Localization of Lipid Droplets in Embryonic Axis Radicle Cells of Soybean Seeds under Various Imbibition Regimes Indicates Their Role in Desiccation Tolerance
Abstract
:1. Introduction
2. Results
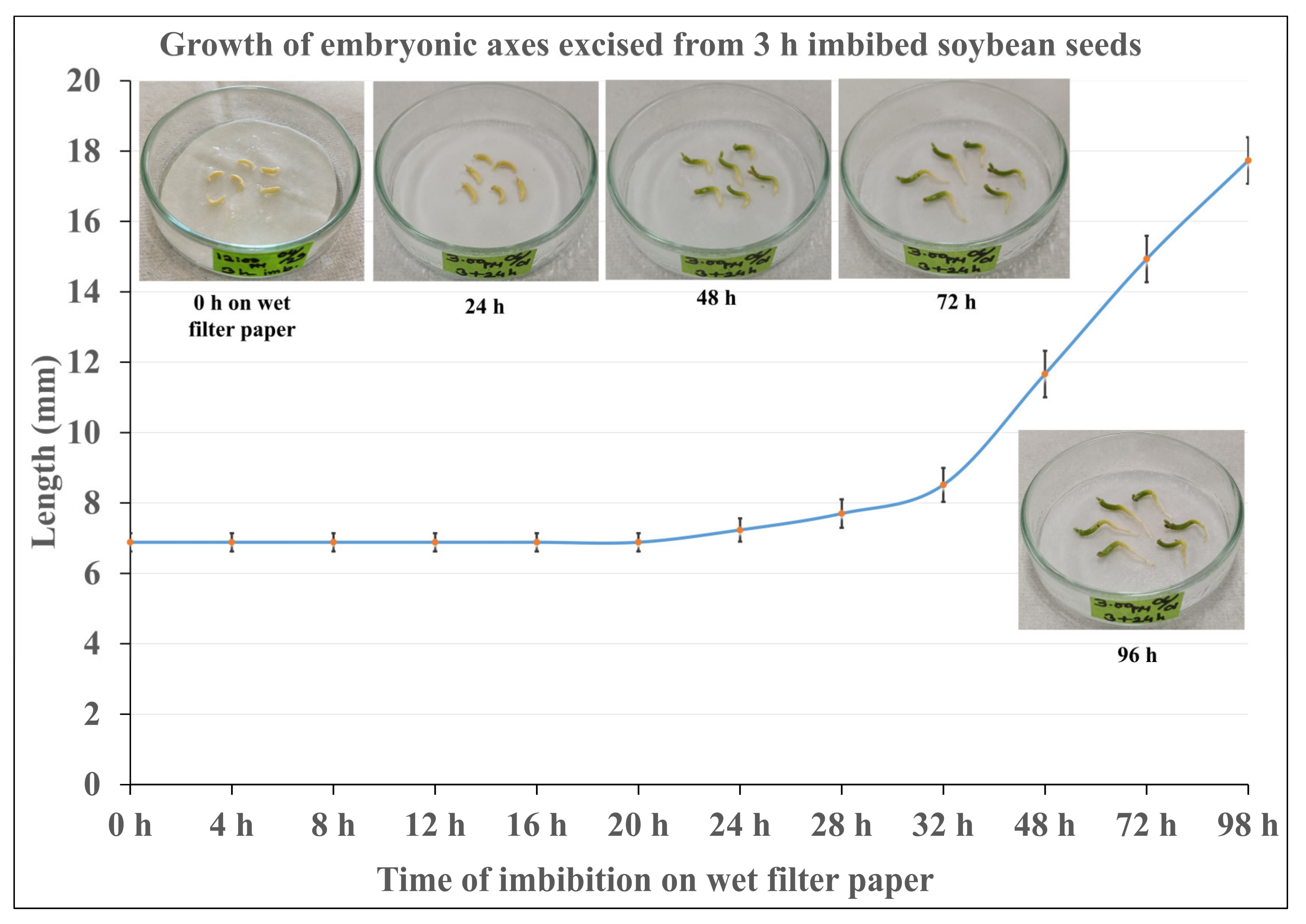
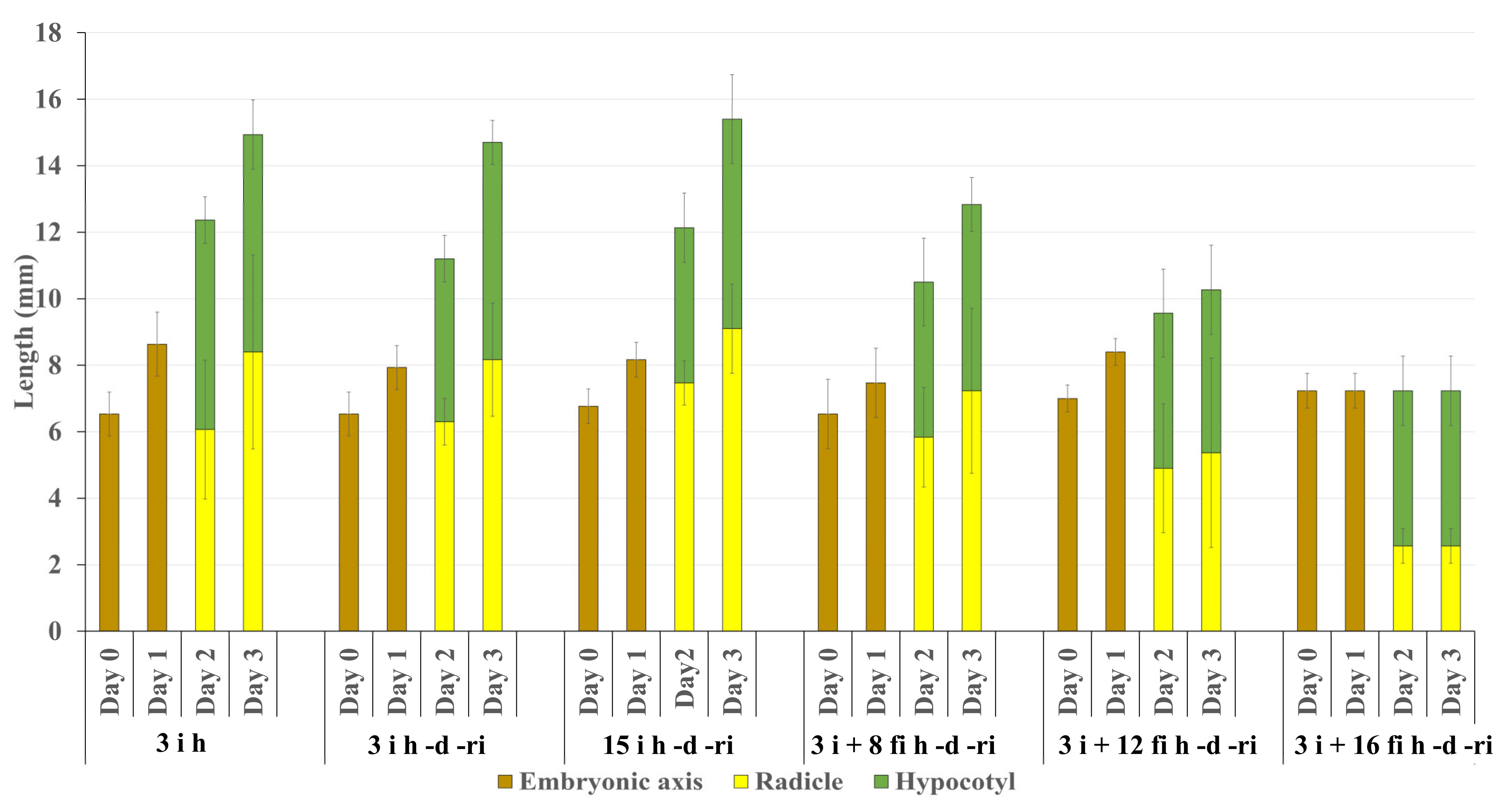
2.1. Observation of the Growth of Embryonic Axes after Re-Imbibition
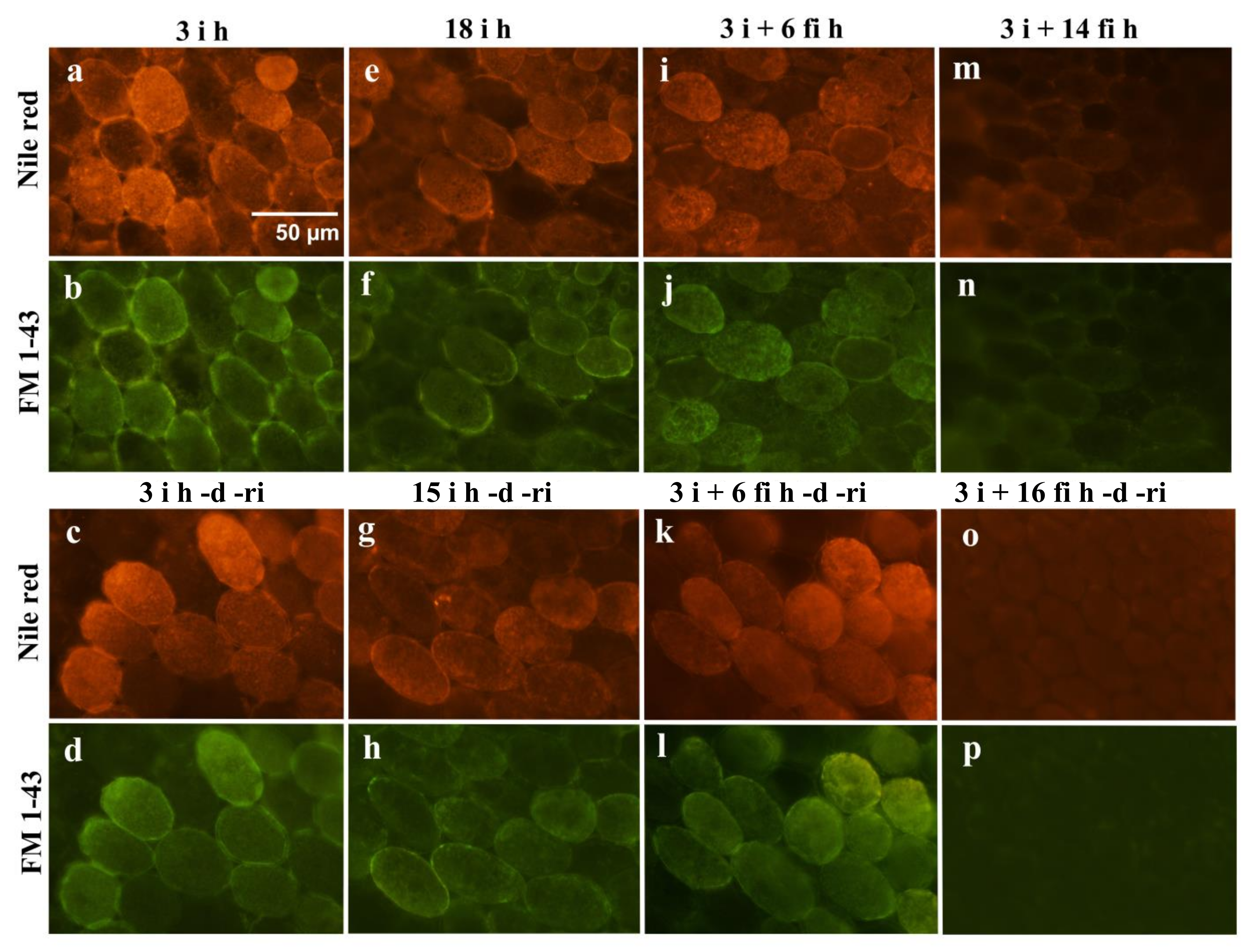
2.2. Fluorescence Microscopy Observation
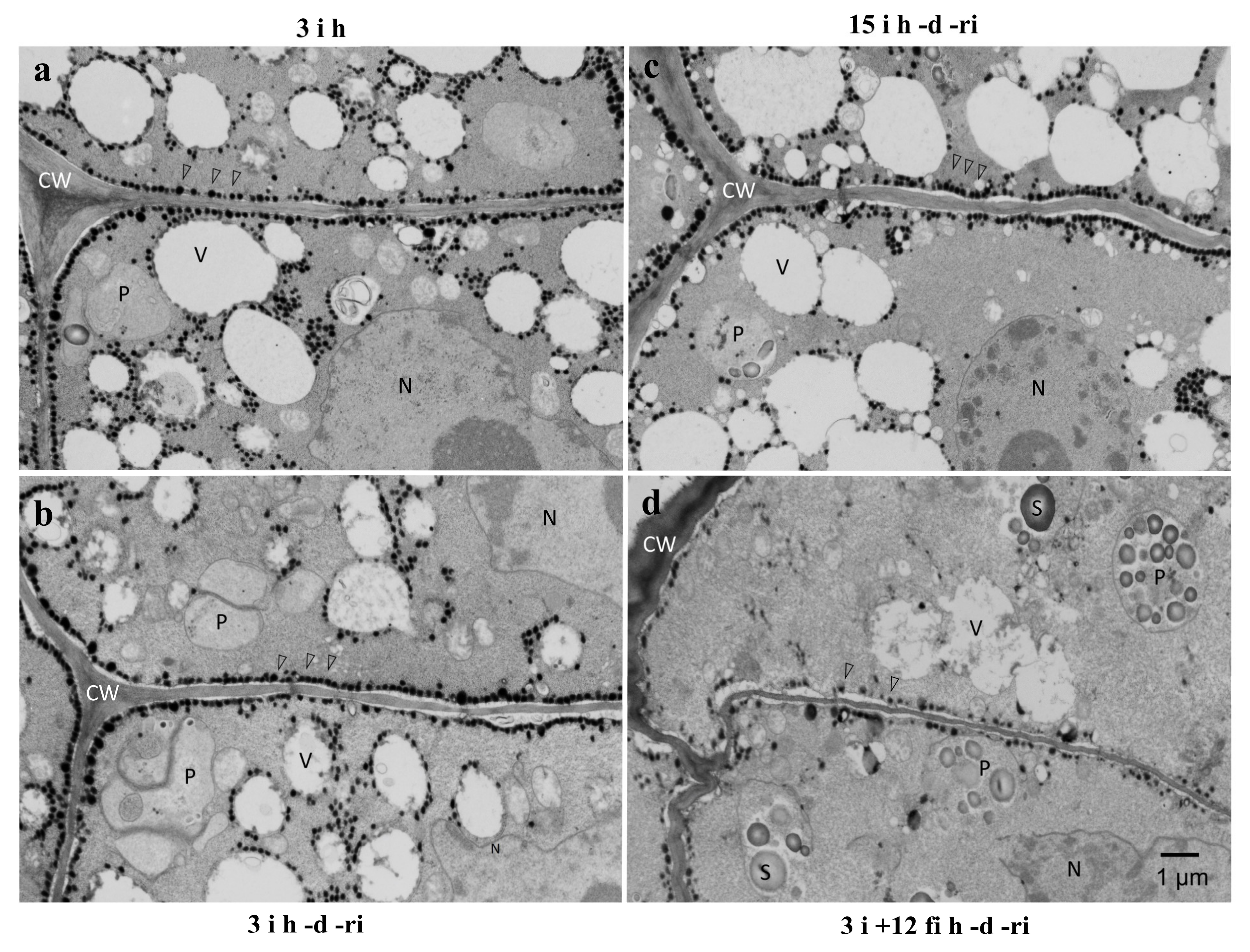
2.3. TEM Observation
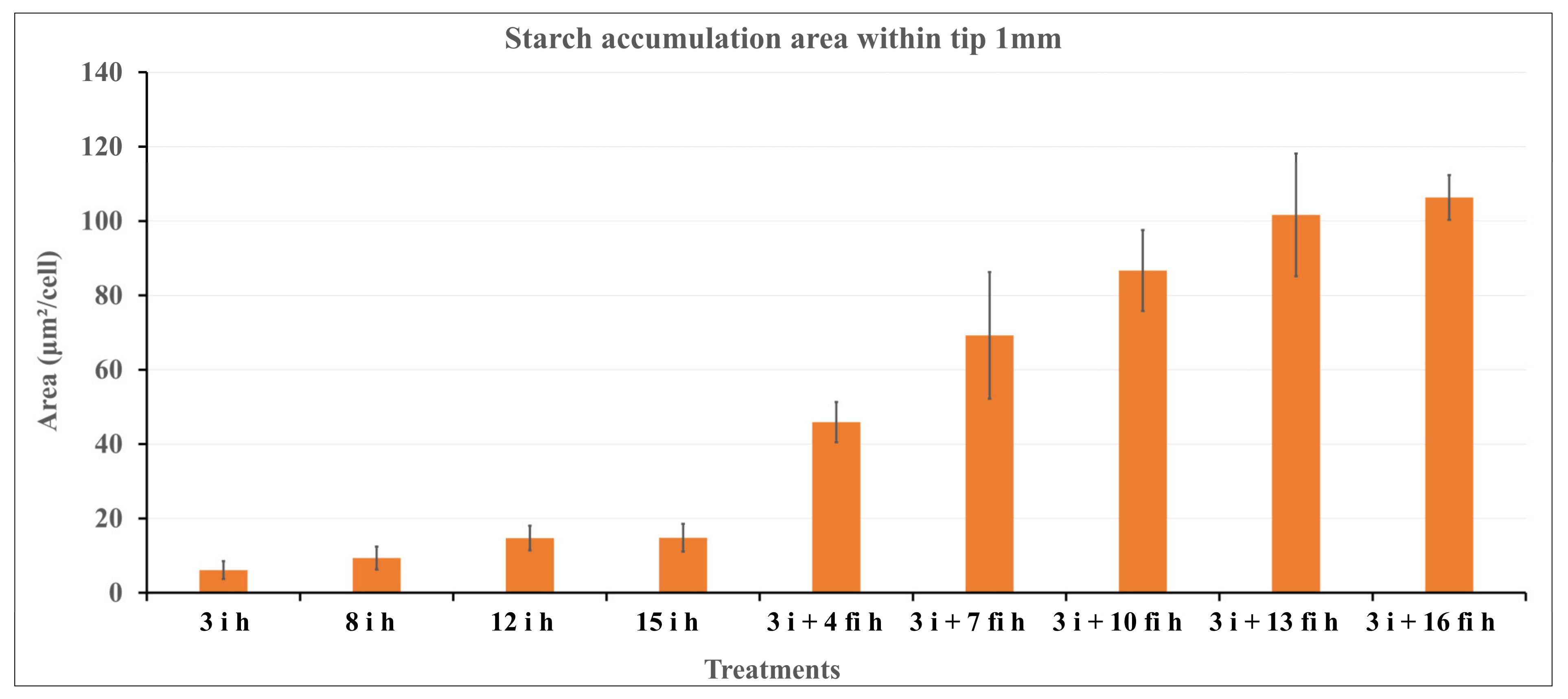
2.4. Light Microscopy Observation
3. Discussion
4. Materials and Methods
4.1. Preparation of Embryonic Axes
4.2. Processing for Fluorescence Microscopy
4.3. Processing for Transmission Electron Microscopy
4.4. Processing for Light Microscopy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matilla, A.J. The Orthodox Dry Seeds Are Alive: A Clear Example of Desiccation Tolerance. Plants 2022, 11, 20. [Google Scholar] [CrossRef]
- Dekkers, B.J.W.; Costa, M.C.D.; Maia, J.; Bentsink, L.; Ligterink, W.; Hilhorst, H.W.M. Acquisition and loss of desiccation tolerance in seeds: From experimental model to biological relevance. Planta 2015, 241, 563–577. [Google Scholar] [CrossRef]
