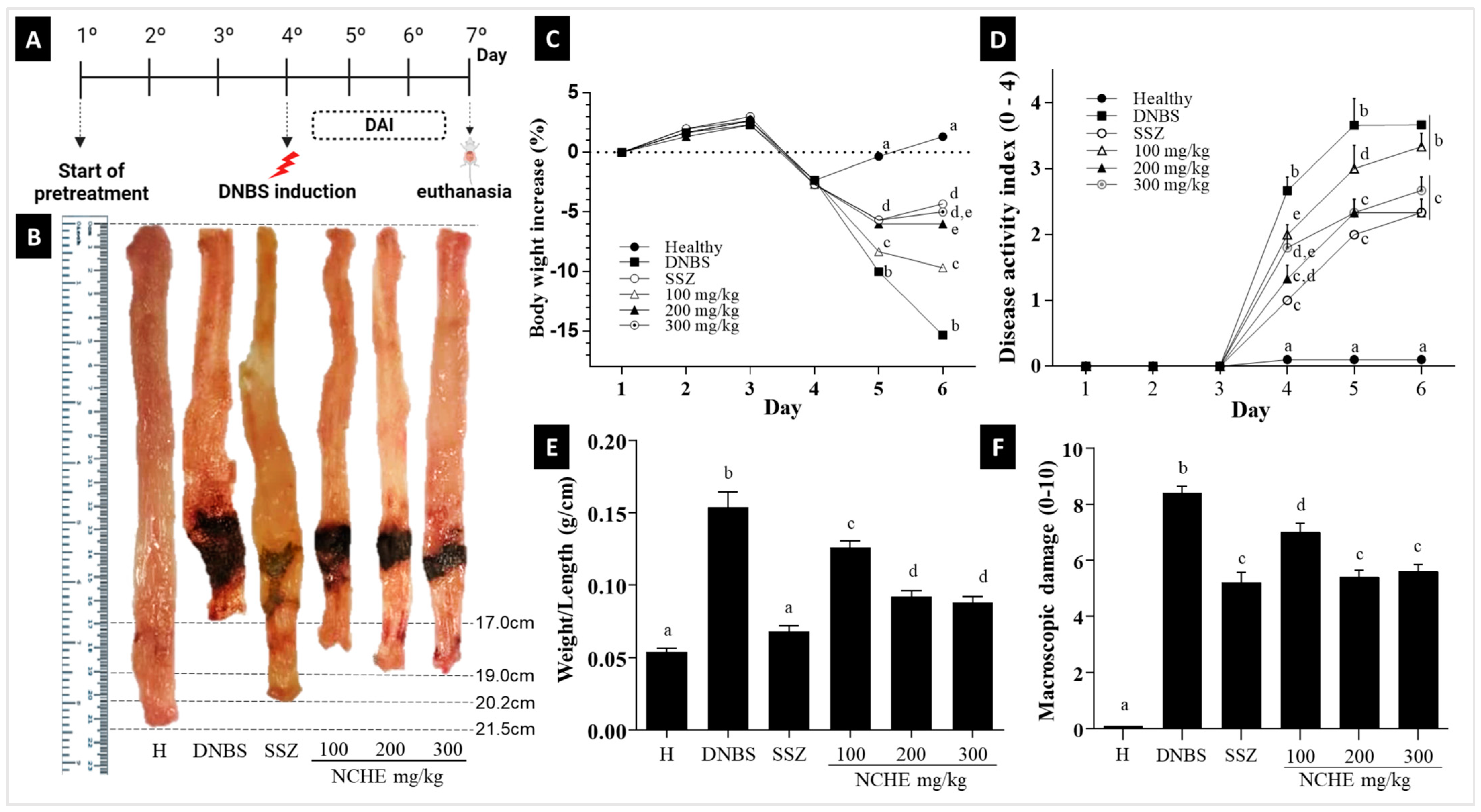
2.3. Effect of Nopalea cochenillifera Extract on DNBS-Induced Colitis in Rats
2.3.1. The Disease Activity Index (DAI), Macroscopic Score and Weight/Colonic Length Ratio
2.3.2. Effect of Nopalea cochenillifera Extract on Malondialdehyde Levels and Myeloperoxidase Activity
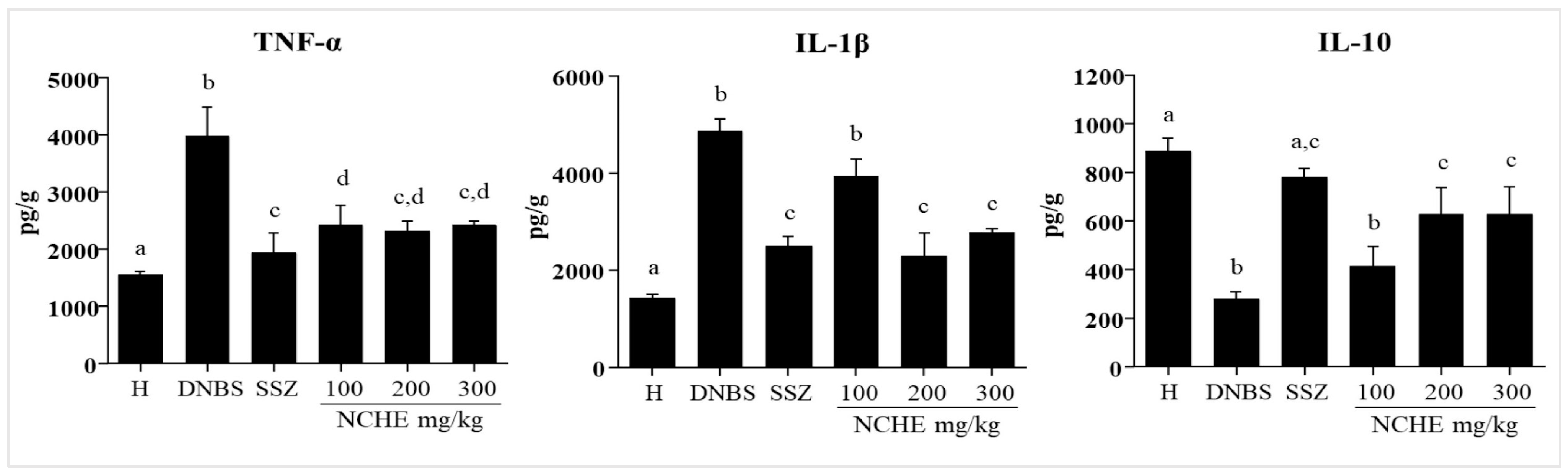
2.3.3. Effect of the Nopalea cochenillifera Extract on the Colonic Levels of TNF-α, IL-1β, and IL-10
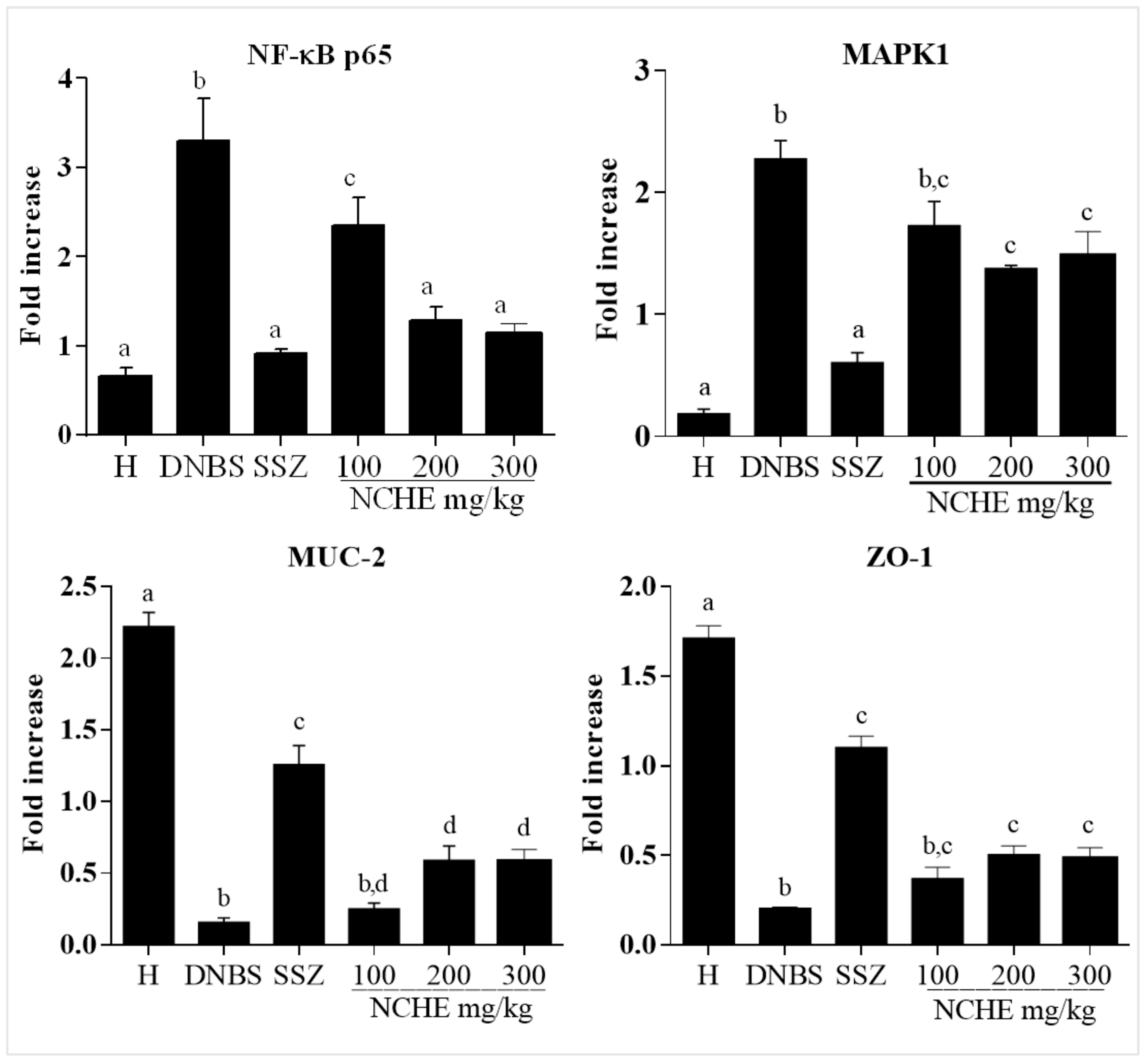
2.3.4. Effect of the Nopalea cochenillifera Extract on the Gene Expression of MAPK1, NF-κB p65, MUC-2 and ZO-1
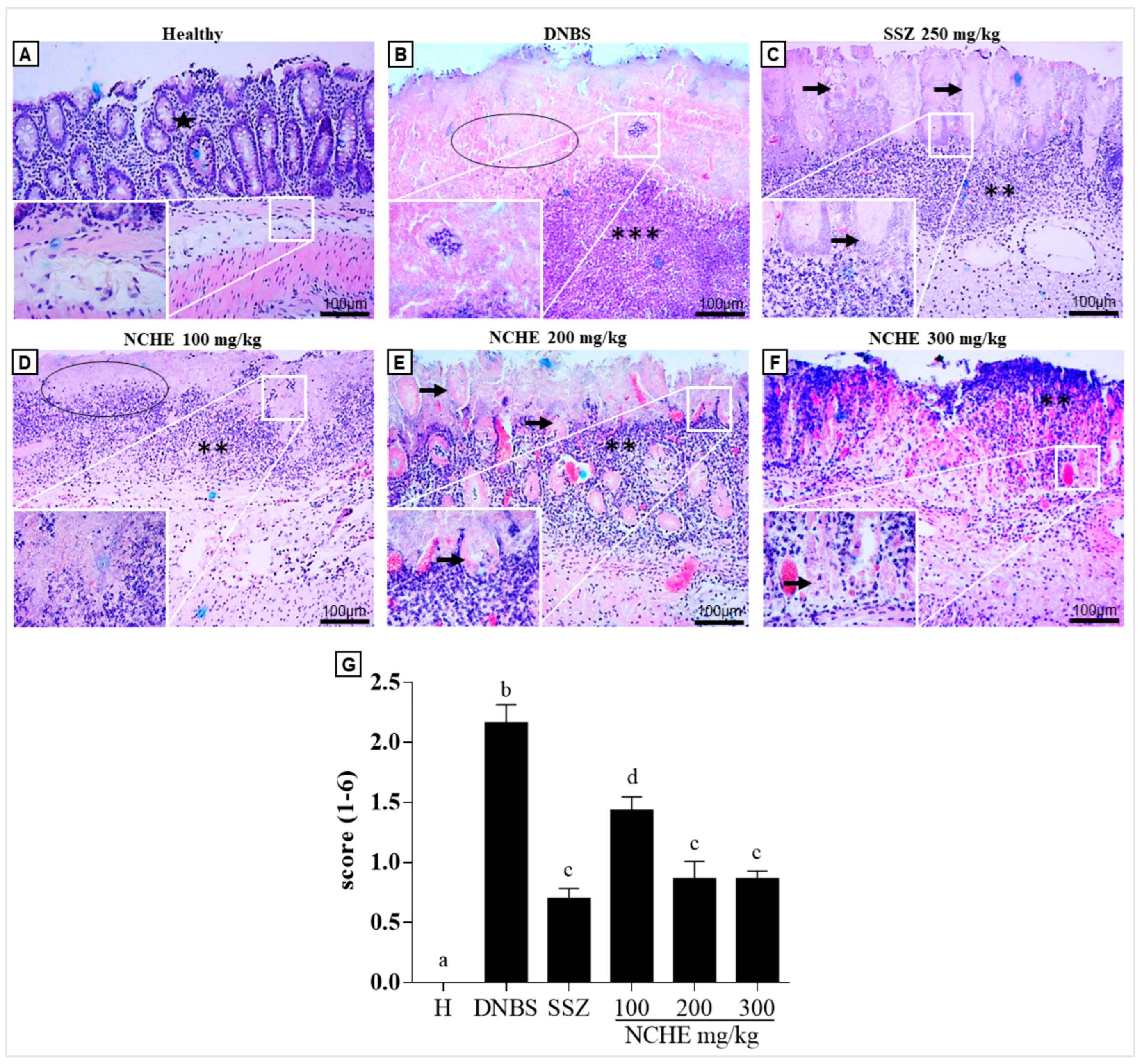
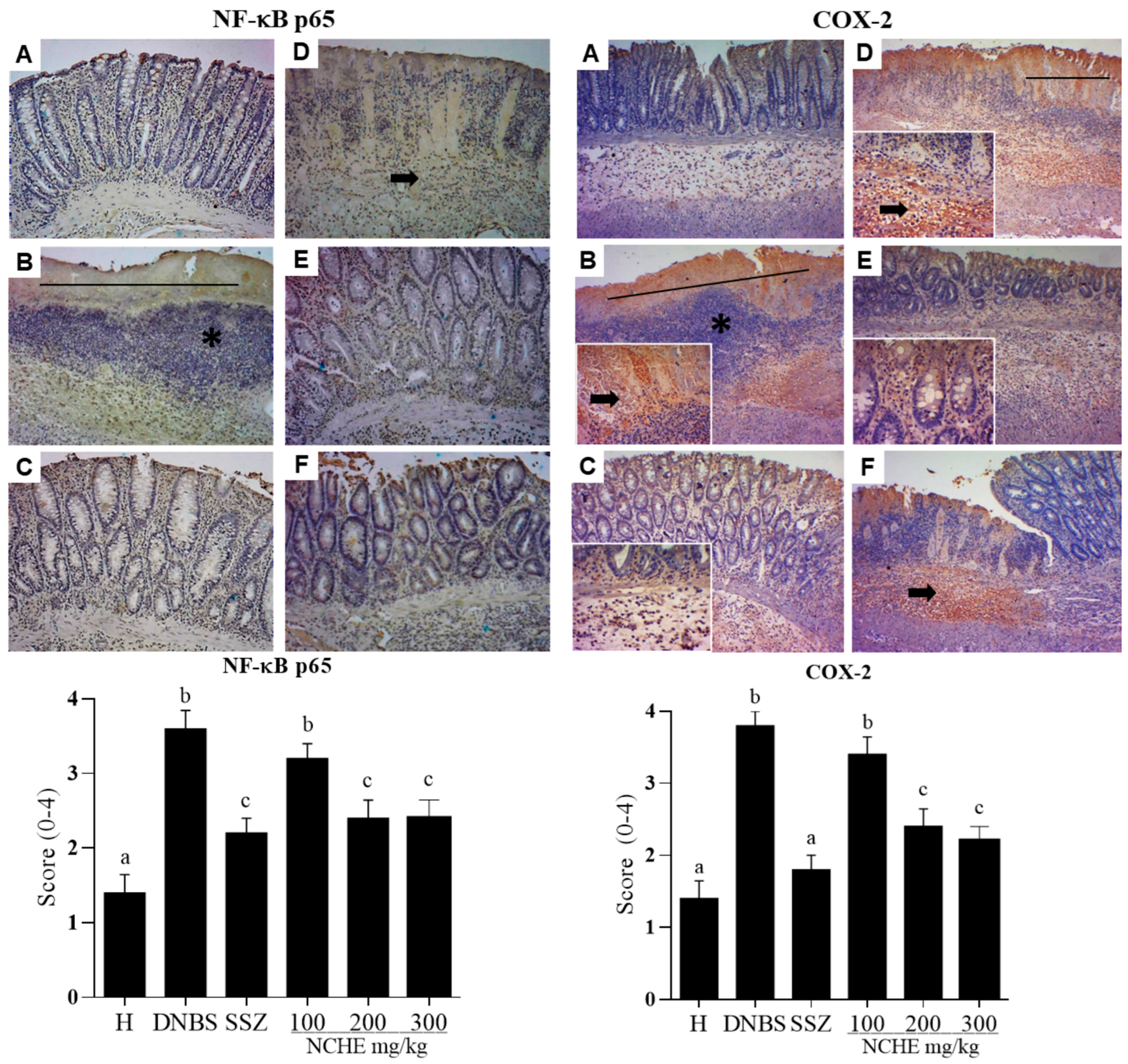
2.3.5. Histological and Immunohistochemical Analysis
3. Discussion
3.1. The Physicochemical and Phytochemical Characterization of Nopalea cochenillifera Extract Identifies the Presence of Compounds Beneficial to Intestinal Health
3.2. Nopalea cochenillifera Hydroethanolic Extract did Not Produce Toxic Effects in Rats after Acute Treatment
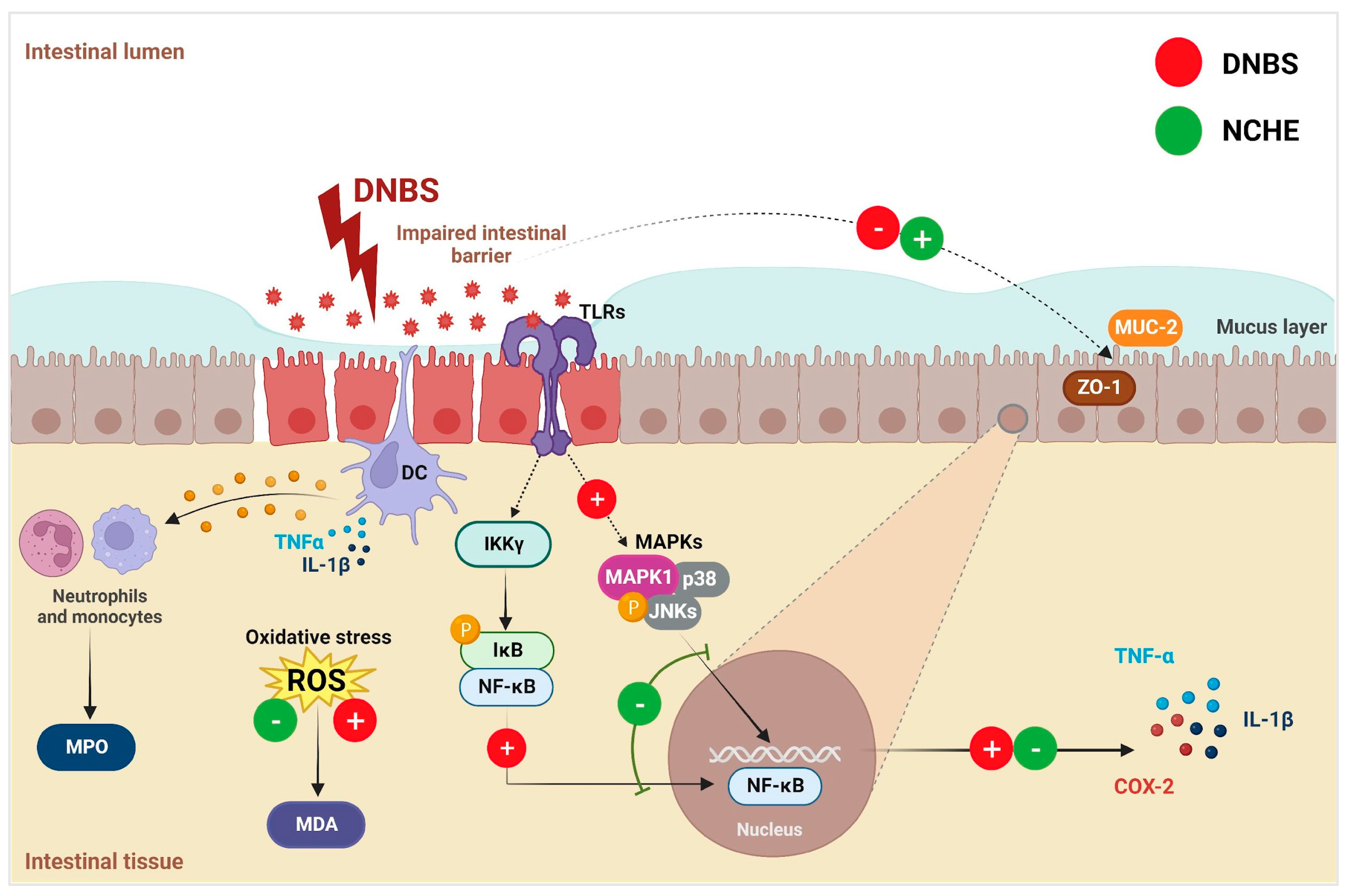
3.3. Effects of Nopalea cochenillifera Hydroethanolic Extract on Inflammatory Markers, Oxidative Stress and Intestinal Permeability in Rats with Induced Colitis
4. Materials and Methods
4.1. Reagents and Plant Material
4.2. Preparation of Nopalea cochenillifera Extract
4.3. Physicochemical Analysis of Nopalea cochenillifera Extract
4.3.1. Determination of Total Phenolic and Flavonoids Content
4.3.2. Phytochemical Analysis by HPLC-ESI-MSn of Nopalea cochenillifera Extract
4.4. Animals
4.5. Acute Toxicity
4.5.1. Behavioral Evaluation (Hippocratic Screening and Open Field Test) and Motor (Rotarod Test)
4.5.2. Evaluation of Hematological, Biochemical and Anatomopathological Parameters
4.6. DNBS-Induced Colitis: Experimental Design and Treatment Protocol
4.6.1. Evaluation of Disease Activity Index (DAI), Weight/Length Ratio and Macroscopic Damage Index Colonic on DNBS Induced
4.6.2. Determination of Myeloperoxidase (MPO) Activity and Malondialdehyde (MDA) Levels in the Intestine
4.6.3. Measurement of Cytokine Production in the Intestine
4.6.4. Analysis of RNA Transcripts by RT-qPCR
4.6.5. Histopathology and Immunohistochemical Analysis
4.6.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The Global, Regional, and National Burden of Inflammatory Bowel Disease in 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oli, A.K.; Maidur, R.N.; Hurkadli, P.S.; Javalgi, A.P.; Javaregowda, P.K.; Goni, M. Incidence of Inflammatory Bowel Disease: A Single Centre Retrospective Study. Arq. Gastroenterol. 2022, 59, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Ramos, G.P.; Papadakis, K.A. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin. Proc. 2019, 94, 155–165. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Ayyagari, R.; Shah, R.; Herrera, H.; Swaroop, P. The Use of Oral Complementary and Alternative Medicine in Patients with Inflammatory Bowel Disease. Am. J. Gastroenterol. 2012, 107, S684–S685. [Google Scholar] [CrossRef]
- Pagnini, C.; Pizarro, T.T.; Cominelli, F. Novel Pharmacological Therapy in Inflammatory Bowel Diseases: Beyond Anti-Tumor Necrosis Factor. Front. Pharmacol. 2019, 10, 671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henriques, D.P.; Oliveira, R.R.d.; Vanni, J.; Lima, H.P.d.; Otiti, J.V.; Neves, F.R.M.; Hammerle, M.B.; Sassaki, L.Y.; Zaltman, C. Complementary and Alternative Medicine Use in Brazilian Patients with Inflammatory Bowel Disease. Arq. Gastroenterol. 2022, 59, 375–382. [Google Scholar] [CrossRef]
- Holleran, G.; Scaldaferri, F.; Gasbarrini, A.; Currò, D. Herbal Medicinal Products for Inflammatory Bowel Disease: A Focus on Those Assessed in Double-blind Randomised Controlled Trials. Phyther. Res. 2020, 34, 77–93. [Google Scholar] [CrossRef]
- Hagan, M.; Hayee, B.H.; Rodriguez-Mateos, A. (Poly)Phenols in Inflammatory Bowel Disease and Irritable Bowel Syndrome: A Review. Molecules 2021, 26, 1843. [Google Scholar] [CrossRef]
- Chen, C.; Wang, H.; Hong, T.; Huang, X.; Xia, S.; Zhang, Y.; Chen, X.; Zhong, Y.; Nie, S. Effects of Tea Polysaccharides in Combination with Polyphenols on Dextran Sodium Sulfate-Induced Colitis in Mice. Food Chem. X 2022, 13, 100190. [Google Scholar] [CrossRef]
