The Response of Chromosomally Engineered Durum Wheat-Thinopyrum ponticum Recombinant Lines to the Application of Heat and Water-Deficit Stresses: Effects on Physiological, Biochemical and Yield-Related Traits
Abstract
1. Introduction
2. Materials and Methods
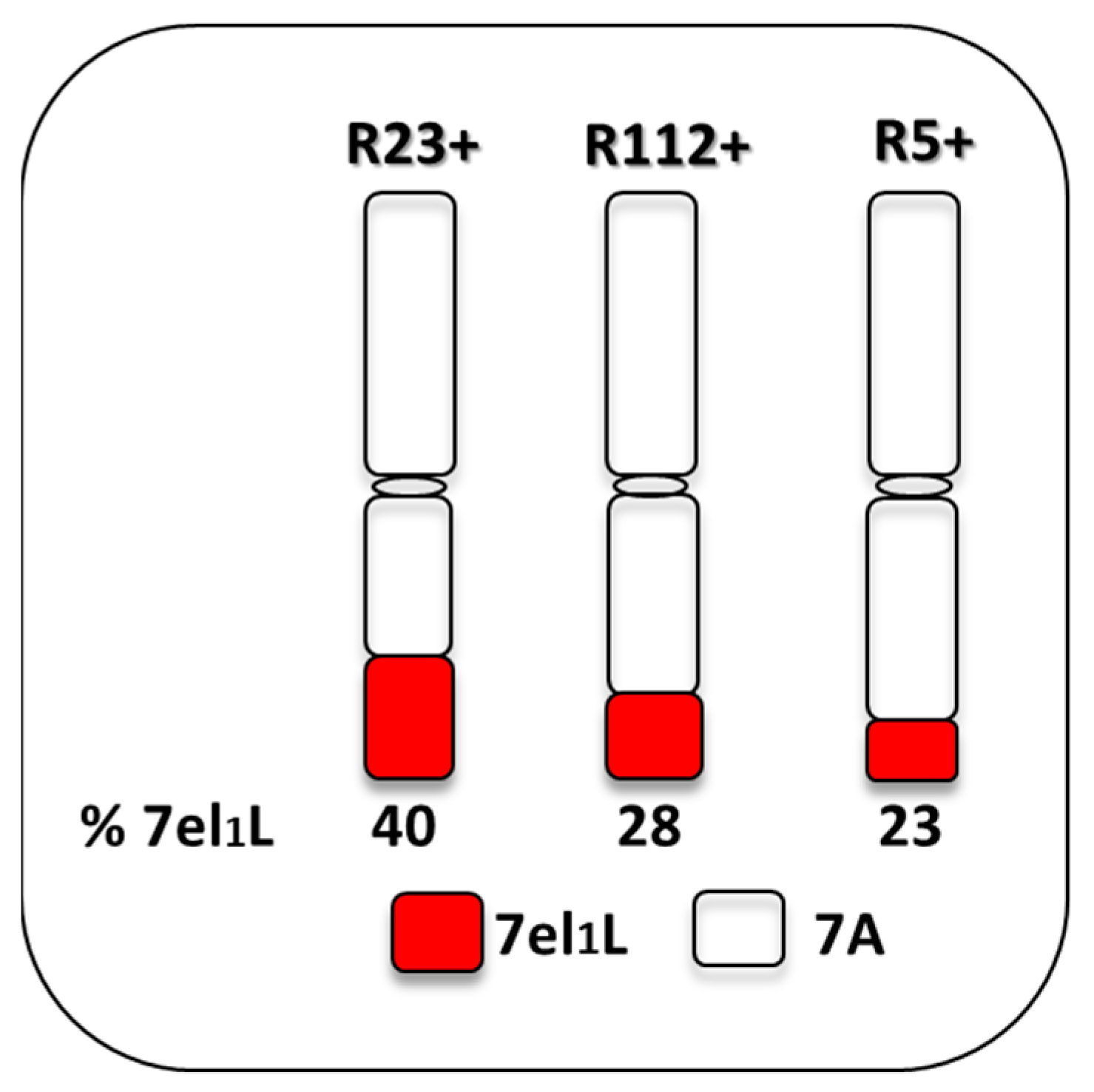
2.1. Plant Materials
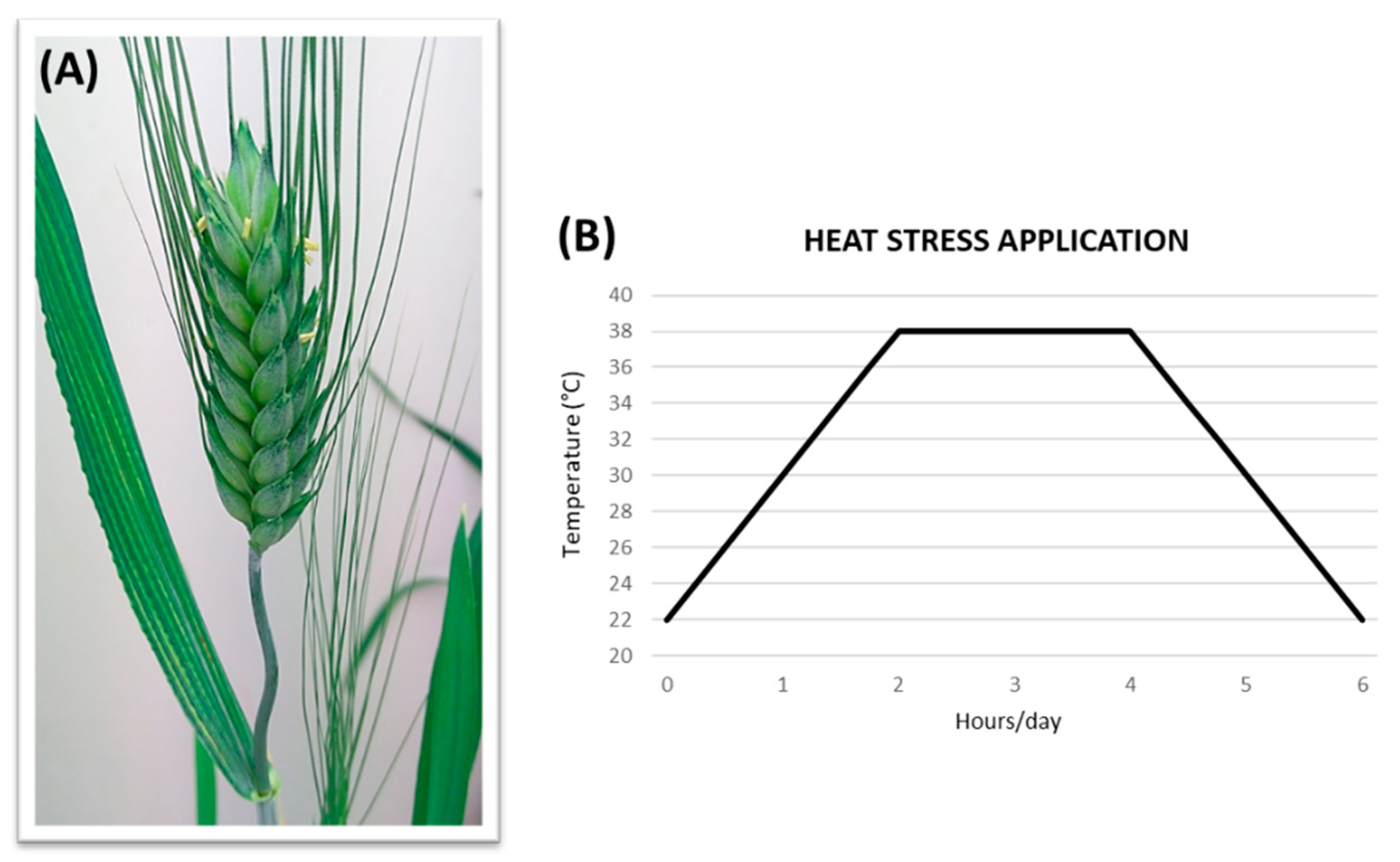
2.2. Plant Growing Conditions and Stress Treatments
2.3. Physiological and Biochemical Measurements
2.3.1. Physiological Parameters
2.3.2. Proline Quantification in Leaves and Spikes
2.4. Evaluation of Yield-Related Parameters
2.5. Stress Indices
2.6. Statistical Analysis
3. Results
3.1. Heat Stress (HS) Application
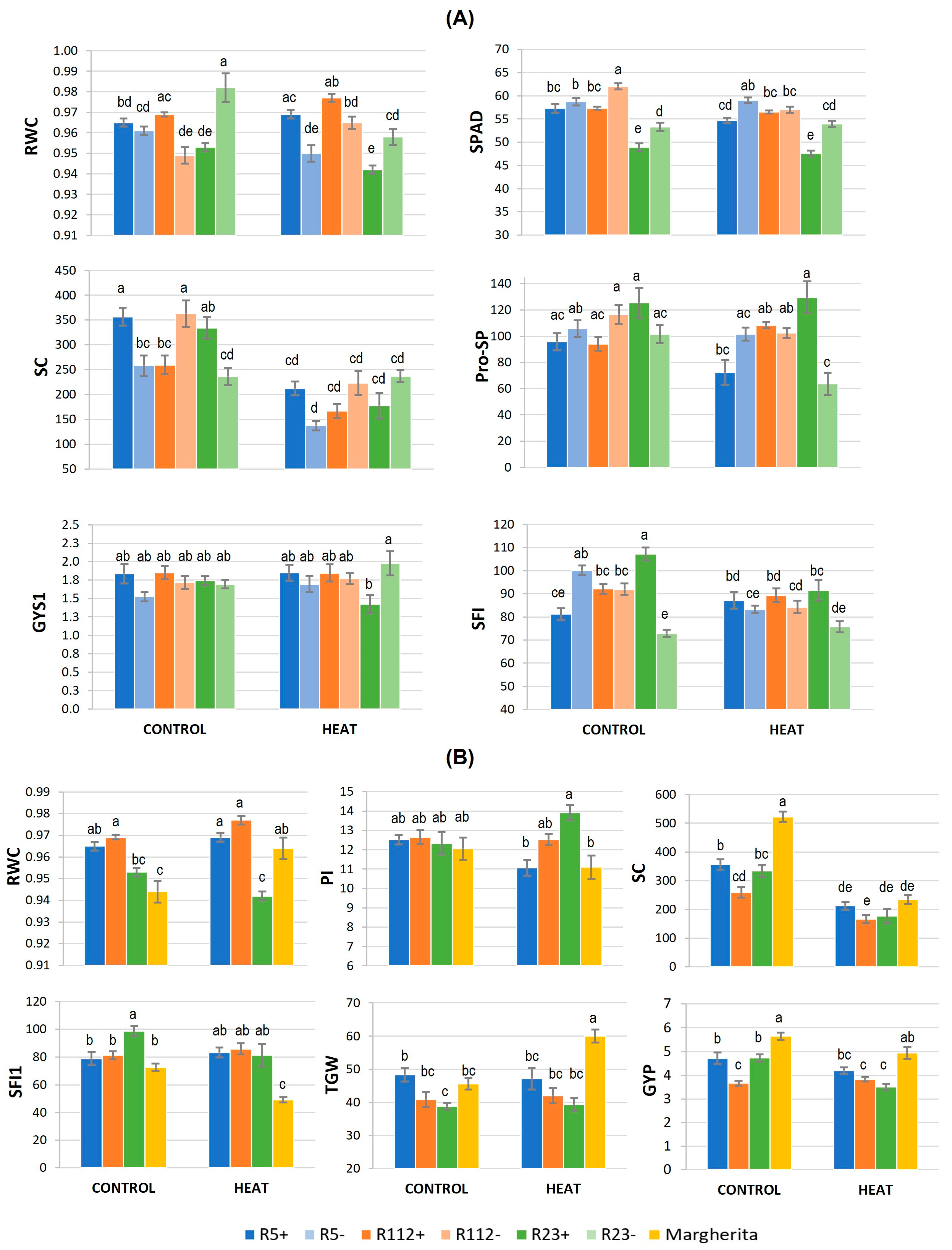
3.1.1. Physiological and Biochemical Response to HS
3.1.2. Main Fertility and Yield-Related Traits Involved in HS Response
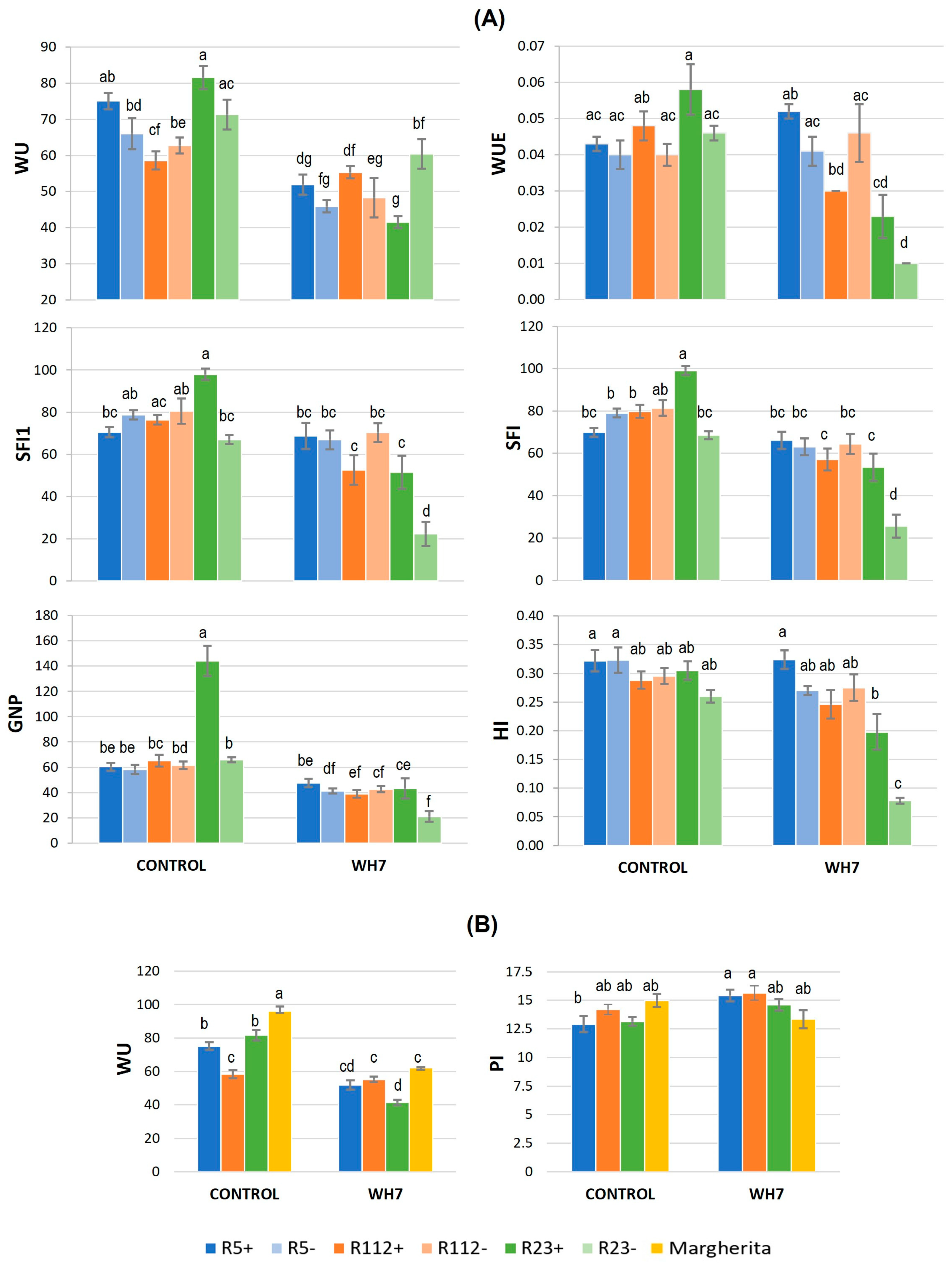
3.2. Combined Water-Deficit and Heat Stress Application
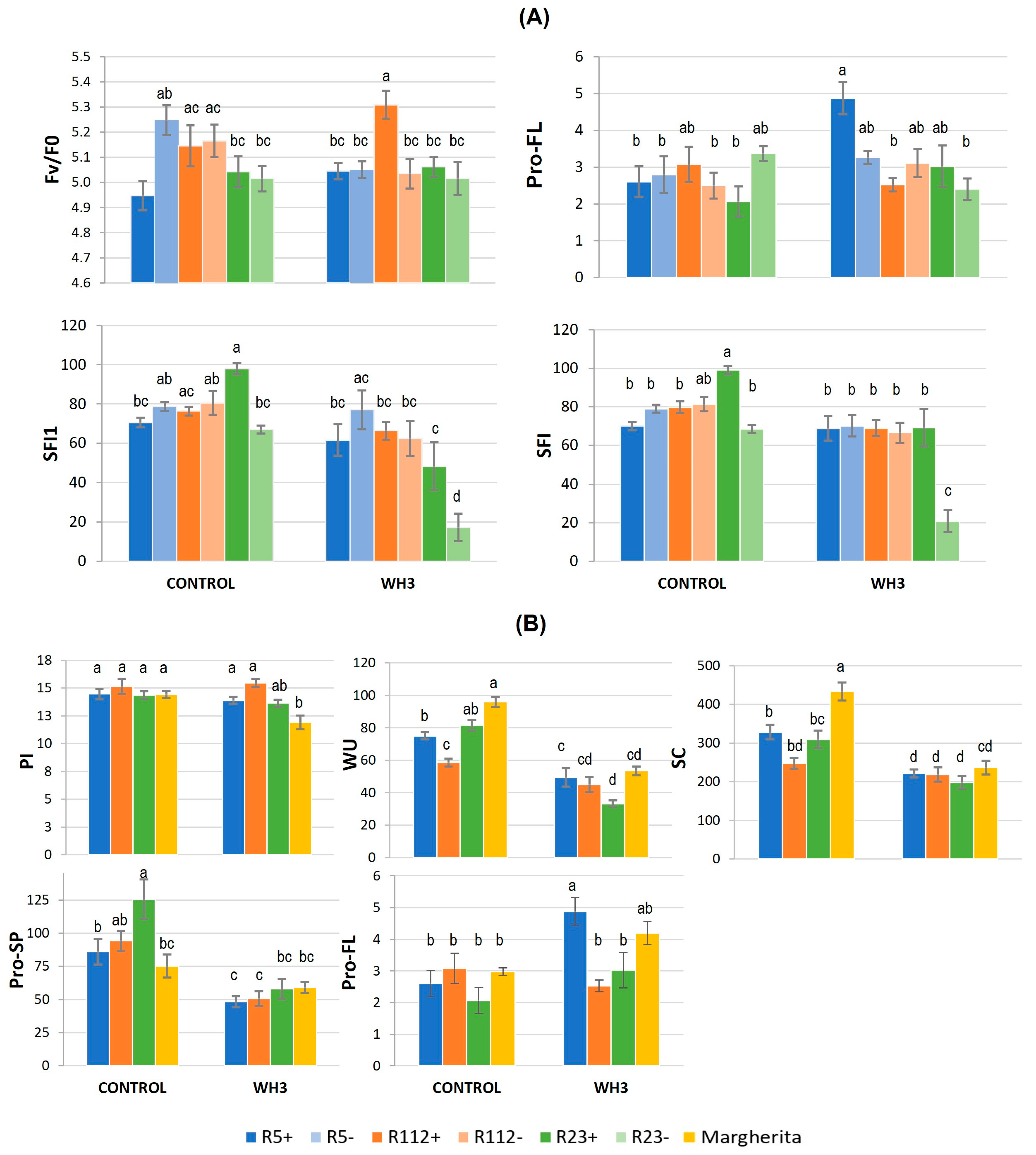
3.2.1. Physiological and Biochemical Response to Combined Stress
3.2.2. Influence of Combined Stress on Spike Fertility and Plant Production
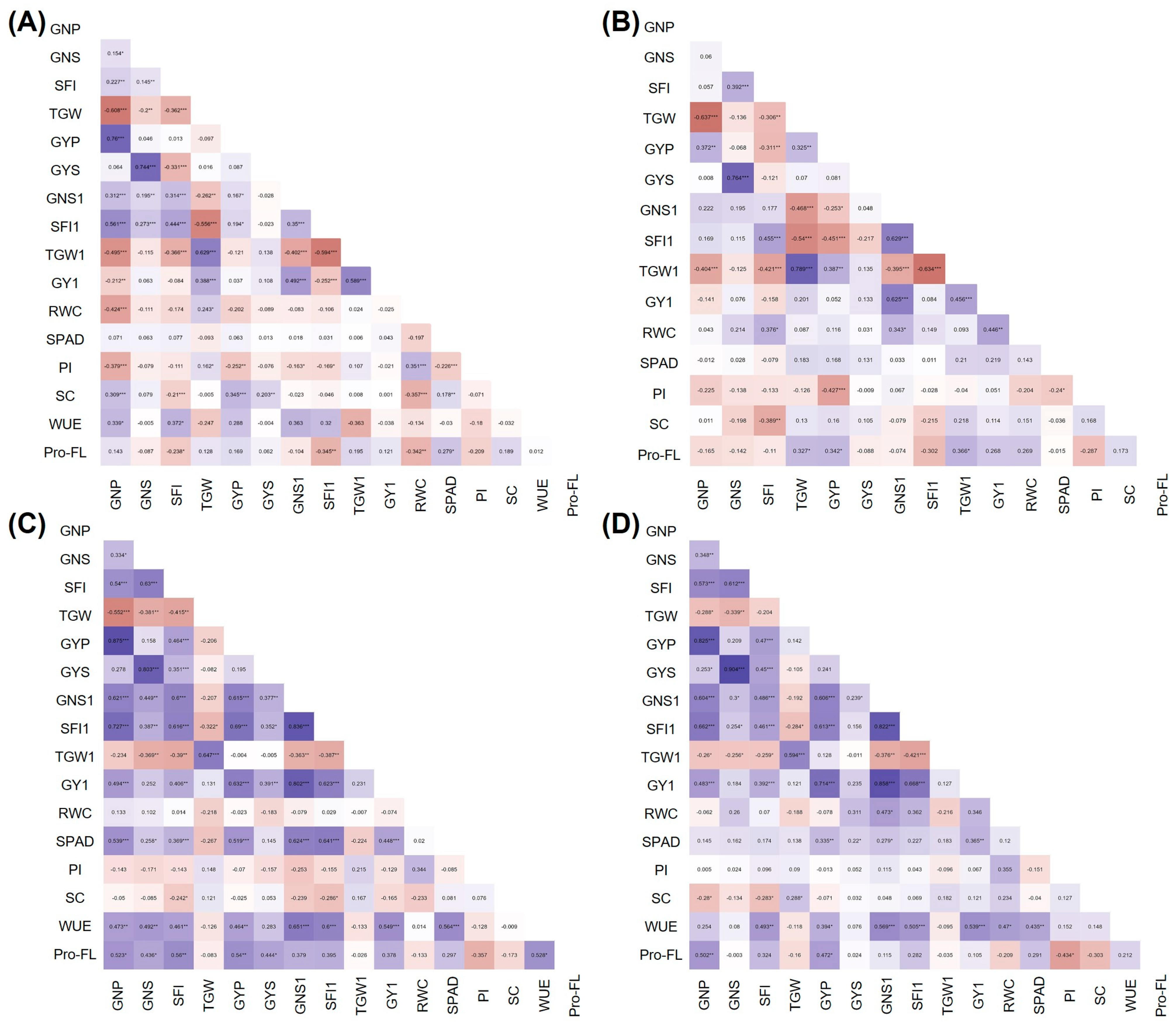
3.3. Correlations between Yield and Physiological Traits under Stress
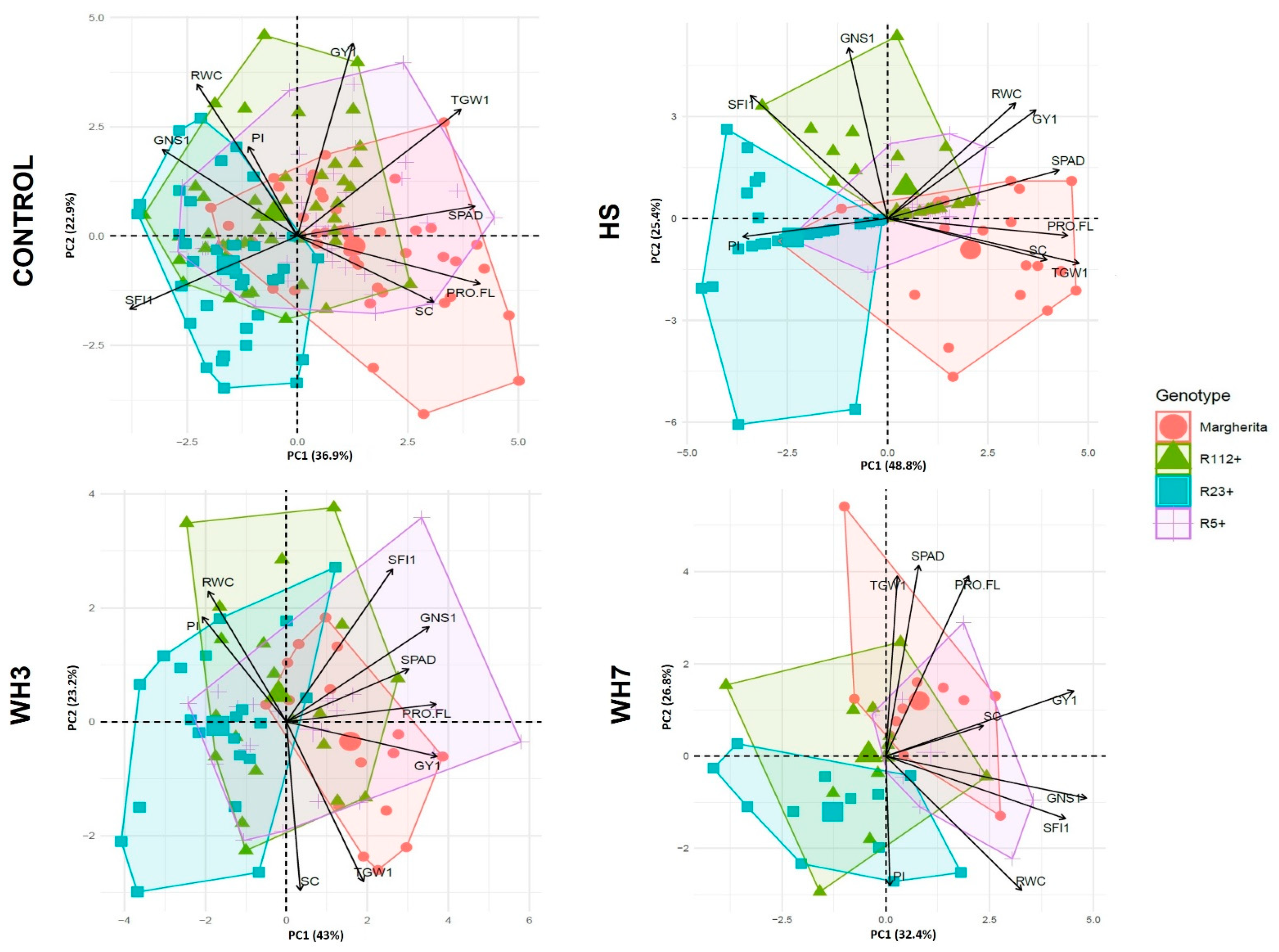
3.4. Principal Components Analysis (PCA) of Yield-Related Traits under Stress
3.5. Stress Indices and Tolerance Ranking
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef]
- Dettori, M.; Cesaraccio, C.; Duce, P. Simulation of climate change impacts on production and phenology of durum wheat in Mediterranean environments using CERES-Wheat model. Field Crop. Res. 2017, 206, 43–53. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef] [PubMed]
- Cramer, W.; Guiot, J.; Fader, M.; Garrabou, J.; Gattuso, J.P.; Iglesias, A.; Lange, M.A.; Lionello, P.; Llasat, M.C.; Paz, S.; et al. Climate change and interconnected risks to sustainable development in the Mediterranean. Nat. Clim. Change 2018, 8, 972–980. [Google Scholar] [CrossRef]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef]
- Zampieri, M.; Ceglar, A.; Dentener, F.; Toreti, A. Wheat yield loss attributable to heat waves, drought and water excess at the global, national and subnational scales. Environ. Res. Lett. 2017, 12, 064008. [Google Scholar] [CrossRef]
- Sall, A.T.; Chiari, T.; Legesse, W.; Seid-Ahmed, K.; Ortiz, R.; van Ginkel, M.; Bassi, F.M. Durum wheat (Triticum durum Desf.): Origin, cultivation and potential expansion in Sub-Saharan Africa. Agronomy 2019, 9, 263. [Google Scholar] [CrossRef]
- Martínez-Moreno, F.; Solís, I.; Noguero, D.; Blanco, A.; Özberk, I.; Nsarellah, N.; Elias, E.; Mylonas, I.; Soriano, J.M. Durum wheat in the Mediterranean Rim: Historical evolution and genetic resources. Genet. Resour. Crop Evol. 2020, 67, 1415–1436. [Google Scholar] [CrossRef]
- Zampieri, M.; Toreti, A.; Ceglar, A.; Naumann, G.; Turco, M.; Tebaldi, C. Climate resilience of the top ten wheat producers in the Mediterranean and the Middle East. Reg. Environ. Change 2020, 20, 41. [Google Scholar] [CrossRef]
- Lidon, F.C.; Almeida, A.S.; Leitão, A.L.; Silva, M.M.; Pinheiro, N.; Maçãs, B.; Costa, R. A synoptic overview of durum wheat production in the Mediterranean region and processing following the European Union requirements. Emir. J. Food Agric. 2014, 26, 693–705. [Google Scholar] [CrossRef]
- Xynias, I.N.; Mylonas, I.; Korpetis, E.G.; Ninou, E.; Tsaballa, A.; Avdikos, I.D.; Mavromatis, A.G. Durum wheat breeding in the Mediterranean region: Current status and future prospects. Agronomy 2020, 10, 432. [Google Scholar] [CrossRef]
- Zhang, H.; Mittal, N.; Leamy, L.J.; Barazani, O.; Song, B.-H. Back into the wild—Apply untapped genetic diversity of wild relatives for crop improvement. Evol. Appl. 2017, 10, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Choudhary, A.; Kaur, H.; Mehta, S. A walk towards wild grasses to unlock the clandestine of gene pools for wheat improvement: A review. Plant Stress 2022, 3, 100048. [Google Scholar] [CrossRef]
- Haudry, A.; Cenci, A.; Ravel, C.; Bataillon, T.; Brunel, D.; Poncet, C.; Hochu, I.; Poirier, S.; Santoni, S.; Glémin, S.; et al. Grinding up wheat: A massive loss of nucleotide diversity since domestication. Mol. Biol. Evol. 2007, 24, 1506–1517. [Google Scholar] [CrossRef]
