Silicon Actuates Poplar Calli Tolerance after Longer Exposure to Antimony
Abstract
:1. Introduction
2. Results
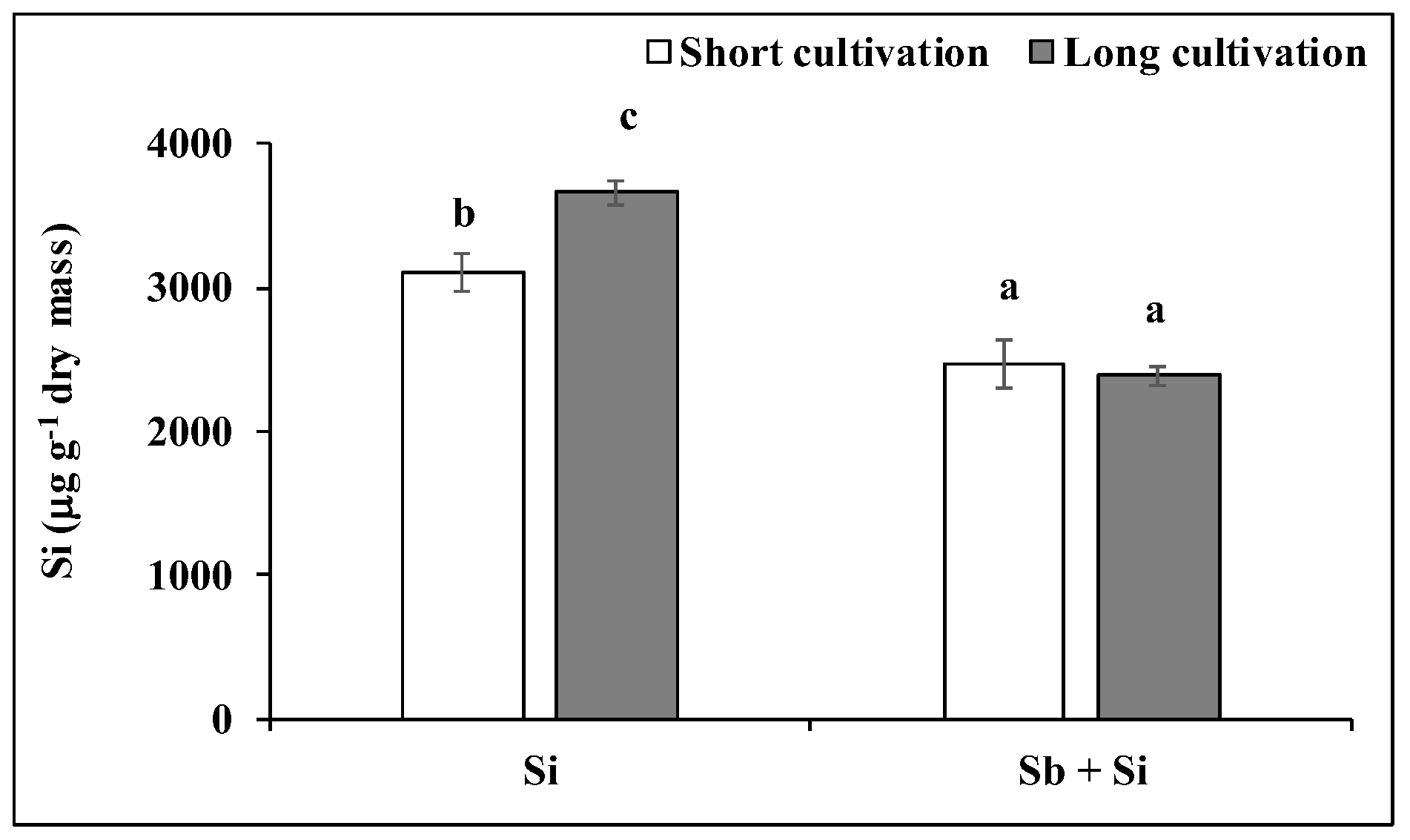
2.1. Effect of Sb and/or Si Treatments on the Concentration of Si and Sb
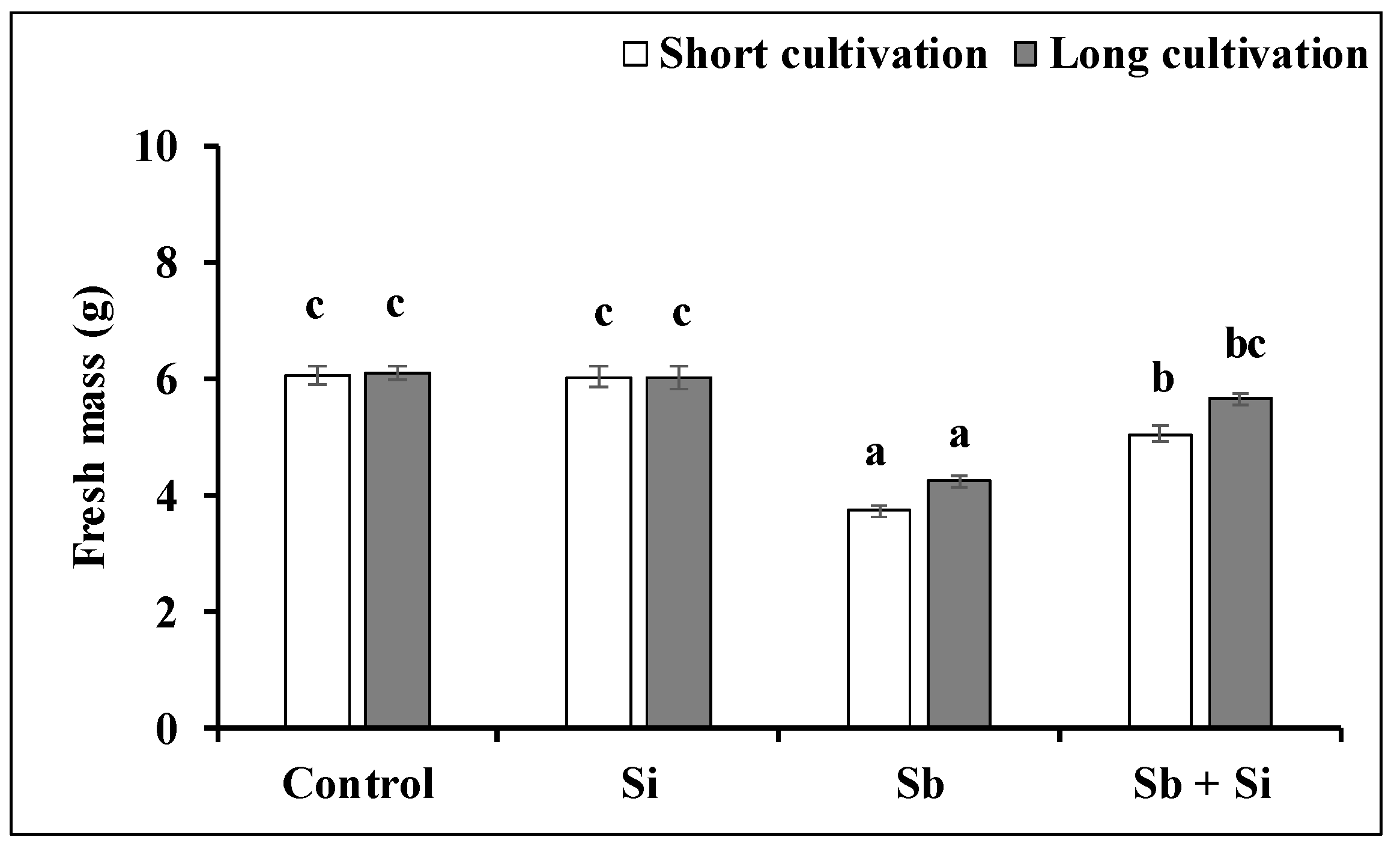
2.2. The Growth of the Callus Exposed to Sb or/and Si
2.3. The Influence of Sb and/or Si on the Concentration of Photosynthetic Pigments
2.4. Effect of Sb and/or Si on the Activity of Antioxidant Enzymes
2.5. Effect of Sb and/or Si on the Concentration of the Mineral Nutrients
3. Discussion
4. Materials and Methods
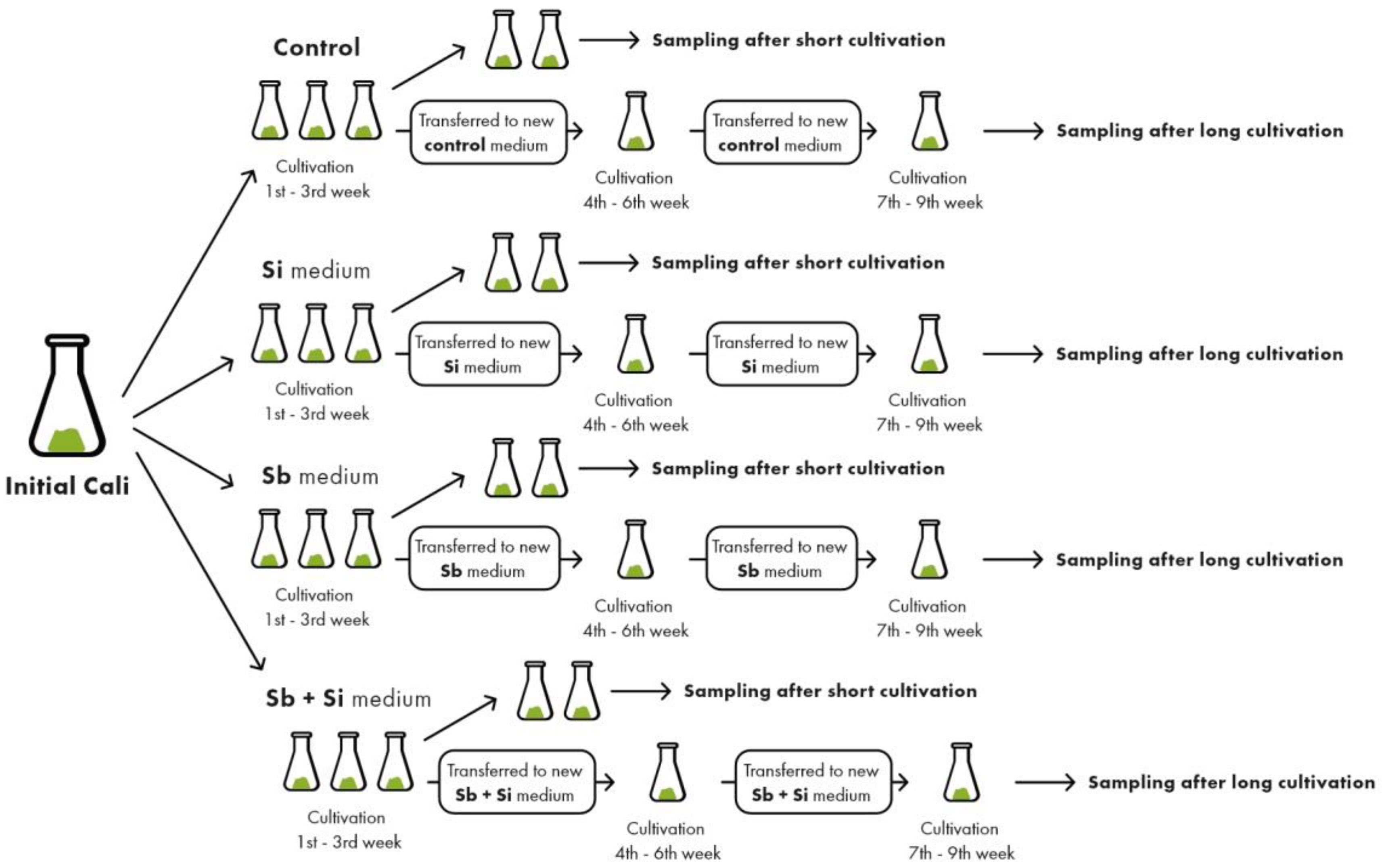
4.1. Plant Material Cultivation
4.2. Determination of Sb and/or Si Concentrations
4.3. Determination of the Fresh and Dry Weight
4.4. Determination of the Photosynthetic Pigment Concentration
4.5. Determination of the Antioxidant Enzyme Activity
4.6. Determination of the Mineral Nutrient Concentrations
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vaculíková, M.; Vaculík, M.; Šimková, L.; Fialová, I.; Kochanová, Z.; Sedláková, B.; Luxová, M. Influence of silicon on maize roots exposed to antimony–Growth and antioxidative response. Plant Physiol. Biochem. 2014, 83, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, B.; Han, F.; Xiao, E.; Xiao, T.; Sun, W. Impacts of arsenic and antimony co-contamination on sedimentary microbial communities in rivers with different pollution gradient. Microb. Ecol. 2019, 78, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Gebel, T. Arsenic and antimony: Comparative approach on mechanistic toxicology. Chem. Biol. Interact. 1997, 107, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Chakravarty, J. Antimony toxicity. Int. J. Environ. Res. Public Health. 2010, 7, 4267–4277. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Su, H.; Li, H.; Zhu, Y.; Shi, D.; Wu, F.; Sun, F. Ecological and human health risk assessment of antimony (Sb) in surface and drinking water in China. J. Clean. Prod. 2021, 318, 128514. [Google Scholar] [CrossRef]
- Lai, Z.; He, M.; Lin, C.; Ouyang, W.; Liu, X. Interactions of antimony with biomolecules and its effects on human health. Ecotoxicol. Environ. Saf. 2022, 233, 113317. [Google Scholar] [CrossRef]
- Herath, I.; Vithanage, M.; Bundschuh, J. Antimony as a global dilemma: Geochemistry, mobility, fate and transport. Environ. Pollut. 2017, 223, 545–559. [Google Scholar] [CrossRef]