- Aswathi, K.P.R.; Kalaji, H.M.; Puthur, J.T. Seed priming of plants aiding in drought stress tolerance and faster recovery: A review. Plant Growth Regul. 2022, 97, 235–253. [Google Scholar] [CrossRef]
- Chen, K.; Arora, R. Priming memory invokes seed stress-tolerance. Environ. Exp. Bot. 2013, 94, 33–45. [Google Scholar] [CrossRef]
- Devika, O.S.; Singh, S.; Sarkar, D.; Barnwal, P.; Suman, J.; Rakshit, A. Seed priming: A potential supplement in integrated resource management under fragile intensive ecosystems. Front. Sustain. Food Syst. 2021, 5, 209–219. [Google Scholar] [CrossRef]
- McDonald, M.B. Seed priming. In Seed Technology and Its Biological Basis; Black, M., Bewley, J.D., Eds.; Sheffield Academic Press: Sheffield, UK, 2000; pp. 287–325. [Google Scholar]
- Crowe, J.H.; Crowe, L.M.; Carpenter, J.F.; Rudolph, A.S.; Aurell Wistrom, C.; Spargo, B.J.; Anchordoguy, T.J. Interactions of sugars with membranes. Biochim. Biophys. Acta 1988, 947, 367–384. [Google Scholar] [CrossRef]
- Koster, K.L.; Leopold, A.C. Sugars and desiccation tolerance in seeds. Plant Physiol. 1988, 88, 829–832. [Google Scholar] [CrossRef]