- Maccaferri, M.; Harris, N.S.; Twardziok, S.O.; Pasam, R.K.; Gundlach, H.; Spannagl, M.; Ormanbekova, D.; Lux, T.; Prade, V.M.; Milner, S.G.; et al. Durum wheat genome highlights past domestication signatures and future improvement targets. Nat. Genet. 2019, 51, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, W.; Sanchez-Garcia, M.; Assefa, S.G.; Amri, A.; Bishaw, Z.; Ogbonnaya, F.C.; Baum, M. Genetic gains in wheat breeding and its role in feeding the world. Crop Breed. Genet. Genom. 2019, 1, e190005. [Google Scholar] [CrossRef]
- Aberkane, H.; Amri, A.; Belkadi, B.; Filali-Maltouf, A.; Kehel, Z.; Tahir, I.S.A.; Meheesi, S.; Tsivelikas, A. Evaluation of durum wheat lines derived from interspecific crosses under drought and heat stress. Crop Sci. 2021, 61, 119–136. [Google Scholar] [CrossRef]
- Ayed, S.; Bouhaouel, I.; Othmani, A.; Bassi, F.M. Use of wild relatives in durum wheat (Triticum turgidum L. var. durum Desf.) breeding program: Adaptation and stability in context of contrasting environments in Tunisia. Agronomy 2021, 11, 1782. [Google Scholar] [CrossRef]
- Ceoloni, C.; Kuzmanović, L.; Forte, P.; Gennaro, A.; Bitti, A. Targeted exploitation of gene pools of alien Triticeae species for sustainable and multi-faceted improvement of the durum wheat crop. Crop Pasture Sci. 2014, 65, 96–111. [Google Scholar] [CrossRef]
- Ceoloni, C.; Kuzmanović, L.; Ruggeri, R.; Rossini, F.; Forte, P.; Cuccurullo, A.; Bitti, A. Harnessing genetic diversity of wild gene pools to enhance wheat crop production and sustainability: Challenges and opportunities. Diversity 2017, 9, 55. [Google Scholar] [CrossRef]
- Ceoloni, C.; Kuzmanović, L.; Forte, P.; Virili, M.E.; Bitti, A. Wheat-perennial Triticeae introgressions: Major achievements and prospects. In Alien Introgression in Wheat; Molnár-Láng, M., Ceoloni, C., Doležel, J., Eds.; Springer: Cham, Switzerland, 2015; pp. 273–313. [Google Scholar] [CrossRef]
- Kuzmanović, L.; Mandalà, G.; Tundo, S.; Ciorba, R.; Frangella, M.; Ruggeri, R.; Rossini, F.; Gevi, F.; Rinalducci, S.; Ceoloni, C. Equipping durum wheat—Thinopyrum ponticum recombinant lines with a Thinopyrum elongatum major QTL for resistance to Fusarium diseases through a cytogenetic strategy. Front. Plant Sci. 2019, 10, 1324. [Google Scholar] [CrossRef] [PubMed]
- Kuzmanović, L.; Ruggeri, R.; Able, J.A.; Bassi, F.M.; Maccaferri, M.; Tuberosa, R.; De Vita, P.; Rossini, F.; Ceoloni, C. Yield of chromosomally engineered durum wheat-Thinopyrum ponticum recombinant lines in a range of contrasting rain-fed environments. Field Crop. Res. 2018, 228, 147–157. [Google Scholar] [CrossRef]
- Kuzmanović, L.; Giovenali, G.; Ruggeri, R.; Rossini, F.; Ceoloni, C. Small “nested” introgressions from wild Thinopyrum species, conferring effective resistance to Fusarium diseases, positively impact durum wheat yield potential. Plants 2021, 10, 579. [Google Scholar] [CrossRef] [PubMed]
- Colmer, T.D.; Flowers, T.J.; Munns, R. Use of wild relatives to improve salt tolerance in wheat. J. Exp. Bot. 2006, 57, 1059–1078. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Z.; Hu, Y.G. Drought resistance of wheat alien chromosome addition lines evaluated by membership function value based on multiple traits and drought resistance index of grain yield. Field Crop. Res. 2015, 179, 103–112. [Google Scholar] [CrossRef]
- Shu, Y.; Zhang, J.; Ao, Y.; Song, L.; Guo, C. Analysis of the Thinopyrum elongatum transcriptome under water deficit stress. Int. J. Genom. 2015, 2015, 265791. [Google Scholar] [CrossRef]
- Monneveux, P.; Reynolds, M.P.; González Aguilar, J.; Singh, R.P.; Weber, W.E. Effects of the 7DL.7Ag translocation from Lophopyrum elongatum on wheat yield and related morphophysiological traits under different environments. Plant Breed. 2003, 122, 379–384. [Google Scholar] [CrossRef]
- Placido, D.F.; Campbell, M.T.; Folsom, J.J.; Cui, X.; Kruger, G.R.; Baenziger, P.S.; Walia, H. Introgression of novel traits from a wild wheat relative improves drought adaptation in wheat. Plant Physiol. 2013, 161, 1806–1819. [Google Scholar] [CrossRef]
- Porter, J.R.; Gawith, M. Temperatures and the growth and development of wheat: A review. Eur. J. Agron. 1999, 10, 23–36. [Google Scholar] [CrossRef]
