Plant Growth Promotion, Phytohormone Production and Genomics of the Rhizosphere-Associated Microalga, Micractinium rhizosphaerae sp. nov.
Abstract
1. Introduction
2. Results and Discussion
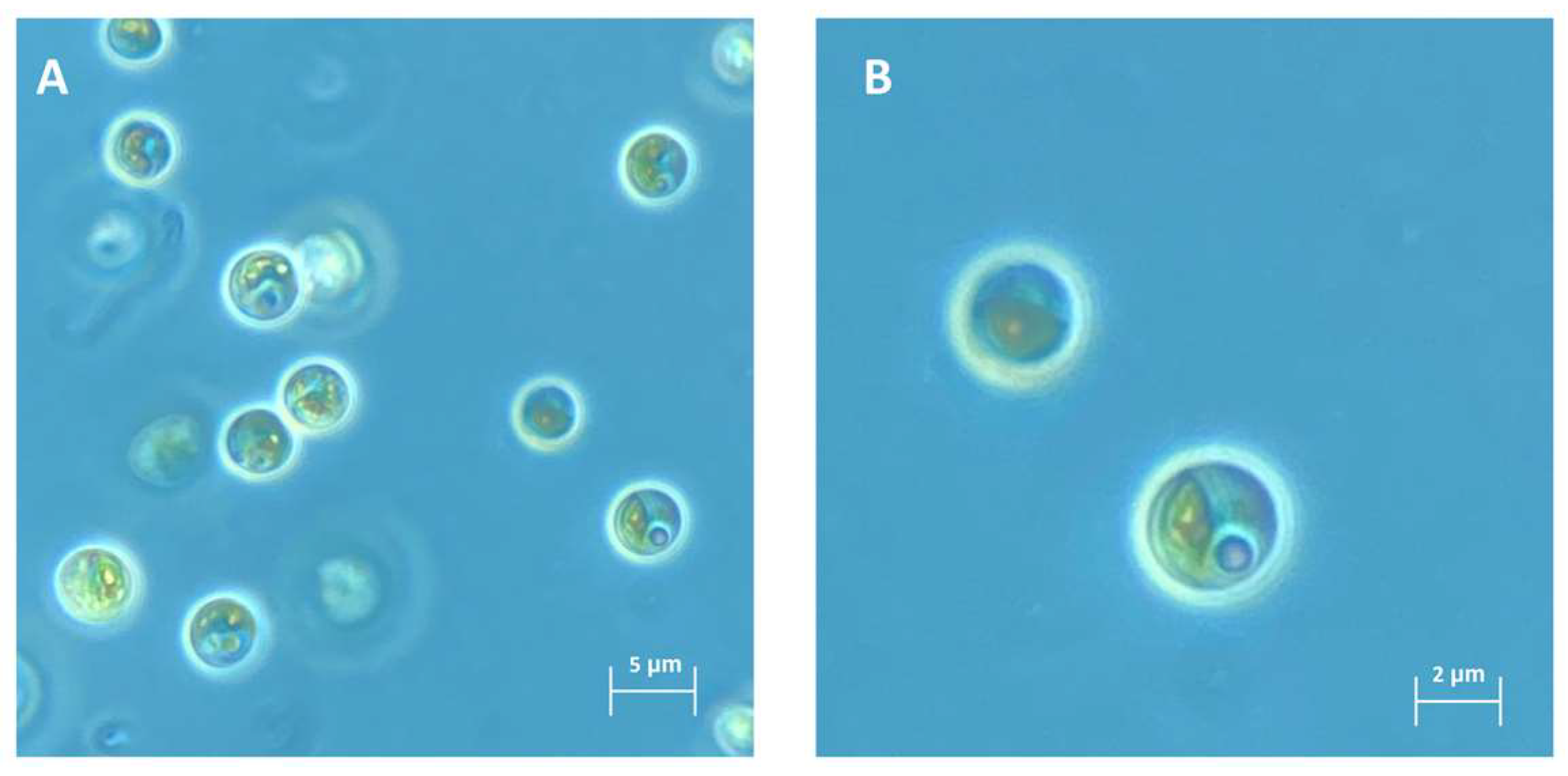
2.1. Characterization of Micractinium rhizosphaerae sp. nov.
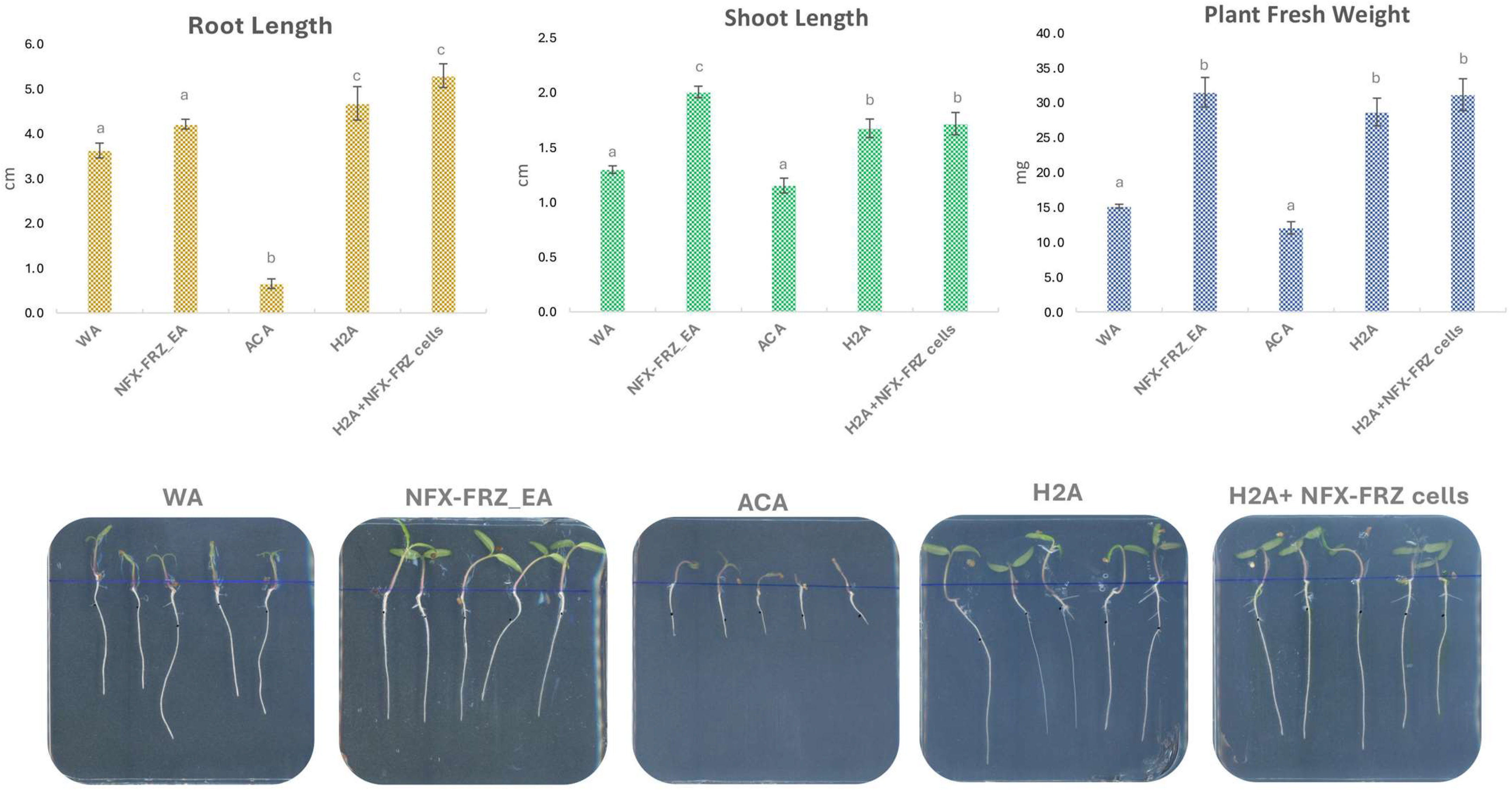
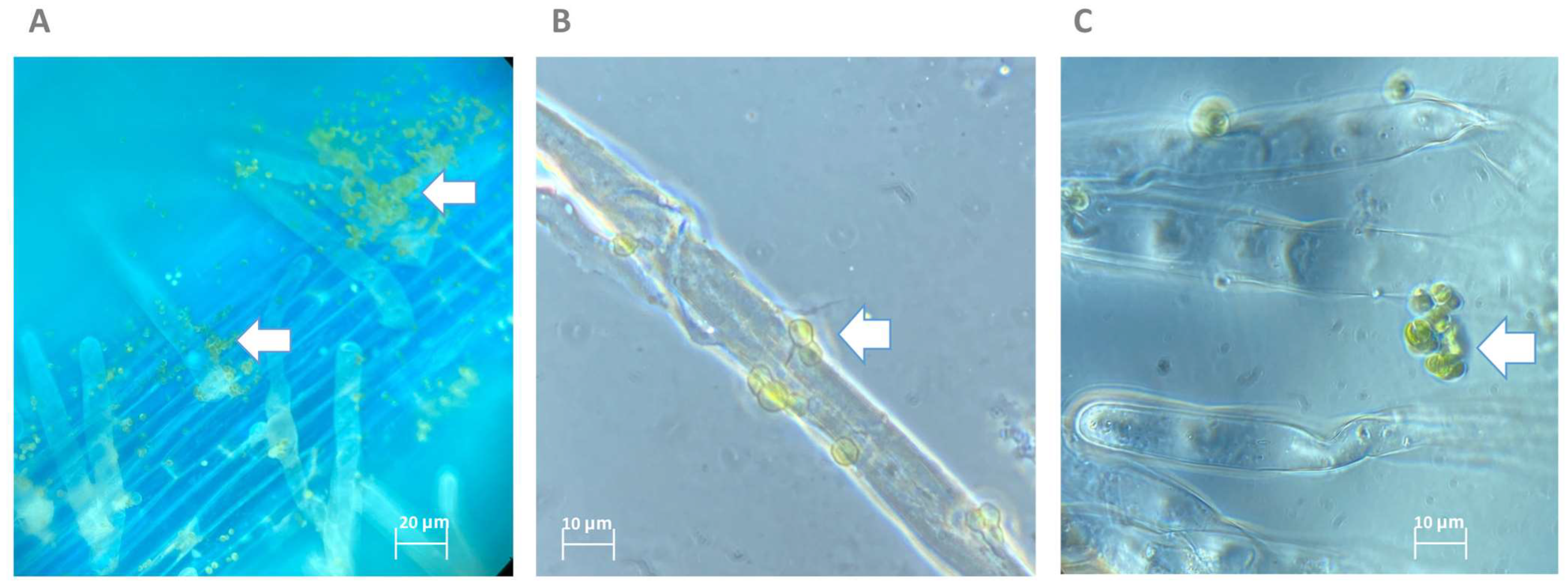
2.2. Micractinium rhizosphaerae NFX-FRZ Promotes Tomato Plant Growth and Actively Binds to Plant Roots
2.3. Metabolomic Analysis of Micractinium rhizosphaerae NFX-FRZ Exudates Revealed the Presence of Several Phytohormones and Plant Growth-Promoting Compounds
2.3.1. General Untargeted Metabolomic Analysis
2.3.2. Phytohormones
2.4. Micractinium rhizosphaerae NFX-FRZ Genomic Properties
2.5. Genomic Insights into Micractinium rhizosphaerae NFX-FRZ Phytohormone Production Abilities
2.5.1. Auxins, IAA
2.5.2. Salicylic Acid
2.5.3. Jasmonic Acid
2.5.4. Abscisic Acid
3. Material and Methods
3.1. Strain Isolation, Identification, and Characterization
3.2. Phylogenetic Analysis
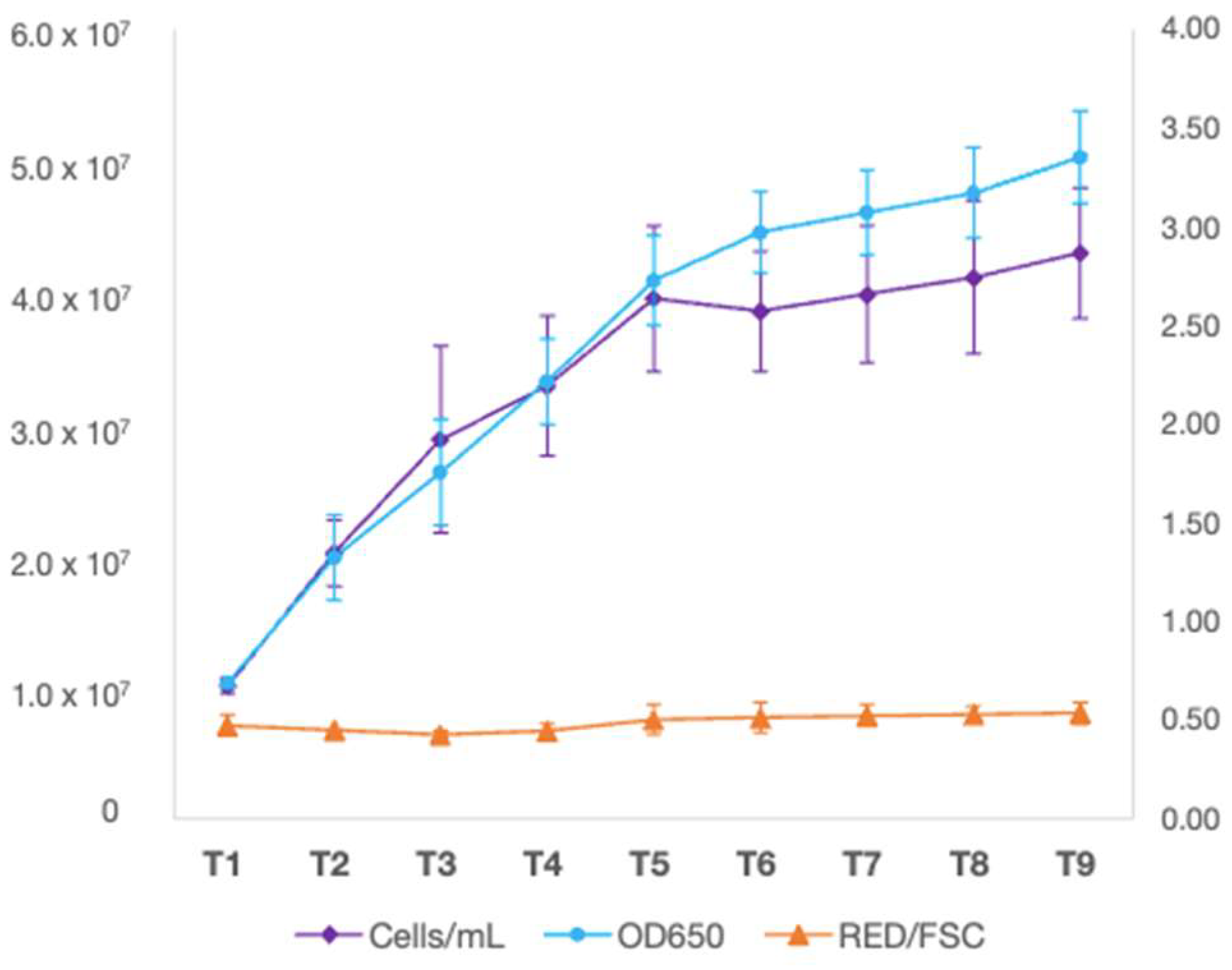
3.3. Growth Kinetics under Autotrophic Conditions
3.4. Tomato Plant Growth Promotion Assays
3.5. Untargeted Metabolomic Analysis of NFX-FRZ Exudates
3.6. Genome Sequencing and Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geada, P.; Moreira, C.; Silva, M.; Nunes, R.; Madureira, L.; Rocha, C.M.R.; Pereira, R.N.; Vicente, A.A.; Teixeira, J.A. Algal proteins: Production strategies and nutritional and functional properties. Bioresour. Technol. 2021, 332, 125125. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Bhalamurugan, G.L.; Valerie, O.; Mark, L. Valuable bioproducts obtained from microalgal biomass and their commercial applications: A review. Environ. Eng. Res. 2018, 23, 229–241. [Google Scholar] [CrossRef]
- Ruiz, J.; Olivieri, G.; de Vree, J.; Bosma, R.; Willems, P.; Reith, J.H.; Eppink, M.H.M.; Kleinegris, D.M.M.; Wijffels, R.H.; Barbosa, M.J. Towards industrial products from microalgae. Energy Environ. Sci. 2016, 9, 3036–3043. [Google Scholar] [CrossRef]
- Sasso, S.; Pohnert, G.; Lohr, M.; Mittag, M.; Hertweck, C. Microalgae in the postgenomic era: A blooming reservoir for new natural products. FEMS Microbiol. Rev. 2012, 36, 761–785. [Google Scholar] [CrossRef] [PubMed]
- Araújo, R.; Vázquez Calderón, F.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Garcia Tasende, M.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current Status of the Algae Production Industry in Europe: An Emerging Sector of the Blue Bioeconomy. Front. Mar. Sci. 2021, 7, 1247. [Google Scholar] [CrossRef]
