Analysis of Fast Fluorescence Kinetics of a Single Cyanobacterium Trapped in an Optical Microcavity
Abstract
:1. Introduction
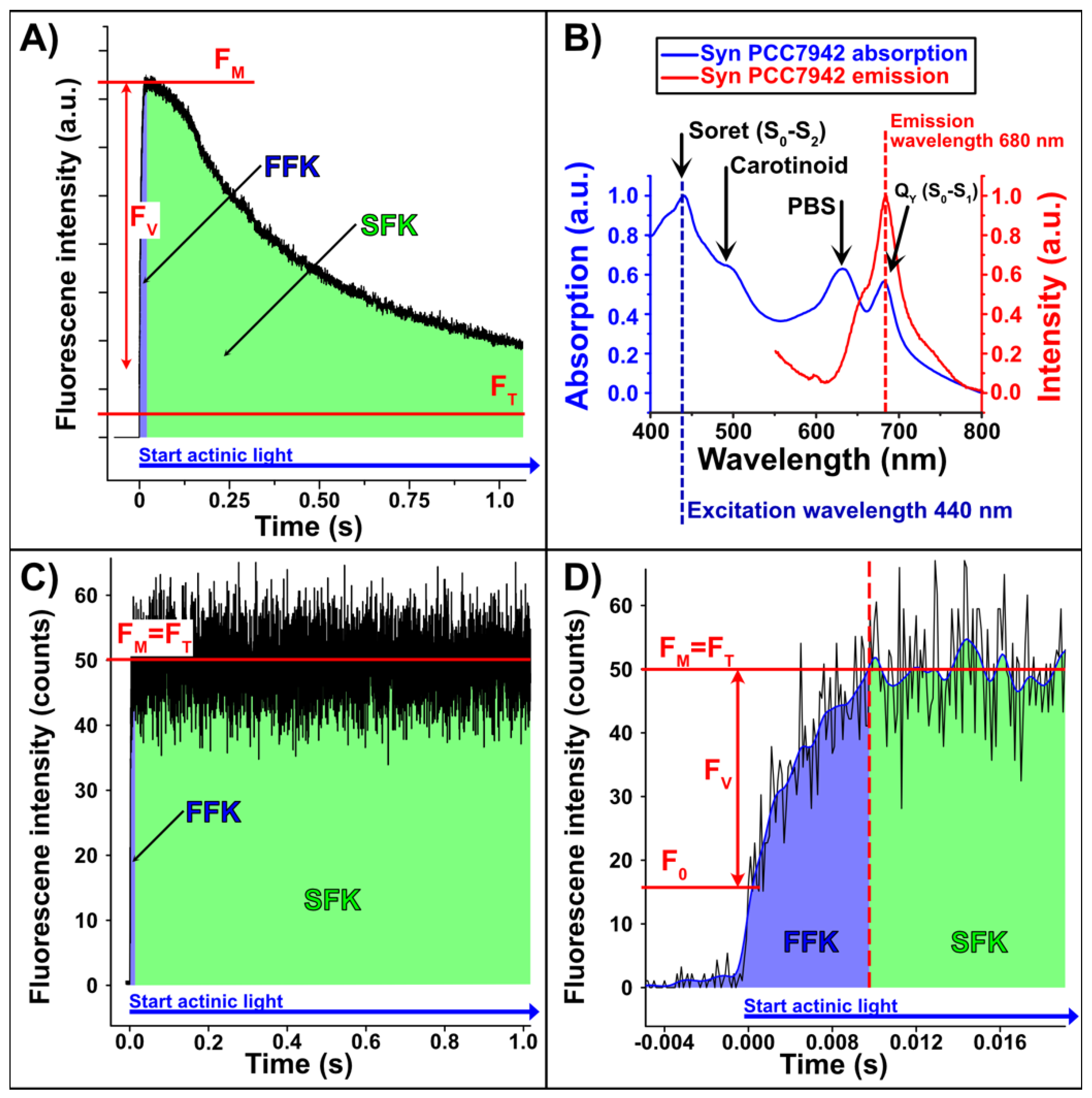
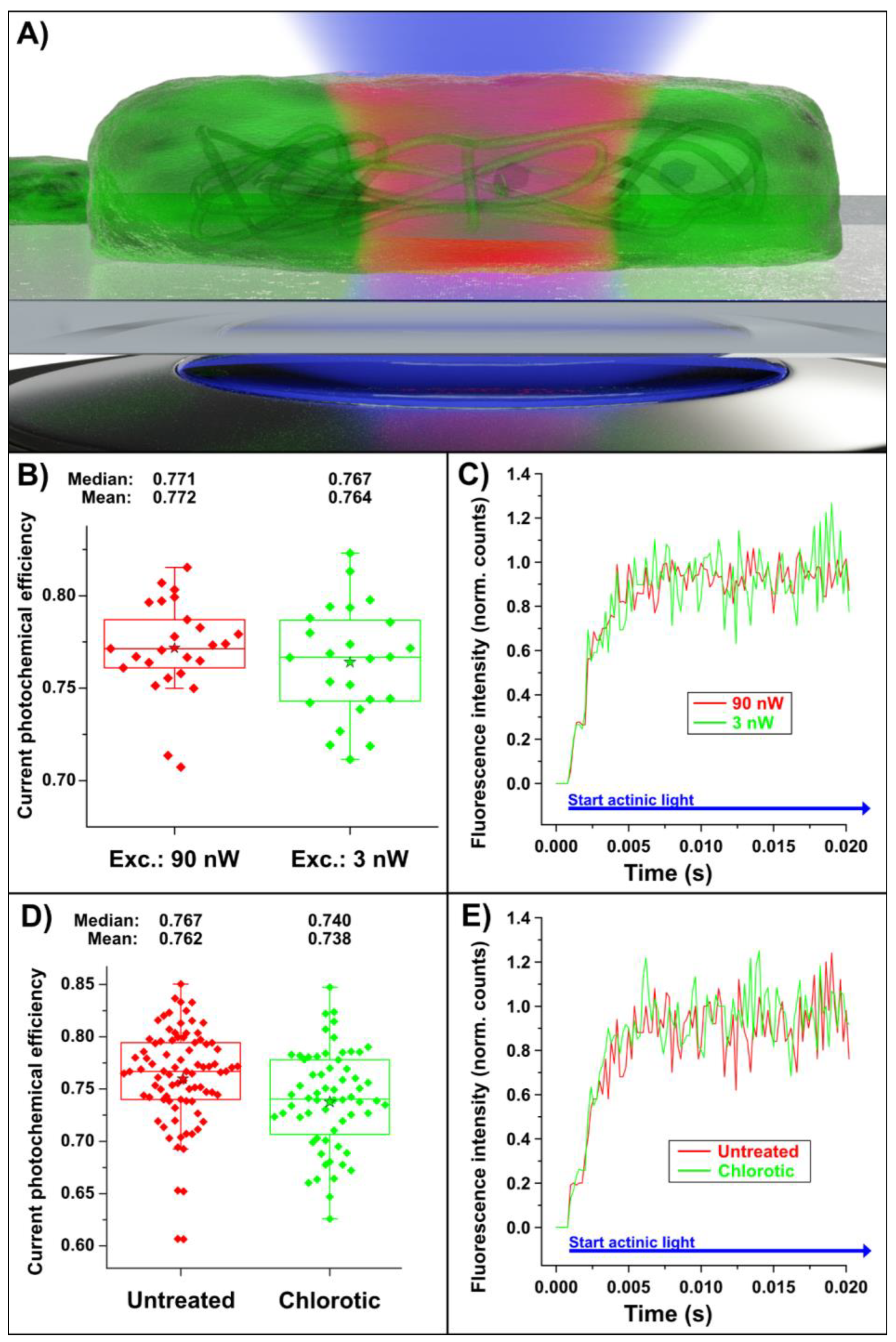
2. Results
2.1. Theoretical Introduction and Technical Challenges
2.2. Measurement Results
3. Discussion
4. Materials and Methods
4.1. Experimental Set-Up
4.2. Preparation of Microcavity Mirrors
4.3. Light Intensity Measurements
4.4. Bacterial Cultivation Conditions
4.5. Bacteria Preparation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schirrmeister, B.E.; de Vos, J.M.; Antonelli, A.; Bagheri, H.C. Evolution of Multicellularity Coincided with Increased Diversification of Cyanobacteria and the Great Oxidation Event. Proc. Natl. Acad. Sci. USA 2013, 110, 1791–1796. [Google Scholar] [CrossRef] [Green Version]
- Holland, H.D. The Oxygenation of the Atmosphere and Oceans. Philos. Trans. R. Soc. B 2006, 361, 903–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keeling, P.J. The Number, Speed, and Impact of Plastid Endosymbioses in Eukaryotic Evolution. Annu. Rev. Plant Biol. 2013, 64, 583–607. [Google Scholar] [CrossRef] [Green Version]
- Mančal, T. A Decade with Quantum Coherence: How Our Past Became Classical and the Future Turned Quantum. Chem. Phys. 2020, 532, 110663. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Küpper, H.; Benedikty, Z.; Morina, F.; Andresen, E.; Mishra, A.; Trtílek, M. Analysis of OJIP Chlorophyll Fluorescence Kinetics and QA Reoxidation Kinetics by Direct Fast Imaging. Plant Physiol. 2019, 179, 369–381. [Google Scholar] [CrossRef] [Green Version]
- Kautsky, H.; Hirsch, A. Neue Versuche zur Kohlensäureassimilation. Naturwissenschaften 1931, 19, 964. [Google Scholar] [CrossRef]
- Rammler, T.; Wackenhut, F.; zur Oven-Krockhaus, S.; Rapp, J.; Forchhammer, K.; Harter, K.; Meixner, A.J. Strong Coupling between an Optical Microcavity and Photosystems in Single Living Cyanobacteria. J. Biophotonics 2022, 15, e202100136. [Google Scholar] [CrossRef] [PubMed]