- Shahid Muhammad, N.; Khalid, S.; Dumat, C.; Pierart, A.; Khan Nabel, L. Biogeochemistry of antimony in soil-plant system: Ecotoxicology and human health. Appl. Geochem. 2019, 106, 45–59. [Google Scholar] [CrossRef]
- Filella, M.; Belzile, N.; Chenm, Y. Antimony in the environment: A review focused on natural water: II. Relevant solution chemistry. Earth-Sci. Rev. 2002, 59, 265–285. [Google Scholar] [CrossRef]
- Chirappurathu Sukumaran-Nair, V.; Shetty, R.; Vaculíková, M.; Vaculík, M. Antimony toxicity in soils and plants, and mechanisms of its alleviation. Environ. Exp. Bot. 2022, 202, 104966. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, C.; Zhu, P.; Liu, F. Effects on antimony stress on photosynthesis and growth of Acorus calamus. Front. Plant. Sci. 2018, 9, 579. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.; Kumar, M.; Singh, E.; Kumar, A.; Singh, L.; Kumar, S.; Keerthanan, S.; Hoang, S.A.; El-Naggar, A.; Vithanage, M.; et al. Antimony contamination and its risk management in complex environmental settings: A review. Environ. Int. 2022, 158, 106908. [Google Scholar] [CrossRef] [PubMed]
- Vaculík, M.; Mrázová, A.; Lux, A. Antimony (SbIII) reduces growth, declines photosynthesis, and modifies leaf tissue anatomy in sunflower (Helianthus annuus L.). Environ. Sci. Pollut. Res. 2015, 22, 18699–18706. [Google Scholar] [CrossRef]
- Tschan, M.; Robinson, B.; Johnson, C.A.; Bürgi, A.; Schulin, R. Antimony uptake and toxicity in sunflower and maize growing in SbIII and SbV contaminated soil. Plant Soil 2010, 334, 235–245. [Google Scholar] [CrossRef]
- Feng, R.; Wei, C.; Tu, S.; Ding, Y.; Wang, R.; Guo, J. The uptake and detoxification of antimony by plants: A review. Environ. Exp. Bot. 2013, 96, 28–34. [Google Scholar] [CrossRef]
- Feng, R.; Lei, L.; Su, J.; Zhang, R.; Zhu, Y.; Chen, W.; Wang, L.; Wang, R.; Dai, J.; Lin, Z.; et al. Toxicity of different forms of antimony to rice plant: Effects on root exudates, cell wall components, endogenous hormones and antioxidant system. Sci. Total Environ. 2020, 711, 134589. [Google Scholar] [CrossRef]
- Espinosa-Vellarino, F.L.; Garrido, I.; Ortega, A.; Casimiro, I.; Espinosa, F. Response to antimony toxicity in Dittrichia viscosa plants: ROS, NO, H2S, and the antioxidant system. Antioxidants 2021, 10, 1698. [Google Scholar] [CrossRef]
- Wang, X.; Li, F.; Yuan, C.; Li, B.; Liu, T.; Liu, C.; Du, Y.; Liu, C. The translocation of antimony in soil-rice system with comparisons to arsenic: Alleviation of their accumulation in rice by simultaneous use of Fe(II) and NO3−. Sci. Total Environ. 2019, 650, 633–641. [Google Scholar] [CrossRef]