- Bérard, A.; Dorigo, U.; Humbert, J.F.; Martin-Laurent, F. Microalgae community structure analysis based on 18S rDNA amplification from DNA extracted directly from soil as a potential soil bioindicator. Agron. Sustain. Dev. 2005, 25, 285–291. [Google Scholar] [CrossRef]
- Davey, M.C.; Rothery, P. Primary Colonization by Microalgae in Relation to Spatial Variation in Edaphic Factors on Antarctic Fellfield Soils. J. Ecol. 1993, 81, 335. [Google Scholar] [CrossRef]
- Cullimore, D.R.; McCann, A.E. Influence of four herbicides on the algal flora of a prairie soil. Plant Soil 1977, 46, 499–510. [Google Scholar] [CrossRef]
- Metting, B. The systematics and ecology of soil algae. Bot. Rev. 1981, 47, 195–312. [Google Scholar] [CrossRef]
- Lee, S.M.; Ryu, C.M. Algae as New Kids in the Beneficial Plant Microbiome. Front. Plant Sci. 2021, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Zhou, Q. Co-cultivation of Chlorella spp and tomato in a hydroponic system. Biomass Bioenergy 2017, 97, 132–138. [Google Scholar] [CrossRef]
- Kholssi, R.; Marks, E.A.N.; Miñón, J.; Montero, O.; Debdoubi, A.; Rad, C. Biofertilizing Effect of Chlorella sorokiniana Suspensions on Wheat Growth. J. Plant Growth Regul. 2018, 38, 644–649. [Google Scholar] [CrossRef]
- Tian, S.L.; Khan, A.; Zheng, W.N.; Song, L.; Liu, J.H.; Wang, X.Q.; Li, L. Effects of Chlorella extracts on growth of Capsicum annuum L. seedlings. Sci. Rep. 2022, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Shim, C.-K.; Kim, Y.-K.; Hong, S.-J.; Park, J.-H.; Han, E.-J.; Jee, H.-J.; Lee, S.-B.; Kim, S.-C. Effect of Chlorella sp. on Improving Antioxidant Activities and Growth Promotion in Organic Soybean Sprout Cultivation. Korean J. Org. Agric. 2015, 23, 939–950. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, B.; Shim, C.K.; Chang, Y.K.; Ryu, C.M. Plant anti-aging: Delayed flower and leaf senescence in Erinus alpinus treated with cell-free Chlorella cultivation medium. Plant Signal. Behav. 2020, 15, 1763005. [Google Scholar] [CrossRef]
- Perveen, K.; Bukhari, N.A.; Al Masoudi, L.M.; Alqahtani, A.N.; Alruways, M.W.; Alkhattaf, F.S. Antifungal potential, chemical composition of Chlorella vulgaris and SEM analysis of morphological changes in Fusarium oxysporum. Saudi J. Biol. Sci. 2022, 29, 2501. [Google Scholar] [CrossRef]
- Al-Nazwani, M.S.; Aboshosha, S.S.; El-Saedy, M.A.M.; Ghareeb, R.Y.; Komeil, D.A. Antifungal activities of Chlorella vulgaris extract on black scurf disease, growth performance and quality of potato. Arch. Phytopathol. Plant Prot. 2021, 54, 2171–2190. [Google Scholar] [CrossRef]
