Agroecological Management of the Grey Mould Fungus Botrytis cinerea by Plant Growth-Promoting Bacteria
Abstract
:1. Introduction
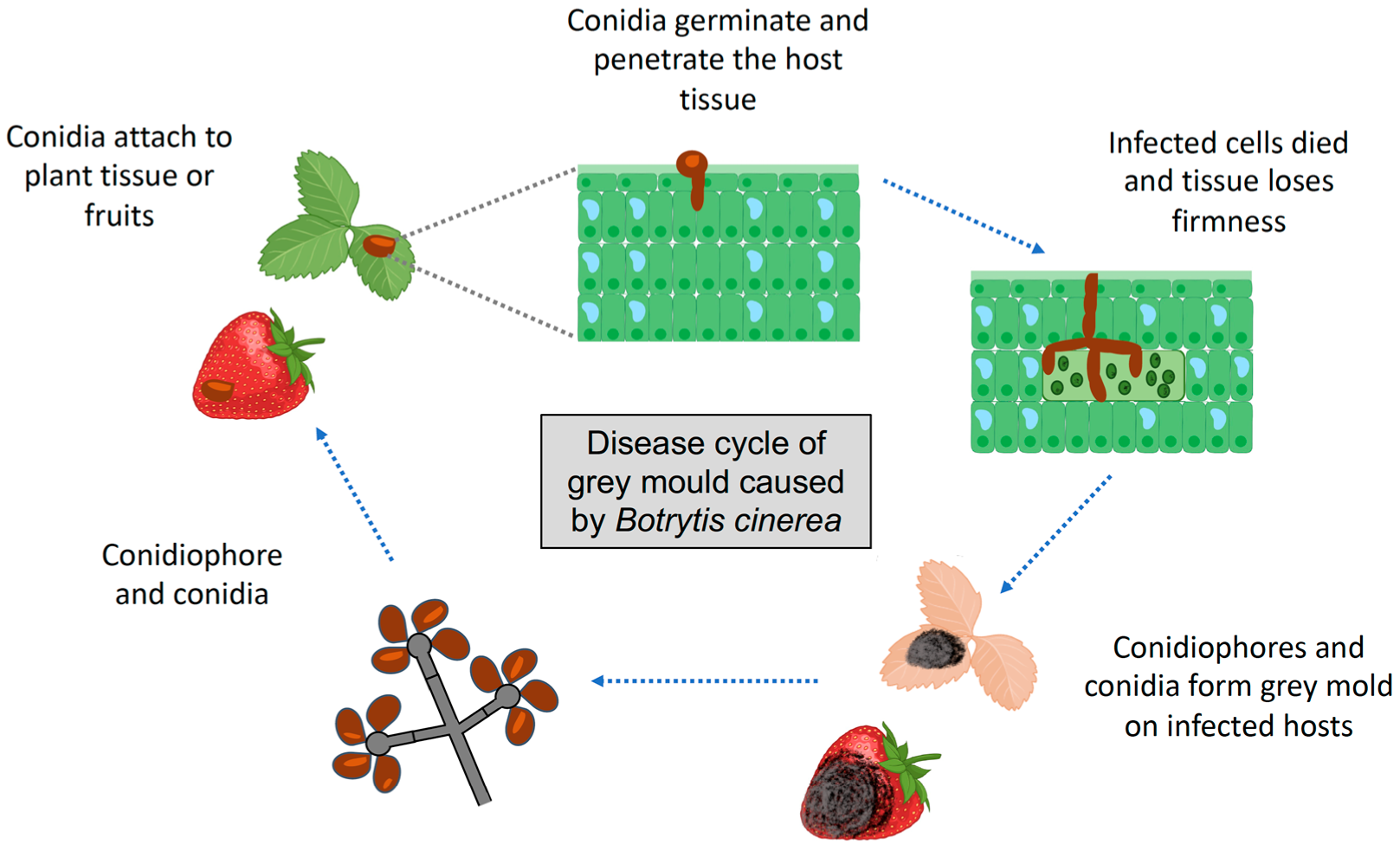
2. Botrytis cinerea Life Cycle and Infection Stages
3. Antifungal Mechanisms of Plant Growth-Promoting Bacteria (PGPB)
3.1. Antibiosis
3.2. Siderophores and Space Occupation
3.3. Ethylene and 1-aminocyclopropane-1-carboxylate (ACC) Deaminase Activity
3.4. Induced Systemic Resistance
4. Antagonistic Effects of PGPB on Botrytis cinerea
5. Biological Control of Botrytis cinerea by PGPB
6. PGPB Consortia to Control Botrytis cinerea
| Biocontrol Agent | Protected Host | Mechanisms of Action Exerted | Antagonistic Effect on B. cinerea/Benefit on Plant | Reference |
|---|---|---|---|---|
| Pseudomonas antimicrobica | None | Antifungal metabolites | Affectations on germ tube production and extension | [90] |
| P. aeruginosa strain LV | None | Phenazine-1-carboxylic acid (PCA) produced | Damage on the hyphae; mycelial growth inhibition | [92] |
| P. fluorescens strain QBA5 | Tomato fruit and plant leaves (cv. Laifen No.1) | Supernatant bioactive compounds | Damage in the conidia germination and plasma membrane; plant and fruit ripening protection | [91] |
| Bacillus cereus strain B-02 | None | Supernatant bioactive compounds | Changes on cell morphology (distortion, shrinking, and swelling) | [94] |
| Pseudomonas sp. strain PsJN | Plantlets of V. vinifera L. ‘Chardonnay’ | Diffusible antagonistic compounds | Growth disruption of fungal mycelium, coagulation, and leakage of protoplasm; plant protection | [97] |
| Bacillus subtilis strain QST713 | Vitis vinifera L. cv. Tempranillo | ND | ND; positive influence on grape production and oenological parameters | [99] |
| P. fluorescens PTA-268 and PTA-CT2, Bacillus subtilis PTA-271, Pantoea agglomerans PTA-AF1 and PTA-AF2, and Acinetobacter lwoffii PTA- 113 and PTA-152 | Vitis vinifera | Induced systemic resistance (ISR) | ND; stimulation in the leaves of vine plants included the activities of lipoxygenase, phenylalanine ammonia-lyase, and chitinase | [100] |
| Arthrobacter agilis UMCV2 | None | Dimethylhexadecylamine (DMHDA) | Mycelial growth inhibition | [101] |
| Aureobasidium pullulans, Bacillus amyloliquefaciens, Bacillus amyloliquefaciens plantarum, Bacillus subtilis, Pythium oligandrum, and Trichoderma atroviride | Grape berries | ND | ND; reduction on Botrytis bunch rot (BBR) disease | [102] |
| B. velezensis strains, 5YN8 and DSN012 | Pepper (Capsicum frutescens) | Secondary (diffusible) metabolites and volatile organic compounds (VOCs) | Suppression of the growth and spore formation; plant growth promotion | [109] |
| B. velezensis strain XT1 | Tomato and strawberry plants | Direct foliar and radicular application | ND; activation of the defence system through phytohormonal regulation | [110] |
| B. amyloliquefaciens strains BBC023 and BBC047 | Tomato plants | Phyllosphere colonization good capacity | ND; stimulation of tomato plant growth | [51] |
| P. fluorescens strains UM16, UM240, UM256, and UM270 | Medicago truncatula plants | Potential diffusible compounds (phenazines, cyanogens, and ACC 1-aminocyclopropane-1-carboxylate deaminase, production of biofilm, siderophores, proteases, indole-3-acetic acid), and volatiles such as dimethyl disulfide, dimethylhexadecylamine, and hydrogen cyanide | Promotion of Medicago truncatula plant biomass and chlorophyll content | [24] |
| Bacillus subtilis, Trichoderma atroviride, and Aureobasidium pullulans | Commercial vineyards in three locations in Italy | Direct application of mixture of biocontrol microorganisms | Biocontrol of the grey mould disease | [115] |
| Streptomyces spp. strains AUR2, AUR4, and ARR4, Mesorhizobium ciceri | Chickpea plants | Single and mixed inoculation in planta | Biocontrol of the grey mould disease; plant protection and induction antioxidant enzymes; enhanced nodulation and nitrogenase activity | [124] |
| Streptomyces spp. strains ATIRS43, ATIRS65, and ARRS10 | Chickpea plants; marigold (Tagetes erecta L.) flower | Potential action of HCN, ammonia (except ATIRS65), ß-1,3-glucanase, chitinase, cellulase (except ATIRS 65), protease, lipase, and siderophores (except ATIRS65) | Reduction on the grey mould disease incidence; stimulation of the plant growth and flower number | [120] |
7. Biocontrol Strategies to Prevent Botrytis cinerea Infection
8. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Choquer, M.; Fournier, E.; Kunz, C.; Levis, C.; Pradier, J.M.; Simon, A.; Viaud, M. Botrytis cinerea virulence factors: New insights into a necrotrophic and polyphageous pathogen. FEMS Microbiol. Lett. 2007, 277, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bi, K.; Liang, Y.; Mengiste, T.; Sharon, A. Killing softly: A roadmap of Botrytis cinerea pathogenicity. Trends Plant Sci. 2022, 28, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Elad, Y.; Stewart, A. Microbial control of Botrytis spp. In Botrytis: Biology, Pathology and Control; Springer: Dordrecht, The Netherlands, 2007; pp. 223–241. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Fillinger, S.; Elad, Y. Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Springer: Cham, Switzerland, 2015; pp. 1–486. [Google Scholar] [CrossRef]
- Risoli, S.; Cotrozzi, L.; Sarrocco, S.; Nuzzaci, M.; Pellegrini, E.; Vitti, A. Trichoderma-Induced Resistance to Botrytis cinerea in Solanum Species: A Meta-Analysis. Plants 2022, 11, 180. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, E.; Gonçalves, B.; Cortez, I.; Castro, I. The Role of Biostimulants as Alleviators of Biotic and Abiotic Stresses in Grapevine: A Review. Plants 2022, 11, 396. [Google Scholar] [CrossRef]
- Wang, R.; Liu, K.; Chen, B.; Ding, W.; Li, Y. Genetic and pathogenic variation of Botrytis cinerea, the causal agent of grey mould on Panax ginseng in China. Can. J. Plant Pathol. 2022, 44, 744–753. [Google Scholar] [CrossRef]
- Leroux, P. Chemical control of Botrytis and its resistance to chemical fungicides. In Botrytis: Biology, Pathology and Control; Springer: Dordrecht, The Netherlands, 2007; pp. 195–222. [Google Scholar] [CrossRef]
- Romanazzi, G.; Feliziani, E. Botrytis Cinerea (Gray Mold); Elsevier: Waltham, MA, USA, 2014; ISBN 9780124115682. [Google Scholar]
- Abbey, J.A.; Percival, D.; Abbey, L.; Asiedu, S.K.; Prithiviraj, B.; Schilder, A. Biofungicides as alternative to synthetic fungicide control of grey mould (Botrytis cinerea)–prospects and challenges. Biocontrol Sci. Technol. 2019, 29, 241–262. [Google Scholar] [CrossRef]
- Leroux, P.; Fritz, R.; Debieu, D.; Albertini, C.; Lanen, C.; Bach, J.; Gredt, M.; Chapeland, F. Mechanisms of resistance to fungicides in field strains of Botrytis cinerea. Pest Manag. Sci. 2002, 58, 876–888. [Google Scholar] [CrossRef]
- Iwaniuk, P.; Lozowicka, B. Biochemical compounds and stress markers in lettuce upon exposure to pathogenic Botrytis cinerea and fungicides inhibiting oxidative phosphorylation. Planta 2022, 255, 61. [Google Scholar] [CrossRef]
- Harper, L.A.; Paton, S.; Hall, B.; McKay, S.; Oliver, R.P.; Lopez-Ruiz, F.J. Fungicide resistance characterized across seven modes of action in Botrytis cinerea isolated from Australian vineyards. Pest Manag. Sci. 2022, 78, 1326–1340. [Google Scholar] [CrossRef]
- Rodríguez, A.; Acosta, A.; Rodríguez, C. Fungicide resistance of Botrytis cinerea in tomato greenhouses in the Canary Islands and effectiveness of non-chemical treatments against gray mold. World J. Microbiol. Biotechnol. 2014, 30, 2397–2406. [Google Scholar] [CrossRef]
- Jacometti, M.A.; Wratten, S.D.; Walter, M. Review: Alternatives to synthetic fungicides for Botrytis cinerea management in vineyards. Aust. J. Grape Wine Res. 2010, 16, 154–172. [Google Scholar] [CrossRef]
- Elad, Y. Biological control of foliar pathogens by means of Trichoderma harzianum and potential modes of action. Crop Prot. 2000, 19, 709–714. [Google Scholar] [CrossRef]
- Motlagh, M.R.S.; Jafari, N. Biological control of Botrytis cinerea, the causal agent of rose gray mold disease by antagonistic fungi. Int. J. Pest Manag. 2020, 68, 167–174. [Google Scholar] [CrossRef]
- Köhl, J.; Molhoek, W.M.L. Effect of water potential on conidial germination and antagonism of Ulocladium atrum against Botrytis cinerea. Phytopathology 2001, 91, 485–491. [Google Scholar] [CrossRef]
- Gong, C.; Liu, Y.; Liu, S.Y.; Cheng, M.Z.; Zhang, Y.; Wang, R.H.; Chen, H.Y.; Li, J.F.; Chen, X.L.; Wang, A.X. Analysis of Clonostachys rosea-induced resistance to grey mould disease and identification of the key proteins induced in tomato fruit. Postharvest Biol. Technol. 2017, 123, 83–93. [Google Scholar] [CrossRef]
- Parafati, L.; Vitale, A.; Restuccia, C.; Cirvilleri, G. Biocontrol ability and action mechanism of food-isolated yeast strains against Botrytis cinerea causing post-harvest bunch rot of table grape. Food Microbiol. 2015, 47, 85–92. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Soliman, S.M.; Salem, H.M.; Ahmed, A.I.; Mahmood, M.; El-Tahan, A.M.; Ebrahim, A.A.M.; Abd El-Mageed, T.A.; Negm, S.H.; et al. Plant growth-promoting microorganisms as biocontrol agents of plant diseases: Mechanisms, challenges and future perspectives. Front. Plant Sci. 2022, 13, 1–19. [Google Scholar] [CrossRef]
- Bolívar-Anillo, H.J.; Garrido, C.; Collado, I.G. Endophytic microorganisms for biocontrol of the phytopathogenic fungus Botrytis cinerea. Phytochem. Rev. 2020, 19, 721–740. [Google Scholar] [CrossRef]