- Purcell, E.M. Spontaneous Emission Probabilities at Radio Frequencies. In Confined Electrons and Photons: New Physics and Applications; Burstein, E., Weisbuch, C., Eds.; Springer: Boston, MA, USA, 1995; p. 839. ISBN 978-1-4615-1963-8. [Google Scholar]
- Chizhik, A.I.; Chizhik, A.M.; Khoptyar, D.; Bär, S.; Meixner, A.J.; Enderlein, J. Probing the Radiative Transition of Single Molecules with a Tunable Microresonator. Nano Lett. 2011, 11, 1700–1703. [Google Scholar] [CrossRef]
- Chizhik, A.; Schleifenbaum, F.; Gutbrod, R.; Chizhik, A.; Khoptyar, D.; Meixner, A.J.; Enderlein, J. Tuning the Fluorescence Emission Spectra of a Single Molecule with a Variable Optical Subwavelength Metal Microcavity. Phys. Rev. Lett. 2009, 102, 073002. [Google Scholar] [CrossRef]
- Simis, S.G.H.; Huot, Y.; Babin, M.; Seppälä, J.; Metsamaa, L. Optimization of Variable Fluorescence Measurements of Phytoplankton Communities with Cyanobacteria. Photosynth. Res. 2012, 112, 13–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casella, S.; Huang, F.; Mason, D.; Zhao, G.-Y.; Johnson, G.N.; Mullineaux, C.W.; Liu, L.-N. Dissecting the Native Architecture and Dynamics of Cyanobacterial Photosynthetic Machinery. Mol. Plant 2017, 10, 1434–1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rexroth, S.; Nowaczyk, M.M.; Rögner, M. Cyanobacterial Photosynthesis: The Light Reactions. In Modern Topics in the Phototrophic Prokaryotes: Metabolism, Bioenergetics, and Omics; Hallenbeck, P.C., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 163–191. ISBN 978-3-319-51365-2. [Google Scholar]
- Kondou, Y.; Nakazawa, M.; Higashi, S.; Watanabe, M.; Manabe, K. Equal-Quantum Action Spectra Indicate Fluence-Rate-Selective Action of Multiple Photoreceptors for Photomovement of the Thermophilic Cyanobacterium Synechococcus Elongatus. Photochem. Photobiol. 2007, 73, 90–95. [Google Scholar] [CrossRef]