- Lee, S.M.; Kim, S.K.; Lee, N.; Ahn, C.Y.; Ryu, C.M. d-Lactic acid secreted by Chlorella fusca primes pattern-triggered immunity against Pseudomonas syringae in Arabidopsis. Plant J. 2020, 102, 761–778. [Google Scholar] [CrossRef]
- Wang, M.; Kuo-Dahab, W.C.; Dolan, S.; Park, C. Kinetics of nutrient removal and expression of extracellular polymeric substances of the microalgae, Chlorella sp. and Micractinium sp., in wastewater treatment. Bioresour. Technol. 2014, 154, 131–137. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.; Yoon, Y.; Park, Y.; Lee, T. Blending water- and nutrient-source wastewaters for cost-effective cultivation of high lipid content microalgal species Micractinium inermum NLP-F014. Bioresour. Technol. 2015, 198, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Arriola, M.B.; Velmurugan, N.; Zhang, Y.; Plunkett, M.H.; Hondzo, H.; Barney, B.M. Genome sequences of Chlorella sorokiniana UTEX 1602 and Micractinium conductrix SAG 241.80: Implications to maltose excretion by a green alga. Plant J. 2018, 93, 566–586. [Google Scholar] [CrossRef] [PubMed]
- Pröschold, T.; Pitsch, G.; Darienko, T. Micractinium tetrahymenae (Trebouxiophyceae, Chlorophyta), a New Endosymbiont Isolated from Ciliates. Diversity 2020, 12, 200. [Google Scholar] [CrossRef]
- Hoshina, R.; Iwataki, M.; Imamura, N. Chlorella variabilis and Micractinium reisseri sp. nov. (Chlorellaceae, Trebouxiophyceae): Redescription of the endosymbiotic green algae of Paramecium bursaria (Peniculia, Oligohymenophorea) in the 120th year. Phycol. Res. 2010, 58, 188–201. [Google Scholar] [CrossRef]
- Agrawal, B.; Lakshmanan, V.; Kaushik, S.; Bais, H.P. Natural variation among Arabidopsis accessions reveals malic acid as a key mediator of Nickel (Ni) tolerance. Planta 2012, 236, 477–489. [Google Scholar] [CrossRef]
- Yao, H.; Zhang, S.; Zhou, W.; Liu, Y.; Liu, Y.; Wu, Y. The effects of exogenous malic acid in relieving aluminum toxicity in Pinus massoniana. Int. J. Phytoremediation 2020, 22, 669–678. [Google Scholar] [CrossRef]
- Šimonová, E.; Henselová, M.; Zahradnik, P. Benzothiazole derivatives substituted in position 2 as biologically active substances with plant growth regulation activity. Plant Soil Environ. 2005, 51, 496–505. [Google Scholar] [CrossRef]
- Teale, W.D.; Paponov, I.A.; Palme, K. Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 2006, 7, 847–859. [Google Scholar] [CrossRef]
- Mazur, H.; Konop, A.; Synak, R. Indole-3-acetic acid in the culture medium of two axenic green microalgae. J. Appl. Phycol. 2001, 13, 35–42. [Google Scholar] [CrossRef]