- Hernández-León, R.; Rojas-Solís, D.; Contreras-Pérez, M.; Orozco-Mosqueda, M. del C.; Macías-Rodríguez, L.I.; Reyes-de la Cruz, H.; Valencia-Cantero, E.; Santoyo, G. Characterization of the antifungal and plant growth-promoting effects of diffusible and volatile organic compounds produced by Pseudomonas fluorescens strains. Biol. Control 2015, 81, 83–92. [Google Scholar] [CrossRef]
- Martínez-Absalón, S.; Rojas-Solís, D.; Hernández-León, R.; Prieto-Barajas, C.; Orozco-Mosqueda, M.D.C.; Peña-Cabriales, J.J.; Sakuda, S.; Valencia-Cantero, E.; Santoyo, G. Potential use and mode of action of the new strain Bacillus thuringiensis UM96 for the biological control of the grey mould phytopathogen Botrytis cinerea. Biocontrol Sci. Technol. 2014, 24, 1349–1362. [Google Scholar] [CrossRef]
- Boukaew, S.; Prasertsan, P.; Troulet, C.; Bardin, M. Biological control of tomato gray mold caused by Botrytis cinerea by using Streptomyces spp. BioControl 2017, 62, 793–803. [Google Scholar] [CrossRef]
- Poveda, J.; Barquero, M.; González-Andrés, F. Insight into the microbiological control strategies against Botrytis cinerea using systemic plant resistance activation. Agronomy 2020, 10, 1832. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Urón, P.; Glick, B.R.; Giachini, A.; Rossi, M.J. Genomic Analysis of the 1-Aminocyclopropane-1-Carboxylate Deaminase-Producing Pseudomonas thivervalensis SC5 Reveals Its Multifaceted Roles in Soil and in Beneficial Interactions With Plants. Front. Microbiol. 2021, 12, 752288. [Google Scholar] [CrossRef]
- Khatoon, Z.; Huang, S.; Rafique, M.; Fakhar, A.; Kamran, M.A.; Santoyo, G. Unlocking the potential of plant growth-promoting rhizobacteria on soil health and the sustainability of agricultural systems. J. Environ. Manage. 2020, 273, 111118. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Santoyo, G.; Yadav, A.N.; Babalola, O.O. Efforts towards overcoming drought stress in crops: Revisiting the mechanisms employed by plant growth-promoting bacteria. Front. Microbiol. 2022, 13, 962427. [Google Scholar] [CrossRef]
- van Kan, J.A.L.; Shaw, M.W.; Grant-Downton, R.T. Botrytis species: Relentless necrotrophic thugs or endophytes gone rogue? Mol. Plant Pathol. 2014, 15, 957–961. [Google Scholar] [CrossRef]
- Veloso, J.; van Kan, J.A.L. Many Shades of Grey in Botrytis–Host Plant Interactions. Trends Plant Sci. 2018, 23, 613–622. [Google Scholar] [CrossRef]
- Kan, J.A.L. Van Infection Strategies of Botrytis cinerea. Acta Hortic. 2005, 660, 77–90. [Google Scholar] [CrossRef]
- Govrin, E.M.; Levine, A. The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr. Biol. 2000, 10, 751–757. [Google Scholar] [CrossRef]
- Elmer, P.A.G.; Michailides, T.J. Epidemiology of Botrytis cinerea in orchard and vine crops. In Botrytis: Biology, Pathology and Control; Springer: Dordrecht, The Netherlands, 2007; pp. 243–272. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.d.C.; Rocha-Granados, M.d.C.; Glick, B.R.; Santoyo, G. Microbiome engineering to improve biocontrol and plant growth-promoting mechanisms. Microbiol. Res. 2018, 208, 25–31. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsieb, G.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- Lugtenberg, B.J.J.; Dekkers, L.; Bloemberg, G. V C Olonization By P Seudomonas. Annu. Rev. Phytopathol. 2001, 39, 461–490. [Google Scholar] [CrossRef]
- Hartmann, A.; Rothballer, M.; Schmid, M. Lorenz Hiltner, a pioneer in rhizosphere microbial ecology and soil bacteriology research. Plant Soil 2008, 312, 7–14. [Google Scholar] [CrossRef]
- Raklami, A.; Bechtaoui, N.; Tahiri, A.I.; Anli, M.; Meddich, A.; Oufdou, K. Use of rhizobacteria and mycorrhizae consortium in the open field as a strategy for improving crop nutrition, productivity and soil fertility. Front. Microbiol. 2019, 10, 1106. [Google Scholar] [CrossRef]
- Enebe, M.C.; Babalola, O.O. The influence of plant growth-promoting rhizobacteria in plant tolerance to abiotic stress: A survival strategy. Appl. Microbiol. Biotechnol. 2018, 102, 7821–7835. [Google Scholar] [CrossRef] [Green Version]
- Adesemoye, A.O.; Kloepper, J.W. Plant-microbes interactions in enhanced fertilizer-use efficiency. Appl. Microbiol. Biotechnol. 2009, 85, 1–12. [Google Scholar] [CrossRef]
- Liu, H.; Brettell, L.E.; Singh, B. Linking the Phyllosphere Microbiome to Plant Health. Trends Plant Sci. 2020, 25, 841–844. [Google Scholar] [CrossRef]
- Morales-Cedeño, L.R.; Orozco-Mosqueda, M. del C.; Loeza-Lara, P.D.; Parra-Cota, F.I.; de los Santos-Villalobos, S.; Santoyo, G. Plant growth-promoting bacterial endophytes as biocontrol agents of pre- and post-harvest diseases: Fundamentals, methods of application and future perspectives. Microbiol. Res. 2021, 242, 126612. [Google Scholar] [CrossRef]
