Root Exudates Mediate the Processes of Soil Organic Carbon Input and Efflux
Abstract
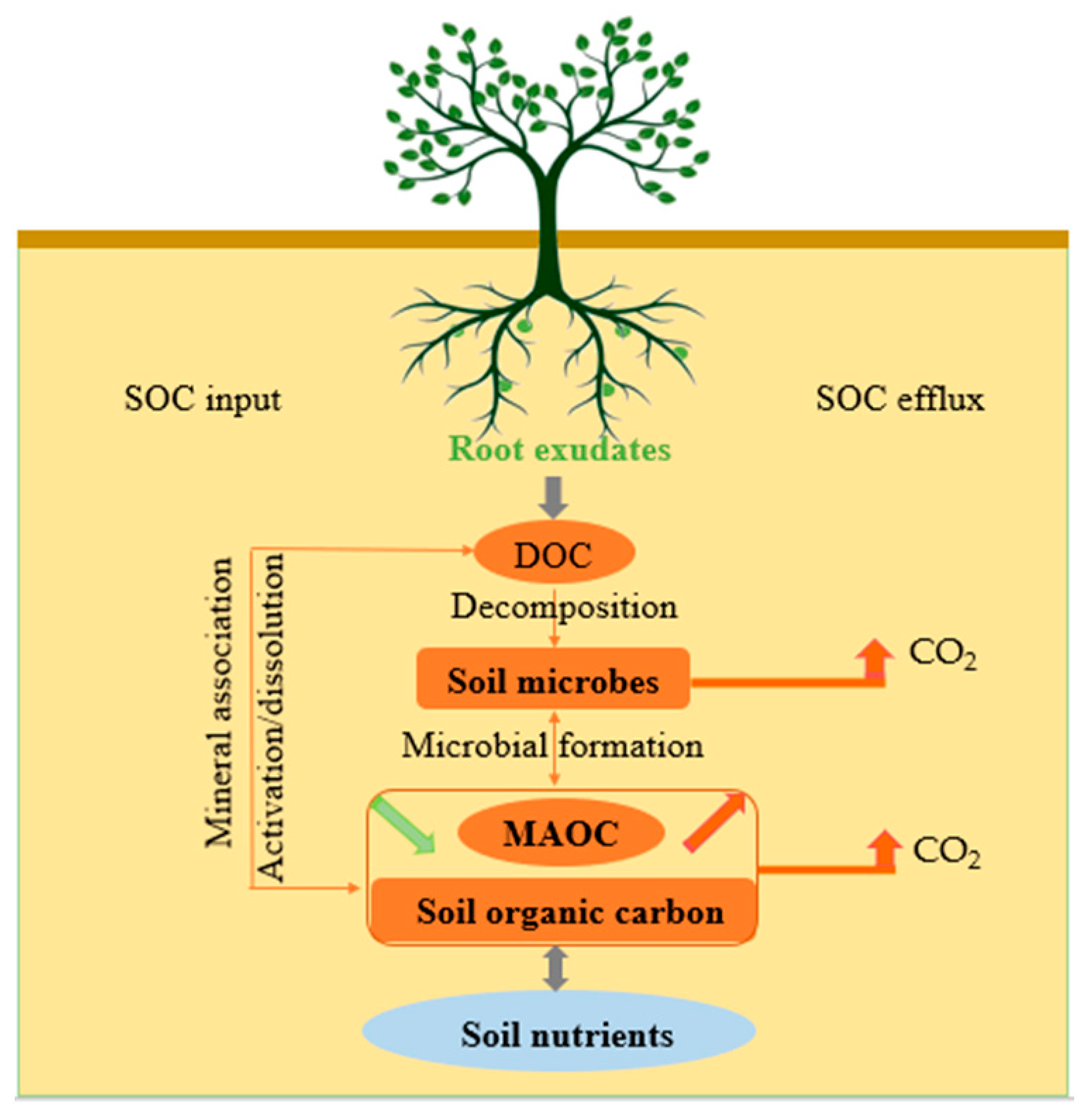
:1. Introduction
2. Root Exudates and Soil Organic Carbon Input
3. Root Exudates and Soil Organic Carbon Efflux
4. Mechanism of Root Exudates Affecting Soil Organic Carbon Sequestration
5. Conclusions and Prospects
- (1)
- The relationship between root exudates and root functional traits in different species
- (2)
- The interaction mechanism between root exudates and soil abiotic factors
- (3)
- The effects of root exudates on soil carbon function
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| C | carbon |
| SOC | soil organic carbon |
| POC | particulate organic carbon |
| MAOC | mineral-associated organic carbon |
| DOC | dissolved organic carbon |
| RD | root diameter |
| RTD | root tissue density |
| RNC | root nitrogen concentration |
References
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, A.C.; Siwar, C.; Shaharudin, M.I.; Anizan, I. Carbon Sequestration in Soils: The Opportunities and Challenges. Carbon Capture Util. Sequestration 2018. [Google Scholar] [CrossRef] [Green Version]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Chang. Biol. 2020, 26, 261–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, W.; Huang, W.; Weintraub-Leff, S.R.; Hall, S.J. Where and why do particulate organic matter (POM) and mineral-associated organic matter (MAOM) differ among diverse soils? Soil Bio. Biochem. 2022, 172, 108756. [Google Scholar] [CrossRef]
- Chen, L.; Fang, K.; Wei, B.; Qin, S.; Feng, X.; Hu, T.; Ji, C.; Yang, Y.; Cleland, E. Soil carbon persistence governed by plant input and mineral protection at regional and global scales. Ecol. Lett. 2021, 24, 1018–1028. [Google Scholar] [CrossRef]
- Sokol, W.N.; Kuebbing, S.E.; Karlsen-Ayala, E.; Bradford, M.A. Evidence for the primacy of living root inputs, not root or shoot litter, in forming soil organic carbon. New Phytol. 2019, 221, 233–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hicks, P.; Caitlin, E.; Bird, J.; Castanha, C.; Hatton, P.J.; Torn, M.S. Long term decomposition: The influence of litter type and soil horizon on retention of plant carbon and nitrogen in soils. Biogeochemistry 2017, 134, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Jackson, R.B.; Lajtha, K.; Crow, S.E.; Hugelius, G.; Kramer, M.G.; Piñeiro, G. The Ecology of Soil Carbon: Pools, Vulnerabilities, and Biotic and Abiotic Controls. Ann. Rev. Ecol. 2017, 48, 419–445. [Google Scholar] [CrossRef] [Green Version]
- Clemmensen, K.E.; Bahr, A.; Ovaskainen, O.; Dahlberg, A.; Ekblad, A.; Wallander, H.; Stenlid, J.; Finlay, R.D.; Wardle, D.A.; Lindahl, B.D. Roots and Associated Fungi Drive Long-Term Carbon Sequestration in Boreal Forest. Science 2013, 339, 1615–1618. [Google Scholar] [CrossRef]