- Wu, C.; Li, F.; Xu, H.; Zeng, W.; Yu, R.; Wu, X.; Shen, L.; Liu, Y.; Li, J. The potential role of brassinosteroids (BRs) in alleviating antimony (Sb) stress in Arabidopsis thaliana. Plant Physiol. Biochem. 2019, 141, 51–59. [Google Scholar] [CrossRef]
- Shetty, R.; Vidya, C.S.N.; Weidinger, M.; Vaculík, M. Silicon alleviates antimony phytoxicity in giant reed (Arundo donax L.). Planta 2021, 254, 100. [Google Scholar] [CrossRef]
- Farooq, M.A.; Ali, S.; Hameed, A.; Ishaque, W.; Mahmood, K.; Iqbal, Z. Alleviation of cadmium toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes; supressed cadmium uptake and oxidative stress in cotton. Ecotoxicol. Environ. Saf. 2013, 96, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Gheshlaghpour, J.; Asghari, B.; Khademian, R.; Sedaghati, B. Silicon alleviates cadmium stress in basil (Ocimum basilicum L.) through alteration of phytochemical and physiological characteristics. Ind. Crops Prod. 2021, 163, 113338. [Google Scholar] [CrossRef]
- Adrees, M.; Ali, S.; Rizwan, M.; Rehman, M.Z.; Ibrahim, M.; Abbas, F.; Farid, M.; Qayyum, M.F.; Irshad, M.K. Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: A review. Ecotoxicol. Environ. Saf. 2015, 119, 186–197. [Google Scholar] [CrossRef]
- Luyckx, M.; Hausman, J.; Blanquet, M.; Guerriero, G.; Lutts, S. Silicon reduces cadmium absorption and increases root-to-shoot translocation without impacting growth in young plants of hemp (Cannabis sativa L.) on a short-term basis. Environ. Sci. Pollut. Res. 2021, 28, 37963–37977. [Google Scholar] [CrossRef]
- Pilon, C.; Sorrato, R.P.; Moreno, L.A. Effects of soil and foliar application of soluble silicon on mineral nutrition, gas exchange, and growth of potato plants. Crop Sci. 2013, 53, 1605–1614. [Google Scholar] [CrossRef]
- Suori, Z.; Khanna, K.; Karimi, N.; Ahmad, P. Silicon and plants: Current knowledge and future prospects. J. Plant Growth Regul. 2021, 40, 906–925. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Rizwan, M.; Brestic, M.; Xie, W. Silicon: An essential element form plant nutrition and phytohormones signalling mechanism under stressful conditions. Plant Growth Regul. 2022. [Google Scholar] [CrossRef]
- Labancová, E.; Vivodová, Z.; Kučerová, D.; Lišková, D.; Kollárová, K. The cadmium tolerance development of poplar callus is influenced by silicon. Ecotoxicology 2020, 29, 987–1002. [Google Scholar] [CrossRef]