- Yaish, M.W.; Antony, I.; Glick, B.R. Isolation and characterization of endophytic plant growth-promoting bacteria from date palm tree (Phoenix dactylifera L.) and their potential role in salinity tolerance. Antonie van Leeuwenhoek 2015, 107, 1519–1532. [Google Scholar] [CrossRef]
- Girsowicz, R.; Moroenyane, I.; Steinberger, Y. Bacterial seed endophyte community of annual plants modulated by plant photosynthetic pathways. Microbiol. Res. 2019, 223–225, 58–62. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, M.; Erb, M.; Ton, J.; Brandenburg, A.; Karlen, D.; Zopfi, J.; Turlings, T.C.J. Volatiles produced by soil-borne endophytic bacteria increase plant pathogen resistance and affect tritrophic interactions. Plant Cell Environ. 2014, 37, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Holopainen, J.K.; Gershenzon, J. Multiple stress factors and the emission of plant VOCs. Trends Plant Sci. 2010, 15, 176–184. [Google Scholar] [CrossRef]
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef] [PubMed]
- Rojas-sánchez, B.; Guzmán-guzmán, P.; Orozco-mosqueda, M.D.C.; Rojas-s, B.; Guzm, P.; Morales-cedeño, L.R.; Orozco-mosqueda, M.C.; Saucedo-mart, B.C.; Juan, M.S.; Fadiji, A.E.; et al. Bioencapsulation of Microbial Inoculants: Mechanisms, Formulation Types and Application Techniques. Appl. Biosci. 2022, 1, 198–220. [Google Scholar] [CrossRef]
- Salvatierra-Martinez, R.; Arancibia, W.; Araya, M.; Aguilera, S.; Olalde, V.; Bravo, J.; Stoll, A. Colonization ability as an indicator of enhanced biocontrol capacity—An example using two Bacillus amyloliquefaciens strains and Botrytis cinerea infection of tomatoes. J. Phytopathol. 2018, 166, 601–612. [Google Scholar] [CrossRef]
- Kiesewalter, H.T.; Lozano-Andrade, C.N.; Wibowo, M.; Strube, M.L.; Maróti, G.; Snyder, D.; Jørgensen, T.S.; Larsen, T.O.; Cooper, V.S.; Weber, T.; et al. Genomic and Chemical Diversity of Bacillus subtilis Secondary Metabolites against Plant Pathogenic Fungi. mSystems 2021, 6, e00770-20. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B.R. Bacterial modulation of plant ethylene levels. Plant Physiol. 2015, 169, 13–22. [Google Scholar] [CrossRef]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef]
- Chowdhury, S.P.; Hartmann, A.; Gao, X.W.; Borriss, R. Biocontrol mechanism by root-associated Bacillus amyloliquefaciens FZB42—A review. Front. Microbiol. 2015, 6, 780. [Google Scholar] [CrossRef]
- Bakker, P.A.H.M.; Pieterse, C.M.J.; Van Loon, L.C. Induced systemic resistance by fluorescent Pseudomonas spp. Phytopathology 2007, 97, 239–243. [Google Scholar] [CrossRef]
- Whipps, J.M. Microbial interactions and biocontrol in the rhizosphere. J. Exp. Bot. 2001, 52, 487–511. [Google Scholar] [CrossRef]
- Romero, D.; De Vicente, A.; Rakotoaly, R.H.; Dufour, S.E.; Veening, J.W.; Arrebola, E.; Cazorla, F.M.; Kuipers, O.P.; Paquot, M.; Pérez-García, A. The iturin and fengycin families of lipopeptides are key factors in antagonism of Bacillus subtilis toward Podosphaera fusca. Mol. Plant-Microbe Interact. 2007, 20, 430–440. [Google Scholar] [CrossRef]
- Valenzuela Ruiz, V.; Gálvez Gamboa, G.T.; Villa Rodríguez, E.D.; Parra Cota, F.I.; Santoyo, G.; De los Santos Villalobos, S. Lipopéptidos producidos por agentes de control biológico del género Bacillus: Revisión de herramientas analíticas utilizadas para su estudio. Rev. Mex. Ciencias Agrícolas 2020, 11, 419–432. [Google Scholar] [CrossRef]
- Ueda, H.; Kikuta, Y.; Matsuda, K. Plant communication. Plant Signal. Behav. 2012, 7, 222–226. [Google Scholar] [CrossRef]
- Fernando, W.G.D.; Ramarathnam, R.; Krishnamoorthy, A.S.; Savchuk, S.C. Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biol. Biochem. 2005, 37, 955–964. [Google Scholar] [CrossRef]
- Martínez-Cámara, R.; Montejano-Ramírez, V.; Moreno-Hagelsieb, G.; Santoyo, G.; Valencia-Cantero, E. The volatile organic compound dimethylhexadecylamine affects bacterial growth and swarming motility of bacteria. Folia Microbiol. (Praha) 2020, 65, 523–532. [Google Scholar] [CrossRef]
- Chávez-moctezuma, M.P.; Martínez-cámara, R.; Hernández-salmerón, J.; Valencia-cantero, E. Comparative genomic and functional analysis of Arthrobacter sp. UMCV2 reveals the presence of luxR -related genes inducible by the biocompound N, N -dimethylhexadecilamine. Front. Microbiol. 2022, 13, 1040932. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Leong, J.; Teintze, M.; Schroth, M.N. Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 1980, 286, 885–886. [Google Scholar] [CrossRef]
- de los Santos-Villalobos, S.; Barrera-Galicia, G.C.; Miranda-Salcedo, M.A.; Peña-Cabriales, J.J. Burkholderia cepacia XXVI siderophore with biocontrol capacity against Colletotrichum gloeosporioides. World J. Microbiol. Biotechnol. 2012, 28, 2615–2623. [Google Scholar] [CrossRef]
- Compant, S.; Clément, C.; Sessitsch, A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 871, 1473. [Google Scholar] [CrossRef] [PubMed]