- Labeeuw, L.; Khey, J.; Bramucci, A.R.; Atwal, H.; De La Mata, A.P.; Harynuk, J.; Case, R.J. Indole-3-acetic acid is produced by Emiliania huxleyi coccolith-bearing cells and triggers a physiological response in bald cells. Front. Microbiol. 2016, 7, 1–16. [Google Scholar] [CrossRef]
- Pichler, G.; Stöggl, W.; Candotto Carniel, F.; Muggia, L.; Ametrano, C.G.; Holzinger, A.; Tretiach, M.; Kranner, I. Abundance and Extracellular Release of Phytohormones in Aero-terrestrial Microalgae (Trebouxiophyceae, Chlorophyta) As a Potential Chemical Signaling Source1. J. Phycol. 2020, 56, 1295–1307. [Google Scholar] [CrossRef] [PubMed]
- Stirk, W.A.; van Staden, J. Potential of phytohormones as a strategy to improve microalgae productivity for biotechnological applications. Biotechnol. Adv. 2020, 44, 107612. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.Y.; Kuo, C.Y.; Lin, W.J.; Wang, W.L.; Chou, J.Y. Indole-3-acetic-acid-induced phenotypic plasticity in Desmodesmus algae. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Li, Y.; Hill, R.T. Microalgal and bacterial auxin biosynthesis: Implications for algal biotechnology. Curr. Opin. Biotechnol. 2022, 73, 300–307. [Google Scholar] [CrossRef]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef]
- Fu, L.; Li, Q.; Chen, C.; Zhang, Y.; Liu, Y.; Xu, L.; Zhou, Y.; Li, C.; Zhou, D.; Rittmann, B.E. Benzoic and salicylic acid are the signaling molecules of Chlorella cells for improving cell growth. Chemosphere 2021, 265, 129084. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, M.; Yan, H.; Li, C. Effects of Salicylic Acid on Heavy Metal Resistance in Eukaryotic Algae and Its Mechanisms. Int. J. Environ. Res. Public Health 2022, 19, 13415. [Google Scholar] [CrossRef]
- Hu, Q.; Huang, D.; Li, A.; Hu, Z.; Gao, Z.; Yang, Y.; Wang, C. Transcriptome-based analysis of the effects of salicylic acid and high light on lipid and astaxanthin accumulation in Haematococcus pluvialis. Biotechnol. Biofuels 2021, 14, 1–20. [Google Scholar] [CrossRef]
- Wu, G.; Gao, Z.; Du, H.; Lin, B.; Yan, Y.; Li, G.; Guo, Y.; Fu, S.; Wei, G.; Wang, M.; et al. The effects of abscisic acid, salicylic acid and jasmonic acid on lipid accumulation in two freshwater Chlorella strains. J. Gen. Appl. Microbiol. 2018, 64, 42–49. [Google Scholar] [CrossRef]
- Wasternack, C.; Strnad, M. Jasmonates: News on Occurrence, Biosynthesis, Metabolism and Action of an Ancient Group of Signaling Compounds. Int. J. Mol. Sci. 2018, 19, 2539. [Google Scholar] [CrossRef]
