Phenolic Profile and Cholinesterase Inhibitory Properties of Three Chilean Altiplano Plants: Clinopodium gilliesii (Benth.) Kuntze [Lamiaceae], Mutisia acuminata Ruiz & Pav. var. hirsuta (Meyen) Cabrera, and Tagetes multiflora (Kunth) [Asteraceae]
Abstract
1. Introduction
2. Results and Discussion
2.1. Extraction Yield
2.2. Tentative Identification and Quantification of Compounds
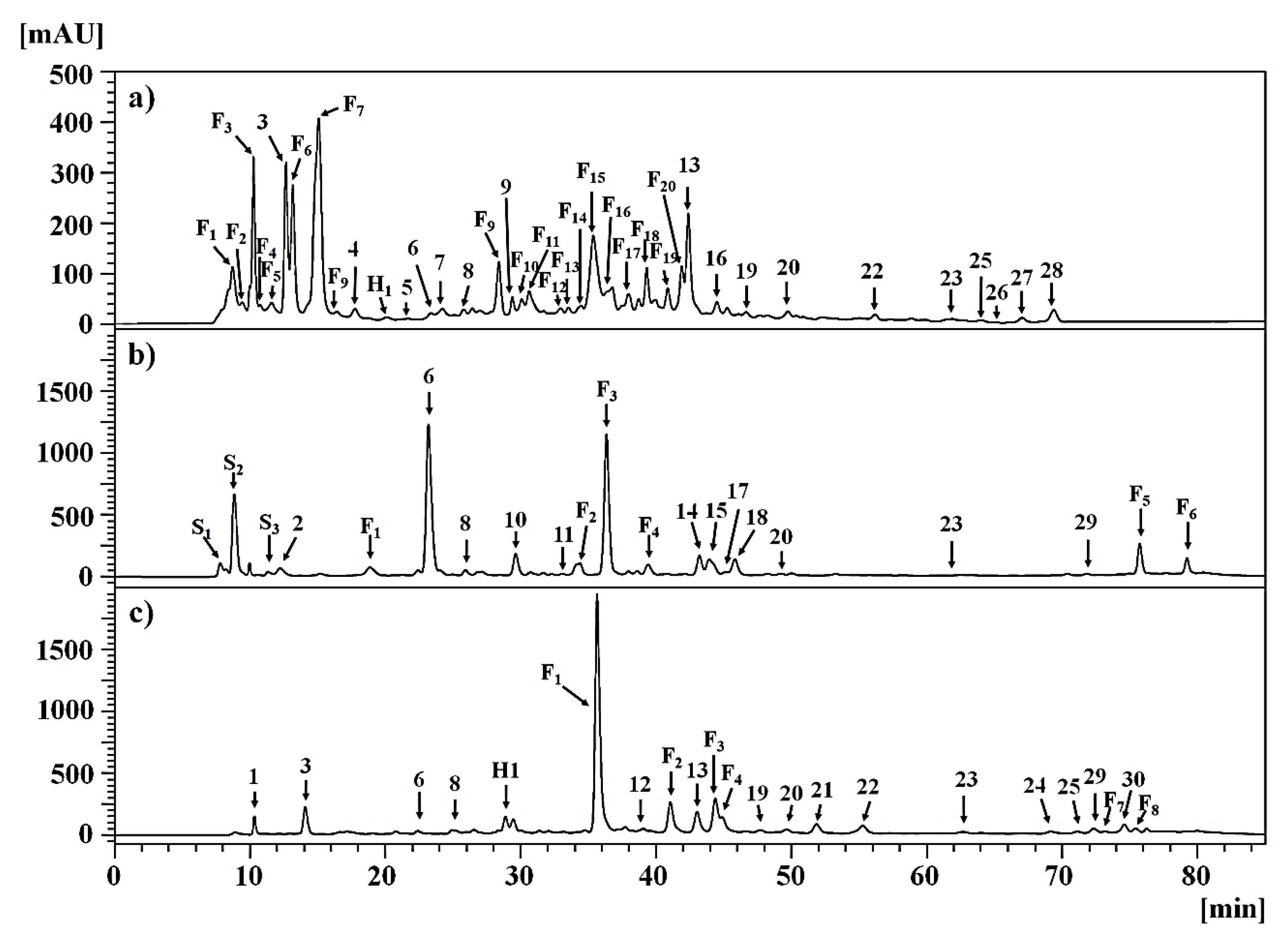
2.2.1. Clinopodium gilliesii Kuntze [Lamiaceae]
2.2.2. Mutisia acuminata Ruiz & Pav. var. hirsuta (Meyen) Cabrera [Asteraceae]
2.2.3. Tagetes multiflora Kunth [Asteraceae]
2.3. Cholinesterase Inhibitory Activities
2.4. Molecular Docking
3. Materials and Methods
3.1. Standards and Reagents
3.2. Plant Material and Preparation of Extracts
3.3. HPLC-DAD Analysis and Quantification
3.4. Determination of Cholinesterase Inhibition
3.5. Molecular Docking
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lima, B.; López, S.; Luna, L.; Agüero, M.B.; Aragón, L.; Tapia, A.; Zacchino, S.; López, M.L.; Zygadlo, J.; Feresin, G.E. Essential oils of medicinal plants from the central andes of Argentina: Chemical composition, and antifungal, antibacterial, and insect-repellent activities. Chem. Biodivers. 2011, 8, 924–936. [Google Scholar] [CrossRef] [PubMed]
- Bessada, S.M.F.; Barreira, J.C.M.; Oliveira, M.B.P.P. Asteraceae species with most prominent bioactivity and their potential applications: A review. Ind. Crops Prod. 2015, 76, 604–615. [Google Scholar] [CrossRef]
- Mamadalieva, N.Z.; Akramov, D.K.; Wessjohann, L.A.; Hussain, H.; Long, C.; Tojibaev, K.S.; Alshammari, E.; Ashour, M.L.; Wink, M. The Genus Lagochilus (Lamiaceae): A Review of Its Diversity, Ethnobotany, Phytochemistry, and Pharmacology. Plants 2021, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Rolnik, A.; Olas, B. The Plants of the Asteraceae Family as Agents in the Protection of Human Health. Int. J. Mol. Sci. 2021, 22, 3009. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, S.; Lecsö-Bornet, M.; Michel, S.; Grougnet, R.; Boutefnouchet, S. Chemical composition and biological activity of essential oils from Artemisia copa Phil. var. copa (Asteraceae) and Aloysia deserticola (Phil.) Lu-Irving & O’Leary (Verbenaceae), used in the Chilean Atacama’s Taira Community (Antofagasta, Chile). J. Essent. Oil Res. 2019, 31, 425–431. [Google Scholar] [CrossRef]
- Brandenburg, M.M.; Rocha, F.G.; Pawloski, P.L.; Soley, B.d.S.; Rockenbach, A.; Scharf, D.R.; Heiden, G.; Ascari, J.; Cabrini, D.A.; Otuki, M.F. Baccharis dracunculifolia (Asteraceae) essential oil displays anti-inflammatory activity in models of skin inflammation. J. Ethnopharmacol. 2020, 259, 112840. [Google Scholar] [CrossRef] [PubMed]
- Paredes, A.; Leyton, Y.; Riquelme, C.; Morales, G. A plant from the altiplano of Northern Chile Senecio nutans, inhibits the Vibrio cholerae pathogen. Springerplus 2016, 5, 1788. [Google Scholar] [CrossRef]