- Sasse, J.; Martinoia, E.; Northen, T. Feed Your Friends: Do Plant Exudates Shape the Root Microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, G.; del Orozco-Mosqueda, M.C.; Govindappa, M. Mechanisms of biocontrol and plant growth-promoting activity in soil bacterial species of Bacillus and Pseudomonas: A review. Biocontrol Sci. Technol. 2012, 22, 855–872. [Google Scholar] [CrossRef]
- Islam, M.T.; Rahman, M.; Pandey, P.; Jha, C.K.; Aeron, A. Bacilli and Agrobiotechnology; Springer: Cham, Switzerland, 2017; ISBN 9783319444093. [Google Scholar]
- Gamalero, E.; Trotta, A.; Massa, N.; Copetta, A.; Martinotti, M.G.; Berta, G. Impact of two fluorescent pseudomonads and an arbuscular mycorrhizal fungus on tomato plant growth, root architecture and P acquisition. Mycorrhiza 2004, 14, 185–192. [Google Scholar] [CrossRef]
- Kuzmanović, N.; Eltlbany, N.; Ding, G.; Baklawa, M.; Min, L.; Wei, L.; Smalla, K. Analysis of the genome sequence of plant beneficial strain Pseudomonas sp. RU47. J. Biotechnol. 2018, 281, 183–192. [Google Scholar] [CrossRef]
- Ahmad, P.; Prasad, M.N.V. Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–515. [Google Scholar] [CrossRef]
- Ryu, H.; Cho, Y.G. Plant hormones in salt stress tolerance. J. Plant Biol. 2015, 58, 147–155. [Google Scholar] [CrossRef]
- Dubois, M.; Van den Broeck, L.; Inzé, D. The Pivotal Role of Ethylene in Plant Growth. Trends Plant Sci. 2018, 23, 311–323. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Rossi, M.J.; Soares, C.R.F.S.; McConkey, B.J.; Glick, B.R. New insights into 1-Aminocyclopropane-1-Carboxylate (ACC) deaminase phylogeny, evolution and ecological significance. PLoS ONE 2014, 9, e99168. [Google Scholar] [CrossRef]
- Ali, S.Z.; Sandhya, V.; Rao, L.V. Isolation and characterization of drought-tolerant ACC deaminase and exopolysaccharide-producing fluorescent Pseudomonas sp. Ann. Microbiol. 2014, 64, 493–502. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Gaurav, A.K.; Srivastava, S.; Verma, J.P. Plant Growth-Promoting Bacteria: Biological Tools for the Mitigation of Salinity Stress in Plants. Front. Microbiol. 2020, 11, 1216. [Google Scholar] [CrossRef]
- Forni, C.; Duca, D.; Glick, B.R. Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil 2017, 410, 335–356. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.D.C.; Glick, B.R.; Santoyo, G. ACC deaminase in plant growth-promoting bacteria (PGPB): An efficient mechanism to counter salt stress in crops. Microbiol. Res. 2020, 235, 126439. [Google Scholar] [CrossRef]
- Walters, D.R.; Havis, N.D.; Paterson, L.; Taylor, J.; Walsh, D.J.; Sablou, C. Control of foliar pathogens of spring barley using a combination of resistance elicitors. Front. Plant Sci. 2014, 5, 241. [Google Scholar] [CrossRef]
- Jung, H.W.; Tschaplinski, T.J.; Wang, L.; Glazebrook, J.; Greenberg, J.T. Priming in systemic plant immunity. Science 2009, 324, 89–91. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Ryu, C.M.; Zhang, S. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 2004, 94, 1259–1266. [Google Scholar] [CrossRef]
- Yu, Y.; Gui, Y.; Li, Z.; Jiang, C.; Guo, J.; Niu, D. Induced Systemic Resistance for Improving Plant Immunity by Beneficial Microbes. Plants 2022, 11, 386. [Google Scholar] [CrossRef]
- Iqbal, A.; Khan, R.S.; Shehryar, K.; Imran, A.; Ali, F.; Attia, S.; Shah, S.; Mii, M. Antimicrobial peptides as effective tools for enhanced disease resistance in plants. Plant Cell. Tissue Organ Cult. 2019, 139, 1–15. [Google Scholar] [CrossRef]
- Mahesh, H.M.; Murali, M.; Anup Chandra Pal, M.; Melvin, P.; Sharada, M.S. Salicylic acid seed priming instigates defense mechanism by inducing PR-Proteins in Solanum melongena L. upon infection with Verticillium dahliae Kleb. Plant Physiol. Biochem. 2017, 117, 12–23. [Google Scholar] [CrossRef]
- Nie, P.; Li, X.; Wang, S.; Guo, J.; Zhao, H.; Niu, D. Induced systemic resistance against Botrytis cinerea by Bacillus cereus AR156 through a JA/ET- and NPR1-dependent signaling pathway and activates PAMP-triggered immunity in arabidopsis. Front. Plant Sci. 2017, 8, 238. [Google Scholar] [CrossRef]
- Dong, X. NPR1, all things considered. Curr. Opin. Plant Biol. 2004, 7, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Esmaeel, Q.; Jacquard, C.; Sanchez, L.; Clément, C.; Ait Barka, E. The mode of action of plant associated Burkholderia against grey mould disease in grapevine revealed through traits and genomic analyses. Sci. Rep. 2020, 10, 19393. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.; Innes, I.; Allan, A. The potential biocontrol agent Pseudomonas antimicrobica inhibits germination of conidia and outgrowth of Botrytis cinerea. Lett. Appl. Microbiol. 2001, 32, 346–348. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Qin, J.; Li, D.; Zhou, S. Inhibitory effect and possible mechanism of a Pseudomonas strain QBA5 against gray mold on tomato leaves and fruits caused by Botrytis cinerea. PLoS ONE 2018, 13, e190932. [Google Scholar] [CrossRef] [PubMed]