- Kučerová, D.; Vivodová, Z.; Kollárová, K. Silicon alleviates the negative effects of arsenic in poplar callus in relation to its nutrient concentrations. Plant Cell Tissue Organ Cult. 2021, 145, 275–289. [Google Scholar] [CrossRef]
- Dai, Z.H.; Guan, D.X.; Bundschuh, J.; Ma, L.Q. Roles of phytohormones in mitigating abiotic stress in plants induced by metal(loid)s As, Cd, Cr, Hg, and Pb. Crit. Rev. Environ. Sci. Technol. 2022, 1–21. [Google Scholar] [CrossRef]
- Al-Mayahi, A.M.W. Effect of calcium and boron on growth and development of callus and shoot regeneration of date palm ‘Barhee’. Can. J. Plant Sci. 2020, 100, 357–364. [Google Scholar] [CrossRef]
- Blevins, D.G.; Lukaszweski, K. Boron in plant structure and function. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 481–500. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.X.; Chen, H.; Xu, W.Z.; He, Z.Y.; Ma, M. Hyperaccumulation of arsenic by callus, sporophytes and gametophytes of Pteris vittata cultured in vitro. Plant Cell Rep. 2007, 26, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Ayub, M.A.; Abbas, M.; Rehman, M.Z. Role of inorganic bio stimulant elements in plant growth. In Sustainable Plant Nutrition; Aftab, T., Hakeem, K.R., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 229–261. ISBN 9780443186752. [Google Scholar] [CrossRef]
- Bakhat, H.F.; Bibi, N.; Zia, Z.; Abbas, S.; Hammad, H.M.; Fahad, S.; Ashraf, M.R.; Shah, G.M.; Saeed, S. Silicon mitigates biotic stresses in crop plants: A review. Crop Prot. 2018, 104, 21–34. [Google Scholar] [CrossRef]
- Thorne, S.J.; Hartley, S.E.; Maathuis, F.J. Is silicon a panacea for alleviating drought and salt stress in crops? Front. Plant. Sci. 2020, 11, 1221. [Google Scholar] [CrossRef]
- Yadav, R.; Arora, P.; Kumar, S.; Chaudhury, A. Perspectives for genetic engineering of poplars for enhanced phytoremediation abilities. Ecotoxicology 2010, 19, 1574–1588. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yang, F.; Liu, J.; Xie, W.; Zhang, L.; Chen, Z.; Peng, Z.; Ou, Y.; Yao, Y. Genome-wide identification of metal tolerance protein genes in Populus trichocarpa and their roles in response to various heavy metal stresses. Int. J. Mol. Sci. 2020, 21, 1680. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Rizwan, M.; Ali, S.; Hassan, M.J.; Brestic, M.; Zhang, X.; Huang, L. Effects of silicon on heavy metal uptake at the soil-plant interphase: A review. Ecotoxicol. Environ. Saf. 2021, 222, 112510. [Google Scholar] [CrossRef]