- Hirsch, R.; Hartung, W.; Gimmler, H. Abscisic Acid Content of Algae under Stress*. Bot. Acta 1989, 102, 326–334. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Abu-Ghosh, S.; Iluz, D.; Dubinsky, Z.; Miller, G. Exogenous Abscisic Acid Confers Salinity Tolerance in Chlamydomonas reinhardtii During Its Life Cycle. J. Phycol. 2021, 57, 1323–1334. [Google Scholar] [CrossRef]
- Norlina, R.; Norashikin, M.N.; Loh, S.H.; Aziz, A.; Cha, T.S. Exogenous Abscisic Acid Supplementation at Early Stationary Growth Phase Triggers Changes in the Regulation of Fatty Acid Biosynthesis in Chlorella vulgaris UMT-M1. Appl. Biochem. Biotechnol. 2020, 191, 1653–1669. [Google Scholar] [CrossRef] [PubMed]
- Duca, D.R.; Glick, B.R. Indole-3-acetic acid biosynthesis and its regulation in plant-associated bacteria. Appl. Microbiol. Biotechnol. 2020, 104, 8607–8619. [Google Scholar] [CrossRef]
- Mano, Y.; Nemoto, K. The pathway of auxin biosynthesis in plants. J. Exp. Bot. 2012, 63, 2853–2872. [Google Scholar] [CrossRef]
- Duan, J.; Jiang, W.; Cheng, Z.; Heikkila, J.J.; Glick, B.R. The complete genome sequence of the plant growth-promoting bacterium Pseudomonas sp. UW4. PLoS ONE 2013, 8, e58640. [Google Scholar] [CrossRef]
- Duca, D.; Lorv, J.; Patten, C.L.; Rose, D.; Glick, B.R. Indole-3-acetic acid in plant-microbe interactions. Antonie Van Leeuwenhoek 2014, 106, 85–125. [Google Scholar] [CrossRef]
- Barbez, E.; Kubeš, M.; Rolčík, J.; Béziat, C.; Pěnčík, A.; Wang, B.; Rosquete, M.R.; Zhu, J.; Dobrev, P.I.; Lee, Y.; et al. A novel putative auxin carrier family regulates intracellular auxin homeostasis in plants. Nature 2012, 485, 119–122. [Google Scholar] [CrossRef]
- De Billy, F.; Grosjean, C.; May, S.; Bennett, M.; Cullimore, J.V. Expression studies on AUX1-like genes in Medicago truncatula suggest that auxin is required at two steps in early nodule development. Mol. Plant. Microbe. Interact. 2001, 14, 267–277. [Google Scholar] [CrossRef]
- Jones, A.M.; Im, K.-H.; Savka, M.A.; Wu, M.-J.; DeWitt, N.G.; Shillito, R.; Binns, A.N. Auxin-dependent cell expansion mediated by overexpressed auxin-binding protein 1. Science 1998, 282, 1114–1117. [Google Scholar] [CrossRef] [PubMed]
- Khasin, M.; Cahoon, R.R.; Nickerson, K.W.; Riekhof, W.R. Molecular machinery of auxin synthesis, secretion, and perception in the unicellular chlorophyte alga Chlorella sorokiniana UTEX 1230. PLoS ONE 2018, 13, e0205227. [Google Scholar] [CrossRef] [PubMed]
