The Amelioration of Grazing through Physiological Integration by a Clonal Dune Plant
Abstract
1. Introduction
2. Methods
2.1. Species Description
2.2. Field Grazing Assessment
2.3. Simulated Grazing and Integration Experiment
2.4. Statistical Analyses
3. Results
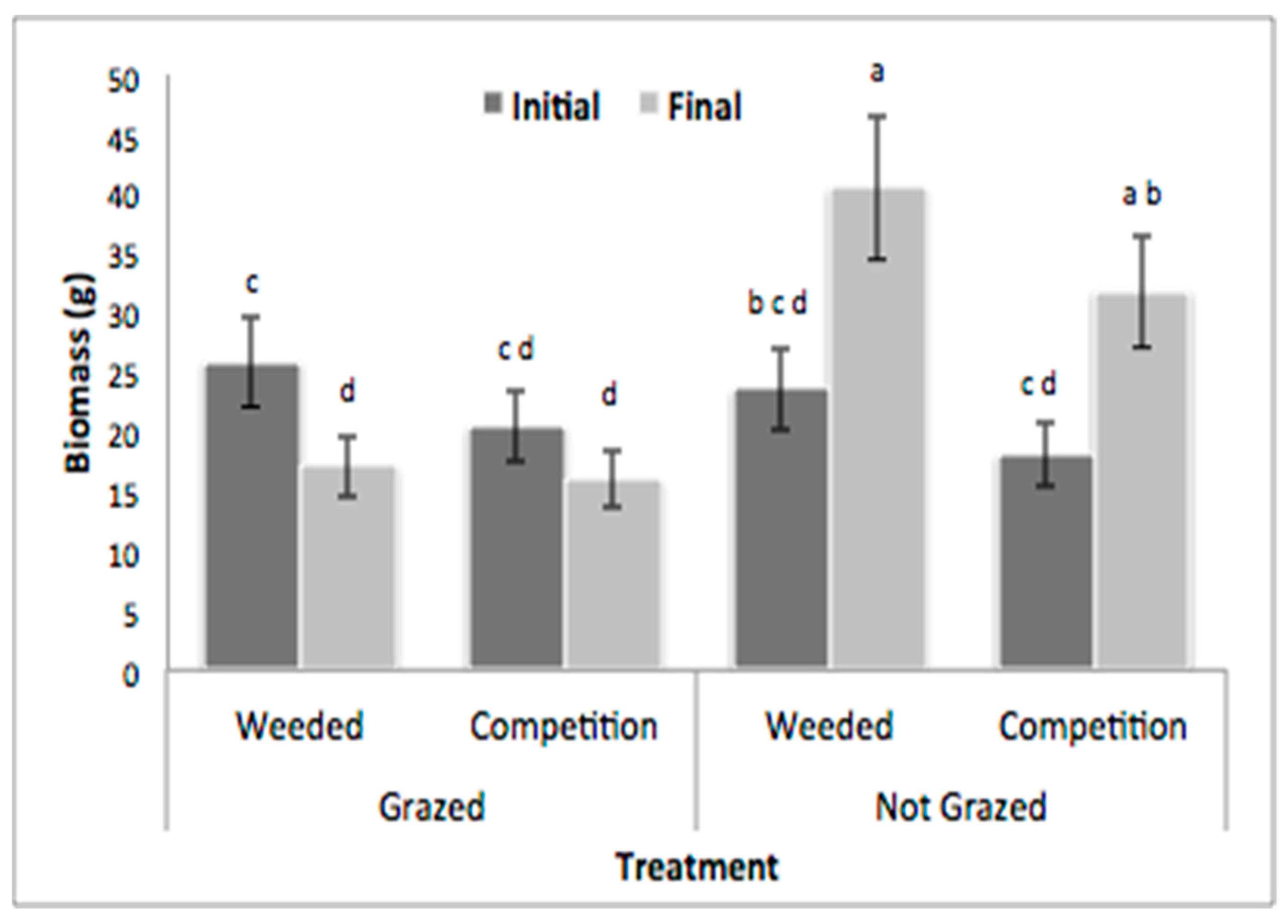
3.1. Field Herbivory Assessment
3.2. Simulated Grazing and Integration Experiment
3.2.1. Severing Effect
3.2.2. Biomass Production
3.2.3. Leaf and Inflorescence Production
3.2.4. Ramet Production and Main Rhizome Length
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, J.P. The effect of local resource availability and clonal integration on ramet functional morphology in Hydrocotyle bonariensis. Oecologia 1992, 89, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Fahrig, L.; Coffin, D.P.; Lauenroth, W.K.; Shugart, H. The advantage of long-distance clonal spreading in highly disturbed habitats. Evol. Ecol. 1994, 8, 172–187. [Google Scholar] [CrossRef]
- Pan, J.J.; Price, J.S. Fitness and evolution in clonal plants: The impact of clonal growth. Evol. Ecol. 2001, 15, 583–600. [Google Scholar] [CrossRef]
- Liu, F.H.; Liu, J.; Dong, M. Ecological consequences of clonal integration in plants. Front. Plant Sci. 2016, 7, 770. [Google Scholar] [CrossRef] [PubMed]
- Schmid, B.; Bazzaz, F.A. Clonal integration and population structure in perennials: Effects of severing rhizome connections. Ecology 1987, 68, 2016–2022. [Google Scholar] [CrossRef]
- Alpert, P. Nitrogen sharing among ramets increases clonal growth in Fragaria chiloensis. Ecology 1991, 72, 69–80. [Google Scholar] [CrossRef]
- Evans, J.P. The effect of resource integration on fitness related traits in a clonal dune perennial, Hydrocotyle bonariensis. Oecologia 1991, 86, 268–275. [Google Scholar] [CrossRef]
- Hartnett, D.C.; Bazzaz, F.A. Physiological integration among intraclonal ramets in Solidago canadensis. Ecology 1983, 64, 779–788. [Google Scholar] [CrossRef]
- Hutchings, M.J.; Bradbury, I.K. Ecological perspectives on clonal perennial herbs. BioScience 1986, 36, 178–182. [Google Scholar] [CrossRef]
- Stuefer, J.F.; During, H.J.; de Kroon, H. High benefits of clonal integration in two stoloniferous species, in response to heterogeneous light environments. J. Ecol. 1994, 82, 511–518. [Google Scholar] [CrossRef]