- Kyselova, Z. Toxicological Aspects of the Use of Phenolic Compounds in Disease Prevention. Interdiscip. Toxicol. 2011, 4, 173–183. [Google Scholar] [CrossRef]
- Ambriz-Pérez, D.L.; Leyva-López, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B. Phenolic Compounds: Natural Alternative in Inflammation Treatment. A Review. Cogent Food Agric. 2016, 2, 1131412. [Google Scholar] [CrossRef]
- Ferraz, C.R.; Carvalho, T.T.; Manchope, M.F.; Artero, N.A.; Rasquel-Oliveira, F.S.; Fattori, V.; Casagrande, R.; Verri, W.A. Therapeutic Potential of Flavonoids in Pain and Inflammation: Mechanisms of Action, Pre-Clinical and Clinical Data, and Pharmaceutical Development. Molecules 2020, 25, 762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef]
- Hanai, H.; Iida, T.; Takeuchi, K.; Watanabe, F.; Maruyama, Y.; Andoh, A.; Tsujikawa, T.; Fujiyama, Y.; Mitsuyama, K.; Sata, M.; et al. Curcumin Maintenance Therapy for Ulcerative Colitis: Randomized, Multicenter, Double-Blind, Placebo-Controlled Trial. Clin. Gastroenterol. Hepatol. 2006, 4, 1502–1506. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.; Salomon, N.; Wu, J.C.Y.; Kopylov, U.; Lahat, A.; Har-Noy, O.; Ching, J.Y.L.; Cheong, P.K.; Avidan, B.; Gamus, D.; et al. Curcumin in Combination With Mesalamine Induces Remission in Patients With Mild-to-Moderate Ulcerative Colitis in a Randomized Controlled Trial. Clin. Gastroenterol. Hepatol. 2015, 13, 1444–1449. [Google Scholar] [CrossRef]
- Vaghari-Tabari, M.; Alemi, F.; Zokaei, M.; Moein, S.; Qujeq, D.; Yousefi, B.; Farzami, P.; Hosseininasab, S.S. Polyphenols and Inflammatory Bowel Disease: Natural Products with Therapeutic Effects? Crit. Rev. Food Sci. Nutr. 2022, 10, 1–24. [Google Scholar] [CrossRef]
- Pang, B.; Xu, X.; Lu, Y.; Jin, H.; Yang, R.; Jiang, C.; Shao, D.; Liu, Y.; Shi, J. Prediction of New Targets and Mechanisms for Quercetin in the Treatment of Pancreatic Cancer, Colon Cancer, and Rectal Cancer. Food Funct. 2019, 10, 5339–5349. [Google Scholar] [CrossRef]
- Darband, S.G.; Kaviani, M.; Yousefi, B.; Sadighparvar, S.; Pakdel, F.G.; Attari, J.A.; Mohebbi, I.; Naderi, S.; Majidinia, M. Quercetin: A Functional Dietary Flavonoid with Potential Chemo-preventive Properties in Colorectal Cancer. J. Cell. Physiol. 2018, 233, 6544–6560. [Google Scholar] [CrossRef] [PubMed]
- Arya, V.S.; Kanthlal, S.K.; Linda, G. The Role of Dietary Polyphenols in Inflammatory Bowel Disease: A Possible Clue on the Molecular Mechanisms Involved in the Prevention of Immune and Inflammatory Reactions. J. Food Biochem. 2020, 44, e13369. [Google Scholar] [CrossRef] [PubMed]
- Danesi, F.; Ferguson, L. Could Pomegranate Juice Help in the Control of Inflammatory Diseases? Nutrients 2017, 9, 958. [Google Scholar] [CrossRef]
- Catalkaya, G.; Venema, K.; Lucini, L.; Rocchetti, G.; Delmas, D.; Daglia, M.; De Filippis, A.; Xiao, H.; Quiles, J.L.; Xiao, J.; et al. Interaction of Dietary Polyphenols and Gut Microbiota: Microbial Metabolism of Polyphenols, Influence on the Gut Microbiota, and Implications on Host Health. Food Front. 2020, 1, 109–133. [Google Scholar] [CrossRef]
- Domínguez-Avila, J.A.; Villa-Rodriguez, J.A.; Montiel-Herrera, M.; Pacheco-Ordaz, R.; Roopchand, D.E.; Venema, K.; González-Aguilar, G.A. Phenolic Compounds Promote Diversity of Gut Microbiota and Maintain Colonic Health. Dig. Dis. Sci. 2021, 66, 3270–3289. [Google Scholar] [CrossRef] [PubMed]
- Araújo, D.F.S.; de Oliveira, M.E.G.; de Carvalho, P.O.A.A.; Tavares, E.A.; Guerra, G.C.B.; Queiroga, R.C.R.E.; Langassner, S.M.Z.; Bezerril, F.F.; Martins, A.C.S.; Medeiros, G.K.V.V.; et al. Food Plants in the Caatinga. In Local Food Plants of Brazil; Springer: Cham, Switzerland, 2021; pp. 225–250. [Google Scholar]
- Gomez-Flores, R.; Tamez-Guerra, P.; Tamez-Guerra, R.; Rodriguez-Padilla, C.; Monreal-Cuevas, E.; Hauad-Marroquin, L.A.; Cordova-Puente, C.; Rangel-Llanas, A. In Vitro Antibacterial and Antifungal Activities of Nopalea Cochenillifera Pad Extracts. Am. J. Infect. Dis. 2006, 2, 1–8. [Google Scholar] [CrossRef]
- Necchi, R.M.M.; Alves, I.A.; Alves, S.H.; Manfron, M.P.; Farmacêuticas, D.P.C. In Vitro Antimicrobial Activity, Total Polyphenols and Flavonoids Contents of Nopalea Cochenillifera (L.) Salm-Dyck (Cactaceae). Res. Pharm. 2012, 2, 1–7. [Google Scholar]
