Cytogenetic Characterization and Metabolomic Differences of Full-Sib Progenies of Saccharum spp.
Abstract
:1. Introduction
2. Results
2.1. Chromosome Counting for Identification of Zhongzhe 1 Sugarcane and G160 Sugarcane, Which Are Derived from the Same Parents
2.2. GISH of F1 Hybrids Resulting from ROC25 × Yunzhe89-7
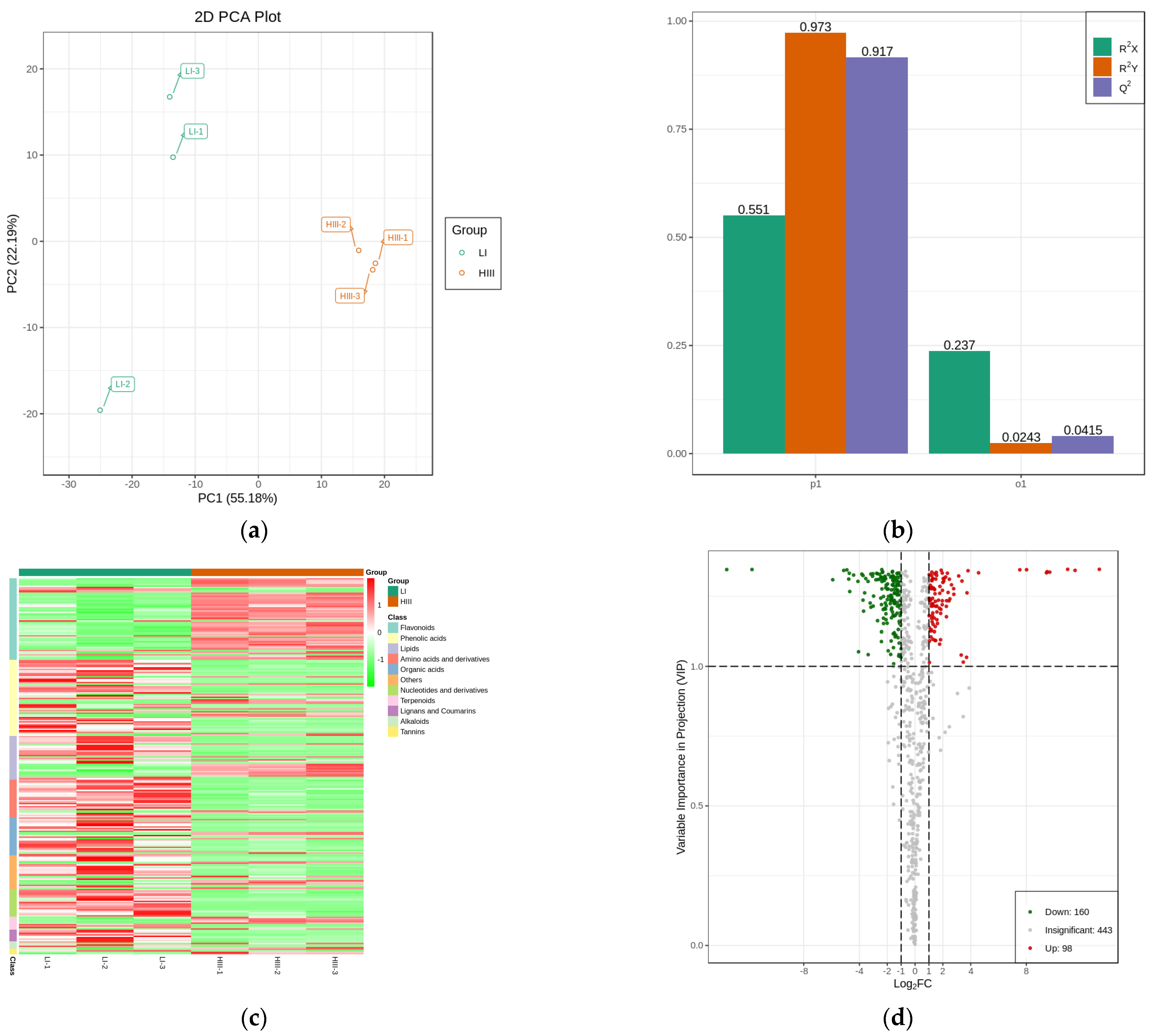
2.3. Metabolomic Differences between the Hybrids Resulting from ROC25 × Yunzhe89-7
2.4. Differentially Enriched Metabolic Pathway Analysis
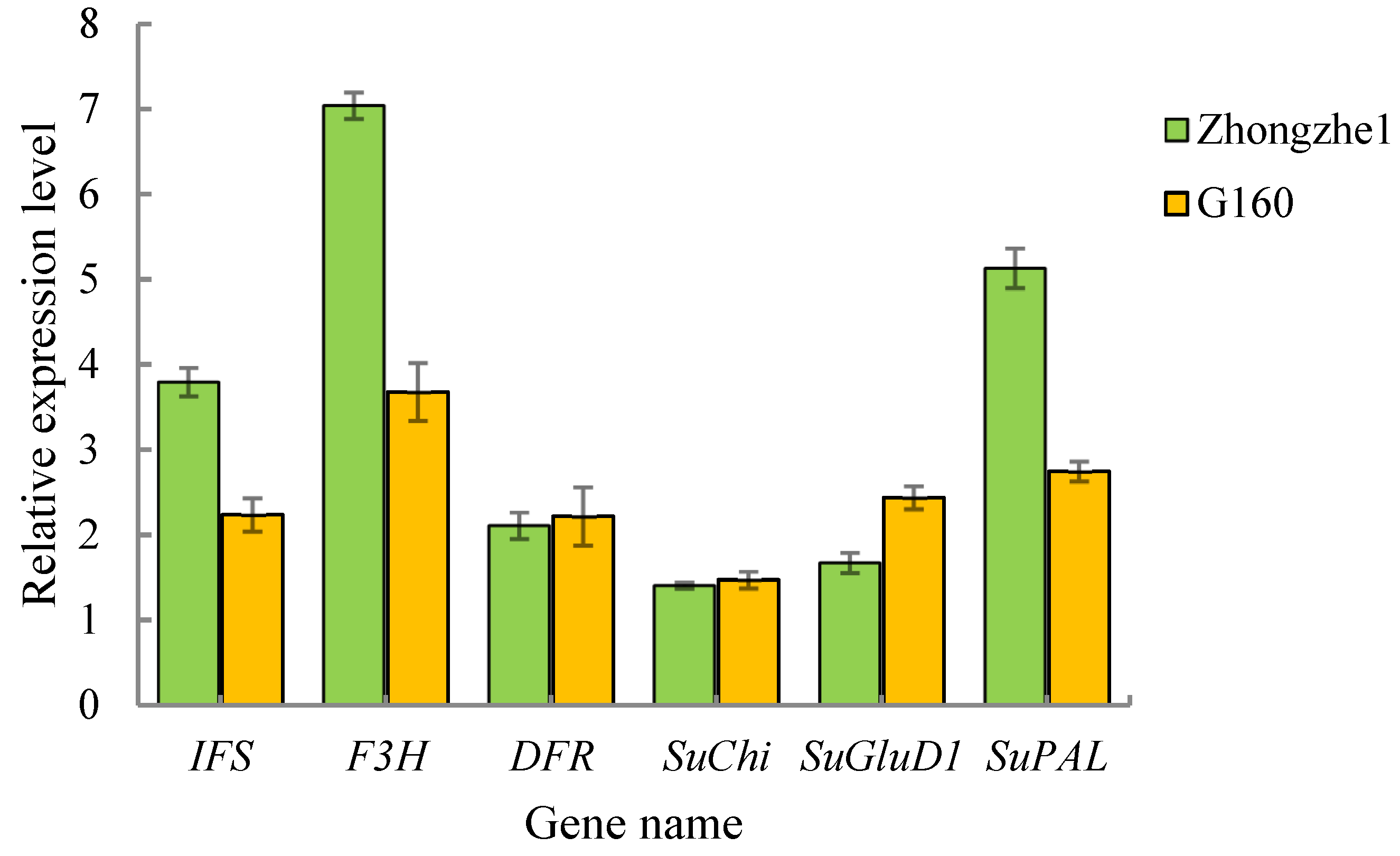
2.5. Analysis of the Validation of Key Genes Involving Metabolites
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Root Tip Collection and Pretreatment
4.3. GISH Procedure
4.4. LC–ESI–MS/MS Analysis and Differentially Accumulated Metabolite Identification
4.5. Quantitative Real-Time PCR
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, N.; Somai, B.M.; Pillay, D. Smut disease assessment by PCR and microscopy in inoculated tissue cultured sugarcane cultivars. Plant Sci. 2004, 167, 987–994. [Google Scholar] [CrossRef]
- Msechu, Z.E.; Keswani, C.L. Effect of sugarcane smut on yield and yield components of sugarcane varieties in Tanzania. Trop. Agric. 1982, 59, 243–247. [Google Scholar]
- Croft, B.J.; Magarey, R.C.; Allsopp, P.G.; Cox, M.C.; Willcox, T.G.; Milford, B.J.; Wallis, E.S. Sugarcane smut in Queensland: Arrival and emergency response. Australas. Plant Pathol. 2008, 37, 26–34. [Google Scholar] [CrossRef]
- Sundar, A.R.; Barnabas, E.L.; Malathi, P.; Viswanathan, R. A Mini-Review on Smut Disease of Sugarcane Caused by Sporisorium scitamineum. In Botany; Mworia, K.J., Ed.; Intech Open: London, UK, 2012; pp. 109–128. [Google Scholar]
- Xiong, G.R.; Zhang, S.Z. Study on sugarcane smut. Plant Dis. Pests 2012, 3, 12–14. [Google Scholar]
- Song, X.P.; Tian, D.D.; Chen, M.H.; Qin, Z.Q.; Wei, J.J.; Wei, C.Y.; Zhang, X.Q.; Li, D.W.; Yang, L.T.; Li, Y.R. Cloning and Identification of Differentially Expressed Genes Associated with Smut in Sugarcane. Sugar Tech. 2018, 20, 717–724. [Google Scholar] [CrossRef]