- McNaughton, S.J.; Georgiadis, N.J. Ecology of African grazing and browsing mammals. Annu. Rev. Ecol. Syst. 1986, 17, 39–66. [Google Scholar] [CrossRef]
- Edwards, P.J.; Wratten, S.D. Induced plant defenses against insect grazing: Fact or artifact? Oikos 1985, 44, 70–74. [Google Scholar] [CrossRef]
- Strauss, S.Y.; Agrawal, A.A. The ecology and evolution of plant tolerance to herbivory. Trends Ecol. Evol. 1999, 14, 179–185. [Google Scholar] [CrossRef]
- Ferraro, D.O.; Oesterheld, M. Effect of defoliation on grass growth. A quantitative review. Oikos 2002, 98, 125–133. [Google Scholar] [CrossRef]
- Parker, M.A.; Root, R.B. Insect herbivores limit habitat distribution of a native composite, Machaeranthera canescens. Ecology 1981, 62, 1390–1392. [Google Scholar] [CrossRef]
- Liu, J.; Chen, C.; Pan, Y.; Zhang, Y.; Gao, Y. The intensity of simulated grazing modifies costs and benefits of physiological integration in a rhizomatous clonal plant. Int. J. Environ. Res. Public Health 2020, 17, 2724. [Google Scholar] [CrossRef]
- Svensson, B.M.; Rydin, H.; Carlsson, B.Å. Clonality in the Plant Community. In Vegetation Ecology; van der Maarel, E., Franklin, J., Eds.; John Wiley & Sons: New York, NY, USA, 2013; pp. 141–163. [Google Scholar]
- Bittebiere, A.; Benot, M.; Mony, C. Clonality as a key but overlooked driver of biotic interactions in plants. Perspect. Plant Ecol. Evol. Syst. 2020, 43, 125510. [Google Scholar] [CrossRef]
- Gruenberg, B.U. The Wild Horse Dilemma: Conflicts and Controversies of the Atlantic Coast Herds; Synclitic Media LLC.: New Providence, PA, USA, 2016. [Google Scholar]
- Boyce, P.N.; Hennig, J.D.; Brook, R.K.; McLoughlin, P.D. Causes and consequences of lags in basic and applied research into feral wildlife ecology: The case for feral horses. Basic Appl. Ecol. 2021, 53, 154–163. [Google Scholar] [CrossRef]
- Baron, J. Effects of feral hogs (Sus scrofa) on the vegetation of Horn Island, Mississippi. Am. Midl. Nat. 1982, 107, 202–205. [Google Scholar] [CrossRef]
- Turner, M.G.; Bratton, S.P. Fire, grazing, and the landscape heterogeneity of a Georgia barrier island. In Landscape Heterogeneity and Disturbance; Springer: New York, NY, USA, 1987; pp. 85–101. [Google Scholar]