- Senapati, N.; Halford, N.G.; Semenov, M.A. Vulnerability of European wheat to extreme heat and drought around flowering under future climate. Environ. Res. Lett. 2021, 16, 024052. [Google Scholar] [CrossRef]
- Liu, H.; Searle, I.R.; Mather, D.E.; Able, A.J.; Able, J.A. Morphological, physiological and yield responses of durum wheat to pre-anthesis water-deficit stress are genotype-dependent. Crop Pasture Sci. 2015, 66, 1024–1038. [Google Scholar] [CrossRef]
- Vahamidis, P.; Karamanos, A.J.; Economou, G. Grain number determination in durum wheat as affected by drought stress: An analysis at spike and spikelet level. Ann. Appl. Biol. 2019, 174, 190–208. [Google Scholar] [CrossRef]
- Khadka, K.; Earl, H.J.; Raizada, M.N.; Navabi, A. A physio-morphological trait-based approach for breeding drought tolerant wheat. Front. Plant. Sci. 2020, 11, 715. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sonnewald, U. Differences and commonalities of plant responses to single and combined stresses. Plant J. 2017, 90, 839–885. [Google Scholar] [CrossRef]
- Tricker, P.J.; ElHabti, A.; Schmidt, J.; Fleury, D. The physiological and genetic basis of combined drought and heat tolerance in wheat. J. Exp. Bot. 2018, 69, 3195–3210. [Google Scholar] [CrossRef]
- Ru, C.; Hu, X.; Chen, D.; Wang, W.; Jingbo Zhen, J. Photosynthetic, antioxidant activities, and osmoregulatory responses in winter wheat differ during the stress and recovery periods under heat, drought, and combined stress. Plant Sci. 2023, 327, 111557. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Lukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Sharma, D.K.; Andersen, S.B.; Ottosen, C.O.; Rosenqvist, E. Wheat cultivars selected for high Fv/Fm under heat stress maintain high photosynthesis, total chlorophyll, stomatal conductance, transpiration and dry matter. Physiol. Plant. 2014, 153, 284–298. [Google Scholar] [CrossRef]
- Živčák, M.; Brestič, M.; Olšovská, K.; Slamka, P. Performance index as a sensitive indicator of water stress in Triticum aestivum L. Plant Soil Environ. 2008, 54, 133–139. [Google Scholar] [CrossRef]
- Faralli, M.; Matthews, J.; Lawson, T. Exploiting natural variation and genetic manipulation of stomatal conductance for crop improvement. Curr. Opin. Plant Biol. 2019, 49, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ramya, K.T.; Jain, N.; Amasiddha, B.; Singh, P.K.; Arora, A.; Singh, G.P.; Prabhu, K.V. Genotypic response for stomatal conductance due to terminal heat stress under late sown condition in wheat (Triticum aestivum L.). Indian. J. Genet. Plant Breed. 2016, 76, 255–265. [Google Scholar] [CrossRef][Green Version]
- Bahar, B.; Yildirim, M.; Yucel, C. Heat and drought resistance criteria in spring bread wheat (Triticum aestivum L.): Morpho-physiological parameters for heat tolerance. Sci. Res. Essays 2011, 6, 2212–2220. [Google Scholar] [CrossRef]
- Aparecido, L.M.T.; Woo, S.; Suazo, C.; Hultine, K.R.; Blonder, B. High water use in desert plants exposed to extreme heat. Ecol. Lett. 2020, 23, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Marchin, R.M.; Backes, D.; Ossola, A.; Leishman, M.R.; Tjoelker, M.G.; Ellsworth, D.S. Extreme heat increases stomatal conductance and drought-induced mortality risk in vulnerable plant species. Glob. Change Biol. 2022, 28, 1133–1146. [Google Scholar] [CrossRef]
- Cruz De Carvalho, M.H. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Mahmud, J.A.; Anee, T.I.; Nahar, K.; Islam, M.T. Drought stress tolerance in wheat: Omics approaches in understanding and enhancing antioxidant defense. In Abiotic Stress-Mediated Sensing and Signaling in Plants: An Omics Perspective; Springer: Singapore, 2018; pp. 267–307. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Siddique, A.; Kandpal, G.; Kumar, P. Proline accumulation and its defensive role under diverse stress condition in plants: An overview. J. Pure Appl. Microbiol. 2018, 12, 1655–1659. [Google Scholar] [CrossRef]
- Barunawati, N.; Maghfoer, M.D.; Kendarini, N.; Aini, N. Proline and specific root length as response to drought of wheat lines (Triticum aestivum L.). J. Agric. Sci. 2016, 38, 296–302. [Google Scholar] [CrossRef][Green Version]