- Lefevere, H.; Bauters, L.; Gheysen, G. Salicylic Acid Biosynthesis in Plants. Front. Plant Sci. 2020, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.; Won, K.C.; Lezhneva, L.; Falk, J.; Krupinska, K.; Shinozaki, K.; Seki, M.; Herrmann, R.G.; Meurer, J. A plant locus essential for phylloquinone (vitamin K1) biosynthesis originated from a fusion of four eubacterial genes. J. Biol. Chem. 2006, 281, 17189–17196. [Google Scholar] [CrossRef]
- Lohmann, A.; Schöttler, M.A.; Bréhélin, C.; Kessler, F.; Bock, R.; Cahoon, E.B.; Dörmann, P. Deficiency in phylloquinone (vitamin K1) methylation affects prenyl quinone distribution, photosystem I abundance, and anthocyanin accumulation in the Arabidopsis AtmenG mutant. J. Biol. Chem. 2006, 281, 40461–40472. [Google Scholar] [CrossRef]
- Wasternack, C.; Song, S. Jasmonates: Biosynthesis, metabolism, and signaling by proteins activating and repressing transcription. J. Exp. Bot. 2017, 68, 1303–1321. [Google Scholar] [CrossRef]
- Brash, A.R.; Baertschi, S.W.; Ingram, C.D.; Harris, T.M. Isolation and characterization of natural allene oxides: Unstable intermediates in the metabolism of lipid hydroperoxides. Proc. Natl. Acad. Sci. USA 1988, 85, 3382–3386. [Google Scholar] [CrossRef]
- Han, G.Z. Evolution of jasmonate biosynthesis and signaling mechanisms. J. Exp. Bot. 2017, 68, 1323–1331. [Google Scholar] [CrossRef]
- Finkelstein, R. Abscisic Acid synthesis and response. Arab. Book 2013, 11, e0166. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Stecher, G.; Tamura, K.; Kumar, S. Molecular Evolutionary Genetics Analysis (MEGA) for macOS. Mol. Biol. Evol. 2020, 37, 1237–1239. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An Integrated Tool for Comprehensive Microbial Variant Detection and Genome Assembly Improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.-J.; Yu, W.-B.; Yang, J.-B.; Song, Y.; dePamphilis, C.W.; Yi, T.-S.; Li, D.-Z. GetOrganelle: A fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 2020, 21, 1–31. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Hoff, K.J.; Stanke, M. WebAUGUSTUS—A web service for training AUGUSTUS and predicting genes in eukaryotes. Nucleic Acids Res. 2013, 41, W123–W128. [Google Scholar] [CrossRef]
- Tillich, M.; Lehwark, P.; Pellizzer, T.; Ulbricht-Jones, E.S.; Fischer, A.; Bock, R.; Greiner, S. GeSeq–versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 2017, 45, W6–W11. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Lim, J.M.; Park, J.; Sim, Y.M.; Choi, H.G.; Lee, J.; Jeong, W.J. Plastid and mitochondrion genomic sequences from Arctic Chlorella sp. ArM0029B. BMC Genom. 2014, 15, 286. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, O.; Hara, Y.; Kuraku, S. Evaluating genome assemblies and gene models using gVolante. Methods Mol. Biol. 2019, 1962, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Seppey, M.; Manni, M.