- Williams, R.J.; Leopold, A.C. The glassy state in corn embryos. Plant Physiol. 1989, 89, 977–981. [Google Scholar] [CrossRef]
- Bruni, F.; Leopold, A.C. Glass transitions in soybean seed: Relevance to anhydrous biology. Plant Physiol. 1991, 96, 660–663. [Google Scholar] [CrossRef]
- Senaratna, T.; McKersie, B.D.; Stinson, R.H. Simulation of dehydration injury to membranes from soybean axes by free radicals. Plant Physiol. 1985, 77, 472–474. [Google Scholar] [CrossRef] [Green Version]
- Blackman, S.A.; Obendorf, R.L.; Leopold, A.C. Desiccation tolerance in developing soybean seeds: The role of stress proteins. Physiol. Plant. 1995, 93, 630–638. [Google Scholar] [CrossRef]
- Cordova-Tellez, L.; Burris, J.S. Seed physiology, production & technology—Alignment of lipid bodies along the plasma membrane during the acquisition of desiccation tolerance in maize seed. Crop Sci. 2002, 42, 1982–1988. [Google Scholar] [CrossRef]
- Senaratna, T.; McKersie, B.D. Dehydration injury in germinating soybean (Glycine max L. Merr.) Seeds. Plant Physiol. 1983, 72, 620–624. [Google Scholar] [CrossRef]