- Yu, F.; Wang, P.; Li, X.T.; Huang, Y.J.; Wang, Q.N.; Luo, L.; Jing, Y.F.; Liu, X.L.; Deng, Z.H.; Wu, J.Y.; et al. Characterization of chromosome composition of sugarcane in nobilization by using genomic in situ hybridization. Mol. Cytogenet. 2018, 11, 35. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, P.; Xian, L.R.; Li, R.; Huang, Y.Z.; Chen, B.S. Identification of Smut Resistance in Sugarcane Zhongzhe Cultivars. Sugar Crops China 2019, 41, 37–40. [Google Scholar]
- Lai, X.Q.; Huang, D.Y.; Yao, X.; Chen, Y.J.; Yao, Z.T.; Zou, C.W.; Chen, B.S. Identification of the F1 Hybrids Facticity from Cross between “ROC” 25 and Yunzhe89-7 using SSR Molecular Markers. Sugar Crops China 2021, 43, 7–12. [Google Scholar]
- Cang, X.Y.; Xia, H.M.; Li, W.F.; Wang, X.Y.; Shan, H.L.; Wang, C.M.; Li, J.; Zhang, R.Y.; Huang, Y.K. Evaluation of natural resistance to smut in elite sugarcane varieties (lines). Acta Agron. Sin. 2021, 47, 2290–2296. [Google Scholar] [CrossRef]
- Mcneil, M.D.; Bhuiyan, S.A.; Berkman, P.J.; Croft, B.J.; Aitken, K.S. Analysis of the resistance mechanisms in sugarcane during Sporisorium scitamineum infection using RNA-seq and microscopy. PLoS ONE 2018, 13, e0197840. [Google Scholar] [CrossRef]
- Zhu, G.N.; Deng, Y.Z.; Cai, E.P.; Yan, M.X.; Cui, G.B.; Wang, Z.Q.; Zou, C.W.; Zhang, B.; Xi, P.G.; Chang, C.Q.; et al. Identification and functional analysis of the pheromone response factor gene of Sporisorium scitamineum. Front. Microbiol. 2019, 10, 2115. [Google Scholar] [CrossRef] [PubMed]
- Gabriella, L.; Eszter, G.; István, M.; Diana, I.; Ekaterina, B.; Márta, M.L. Molecular cytogenetic (FISH) and genome analysis of diploid wheatgrasses and their phylogenetic relationship. PLoS ONE 2017, 12, e0173623. [Google Scholar]
- Singh, A.K.; Zhang, P.; Dong, C.; Li, J.B.; Singh, S.; Trethowan, R.M.; Sharp, P.J. Development and molecular cytogenetic characterization of Thinopyrum bessarabicum introgression lines in hexaploid and tetraploid wheats. Theor. Appl. Genet. 2020, 133, 2117–2130. [Google Scholar] [CrossRef] [PubMed]
- Patokar, C.; Sepsi, A.; Schwarzacher, T.; Kishii, M.; Heslop-Harrison, J.S. Molecular cytogenetic characterization of novel wheat-Thinopyrum bessarabicum recombinant lines carrying intercalary translocations. Chromosoma 2016, 125, 163–172. [Google Scholar] [CrossRef]
- Srinivasan, S.; Shariff, M.; Bartlett, S.E. The role of the glucocorticoids in developing resilience to stress and addiction. Front. Psychiatry 2013, 1, 68. [Google Scholar] [CrossRef]