- Waller, S.B.; Cleff, M.B.; Serra, E.F.; Silva, A.L.; Gomes, A.d.R.; de Mello, J.R.B.; de Faria, R.O.; Meireles, M.C.A. Plants from Lamiaceae family as source of antifungal molecules in humane and veterinary medicine. Microb. Pathog. 2017, 104, 232–237. [Google Scholar] [CrossRef]
- Elso, O.G.; Clavin, M.; Hernandez, N.; Sgarlata, T.; Bach, H.; Catalan, C.A.N.; Aguilera, E.; Alvarez, G.; Sülsen, V.P. Antiprotozoal Compounds from Urolepis hecatantha (Asteraceae). Evidence-Based Complement. Altern. Med. 2021, 2021, 6622894. [Google Scholar] [CrossRef]
- Carvajal, F.; Huanca, A.; González-Teuber, M.; Urzúa, A.; Echeverría, J. Uses of hazardous medicinal plants: Composition of the essential oil of Clinopodium gilliesii (Benth.) Kuntze (Lamiaceae), collected in Chile. Bol. Latinoam. Y Del Caribe Plantas Med. Y Aromat. 2017, 16, 486–492. [Google Scholar]
- Juárez, B.E.; Mendiondo, M.E. Flavonoids from Mutisia acuminata. Pharm. Biol. 2008, 41, 291–292. [Google Scholar] [CrossRef]
- Velásquez, D.; Gallardo-Jugo, T.; Retuerto-Figueroa, M.G.; Ramos-Llica, E.; Calixto, M.; De La Cruz, C.V.; Ortega-Romero, E.C.; Islam, M.S. Evaluation of antimicrobial activity antioxidant and cytotoxicity of ethanolic and aqueous extracts of Tagetes multiflora Kunth chinche. Ann. Trop. Med. Public Health 2020, 23, 786–800. [Google Scholar] [CrossRef]
- Catalano, S.; Cioni, P.L.; Flamini, G.; De Feo, V.; Morelli, I. Chemical Investigation of the Aerial Parts of Mutisia acuminata. Int. J. Pharmacogn. 2008, 33, 73–74. [Google Scholar] [CrossRef]
- Barbieri, N.; Costamagna, M.; Gilabert, M.; Perotti, M.; Schuff, C.; Isla, M.I.; Benavente, A. Antioxidant activity and chemical composition of essential oils of three aromatic plants from La Rioja province. Pharm. Biol. 2015, 54, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Cabana, R.; Silva, L.R.; Valentão, P.; Viturro, C.I.; Andrade, P.B. Effect of different extraction methodologies on the recovery of bioactive metabolites from Satureja parvifolia (Phil.) Epling (Lamiaceae). Ind. Crops Prod. 2013, 48, 49–56. [Google Scholar] [CrossRef]
- Debenedetti, S.; Muschietti, L.; Van Baren, C.; Clavin, M.; Broussalis, A.; Martino, V.; Houghton, P.J.; Warhurst, D.; Steele, J. In vitro antiplasmodial activity of extracts of Argentinian plants. J. Ethnopharmacol. 2002, 80, 163–166. [Google Scholar] [CrossRef]
- van Baren, C.; Martino, V.; Di Leo Lira, P.; Anao, I.; Houghton, P.; Debenedetti, S.; Croft, S. Triterpenic Acids and Flavonoids from Satureja parvifolia. Evaluation of their Antiprotozoal Activity. Zeitschrift Fur Naturforsch.-Sect. C J. Biosci. 2006, 61, 189–192. [Google Scholar] [CrossRef]
- Catalano, S.; Cioni, P.L.; Panizzi, L.; Morelli, I. Antimicrobial activity of extracts of Mutisia acuminata var. acuminata. J. Ethnopharmacol. 1998, 59, 207–209. [Google Scholar] [CrossRef]