- Dean, A.; Wigg, K.; Zambiazzi, E.; Christian, E.; Goggi, S.; Schwarte, A.; Johnson, J.; Cabrera, E. Migration of Oil Bodies in Embryo Cells during Acquisition of Desiccation Tolerance in Chemically Defoliated Corn (Zea mays L.) Seed Production Fields. Agriculture 2021, 11, 129. [Google Scholar] [CrossRef]
- Perdomo, A.; Burris, J.S. Histochemical, physiological, and ultrastructural changes in the maize embryo during artificial drying. Crop Sci. 1998, 38, 1236–1244. [Google Scholar] [CrossRef]
- Yang, Y.; Benning, C. Functions of triacylglycerols during plant development and stress. Curr. Opin. Biotechnol. 2018, 49, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Ischebeck, T.; Krawczyk, H.E.; Mullen, R.T.; Dyer, J.M.; Chapman, K.D. Lipid droplets in plants and algae: Distribution, formation, turnover and function. Semin. Cell Dev. Biol. 2020, 108, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.J.; Hernández-Pinzón, I.; Patel, K. Roles of lipid bodies and lipid-body proteins in seeds and other tissues. J. Plant Physiol. 2001, 158, 471–478. [Google Scholar] [CrossRef]
- Gasulla, F.; Vom Dorp, K.; Dombrink, I.; Zähringer, U.; Gisch, N.; Dörmann, P.; Bartels, D. The role of lipid metabolism in the acquisition of desiccation tolerance in Craterostigma plantagineum: A comparative approach. Plant J. 2013, 75, 726–741. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Huang, G.; Feng, F.; Liu, X.; Guo, R.; Gu, F.; Zhong, X.; Mei, X. Dynamic changes in membrane lipid composition of leaves of winter wheat seedlings in response to PEG-induced water stress. BMC Plant Biol. 2020, 20, 84. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Fan, J.; Shanklin, J. Metabolic and functional connections between cytoplasmic and chloroplast triacylglycerol storage. Prog. Lipid Res. 2020, 80, 101069. [Google Scholar] [CrossRef]
- Perlikowski, D.; Lechowicz, K.; Skirycz, A.; Michaelis, Ä.; Pawłowicz, I.; Kosmala, A. The Role of Triacylglycerol in the Protection of Cells against Lipotoxicity under Drought in Lolium multiflorum/Festuca arundinacea Introgression Forms. Plant Cell Physiol. 2022, 63, 353–368. [Google Scholar] [CrossRef]
- Kummerow, F.A.; Mahfouz, M.; Zhou, Q.; Masterjohn, C. Effects of trans fats on prostacyclin production. Scand. Cardiovasc. J. 2013, 47, 377–382. [Google Scholar] [CrossRef]