- Kennis, J.T.M.; Gobets, B.; van Stokkum, I.H.M.; Dekker, J.P.; van Grondelle, R.; Fleming, G.R. Light Harvesting by Chlorophylls and Carotenoids in the Photosystem I Core Complex of Synechococcus Elongatus: A Fluorescence Upconversion Study. J. Phys. Chem. B 2001, 105, 4485–4494. [Google Scholar] [CrossRef]
- Lahmi, R.; Sendersky, E.; Perelman, A.; Hagemann, M.; Forchhammer, K.; Schwarz, R. Alanine Dehydrogenase Activity Is Required for Adequate Progression of Phycobilisome Degradation during Nitrogen Starvation in Synechococcus Elongatus PCC 7942. J. Bacteriol. 2006, 188, 5258–5265. [Google Scholar] [CrossRef] [Green Version]
- Damjanović, A.; Vaswani, H.M.; Fromme, P.; Fleming, G.R. Chlorophyll Excitations in Photosystem I of Synechococcus Elongatus. J. Phys. Chem. B 2002, 106, 10251–10262. [Google Scholar] [CrossRef]
- Pfündel, E.E.; Klughammer, C.; Meister, A.; Cerovic, Z.G. Deriving Fluorometer-Specific Values of Relative PSI Fluorescence Intensity from Quenching of F0 Fluorescence in Leaves of Arabidopsis Thaliana and Zea Mays. Photosynth. Res. 2013, 114, 189–206. [Google Scholar] [CrossRef]
- Krause, G.H.; Weis, E. Chlorophyll Fluorescence and Photosynthesis: The Basics. Annu. Rev. Plant Biol. 1991, 42, 313–349. [Google Scholar] [CrossRef]
- Zouni, A.; Witt, H.-T.; Kern, J.; Fromme, P.; Krauss, N.; Saenger, W.; Orth, P. Crystal Structure of Photosystem II from Synechococcus Elongatus at 3.8 Å Resolution. Nature 2001, 409, 739–743. [Google Scholar] [CrossRef]
- van Kooten, O.; Snel, J.F.H. The Use of Chlorophyll Fluorescence Nomenclature in Plant Stress Physiology. Photosynth. Res. 1990, 25, 147–150. [Google Scholar] [CrossRef]
- Lohscheider, J.N.; Strittmatter, M.; Küpper, H.; Adamska, I. Vertical Distribution of Epibenthic Freshwater Cyanobacterial Synechococcus Spp. Strains Depends on Their Ability for Photoprotection. PLoS ONE 2011, 6, e20134. [Google Scholar] [CrossRef] [Green Version]