- Mustafa, T.; Sattar, A.; Sher, A.; Ul-Allah, S.; Ijaz, M.; Irfan, M.; Butt, M.; Cheema, M. Exogenous application of silicon improves the performance of wheat under terminal heat stress by triggering physio-biochemical mechanisms. Sci. Rep. 2021, 11, 23170. [Google Scholar] [CrossRef] [PubMed]
- Ceoloni, C.; Forte, P.; Gennaro, A.; Micali, S.; Carozza, R.; Bitti, A. Recent developments in durum wheat chromosome engineering. Cytogenet. Genome Res. 2005, 109, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Sall, A.T.; Cisse, M.; Gueye, H.; Kabbaj, H.; Ndoye, I.; Filali-Maltouf, A.; Belkadi, B.; El-Mourid, M.; Ortiz, R.; Bassi, F.M. Heat tolerance of durum wheat (Triticum durum Desf.) elite germplasm tested along the Senegal river. J. Agric. Sci. 2018, 10, 217. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Morison, J.I.L.; Baker, N.R.; Mullineaux, P.M.; Davies, W.J. Improving water use in crop production. Philos. Trans. R. Soc. B 2008, 363, 639–658. [Google Scholar] [CrossRef]
- Mega, R.; Abe, F.; Kim, J.S.; Tsuboi, Y.; Tanaka, K.; Kobayashi, H.; Sakata, Y.; Hanada, K.; Tsujimoto, H.; Kikuchi, J.; et al. Tuning water-use efficiency and drought tolerance in wheat using abscisic acid receptors. Nat. Plants 2019, 5, 153–159. [Google Scholar] [CrossRef]
- Abrahám, E.; Hourton-Cabassa, C.; Erdei, L.; Szabados, L. Methods for determination of proline in plants. Methods Mol. Biol. 2010, 639, 317–331. [Google Scholar] [CrossRef]
- Pour-Aboughadareh, A.; Yousefian, M.; Moradkhani, H.; Moghaddam Vahed, M.; Poczai, P.; Siddique, K.H.M. iPASTIC: An online toolkit to estimate plant abiotic stress indices. Appl. Plant Sci. 2019, 7, e11278. [Google Scholar] [CrossRef]
- Aberkane, H.; Belkadi, B.; Kehel, Z.; Filali-Maltouf, A.; Tahir, I.S.A.; Meheesi, S.; Amri, A. Assessment of drought and heat tolerance of durum wheat lines derived from interspecific crosses using physiological parameters and stress indices. Agronomy 2021, 11, 695. [Google Scholar] [CrossRef]
- Khan, A.A.; Kabir, M.R. Evaluation of spring wheat genotypes (Triticum aestivum L.) for heat stress tolerance using different stress tolerance indices. Cercet. Agron. Mold. 2015, 47, 49–63. [Google Scholar] [CrossRef]
- Kuzmanović, L.; Gennaro, A.; Benedettelli, S.; Dodd, I.C.; Quarrie, S.A.; Ceoloni, C. Structural-functional dissection and characterization of yield-contributing traits originating from a group 7 chromosome of the wheatgrass species Thinopyrum ponticum after transfer into durum wheat. J. Exp. Bot. 2014, 65, 509–525. [Google Scholar] [CrossRef] [PubMed]
- Kuzmanović, L.; Ruggeri, R.; Virili, M.E.; Rossini, F.; Ceoloni, C. Effects of Thinopyrum ponticum chromosome segments transferred into durum wheat on yield components and related morpho-physiological traits in Mediterranean rain-fed conditions. Field Crop. Res. 2016, 186, 86–98. [Google Scholar] [CrossRef]
- Tyagi, M.; Pandey, G.C. Physiology of heat and drought tolerance in wheat: An overview. J. Cereal Res. 2022, 14, 13–25. [Google Scholar] [CrossRef]
- Fernie, E.; Tan, D.K.Y.; Liu, S.Y.; Ullah, N.; Khoddami, A. Post-anthesis heat influences grain yield, physical and nutritional quality in wheat: A review. Agriculture 2022, 12, 886. [Google Scholar] [CrossRef]
- Dolferus, R.; Ji, X.; Richards, R.A. Abiotic stress and control of grain number in cereals. Plant Sci. 2011, 181, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Nadeem, F.; Nawaz, A.; Siddique, K.H.M.; Farooq, M. Heat stress effects on the reproductive physiology and yield of wheat. J. Agron. Crop Sci. 2022, 208, 1–17. [Google Scholar] [CrossRef]
- Reynolds, M.P.; Calderini, D.F.; Condon, A.G.; Rajaram, S. Physiological basis of yield gains in wheat associated with the Lr19 translocation from Agropyron elongatum. Euphytica 2001, 119, 137–141. [Google Scholar] [CrossRef]
- Urban, O.; Hlaváčováa, M.; Klema, K.; Novotnáa, K.; Rapantováa, B.; Smutná, P.; Horáková, V.; Hlavinka, P.; Škarpa, P.; Trnka, M. Combined effects of drought and high temperature on photosynthetic characteristics in four winter wheat genotypes. Field Crop. Res. 2018, 223, 137–149. [Google Scholar] [CrossRef]
- Barnabas, B.; Jager, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef]