- Fabela-Illescas, H.E.; Castro-Mendoza, M.P.; Montalvo-González, E.; Anaya-Esparza, L.M.; Vargas-Torres, A.; Betanzos-Cabrera, G.; Hernandez-Uribe, J.P. Bioactive Compounds Identification and Physicochemical Characterization from Nopalea Cochenillifera (L.) Salm-Dyck Cladodes Flour. Biotecnia 2022, 24, 46–54. [Google Scholar] [CrossRef]
- Da Silva, R.R.; Sampaio, E.V.d.S.B. Palmas Forrageiras Opuntia Fícus-Indica e Nopalea Cochenillifera: Sistemas de Produção e Usos. Rev. GEAMA 2015, 1, 151–161. [Google Scholar]
- Andrade-Cetto, A.; Heinrich, M. Mexican Plants with Hypoglycaemic Effect Used in the Treatment of Diabetes. J. Ethnopharmacol. 2005, 99, 325–348. [Google Scholar] [CrossRef]
- Lans, C.A. Ethnomedicines Used in Trinidad and Tobago for Urinary Problems and Diabetes Mellitus. J. Ethnobiol. Ethnomed. 2006, 2, 45. [Google Scholar] [CrossRef] [Green Version]
- Magaña-Cerino, J.M.; Guzmán, T.J.; Soto-Luna, I.C.; Betanzos-Cabrera, G.; Gurrola-Díaz, C.M. Cladodes from Nopalea Cochenillifera (L.) Salm-Dyck (Cactaceae) Attenuate Postprandial Glycaemia without Markedly Influencing α-Glucosidase Activity. Nat. Prod. Res. 2022, 36, 1105–1108. [Google Scholar] [CrossRef]
- Fabela-Illescas, H.E.; Ávila-Domínguez, R.; Hernández-Pacheco, A.; Ariza Ortega, J.A.; Betanzos-Cabrera, G. Effect of a beverage made from cactus pear (Nopalea cochenillifera (L) SALM-DYCK) in a rural population of hidalgo, México; a pilot clinical trial. Nutr. Hosp. 2015, 32, 2710–2714. [Google Scholar] [CrossRef]
- Necchi, R.; Maki, T.; Canto, G.; Moresco, R.; Dalmora, S.; Manfron, M. Antiinflammatory Activity and Biochemical Parameters of the Ethanol Extract of Nopalea Cochenillifera (L.) Salm-Dyck (Cactaceae). Lat. Am. J. Pharm. 2011, 30, 786–789. [Google Scholar]
- Tripodo, G.; Ibáñez, E.; Cifuentes, A.; Gilbert-López, B.; Fanali, C. Optimization of Pressurized Liquid Extraction by Response Surface Methodology of Goji Berry (Lycium Barbarum L.) Phenolic Bioactive Compounds. Electrophoresis 2018, 39, 1673–1682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felipe, D.F.; Brambilla, L.Z.; Porto, C.; Pilau, E.J.; Cortez, D.A. Phytochemical Analysis of Pfaffia Glomerata Inflorescences by LC-ESI-MS/MS. Molecules 2014, 19, 15720–15734. [Google Scholar] [CrossRef] [PubMed]
- Tikunov, Y.M.; de Vos, R.C.H.; González Paramás, A.M.; Hall, R.D.; Bovy, A.G. A Role for Differential Glycoconjugation in the Emission of Phenylpropanoid Volatiles from Tomato Fruit Discovered Using a Metabolic Data Fusion Approach. Plant Physiol. 2009, 152, 55–70. [Google Scholar] [CrossRef] [Green Version]
- Yakubu, Y.; Lee, S.Y.; Shaari, K. Chemical Profiles of Terminalia Catappa LINN Nut and Terminalia Subspathulata KING Fruit. Pertanika J. Trop. Agric. Sci. 2021, 44, 795–823. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Arráez-Román, D.; Segura-Carretero, A. HPLC–DAD–ESI-MS/MS Screening of Bioactive Components from Rhus Coriaria L. (Sumac) Fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef] [Green Version]
- Tuytten, R.; Lemière, F.; Van Dongen, W.; Esmans, E.L.; Witters, E.; Herrebout, W.; Van Der Veken, B.; Dudley, E.; Newton, R.P. Intriguing Mass Spectrometric Behavior of Guanosine Under Low Energy Collision-Induced Dissociation: H2O Adduct Formation and Gas-Phase Reactions in the Collision Cell. J. Am. Soc. Mass Spectrom. 2005, 16, 1291–1304. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Ye, M.; Qiao, X.; Xu, M.; Wang, B.; Guo, D.-A. Characterization of Phenolic Compounds in the Chinese Herbal Drug Artemisia Annua by Liquid Chromatography Coupled to Electrospray Ionization Mass Spectrometry. J. Pharm. Biomed. Anal. 2008, 47, 516–525. [Google Scholar] [CrossRef]
- Ben Said, R.; Hamed, A.I.; Mahalel, U.A.; Al-Ayed, A.S.; Kowalczyk, M.; Moldoch, J.; Oleszek, W.; Stochmal, A. Tentative Characterization of Polyphenolic Compounds in the Male Flowers of Phoenix dactylifera by Liquid Chromatography Coupled with Mass Spectrometry and DFT. Int. J. Mol. Sci. 2017, 18, 512. [Google Scholar] [CrossRef]
