Abstract
Marine seaweeds synthesize a plethora of bioactive metabolites, of which phlorotannins of brown algae currently attract special attention due to their high antibiotic and cytotoxic capacities. Here we measured the minimum inhibitory concentrations (MICs) of several semi-purified phlorotannin preparations of different origins and molecular composition using a set of model unicellular organisms, such as Escherichia coli, Saccharomyces cerevisiae, Chlamydomonas reinhardtii, etc. For the first time, MIC values were evaluated for phlorotannin-enriched extracts of brown algae of the orders Ectocarpales and Desmarestiales. Phlorotannin extracts of Desmarestia aculeata, Fucus vesiculosus, and Ectocarpus siliculosus showed the lowest MIC values against most of the treated organisms (4–25 μg/mL for bacteria and yeast). Analysis of the survival curves of E. coli showed that massive loss of cells started after 3–4 h of exposure. Microalgae were less susceptible to activity of phlorotannin extracts, with the highest MIC values (≥200 µg/mL) measured for Chlorella vulgaris cells. D. aculeata, E. siliculosus, and three fucalean algae accumulate considerable amounts (4–16% of dry weight) of phlorotannins with MIC values similar to those widely used antibiotics. As these species grow abundantly in polar and temperate seas and have considerable biomass, they may be regarded as promising sources of phlorotannins.
1. Introduction
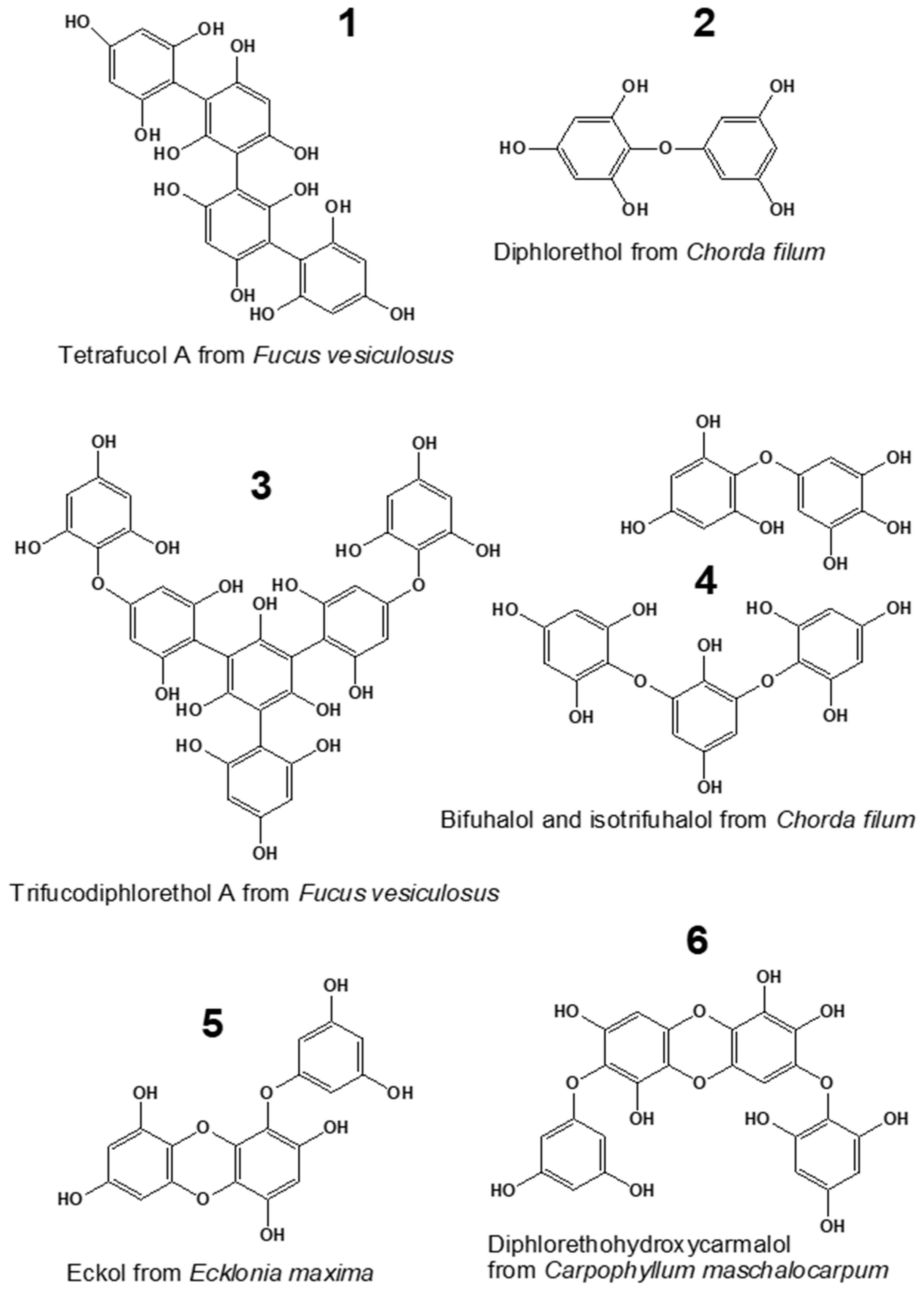
Marine seaweeds are known as rich sources of specific bioactive substances, such as carrageenans, alginates, fucoidans, and phenols [1,2]. Phlorotannins, the unique phenolic compounds produced by brown algae, represent one of the most interesting classes of bioactive algal metabolites. These molecules are oligomers and polymers of phloroglucinol (1,3,5-trihydroxybenzene), widely varying in structure and degree of polymerization (DP). Among these compounds, up to six structural classes can be distinguished, depending on the bond type between the phloroglucinol moieties (aryl-aryl, ether, or combination of aryl and ether bonds) and the presence of additional hydroxyl groups (Figure 1) [3,4]. The molecular profiles of phlorotannins are very complex and species-specific. Considerable differences in polyphenol molecular structure and DP were shown even for closely related species of brown algae [5,6].
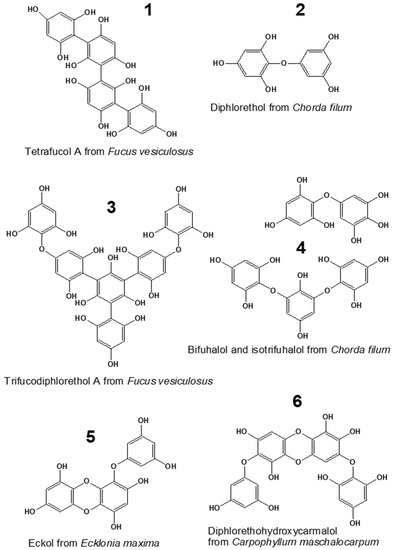
Figure 1.
Compounds representing six major phlorotannin classes: fucols (1), phlorethols (2), fucophlorethols (3), fuhalols (4), eckols (5), and carmalols (6) [3,7,8].
The content of phlorotannins in the cells of brown algae is currently studied in detail only for several species of the orders Laminariales, Fucales, and Dictyotales [9]. All these algae contain considerable amounts of polyphenols, varying from 0.5 to 30% of dry weight (DW), with the highest values reported from fucalean algae such as Fucus, Ascophyllum (Fucaceae), and Carpophyllum (Sargassaceae) [9,10,11]. In addition to interspecies variation, the content of phlorotannins also depends on some environmental and physiological factors, such as developmental stage, season, location, nutrient availability, and activity of biofoulers and grazers [12,13,14,15,16,17,18]. Studies of natural variations of phlorotannin content in brown algae are hampered by the absence of a universal protocol for the extraction of these metabolites. The diversity of established extraction techniques was comprehensively reviewed by Cassani et al. [19]. Mostly, extraction procedures include using some polar organic solvents with low boiling points (acetone, ethanol, methanol, ethyl acetate) and various physical treatments (ultrasound, microwaves, high temperature, high pressure, shaking). One of the most successful and robust protocols by Koivikko et al. [20,21] was employed for this study.
Phlorotannins have both primary and secondary metabolic roles in algal cells [22,23,24]. They are structural constituents of the cell walls of brown algae and contribute to the processes of embryogenesis [25,26]. Moreover, phlorotannins possess high antioxidative activity [27,28,29], scavenge heavy metals [30,31], and participate in wound-healing processes in brown algae [32]. The most pronounced and extensively studied function of phlorotannins is their contribution to the chemical defense of brown seaweeds against diverse deleterious microorganisms, biofoulers, and grazers [13,22,33,34,35,36]. This function is conferred by the considerable toxicity of these phenolic compounds, especially for unicellular organisms. Thus, phlorotannins have antibiotic effects against Gram-negative and Gram-positive bacteria, human pathogenic yeast and other microfungi, and microalgae [36,37,38,39,40]. In antimicrobial tests, several phlorotannin extracts demonstrated minimum inhibitory concentration (MIC) values close to those of widely used natural and synthetic antibiotics; moreover, some polyphenol preparations were effective even against antibiotic-resistant bacterial strains [40,41,42,43,44]. Currently, MIC values are measured precisely only for phlorotannins isolated from several fucalean and laminarialean species (Ascophyllum nodosum, Eisenia bicyclis, Ecklonia cava) [18,41,45]. Unfortunately, a considerable portion of the literature describing antibiotic effects of phlorotannins cannot be used for adequate comparison, as this data is either phenomenological or the authors used crude extracts without measuring phlorotannin content and thus could not attribute the biological effect to the amount of these specific metabolites [46]. Another factor contributing to data inconsistency is the use of incomparable methods (e.g., agar disk diffusion and broth microdilution) and different breakpoints for MIC or minimum bactericidal concentration evaluation [47].