- Qaseem, M.F.; Qureshi, R.; Shaheen, H. Effects of pre-anthesis drought, heat and their combination on the growth, yield and physiology of diverse wheat (Triticum aestivum L.) genotypes varying in sensitivity to heat and drought stress. Sci. Rep. 2019, 9, 6955. [Google Scholar] [CrossRef]
- Kuzmanović, L.; Rossini, F.; Ruggeri, R.; Pagnotta, M.A.; Ceoloni, C. Engineered durum wheat germplasm with multiple alien introgressions: Agronomic and quality performance. Agronomy 2020, 10, 486. [Google Scholar] [CrossRef]
- Liu, H.; Able, A.J.; Able, J.A. Genotypic performance of Australian durum under single and combined water-deficit and heat stress during reproduction. Sci. Rep. 2019, 9, 14986. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, J.L.; Dold, C. Water-use efficiency: Advances and challenges in a changing climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.P.; Mirabella, N.E.; Panelo, J.S.; Cendoya, M.G.; Pontaroli, A.C. Selection for high spike fertility index increases genetic progress in grain yield and stability in bread wheat. Euphytica 2018, 214, 112. [Google Scholar] [CrossRef]
- Pradhan, S.; Babar, M.A.; Robbins, K.; Bai, G.; Mason, R.E.; Khan, J.; Shahi, D.; Avci, M.; Guo, J.; Hossain, M.M.; et al. Understanding the genetic basis of spike fertility to improve grain number, harvest index, and grain yield in wheat under high temperature stress environments. Front. Plant Sci. 2019, 10, 1481. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Liu, G.; Tang, Y.; Zhou, C.; Zhang, L.; Lv, J. The spike plays important roles in the drought tolerance as compared to the flag leaf through the phenylpropanoid pathway in wheat. Plant Physiol. Bioch. 2020, 152, 100–111. [Google Scholar] [CrossRef]
- Frimpong, F.; Windt, C.W.; van Dusschoten, D.; Naz, A.A.; Frei, M.; Fiorani, F. A wild allele of pyrroline-5-carboxylate synthase1 leads to proline accumulation in spikes and leaves of barley contributing to improved performance under reduced water availability. Front. Plant Sci. 2021, 12, 633448. [Google Scholar] [CrossRef]
- Rizhsky, L.; Liang, H.; Shuman, J.; Shulaev, V.; Davletova, S.; Mittler, R. When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 2004, 134, 1683–1696. [Google Scholar] [CrossRef]
- Jin, R.; Wang, Y.; Liu, R.; Gou, J.; Chan, Z. Physiological and metabolic changes of purslane (Portulaca oleracea L.) in response to drought, heat, and combined stresses. Front. Plant Sci. 2016, 6, 1123. [Google Scholar] [CrossRef]
- Raja, V.; Qadir, S.U.; Alyemeni, M.N.; Ahmad, P. Impact of drought and heat stress individually and in combination on physio-biochemical parameters, antioxidant responses, and gene expression in Solanum lycopersicum. 3 Biotech 2020, 10, 208. [Google Scholar] [CrossRef]
- Vicente, R.; Vergara-Díaz, O.; Medina, S.; Chairi, F.; Kefauver, S.C.; Bort, J.; Serret, M.D.; Aparicio, N.; Araus, J.L. Durum wheat ears perform better than the flag leaves under water stress: Gene expression and physiological evidence. Environ. Exp. Bot. 2018, 153, 271–285. [Google Scholar] [CrossRef]
- Kuzmanović, L.; Menasria, H.; Rouabhi, A.; Giovenali, G.; Capoccioni, A.; Saveriano, M.; Di Romana, M.; Ruggeri, R.; Ceoloni, C. Performance of locally adapted durum wheat germplasm in the Mediterranean basin and recombinant lines with Thinopyrum spp. introgressions across Algerian and Italian environments with different water availability. In Proceedings of the From Seed To Pasta IV International Conference, Bologna, Italy, 26–29 October 2022; Available online: https://www.fromseedtopasta.com/wp-content/uploads/2022/10/45_Ceoloni.pdf (accessed on 16 November 2022).
- Ruggeri, R.; Özberk, I.; Kuzmanović, L.; Özberk, F.; Ayhan, H.; Rossini, F.; Ceoloni, C. Assessing yield and quality traits of durum wheat varieties, landraces and recombinant lines with alien introgressions in Turkish and Italian environments under rainfed and irrigated conditions. In Proceedings of the From Seed To Pasta IV International Conference, Bologna, Italy, 26–29 October 2022; Available online: https://www.fromseedtopasta.com/wp-content/uploads/2022/10/46_Ruggeri-et-al..pdf (accessed on 16 November 2022).
- Powell, N.; Ji, X.; Ravash, R.; Edlington, J.; Dolferus, R. Yield stability for cereals in a changing climate. Funct. Plant Biol. 2012, 39, 539–552. [Google Scholar] [CrossRef] [PubMed]