- Simionato, A.S.; Navarro, M.O.P.; de Jesus, M.L.A.; Barazetti, A.R.; da Silva, C.S.; Simões, G.C.; Balbi-Peña, M.I.; de Mello, J.C.P.; Panagio, L.A.; de Almeida, R.S.C.; et al. The effect of phenazine-1-carboxylic acid on mycelial growth of Botrytis cinerea produced by Pseudomonas aeruginosa LV strain. Front. Microbiol. 2017, 8, 1102. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Herrera-Balandrano, D.D.; Wang, Y.-X.; Shi, X.-C.; Chen, X.; Jin, Y.; Liu, F.-Q.; Laborda, P. Biocontrol Ability of the Bacillus amyloliquefaciens Group, B. amyloliquefaciens, B. velezensis, B. nakamurai, and B. siamensis, for the Management of Fungal Postharvest Diseases: A Review. J. Agric. Food Chem. 2022, 70, 6591–6616. [Google Scholar] [CrossRef]
- Li, F.X.; Ma, H.Q.; Liu, J.; Zhang, C. Antagonistic effects of Bacillus cereus strain B-02 on morphology, ultrastructure and cytophysiology of Botrytis cinerea. Polish J. Microbiol. 2012, 61, 119–128. [Google Scholar] [CrossRef]
- Bhagwat, A.; Collins, C.H.; Dordick, J.S. Selective antimicrobial activity of cell lytic enzymes in a bacterial consortium. Appl. Microbiol. Biotechnol. 2019, 103, 7041–7054. [Google Scholar] [CrossRef]
- Bodhankar, S.; Grover, M.; Hemanth, S.; Reddy, G.; Rasul, S.; Yadav, S.K.; Desai, S.; Mallappa, M.; Mandapaka, M.; Srinivasarao, C. Maize seed endophytic bacteria: Dominance of antagonistic, lytic enzyme-producing Bacillus spp. 3 Biotech 2017, 7, 232. [Google Scholar] [CrossRef]
- Ait Barka, E.; Gognies, S.; Nowak, J.; Audran, J.C.; Belarbi, A. Inhibitory effect of endophyte bacteria on Botrytis cinerea and its influence to promote the grapevine growth. Biol. Control 2002, 24, 135–142. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Jha, D.K. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef]
- Escribano-Viana, R.; Portu, J.; Garijo, P.; Gutiérrez, A.R.; Santamaría, P.; López-Alfaro, I.; López, R.; González-Arenzana, L. Evaluating a preventive biological control agent applied on grapevines against Botrytis cinerea and its influence on winemaking. J. Sci. Food Agric. 2018, 98, 4517–4526. [Google Scholar] [CrossRef]
- Trotel-Aziz, P.; Couderchet, M.; Biagianti, S.; Aziz, A. Characterization of new bacterial biocontrol agents Acinetobacter, Bacillus, Pantoea and Pseudomonas spp. mediating grapevine resistance against Botrytis cinerea. Environ. Exp. Bot. 2008, 64, 21–32. [Google Scholar] [CrossRef]
- Velázquez-Becerra, C.; Macías-Rodríguez, L.I.; López-Bucio, J.; Flores-Cortez, I.; Santoyo, G.; Hernández-Soberano, C.; Valencia-Cantero, E. The rhizobacterium Arthrobacter agilis produces dimethylhexadecylamine, a compound that inhibits growth of phytopathogenic fungi in vitro. Protoplasma 2013, 250, 1251–1262. [Google Scholar] [CrossRef]
- Fedele, G.; Brischetto, C.; Rossi, V. Biocontrol of Botrytis cinerea on Grape Berries as Influenced by Temperature and Humidity. Front. Plant Sci. 2020, 11, 1232. [Google Scholar] [CrossRef]
- Lahlali, R.; Peng, G.; McGregor, L.; Gossen, B.D.; Hwang, S.F.; McDonald, M. Mechanisms of the biofungicide Serenade (Bacillus subtilis QST713) in suppressing clubroot. Biocontrol Sci. Technol. 2011, 21, 1351–1362. [Google Scholar] [CrossRef]
- Punja, Z.K.; Tirajoh, A.; Collyer, D.; Ni, L. Efficacy of Bacillus subtilis strain QST 713 (Rhapsody) against four major diseases of greenhouse cucumbers. Crop Prot. 2019, 124, 104845. [Google Scholar] [CrossRef]
- Meziane, H.; Van Der Sluis, I.; Van Loon, L.C.; Höfte, M.; Bakker, P.A. Determinants of Pseudomonas putida WCS358 involved in inducing. Mol. Plant Pathol. 2005, 6, 177–185. [Google Scholar] [CrossRef]
- Elanchezhiyan, K.; Keerthana, U.; Nagendran, K.; Prabhukarthikeyan, S.R.; Prabakar, K.; Raguchander, T.; Karthikeyan, G. Multifaceted benefits of Bacillus amyloliquefaciens strain FBZ24 in the management of wilt disease in tomato caused by Fusarium oxysporum f. sp. lycopersici. Physiol. Mol. Plant Pathol. 2018, 103, 92–101. [Google Scholar] [CrossRef]
- Yao, A.; Bochow, H.; Karimov, S.; Boturov, U.; Sanginboy, S.; Sharipov, A. Effect of FZB 24® Bacillus subtilis as a biofertilizer on cotton yields in field tests. Arch. Phytopathol. Plant Prot. 2006, 39, 323–328. [Google Scholar] [CrossRef]
- Idris, E.S.E.; Iglesias, D.J.; Talon, M.; Borriss, R. Tryptophan-dependent production of Indole-3-Acetic Acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol. Plant-Microbe Interact. 2007, 20, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.H.; Liao, M.J.; Wang, H.K.; Zheng, M.Z.; Xu, J.J.; Guo, J.H. Bacillus velezensis, a potential and efficient biocontrol agent in control of pepper gray mold caused by Botrytis cinerea. Biol. Control 2018, 126, 147–157. [Google Scholar] [CrossRef]