- Nwugo, C.C.; Huerta, A.J. Effects of silicon nutrition on cadmium uptake, growth and photosynthesis of rice plants exposed to low-level cadmium. Plant Soil 2008, 311, 73–86. [Google Scholar] [CrossRef]
- Mhamdi, A.; Van Breusegem, F. Reactive oxygen species in plant development. Development 2018, 145, dev164376. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Kumar, A.; Latowski, D.; Kuczynska, P.; Strzałka, K.; Prasad, M.N.V. Heavy metal-induced oxidative damage, defense reactions, and detoxification mechanisms in plants. Acta Physiol. Plant. 2013, 35, 985–999. [Google Scholar] [CrossRef]
- Yan, G.; Fan, X.; Peng, M.; Yin, C.; Xiao, Z.; Liang, Y. Silicon improves rice salinity resistance by alleviating ionic toxicity and osmotic constraint in an organ-specific pattern. Front. Plant Sci. 2020, 11, 260. [Google Scholar] [CrossRef] [PubMed]
- Piršelová, B.; Kuna, R.; Libantová, J.; Moravčíková, J.; Matušíková, I. Biochemical and physiological comparison of heavy metal-triggered defense responses in the monocot maize and dicot soybean roots. Mol. Biol. Rep. 2011, 38, 3437–3446. [Google Scholar] [CrossRef]
- Gutsch, A.; Keunen, E.; Guerriero, G.; Renaut, J.; Cuypers, A.; Hausman, J.F.; Sergeant, K. Long-term cadmium exposure influences the abundance of proteins that impact the cell wall structure in Medicago sativa stems. Plant Biol. 2018, 20, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Marjamaa, K.; Kukkola, E.M.; Fagerstedt, K.V. The role of xylem class III peroxidases in lignification. J. Exp. Bot. 2009, 60, 367–376. [Google Scholar] [CrossRef]
- Alayón-Luaces, P.; Ponce, N.M.; Mroginski, L.A.; Stortz, C.A.; Sozzi, G.O. Compositional changes in cell wall polysaccharides from apple fruit callus cultures modulated by different plant growth regulators. Plant Sci. 2012, 185, 169–175. [Google Scholar] [CrossRef]
- Reid, R.J.; Zhang, Q.; Sekimoto, H. Plant Nutrition. In Developments in Plant and Soil Sciences; Springer: Dordrecht, Germany, 2001; pp. 189–199. ISBN 978-0-306-47624-2. [Google Scholar]
- Kučerová, D.; Labancová, E.; Vivodová, Z.; Kollárová, K. The modulation of ion homeostasis by silicon in cadmium treated poplar callus cells. Environ. Sci. Pollut. Res. 2020, 27, 2857–2867. [Google Scholar] [CrossRef]