| Index | Formula |
|---|---|
| Tolerance index (TOL) | TOL = Yp − Ys |
| Mean productivity (MP) | MP = (Yp + Ys)/2 |
| Geometric mean productivity (GMP) | GMP = √(Ys × Yp) |
| Harmonic mean (HM) | HM= 2 (Ys × Yp)/(Ys + Yp) |
| Stress susceptibility index (SSI) | SSI = 1 − (Ys/Yp)/1 − (Ȳs/Ȳp) |
| Stress tolerance index (STI) | STI = (Ys × Yp)/(Ȳp)² |
| Yield index (YI) | YI = Ys/Ȳs |
| Yield stability index (YSI) | YSI = Ys/Yp |
| Relative stress index (RSI) | RSI = (Ys/Yp)/(Ȳs/Ȳp) |
| A | B | |||||||
|---|---|---|---|---|---|---|---|---|
| Factors | G | T | G × T | Residuals | G | T | G × T | Residuals |
| df | 5 | 1 | 5 | 3 | 1 | 3 | ||
| SPAD | 718.3 *** | 149.9 *** | 52.1 *** | 11.2 | 750.6 *** | 119.3 ** | 7.3 | 13.4 |
| SC | 24341.2 *** | 280889.3 *** | 14015.7 ** | 3489.9 | 105054.2 *** | 491996.4 *** | 38365.4 *** | 3525.7 |
| TL | 1.12 | 2.15 | 2.14 | 1.02 | 2.40 * | 9.71 *** | 9.58 *** | 0.76 |
| Fv/Fm | 0.00008 | 0.00028 * | 0.00005 | 0.00006 | 0.00004 | 0.00004 | 0.00010 | 0.00006 |
| Fv/F0 | 0.15 | 0.29 * | 0.07 | 0.07 | 0.07 | 0.06 | 0.09 | 0.06 |
| PI | 15.2 *** | 1.41 | 7.50 * | 2.38 | 9.47 ** | 1.03 | 8.11 ** | 1.87 |
| RWC | 0.001 *** | 0.0001 | 0.0005 *** | 0.00005 | 0.001 *** | 0.0003 * | 0.0005 *** | 0.0001 |
| Pro-FL | 9.47 | 145.1 *** | 5.65 | 4.61 | 34.9 ** | 196.4 *** | 6.80 | 5.62 |
| Pro-SP | 2737.6 *** | 1468.1 * | 803.9 * | 293.4 | 6369.7 *** | 149.3 | 631.0 | 380.4 |
| GNS1 | 60.7 | 25.4 | 158.2 ** | 36.5 | 162.9 ** | 252.8 ** | 169.50** | 31.2 |
| GNSL1 | 0.73 *** | 0.10 | 0.50 * | 0.17 | 0.39 | 0.73 * | 0.58 * | 0.16 |
| SFI1 | 1884.6 *** | 291.6 | 555.5 * | 181.9 | 3554.9 *** | 1368.4 ** | 1165.25*** | 181.7 |
| TGW1 | 385.9 *** | 85.6 | 91.9 | 47.5 | 843.3 *** | 166.6 | 257.1 ** | 46.1 |
| GYS1 | 0.34 * | 0.04 | 0.27 * | 0.12 | 0.45 * | 0.12 | 0.16 | 0.11 |
| GNS | 171.4 * | 383.6 * | 123.1 | 65.1 | 183.4 * | 794.5 *** | 195.4 * | 60.9 |
| GNSL | 1.58 *** | 2.07 ** | 0.49 | 0.25 | 1.03 ** | 4.68 *** | 0.77 * | 0.24 |
| SFI | 5718.6 *** | 3357.2 ** | 1506.1 *** | 316.2 | 20441.3 *** | 6067.4 *** | 2997.9 *** | 365.5 |
| GYS | 0.49 * | 0.001 | 0.34 | 0.18 | 1.57 *** | 0.20 | 0.38 | 0.17 |
| TNP | 1.87 * | 6.23 ** | 1.60 * | 0.64 | 0.81 | 3.38 * | 1.30 | 0.49 |
| PTP | 2.94 *** | 1.35 | 0.33 | 0.49 | 3.23 *** | 0.85 | 0.32 | 0.50 |
| GNP | 2809.1 *** | 8015.9 *** | 1224.1 ** | 375.7 | 868.9 | 8335.6 *** | 2391.6 *** | 359.3 |
| TGW | 381.6 *** | 282.1 * | 103.9 | 46.6 | 854.2 *** | 301.1 * | 257.8 ** | 48.7 |
| GYP | 1.74 *** | 4.23 *** | 1.59 *** | 0.36 | 9.07 *** | 7.03 *** | 2.08 *** | 0.32 |
| H | 2191.3 *** | 401.9 *** | 49.8 | 26.3 | 1110.1 *** | 171.2 ** | 39.1 | 17.7 |
| A | B | |||||||
|---|---|---|---|---|---|---|---|---|
| Factors | G | T | G × T | Residuals | G | T | G × T | Residuals |
| df | 5 | 1 | 5 | 3 | 1 | 3 | ||
| SPAD | 1017.7 *** | 168.9 | 126.9 * | 55.6 | 671.1 *** | 408.5 ** | 139.1 | 58.2 |
| SC | 10722.8 | 154764.8 *** | 16595.3 * | 5440.0 | 53776.6 *** | 359666.3 *** | 32983.9 *** | 4722.6 |
| TL | 4.52 | 70.80 *** | 1.64 | 2.17 | 6.09 * | 71.12 *** | 0.64 | 1.66 |
| Fv/Fm | 0.00017 *** | 0.00001 | 0.00005 | 0.00004 | 0.0002 *** | 0.0000001 | 0.0001 * | 0.00004 |
| Fv/F0 | 0.25 *** | 0.003 | 0.15 * | 0.05 | 0.34 *** | 0.08 | 0.09 | 0.05 |
| PI | 11.89** | 3.24 | 2.58 | 3.51 | 23.8 *** | 23.8 ** | 10.7 * | 3.1 |
| RWC | 0.00002 | 0.00015 | 0.0001 | 0.00009 | 0.00003 | 0.00006 | 0.0001 | 0.0001 |
| WU | 137.4 | 8958.2 *** | 439.8 *** | 58.6 | 1049.5 *** | 11465.6 *** | 670.9 *** | 61.4 |
| WUE | 0.0005 ** | 0.0003 | 0.001 *** | 0.0002 | 0.0001 | 0.0001 | 0.0007 * | 0.0002 |
| Pro-FL | 1.15 | 2.12 | 2.13 * | 0.62 | 2.25 * | 6.08 ** | 2.16 * | 0.59 |
| Pro-SP | 701.5 * | 33878.8 *** | 475.6 | 243.0 | 1132.9 * | 15349.5 *** | 996.3 * | 271.1 |
| GNS1 | 375.9 *** | 4700.6 *** | 193.6 *** | 39.0 | 69.5 | 2072.7 *** | 268.6 *** | 32.6 |
| GNSL1 | 2.22 *** | 19.1 *** | 1.00 ** | 0.28 | 0.52 | 6.83 *** | 1.23 ** | 0.27 |
| SFI1 | 2616.9 *** | 12260.6 *** | 1778.0 *** | 268.3 | 159.9 | 5843.2 *** | 1596.3 *** | 209.5 |
| TGW1 | 482.7 *** | 803.3 *** | 80.6 | 53.1 | 455.7 *** | 32.1 | 8.73 | 54.8 |
| GYS1 | 0.90 *** | 6.17 *** | 0.40 ** | 0.12 | 0.82 *** | 3.21 *** | 0.21 | 0.11 |