- Pausch, J.; Kuzyakov, Y. Carbon input by roots into the soil: Quantification of rhizodeposition from root to ecosystem scale. Glob. Chang. Biol. 2018, 24, 1–12. [Google Scholar] [CrossRef]
- Huang, J.; Liu, W.; Yang, S.; Yang, L.; Peng, Z.; Deng, M.; Xu, S.; Zhang, B.; Ahirwal, J.; Liu, L. Plant carbon inputs through shoot, root, and mycorrhizal pathways affect soil organic carbon turnover differently. Soil Biol. Biochem. 2021, 160, 108322. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; Zhu, B.; Cheng, W. Root effects on soil organic carbon: A double-edged sword. New Phytol. 2021, 230, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tilman, D.; Furey, G.; Lehman, C. Soil carbon sequestration accelerated by restoration of grassland biodiversity. Nat. Commun. 2019, 10, 718. [Google Scholar] [CrossRef] [Green Version]
- Freschet, G.T.; Roumet, C.; Treseder, K. Sampling roots to capture plant and soil functions. Funct. Ecol. 2017, 31, 1506–1518. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Liu, W.; Deng, M.; Wang, X.; Wang, Z.; Yang, L.; Liu, L. Allocation and turnover of rhizodeposited carbon in different soil microbial groups. Soil Biol. Biochem. 2020, 150, 107973. [Google Scholar] [CrossRef]
- Gargallo-Garriga, A.; Preece, C.; Sardans, J.; Oravec, M.; Urban, O.; Penuelas, J. Root exudate metabolomes change under drought and show limited capacity for recovery. Sci. Rep. 2018, 8, 12696. [Google Scholar] [CrossRef] [Green Version]
- Xiao, C.; Yang, F.; Zhou, Y.; Su, J.; Liang, Y.; Pei, Z. Research progress on belowground carbon input and efflux processes in terrestrial ecosystems. Chin. Bull. Bot. 2017, 52, 652–668. [Google Scholar]
- Shen, X.; Yang, F.; Xiao, C.; Zhou, Y. Increased contribution of root exudates to soil carbon input during grassland degradation. Soil Biol. Biochem. 2020, 146, 107817. [Google Scholar] [CrossRef]
- Keiluweit, M.; Bougoure, J.J.; Nico, P.S.; Pett-Ridge, J.; Weber, P.K.; Kleber, M. Mineral protection of soil carbon counteracted by root exudates. Nat. Clim. Chang. 2015, 5, 588–595. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhu, L.; Cheng, Y.; Xing, Q.; Lan, Y. Current situation and trend of root exudates research-knowledge mapping analysis based on citespace. Jiangsu Agric. Sci. 2022, 50, 34–45. [Google Scholar]
- Yin, H.; Zhang, Z.; Liu, Q. Root exudates and their ecological consequences in forest ecosystems: Problems and perspective. Chin. J. Plant Ecol. 2018, 42, 1055–1070. [Google Scholar] [CrossRef]
- Haichar, F.Z.; Santaella, C.; Heulin, T.; Achouak, W. Root exudates mediated interactions belowground. Soil Bio. Biochem. 2014, 77, 69–80. [Google Scholar] [CrossRef]
- Li, J.; Fan, M.; Shang-Guan, Z. Research progress on main ecological functions of plant root exudates. Chin. Bull. Bot. 2020, 55, 788–796. [Google Scholar]