- Mo, X.H.; Zhang, M.K.; Liang, C.Y.; Cai, Y.L.; Jiang, T. Integration of metabolome and transcriptome analyses highlights soybean roots responding to phosphorus deficiency by modulating phosphorylated metabolite processes. Plant Physiol. Biochem. 2019, 139, 697–706. [Google Scholar] [CrossRef]
- Treutter, D. Signifcance of favonoids in plant resistance and enhancement of their biosynthesis. Plant Biol. 2005, 7, 581–591. [Google Scholar] [CrossRef]
- Zhang, J.; Subramanian, S.; Stacey, G.; Yu, O. Flavones and favonols play distinct critical roles during nodulation of Medicago truncatula by Sinorhizobium meliloti. Plant J. 2009, 57, 171–183. [Google Scholar] [CrossRef]
- Zhao, X.; Yan, Y.; Zhou, W.H.; Feng, R.Z.; Shuai, Y.K.; Li, Y.; Liu, M.J.; He, X.Y.; Wei, Q. Transcriptome and metabolome reveal the accumulation of secondary metabolites in different varieties of Cinnamomum longepaniculatum. BMC Plant Biol. 2022, 22, 243. [Google Scholar] [CrossRef]
- Schaker, P.D.C.; Peters, L.P.; Cataldi, T.R.; Labate, C.A.; Caldana, C.; Monteiro-Vitorello, C.B. Metabolome Dynamics of Smutted Sugarcane Reveals Mechanisms Involved in Disease Progression and Whip Emission. Front. Plant Sci. 2017, 8, 882. [Google Scholar] [CrossRef]
- Völz, R.; Park, J.Y.; Harris, W.; Hwang, S.; Lee, Y.H. Lyso–phosphatidylethanolamine primes the plant immune system and promotes basal resistance against hemibiotrophic pathogens. BMC Biotechnol. 2021, 21, 12. [Google Scholar] [CrossRef]
- Ambriz-Pérez, D.L.; Leyva-López, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B. Phenolic compounds: Natural alternative in inflammation treatment. A Review. Cogent Food Agric. 2016, 2, 1131412. [Google Scholar]
- Pachakkil, B.; Terajima, Y.; Ohmido, N.; Ebina, M.; Irei, S.; Hayashi, H.; Takagi, H. Cytogenetic and agronomic characterization of intergeneric hybrids between Saccharum spp. hybrid and Erianthus Arundinaceus. Sci. Rep. 2019, 9, 1748. [Google Scholar] [PubMed]
- Huang, N.; Ling, H.; Su, Y.C.; Liu, F.; Xu, L.P.; Su, W.H.; Wu, Q.B.; Guo, J.L.; Gao, S.W.; Que, Y.X. Transcriptional analysis identifies major pathways as response components to Sporisorium scitamineum stress in sugarcane. Gene 2018, 678, 207–218. [Google Scholar] [CrossRef]