- Papageorgiou, G.C.; Govindjee (Eds.) Chlorophyll a Fluorescence: A Signature of Photosynthesis; Advances in Photosynthesis and Respiration; Kluwer Academic: Dordrecht, The Netherlands, 2004; ISBN 978-1-4020-3217-2. [Google Scholar]
- Komenda, J.; Koblıžek, M.; Prašil, O. Characterization of Processes Responsible for the Distinct Effect of Herbicides DCMU and BNT on Photosystem II Photoinactivation in Cells of the Cyanobacterium Synechococcus Sp. PCC 7942. Photosynth. Res. 2000, 63, 135–144. [Google Scholar] [CrossRef]
- Baker, N.R. A Possible Role for Photosystem II in Environmental Perturbations of Photosynthesis. Physiol. Plant 1991, 81, 563–570. [Google Scholar] [CrossRef]
- Björkman, O.; Demmig, B. Photon Yield of O2 Evolution and Chlorophyll Fluorescence Characteristics at 77 K among Vascular Plants of Diverse Origins. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Matyssek, R.; Herppich, W.B. Experimentelle Pflanzenökologie: Chlorophyllfluoreszenzanalyse. In Handbuch der Geodäsie; Freeden, W., Rummel, R., Eds.; Springer Reference Naturwissenschaften; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–56. ISBN 978-3-662-46900-2. [Google Scholar]
- Ogawa, T.; Sonoike, K. Effects of Bleaching by Nitrogen Deficiency on the Quantum Yield of Photosystem II in Synechocystis sp. PCC 6803 Revealed by Chl Fluorescence Measurements. Plant Cell Physiol. 2016, 57, 558–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santabarbara, S.; Villafiorita Monteleone, F.; Remelli, W.; Rizzo, F.; Menin, B.; Casazza, A.P. Comparative Excitation-emission Dependence of the FV/FM Ratio in Model Green Algae and Cyanobacterial Strains. Physiol. Plant. 2019, 166, 351–364. [Google Scholar] [CrossRef]
- Görl, M.; Sauer, J.; Baier, T.; Forchhammer, K. Nitrogen-Starvation-Induced Chlorosis in Synechococcus PCC 7942: Adaptation to Long-Term Survival. Microbiology 1998, 144, 2449–2458. [Google Scholar] [CrossRef] [Green Version]