- Canarini, A.; Kaiser, C.; Merchant, A.; Richter, A.; Wanek, W. Root Exudation of Primary Metabolites: Mechanisms and Their Roles in Plant Responses to Environmental Stimuli. Front. Plant Sci. 2019, 10, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Yang, F.; Han, P.; Zhou, W.; Wang, J.; Yan, X.; Lin, J. Research progress on the mechanism of root exudates in response to abiotic stresses. Chin. J. Appl. Environ. Biol. 2021, 28, 1384–1392. [Google Scholar]
- Zhou, S.; Lin, J.; Wang, P.; Zhu, P.; Zhu, B. Resistant soil organic carbon is more vulnerable to priming by root exudate fractions than relatively active soil organic carbon. Plant Soil 2022, in press. [Google Scholar] [CrossRef]
- Pett-Ridge, J.; Firestone, M.K. Using stable isotopes to explore root-microbe-mineral interactions in soil. Rhizosphere 2017, 3, 244–253. [Google Scholar] [CrossRef]
- Baumert, V.L.; Vasilyeva, N.A.; Vladimirov, A.; Kallenbach, C.M.; Frey, S.D.; Grandy, A.S. Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat. Commun. 2016, 7, 13630. [Google Scholar]
- Baumert, V.L.; Vasilyeva, N.A.; Vladimirov, A.A.; Meier, I.C.; Kögel-Knabner, I.; Mueller, C.W. Root Exudates Induce Soil Macroaggregation Facilitated by Fungi in Subsoil. Front. Environ. Sci. 2018, 6, 140. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Soong, J.L.; Horton, A.J.; Campbell, E.E.; Haddix, M.L.; Wall, D.H.; Parton, W.J. Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat. Geosci. 2015, 8, 776–779. [Google Scholar] [CrossRef]
- Gao, X. Root Exudates of Dominant Plants and the Effects of Their Main Components on Soil Microorganisms in Stipa Breviflora Desert Steppe. Doctoral Dissertation, Inner Mongolia Agricultural University, Huhhot, China, 2017. [Google Scholar]
- Williams, A.; Langridge, H.; Straathof, A.L.; Muhamadali, H.; Hollywood, K.A.; Goodacre, R.; Vries, F.T. Root functional traits explain root exudation rate and composition across a range of grassland species. J. Ecol. 2021, 110, 21–33. [Google Scholar] [CrossRef]
- Poirier, V.; Roumet, C.; Munson, A.D. The root of the matter: Linking root traits and soil organic matter stabilization processes. Soil Bio. Biochem. 2018, 120, 246–259. [Google Scholar] [CrossRef]
- Guyonnet, J.P.; Cantarel, A.A.M.; Simon, L.; Haichar, F.E. Root exudation rate as functional trait involved in plant nutrient-use strategy classification. Ecol. Evol. 2018, 8, 8573–8581. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Ataka, M.; Han, M.; Han, Y.; Gan, D.; Xu, T.; Guo, Y.; Zhu, B. Root exudation as a major competitive fine-root functional trait of 18 coexisting species in a subtropical forest. New Phytol. 2021, 229, 259–271. [Google Scholar] [CrossRef]
- Kramer-Walter, K.R.; Bellingham, P.J.; Millar, T.R.; Smissen, R.D.; Richardson, S.J.; Laughlin, D.C. Root traits are multidimensional: Specific root length is independent from root tissue density and the plant economic spectrum. J. Ecol. 2016, 104, 1299–1310. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Cavagnaro, T.R.; Jiang, Y.; Keitel, C.; Dijkstra, F.A. Carbon allocation to the rhizosphere is affected by drought and nitrogen addition. J. Ecol. 2021, 109, 3699–3709. [Google Scholar] [CrossRef]