- Hayun, H.; Gavrila, I.; Silviana, S.; Siahaan, A.E.K.; Vonna, R.F.; Latifah, M.I. Synthesis and antioxidant activity study of new mannich bases derived from vanillic acid. Rasayan J. Chem. 2020, 13, 131–138. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Lu, S.; Harnly, J.M. Detection and Quantification of Glycosylated Flavonoid Malonates in Celery, Chinese Celery, and Celery Seed by LC-DAD-ESI/MS. J. Agric. Food Chem. 2007, 55, 1321–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, P.; Harnly, J.M.; Sun, J.; Zhang, M.; Chen, P. Feruloyl Dopamine-O-Hexosides Are Efficient Marker Compounds as Orthogonal Validation for Authentication of Black Cohosh (Actaea Racemosa)—An UHPLC-HRAM-MS Chemometrics Study. Anal. Bioanal. Chem. 2017, 409, 2591–2600. [Google Scholar] [CrossRef] [Green Version]
- Nikolić, D.; Gödecke, T.; Chen, S.-N.; White, J.; Lankin, D.C.; Pauli, G.F.; van Breemen, R.B. Mass Spectrometric Dereplication of Nitrogen-Containing Constituents of Black Cohosh (Cimicifuga Racemosa L.). Fitoterapia 2012, 83, 441–460. [Google Scholar] [CrossRef] [PubMed]
- Spínola, V.; Llorent-Martínez, E.J.; Gouveia-Figueira, S.; Castilho, P.C. Ulex Europaeus: From Noxious Weed to Source of Valuable Isoflavones and Flavanones. Ind. Crops Prod. 2016, 90, 9–27. [Google Scholar] [CrossRef]
- Lima, C.M.; Lima, A.K.; Melo, M.G.D.; Dória, G.A.A.; Serafini, M.R.; Albuquerque-Júnor, R.L.C.; Araújo, A.A.S. Valores de Referência Hematológicos e Bioquímicos de Ratos (Rattus Novergicus Linhagem Wistar) Provenientes Do Biotério Da Universidade Tiradentes. Sci. Plena 2014, 10. [Google Scholar]
- Spinelli, M.O.; Motta, M.C.; Cruz, R.J.; Santos, C.M.; Godoy, C. Reference Intervals for Hematological Parameters of Animals Bred and Kept at the Vivarium of the Faculty of Medicine of the State University of São Paulo. Acta Sci. Health Sci. 2014, 36, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.P.G.d.; Souza, C.C.E.d.; Ribeiro, J.E.S.; Santos, M.C.G.d.; Pontes, A.L.d.S.; Madruga, M.S. Características Físicas, Químicas e Bromatológicas de Palma Gigante (Opuntia Ficus-Indica) e Miúda (Nopalea Cochenillifera) Oriundas Do Estado Da Paraíba. Rev. Bras. Tecnol. Agroind. 2015, 9, 1810–1820. [Google Scholar] [CrossRef] [Green Version]
- Lima, V.R.d.N.; Silva, Á.G.F.d.; Cruz, R.R.P.; Barbosa, L.d.S.; Junior, N.R.d.S.; Sales, G.N.B.; Limão, M.A.R.; Costa, F.B.d.; Souza, P.A.d.; Lopes, K.P.; et al. Nopalea Cochenillifera Biomass as Bioadsorbent in Water Purification. Water 2021, 13, 2012. [Google Scholar] [CrossRef]
- Alves, F.A.L.; Andrade, A.P.d.; Bruno, R.d.L.A.; Silva, M.G.d.V.; Souza, M.d.F.V.d.; Santos, D.C.d. Seasonal Variability of Phenolic Compounds and Antioxidant Activity in Prickly Pear Cladodes of Opuntia and Nopalea Genres. Food Sci. Technol. 2017, 37, 536–543. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.M.P.; Inácio, M.R.C.; Silva, G.G.C.d.; Silva, J.M.d.S.e; Luz, J.R.D.d.; Almeida, M.d.G.; Moraes, E.P.; Esposito, D.; Ferreira, L.D.S.; Zucolotto, S.M. The First Optimization Process from Cultivation to Flavonoid-Rich Extract from Moringa Oleifera Lam. Leaves in Brazil. Foods 2022, 11, 1452. [Google Scholar] [CrossRef]
- Cobaleda-Velasco, M.; Alanis-Bañuelos, R.E.; Almaraz-Abarca, N.; Rojas-López, M.; González-Valdez, L.S.; Ávila-Reyes, J.A.; Rodrigo, S. Phenolic Profiles and Antioxidant Properties of Physalis Angulata L. as Quality Indicators. J. Pharm. Pharmacogn. Res. 2017, 5, 114–128. [Google Scholar]
- Cuyckens, F.; Claeys, M. Mass Spectrometry in the Structural Analysis of Flavonoids. J. Mass Spectrom. 2004, 39, 1–15. [Google Scholar] [CrossRef]
- Dantas-Medeiros, R.; Furtado, A.A.; Zanatta, A.C.; Torres-Rêgo, M.; Guimarães Lourenço, E.M.; Ferreira Alves, J.S.; Galinari, É.; Alexandre de Oliveira Rocha, H.; Bernardo Guerra, G.C.; Vilegas, W.; et al. Mass Spectrometry Characterization of Commiphora Leptophloeos Leaf Extract and Preclinical Evaluation of Toxicity and Anti-Inflammatory Potential Effect. J. Ethnopharmacol. 2021, 264, 113229. [Google Scholar] [CrossRef] [PubMed]
- Justesen, U. Collision-Induced Fragmentation of Deprotonated Methoxylated Flavonoids, Obtained by Electrospray Ionization Mass Spectrometry. J. Mass Spectrom. 2001, 36, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Zanatta, A.C.; Mari, A.; Masullo, M.; Zeppone Carlos, I.; Vilegas, W.; Piacente, S.; Campaner dos Santos, L. Chemical Metabolome Assay by High-Resolution Orbitrap Mass Spectrometry and Assessment of Associated Antitumoral Activity of Actinocephalus Divaricatus. Rapid Commun. Mass Spectrom. 2018, 32, 241–250. [Google Scholar] [CrossRef]