Despite numerous studies of phlorotannin cytotoxicity, our understanding of the underlying mechanisms is still very limited. Traditionally, antibiotic effects of phlorotannins were regarded as the consequence of their ability to precipitate proteins, a general feature of phenolic compounds [48,49]. This implies that phlorotannin toxicity is non-specific. However, a considerable variation was shown for the antibiotic activity of polyphenols having different molecular profiles and isolated from different brown algae. Moreover, even phlorotannin molecules of the same structural class may differ in their biological effects. Thus, phlorofucofuroeckol-A isolated from E. bicyclis is especially toxic for microalgae and methicillin-resistant strains of Staphylococcus aureus [37,41]. At the same time, fucofuroeckol-A of the same origin is more effective against Candida species, streptomycin-resistant Listeria monocytogenes, and acne-related bacteria [43,44,50]. The antibiotic effect of phlorotannins may be not only bactericidal but also bacteriostatic [51]. In addition, a variety of specific responses was reported for eukaryotic unicellular organisms. e.g., phlorotannins inhibit the dimorphic transition in pathogenic yeast and reduce the buoyancy of microalgae [37,39]. Altogether, this data implies that the antibiotic activity of phlorotannins is complex and specific and that investigations on the molecular level are needed to reveal its mechanisms. Currently, practically focused investigations not considering the underlying mechanisms of phlorotannin toxicity are highly predominant among the studies of the biological effects of these metabolites (reviewed in: [52]). As a result, different data is obtained by different methods (often incomparable), mostly on pathogenic microorganisms, which are sometimes still poorly studied from a molecular and genetic perspective. To create a foundation for deeper mechanistic investigations in this field, a more systematic approach is needed. Phlorotannins of different origins should be extracted and quantified by the same protocol and then tested on the model objects of molecular biology and genetics, representing different taxa of unicellular organisms (e.g., Escherichia coli for prokaryotes, Saccharomyces cerevisiae for fungi, Chlamydomonas reinhardtii for microalgae, etc.). The benefits of this approach include: (1) the assessment of specific responses of organisms with different cell structures and surface composition to a variety of phlorotannin extracts from different algal species and (2) the possibility of using well-established, standardized methods on model organisms for further deeper molecular studies and revealing the mechanisms of phlorotannin action.
As the first and crucial step in every toxicological research is usually a precise determination of MIC values, the objective of our study is measuring MICs of several phlorotannin preparations of different origins using a set of model unicellular organisms. In addition, we analyzed the total phenolic content of the easily extractable intracellular fraction of phlorotannins in several brown algae representing different taxonomic groups.
2. Results
2.1. Phlorotannin Content and Chemical Characterization
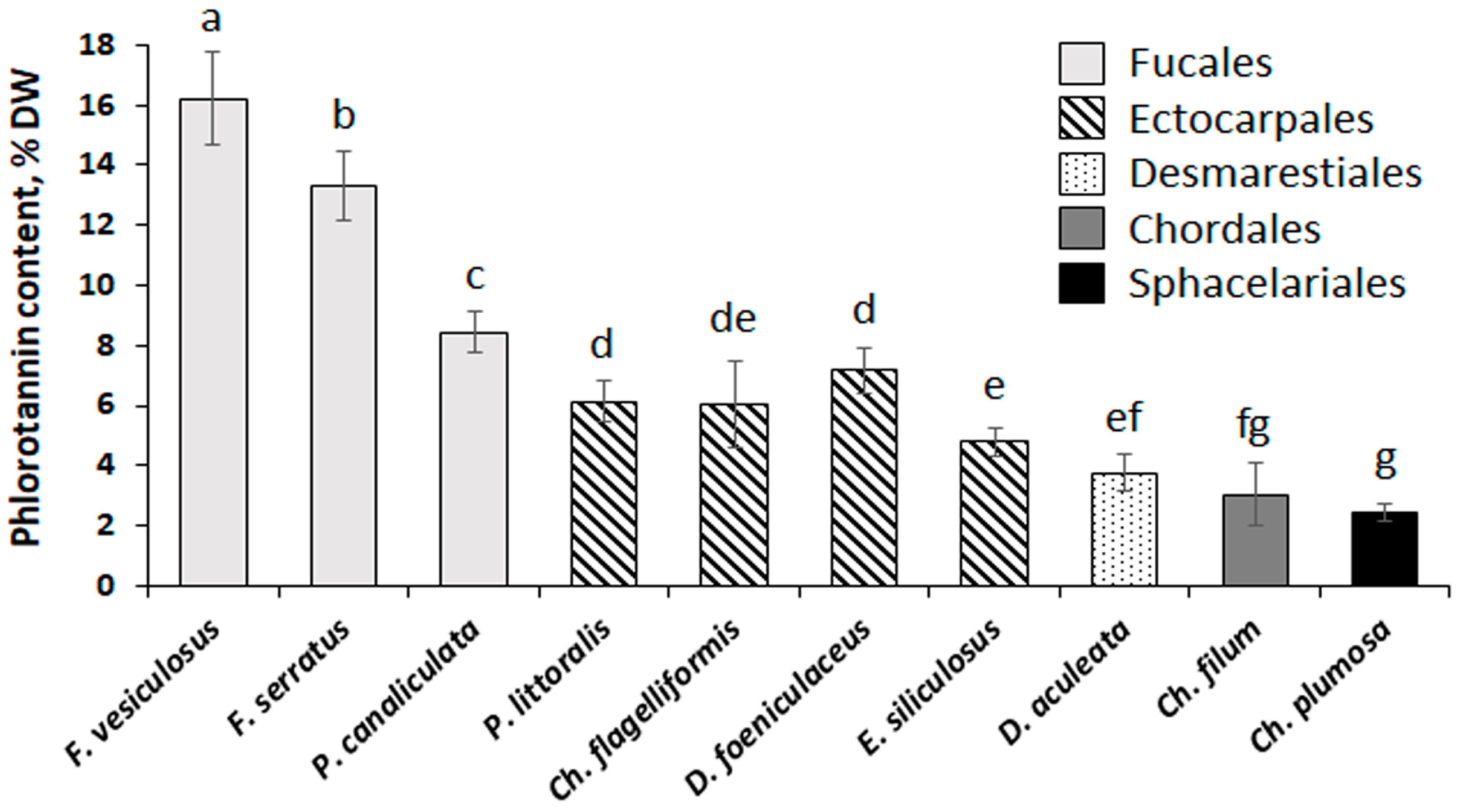
The total content of phlorotannins in the studied brown algae varied from 2.4% DW (Ch. plumosa) to 16.2% DW (F. vesiculosus) (Figure 2). Representatives of the Fucales contained the highest amounts of phenolic substances, ectocarpalean species showed medium phlorotannin content (4.8–7.2% DW), and Desmarestia, Chorda, and Chaetopteris featured the lowest phlorotannin content (2.4–3.8% DW).
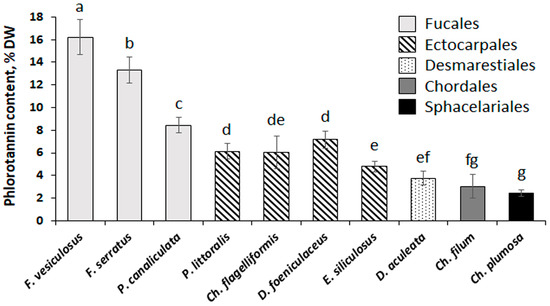
Figure 2.
Content of phlorotannins in the algae representing five orders of Phaeophyceae (Fucales, Ectocarpales, Desmarestiales, Chordales, and Sphacelariales). Bars represent the mean ± SD (n = 6–12). Different letters indicate significant differences (p < 0.05, Student’s t-test).
A comprehensive HPLC-MS analysis of three of the most biologically active extracts (F. vesiculosus, F. serratus, and P. canaliculata) carried out in our previous study [6] confirmed the effectivity of the extraction procedures, demonstrating that phlorotannins are the major constituents of the extracts and revealing specific phlorotannin profiles (Figures S1 and S2).