- Pichette, A.; Garneau, F.X.; Collin, G.; Jean, F.I.; Gagno, H.; Arze, J.B.L. Essential Oils from Bolivia. IV. Compositae: Tagetes aff. maxima Kuntze and Tagetes multiflora H.B.K. J. Essent. Oil Res. 2011, 17, 27–28. [Google Scholar] [CrossRef]
- Khan, H.; Marya; Amin, S.; Kamal, M.A.; Patel, S. Flavonoids as acetylcholinesterase inhibitors: Current therapeutic standing and future prospects. Biomed. Pharmacother. 2018, 101, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.H.; Eom, B.H.; Ryu, H.W.; Kang, M.G.; Park, J.E.; Kim, D.Y.; Kim, J.H.; Park, D.; Oh, S.R.; Kim, H. Acetylcholinesterase and butyrylcholinesterase inhibitory activities of khellactone coumarin derivatives isolated from Peucedanum japonicum Thurnberg. Sci. Rep. 2020, 10, 21695. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Khan, S.T.; Zargaham, M.K.; Khan, A.U.; Khan, S.; Hussain, A.; Uddin, J.; Khan, A.; Al-Harrasi, A. Potential therapeutic natural products against Alzheimer’s disease with Reference of Acetylcholinesterase. Biomed. Pharmacother. 2021, 139, 111609. [Google Scholar] [CrossRef]
- Essa, M.M.; Vijayan, R.K.; Castellano-Gonzalez, G.; Memon, M.A.; Braidy, N.; Guillemin, G.J. Neuroprotective effect of natural products against Alzheimer’s disease. Neurochem. Res. 2012, 37, 1829–1842. [Google Scholar] [CrossRef] [PubMed]
- Larrazábal-Fuentes, M.J.; Fernández-Galleguillos, C.; Palma-Ramírez, J.; Romero-Parra, J.; Sepúlveda, K.; Galetovic, A.; González, J.; Paredes, A.; Bórquez, J.; Simirgiotis, M.J.; et al. Chemical Profiling, Antioxidant, Anticholinesterase, and Antiprotozoal Potentials of Artemisia copa Phil. (Asteraceae). Front. Pharmacol. 2020, 11, 594174. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, X.; He, W.H.; Xu, H.G.; Yuan, F.; Gao, Y.X. Investigation into the antioxidant activity and chemical composition of alcoholic extracts from defatted marigold (Tagetes erecta L.) residue. Fitoterapia 2012, 83, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, H.; Chen, H.; Tai, K.; Liu, F.; Gao, Y. In vitro antioxidant, anti-diabetic and antilipemic potentials of quercetagetin extracted from marigold (Tagetes erecta L.) inflorescence residues. J. Food Sci. Technol. 2016, 53, 2614–2624. [Google Scholar] [CrossRef]
- Zgórka, G.; Glowniak, K. Variation of free phenolic acids in medicinal plants belonging to the Lamiaceae family. J. Pharm. Biomed. Anal. 2001, 26, 79–87. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical Scheme for LC-MSn Identification of Chlorogenic Acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef] [PubMed]
- de Souza, L.M.; Cipriani, T.R.; Iacomini, M.; Gorin, P.A.J.; Sassaki, G.L. HPLC/ESI-MS and NMR analysis of flavonoids and tannins in bioactive extract from leaves of Maytenus ilicifolia. J. Pharm. Biomed. Anal. 2008, 47, 59–67. [Google Scholar] [CrossRef]