- Toral, L.; Rodríguez, M.; Béjar, V.; Sampedro, I. Crop protection against Botrytis cinerea by rhizhosphere biological control agent bacillus velezensis XT1. Microorganisms 2020, 8, 992. [Google Scholar] [CrossRef] [PubMed]
- Mikani, A.; Etebarian, H.R.; Sholberg, P.L.; O’Gorman, D.T.; Stokes, S.; Alizadeh, A. Biological control of apple gray mold caused by Botrytis mali with Pseudomonas fluorescens strains. Postharvest Biol. Technol. 2008, 48, 107–112. [Google Scholar] [CrossRef]
- Bonfante, P.; Anca, I.A. Plants, mycorrhizal fungi, and bacteria: A network of interactions. Annu. Rev. Microbiol. 2009, 63, 363–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassani, M.A.; Durán, P.; Hacquard, S. Microbial interactions within the plant holobiont. Microbiome 2018, 6, 58. [Google Scholar] [CrossRef]
- Santoyo, G.; Guzm, P.; Parra-cota, F.I.; Santos-villalobos, S.D.L.; Orozco-mosqueda, M.C.; Glick, B.R. Plant Growth Stimulation by Microbial Consortia. Agronomy 2021, 11, 219. [Google Scholar] [CrossRef]
- Pertot, I.; Giovannini, O.; Benanchi, M.; Caffi, T.; Rossi, V.; Mugnai, L. Combining biocontrol agents with different mechanisms of action in a strategy to control Botrytis cinerea on grapevine. Crop Prot. 2017, 97, 85–93. [Google Scholar] [CrossRef]
- Chauhan, H.; Bagyaraj, D.J. Inoculation with selected microbial consortia not only enhances growth and yield of French bean but also reduces fertilizer application under field condition. Sci. Hortic. (Amsterdam) 2015, 197, 441–446. [Google Scholar] [CrossRef]
- Sarma, B.K.; Yadav, S.K.; Singh, S.; Singh, H.B. Microbial consortium-mediated plant defense against phytopathogens: Readdressing for enhancing efficacy. Soil Biol. Biochem. 2015, 87, 25–33. [Google Scholar] [CrossRef]
- Santoyo, G.; Gamalero, E.; Glick, B.R. Mycorrhizal-Bacterial Amelioration of Plant Abiotic and Biotic Stress. Front. Sustain. Food Syst. 2021, 5, 672881. [Google Scholar] [CrossRef]
- Durgadevi, D.; Srivignesh, S.; Sankaralingam, A. Effect of Consortia Bioformulation of Rhizobacteria on Induction of Systemic Resistance in Tuberose Against Peduncle Blight Disease. Int. J. Bio-Resour. Stress Manag. 2018, 9, 510–517. [Google Scholar] [CrossRef]
- Vijayabharathi, R.; Gopalakrishnan, S.; Sathya, A.; Vasanth Kumar, M.; Srinivas, V.; Mamta, S. Streptomyces sp. as plant growth-promoters and host-plant resistance inducers against Botrytis cinerea in chickpea. Biocontrol Sci. Technol. 2018, 28, 1140–1163. [Google Scholar] [CrossRef]
- Rojas-Solís, D.; Zetter-Salmón, E.; Contreras-Pérez, M.; Rocha-Granados, M.D.C.; Macías-Rodríguez, L.; Santoyo, G. Pseudomonas stutzeri E25 and Stenotrophomonas maltophilia CR71 endophytes produce antifungal volatile organic compounds and exhibit additive plant growth-promoting effects. Biocatal. Agric. Biotechnol. 2018, 13, 46–52. [Google Scholar] [CrossRef]
- Rojas-Solis, D.; Hernandez-Pacheco, C.E.; Santoyo, G. Evaluation of Bacillus and Pseudomonas to colonize the rhizosphere and their effect on growth promotion in tomato (Physalis ixocarpa Brot. ex Horm.). Rev. Chapingo, Ser. Hortic. 2016, 22, 45–58. [Google Scholar] [CrossRef]
- Balthazar, C.; Cantin, G.; Novinscak, A.; Joly, D.L.; Filion, M. Expression of Putative Defense Responses in Cannabis Primed by Pseudomonas and/or Bacillus Strains and Infected by Botrytis cinerea. Front. Plant Sci. 2020, 11, 1873. [Google Scholar] [CrossRef]
- Vijayabharathi, R.; Gopalakrishnan, S.; Sathya, A.; Srinivas, V.; Sharma, M. Deciphering the tri-dimensional effect of endophytic Streptomyces sp. on chickpea for plant growth promotion, helper effect with Mesorhizobium ciceri and host-plant resistance induction against Botrytis cinerea. Microb. Pathog. 2018, 122, 98–107. [Google Scholar] [CrossRef]
- Bunster, L.; Fokkema, N.J.; Schippers, B. Effect of Surface-Active Pseudomonas spp. on Leaf Wettability. Appl. Environ. Microbiol. 1989, 55, 1340–1345. [Google Scholar] [CrossRef]
- Edwards, S.G.; Young, J.P.W.; Fitter, A.H. Interactions between Pseudomonas fluorescens biocontrol agents and Glomus mosseae, an arbuscular mycorrhizal fungus, within the rhizosphere. FEMS Microbiol. Lett. 1998, 166, 297–303. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M. The fruit microbiome: A new frontier for postharvest biocontrol and postharvest biology. Postharvest Biol. Technol. 2018, 140, 107–112. [Google Scholar] [CrossRef]
- Adaskaveg, J.E.; Förster, H.; Thompson, D.F. Identification and etiology of visible quiescent infections of Monilinia fructicola and Botrytis cinerea in sweet cherry fruit. Plant Dis. 2000, 84, 328–333. [Google Scholar] [CrossRef]