- Wood, G.W.; Mengak, M.T.; Murphy, M. Ecological importance of feral ungulates at Shackleford Banks, North Carolina. Am. Midl. Nat. 1987, 118, 236–244. [Google Scholar] [CrossRef]
- Rheinhardt, R.D.; Rheinhardt, M.C. Feral horse seasonal habitat use on a coastal barrier spit. J. Range Manag. 2004, 57, 253–258. [Google Scholar] [CrossRef]
- Porter, K.M.; DePerno, C.S.; Krings, A.; Krachey, M.; Braham, R. Vegetative impact of feral horses, feral pigs, and white-tailed deer on the Currituck National Wildlife Refuge, North Carolina. Castanea 2014, 79, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Seliskar, D.M. The response of Ammophila breviligulata and Spartina patens (Poaceae) to grazing by feral horses on a dynamic mid-Atlantic barrier island. Am. J. Bot. 2003, 90, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.P. Nitrogen translocation in a clonal dune perennial, Hydrocotyle bonariensis. Oecologia 1988, 77, 64–68. [Google Scholar] [CrossRef]
- Evans, J.P.; Whitney, S. Clonal integration across a salt gradient by a nonhalophyte, Hydrocotyle bonariensis (Apiaceae). Am. J. Bot. 1992, 79, 1344–1347. [Google Scholar] [CrossRef]
- Duncan, W.H.; Duncan, M.B. The Smithsonian guide to seaside plants of the Gulf and Atlantic coasts. Smithsonian Institution Press: Washington, DC, USA, 1988; 409p. [Google Scholar]
- Evans, J.P. Seedling establishment and genet recruitment in Hydrocotyle bonariensis. In Barrier Island Ecology of the Mid-Atlantic Coast: A Symposium; Cole, C.A., Turner, K., Eds.; National Park Service, SE Regional Office: Atlanta, GA, USA, 1992; pp. 75–84. [Google Scholar]
- Taggart, J.B. Management of feral horses at the North Carolina National Estuarine Research Reserve. Nat. Areas J. 2008, 28, 187–195. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Stroup, W.W. Generalized Linear Mixed Models: Modern Concepts, Methods and Applications; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- SAS Institute Inc. SAS/STAT Software, Version 9.4; SAS Institute Inc: Cary, NC, USA, 2002–2012. Available online: http://www.sas.com/ (accessed on 3 January 2023).