- Williams, A.; de Vries, F.T. Plant root exudation under drought: Implications for ecosystem functioning. New Phytol. 2020, 225, 1899–1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuzyakov, Y. Priming effects: Interactions between living and dead organic matter. Soil Biol. Biochem. 2010, 42, 1363–1371. [Google Scholar] [CrossRef]
- Zhou, J.; Guillaume, T.; Wen, Y.; Blagodatskaya, E.; Shahbaz, M.; Zeng, Z.; Peixoto, L.; Zang, H.; Kuzyakov, Y. Frequent carbon input primes decomposition of decadal soil organic matter. Soil Biol. Biochem. 2022, 175, 108850. [Google Scholar] [CrossRef]
- Henneron, L.; Cros, C.; Picon-Cochard, C.; Rahimian, V.; Fontaine, S. Plant economic strategies of grassland species control soil carbon dynamics through rhizodeposition. J. Ecol. 2019, 108, 528–545. [Google Scholar] [CrossRef]
- Li, J.; Zhang, R.; Cheng, B.; Ye, L.; Li, W.; Shi, X. Effects of nitrogen and phosphorus additions on decomposition and accumulation of soil organic carbon in alpine meadows on the Tibetan Plateau. Land Degrad. Dev. 2021, 32, 1467–1477. [Google Scholar] [CrossRef]
- Han, M.; Sun, L.; Gan, D.; Fu, L.; Zhu, B. Root functional traits are key determinants of the rhizosphere effect on soil organic matter decomposition across 14 temperate hardwood species. Soil Biol. Biochem. 2020, 151, 108019. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Qin, S.; Yang, G.; Fang, K.; Zhu, B.; Kuzyakov, Y.; Chen, P.; Xu, Y.; Yang, Y. Regulation of priming effect by soil organic matter stability over a broad geographic scale. Nat. Commun. 2019, 10, 5112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Feng, J.; Li, J.; Huang, C.; Shen, Y.; Cheng, W.; Zhu, B. A distinct sensitivity to the priming effect between labile and stable soil organic carbon. New Phytol. 2022, 237, 88–99. [Google Scholar] [CrossRef]
- Finzi, A.C.; Abramoff, R.Z.; Spiller, K.S.; Brzostek, E.R.; Darby, B.A.; Kramer, M.A.; Phillips, R.P. Rhizosphere processes are quantitatively important components of terrestrial carbon and nutrient cycles. Glob. Chang. Biol. 2015, 21, 2082–2094. [Google Scholar] [CrossRef]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loque, D.; Bowen, B.P.; et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, N.; Schaefer, D.; Blagodatskaya, E.; Zou, X.; Xu, X.; Kuzyakov, Y. Labile carbon retention compensates for CO2 released by priming in forest soils. Glob. Chang. Biol. 2014, 20, 1943–1954. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Bicharanloo, B.; Shirvan, M.B.; Cavagnaro, T.R.; Jiang, Y.; Keitel, C.; Dijkstra, F.A. A novel 13C pulse-labelling method to quantify the contribution of rhizodeposits to soil respiration in a grassland exposed to drought and nitrogen addition. New Phytol. 2021, 230, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Dijkstra, F.A.; Wang, P.; Zhu, B.; Cheng, W. Rhizosphere priming effects on soil carbon and nitrogen dynamics among tree species with and without intraspecific competition. New Phytol. 2018, 218, 1036–1048. [Google Scholar] [CrossRef] [Green Version]