- Mabry, T.J. The structure analysis of flavonoids by proton nuclear magnetic resonance spectroscopy. Syst. Identif. Flavonoids. 1970, 40, 298. [Google Scholar]
- Chirinos, R.; Huamán, M.; Betalleluz-Pallardel, I.; Pedreschi, R.; Campos, D. Characterisation of phenolic compounds of Inca muña (Clinopodium bolivianum) leaves and the feasibility of their application to improve the oxidative stability of soybean oil during frying. Food Chem. 2011, 128, 711–716. [Google Scholar] [CrossRef]
- Daily, A.; Seligmann, O.; Nonnenmacher, G.; Fessler, B.; Wong, S.M.; Wagner, H. New chromone, coumarin, and coumestan derivatives from Mutisia acuminata var. hirsuta. Planta Med. 1988, 54, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Escarpa, A.; González, M.C. Optimization strategy and validation of one chromatographic method as approach to determine the phenolic compounds from different sources. J. Chromatogr. A 2000, 897, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Grassi-Zampieron, R.; França, L.V.; Carollo, C.A.; do Carmo Vieira, M.; Oliveros-Bastidas, A.; de Siqueira, J.M. Comparative profiles of Achyrocline alata (Kunth) DC. and A. satureioides (Lam.) DC., Asteraceae, applying HPLC-DAD-MS. Rev. Bras. Farmacogn. 2010, 20, 575–579. [Google Scholar] [CrossRef]
- Viturro, C.; Molina, A.; Schmeda-Hirschmann, G. Free radical scavengers from Mutisia fiesiana (Asteraceae) and Sanicula graveolens (Apiaceae). Phyther. Res. 1999, 13, 422–424. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G.; Quispe, C.; González, B. Phenolic profiling of the South American “baylahuen” tea (Haplopappus spp., Asteraceae) by HPLC-DAD-ESI-MS. Molecules 2015, 20, 913–928. [Google Scholar] [CrossRef] [PubMed]
- Aquino, R.; Cáceres, A.; Morelli, S.; Rastrelli, L. An Extract of Tagetes lucida and Its Phenolic Constituents as Antioxidants. J. Nat. Prod. 2002, 65, 1773–1776. [Google Scholar] [CrossRef]
- Tereschuk, M.L.; Riera, M.V.Q.; Castro, G.R.; Abdala, L.R. Antimicrobial activity of flavonoids from leaves of Tagetes minuta. J. Ethnopharmacol. 1997, 56, 227–232. [Google Scholar] [CrossRef]
- Parejo, I.; Bastida, J.; Viladomat, F.; Codina, C. Acylated quercetagetin glycosides with antioxidant activity from Tagetes maxima. Phytochemistry 2005, 66, 2356–2362. [Google Scholar] [CrossRef]
- Shahzadi, I.; Shah, M.M. Acylated flavonol glycosides from Tagetes minuta with antibacterial activity. Front. Pharmacol. 2015, 6, 195. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.W.; Chen, J.; Qi, H.Y.; Shi, Y.P. Phytochemicals and Their Biological Activities of Plants in Tagetes L. Chin. Herb. Med. 2012, 4, 103–117. [Google Scholar] [CrossRef]
- Abdala, L.R. Chemosystematic interpretations of the flavonoids identified in Tagetes gracilis (Asteraceae). Biochem. Syst. Ecol. 2003, 3, 323–325. [Google Scholar] [CrossRef]