- Deng, Z.H.; Zhang, M.Q.; Lin, W.L.; Cheng, F.; Zhang, C.M.; Li, Y.C.; Lai, L.P.; Lin, Y.Q.; Chen, R.K. Analysis of disequilibrium hybridization in hybrid and backcross progenies of Saccharum officinarum × Erianthus arundinaceus. Agric. Sci. China 2010, 9, 1271–1277. [Google Scholar] [CrossRef]
- Chen, W.; Gong, L.; Guo, Z.L.; Wang, W.S.; Zhang, H.Y.; Liu, X.Q.; Yu, S.B.; Xiang, L.Z.; Luo, J. A novel integrated method for large-scale detection, identification, and quantification of widely targeted metabolites: Application in the study of rice metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef]
- Zhu, G.T.; Wang, S.C.; Huang, Z.J.; Zhang, S.; Liao, Q.G.; Zhang, C.Z.; Lin, T.; Qin, M.; Peng, M.; Yang, C.K.; et al. Rewiring of the fruit metabolome in tomato breeding. Cell 2018, 172, 249–261. [Google Scholar] [CrossRef]
- Berger, A.; Latimer, S.; Stutts, L.R.; Soubeyrand, E.; Block, A.K.; Basset, G.J. Kaempferol as a precursor for ubiquinone (coenzyme Q) biosynthesis: An atypical node between specialized metabolism and primary metabolism. Curr. Opin. Plant Biol. 2022, 66, 102165. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cheng, J.; Jiang, W.; Chen, S. Metabolomics study of flavonoids in Coreopsis tinctoria of different origins by UPLC–MS/MS. PeerJ 2022, 10, e14580. [Google Scholar] [CrossRef]
- Lee, S.; Seol, H.S.; Eom, S.; Lee, J.; Kim, C.; Park, J.H.; Kim, T.H.; Lee, J.H. Hydroxy Pentacyclic triter–pene acid, Kaempferol, inhibits the human 5–Hydroxytryptamine type 3A receptor activity. Int. J. Mol. Sci. 2022, 23, 544. [Google Scholar] [CrossRef]
- Elarabi, N.I.; Abdelhadi, A.A.; Sief-Eldein, A.G.M.; Ismail, A.I.; Abdallah, N.A. Overexpression of chalcone isomerase a gene in Astragalus trigonus for stimulating apigenin. Sci. Rep. 2021, 11, 24176. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Vyas, S.; Bhattacharya, S.; Sharma, M. Catechin prodrugs and analogs: A new array of chemical entities with improved pharmacological and pharmacokinetic properties. Nat. Prod. Rep. 2013, 30, 1438–1454. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.H.; Wei, Q.; Feng, R.Z.; Liu, Y.; Liang, H.Q.; Li, J.; Yan, K. Diversity and spatial distribution of endophytic fungi in Cinnamomum longepaniculatum of Yibin, China. Arch. Microbiol. 2021, 203, 3361–3372. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, W.; Cheng, J.S.; He, X.G.; Chu, G.H. Differential analysis of flavonoid metabolites and key enzyme gene expressions in Coreopsis tinctoria collected from plateau and plain areas. J. Food Saf. Qual. 2022, 13, 6956–6963. [Google Scholar]