- Meier, I.C.; Finzi, A.C.; Phillips, R.P. Root exudates increase N availability by stimulating microbial turnover of fast-cycling N pools. Soil Biol. Biochem. 2017, 106, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Phillips, R.P.; Meier, I.C.; Bernhardt, E.S.; Grandy, A.S.; Wickings, K.; Finzi, A.C. Roots and fungi accelerate carbon and nitrogen cycling in forests exposed to elevated CO2. Ecol Lett. 2012, 15, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Kuzyakov, Y. Sources of CO2 efflux from soil and review of partitioning methods. Soil Biol. Biochem. 2006, 38, 425–448. [Google Scholar] [CrossRef]
- Werth, M.; Kuzyakov, Y. 13C fractionation at the root–microorganisms–soil interface: A review and outlook for partitioning studies. Soil Biol. Biochem. 2010, 42, 1372–1384. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L.; Zheng, S.; Chen, Z.; Cao, Y.; Wen, X.; He, N. Temperature sensitivity of soil microbial respiration in soils with lower substrate availability is enhanced more by labile carbon input. Soil Biol. Biochem. 2021, 154, 108148. [Google Scholar] [CrossRef]
- Castellano, M.J.; Mueller, K.E.; Olk, D.C.; Sawyer, J.E.; Six, J. Integrating plant litter quality, soil organic matter stabilization, and the carbon saturation concept. Glob. Chang. Biol. 2015, 21, 3200–3209. [Google Scholar] [CrossRef] [Green Version]
- Rocci, K.S.; Lavallee, J.M.; Stewart, C.E.; Cotrufo, M.F. Soil organic carbon response to global environmental change depends on its distribution between mineral-associated and particulate organic matter: A meta-analysis. Sci. Total Environ. 2021, 793, 148569. [Google Scholar] [CrossRef]
- Lugato, E.; Lavallee, J.M.; Haddix, M.L.; Panagos, P.; Cotrufo, M.F. Different climate sensitivity of particulate and mineral-associated soil organic matter. Nat. Geosci. 2021, 14, 295–300. [Google Scholar] [CrossRef]
- Feng, J.; He, K.; Zhang, Q.; Han, M.; Zhu, B. Changes in plant inputs alter soil carbon and microbial communities in forest ecosystems. Glob. Chang. Biol. 2022, 28, 3426–3440. [Google Scholar] [CrossRef]
- Villarino, S.; Pinto, P.; Jackson, R.B.; Piñeiro, G. Plant rhizodeposition a key factor for soil organic matter formation in stable fractions. Sci. Adv. 2021, 7, 3176. [Google Scholar] [CrossRef]
- Sokol, N.W.; Bradford, M.A. Microbial formation of stable soil carbon is more efficient from belowground than aboveground input. Nat. Geosci. 2018, 12, 46–53. [Google Scholar] [CrossRef]