- Caruso, F.; Saikin, S.K.; Solano, E.; Huelga, S.F.; Aspuru-Guzik, A.; Plenio, M.B. Probing Biological Light-Harvesting Phenomena by Optical Cavities. Phys. Rev. B 2012, 85, 125424. [Google Scholar] [CrossRef] [Green Version]
- Boca, A.; Miller, R.; Birnbaum, K.M.; Boozer, A.D.; McKeever, J.; Kimble, H.J. Observation of the Vacuum Rabi Spectrum for One Trapped Atom. Phys. Rev. Lett. 2004, 93, 233603. [Google Scholar] [CrossRef] [Green Version]
- Konrad, A.; Metzger, M.; Kern, A.M.; Brecht, M.; Meixner, A.J. Controlling the Dynamics of Förster Resonance Energy Transfer inside a Tunable Sub-Wavelength Fabry—Pérot-Resonator. Nanoscale 2015, 7, 10204–10209. [Google Scholar] [CrossRef]
- Rippka, R.; Stanier, R.Y.; Deruelles, J.; Herdman, M.; Waterbury, J.B. Generic Assignments, Strain Histories and Properties of Pure Cultures of Cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef] [Green Version]
- Klotz, A.; Georg, J.; Bučinská, L.; Watanabe, S.; Reimann, V.; Januszewski, W.; Sobotka, R.; Jendrossek, D.; Hess, W.R.; Forchhammer, K. Awakening of a Dormant Cyanobacterium from Nitrogen Chlorosis Reveals a Genetically Determined Program. Curr. Biol. 2016, 26, 2862–2872. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rammler, T.; Wackenhut, F.; Rapp, J.; zur Oven-Krockhaus, S.; Forchhammer, K.; Meixner, A.J.; Harter, K. Analysis of Fast Fluorescence Kinetics of a Single Cyanobacterium Trapped in an Optical Microcavity. Plants 2023, 12, 607. https://doi.org/10.3390/plants12030607
Rammler T, Wackenhut F, Rapp J, zur Oven-Krockhaus S, Forchhammer K, Meixner AJ, Harter K. Analysis of Fast Fluorescence Kinetics of a Single Cyanobacterium Trapped in an Optical Microcavity. Plants. 2023; 12(3):607. https://doi.org/10.3390/plants12030607
Chicago/Turabian StyleRammler, Tim, Frank Wackenhut, Johanna Rapp, Sven zur Oven-Krockhaus, Karl Forchhammer, Alfred J. Meixner, and Klaus Harter. 2023. "Analysis of Fast Fluorescence Kinetics of a Single Cyanobacterium Trapped in an Optical Microcavity" Plants 12, no. 3: 607. https://doi.org/10.3390/plants12030607