2.2. Minimum Inhibitory Concentrations of Phlorotannin Preparations
The toxicity of phlorotannin extracts varied considerably depending on both the source of polyphenols and the organism used as the test object (Table 1). Extracts of D. aculeata demonstrated the strongest antibiotic effect against all treated microorganisms, with MIC values ranging from 4 (for S. cerevisiae) to 300 (for Chlorella) µg/mL. Phlorotannins extracted from fucoid algae also had relatively low MIC values (5–20 µg/mL) for bacteria and yeast but were considerably less toxic for microalgae. Polyphenols of the Ectocarpales demonstrated high variation in their toxicity: extract of E. siliculosus had the lowest MIC values, comparable to those of fucoid algae, extracts D. foeniculaceus and Ch. flagelliformis showed moderate antibiotic activity, and extracts of P. littoralis were the least toxic against all the tested microorganisms.

Table 1.
Minimum inhibitory concentrations (MIC, µg/mL) of phloroglucinol and phlorotannin-enriched extracts of ten brown algal species against the unicellular test organisms.
Generally, microalgae were less susceptible to phlorotannins compared to bacteria and yeast (Table 1). Chlorella demonstrated the highest tolerance (all MIC values ≥ 200 µg/mL). The lowest MIC values of most phlorotannin preparations were registered in the experiments with yeast.
Phloroglucinol, the monomer of phlorotannins, at concentrations up to 1 mg/mL, was not toxic to any tested organism (Table 1).
2.3. Antibiotic Activity of Phlorotannin extracts against E. coli
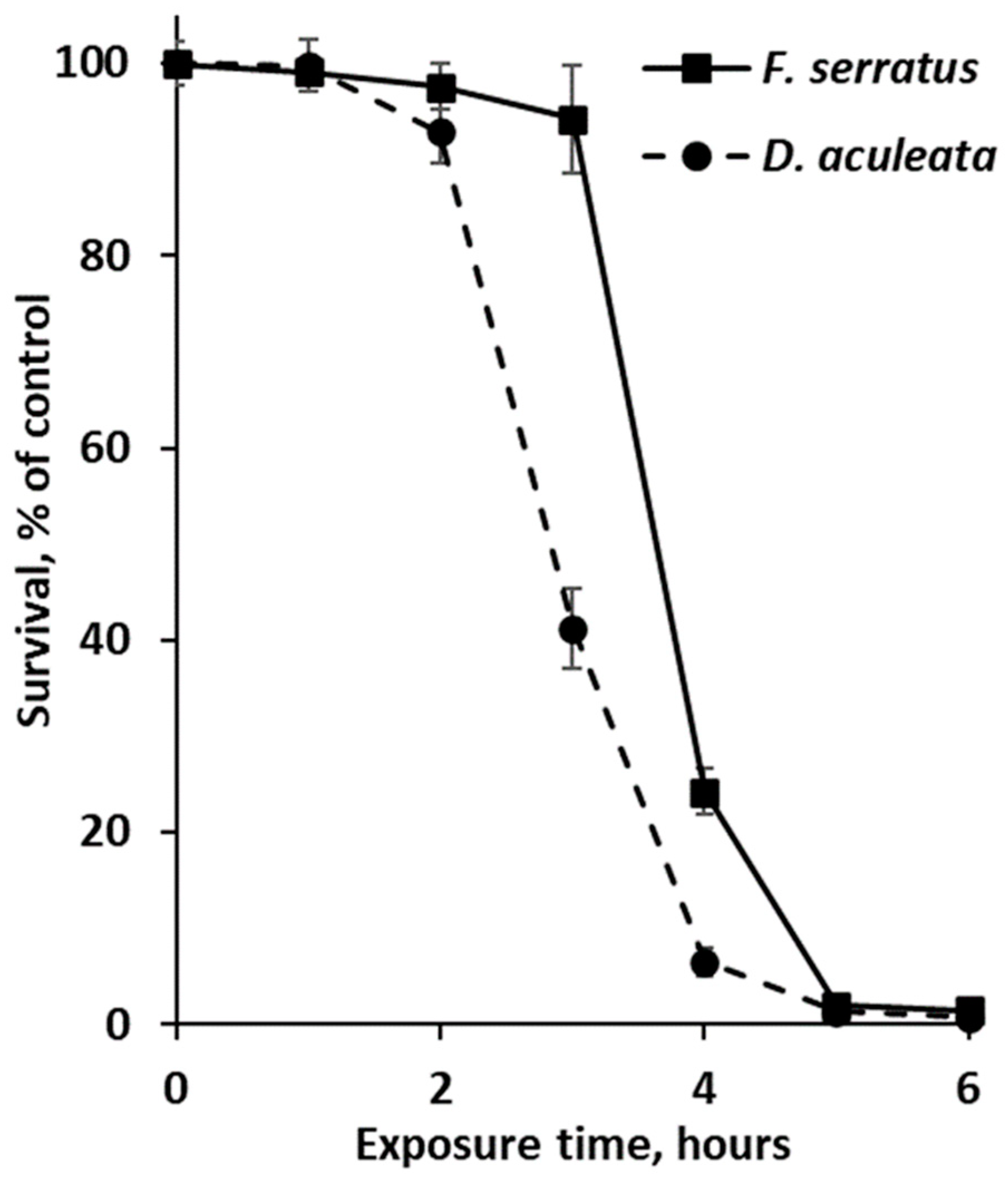
As phlorotannin-enriched extracts of Desmarestia and fucoid algae showed the highest antibiotic activity, these preparations were additionally tested in experiments with non-growing and growing cultures of E. coli. The results of a time-kill assay with non-dividing bacterial cells (incubated in 0.9% NaCl solution) are shown in Figure 3. According to the survival curves, the toxic effect of phlorotannins was manifested after 3 (for D. aculeata) or 4 (for F. serratus) hours of exposure, and more than 98% of E. coli cells died after 5 h-long exposure to any of the phlorotannin preparations (Figure 3).
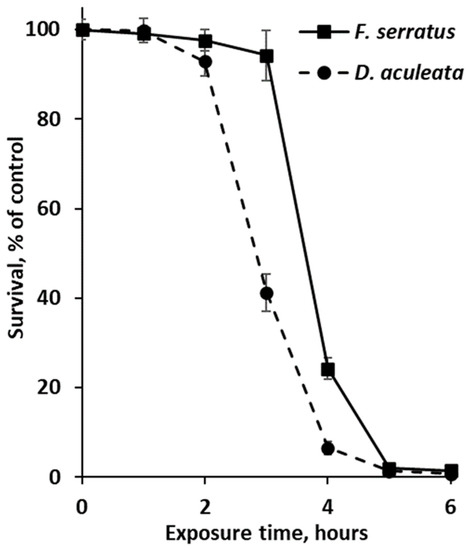
Figure 3.
Survival curves of Escherichia coli cells in 0.9% NaCl solution supplemented with phlorotannin-enriched extracts of Fucus serratus and Desmarestia aculeata at the minimum inhibitory concentrations (20 and 5 μg/mL, respectively). Means are given with ±SD (n = 4).