- Smolikova, G.; Leonova, T.; Vashurina, N.; Frolov, A.; Medvedev, S. Desiccation tolerance as the basis of long-term seed viability. Int. J. Mol. Sci. 2021, 22, 101. [Google Scholar] [CrossRef]
- Reisdorph, N.A.; Koster, K.L. Progressive loss of desiccation tolerance in germinating pea (Pisum sativum) seeds. Physiol. Plant. 1999, 105, 266–271. [Google Scholar] [CrossRef]
- Buitink, J.; Vu, B.L.; Satour, P.; Leprince, O. The re-establishment of desiccation tolerance in germinated radicles of Medicago truncatula Gaertn. seeds. Seed Sci. Res. 2003, 13, 273–286. [Google Scholar] [CrossRef]
- Leprince, O.; Pellizzaro, A.; Berriri, S.; Buitink, J. Late seed maturation: Drying without dying. J. Exp. Bot. 2017, 68, 827–841. [Google Scholar] [CrossRef] [PubMed]
- Yoo, B.Y. Ultrastructural changes in cells of pea embryo radicles during germination. J. Cell Biol. 1970, 45, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Keegstra, K. Plastid biogenesis in embryonic pea leaf cells during early germination. Protoplasma 1996, 195, 59–67. [Google Scholar] [CrossRef]