| GNS | 364.5 *** | 6432.5 *** | 216.9 ** | 57.9 | 47.9 | 2614.0 *** | 252.2 ** | 57.8 |
| GNSL | 1.94 *** | 25.1 *** | 0.84 * | 0.31 | 0.24 | 9.80 *** | 0.78 | 0.32 |
| SFI | 4931.4 *** | 14469.5 *** | 1991.1 *** | 281.6 | 1746.5 *** | 5137.5 *** | 1217.9 ** | 293.6 |
| GYS | 0.61 ** | 9.00 *** | 0.31 | 0.16 | 0.71 ** | 3.74 *** | 0.13 | 0.15 |
| TNP | 3.40 *** | 0.31 | 4.22 *** | 0.50 | 6.25*** | 5.11 ** | 3.93 ** | 0.67 |
| PTP | 3.45 *** | 2.22 * | 2.84 *** | 0.48 | 6.13 *** | 6.08 ** | 2.52 ** | 0.60 |
| GNP | 4203.4 *** | 22969.3 *** | 5385.4 *** | 204.2 | 5182.7 *** | 24323.4 *** | 6268.4 *** | 268.2 |
| TGW | 357.3 *** | 734.1 *** | 22.6 | 30.9 | 298.4 *** | 294.2 ** | 17.1 | 32.9 |
| BM | 45.8 *** | 114.5 *** | 16.3 ** | 3.97 | 61.7 *** | 173.5 *** | 13.3 ** | 3.69 |
| HI | 0.06 *** | 0.05 *** | 0.03 *** | 0.004 | 0.04 *** | 0.07 *** | 0.02 ** | 0.003 |
| GYP | 4.54 *** | 34.2 *** | 8.44 *** | 0.71 | 7.03 *** | 41.3 *** | 7.9 *** | 1.02 |
| H | 1002.7 *** | 52.4 | 22.9 | 16.4 | 765.9 *** | 33.5 | 55.1 * | 16.1 |
| A | B | |||||||
|---|---|---|---|---|---|---|---|---|
| Factors | G | T | G × T | Residuals | G | T | G × T | Residuals |
| df | 5 | 1 | 5 | 3 | 1 | 3 | ||
| SPAD | 515.6 *** | 583.5 *** | 84.9 * | 28.1 | 265.2 *** | 602.4 *** | 48.7 | 36.5 |
| SC | 15069.4 ** | 760720.9 *** | 9298.6 * | 3700.3 | 34393.1 *** | 614880.3 *** | 10975.7 | 4858.0 |
| TL | 2.02 | 73.7 *** | 7.08 * | 2.94 | 10.0 ** | 52.4 *** | 3.12 | 2.46 |
| Fv/Fm | 0.00004 | 0.0005 ** | 0.00005 | 0.00006 | 0.00007 | 0.0003 * | 0.00005 | 0.00006 |
| Fv/F0 | 0.09 | 0.68 ** | 0.06 | 0.07 | 0.13 | 0.47 * | 0.05 | 0.07 |
| PI | 4.64 | 46.50 *** | 3.05 | 3.37 | 3.94 | 18.5 * | 17.9 ** | 3.80 |
| RWC | 0.0004 * | 0.00004 | 0.0002 | 0.0001 | 0.0002 | 0.001 ** | 0.0002 | 0.0001 |
| WU | 205.2 ** | 5666.1 *** | 419.7 *** | 54.0 | 1033.9 *** | 6972.3 *** | 678.1 *** | 33.4 |
| WUE | 0.0004 ** | 0.002 *** | 0.001 *** | 0.0001 | 0.0001 | 0.003 *** | 0.0007 *** | 0.0001 |
| Pro-FL | 3.28 * | 23.8 *** | 2.10 | 1.01 | 5.17 * | 37.3 *** | 1.18 | 1.40 |
| GNS1 | 335.8 *** | 6199.9 *** | 209.9 *** | 39.4 | 12.8 | 4117.6 *** | 225.2 ** | 43.6 |
| GNSL1 | 2.27 *** | 25.6 *** | 1.00 *** | 0.20 | 0.30 | 17.7 *** | 0.80 ** | 0.25 |
| SFI1 | 2620.6 *** | 14652.8 *** | 1660.3 *** | 220.6 | 313.2 | 6230. 6 *** | 2093.9 *** | 235.9 |
| TGW1 | 489.9 *** | 28.4 | 16.4 | 37.9 | 590.0 *** | 0.34 | 2.60 | 42.7 |
| GYS1 | 0.82 *** | 12.7 *** | 0.40 ** | 0.09 | 0.87 *** | 8.61 *** | 0.23 | 0.09 |
| GNS | 221.7 ** | 9992.5 *** | 190.3 * | 64.1 | 42.3 | 9909.4 *** | 209.3 * | 65.3 |
| GNSL | 1.64 *** | 40.3 *** | 0.72 * | 0.29 | 0.13 | 41.3 *** | 0.73 | 0.33 |
| SFI | 3232.2 *** | 28549.0 *** | 2528.4 *** | 276.6 | 1141.1 * | 19765.9 *** | 3232.8 *** | 331.7 |
| GYS | 0.78 *** | 18.6 *** | 0.25 | 0.14 | 0.76 ** | 17.7 *** | 0.17 | 0.14 |
| TNP | 5.17 *** | 1.39 | 3.35 *** | 0.46 | 5.78 *** | 3.08 * | 5.08 *** | 0.60 |
| PTP | 4.72 *** | 3.43 ** | 2.55 *** | 0.42 | 4.83 *** | 4.23 ** | 3.82 *** | 0.53 |
| GNP | 5414.3 *** | 35354.0 *** | 4694.8 *** | 200.9 | 6196.3 *** | 36513.4 *** | 6394.2 *** | 282.2 |
| TGW | 412.3 *** | 194.3 ** | 5.6 | 18.8 | 515.4 *** | 155.3 ** | 3.83 | 17.5 |
| BM | 20.7 *** | 177.2 *** | 32.6 *** | 3.5 | 19.2 ** | 279.3 *** | 37.3 *** | 3.76 |
| HI | 0.03 *** | 0.07 *** | 0.01 *** | 0.002 | 0.01 ** | 0.05 *** | 0.01 | 0.003 |
| GYP | 3.57 *** | 69.5 *** | 9.6 *** | 0.58 | 4.13 ** | 78.0 *** | 12.6 *** | 0.85 |
| H | 860.7 *** | 251.0 *** | 59.3 * | 19.6 | 741.4 *** | 495.3 *** | 49.1 * | 16.6 |
| Treatment | Genotype | Stress Tolerance Indices | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yp | Ys | TOL | MP | GMP | HM | SSI | STI | YI | YSI | RSI | SR | SD | AR | |||||||||||||
| Value | Rank | Value | Rank | Value | Rank | Value | Rank | Value | Rank | Value | Rank | Value | Rank | Value | Rank | Value | Rank | Value | Rank | Value | Rank | |||||
| HS | Margherita | 5.65 | 1 | 4.94 | 1 | 0.71 | 6 | 5.30 | 1 | 5.29 | 1 | 5.27 | 1 | 1.38 | 6 | 1.48 | 1 | 1.25 | 1 | 0.87 | 6 | 0.96 | 6 | 31 | 2.52 | 2.82 |
| R5+ | 4.72 | 3 | 4.20 | 2 | 0.52 | 5 | 4.46 | 2 | 4.45 | 2 | 4.45 | 2 | 1.22 | 5 | 1.05 | 2 | 1.06 | 2 | 0.89 | 5 | 0.98 | 5 | 35 | 1.47 | 3.18 | |
| R5- | 3.75 | 6 | 3.76 | 4 | −0.01 | 2 | 3.76 | 5 | 3.75 | 5 | 3.75 | 5 | −0.04 | 2 | 0.75 | 5 | 0.95 | 4 | 1.00 | 2 | 1.10 | 2 | 42 | 1.54 | 3.82 | |