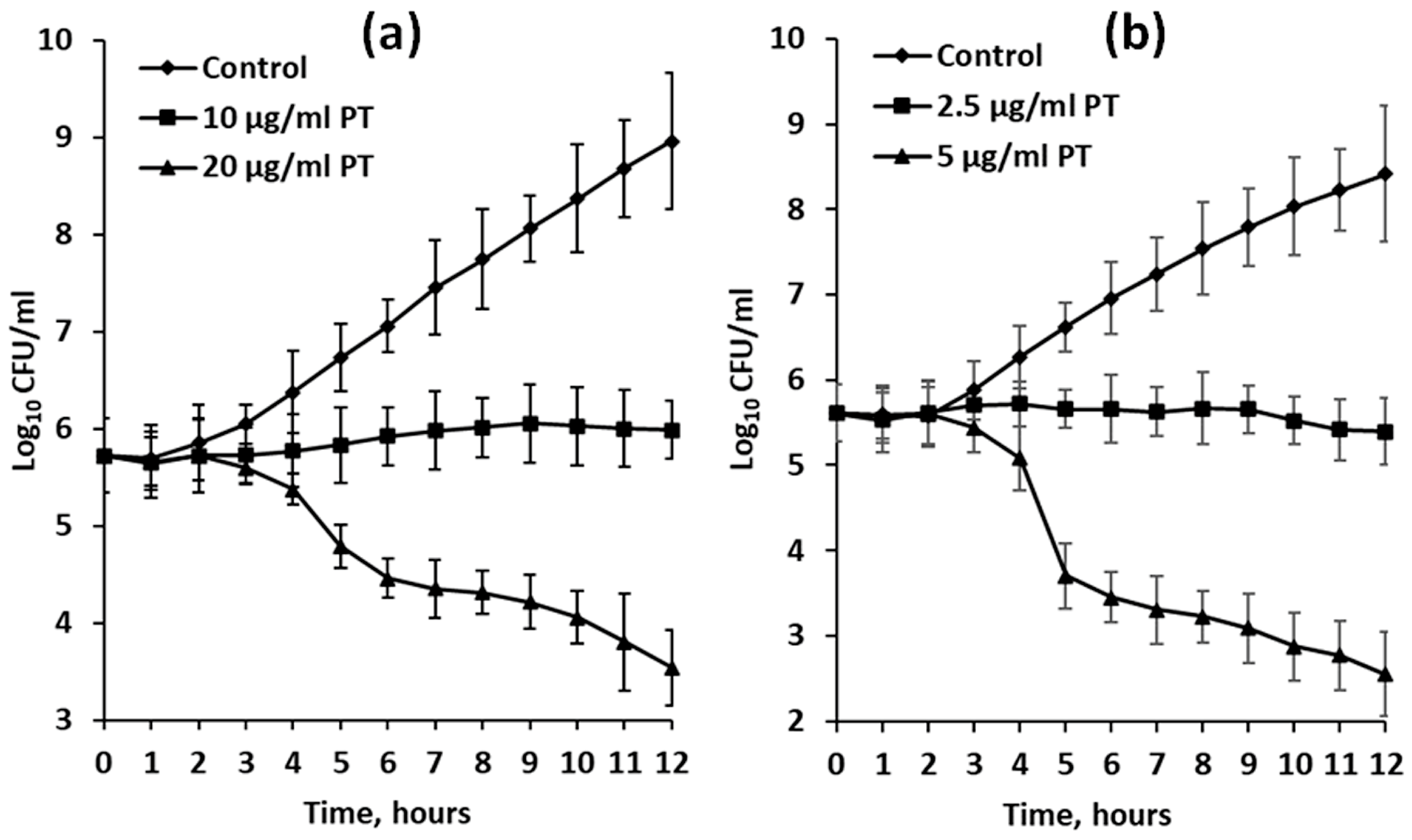
In the experiments with dividing bacterial cells, growth inhibition was visible after 3 h of exposure (Figure 4). When treated with phlorotannin extracts at half-MIC dosage (10 and 2.5 μg/mL for F. serratus and D. aculeata, respectively), cultures of E. coli stopped growing completely for the first 12 h (Figure 4). However, after 24 h of exposure, slight growth was visible (data not shown).
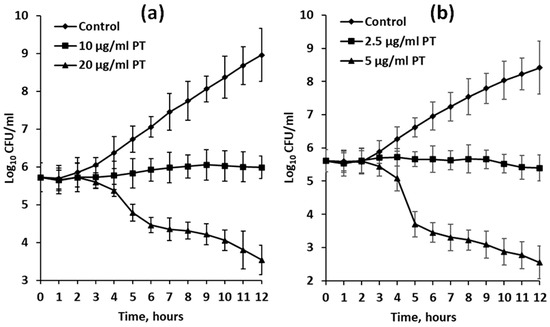
Figure 4.
Growth curves of Escherichia coli cultures in VB medium (control) or VB medium supplemented with phlorotannin-enriched extracts (PT) of Fucus serratus (a) and Desmarestia aculeata (b). Means are given with ±SD (n = 4).
3. Discussion
Our results show that total phenolic content varies considerably in brown algae representing different taxonomic groups (Figure 2). As we measured this parameter in crude extracts, the values of total phenolic content may be used only as a proxy for the total content of phlorotannins in the algal thalli. However, phlorotannins are highly dominant in the phenolic profiles of brown algae, and it was shown that non-phenolic compounds contribute to less than 5% of Folin-Ciocalteu reactive substances [53]. This approach does not lead to a considerable overestimation of phlorotannin content. Relatively high amounts of polyphenols in the fucalean species (8–16% DW) correspond well to the literature data: thalli of Fucus spp., P. canaliculata, and Ascophyllum nodosum were shown to contain from 5 to 13% DW of phlorotannins, depending on location and season [31,33,54,55,56]. Different species of Desmarestia contain from 1.1 to 11.7% DW of phlorotannins [16,57,58,59], which is consistent with our results on D. aculeata (Figure 2). The other orders of brown algae are less studied from this perspective. Several ectocarpalean algae, including genera Pylaiella and Dictyosiphon, were reported to contain 0.23–1.7% DW of phlorotannins [60,61,62,63], which is less than in our study (Figure 2). The only studied representative of the order Sphacelariales (Halopteris scoparia) also contained fewer phlorotannins (0.16% DW) compared to our data on Ch. plumosa (Figure 2) [64]. Such discrepancy may be related to both the interspecies and geographical variation of phlorotannin content in brown algae. To our knowledge, our study is the first one focused on seaweeds inhabiting the Arctic Ocean. In contrast, all other available data on Ectocarpales and Sphacelariales was obtained from either tropical or temperate regions of the Atlantic and Pacific oceans [61,62,64]. Thus, we may suggest that the Arctic species of Ectocarpales and Sphacelariales are characterized by a higher content of phlorotannins. The possible reasons for this phenomenon warrant further investigation. We could not find any reports on phlorotannin content in Chorda or other species of the order Chordales. This small order combining 10 species was recently separated from the order Laminariales [65]. Given the phylogenetic similarity of these two taxonomic groups, we may compare phlorotannin content in Chorda with those in different laminarialean species. Among the Laminariales, the most studied are representatives of the families Laminariaceae containing 0.6–4% DW of phlorotannins, Lessoniaceae with 4–11% DW of phlorotannins, and Alariaceae containing 2–3% DW of phlorotannins [10,66,67]. Thus, we may conclude that phlorotannin content in Ch. filum is close to those of laminarialean algae (Figure 2).
Table 2 summarizes literature data on the toxicity of phlorotannins extracted from fucalean and laminarialean algae against different unicellular organisms. Analysis of this data shows a considerable discrepancy in the reported MIC values: while some studies showed relatively low (16–900 μg/mL) MICs [41,44,45,68], other publications presented values 2–3 orders of magnitude higher [38,39,50]. In the first group of studies, the tested phlorotannin preparations were carefully purified and separated chromatographically. As a result, authors worked with either individual molecules or mixtures containing several low-molecular-weight (DP 4–6) phlorotannins and quantified the biological activity of the extracts on their dry matter basis. This approach allows attributing the observed biological effects to particular phlorotannins. Another appropriate strategy of MIC assessment includes measuring the total phlorotannin content in purified or partially purified extracts without extensive chromatographic fractionation. Such an approach was used also in our study, as we tested semi-purified phlorotannin extracts containing the whole molecular profile. In this case, the biological activities may be attributed to the dominating phlorotannin species of the tested preparations or, possibly, particular active phlorotannin representatives. Though still very limited, literature data on the molecular composition of natural phlorotannins implies that brown algae do not synthesize the whole plethora of phlorotannins in equal quantities but tend to accumulate specific groups of phenolic molecules. Thus, F. vesiculosus, an alga in which phlorotannins demonstrated high antibiotic activities in our study, contains predominantly low-molecular-weight (DP 6–10) fucols and fucophlorethols, as was reported in our previous work [6]. The MIC values measured in our study using partially purified extracts with known total phlorotannin content (Table 1) are in the same range as data obtained using fractionated phlorotannins (Table 2 [41,44,45,68]), thus confirming that both methodical approaches produce comparable results. Meanwhile, we suppose that MIC values as high as 4000–31,300 μg/mL (Table 2 [38,39,50]) cannot be adequately compared to other literature data presented in Table 2 or to our own data (Table 1). In the studies reporting such high MIC values, crude or semi-purified algal extracts were used without measuring their total phlorotannin content. In this case, calculating the biological activity on the dry matter basis (as it was apparently conducted) may not be appropriate, as phlorotannins may contribute to a rather small portion of extract DW. Such methodology will inevitably lead to the overestimation of MICs, and we suppose this is one of the main reasons for the current inconsistency of literature data.

Table 2.
Minimum inhibitory concentration (MIC, μg/mL) of different phlorotannin preparations against bacteria, fungi, and microalgae according to available literature data. Algal species are named according to the current AlgaeBase classification [69].