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness. Methods Mol. Biol. 2019, 1962, 227–245. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Sato, Y.; Morishima, K. BlastKOALA and GhostKOALA: KEGG Tools for Functional Characterization of Genome and Metagenome Sequences. J. Mol. Biol. 2016, 428, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Apweiler, R.; Bairoch, A.; Wu, C.H.; Barker, W.C.; Boeckmann, B.; Ferro, S.; Gasteiger, E.; Huang, H.; Lopez, R.; Magrane, M.; et al. UniProt: The universal protein knowledgebase. Nucleic Acids Res. 2017, 45, D158–D169. [Google Scholar] [CrossRef]

| Name | Molecular Weight | m/z | RT [min] | Average Normalized Peak Area | Ionization Mode |
|---|---|---|---|---|---|
| Tartaric acid | 150.02 | 149.01 | 0.89 | 10,113.57 | - |
| Stearic acid | 284.27 | 283.26 | 11.43 | 1366.32 | - |
| Phthalic acid | 166.03 | 165.02 | 3.98 | 1069.89 | - |
| Gingerol | 294.18 | 293.18 | 8.37 | 964.23 | - |
| 3-methyl-2-oxovaleric acid | 130.06 | 129.05 | 3.66 | 897.09 | - |
| Lactic acid | 90.03 | 89.02 | 1.23 | 797.01 | - |
| Azelaic acid | 188.10 | 187.10 | 5.36 | 677.12 | - |
| Malic acid | 134.02 | 133.01 | 0.88 | 659.34 | - |
| Pyruvic acid | 88.01 | 87.01 | 0.90 | 638.25 | - |
| 2-Isopropylmalic acid | 176.07 | 175.06 | 3.68 | 611.98 | - |
| 3-Hydroxy-3-methylglutaric acid | 162.05 | 161.04 | 1.59 | 559.06 | - |
| 10-oxo-8-decenoic acid | 184.11 | 183.10 | 5.94 | 434.27 | - |
| 2,4-Dihydroxybenzoic acid | 154.03 | 153.02 | 0.88 | 405.99 | - |
| Indole | 117.06 | 116.05 | 3.27 | 389.51 | - |
| Salicylic acid | 138.03 | 137.02 | 5.67 | 351.88 | - |
| Benzothiazole | 135.01 | 136.02 | 6.37 | 32,851.00 | + |
| 1-Methoxy-1H-indole-3-acetonitrile | 186.08 | 94.05 | 0.72 | 26,810.47 | + |
| 2-Acetylthiazole | 135.01 | 136.02 | 6.37 | 13,111.33 | + |
| Indole-3-acetic acid | 186.08 | 94.05 | 0.72 | 8808.64 | + |
| Levoglucosan | 127.01 | 128.02 | 0.72 | 2797.73 | + |
| S-Aminoethyl-cysteine | 219.03 | 110.52 | 14.65 | 1331.66 | + |
| Hydrocinnamic acid | 162.05 | 163.06 | 1.26 | 1234.93 | + |
| Sedanolide | 164.06 | 83.04 | 0.73 | 994.99 | + |
| Traumatin | 150.07 | 301.14 | 0.81 | 822.69 | + |
| Phenylalanine | 194.13 | 195.14 | 6.68 | 811.84 | + |
| Sphinganine | 212.14 | 213.15 | 6.67 | 672.22 | + |
| Thymoquinone | 165.08 | 166.09 | 2.40 | 368.78 | + |
| Sulfamic acid | 301.30 | 302.30 | 8.12 | 328.02 | + |
| Pyroglutamic acid | 164.08 | 165.09 | 5.21 | 253.56 | + |
| Uridine | 96.98 | 139.02 | 14.64 | 244.76 | + |