- Dos Santos, T.C.; Gomes, T.M.; Pinto, B.A.S.; Camara, A.L.; De Andrade Paes, A.M. Naturally occurring acetylcholinesterase inhibitors and their potential use for Alzheimer’s disease therapy. Front. Pharmacol. 2018, 9, 1192. [Google Scholar] [CrossRef]
- Katalinić, M.; Rusak, G.; Domaćinović Barović, J.; Šinko, G.; Jelić, D.; Antolović, R.; Kovarik, Z. Structural aspects of flavonoids as inhibitors of human butyrylcholinesterase. Eur. J. Med. Chem. 2010, 45, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, S.; Bec, N.; Larroque, C.; Ramírez, J.; Sgorbini, B.; Bicchi, C.; Cumbicus, N.; Gilardoni, G. A Novel Chemical Profile of a Selective In Vitro Cholinergic Essential Oil from Clinopodium taxifolium (Kunth) Govaerts (Lamiaceae), a Native Andean Species of Ecuador. Molecules 2020, 26, 45. [Google Scholar] [CrossRef] [PubMed]
- Matailo, A.; Bec, N.; Calva, J.; Ramírez, J.; Andrade, J.M.; Larroque, C.; Vidari, G.; Armijos, C. Selective BuChE inhibitory activity, chemical composition, and enantiomer content of the volatile oil from the Ecuadorian plant Clinopodium brownei. Rev. Bras. Farmacogn. 2020, 29, 749–754. [Google Scholar] [CrossRef]
- Bektašević, M.; Politeo, O.; Roje, M.; Jurin, M. Polyphenol Composition, Anticholinesterase and Antioxidant Potential of the Extracts of Clinopodium vulgare L. Chem. Biodivers. 2022, 19, e202101002. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Ozer, M.S.; Tepe, B.; Dilek, E.; Ceylan, O. Phenolic composition, antioxidant and enzyme inhibitory activities of acetone, methanol and water extracts of Clinopodium vulgare L. subsp. vulgare L. Ind. Crops Prod. 2015, 76, 961–966. [Google Scholar] [CrossRef]
- Beddiar, H.; Boudiba, S.; Benahmed, M.; Tamfu, A.N.; Ceylan, Ö.; Hanini, K.; Kucukaydin, S.; Elomari, A.; Bensouici, C.; Laouer, H.; et al. Chemical Composition, Anti-Quorum Sensing, Enzyme Inhibitory, and Antioxidant Properties of Phenolic Extracts of Clinopodium nepeta L. Kuntze. Plants 2021, 10, 1955. [Google Scholar] [CrossRef] [PubMed]
- Moliner, C.; Barros, L.; Dias, M.I.; López, V.; Langa, E.; Ferreira, I.C.F.R.; Gómez-Rincón, C. Edible Flowers of Tagetes erecta L. as Functional Ingredients: Phenolic Composition, Antioxidant and Protective Effects on Caenorhabditis elegans. Nutrients 2018, 10, 2002. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, A.; Arumugam, M.; George, S. Combining in silico and in vitro approaches to evaluate the acetylcholinesterase inhibitory profile of some commercially available flavonoids in the management of Alzheimer’s disease. Int. J. Biol. Macromol. 2017, 95, 199–203. [Google Scholar] [CrossRef]
- Khan, M.T.H.; Orhan, I.; Şenol, F.S.; Kartal, M.; Şener, B.; Dvorská, M.; Šmejkal, K.; Šlapetová, T. Cholinesterase inhibitory activities of some flavonoid derivatives and chosen xanthone and their molecular docking studies. Chem. Biol. Interact. 2009, 181, 383–389. [Google Scholar] [CrossRef]