In general, though the literature describing the biological effects of phlorotannins is abundant, to date, only polyphenols of a limited number of algal species were tested for their MIC against bacteria and fungi (Table 2). Two laminarialean algae of the family Lessoniaceae (E. bicyclis and E. cava) gained particular interest due to the high toxicity of their phlorotannins. The dominating phlorotannin molecules of these algae are low-molecular-weight eckols (Figure 1), and the lowest MIC values against bacteria (16–32 μg/mL) were reported for fucofuroeckol-A from E. bicyclis [43,44]. In our study, phlorotannin-enriched extracts of Chorda filum, the species phylogenetically close to the Laminariales, demonstrated MIC values against Gram-negative bacteria, fungi, and microalgae in the same range as purified phlorotannin preparations of E. bicyclis and E. cava (Table 1 and Table 2). The analysis of the molecular composition of phlorotannins isolated from Ch. filum showed that they comprise low-molecular-weight fuhalols, isofuhalols, and phlorethols, with bifuhalol as the dominant compound (Figure 1) [8]. The other taxonomic group of brown algae accumulating phlorotannins with relatively low MIC values in toxicity tests is the order of Fucales. Among these algae, the lowest MICs against E. coli (25–50 μg/mL) were reported for extracts of A. nodosum [51]. We measured very close MIC values for three other fucalean algae, Fucus vesiculosus, F. serratus, and Pelvetia canaliculata, with extracts of F. vesiculosus being the most toxic (Table 1). In this case, we may attribute the toxic effect to low-molecular-weight fucols and fucophlorethols (Figure 1), which preferably accumulate in the cells of fucalean algae and are especially abundant in F. vesiculosus, according to our previously published data (Figure S1) [6].
Compared to polyphenols of laminarealen and fucalean algae, phlorotannins of the Desmarestiales, Ectocarpales, and Sphacelariales are much less studied. It was shown that phlorotannin extracts of Antarctic desmarestialean species, Desmarestia anceps, and Phaeurus antarcticus, had a relatively weak antibiotic effect against four marine bacterial strains and a stronger effect against several fouling diatom algae [75]. However, the MIC values of these phlorotannin preparations have not been measured, and we cannot directly compare these data to our results. In the present study, extracts of D. aculeata demonstrated the lowest MIC values against most of the tested organisms (Table 1). Unfortunately, the molecular profiles of phlorotannins of the Desmarestiales are still not studied, and thus further investigations are needed to reveal which phlorotannin species might confer such high toxicity.
Toxicological studies of phlorotannins isolated from Ectocarpales are also very scarce. We found only one research study reporting a considerable algicidal activity of the extracts of Adenocystis utricularis (Adenocystaceae) against marine fouling diatoms [75]. Unlike preparations of three fucalean algae having similar MIC values in our study, phlorotannin-enriched extracts of four tested ectocarpalean species demonstrated a wide variation in toxicity, with MIC values differing up to 40 times (Table 1). Ectocarpales is the largest order of brown algae, comprising several subordinate taxa of questionable position [76]. As a result, considerable differences in the biochemical composition may characterize the species belonging to this order. Indeed, the study of McInnes et al. [77] showed that representatives of different families of ectocarpalean algae preferentially synthesize phlorotannins of different classes. Thus, Dictyosiphon foeniculaceus and Chordaria flagelliformis (Chordariaceae) contain polyphenols with ether-linked phloroglucinol units with a high degree of hydroxylation (fuhalols), whereas Pylaiella littoralis (Acinetosporaceae) synthesizes almost exclusively phlorotannins with aryl linkages (fucols). Though the mentioned study was focused on high-molecular-weight polyphenols, the authors noted that all available data indicated a structural similarity of low- and high-molecular-weight phlorotannins of the same origin [77]. Combining this information with our data on the same three species (Table 1), we may suggest that fuhalol-enriched phlorotannin mixtures are generally more toxic compared to fucol-enriched ones, most probably due to the higher proportion of hydroxyl groups, in particular in ortho-position. It was shown that the antibiotic activity of polyphenols of higher plants (hydrolyzable and condensed tannins) depends on the relative content of the ortho-phenolic hydroxyl groups, as these groups mostly confer the affinity to bacterial surface proteins [78,79]. Unfortunately, the composition of phlorotannins of Ectocarpus is still not studied, though given their high antibiotic activity (Table 1), such an investigation would be in high demand.
The phlorotannin-enriched extract of the only representative of the order Sphacelariales used in this study (Chaetopteris plumosa) showed moderate toxicity comparable to that of some ectocarpalean species (D. foeniculaceus, Ch. flagelliformis) (Table 1). This result differs dramatically from data in the study by Lopes et al. [38], who reported MIC values of more than 30 mg/mL for extracts of three other sphacelarialean species (Cladostephus spongiosus, Halopteris filicina, H. scoparia). We may suggest two possible reasons for this discrepancy. First, sphacelarialean algae may differ considerably in both structure and biological activity of their phlorotannins, as shown for the Ectocarpales. Then, data inconsistency may be a result of different methods of MIC calculation, as discussed above.
According to our data, we may select five brown algal species (F. vesiculosus, F. serratus, P. canaliculata, D. aculeata, and E. siliculosus) demonstrating MIC values similar to those of widely used antibiotics, fungicides and algicides. For comparison, MIC of tetracycline, ampicillin, amoxicillin, and ceftiofur is 0.5–128 μg/mL for different isolates and strains of E. coli [80,81], MIC of fluconazole and amphotericin B towards different isolates of S. cerevisiae are 0.25–8 μg/mL [82], and MIC of diuron and atrazine for different species of Chlamydomonas and Chlorella are 2–11 μg/mL [83]. Moreover, even half-MIC dosage of the most active phlorotannin preparations tested in our study, though it did not kill bacterial cells, produced a prolonged bacteriostatic effect (Figure 4). While fucalean algae have already been reported as sources of highly toxic phlorotannins [51], MICs of D. aculeata and E. siliculosus were determined here for the first time.
In studies using semi-purified extracts containing the whole phlorotannin profile, it is desirable to minimize the potential interference of other biologically active compounds. Among the brown algal constituents extractable by organic polar solvents and occurring in the cells at concentrations comparable to those of phlorotannins, are photosynthetic pigments, such as chlorophyll a and fucoxanthin, and storage carbohydrates of low molecular weight, such as mannitol and volemitol. Multi-step washing with dichloromethane provided the elimination of lipids and pigments from our extracts. Cerantola et al. [84] and Audibert et al. [85], using similar protocols of phlorotannin extraction with polar organic solvents, reported that mannitol was still a major contaminant after the successive liquid-liquid separation with ethyl acetate. Pelvetia extracts may also contain some volemitol, a specific carbohydrate of this species [86]. However, both mannitol and volemitol are not toxic and, thus, are not expected to inhibit the growth of microorganisms to the observed extent when applied in concentrations comparable to MIC values measured in our study (Table 1). Moreover, our chromatograms of fucoid algae showed that apart from clear and abundant phlorotannin-related signals, any signal background was at least more than factor ten lower (Figure S2), so it can be reasoned that no other abundant compound could be detected in the used extracts during our earlier LC-MS analyses [6]. Thus, we reasonably suggest that the observed toxic effects of studied algal extracts may be attributed mainly to phlorotannins. However, further studies are underway to characterize the extracts with the most interesting activity in more detail.
To date, information about the mechanisms of phlorotannin toxicity is still very limited. As the relatively large hydrophilic molecules of phlorotannins cannot penetrate the intact cell membrane, the cell surface structures are obviously their first targets, and all available literature data agrees that phlorotannins damage the plasma membrane, thus resembling the biological effects of condensed and hydrolyzable tannins of vascular plants [18,51,87]. It was reported that cells of E. coli treated with phlorotannins had an altered surface structure: electron microphotographs showed surface loosening and disorganization, accompanied by the formation of some electron-dense precipitated deposits. The same effect, though to a lesser extent, was produced by hydrolyzable and condensed tannins of Rhus semialata and Schinopsis balansae [51]. Measurements of electrical conductivity in the suspensions of E. coli, Salmonella agona, Vibrio parahaemolyticus, and Streptococcus suis showed that phlorotannins caused a considerable increase in cell membrane permeability after 2–6 h of exposure [18,74]. This is consistent with our data on survival and growth curves of E. coli exposed to phlorotannins (Figure 3 and Figure 4). The increase in electrical conductivity in the bacterial suspensions is accompanied by leakage of intracellular and periplasmic proteins and a decrease in ATP content, which suggests the inhibition of oxidative phosphorylation, another membrane-associated process [18,74]. The inhibition of oxidative phosphorylation was also reported for the bacteria exposed to the tannins of vascular plants [88]. The responses of eukaryotic cells to phlorotannin treatment also mainly include alterations of surface structures. Phlorotannins of Gongolaria spp. reduced the ergosterol content in the plasma membrane of the yeast Candida albicans and dermatophyte fungus Trichophyton rubrum, thus compromising membrane integrity, and polyphenols of Fucus spiralis reduced the amount of chitin in the cell walls of T. rubrum. Moreover, these phlorotannin preparations inhibited adherence of C. albicans to epithelial cells [39].