- Su, Y.C.; Xu, L.P.; Fu, Z.W.; Yang, Y.T.; Guo, J.L.; Wang, S.S.; Que, Y.X. Scchi encoding an acidic class III chitinase of sugarcane confers positive responses to biotic and abiotic stresses in sugarcane. Int. J. Mol. Sci. 2014, 15, 2738–2760. [Google Scholar] [CrossRef]
- Su, Y.C.; Xu, L.P.; Xue, B.T.; Wu, Q.B.; Guo, J.L.; Wu, L.G.; Que, Y.X. Molecular cloning and characterization of two pathogenesis related ß-13-glucanase genes ScGluA1 and ScGluD1 from sugarcane infected by Sporisorium scitamineum. Plant Cell Rep. 2013, 32, 1503–1519. [Google Scholar] [CrossRef]
- Song, X.P.; Huang, X.; Mo, F.L.; Tian, D.D.; Yang, L.T.; Li, Y.R.; Chen, B.S. Cloning and expression analysis of sugarcane Phenylalanin Ammonia-lyase (PAL). Sci. Agric. Sin. 2013, 46, 2856–2868. [Google Scholar]
- Soo, J.W.; So, Y.S.; Kyoungwon, C.; Myung, H.N.; Ky, Y.P. Lysophosphatidylcholine enhances susceptibility in signaling pathway against pathogen infection through biphasic production of reactive oxygen species and ethylene in tobacco plants. Phytochemistry 2014, 104, 48–59. [Google Scholar]
- Huang, Y.; Xie, F.J.; Cao, X.; Li, M.Y. Research progress in biosynthesis and regulation of plant terpenoids. Biotechnol. Biotechnol. Equip. 2021, 35, 1799–1808. [Google Scholar] [CrossRef]
- Tholl, D. Biosynthesis and biological functions of terpenoids in plants. Adv. Biochem. Eng. Biotechnol. 2015, 148, 63–106. [Google Scholar]
- Han, X.; Zhang, Y.; Wang, M. Discovery and engineering of cytochrome P450s for terpenoid biosynthesis. Trends Biotechnol. 2019, 37, 618–631. [Google Scholar]
- Islam, F.M.A.; Rengifo, J.; Redden, R.J.; Basford, K.E.; Beebe, S.E. Association between seed coat polyphenolics (tannins) and disease resistance in common bean. Plant Foods Hum. Nutr. 2003, 58, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Hassan, O.; Chang, T.; Hossain, A. Changes in the secondary compounds of persimmon leaves as a defense against circular leaf spot caused by Plurivorosphaerella nawae. PLoS ONE 2020, 15, e0230286. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, G.; Ownley, B.H.; Augé, R.M.; Toler, H.; Dee, M. Colonization by arbuscular mycorrhizal and endophytic fungi enhanced terpene production in tomato plants and their defense against a herbivorous insect. Symbiosis 2015, 65, 65–74. [Google Scholar] [CrossRef]