- Kostic, L.; Nikolic, N.; Bosnic, D.; Samardzic, J.; Nikolic, M. Silicon increases phosphorus (P) uptake by wheat under low P acid soil conditions. Plant Soil 2017, 419, 447–455. [Google Scholar] [CrossRef]
- Schaller, J.; Faucherre, S.; Joss, H.; Obst, M.; Goeckede, M.; Planer-Friedrich, B.; Peiffer, S.; Gilfedder, B.; Elberling, B. Silicon increases the phosphorus availability of Arctic soils. Sci. Rep. 2019, 9, 449. [Google Scholar] [CrossRef] [Green Version]
- Pavlovic, J.; Samardzic, J.; Kostic, L.; Laursen, K.H.; Natic, M.; Timotijevic, G.; Schjoerring, J.K.; Nikolic, M. Silicon enhances leaf remobilization of iron in cucumber under limited iron conditions. Ann. Bot. 2016, 118, 271–280. [Google Scholar] [CrossRef]
- Poirier, Y.; Bucher, M. Phosphate transport and homeostasis in Arabidopsis. Arab. Book 2002, 1, e0024. [Google Scholar] [CrossRef]
- Bose, J.; Babourina, O.; Rengel, Z. Role of magnesium in alleviation of aluminium toxicity in plants. J. Exp. Bot. 2011, 62, 2251–2264. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Khan, N.A.; Jahan, B.; Goyal, V.; Hamid, J.; Khan, S.; Iqbal, N.; Alamri, S.; Siddiqui, M.H. Phosphorus supplementation modulates nitric oxide biosynthesis and stabilizes the defence system to improve arsenic stress tolerance in mustard. Plant Biol. 2020, 23, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Venugopalan, V.; Nath, R.; Sengupta, K.; Pal, A.; Banerjee, S.; Banerjee, P.; Chandran, M.A.S.; Roy, S.; Sharma, L.; Hossain, A.; et al. Foliar spray of micronutrients alleviates heat and moisture stress in lentil (Lens culinaris Medik) grown under rainfed field conditions. Front. Plant. Sci. 2022, 13, 847743. [Google Scholar] [CrossRef]
- Kumari, V.V.; Banerjee, P.; Verma, V.C.; Sukumaran, S.; Chandran, M.A.S.; Gopinath, K.A.; Venkatesh, G.; Yadav, S.K.; Singh, V.K.; Awasthi, N.K. Plant nutrition: An effective way to alleviate abiotic stress in agricultural crops. Int. J. Mol. Sci. 2022, 23, 8519. [Google Scholar] [CrossRef]
- Liu, J.; Ma, J.; He, C.; Li, X.; Zhang, W.; Xu, F.; Lin, Y.; Wang, L. Inhibition of cadmium ion uptake in rice (Oryza sativa) cells by a wall-bound form of silicon. New Phytol. 2013, 200, 691–699. [Google Scholar] [CrossRef]
- Liang, Y.; Sun, W.; Zhu, Y.G.; Christie, P. Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: A review. Environ. Pollut. 2007, 147, 422–428. [Google Scholar] [CrossRef]