Most probably, the first step in the progress of the reported damage to cell surface structures is the precipitation of surface-exposed proteins by phlorotannin oligomers. The ability of phlorotannins to precipitate proteins is higher compared to that of the tannins of terrestrial plants. Due to their susceptibility to spontaneous oxidation, phlorotannins, under natural conditions, cannot only form hydrogen bonds and non-polar interactions with proteins but also precipitate them via covalent bonds [48]. It was shown that the type and strength of phlorotannin-protein interactions considerably depend on the molecular structure of both phlorotannins and target proteins as well as on their proportion [48]. Thus, the most reactive phlorotannin species should be the ether-linked molecules that have ortho-substituted hydroxyl groups, making them prone to oxidation, and the most susceptible cells—those having the highest content of functional proteins exposed to the surface. This is consistent with our data on the MIC values, which were lowest for E. coli and yeast (Table 1). The outer membrane of E. coli consists of about 50% of proteins, playing key roles in the control of membrane permeability and stress resistance [89,90]. Moreover, the outer membrane proteins interact with peptidoglycans, the major constituents of the E. coli cell wall, which also may be targeted for phlorotannins [90]. The cell wall of S. cerevisiae also has a high content of specific proteins (about 15% of the wall constituents). In particular, a set of mannoproteins linked to the cell wall polysaccharides forms the outer layer of the wall, the first structure facing the phlorotannin impact [91,92]. Mannoproteins of S. cerevisiae are also known to interact readily with tannins of vascular plants, and this precipitation reaction is widely exploited in wineries (e.g., [93]). Apparently, the resistance of the chlorococcalean microalga Chlorella vulgaris to phlorotannin treatment (Table 1) is also enabled by the specific features of the surface structure. Cell walls of many representatives of the Chlorococcales, including Chlorella spp., are extremely thick, robust, and chemically resistant [94]. Notably, among the Chlorococcales, the cell walls of Chlorella spp. have the lowest protein content (1.7–4.5%). The major constituents of the walls of Ch. vulgaris are neutral sugars, gluconic acids, and glucosamine [95].
According to the comprehensive study of Stern et al. [48], phlorotannins interact with proteins so fast that precipitation resulting in loss of protein functionality takes no more than several minutes. At the same time, analysis of the time-kill curves shows that bacterial cells exposed to phlorotannin-enriched extracts maintain viability for 2–3 h (Figure 3). This data suggests that though protein precipitation may be a crucial and, obviously, the first step in the phlorotannin action, it is accompanied by some other, more prolonged pathologic processes, presumably triggered by the phlorotannin entry into the cells through the damaged membranes.
4. Materials and Methods
4.1. Algal Material Collection
Brown algae (Fucus vesiculosus L., F. serratus L., Pelvetia canaliculata (L.) Dcne and Thur., Pylaiella littoralis (L.) Kjellm., Chordaria flagelliformis (O. F. Müll.) C. Ag., Dictyosiphon foeniculaceus (Huds.) Grev., Ectocarpus siliculosus (Dillw.) Lyngb., Desmarestia aculeata (L.) J. V. Lamour., Chorda filum (L.) Stackh., and Chaetopteris plumosa (Lyngb.) Kütz.) were collected in the Keret Archipelago (Kandalaksha Bay, White Sea; 66°17′28.76″ N 33°40′03.46″ E) in July–August 2020–2022. All algal species are named according to AlgaeBase [67]. Mature thalli were collected from the typical habitats of each species, cleaned from the epiphytes, rinsed with distilled water, carefully wiped with filter paper, and then immediately used for phlorotannin extraction or frozen and kept at –80 °C.
4.2. Phlorotannin Extraction
Phlorotannin extraction was carried out based on the standard protocol of Koivikko et al. [20,21]. For analysis of the total content of intracellular phlorotannins in the algal thalli, 20 mg of fresh algal material was poured with acetone:water (70:30, v/v) mixture, ground with mortar and pestle and left soaking in 1 mL aqueous acetone for one hour to extract intracellular phenolics. Then, the extract was centrifuged (5000× g, 10 min), the supernatant was transferred to another tube, and the pellet was re-extracted with another 1 mL of aqueous acetone. The supernatants of five extraction rounds were combined.
For toxicity tests, concentrated and semi-purified extracts were used. Samples of 1–2 g frozen algal material were homogenized using a cryogenic laboratory mill Freezer/Mill 6870 (SPEX SamplePrep, Metuchen, NJ, USA, Germany), transferred to the falcon tubes, poured with 10 mL of acetone:water (70:30, v/v) and left soaking for one hour. Then, each extract was centrifuged (5000× g, 10 min), the supernatant was transferred to another tube, and the pellet was re-extracted with another 10 mL of aqueous acetone. The supernatants of five extraction rounds were combined, and acetone was evaporated in a speed vac (CentriVap vacuum concentrator system, Labconco, Kansas City, MO, USA). Then the extracts were defatted three times, partitioning against dichloromethane (1:1, v/v), and phlorotannins were extracted by five successive portions of ethyl acetate (1:1, v/v). Ethyl acetate extracts were dried and resuspended in 1 mL water.
A comprehensive HPLC-MS analysis of three exemplary extracts (F. vesiculosus, F. serratus, and P. canaliculata) was carried out in our previous study [6], where we confirmed that phlorotannins are the principal constituents of the extracts and showed their specific molecular profiles. Koivikko et al. used a similar protocol of phlorotannin extraction and did not detect any major constituents besides phlorotannins by a combination of HPLC with UV- and MS-detection and NMR-analysis [21]. Improving chemical analysis to further and better characterize the phlorotannin profile of more species is an ongoing objective in our current research.
4.3. Analysis of Phlorotannin Content
A modification of the Folin–Ciocalteu micro-method was used to measure the total phlorotannin content in the crude and purified extracts [96]. Phloroglucinol (Sigma-Aldrich 79330) was used as the standard. The reaction mixture containing 0.3 mL of sample, 0.3 mL of Folin reagent, and 2.4 mL of 5% (w/v) aqueous Na2CO3 was incubated for 20 min at 45 °C, and then the absorbance was measured at 750 nm using a SPEKOL 1300 spectrophotometer (Analytik Jena AG, Jena, Germany).
4.4. Test Organisms and Growth Media
The following unicellular organisms were used for the bioassays: Gram-negative bacteria Escherichia coli strain KA796 (ara thi D(pro-lac)) [97], ascomycete yeast Saccharomyces cerevisiae haploid strain LAN201-ura3Δ (MATa ade5-1 ura3Δ lys2-Tn5-13 trp1-289 his7-2 leu2-3,112) [98], euglenoid protist Euglena gracilis Klebs strain Z, and two green microalgae, Chlamydomonas reinhardtii P. A. Dang. strain CC-124 and Chlorella vulgaris Beijer. strain Pringsheim. E. coli, S. cerevisiae, and Ch. reinhardtii were obtained from the collection of the Department of Genetics and Biotechnology, St. Petersburg State University. E. gracilis and Ch. vulgaris were obtained from the Resource Centre “Culture Collection of Microorganisms” of St. Petersburg State University.
E. coli was cultured in a complete LB medium [99] or minimal Vogel-Bonner medium containing proline (25 mg/L) [100]. S. cerevisiae was grown in complete YPD medium or minimal SD media (Yeast Nitrogen Base 6.7 g/L; glucose 2%) containing adenine (20 mg/L), uracil (20 mg/L), lysine (30 mg/L), tryptophan (20 mg/L), histidine (20 mg/L), and leucine (60 mg/L) [101]. All treatments with phlorotannin extracts were carried out in minimal media. Complete media were used to obtain stock cultures of microorganisms and for growing bacteria and yeast after phlorotannin exposure. Euglena, Chlamydomonas, and Chlorella were grown phototrophycally under continuous light (50 μM photons/m2c) in the mineral Cramer-Myers, TAP, and BBM media, respectively [102,103,104].
4.5. Measurement of Minimum Inhibitory Concentrations
MIC values were determined as the lowest concentrations of tested phlorotannin extracts, completely inhibiting the growth of the microorganism cultures. In addition to phlorotannins isolated from brown algae, an aqueous solution of phloroglucinol was also tested. The broth dilution method was used for the MIC assays [105]. First, the stock solutions of phlorotannin extracts were made in the corresponding growth media, and serial 2-fold dilutions of the stock were prepared in 96-well microtiter plates (100 μL per well). Then, 100 μL aliquots of cell suspensions containing approximately 104 cells/mL of bacteria and yeast, and 105 cells/mL of microalgae, respectively, were inoculated into each well. The final concentration of phlorotannin extracts/phloroglucinol ranged from 1 to 1000 μg/mL; pure growth medium was used as a negative control. For E. coli and S. cerevisiae, the MIC values were visually determined after 24 or 48 h of incubation, respectively. For microalgae, the MIC values were estimated after 4 days of incubation by counting the cells in a hemocytometer.
4.6. Antibiotic Activity Assay
Two variants of bioassays were carried out for a more detailed study of the antibiotic effect of phlorotannins extracted from F. serratus and D. aculeata against E. coli.
Bactericidal activity was tested using a time-kill assay for non-dividing E. coli cells. An overnight culture of E. coli was diluted 4 × 105 times with 0.9% NaCl solution supplemented with phlorotannin-enriched extracts at the minimum inhibitory concentrations (20 μg/mL for F. serratus extract and 5 μg/mL for D. aculeata extract). Pure NaCl solution was used as a negative control. Then, the cell suspensions were incubated at 37 °C for 6 h, and 50 μL aliquots were plated on LB media every hour. The plates were incubated overnight at 37 °C, and the viable colony-forming units (CFU) were counted.