- Reynaud, J.; Guilet, D.; Terreux, R.; Lussignol, M.; Walchshofer, N. Isoflavonoids in Non-Leguminous Families: An Update. Nat. Prod. Rep. 2005, 22, 504–515. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhao, C.; Zhao, X.; Xia, Y.; Sun, X.; Xie, W.; Ye, Y.; Lu, X.; Xu, G. Deep Annotation of Hydroxycinnamic Acid Amides in Plants Based on Ultra-High-Performance Liquid Chromatography–High-Resolution Mass Spectrometry and Its In Silico Database. Anal. Chem. 2018, 90, 14321–14330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glabska, D.; Guzek, D.; Grudzińska, D.; Lech, G. Influence of Dietary Isoflavone Intake on Gastrointestinal Symptoms in Ulcerative Colitis Individuals in Remission. World J. Gastroenterol. 2017, 23, 5356–5363. [Google Scholar] [CrossRef] [PubMed]
- Skolmowska, D.; Glabska, D.; Guzek, D.; Lech, G. Association between Dietary Isoflavone Intake and Ulcerative Colitis Symptoms in Polish Caucasian Individuals. Nutrients 2019, 11, 1936. [Google Scholar] [CrossRef] [Green Version]
- Teo, S.; Stirling, D.; Thomas, S.; Hoberman, A.; Kiorpes, A.; Khetani, V. A 90-Day Oral Gavage Toxicity Study of d-Methylphenidate and d,l-Methylphenidate in Sprague–Dawley Rats. Toxicology 2002, 179, 183–196. [Google Scholar] [CrossRef]
- Michael, B.; Yano, B.; Sellers, R.S.; Perry, R.; Morton, D.; Roome, N.; Johnson, J.K.; Schafer, K. Evaluation of Organ Weights for Rodent and Non-Rodent Toxicity Studies: A Review of Regulatory Guidelines and a Survey of Current Practices. Toxicol. Pathol. 2007, 35, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.B.; Sowemimo, A.A.; Sofidiya, M.O.; Badmos, K.B.; Fageyinbo, M.S.; Abdulkareem, F.B.; Odukoya, O.A. Sub-Acute and Chronic Toxicity Profiles of Markhamia Tomentosa Ethanolic Leaf Extract in Rats. J. Ethnopharmacol. 2016, 193, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Olson, H.; Betton, G.; Robinson, D.; Thomas, K.; Monro, A.; Kolaja, G.; Lilly, P.; Sanders, J.; Sipes, G.; Bracken, W.; et al. Concordance of the Toxicity of Pharmaceuticals in Humans and in Animals. Regul. Toxicol. Pharmacol. 2000, 32, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Osei-Bimpong, A.; McLean, R.; Bhonda, E.; Lewis, S.M. The Use of the White Cell Count and Haemoglobin in Combination as an Effective Screen to Predict the Normality of the Full Blood Count. Int. J. Lab. Hematol. 2012, 34, 91–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OCDE Guideline for the testing of chemicals. Test No. 425: Acute Oral Toxicity: Up-and-Down Procedure. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2001. [Google Scholar] [CrossRef]
- Vezza, T.; Rodríguez-nogales, A.; Algieri, F.; Utrilla, M.P.; Rodriguez-cabezas, M.E.; Galvez, J. Flavonoids in Inflammatory Bowel Disease: A Review. Nutrients 2016, 8, 211. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Zhao, P.; Li, X.; Guo, L.; Gao, W. The Potential Roles of Natural Plant Polysaccharides in Inflammatory Bowel Disease: A Review. Carbohydr. Polym. 2022, 277, 118821. [Google Scholar] [CrossRef]
- Morampudi, V.; Bhinder, G.; Wu, X.; Dai, C.; Sham, H.P.; Vallance, B.A.; Jacobson, K. DNBS/TNBS Colitis Models: Providing Insights Into Inflammatory Bowel Disease and Effects of Dietary Fat. J. Vis. Exp. 2014, 84, e51297. [Google Scholar] [CrossRef] [Green Version]
- Chami, B.; Martin, N.J.J.; Dennis, J.M.; Witting, P.K. Myeloperoxidase in the Inflamed Colon: A Novel Target for Treating Inflammatory Bowel Disease. Arch. Biochem. Biophys. 2018, 645, 61–71. [Google Scholar] [CrossRef]
- Draper, H.H.; Hadley, M. Malondialdehyde Determination as Index of Lipid Peroxidation. Methods Enzymol. 1990, 186, 421–431. [Google Scholar]
- Kaulmann, A.; Bohn, T. Bioactivity of Polyphenols: Preventive and Adjuvant Strategies toward Reducing Inflammatory Bowel Diseases—Promises, Perspectives, and Pitfalls. Oxid. Med. Cell. Longev. 2016, 2016, 9346470. [Google Scholar] [CrossRef] [Green Version]