- Meharg, C.; Meharg, A.A. Silicon, the silver bullet for mitigating biotic and abiotic stress, and improving grain quality in rice? Environ. Exp. Bot. 2015, 120, 8–17. [Google Scholar] [CrossRef]
- Diaz-Colon, J.D.; Bovey, R.W.; Davis, F.S.; Baur, J.R. Comparative effects and concentration of picloram, 2,4,5-T and dicamba in tissue culture. Physiol. Plant. 1972, 27, 60–64. [Google Scholar] [CrossRef]
- Šípošová, K.; Labancová, E.; Kučerová, D.; Kollárová, K.; Vivodová, Z. Effects of exogenous application of indole-3-butyric acid on maize plants cultivated in the presence or absence of cadmium. Plants 2021, 10, 2503. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods. Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Madamanchi, N.R.; Donahue, J.L.; Cramer, C.L.; Alscher, R.G.; Pedersen, K. Differential response of Cu, Zn superoxide dismutases in two pea cultivars during a short-term exposure to sulfur dioxide. Plant Mol. Biol. 1994, 26, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Hodges, D.M.; Andrews, C.J.; Johnson, D.A.; Hamilton, R.I. Antioxidant enzyme responses to chilling stress in differentially sensitive inbred maize lines. J. Exp. Bot. 1997, 48, 1105–1113. [Google Scholar] [CrossRef]
- Frič, F.; Fuchs, W.H. Veränderungen der Aktivität einiger enzyme im Weizenblatt in Abhängigkeit von Puccinia graminis tritici. Phytopathol 1970, 67, 161–174. [Google Scholar] [CrossRef]



| Photosynthetic Pigments (μg g−1 Fresh Mass) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Chlorophyll a | Chlorophyll b | Carotenoids | ||||||||
| Control | short cultivation | 127.92 | ± | 1.46 cd | 48.01 | ± | 1.50 bc | 37.75 | ± | 0.64 d |
| Si | 125.39 | ± | 2.23 bc | 44.23 | ± | 0.44 b | 36.24 | ± | 1.24 d | |
| Sb | 42.20 | ± | 4.38 a | 15.14 | ± | 15.14 a | 13.53 | ± | 1.05 a | |
| Sb + Si | 111.21 | ± | 3.83 b | 39.69 | ± | 0.73 b | 29.99 | ± | 0.46 c | |
| Control | long cultivation | 139.87 | ± | 0.95 cd | 58.05 | ± | 2.62 d | 27.47 | ± | 1.01 bc |
| Si | 142.38 | ± | 1.45 d | 61.61 | ± | 1.85 d | 30.91 | ± | 0.93 c | |
| Sb | 30.14 | ± | 4.73 a | 14.78 | ± | 1.69 a | 11.24 | ± | 1.09 a | |
| Sb + Si | 113.12 | ± | 1.91 b | 54.32 | ± | 2.79 cd | 24.33 | ± | 0.79 b | |
| Concentration of the Macroelements (mg/g Dry Mass) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | K | Ca | Mg | ||||||||||
| Control | short cultivation | 2.97 | ± | 0.06 bc | 54.23 | ± | 0.22 c | 6.31 | ± | 0.02 b | 2.27 | ± | 0.03 bc |
| Si | 2.93 | ± | 0.05 bc | 52.43 | ± | 0.70 bc | 6.33 | ± | 0.06 b | 2.23 | ± | 0.01 b | |