To test both the bacteriostatic and bactericidal activity of phlorotannins for the growing microbial culture, the growth curves of E. coli were analyzed. Overnight culture of E. coli was diluted 4 × 105 times with fresh media supplemented with phlorotannins at half-MIC and MIC (10–20 μg/mL for F. serratus extract and 2.5–5 μg/mL for D. aculeata extract). A pure growth medium was used as a negative control. Then, cell suspensions were incubated at 37 °C for 12 h, and 100 μL aliquots (diluted to contain ~400 CFU) were plated on LB media every hour. The plates were incubated overnight at 37 °C, and the number of viable CFU was counted.
4.7. Data Analysis
Measurements were performed with four (survival and growth curves) to twelve (total phenolic content) biological replicates. An individual seaweed organism was used to obtain each analyzed phlorotannin sample. Excel 2016 (Microsoft, Redmond, WA, USA), Photoshop CS2 (Adobe Inc., San Jose, CA, USA), and ChemDraw Ultra (CambridgeSoft, Cambridge, MA, USA) software was used for data processing and creating of figures. Where appropriate, data were tested for normality using the Kolmogorov–Smirnov test and for homogeneity of variance using the Levene test. Student’s t-test was used to confirm the significant differences between the means. All values are expressed as means and standard deviations.
5. Conclusions
In our study, we investigated MICs of several partially purified phlorotannin preparations of different origins using a set of unicellular model organisms: E. coli, S. cerevisiae, Ch. reinhardtii, E. gracilis, and Ch. vulgaris. For the first time, MIC values were evaluated for phlorotannin-enriched extracts of the brown algae of the orders Ectocarpales and Desmarestiales. Five of the tested algal extracts (those of D. aculeata, E. siliculosus, and three fucalean algae) demonstrated high toxicity with MIC values similar to those of widely used antibiotics. As all these species grow abundantly in polar and temperate seas and have considerable biomass and relatively high phlorotannin content, they may be regarded as promising sources of these valuable metabolites. Using common model organisms of molecular biology and genetics for the toxicological assays established the basis required for further mechanistic investigations of phlorotannin bioactivity. Certainly, one of the future imperatives of phlorotannin research should also be the production and availability of single phlorotannin standards for more systematic investigations of structure-activity relationships (SAR).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12040821/s1, Figure S1: Relative contribution of eight major types of phlorotannin molecules to the total pool of phlorotannins in the brown algae Fucus vesiculosus, F. serratus, and Pelvetia canaliculata; Figure S2: HPLC-MS analysis of the phlorotannin-enriched extract of brown alga Fucus vesiculosus. Reference [6] is cited in the supplementary materials.
Author Contributions
Conceptualization, E.T.; Data curation, C.B. and E.T.; Formal analysis, E.S. and E.T.; Funding acquisition, E.T.; Investigation, V.L., R.I., E.S., A.S. and E.T.; Methodology, E.S., C.B. and E.T.; Project administration, E.T.; Resources, E.S., A.S. and E.T.; Supervision, C.B. and E.T.; Validation, C.B. and E.T.; Visualization, V.L., R.I., C.B. and E.T.; Writing—original draft, V.L., E.S. and E.T.; Writing—review and editing, C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation (grant N° 22-24-20039, https://rscf.ru/en/project/22-24-20039/ (accessed on 11 February 2023)) and St. Petersburg Science Foundation (grant N° 35/2022 from 14.04.2022).
Data Availability Statement
The datasets generated and/or analyzed in this study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank the Marine Biological Station “UNB Belomorskaya” and the Research Park of St. Petersburg State University for providing facilities. We also thank Elena Chekunova for providing Chlamydomonas culture and Sofia Ilinykh for technical assistance. All LC-MS analyses were performed in the mass spectrometry core facility at the faculty of chemistry and mineralogy at Leipzig University, MS-UL; the authors thank Susan Billig for technical support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K. Antimicrobial bioactive compounds from marine algae: A mini review. Ind. J. Geo-Mar. Sci. 2016, 45, 1076–1085. [Google Scholar]
- Martinez, J.H.; Castaneda, H.G. Preparation and chromatographic analysis of phlorotannins. J. Chromatogr. Sci. 2013, 51, 825–838. [Google Scholar] [CrossRef]
- Shrestha, S.; Zhang, W.; Smid, S.D. Phlorotannins: A review on biosynthesis, chemistry and bioactivity. Food Biosci. 2021, 39, 100832. [Google Scholar] [CrossRef]
- Steevensz, A.J.; Mackinnon, S.L.; Hankinson, R.; Craft, C.; Connan, S.; Stengel, D.B.; Melanson, J.E. Profiling phlorotannins in brown macroalgae by liquid chromatography-high resolution mass spectrometry. Phytochem. Anal. 2012, 23, 547–553. [Google Scholar] [CrossRef]
- Birkemeyer, C.; Lemesheva, V.; Billig, S.; Tarakhovskaya, E. Composition of intracellular and cell wall-bound phlorotannin fractions in fucoid algae indicates specific functions of these metabolites dependent on the chemical structure. Metabolites 2020, 10, 369. [Google Scholar] [CrossRef] [PubMed]
- Glombitza, K.W.; Rauwald, H.W.; Eckhardt, G. Fucole, Polyhydroxyoligophenyle aus Fucus vesiculosus. Phytochemistry 1975, 14, 1403–1405. [Google Scholar] [CrossRef]
- Grosse-Damhues, J.; Glombitza, K.W. Isofuhalols, a type of phlorotannin from the brown alga Chorda filum. Phytochemistry 1984, 23, 2639–2642. [Google Scholar] [CrossRef]
- Generalić Mekinić, I.; Skroza, D.; Šimat, V.; Hamed, I.; Čagalj, M.; Popović Perković, Z. Phenolic content of brown algae (Pheophyceae) species: Extraction, identification, and quantification. Biomolecules 2019, 9, 244. [Google Scholar] [CrossRef]
- Steinberg, P.D. Biogeographical variation in brown algal polyphenolics and other secondary metabolites: Comparison between temperate Australasia and North America. Oecologia 1989, 78, 373–382. [Google Scholar] [CrossRef]
- Van Alstyne, K.L.; Paul, V.J. The biogeography of polyphenolic compounds in marine macroalgae: Temperate brown algal defenses deter feeding by tropical herbivorous fishes. Oecologia 1990, 84, 158–163. [Google Scholar] [CrossRef]
- Targett, N.M.; Arnold, T.M. Predicting the effects of brown algal phlorotannins on marine herbivores in tropical and temperate oceans. J. Phycol. 1998, 34, 195–205. [Google Scholar] [CrossRef]
- Pavia, H.; Toth, G.B. Inducible chemical resistance to herbivory in the brown seaweed Ascophyllum nodosum. Ecology 2000, 81, 3212–3225. [Google Scholar] [CrossRef]
- Van Alstyne, K.L.; Pelletreau, K.N. Effects of nutrient enrichment on growth and phlorotannin production in Fucus gardneri embryos. Mar. Ecol. Prog. Ser. 2000, 206, 33–43. [Google Scholar] [CrossRef]
- Connan, S.; Delisle, F.; Deslandes, E.; Ar Gall, E. Intra-thallus phlorotannin content and antioxidant activity in Phaeophyceae of temperate waters. Bot. Mar. 2006, 49, 39–46. [Google Scholar] [CrossRef]
- Iken, K.; Amsler, C.D.; Hubbard, J.M.; McClintock, J.B.; Baker, B.J. Allocation patterns of phlorotannins in Antarctic brown algae. Phycologia 2007, 46, 386–395. [Google Scholar] [CrossRef]
- Kamiya, M.; Nishio, T.; Yokoyama, A.; Yatsuya, K.; Nishigaki, T.; Yoshikawa, S.; Ohki, K. Seasonal variation of phlorotannin in sargassacean species from the coast of the Sea of Japan. Phycol. Res. 2010, 58, 53–61. [Google Scholar] [CrossRef]
- Ford, L.; Stratakos, A.C.; Theodoridou, K.; Dick, J.T.; Sheldrake, G.N.; Linton, M.; Corcionivoschi, N.; Walsh, P.J. Polyphenols from brown seaweeds as a potential antimicrobial agent in animal feeds. ACS Omega 2020, 5, 9093–9103. [Google Scholar] [CrossRef] [PubMed]