- Yoon, S.J.; Sukweenadhi, J.; Khorolragchaa, A.; Mathiyalagan, R.; Subramaniyam, S.; Kim, Y.J.; Kim, H.B.; Kim, M.J.; Kim, Y.J.; Yang, D.C. Overexpression of panax ginseng sesquiterpene synthase gene confers tolerance against Pseudomonas syringae pv. tomato in Arabidopsis thaliana. Physiol. Mol. Biol. Plants 2016, 22, 485–495. [Google Scholar] [CrossRef]
- Riedlmeier, M.; Ghirardo, A.; Wenig, M.; Knappe, C.; Koch, K.; Georgii, E.; Dey, S.; Parker, J.E.; Schnitzler, J.P.; Vlot, A.C. Monoterpenes support systemic acquired resistance within and between plants. Plant Cell 2017, 29, 1440–1459. [Google Scholar] [CrossRef]
- Przybylska-Balcerek, A.; Szablewski, T.; Cegielska-Radziejewska, R.; Góral, T.; Kurasiak–Popowska, D.; Stuper-Szablewska, K. Assessment of Antimicrobial Properties of Phenolic Acid Extracts from Grain Infected with Fungi from the Genus Fusarium. Molecules 2022, 27, 1741. [Google Scholar] [CrossRef]
- Yu, H.; Li, H.Y.; Wei, R.F.; Cheng, G.; Zhou, Y.M.; Liu, J.B.; Xie, T.L.; Guo, R.R.; Zhou, S.H. Widely Targeted Metabolomics Profiling Reveals the Effect of Powdery Mildew on Wine Grape Varieties with Different Levels of Tolerance to the Disease. Foods 2022, 11, 2461. [Google Scholar] [CrossRef]
- Hildebrandt, T.M.; Nesi, A.N.; Araújo, W.L.; Braun, H.P. Amino Acid Catabolism in Plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef]
- Straube, H.; Witte, C.P.; Herde, M. Analysis of Nucleosides and Nucleotides in Plants: An Update on Sample Preparation and LC–MS Techniques. Cells 2021, 10, 689. [Google Scholar] [CrossRef]
- Stewart, C.N.J.; Via, L.E. A rapid CTAB DNA isolation technique useful for RAPD fingerprinting and other PCR applications. BioTechniques 1993, 14, 748–750. [Google Scholar]