- Stuefer, J.F.; Gómez, S.; Mölken, T.V. Clonal integration beyond resource sharing: Implications for defence signalling and disease transmission in clonal plant networks. Evol. Ecol. 2004, 18, 647–667. [Google Scholar] [CrossRef]
- Dong, M. Plant clonal growth in heterogeneous habitats: Risk-spreading. Acta Phytoecol. Sin. 1996, 20, 543–548. [Google Scholar]
- Liu, H.D.; Yu, F.H.; He, W.M.; Chu, Y.; Dong, M. Are clonal plants more tolerant to grazing than co-occurring non-clonal plants in inland dunes? Ecol. Res. 2007, 22, 502–506. [Google Scholar] [CrossRef]
- Barbour, M.G.; De Jong, T.M.; Pavlik, B.M. Marine beach and dune plant communities. In Physiological Ecology of North American Plant Communities; Chabot, B., Mooney, H., Eds.; Chapman and Hall: New York, NY, USA, 1985; pp. 296–322. [Google Scholar]
- Ehrenfeld, J.G. Dynamics and processes of barrier-island vegetation. Rev. Aquat. Sci. 1990, 2, 437–480. [Google Scholar]
- Zuo, X.; Zhao, H.; Zhao, X.; Zhang, T.; Guo, Y.; Wang, S.; Drake, S. Spatial pattern and heterogeneity of soil properties in sand dunes under grazing and restoration in Horqin Sandy Land, Northern China. Soil Tillage Res. 2008, 99, 202–212. [Google Scholar] [CrossRef]
- Eldridge, D.J.; Ding, J.; Travers, S.K. Feral horse activity reduces environmental quality in ecosystems globally. Biol. Conserv. 2020, 241, 10836. [Google Scholar] [CrossRef]
- Hobbs, N.T. Modification of ecosystems by ungulates. J. Wildl. Manag. 1996, 60, 695–713. [Google Scholar] [CrossRef]
- Turner, M.G. Effects of grazing by feral horses, clipping, trampling, and burning on a Georgia salt marsh. Estuaries 1987, 10, 54–60. [Google Scholar] [CrossRef]
- Ruess, R.W. Nutrient movement and grazing: Experimental effects of clipping and nitrogen source on nutrient uptake in Kyllinga nervosa. Oikos 1984, 43, 183–188. [Google Scholar] [CrossRef]
- Cain, M.L.; Subler, S.; Evans, J.P.; Fortin, M.J. Sampling spatial and temporal variation in soil nitrogen availability. Oecologia 1999, 118, 397–404. [Google Scholar] [CrossRef]
- Haynes, R.J. Mineral Nitrogen in the Plant-Soil System; Academic Press: London, UK, 1986; 483p. [Google Scholar]
- Jónsdóttir, I.S. Effects of grazing on tiller size and population dynamics in a clonal sedge (Carex bigelowii). Oikos 1991, 62, 177–188. [Google Scholar] [CrossRef]
- Hawkes, C.V.; Sullivan, J.J. The impact of herbivory on plants in different resource conditions: A meta-analysis. Ecology 2001, 82, 2045–2058. [Google Scholar] [CrossRef]
- Price, E.A.; Hutchings, M.J. Studies of growth in the clonal herb Glechoma hederacea. II. The effects of selective defoliation. J. Ecol. 1992, 80, 39–47. [Google Scholar] [CrossRef]
- Bach, C.E. Effects of clonal integration on response to sand burial and defoliation by the dune plant Ipomoea pes-caprae (Convolvulaceae). Aust. J. Bot. 2000, 48, 159–166. [Google Scholar] [CrossRef]
- You, W.H.; Yu, D.; Xie, D.; Han, C.M.; Liu, C.H. The invasive plant Alternanthera philoxeroides benefits from clonal integration in response to defoliation. Flora 2014, 209, 666–673. [Google Scholar] [CrossRef]
- Wang, P.; Li, H.; Pang, X.Y.; Wang, A.; Dong, B.C.; Lei, J.P.; Yu, F.H.; Li, M.H. Clonal integration increases tolerance of a phalanx clonal plant to defoliation. Sci. Total Environ. 2017, 593, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Dong, M.; Krüsi, B. Clonal integration helps Psammochloa villosa survive sand burial in an inland dune. New Phytol. 2004, 162, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yu, F.H.; van Drunen, E.; Schieving, F.; Dong, M.; Anten, N.P. Trampling, defoliation and physiological integration affect growth, morphological and mechanical properties of a root-suckering clonal tree. Ann. Bot. 2012, 109, 1001–1008. [Google Scholar] [CrossRef]
- Wilsey, B. Clonal plants in a spatially heterogeneous environment: Effects of integration on Serengeti grassland response to defoliation and urine-hits from grazing mammals. Plant Ecol. 2002, 159, 15–22. [Google Scholar] [CrossRef]