- Andrade, A.W.L.; Guerra, G.C.B.; de Souza Araújo, D.F.; de Araújo Júnior, R.F.; de Araújo, A.A.; de Carvalho, T.G.; Fernandes, J.M.; Diez-Echave, P.; Hidalgo-García, L.; Rodriguez-Cabezas, M.E.; et al. Anti-Inflammatory and Chemopreventive Effects of Bryophyllum Pinnatum (Lamarck) Leaf Extract in Experimental Colitis Models in Rodents. Front. Pharmacol. 2020, 11, 998. [Google Scholar] [CrossRef] [PubMed]
- Roudsari, N.M.; Lashgari, N.-A.; Momtaz, S.; Farzaei, M.H.; Marques, A.M.; Abdolghaffari, A.H. Natural Polyphenols for the Prevention of Irritable Bowel Syndrome: Molecular Mechanisms and Targets; a Comprehensive Review. Daru: J. Pharm. Sci. 2019, 27, 755–780. [Google Scholar] [CrossRef] [PubMed]
- Mitsuyama, K.; Tomiyasu, N.; Takaki, K.; Masuda, J.; Yamasaki, H.; Kuwaki, K.; Takeda, T.; Kitazaki, S.; Tsuruta, O.; Sata, M. Interleukin-10 in the Pathophysiology of Inflammatory Bowel Disease: Increased Serum Concentrations During the Recovery Phase. Mediators Inflamm. 2006, 2006, 026875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ljuca, F.; Gegic, A.; Salkic, N.N.; Pavlovic-Calic, N. Circulating Cytokines Reflect Mucosal Inflammatory Status in Patients with Crohn’s Disease. Dig. Dis. Sci. 2010, 55, 2316–2326. [Google Scholar] [CrossRef]
- Liu, B.; Li, S.; Sui, X.; Guo, L.; Liu, X.; Li, H.; Gao, L.; Cai, S.; Li, Y.; Wang, T.; et al. Root Extract of Polygonum cuspidatum Siebold & Zucc. Ameliorates DSS-Induced Ulcerative Colitis by Affecting NF-KappaB Signaling Pathway in a Mouse Model via Synergistic Effects of Polydatin, Resveratrol, and Emodin. Front. Pharmacol. 2018, 9, 347. [Google Scholar] [CrossRef]
- Coskun, M.; Olsen, J.; Seidelin, J.B.; Nielsen, O.H. MAP Kinases in Inflammatory Bowel Disease. Clin. Chim. Acta 2011, 412, 513–520. [Google Scholar] [CrossRef]
- Chen, X.; Chen, W.; He, Y.; Zhang, Y.; Chen, C.; Zhu, Z.; Wang, H. The P38 MAPK and NF-ΚB Pathways Are Involved in Cyclic Compressive Force-Induced IL-6 Secretion in MLO-Y4 Cells. Braz. Arch. Biol. Technol. 2018, 61, e18170777. [Google Scholar] [CrossRef]
- Nair, M.P.; Mahajan, S.; Reynolds, J.L.; Aalinkeel, R.; Nair, H.; Schwartz, S.A.; Kandaswami, C. The Flavonoid Quercetin Inhibits Proinflammatory Cytokine (Tumor Necrosis Factor Alpha) Gene Expression in Normal Peripheral Blood Mononuclear Cells via Modulation of the NF-Κβ System. Clin. Vaccine Immunol. 2006, 13, 319–328. [Google Scholar] [CrossRef] [Green Version]
- Manoharan, S.; Rejitharaji, T.; M Prabhakar, M.; Manimaran, A.; B Singh, R. Modulating Effect of Ferulic Acid on NF-KB, COX-2 and VEGF Expression Pattern During 7, 12-Dimethylbenz(a)Anthracene Induced Oral Carcinogenesis. Open Nutraceuticals J. 2014, 7, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Giacomarra, M.; Mangano, A.; Montana, G. The Anti-Inflammatory Activity of Ferulic Acid on NF- ΚB Depends on Keap1. LOJ Pharmacol. Clin. Res. 2020, 2, 168–180. [Google Scholar] [CrossRef]
- Ho, H.-H.; Chang, C.-S.; Ho, W.-C.; Liao, S.-Y.; Wu, C.-H.; Wang, C.-J. Anti-Metastasis Effects of Gallic Acid on Gastric Cancer Cells Involves Inhibition of NF-ΚB Activity and Downregulation of PI3K/AKT/Small GTPase Signals. Food Chem. Toxicol. 2010, 48, 2508–2516. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Li, N.; Zhang, X. Daidzein Ameliorates Dextran Sulfate Sodium-Induced Experimental Colitis in Mice by Regulating NF-ΚB Signaling. J. Environ. Pathol. Toxicol. Oncol. 2019, 38, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Cobo, E.R.; Kissoon-Singh, V.; Moreau, F.; Chadee, K. Colonic MUC2 Mucin Regulates the Expression and Antimicrobial Activity of β-Defensin 2. Mucosal Immunol. 2015, 8, 1360–1372. [Google Scholar] [CrossRef] [Green Version]
- Kuo, W.-T.; Zuo, L.; Turner, J. The Tight Junction Protein ZO-1 Is Dispensable for Barrier Function but Critical for Effective Mucosal Repair. Inflamm. Bowel Dis. 2021, 27, S28. [Google Scholar] [CrossRef]
- Carrasco-Pozo, C.; Morales, P.; Gotteland, M. Polyphenols Protect the Epithelial Barrier Function of Caco-2 Cells Exposed to Indomethacin through the Modulation of Occludin and Zonula Occludens-1 Expression. J. Agric. Food Chem. 2013, 61, 5291–5297. [Google Scholar] [CrossRef] [PubMed]
- WHO Nopalea Cochenillifera (L.) Salm-Dyck. Available online: http://www.worldfloraonline.org/taxon/wfo-0000378923 (accessed on 16 November 2022).