| Sb | 2.49 | ± | 0.02 a | 48.47 | ± | 0.41 a | 5.79 | ± | 0.04 a | 2.05 | ± | 0.03 a | |
| Sb + Si | 2.86 | ± | 0.06 bc | 52.03 | ± | 0.09 bc | 6.19 | ± | 0.04 ab | 2.24 | ± | 0.02 b | |
| Control | long cultivation | 3.14 | ± | 0.10 c | 53.10 | ± | 0.51 bc | 6.47 | ± | 0.19 bc | 2.39 | ± | 0.06 c |
| Si | 3.06 | ± | 0.07 bc | 52.27 | ± | 0.27 bc | 6.91 | ± | 0.12 c | 2.52 | ± | 0.03 d | |
| Sb | 2.46 | ± | 0.04 a | 47.13 | ± | 1.10 a | 5.83 | ± | 0.07 a | 2.08 | ± | 0.01 a | |
| Sb + Si | 2.79 | ± | 0.02 b | 51.50 | ± | 0.38 b | 6.33 | ± | 0.06 b | 2.30 | ± | 0.01 bc | |
| Concentration of the Microelements (μg/g Dry Mass) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Mn | Zn | Cu | ||||||||||
| Control | short cultivation | 155.67 | ± | 3.71 bc | 411.33 | ± | 4.26 c | 107.80 | ± | 5.96 b | 1.3 | ± | 0.01 cd |
| Si | 149.00 | ± | 4.62 b | 412.00 | ± | 0.58 c | 107.60 | ± | 5.06 b | 1.3 | ± | 0.01 cd | |
| Sb | 129.33 | ± | 2.60 a | 343.00 | ± | 2.89 a | 86.00 | ± | 1.25 a | 1.1 | ± | 0.02 a | |
| Sb + Si | 147.33 | ± | 3.18 b | 392.67 | ± | 0.88 bc | 104.80 | ± | 1.51 b | 1.3 | ± | 0.01 bc | |
| Control | long cultivation | 149.33 | ± | 4.67 b | 459.33 | ± | 1.76 d | 114.57 | ± | 0.67 b | 1.5 | ± | 0.02 de |
| Si | 145.33 | ± | 1.20 b | 445.00 | ± | 5.03 d | 105.40 | ± | 1.62 b | 1.5 | ± | 0.09 e | |
| Sb | 129.00 | ± | 1.73 a | 359.00 | ± | 5.51 a | 88.80 | ± | 1.73 a | 1.1 | ± | 0.01 ab | |
| Sb + Si | 168.33 | ± | 2.03 c | 385.33 | ± | 8.01 b | 105.20 | ± | 1.10 b | 1.4 | ± | 0.01 cd | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labancová, E.; Vivodová, Z.; Šípošová, K.; Kollárová, K. Silicon Actuates Poplar Calli Tolerance after Longer Exposure to Antimony. Plants 2023, 12, 689. https://doi.org/10.3390/plants12030689
Labancová E, Vivodová Z, Šípošová K, Kollárová K. Silicon Actuates Poplar Calli Tolerance after Longer Exposure to Antimony. Plants. 2023; 12(3):689. https://doi.org/10.3390/plants12030689
Chicago/Turabian StyleLabancová, Eva, Zuzana Vivodová, Kristína Šípošová, and Karin Kollárová. 2023. "Silicon Actuates Poplar Calli Tolerance after Longer Exposure to Antimony" Plants 12, no. 3: 689. https://doi.org/10.3390/plants12030689
APA StyleLabancová, E., Vivodová, Z., Šípošová, K., & Kollárová, K. (2023). Silicon Actuates Poplar Calli Tolerance after Longer Exposure to Antimony. Plants, 12(3), 689. https://doi.org/10.3390/plants12030689