- Lange, M.; Eisenhauer, N.; Sierra, C.A.; Bessler, H.; Engels, C.; Griffiths, R.I.; Mellado-Vazquez, P.G.; Malik, A.A.; Roy, J.; Scheu, S.; et al. Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 2015, 6, 6707. [Google Scholar] [CrossRef] [Green Version]
- Panchal, P.; Preece, C.; Peñuelas, J.; Giri, J. Soil carbon sequestration by root exudates. Trends Plant Sci. 2022, 27, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Cotrufo, M. Grassland soil carbon sequestration: Current understanding, challenges, and solutions. Sci. Adv. 2022, 377, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Whalen, E.D.; Grandy, A.S.; Sokol, N.W.; Keiluweit, M.; Ernakovich, J.; Smith, R.G.; Frey, S.D. Clarifying the evidence for microbial- and plant-derived soil organic matter, and the path toward a more quantitative understanding. Glob. Chang. Biol. 2022, 28, 7167–7185. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.P.; Finzi, A.C.; Bernhardt, E.S. Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO2 fumigation. Ecol. Lett. 2011, 14, 187–194. [Google Scholar] [CrossRef]
- Islam, M.R.; Singh, B.; Dijkstra, F.A. Stabilisation of soil organic matter: Interactions between clay and microbes. Biogeochemistry 2022, 160, 145–158. [Google Scholar] [CrossRef]
- Chen, X.; Li, Q.; Chen, D.; He, F.; Huo, L.; Zhao, L.; Xiao, C. Reasonable management of perennial planting grassland contributes to positive succession of soil microbial community in Sanjiangyuan of Qinghai-Tibetan Plateau, China. J. Plant Ecol. 2022, 15, 359–371. [Google Scholar] [CrossRef]
- Yu, Y.; Zheng, L.; Zhou, Y.; Sang, W.; Zhao, J.; Liu, L.; Li, C.; Xiao, C. Changes in soil microbial community structure and function following degradation in a temperate grassland. J. Plant Ecol. 2021, 14, 384–397. [Google Scholar] [CrossRef]
- Jeewani, P.H.; Van Zwieten, L.; Zhu, Z.; Ge, T.; Guggenberger, G.; Luo, Y.; Xu, J. Abiotic and biotic regulation on carbon mineralization and stabilization in paddy soils along iron oxide gradients. Soil Biol. Biochem. 2021, 160. [Google Scholar] [CrossRef]
- Kleber, M.; Eusterhues, K.; Keiluweit, M.; Mikutta, C.; Mikutta, R.; Nico, P.S. Mineral–organic associations: Formation, properties, and relevance in soil environments. Adv. Agron. 2015, 130, 1–140. [Google Scholar]
- Wen, T.; Yu, G.; Hong, W.; Yuan, J.; Niu, G.; Xie, P.; Sun, F.; Guo, L.; Kuzyakov, Y.; Shen, Q. Root exudate chemistry affects soil carbon mobilization via microbial community reassembly. Fundam. Res. 2022, 2, 697–707. [Google Scholar] [CrossRef]
- Dong, J.; Hunt, J.; Delhaize, E.; Zheng, S.J.; Jin, C.W.; Tang, C. Impacts of elevated CO2 on plant resistance to nutrient deficiency and toxic ions via root exudates: A review. Sci. Total Environ. 2021, 754, 142434. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Xiao, J.; Zhang, Z.; Qiao, M.; He, W.; Liu, Q.; Yin, H. Effects of night-time warming on the rates and main chemical components of root exudatesproduced by Picea asperata seedlings in subalpine coniferous forests. Acta Ecol. Sin. 2018, 38, 3086–3096. [Google Scholar]