- AOAC 925.10, 65.17, 974.24, 992.16; Official Methods of Analysis. The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000.
- Terra, J.; Antunes, A.M.; Bueno, M.I.M.S.; Prado, M.A. Um Método Verde, Rápido e Simples Para Determinar o Valor Energético de Farinhas e Cereais Matinais. Quim. Nova 2010, 33, 1098–1103. [Google Scholar] [CrossRef] [Green Version]
- Dantas-Medeiros, R.; Zanatta, A.C.; de Souza, L.B.F.C.; Fernandes, J.M.; Amorim-Carmo, B.; Torres-Rêgo, M.; Fernandes-Pedrosa, M.d.F.; Vilegas, W.; Araújo, T.A.d.S.; Michel, S.; et al. Antifungal and Antibiofilm Activities of B-Type Oligomeric Procyanidins From Commiphora Leptophloeos Used Alone or in Combination With Fluconazole Against Candida Spp. Front. Microbiol. 2021, 12, 613155. [Google Scholar] [CrossRef] [PubMed]
- Sousa, E.O.; Miranda, C.M.B.A.; Nobre, C.B.; Boligon, A.A.; Athayde, M.L.; Costa, J.G.M. Phytochemical Analysis and Antioxidant Activities of Lantana Camara and Lantana Montevidensis Extracts. Ind. Crops Prod. 2015, 70, 7–15. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and Community Curation of Mass Spectrometry Data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [Green Version]
- Rutz, A.; Sorokina, M.; Galgonek, J.; Mietchen, D.; Willighagen, E.; Gaudry, A.; Graham, J.G.; Stephan, R.; Page, R.; Vondrášek, J.; et al. The LOTUS Initiative for Open Knowledge Management in Natural Products Research. Elife 2022, 11, e70780. [Google Scholar] [CrossRef]
- Malone, M.H.; Robichaud, R.C. A Hippocratic Screen for Pure or Crude Drug Materials. Lloydia 1962, 25, 320–332. [Google Scholar]
- Pereira, J.R.; da Fonseca, A.G.; de Sena Fernandes, L.L.; Furtado, A.A.; da Silva, V.C.; da Veiga Júnior, V.F.; Sant’Ana, A.E.G.; Oliveira, C.N.; Guerra, G.C.B.; de Freitas Fernandes-Pedrosa, M.; et al. Toxicological and Pharmacological Effects of Pentacyclic Triterpenes Rich Fraction Obtained from the Leaves of Mansoa Hirsuta. Biomed. Pharmacother. 2022, 145, 112478. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.P.; Beck, P.L.; Herridge, M.S.; Depew, W.T.; Szewczuk, M.R.; Wallace, J.L. Hapten-Induced Model of Chronic Inflammation and Ulceration in the Rat Colon. Gastroenterology 1989, 96, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, J.; Garrido, M.; Merlos, M.; Torres, M.I.; Zarzuelo, A. Intestinal Anti-Inflammatory Activity of UR-12746, a Novel 5-ASA Conjugate, on Acute and Chronic Experimental Colitis in the Rat. Br. J. Pharmacol. 2000, 130, 1949–1959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, H.S.; Murthy, S.N.; Shah, R.S.; Sedergran, D.J. Clinicopathologic Study of Dextran Sulfate Sodium Experimental Murine Colitis. Lab. Investig. 1993, 62, 238–247. [Google Scholar]
- Berberat, P.O.; A-Rahim, Y.I.; Yamashita, K.; Warny, M.M.; Csizmadia, E.; Robson, S.C.; Bach, F.H. Heme Oxygenase-1-Generated Biliverdin Ameliorates Experimental Murine Colitis. Inflamm. Bowel Dis. 2005, 11, 350–359. [Google Scholar] [CrossRef]
- Bell, C.J.; Gall, D.G.; Wallace, J.L. Disruption of Colonic Electrolyte Transport in Experimental Colitis. Am. J. Physiol. Liver Physiol. 1995, 268, G622–G630. [Google Scholar] [CrossRef]
- Krawisz, J.E.; Sharon, P.; Stenson, W.F. Quantitative Assay for Acute Intestinal Inflammation Based on Myeloperoxidase Activity. Assessment of Inflammation in Rat and Hamster Models. Gastroenterology 1984, 87, 1344–1350. [Google Scholar] [CrossRef]
- Esterbauer, H.; Cheeseman, K.H. Determination of Aldehydic Lipid Peroxidation Products: Malonaldehyde and 4-Hydroxynonenal. Methods Enzymol. 1990, 186, 407–421. [Google Scholar]
- da Silva, V.; de Araújo, A.; Araújo, D.; Lima, M.; Vasconcelos, R.; de Araújo Júnior, R.; Langasnner, S.; Pedrosa, M.; de Medeiros, C.; Guerra, G. Intestinal Anti-Inflammatory Activity of the Aqueous Extract from Ipomoea Asarifolia in DNBS-Induced Colitis in Rats. Int. J. Mol. Sci. 2018, 19, 4016. [Google Scholar] [CrossRef] [Green Version]
- Zea-Iriarte, W.-L.; Makiyama, K.; Goto, S.; Murase, K.; Urata, Y.; Sekine, I.; Hara, K.; Kondo, T. Impairment of Antioxidants in Colonic Epithelial Cells Isolated from Trinitrobenzene Sulphonic Acid-Induced Colitis Rats Protective Effect of Rebamipide. Scand. J. Gastroenterol. 1996, 31, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Guerra, G.C.B.; de Menezes, M.S.S.; de Araújo, A.A.; de Araújo Júnior, R.F.; de Medeiros, C.A.C.X. Olmesartan Prevented Intra-Articular Inflammation Induced by Zymosan in Rats. Biol. Pharm. Bull. 2016, 39, 1793–1801. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Values |
|---|---|
| pH | 4.50 ± 0.04 |
| Molar acidity (g/100 g) | 4.40 ± 0.00 |
| Moisture (g/100 g) | 12.98 ± 0.51 |
| Ashes (g/100 g) | 0.002 ± 0.00 |
| Ether extract (g/100 g) | 2.78 ± 0.69 |
| Crude fiber (g/100 g) | 63.85 ± 7.32 |
| Protein (g/100 g) | 16.36 ± 0.35 |
| Total carbohydrates (g/100 g) | 67.86 ± 0.00 |
| Total phenolics 1 (mg GAE/g) | 67.85 ± 0.04 |
| Total flavonoids 2 (mg QE/g) | 46.16 ± 0.03 |
| N. | Rt (min) | Adduct | MS (m/z) | MS2 (m/z) | MS3 (m/z) | Metabolite | References |
|---|---|---|---|---|---|---|---|
| 1 | 4.47 | [M+Cl]− | 377 | 341 | 281, 179, 161 | dihexose (maltose or sucrose) | [33] |
| 2 | 4.71 | [M−H]− | 195 | 177, 159, 129 | 101, 85, 57 | gluconic acid | [34] |
| 3 | 4.95 | [M+Cl]− | 539 | 503, 341 | 341, 323 | trihexose (raffinose) | [35] |
| 4 | 5.17 | [M+Cl]− | 377 | 341 | 281, 179, 161 | dihexose (maltose or sucrose) | [33] |
| 5 | 9.32 | [M−H]− | 191 | 173, 111 | 67 | citric acid | [36] |
| 6 | 9.69 | [M+H]+ | 135 | 117, 99 | 99, 71 | malic acid | [37] |
| 7 | 12.19 | [M+H]+ | 284 | 152 | 135, 128, 110 | guanosine | [38] |
| 8 | 19.50 | [M−H]− | 447 | 429, 403, 315, 297, 207, 177, 163 | 269, 195, 163, 153, 119 | dihydroxybenzoic acid O-pentose-hexose | [39] |
| 9 | 21.42 | [M−H]− | 447 | 315 | 153, 123 | dihydroxybenzoic acid O-pentose-hexose | [39] |
| 10 | 22.46 | [M+H]+ | 195 | 177 | 163, 145, 117 | ferulic acid | [40] |
| 11 | 24.36 | [M−H]− | 355 | 295, 265, 235, 217, 193, 175, 160, 134 | 178, 149, 134 | ferulic acid O-hexose | [40] |