| R112+ | 3.67 | 7 | 3.84 | 3 | −0.17 | 1 | 3.75 | 6 | 3.75 | 6 | 3.75 | 6 | −0.52 | 1 | 0.74 | 6 | 0.97 | 3 | 1.05 | 1 | 1.15 | 1 | 41 | 2.49 | 3.73 | |
| R112- | 4.06 | 4 | 3.75 | 5 | 0.31 | 4 | 3.90 | 4 | 3.90 | 4 | 3.90 | 4 | 0.84 | 4 | 0.80 | 4 | 0.95 | 5 | 0.92 | 4 | 1.02 | 4 | 46 | 0.40 | 4.18 | |
| R23+ | 4.73 | 2 | 3.51 | 7 | 1.22 | 7 | 4.12 | 3 | 4.08 | 3 | 4.03 | 3 | 2.84 | 7 | 0.88 | 3 | 0.89 | 7 | 0.74 | 7 | 0.82 | 7 | 56 | 2.21 | 5.09 | |
| R23- | 3.84 | 5 | 3.66 | 6 | 0.19 | 3 | 3.75 | 7 | 3.75 | 7 | 3.75 | 7 | 0.53 | 3 | 0.74 | 7 | 0.93 | 6 | 0.95 | 3 | 1.05 | 3 | 57 | 1.83 | 5.18 | |
| WH3 | Margherita | 4.39 | 2 | 3.06 | 1 | 1.33 | 5 | 3.73 | 2 | 3.67 | 1 | 3.61 | 1 | 0.85 | 4 | 1.10 | 1 | 1.36 | 1 | 0.70 | 4 | 1.08 | 4 | 26 | 1.57 | 2.36 |
| R5+ | 2.95 | 4 | 2.49 | 3 | 0.47 | 3 | 2.72 | 4 | 2.71 | 4 | 2.70 | 4 | 0.44 | 3 | 0.60 | 4 | 1.10 | 3 | 0.84 | 3 | 1.31 | 3 | 38 | 0.52 | 3.45 | |
| R5- | 2.60 | 7 | 2.98 | 2 | −0.38 | 1 | 2.79 | 3 | 2.78 | 3 | 2.78 | 3 | −0.42 | 1 | 0.63 | 3 | 1.33 | 2 | 1.15 | 1 | 1.78 | 1 | 27 | 1.75 | 2.45 | |
| R112+ | 2.94 | 5 | 1.87 | 5 | 1.07 | 4 | 2.40 | 6 | 2.34 | 6 | 2.28 | 6 | 1.03 | 5 | 0.45 | 6 | 0.83 | 5 | 0.63 | 5 | 0.99 | 5 | 58 | 0.65 | 5.27 | |
| R112- | 2.80 | 6 | 2.46 | 4 | 0.34 | 2 | 2.63 | 5 | 2.63 | 5 | 2.62 | 5 | 0.34 | 2 | 0.56 | 5 | 1.09 | 4 | 0.88 | 2 | 1.37 | 2 | 42 | 1.54 | 3.82 | |
| R23+ | 5.62 | 1 | 1.86 | 6 | 3.76 | 7 | 3.74 | 1 | 3.23 | 2 | 2.79 | 2 | 1.88 | 6 | 0.85 | 2 | 0.82 | 6 | 0.33 | 6 | 0.51 | 6 | 45 | 2.43 | 4.09 | |
| R23- | 3.17 | 3 | 1.04 | 7 | 2.13 | 6 | 2.10 | 7 | 1.81 | 7 | 1.56 | 7 | 1.89 | 7 | 0.27 | 7 | 0.46 | 7 | 0.33 | 7 | 0.51 | 7 | 72 | 1.21 | 6.55 | |
| WH7 | Margherita | 4.39 | 2 | 2.12 | 2 | 2.27 | 6 | 3.25 | 2 | 3.05 | 1 | 2.86 | 1 | 1.05 | 5 | 0.76 | 1 | 1.19 | 2 | 0.48 | 5 | 0.95 | 5 | 32 | 1.92 | 2.91 |
| R5+ | 2.95 | 4 | 2.37 | 1 | 0.59 | 1 | 2.66 | 3 | 2.64 | 2 | 2.63 | 2 | 0.40 | 1 | 0.57 | 2 | 1.33 | 1 | 0.80 | 1 | 1.58 | 1 | 19 | 1.01 | 1.73 | |
| R5- | 2.60 | 7 | 1.92 | 4 | 0.68 | 2 | 2.26 | 6 | 2.23 | 6 | 2.21 | 4 | 0.53 | 2 | 0.41 | 6 | 1.08 | 4 | 0.74 | 2 | 1.46 | 2 | 45 | 1.92 | 4.09 | |
| R112+ | 2.94 | 5 | 1.75 | 5 | 1.19 | 4 | 2.34 | 5 | 2.27 | 5 | 2.19 | 5 | 0.82 | 4 | 0.42 | 5 | 0.99 | 5 | 0.59 | 4 | 1.17 | 4 | 51 | 0.50 | 4.64 | |
| R112- | 2.80 | 6 | 2.04 | 3 | 0.76 | 3 | 2.42 | 4 | 2.39 | 4 | 2.36 | 3 | 0.55 | 3 | 0.47 | 4 | 1.15 | 3 | 0.73 | 3 | 1.43 | 3 | 39 | 0.93 | 3.55 | |
| R23+ | 5.62 | 1 | 1.16 | 6 | 4.45 | 7 | 3.39 | 1 | 2.56 | 3 | 1.93 | 6 | 1.61 | 7 | 0.54 | 3 | 0.66 | 6 | 0.21 | 7 | 0.41 | 7 | 54 | 2.43 | 4.91 | |
| R23- | 3.17 | 3 | 1.06 | 7 | 2.11 | 5 | 2.12 | 7 | 1.83 | 7 | 1.59 | 7 | 1.35 | 6 | 0.28 | 7 | 0.60 | 7 | 0.34 | 6 | 0.66 | 6 | 68 | 1.25 | 6.18 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giovenali, G.; Kuzmanović, L.; Capoccioni, A.; Ceoloni, C. The Response of Chromosomally Engineered Durum Wheat-Thinopyrum ponticum Recombinant Lines to the Application of Heat and Water-Deficit Stresses: Effects on Physiological, Biochemical and Yield-Related Traits. Plants 2023, 12, 704. https://doi.org/10.3390/plants12040704
Giovenali G, Kuzmanović L, Capoccioni A, Ceoloni C. The Response of Chromosomally Engineered Durum Wheat-Thinopyrum ponticum Recombinant Lines to the Application of Heat and Water-Deficit Stresses: Effects on Physiological, Biochemical and Yield-Related Traits. Plants. 2023; 12(4):704. https://doi.org/10.3390/plants12040704
Chicago/Turabian StyleGiovenali, Gloria, Ljiljana Kuzmanović, Alessandra Capoccioni, and Carla Ceoloni. 2023. "The Response of Chromosomally Engineered Durum Wheat-Thinopyrum ponticum Recombinant Lines to the Application of Heat and Water-Deficit Stresses: Effects on Physiological, Biochemical and Yield-Related Traits" Plants 12, no. 4: 704. https://doi.org/10.3390/plants12040704
APA StyleGiovenali, G., Kuzmanović, L., Capoccioni, A., & Ceoloni, C. (2023). The Response of Chromosomally Engineered Durum Wheat-Thinopyrum ponticum Recombinant Lines to the Application of Heat and Water-Deficit Stresses: Effects on Physiological, Biochemical and Yield-Related Traits. Plants, 12(4), 704. https://doi.org/10.3390/plants12040704