- Aziz, A.; Trotel-Aziz, P.; Dhuicq, L.; Jeandet, P.; Couderchet, M.; Vernet, G. Chitosan oligomers and copper sulfate induce grapevine defense reactions and resistance to gray mold and downy mildew. Phytopathology 2006, 96, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Sandoval Flores, M.G.; Jiménez Mejía, R.; Santoyo, G.; Alva Murillo, P.N.; López Meza, J.E.; Loeza Lara, P.D. Compósitos de quitosano-ácidos grasos reducen la infección de Botrytis cinerea en fresa en poscosecha (Chitosan-fatty acids composite reduce Botrytis cinerea infection on post-harvest strawberry). Nov. Sci. 2018, 10, 207–227. [Google Scholar] [CrossRef]
- Wang, F.; Feng, G.; Chen, K. Defense responses of harvested tomato fruit to burdock fructooligosaccharide, a novel potential elicitor. Postharvest Biol. Technol. 2009, 52, 110–116. [Google Scholar] [CrossRef]
- Guzmán-Guzmán, P.; Kumar, A.; de los Santos-Villalobos, S.; Parra-Cota, F.I.; Orozco-Mosqueda, M.d.C.; Fadiji, A.E.; Hyder, S.; Babalola, O.O.; Santoyo, G. Trichoderma Species: Our Best Fungal Allies in the Biocontrol of Plant Diseases—A Review. Plants 2023, 12, 432. [Google Scholar] [CrossRef]
- Galindo, E.; Serrano-carreón, L.; Gutiérrez, C.R.; Balderas-ruíz, K.A.; Muñoz-celaya, A.L.; Arroyo-colín, M.M.J. Desarrollo histórico y los retos tecnológicos y legales para comercializar Fungifree AB®, el primer biofungicida 100% mexicano. TIP. Rev. Espec. En Cienc. Químico-Biológicas 2015, 18, 52–60. [Google Scholar] [CrossRef]
- Hurtado-Bautista, E.; Pérez-Sánchez, L.F.; Islas-Robles, Á.; Santoyo, G.; Olmedo-Álvarez, G. Phenotypic plasticity and evolution of thermal tolerance in two lineages of bacteria from temperate and hot environments. PeerJ 2021, 9, e11734. [Google Scholar] [CrossRef]
- Egidi, E.; Delgado-Baquerizo, M.; Plett, J.M.; Wang, J.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K. A few Ascomycota taxa dominate soil fungal communities worldwide. Nat. Commun. 2019, 10, 2369. [Google Scholar] [CrossRef]
- Rascovan, N.; Carbonetto, B.; Perrig, D.; Díaz, M.; Canciani, W.; Abalo, M.; Alloati, J.; González-Anta, G.; Vazquez, M.P. Integrated analysis of root microbiomes of soybean and wheat from agricultural fields. Sci. Rep. 2016, 6, 28084. [Google Scholar] [CrossRef]
- van Kan, J.A.L. Licensed to kill: The lifestyle of a necrotrophic plant pathogen. Trends Plant Sci. 2006, 11, 247–253. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.P. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Santoyo, G. How plants recruit their microbiome? New insights into beneficial interactions. J. Adv. Res. 2021, 40, 45–58. [Google Scholar] [CrossRef]


| Bacterial Species/Strain | Trade Name ® | Company (and/or Country) |
|---|---|---|
| Pantoea agglomerans | Pantovital | IRTA (Spain) |
| Bacillus subtilis | Serenade Max | Bayer, formerly BASF (Germany) |
| Pseudomonas syringae strain ESC-10 | Bio-save | Jet Harvest Solutions (USA) |
| Bacillus amyloliquefaciens | Amylo-X | Biogard CBC (Italy) |
| Bacillus amyloliquefaciens | Double Nickel 55WDG/LC | Certis (USA) |
| Bacillus subtilis GB03 | Companion | Growth Products (USA) |
| Bacillus subtilis IK-1080 | Botokira Wettable Powder | Idemitsu Kosan Inc., Japan |
| Bacillus megaterium | Bio Arc | Sphere Bio-Arc PVT Ltd. (India) |
| Streptomyces griseoviridis strain K61 | Mycostop | Verdera Oy (Finland) |
| Streptomyces lydicus WYEC 108 | Actinovate | Novozymes (Denmark) |
| Bacillus subtilis | Fungifree | Agro & Biotecnia, S. de R.L. de C.V. (México) |
| Bacillus subtilis strain QST 713 | Serenade | AgraQuest (USA) |
| Bacillus pumilus strain QST2808 | Sonata | AgraQuest, Davis-CA (USA) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orozco-Mosqueda, M.d.C.; Kumar, A.; Fadiji, A.E.; Babalola, O.O.; Puopolo, G.; Santoyo, G. Agroecological Management of the Grey Mould Fungus Botrytis cinerea by Plant Growth-Promoting Bacteria. Plants 2023, 12, 637. https://doi.org/10.3390/plants12030637
Orozco-Mosqueda MdC, Kumar A, Fadiji AE, Babalola OO, Puopolo G, Santoyo G. Agroecological Management of the Grey Mould Fungus Botrytis cinerea by Plant Growth-Promoting Bacteria. Plants. 2023; 12(3):637. https://doi.org/10.3390/plants12030637
Chicago/Turabian StyleOrozco-Mosqueda, Ma. del Carmen, Ajay Kumar, Ayomide Emmanuel Fadiji, Olubukola Oluranti Babalola, Gerardo Puopolo, and Gustavo Santoyo. 2023. "Agroecological Management of the Grey Mould Fungus Botrytis cinerea by Plant Growth-Promoting Bacteria" Plants 12, no. 3: 637. https://doi.org/10.3390/plants12030637
APA StyleOrozco-Mosqueda, M. d. C., Kumar, A., Fadiji, A. E., Babalola, O. O., Puopolo, G., & Santoyo, G. (2023). Agroecological Management of the Grey Mould Fungus Botrytis cinerea by Plant Growth-Promoting Bacteria. Plants, 12(3), 637. https://doi.org/10.3390/plants12030637