- Bajda, M.; Wiȩckowska, A.; Hebda, M.; Guzior, N.; Sotriffer, C.A.; Malawska, B. Structure-Based Search for New Inhibitors of Cholinesterases. Int. J. Mol. Sci. 2013, 14, 5608. [Google Scholar] [CrossRef]
- Guo, H.B.; He, F.; Gu, B.; Liang, L.; Smith, J.C. Time-dependent density functional theory assessment of UV absorption of benzoic acid derivatives. J. Phys. Chem. A 2012, 116, 11870–11879. [Google Scholar] [CrossRef]
- Lin, L.Z.; Harnly, J.; Zhang, R.W.; Fan, X.E.; Chen, H.J. Quantitation of the Hydroxycinnamic Acid Derivatives and the Glycosides of Flavonols and Flavones by UV Absorbance after Identification by LC-MS. J. Agric. Food Chem. 2012, 60, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Mieres-Castro, D.; Schmeda-Hirschmann, G.; Theoduloz, C.; Gómez-Alonso, S.; Pérez-Navarro, J.; Márquez, K.; Jiménez-Aspee, F. Antioxidant activity and the isolation of polyphenols and new iridoids from Chilean Gaultheria phillyreifolia and G. poeppigii berries. Food Chem. 2019, 291, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.; Jalil, B.; Abdel-Tawab, M.; Echeverria, J.; Kulić, Ž.; McGaw, L.J.; Pezzuto, J.M.; Potterat, O.; Wang, J.-B. Best Practice in the chemical characterisation of extracts used in pharmacological and toxicological research—The ConPhyMP—Guidelines12. Front. Pharmacol. 2022, 13, 953205. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.; Gary, E.N.; Shiomi, K.; Rosenberry, T.L. Structures of human acetylcholinesterase bound to dihydrotanshinone I and territrem B show peripheral site flexibility. ACS Med. Chem. Lett. 2013, 4, 1091–1096. [Google Scholar] [CrossRef]
- Nachon, F.; Carletti, E.; Ronco, C.; Trovaslet, M.; Nicolet, Y.; Jean, L.; Renard, P.Y. Crystal structures of human cholinesterases in complex with huprine W and tacrine: Elements of specificity for anti-Alzheimer’s drugs targeting acetyl- and butyryl-cholinesterase. Biochem. J. 2013, 453, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Madhavi Sastry, G.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided. Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A New Approach for Rapid, Accurate Docking and Scoring. 1. Method and Assessment of Docking Accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef] [PubMed]

| Peak | Rt (min) | λmax (nm) | Compound Identification | C. gilliesii | M. acuminata | T. multiflora |
|---|---|---|---|---|---|---|
| Simple phenolics | ||||||
| 1 | 9.89 | 272, 245sh, 235 | Gallic acid | ND | ND | 6.28 ± 0.17 |
| 3 | 12.65 | 260, 293, 231 | Vanillic acid | 12.37 ± 0.36 | ND | 7.17 ± 0.32 |
| 4 | 17.74 | 257, 233 | p-Hydroxybenzoic acid | 1.43 ± 0.09 | ND | ND |
| 10 | 29.63 | 301, 245, 233 | Salicylic acid | ND | 15.18 ± 0.03 | ND |
| Total content1 | 13.80 ± 0.45 | 66.99 ± 2.29 | 13.45 ± 0.49 | |||
| Hydroxycinnamic acids | ||||||
| 2 | 12.22 | 324, 300sh, 245 | 5-O-Caffeoylquinic acid | ND | 5.92 ± 0.01 | ND |