- Chabot, J.F.; Leopold, A.C. Ultrastructural changes of membranes with hydration in soybean seeds. Am. J. Bot. 1982, 69, 623–633. [Google Scholar] [CrossRef]
- Eastmond, P.J.; Graham, I.A. Re-examining the role of the glyoxylate cycle in oilseeds. Trends Plant Sci. 2001, 6, 72–78. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanam, S.; Atsuzawa, K.; Kaneko, Y. Localization of Lipid Droplets in Embryonic Axis Radicle Cells of Soybean Seeds under Various Imbibition Regimes Indicates Their Role in Desiccation Tolerance. Plants 2023, 12, 799. https://doi.org/10.3390/plants12040799
Khanam S, Atsuzawa K, Kaneko Y. Localization of Lipid Droplets in Embryonic Axis Radicle Cells of Soybean Seeds under Various Imbibition Regimes Indicates Their Role in Desiccation Tolerance. Plants. 2023; 12(4):799. https://doi.org/10.3390/plants12040799
Chicago/Turabian StyleKhanam, Salma, Kimie Atsuzawa, and Yasuko Kaneko. 2023. "Localization of Lipid Droplets in Embryonic Axis Radicle Cells of Soybean Seeds under Various Imbibition Regimes Indicates Their Role in Desiccation Tolerance" Plants 12, no. 4: 799. https://doi.org/10.3390/plants12040799