- D’hont, A.; Rao, P.; Feldmann, P.; Grivet, L.; Islam-Faridi, N.; Taylor, P.; Glaszmann, J. Identification and characterisation of sugarcane intergeneric hybrids, Saccharum officinarum × Erianthus arundinaceus, with molecular markers and DNA in situ hybridisation. Theor. Appl. Genet. 1995, 91, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Tu, H.; Wan, J.; Chen, W.; Liu, X.Q.; Luo, J.; Xu, J.; Zhang, H.Y. Spatio-temporal distribution and natural variation of metabolites in citrus fruits. Food Chem. 2016, 199, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Singh, R.K.; Song, Q.Q.; Li, H.B.; Guo, D.J.; Malviya, M.K.; Verma, K.K.; Song, X.P.; Lakshmanan, P.; Yang, L.T.; et al. Comparative analysis of protein and differential responses of defenserelated gene and enzyme activity reveals the long-term molecular responses of sugarcane inoculated with Sporisorium scitamineum. J. Plant Interact. 2021, 16, 12–29. [Google Scholar] [CrossRef]




| Category | Phenolic Acids | Flavonoids | Lipids | Organic Acids | Amino Acids and Derivatives | Nucleotides and Derivatives | Alkaloids | Terpenoids | Others |
|---|---|---|---|---|---|---|---|---|---|
| Amounts in each category | 118 | 138 | 98 | 73 | 73 | 38 | 17 | 33 | 113 |
| Amounts of differentially accumulated metabolites | 52 | 56 | 30 | 26 | 26 | 19 | 5 | 9 | 35 |
| Percentages of differentially accumulated metabolites (%) | 44.1 | 40.1 | 30.1 | 35.6 | 35.6 | 50.0 | 29.4 | 27.3 | 31.0 |
| Gene | Upstream Primers (5′→3′) | Downstream Primers (5′→3′) | Reference |
|---|---|---|---|
| IFS | ACAACGGCGGAACATACG | ACACTGCTTGCCACTCACC | [35] |
| F3H | AAGAAGTGGAGCAAGGGAAAG | CGGAGACATTGGTGGAGAAA | [35] |
| DFR | ATGTTGCTACACCGTGGTTAC | AAATCGAAAGATCCCTCCTC | [35] |
| SuChi | ACGGCTACGGCGACAACA | GTCCGCTGACCAGATGAAGAG | [36] |
| SuGluD1 | TGCTACTTCTTATCCACCCTCTG | CGTTGACATAGAAAGGTGAGCC | [37] |
| SuPAL | CTCGAGGAGAACATCAAGAC | GTGATGAGCTCCTTCTCG | [38] |
| β-tubulin | ATGTTCAGGCGCAAGGCTT | TCTGCAACCGGGTCATTCAT | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, R.; Chen, B. Cytogenetic Characterization and Metabolomic Differences of Full-Sib Progenies of Saccharum spp. Plants 2023, 12, 810. https://doi.org/10.3390/plants12040810
Wang Y, Li R, Chen B. Cytogenetic Characterization and Metabolomic Differences of Full-Sib Progenies of Saccharum spp. Plants. 2023; 12(4):810. https://doi.org/10.3390/plants12040810
Chicago/Turabian StyleWang, Yi, Ru Li, and Baoshan Chen. 2023. "Cytogenetic Characterization and Metabolomic Differences of Full-Sib Progenies of Saccharum spp." Plants 12, no. 4: 810. https://doi.org/10.3390/plants12040810