| 6 | 23.19 | 325, 300sh, 240 | 3-O-Caffeoylquinic acid | 1.14 ± 0.04 | 90.03 ± 0.46 | 2.17 ± 0.01 |
| 8 | 25.91 | 323, 300sh, 265, 240 | trans-Caffeic acid | 1.56 ± 0.10 | 2.74 ± 0.01 | 2.05 ± 0.06 |
| 14 | 43.21 | 323, 300sh, 255, 233 | 1,5-Dicaffeoylquinic acid | ND | 9.86 ± 0.02 | ND |
| 18 | 45.83 | 327, 395sh, 257, 233 | 3,5-Dicaffeoylquinic acid | ND | 8.51 ± 0.01 | ND |
| Total content2 | 4.27 ± 0.35 | 117.07 ± 0.50 | 11.74 ± 0.27 | |||
| Flavan-3-ol monomers and polymers | ||||||
| 5 | 21.67 | 282, 249, 231 | (+)-Catechin | 0.40 ± 0.00 | ND | ND |
| 7 | 24.10 | 279, 249, 230 | B-type Procyanidin dimer-(epi)Catechin-(epi)Catechin | 5.75 ± 1.06 | ND | ND |
| Total content3 | 70.00 ± 4.87 | 0.00 ± 0.00 | 0.00 ± 0.00 | |||
| Flavanones | ||||||
| 11 | 33.11 | 293, 330sh, 250 | Pinocembrin | ND | 1.98 ± 0.01 | ND |
| 19 | 46.86 | 283, 330sh, 250 | Naringin | 1.67 ± 0.01 | ND | ND |
| 26 | 65.07 | 289, 330sh, 250 | Naringenin | 0.09 ± 0.00 | ND | ND |
| Total content4 | 93.08 ± 2.75 | 144.01 ± 1.25 | 0.00 ± 0.00 | |||
| Flavonols | ||||||
| 9 | 29.33 | 348, 265sh, 255, 236 | Luteolin-7-O-glucuronide | 1.33 ± 0.01 | ND | ND |
| 12 | 39.06 | 344, 273, 248 | Myricetin-3-O-glucoside | ND | ND | 1.35 ± 0.00 |
| 13 | 42.43 | 348, 267sh, 245, 236 | Luteolin-7-O-glucoside | 15.72 ± 0.17 | ND | 11.18 ± 0.46 |
| 15 | 43.84 | 353, 258sh, 230 | Quercetin-3-O-glucuronide | ND | 17.80 ± 0.20 | ND |
| 16 | 44.52 | 354, 270sh, 250 | Quercetin-3-O-galactoside | 1.53 ± 0.14 | ND | ND |
| 17 | 45.10 | 354, 263sh, 230 | Quercetin-3-O-rutinoside | ND | 2.49 ± 0.08 | 3.36 ± 0.11 |
| 20 | 49.90 | 354, 268sh, 256 | Quercetin-3-O-glucoside | 1.63 ± 0.07 | 1.05 ± 0.06 | 3.93 ± 0.83 |
| 21 | 51.86 | 353, 257, 237 | Quercetin-3-O-arabinoside | ND | ND | 6.07 ± 1.15 |
| 22 | 56.40 | 364, 263, 231 | Kaempferol-3-O-glucoside | 1.20 ± 0.14 | ND | 8.00 ± 0.89 |
| 23 | 62.30 | 365, 290, 231 | Isorhamnetin-3-O-rutinoside | 0.45 ± 0.00 | 1.21 ± 0.00 | ND |
| 24 | 62.68 | 348, 257, 238 | Quercetin-3-O-rhamnoside | ND | ND | 2.27 ± 0.53 |
| 25 | 64.50 | 365, 268, 250 | Isorhamnetin-3-O-glucoside | 0.10 ± 0.00 | ND | ND |
| 27 | 67.40 | 337, 267, 244 | Apigenin-7-O-glucoside | 1.07 ± 0.05 | ND | ND |
| 28 | 69.80 | 347, 296sh, 267, 256 | Luteolin | 3.33 ± 0.25 | ND | ND |
| 29 | 71.82 | 369, 249, 256 | Quercetin | ND | 1.89 ± 0.19 | 5.29 ± 0.20 |
| 30 | 74.59 | 370, 268, 231 | Isorhamnetin | ND | ND | 8.08 ± 0.15 |
| Total content5 | 48.39 ± 2.25 | 24.43 ± 0.54 | 276.90 ± 7.2 |
| AChE | BChE | |||||
|---|---|---|---|---|---|---|
| Interaction Type | Residues | N a | Distance (Å) | Residues | N a | Distance (Å) |
| H-Bond | Phe295 | 6 (86%) | 1.94 ± 0.11 | Gly115 | 6 (86%) | 1.87 ± 0.27 |
| Asp74 | 4 (57%) | 2.00 ± 0.22 | Tyr128 | 6 (86%) | 2.14 ± 0.21 | |
| Ser293 | 3 (43%) | 2.53 ± 0.31 | His438 | 2 (29%) | 1.82 ± 0.06 | |