| 12 | 25.75 | [M−H]− | 355 | 217, 193, 175 | 178, 149, 134 | ferulic acid O-hexose | [40] |
| 13 | 27.53 | [M+H]+ | 169 | 137 | 109, 93, 81 | vanillic acid | [41] |
| 14 | 30.98 | [M+H]+ | 757 | 611, 465, 303 | 285, 257, 229, 165, 153 | quercetin-O-hexose-deoxyhexose | [42] |
| 15 | 31.89 | [M+H]+ | 476 | 314 | 177, 145 | feruloyl tyramine-O- hexose | [43] |
| 16 | 32.98 | [M+H]+ | 771 | 625, 479, 463, 427, 317, 302 | 302, 285, 274, 257, 153 | methylquercetin-O- hexose-deoxyhexose-deoxyhexose | [42] |
| 17 | 34.32 | [M+H]+ | 562 | 386, 314 | 177, 145 | feruloyl tyramine-O- (malonyl)-hexose | [44] |
| 18 | 35.19 | [M+H]+ | 611 | 593, 465, 303 | 285, 257, 229, 165 | quercetin-O-hexose-deoxyhexose (rutin) | [45] |
| 19 | 36.56 | [M−H]− | 445 | 283 | 268 | hydroxy-methoxy- isoflavone-O-hexose | [42] |
| 20 | 37.27 | [M+H]+ | 461 | 299 | 284, 266 | hydroxy-dimethoxy- isoflavone-O-hexose | [42] |
| 21 | 37.46 | [M+H]+ | 314 | 177, 145, 117 | 145, 117 | feruloyltyramine | [44] |
| 22 | 38.15 | [M+H]+ | 595 | 449, 287 | 269, 258, 241, 231, 213, 197, 165, 153, 121 | kaempferol-O-hexose-deoxyhexose | [42] |
| 23 | 38.60 | [M+H]+ | 625 | 479, 317, 302 | 302, 285, 229, 165, 153 | methylquercetin-O-hexose-deoxyhexose | [42] |
| 24 | 38.83 | [M+H]+ | 547 | 299, 284, 266 | 284, 266 | hydroxydimethoxy-isoflavone-O-(malonyl)-hexose | [42] |
| 25 | 39.91 | [M+H]+ | 547 | 299, 284, 266 | 284, 266, 239 | hydroxydimethoxy-isoflavone-O-(malonyl)-hexose | [42] |
| Biochemical Parameters | |||
|---|---|---|---|
| Parameters | Controlns | NCHEns (2000 mg/kg) | Reference Wistar (Females) |
| Glucose (mg/dL) | 159 ± 5.39 | 151 ± 9.26 | 53–172 |
| Triglycerides (mg/dL) | 48 ± 9.91 | 54 ± 5.26 | 23–138 |
| Total Cholesterol (mg/dL) | 69 ± 5.55 | 62 ± 3.33 | 54–96 |
| Urea (mg/dL) | 36 ± 5.55 | 33 ± 5.26 | 24–49 |
| Creatinine (mg/dL) | 0.6 ± 0.05 | 0.5 ± 0.08 | 0.3–1.1 |
| Albumin (mg/dL) | 2.9 ± 0.09 | 3.0 ± 0.04 | 1.3–3.8 |
| AST (U/L) | 99 ± 2.90 | 86 ±8.53 | 51–211 |
| ALT (U/L) | 50 ± 0.69 | 52 ± 0.55 | 32–62 |
| Hematological Parameters | |||
| Erythrocytes (×106/µL) | 7.53 ± 5.39 | 7.28 ± 9.26 | 5.21–8.83 |
| Hemoglobin (g/dL) | 13.7 ± 2.20 | 13.40 ± 1.13 | 11.1–17.10 |
| Hematocrit (%) | 44 ± 2.95 | 43.00 ± 2.43 | 27.00–49.00 |
| MCV (fL) | 51.27 ± 1.55 | 49.86 ± 4.36 | 45.00–56.70 |
| MCH (pg) | 18.41 ± 1.95 | 20.06 ± 1.06 | 16.60–22.80 |
| MCHC (g/dL) | 30.74 ± 0.12 | 31.35 ± 0.98 | 30.40–43.90 |
| Leukocytes (cell/µL) | 6400 ± 1920 | 5900 ± 1150 | 2300–9900 |
| Platelets (×106/µL) | 904 ± 96 | 1.025 ± 89 | 760–1.310 |
| Open Field Test | |||
|---|---|---|---|
| Parameters | Day | Control | NCHE (2000 mg/kg) |
| Total distance traveled (cm) | 1 | 230.80 ± 3.93 | 250.20 ± 5.54 |
| 7 | 240.20 ± 2.15 | 220.20 ± 4.76 | |
| 14 | 260.80 ± 3.50 | 250.80 ± 4.52 | |
| Rearing or climbing behavior (count) | 1 | 14.20 ± 2.25 | 16.40 ± 8.40 |
| 7 | 12.32 ± 5.28 | 12.20 ± 4.38 | |
| 14 | 16.00 ± 6.06 | 19.6 ± 4.76 | |
| Grooming (count) | 1 | 8.60 ± 1.95 | 9.60 ± 2.91 |
| 7 | 7.00 ± 2.80 | 8.74 ± 2.67 | |
| 14 | 7.65 ± 1.54 | 9.80 ± 2.96 | |
| Number of defecations | 1 | 1.00 ± 1.00 a | 3.80 ± 1.00 b |
| 7 | 1.60 ± 1.02 a | 3.80 ± 0.98 b | |
| 14 | 0.80 ± 0.53 a | 4.20 ± 2.00 b | |
| Rotarod Test | |||
| Staying Time (seconds) | 1 | 180.0 ± 0.00 | 179.6 ± 0.40 |
| 7 | 180.0 ± 0.00 | 179.0 ± 1.00 | |
| 14 | 180.0 ± 0.00 | 180.0 ± 0.00 | |
| Relative Organ Weight (g/100 g Body Weight) | ||
|---|---|---|
| Organ | Controlns | NCHEns (2000 mg/kg)ns |
| Liver | 3.80 ± 0.12 | 4.10 ± 0.02 |
| Spleen | 0.28 ± 0.03 | 0.30 ± 0.04 |
| Kidneys | 0.90 ± 0.03 | 0.87 ± 0.04 |
| Heart | 0.35 ± 0.01 | 0.37 ± 0.02 |
| Lung | 0.42 ± 0.09 | 0.42 ± 0.20 |
| Gene | Sequence 5′–3′ | Annealing Temperature (°C) |
|---|---|---|
| β-actin | Fw—CGCACTGCCGCATCCTCT | 58 |
| Rv—GTCGAAGAGAGCCTCGG | ||
| MAPK1 | Fw—CCCAAGTGATGAGCCCATTG | 58 |
| Rv—GGTAAGTCGTCCAGCTCCATGT | ||
| NF-κB p65 | Fw—TCTGCTTCCAGGTGACAGTG | 58 |
| Rv—ATCTTGAGCTCGGCAGTGTT | ||
| ZO-1 | Fw—GGGGCCTACACTGATCAAGA | 56 |
| Rv—TGGAGATGAGGCTTCTGCTT | ||
| MUC-2 | Fw—GCAGTCCTCAGTGGCACCTC | 60 |
| Rv—CACCGTGGGGCTACTGGAGAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavares, E.d.A.; Guerra, G.C.B.; da Costa Melo, N.M.; Dantas-Medeiros, R.; da Silva, E.C.S.; Andrade, A.W.L.; de Souza Araújo, D.F.; da Silva, V.C.; Zanatta, A.C.; de Carvalho, T.G.; et al. Toxicity and Anti-Inflammatory Activity of Phenolic-Rich Extract from Nopalea cochenillifera (Cactaceae): A Preclinical Study on the Prevention of Inflammatory Bowel Diseases. Plants 2023, 12, 594. https://doi.org/10.3390/plants12030594
Tavares EdA, Guerra GCB, da Costa Melo NM, Dantas-Medeiros R, da Silva ECS, Andrade AWL, de Souza Araújo DF, da Silva VC, Zanatta AC, de Carvalho TG, et al. Toxicity and Anti-Inflammatory Activity of Phenolic-Rich Extract from Nopalea cochenillifera (Cactaceae): A Preclinical Study on the Prevention of Inflammatory Bowel Diseases. Plants. 2023; 12(3):594. https://doi.org/10.3390/plants12030594
Chicago/Turabian StyleTavares, Emanuella de Aragão, Gerlane Coelho Bernardo Guerra, Nadja Maria da Costa Melo, Renato Dantas-Medeiros, Elaine Cristine Souza da Silva, Anderson Wilbur Lopes Andrade, Daline Fernandes de Souza Araújo, Valéria Costa da Silva, Ana Caroline Zanatta, Thaís Gomes de Carvalho, and et al. 2023. "Toxicity and Anti-Inflammatory Activity of Phenolic-Rich Extract from Nopalea cochenillifera (Cactaceae): A Preclinical Study on the Prevention of Inflammatory Bowel Diseases" Plants 12, no. 3: 594. https://doi.org/10.3390/plants12030594
APA StyleTavares, E. d. A., Guerra, G. C. B., da Costa Melo, N. M., Dantas-Medeiros, R., da Silva, E. C. S., Andrade, A. W. L., de Souza Araújo, D. F., da Silva, V. C., Zanatta, A. C., de Carvalho, T. G., de Araújo, A. A., de Araújo-Júnior, R. F., & Zucolotto, S. M. (2023). Toxicity and Anti-Inflammatory Activity of Phenolic-Rich Extract from Nopalea cochenillifera (Cactaceae): A Preclinical Study on the Prevention of Inflammatory Bowel Diseases. Plants, 12(3), 594. https://doi.org/10.3390/plants12030594