| Name | Molecular Weight | m/z | RT [min] | Average Normalized Peak Area | Ionization Mode |
|---|---|---|---|---|---|
| Indole-3-acetic acid | 219.03 | 110.52 | 14.65 | 8808.64 | + |
| 3-Indoleacrylic acid | 187.06 | 188.07 | 3.28 | 65.43 | + |
| Salicylic acid | 138.03 | 137.02 | 5.67 | 351.88 | - |
| p-Hydroxysalicylic acid | 154.03 | 153.02 | 0.88 | 405.99 | - |
| Methyl dihydrojasmonate | 226.16 | 225.15 | 9.27 | 173.19 | - |
| Epi-jasmonic acid | 210.13 | 211.13 | 7.64 | 142.77 | + |
| Dihydrojasmonic Acid | 212.14 | 211.13 | 7.39 | 53.98 | - |
| Methyl Jasmonate | 224.14 | 225.15 | 5.52 | 19.33 | + |
| Abscisic acid | 264.14 | 265.14 | 8.66 | 29.13 | + |
| M. rhizosphaerae NFX-FRZ | M. conductrix SAG 241.80 | |
|---|---|---|
| Nuclear genome size (Mbp) | 68.3 | 60.8 |
| Nuclear genome GC% | 65.3% | 67.4% |
| Chloroplast genome size (bp) | 120,168 | 129,445 |
| Chloroplast genome GC% | 32.4 | 34.8 |
| Mitochondrial genome size (bp) | 74,426 | 75,014 |
| Mitochondrial genome GC% | 30.3 | 29.4 |
| Nuclear CDS | 14,335 | 10,070 |
| Annotated CDS | 5085 | 4566 |
| GIP | 1096 + 932 | 980 + 893 |
| CM | 390 | 359 |
| SCP | 373 | 321 |
| EIP | 237 | 168 |
| CP | 224 | 216 |
| EM | 198 | 177 |
| AAM | 190 | 167 |
| MCV | 175 | 184 |
| LM | 154 | 141 |
| GBM | 116 | 75 |
| NM | 94 | 88 |
| MTP | 43 | 39 |
| MAA | 37 | 34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintas-Nunes, F.; Brandão, P.R.; Barreto Crespo, M.T.; Glick, B.R.; Nascimento, F.X. Plant Growth Promotion, Phytohormone Production and Genomics of the Rhizosphere-Associated Microalga, Micractinium rhizosphaerae sp. nov. Plants 2023, 12, 651. https://doi.org/10.3390/plants12030651
Quintas-Nunes F, Brandão PR, Barreto Crespo MT, Glick BR, Nascimento FX. Plant Growth Promotion, Phytohormone Production and Genomics of the Rhizosphere-Associated Microalga, Micractinium rhizosphaerae sp. nov. Plants. 2023; 12(3):651. https://doi.org/10.3390/plants12030651
Chicago/Turabian StyleQuintas-Nunes, Francisco, Pedro R. Brandão, Maria T. Barreto Crespo, Bernard R. Glick, and Francisco X. Nascimento. 2023. "Plant Growth Promotion, Phytohormone Production and Genomics of the Rhizosphere-Associated Microalga, Micractinium rhizosphaerae sp. nov." Plants 12, no. 3: 651. https://doi.org/10.3390/plants12030651
APA StyleQuintas-Nunes, F., Brandão, P. R., Barreto Crespo, M. T., Glick, B. R., & Nascimento, F. X. (2023). Plant Growth Promotion, Phytohormone Production and Genomics of the Rhizosphere-Associated Microalga, Micractinium rhizosphaerae sp. nov. Plants, 12(3), 651. https://doi.org/10.3390/plants12030651