- Canarini, A.; Merchant, A.; Dijkstra, F.A. Drought effects on Helianthus annuus and Glycine max metabolites: From phloem to root exudates. Rhizosphere 2016, 2, 85–97. [Google Scholar] [CrossRef]
- Bobille, H.; Fustec, J.; Robins, R.J.; Cukier, C.; Limami, A.M. Effect of water availability on changes in root amino acids and associated rhizosphere on root exudation of amino acids in Pisum sativum L. Phytochemistry 2019, 161, 75–85. [Google Scholar] [CrossRef]
- de Vries, F.T.; Williams, A.; Stringer, F.; Willcocks, R.; McEwing, R.; Langridge, H.; Straathof, A.L. Changes in root-exudate-induced respiration reveal a novel mechanism through which drought affects ecosystem carbon cycling. New Phytol. 2019, 224, 132–145. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Yang, X.; Xiao, J.; Zhang, Z.; Jiang, Z.; Yuan, Y.; Wang, D.; Liu, Q.; Yin, H. Effects of nitrogen enrichment on root exudative carbon inputs in Sibiraea angustata shrubbery at the eastern fringe of Qinghai-Xizang Plateau. Chin. J. Plant Ecol. 2017, 41, 610–621. [Google Scholar]
- Chari, N.R.; Taylor, B.N. Soil organic matter formation and loss are mediated by root exudates in a temperate forest. Nat. Geosci. 2022, 15, 1011–1016. [Google Scholar] [CrossRef]
- Badri, D.V.; Vivanco, J.M. Regulation and function of root exudates. Plant Cell Environ. 2009, 32, 666–681. [Google Scholar] [CrossRef]
- Oburger, E.; Jones, D.L. Sampling root exudates—Mission impossible. Rhizosphere 2018, 6, 116–133. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, Y.; Luo, L.; Li, Z.; Yang, J.; Zu, C.; Yu, H.; Wu, H.; Zheng, W. Comparative Study on Collection Methods of Plant Root Exudates—Take Betel Nut as an Example. Chin. J. Trop. Agric. 2020, 40, 21–29. [Google Scholar]
- Guo, W.; Zhang, Z.; Liu, Q.; Yin, H. Research progress of root exudates collection technology. Chin. J. Appl. Ecol. 2019, 30, 3951–3962. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, X.; Shen, Y.; Zhao, J.; Huang, J.; Wang, H.; Yu, Y.; Xiao, C. Root Exudates Mediate the Processes of Soil Organic Carbon Input and Efflux. Plants 2023, 12, 630. https://doi.org/10.3390/plants12030630
Lei X, Shen Y, Zhao J, Huang J, Wang H, Yu Y, Xiao C. Root Exudates Mediate the Processes of Soil Organic Carbon Input and Efflux. Plants. 2023; 12(3):630. https://doi.org/10.3390/plants12030630
Chicago/Turabian StyleLei, Xue, Yuting Shen, Jianing Zhao, Jiajia Huang, Hui Wang, Yang Yu, and Chunwang Xiao. 2023. "Root Exudates Mediate the Processes of Soil Organic Carbon Input and Efflux" Plants 12, no. 3: 630. https://doi.org/10.3390/plants12030630
APA StyleLei, X., Shen, Y., Zhao, J., Huang, J., Wang, H., Yu, Y., & Xiao, C. (2023). Root Exudates Mediate the Processes of Soil Organic Carbon Input and Efflux. Plants, 12(3), 630. https://doi.org/10.3390/plants12030630