| Tyr72 | 2 (29%) | 1.90 ± 0.33 | Ala328 | 2 (29%) | 2.25 ± 0.49 | |
| Gly122 | 2 (29%) | 2.26 ± 0.07 | Tyr332 | 2 (29%) | 2.01 ± 0.21 | |
| Ser203 | 2 (29%) | 1.77 ± 0.01 | Glu197 | 1 (14%) | 2.14 ± 0.00 | |
| His287 | 1 (14%) | 2.13 ± 0.00 | Gly116 | 1 (14%) | 2.19 ± 0.00 | |
| π-stacking | Tyr341 | 4 (57%) | 3.84 ± 0.19 | |||
| Trp286 | 3 (43%) | 4.38 ± 0.88 | ||||
| His447 | 2 (29%) | 4.96 ± 0.02 | ||||
| Phe338 | 2 (29%) | 4.75 ± 0.01 | ||||
| Compounds | Binding Energy(kcal/mol) * | |
|---|---|---|
| AChE | BChE | |
| Luteolin-7-O-glucoside (13) | −13.471 | −12.380 |
| Quercetin-3-O-arabinoside (21) | −11.728 | −9.950 |
| Quercetin (29) | −11.359 | −9.863 |
| Isorhamnetin (30) | −9.980 | −8.742 |
| Kaempferol-3-O-glucoside (22) | −9.949 | −8.973 |
| Gallic acid (1) | −5.467 | −6.085 |
| Vanillic acid (3) | −5.060 | −5.543 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Galleguillos, C.; Jiménez-Aspee, F.; Mieres-Castro, D.; Rodríguez-Núñez, Y.A.; Gutiérrez, M.; Guzmán, L.; Echeverría, J.; Sandoval-Yañez, C.; Forero-Doria, O. Phenolic Profile and Cholinesterase Inhibitory Properties of Three Chilean Altiplano Plants: Clinopodium gilliesii (Benth.) Kuntze [Lamiaceae], Mutisia acuminata Ruiz & Pav. var. hirsuta (Meyen) Cabrera, and Tagetes multiflora (Kunth) [Asteraceae]. Plants 2023, 12, 819. https://doi.org/10.3390/plants12040819
Fernández-Galleguillos C, Jiménez-Aspee F, Mieres-Castro D, Rodríguez-Núñez YA, Gutiérrez M, Guzmán L, Echeverría J, Sandoval-Yañez C, Forero-Doria O. Phenolic Profile and Cholinesterase Inhibitory Properties of Three Chilean Altiplano Plants: Clinopodium gilliesii (Benth.) Kuntze [Lamiaceae], Mutisia acuminata Ruiz & Pav. var. hirsuta (Meyen) Cabrera, and Tagetes multiflora (Kunth) [Asteraceae]. Plants. 2023; 12(4):819. https://doi.org/10.3390/plants12040819
Chicago/Turabian StyleFernández-Galleguillos, Carlos, Felipe Jiménez-Aspee, Daniel Mieres-Castro, Yeray A. Rodríguez-Núñez, Margarita Gutiérrez, Luis Guzmán, Javier Echeverría, Claudia Sandoval-Yañez, and Oscar Forero-Doria. 2023. "Phenolic Profile and Cholinesterase Inhibitory Properties of Three Chilean Altiplano Plants: Clinopodium gilliesii (Benth.) Kuntze [Lamiaceae], Mutisia acuminata Ruiz & Pav. var. hirsuta (Meyen) Cabrera, and Tagetes multiflora (Kunth) [Asteraceae]" Plants 12, no. 4: 819. https://doi.org/10.3390/plants12040819
APA StyleFernández-Galleguillos, C., Jiménez-Aspee, F., Mieres-Castro, D., Rodríguez-Núñez, Y. A., Gutiérrez, M., Guzmán, L., Echeverría, J., Sandoval-Yañez, C., & Forero-Doria, O. (2023). Phenolic Profile and Cholinesterase Inhibitory Properties of Three Chilean Altiplano Plants: Clinopodium gilliesii (Benth.) Kuntze [Lamiaceae], Mutisia acuminata Ruiz & Pav. var. hirsuta (Meyen) Cabrera, and Tagetes multiflora (Kunth) [Asteraceae]. Plants, 12(4), 819. https://doi.org/10.3390/plants12040819