- Slade, A.J.; Hutchings, M.J. Clonal integration and plasticity in foraging behaviour in Glechoma hederacea. J. Ecol. 1987, 75, 1023–1036. [Google Scholar] [CrossRef]
- Price, E.; Marshall, C. Clonal plants and environmental heterogeneity: An introduction to the proceedings. Plant Ecol. 1999, 141, 3–7. [Google Scholar] [CrossRef]
- de Kroon, H.; Huber, H.; Stuefer, J.F.; van Groenendael, J.M. A modular concept of phenotypic plasticity in plants. New Phytol. 2005, 166, 73–82. [Google Scholar] [CrossRef]
- Liu, H.D.; Yu, F.H.; He, W.M.; Chu, Y.; Dong, M. Clonal integration improves compensatory growth in heavily grazed ramet populations of two inland-dune grasses. Flora-Morphol. Distrib. Funct. Ecol. Plants 2009, 204, 298–305. [Google Scholar] [CrossRef]
- Wang, Z.W.; Li, L.H.; Han, X.G.; Ming, D. Do rhizome severing and shoot defoliation affect clonal growth of Leymus chinensis at ramet population level? Acta Oecol.Int. J. Ecol. 2004, 26, 255–260. [Google Scholar] [CrossRef]
- Dominiak-Świgoń, M.; Kasprzykowski, Z.; Lembicz, M. Changes in the growth and reproduction of a clonal plant as a result of disruption of mycorrhizal network. Plant Fungal Syst. 2021, 66, 195–200. [Google Scholar] [CrossRef]
- Van der Putten, W.H. Plant defense belowground and spatiotemporal processes in natural vegetation. Ecology 2003, 84, 2269–2280. [Google Scholar] [CrossRef]
- Benot, M.L.; Mony, C.; Puijalon, S.; Mohammad-Esmaeili, M.; van Alphen, J.J.; Bouzillé, J.B.; Bonis, A. Responses of clonal architecture to experimental defoliation: A comparative study between ten grassland species. In Herbaceous Plant Ecology; Springer: Dordrecht, The Netherlands, 2008; pp. 257–266. [Google Scholar]
- Gao, Y.; Wang, D.; Xing, F.; Liu, J.; Wang, L. Combined effects of resource heterogeneity and simulated herbivory on plasticity of clonal integration in a rhizomatous perennial herb. Plant Biol. 2014, 16, 774–782. [Google Scholar] [CrossRef]
- Wang, P.; Alpert, P.; Yu, F.H. Physiological integration can increase competitive ability in clonal plants if competition is patchy. Oecologia 2021, 195, 199–212. [Google Scholar] [CrossRef]
- Evans, J.P.; Cain, M.L. A spatially explicit test of foraging behavior in a clonal plant. Ecology 1995, 76, 1147–1155. [Google Scholar] [CrossRef]
- Bonacic, C.; Almuna, R.; Ibarra, J.T. Biodiversity conservation requires management of feral domestic animals. Trends Ecol. Evol. 2019, 34, 683–686. [Google Scholar] [CrossRef]

| Grazing (G) | Competition (C) | Time (T) | G × C | G × T | C × T | G × C × T | |
|---|---|---|---|---|---|---|---|
| Biomass | 8.06 ** | 3.02 * | 1.56 ns | 0.18 ns | 25.11 *** | 0.3 ns | 0.15 ns |
| Effect | df | Total Biomass | Leaf Area | Leaves | Inflorescences | Main Rhizome Length | Ramets |
|---|---|---|---|---|---|---|---|
| S | 1.54 | 28.83 *** | 68.58 *** | 4.08 * | 20.34 *** | 0.24 ns | 1.15 ns |
| G | 2.54 | 163.23 *** | 164.56 *** | 11.82 *** | 124.03 *** | 14.50 *** | 2.44 ns |
| S × G | 2.54 | 14.68 *** | 32.81 *** | 17.46 *** | 20.62 *** | 0.664 ns | 34.29 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evans, J.P.; Meckstroth, S.; Garai, J. The Amelioration of Grazing through Physiological Integration by a Clonal Dune Plant. Plants 2023, 12, 724. https://doi.org/10.3390/plants12040724
Evans JP, Meckstroth S, Garai J. The Amelioration of Grazing through Physiological Integration by a Clonal Dune Plant. Plants. 2023; 12(4):724. https://doi.org/10.3390/plants12040724
Chicago/Turabian StyleEvans, Jonathan P., Shelby Meckstroth, and Julie Garai. 2023. "The Amelioration of Grazing through Physiological Integration by a Clonal Dune Plant" Plants 12, no. 4: 724. https://doi.org/10.3390/plants12040724
APA StyleEvans, J. P., Meckstroth, S., & Garai, J. (2023). The Amelioration of Grazing through Physiological Integration by a Clonal Dune Plant. Plants, 12(4), 724. https://doi.org/10.3390/plants12040724