- Cassani, L.; Gomez-Zavaglia, A.; Jimenez-Lopez, C.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Seaweed-based natural ingredients: Stability of phlorotannins during extraction, storage, passage through the gastrointestinal tract and potential incorporation into functional foods. Food Res. Int. 2020, 137, 109676. [Google Scholar] [CrossRef]
- Koivikko, R.; Loponen, J.; Honkanen, T.; Jormalainen, V. Contents of cytoplasmic, cell-wall-bound and exudes phlorotannins in the brown alga Fucus vesiculosus, with implications on their ecological functions. J. Chem. Ecol. 2005, 31, 195–209. [Google Scholar] [CrossRef]
- Koivikko, R.; Loponen, J.; Pihlaja, K.; Jormalainen, V. High-performance liquid chromatographic analysis of phlorotannins from the brown alga Fucus vesiculosus. Phytochem. Anal. 2007, 18, 326–332. [Google Scholar] [CrossRef]
- Amsler, C.D.; Fairhead, V.A. Defensive and sensory chemical ecology of brown algae. Adv. Bot. Res. 2006, 43, 1–91. [Google Scholar] [CrossRef]
- Lemesheva, V.; Tarakhovskaya, E. Physiological functions of phlorotannins. Biol. Comm. 2018, 63, 70–76. [Google Scholar] [CrossRef]
- Creis, E.; Ar Gall, E.; Potin, P. Ubiquitous phlorotannins prospects and perspectives. In Blue Biotechnology: Production and Use of Marine Molecules, 1st ed.; La Barre, S., Bates, S.S., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018; pp. 67–116. [Google Scholar] [CrossRef]
- Deniaud-Bouët, E.; Hardouin, K.; Potin, P.; Kloareg, B.; Hervé, C. A review about brown algal cell walls and fucose-containing sulfated polysaccharides: Cell wall context, biomedical properties and key research challenges. Carbohydr. Polym. 2017, 175, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Lemesheva, V.; Birkemeyer, C.; Garbary, D.; Tarakhovskaya, E. Vanadium-dependent haloperoxidase activity and phlorotannin incorporation into the cell wall during early embryogenesis of Fucus vesiculosus (Phaeophyceae). Eur. J. Phycol. 2020, 55, 275–284. [Google Scholar] [CrossRef]
- Ferreres, F.; Lopes, G.; Gil-Izquierdo, A.; Andrade, P.; Sousa, C.; Mouga, T.; Valentão, P. Phlorotannin extracts from Fucales characterized by HPLC-DAD-ESI-MSn: Approaches to hyaluronidase inhibitory capacity and antioxidant properties. Mar. Drugs 2012, 10, 2766–2781. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, N.; Smyth, T.J.; Soler-Villa, A.; Fitzgerald, R.J.; Brunton, N.P. Phenolic content and antioxidant activity of fractions obtained from selected Irish macroalgae species (Laminaria digitata, Fucus serratus, Gracilaria gracilis and Codium fragile). J. Appl. Phycol. 2015, 27, 519–530. [Google Scholar] [CrossRef]
- Hermund, D.B.; Plaza, M.; Turner, C.; Jónsdóttir, R.; Kristinsson, H.G.; Jacobsen, C.; Nielsen, K.F. Structure dependent antioxidant capacity of phlorotannins from Icelandic Fucus vesiculosus by UHPLC-DAD-ECD-QTOFMS. Food Chem. 2018, 240, 904–909. [Google Scholar] [CrossRef]
- Toth, G.; Pavia, H. Lack of phlorotannin induction in the brown seaweed Ascophyllum nodosum in response to increased copper concentrations. Mar. Ecol. Prog. Ser. 2000, 192, 119–126. [Google Scholar] [CrossRef]
- Connan, S.; Stengel, D.B. Impacts of ambient salinity and copper on brown algae: 2. Interactive effects on phenolic pool and assessment of metal binding capacity of phlorotannin. Aquat. Toxicol. 2011, 104, 1–13. [Google Scholar] [CrossRef]
- Lüder, U.H.; Clayton, M.N. Induction of phlorotannins in the brown macroalga Ecklonia radiata (Laminariales, Phaeophyta) in response to simulated herbivory—The first microscopic study. Planta 2004, 218, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Wikström, S.A.; Pavia, H. Chemical settlement inhibition versus post-settlement mortality as an explanation for differential fouling of two congeneric seaweeds. Oecologia 2004, 138, 223–230. [Google Scholar] [CrossRef]
- Kubanek, J.; Lester, S.E.; Fenical, W.; Hay, M.E. Ambiguous role of phlorotannins as chemical defenses in the brown alga Fucus vesiculosus. Mar. Ecol. Prog. Ser. 2004, 277, 79–93. [Google Scholar] [CrossRef]
- Honkanen, T.; Jormalainen, V. Genotypic variation in tolerance and resistance to fouling in the brown alga Fucus vesiculosus. Oecologia 2005, 144, 196–205. [Google Scholar] [CrossRef]
- Negara, B.F.S.P.; Sohn, J.H.; Kim, J.S.; Choi, J.S. Antifungal and larvicidal activities of phlorotannins from brown seaweeds. Mar. Drugs 2021, 19, 223. [Google Scholar] [CrossRef]
- Nagayama, K.; Shibata, T.; Fujimoto, K.; Honjo, T.; Nakamura, T. Algicidal effect of phlorotannins from the brown alga Ecklonia kurome on red tide microalgae. Aquaculture 2003, 218, 601–611. [Google Scholar] [CrossRef]
- Lopes, G.; Sousa, C.; Silva, L.R.; Pinto, E.; Andrade, P.B.; Bernardo, J.; Mouga, T.; Valentão, P. Can phlorotannins purified extracts constitute a novel pharmacological alternative for microbial infections with associated inflammatory conditions? PLoS ONE 2012, 7, e31145. [Google Scholar] [CrossRef]
- Lopes, G.; Pinto, E.; Andrade, P.B.; Valentaõ, P. Antifungal activity of phlorotannins against dermatophytes and yeasts: Approaches to the mechanism of action and influence on Candida albicans virulence factor. PLoS ONE 2013, 8, e72203. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.H.; Kim, Y.M.; Kim, S.K. Antimicrobial effect of phlorotannins from marine brown algae. Food Chem. Toxicol. 2012, 50, 3251–3255. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.H.; Kim, D.H.; Lee, S.H.; Yoon, N.Y.; Kim, J.H.; Kim, T.H.; Chung, Y.H.; Kim, S.B.; Kim, Y.M.; Kim, H.W.; et al. In vitro antibacterial activity and synergistic antibiotic effects of phlorotannins isolated from Eisenia bicyclis against methicillin-resistant Staphylococcus aureus. Phytother. Res. 2013, 27, 1260–1264. [Google Scholar] [CrossRef]
- Eom, S.H.; Kang, M.S.; Kim, Y.M. Antibacterial activity of the Phaeophyta Ecklonia stolonifera on methicillin-resistant Staphylococcus aureus. Fish. Aquatic Sci. 2008, 11, 1–6. [Google Scholar] [CrossRef][Green Version]
- Lee, J.H.; Eom, S.H.; Lee, E.H.; Jung, Y.J.; Kim, H.J.; Jo, M.R.; Son, K.T.; Lee, H.J.; Kim, J.H.; Lee, M.S. In vitro antibacterial and synergistic effect of phlorotannins isolated from edible brown seaweed Eisenia bicyclis against acne-related bacteria. Algae 2014, 29, 47–55. [Google Scholar] [CrossRef]
- Kim, H.J.; Dasagrandhi, C.; Kim, S.H.; Kim, B.G.; Eom, S.H.; Kim, Y.M. In vitro antibacterial activity of phlorotannins from edible brown algae, Eisenia bicyclis against streptomycin-resistant Listeria monocytogenes. Ind. J. Microbiol. 2018, 58, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, K.; Iwamura, Y.; Shibata, T.; Hirayama, I.; Nakamura, T. Bactericidal activity of phlorotannins from the brown alga Ecklonia kurome. J. Antimicrob. Chemother. 2002, 50, 889–893. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Antibacterial derivatives of marine algae: An overview of pharmacological mechanisms and applications. Mar. Drugs 2016, 14, 81. [Google Scholar] [CrossRef]
- Tan, J.B.L.; Lim, Y.Y. Critical analysis of current methods for assessing the in vitro antioxidant and antibacterial activity of plant extracts. Food Chem. 2015, 172, 814–822. [Google Scholar] [CrossRef]
- Stern, J.; Hagerman, A.; Steinberg, P.; Mason, P. Phlorotannin–protein interactions. J. Chem. Ecol. 1996, 22, 1877–1899. [Google Scholar] [CrossRef]
- Pizzi, A. Tannins medical/pharmacological and related applications: A critical review. Sustain. Chem. Pharm. 2021, 22, 100481. [Google Scholar] [CrossRef]
- Kim, K.H.; Eom, S.H.; Kim, H.J.; Lee, D.S.; Nshimiyumukiza, O.; Kim, D.; Kim, Y.M.; Lee, M.S. Antifungal and synergistic effects of an ethyl acetate extract of the edible brown seaweed Eisenia bicyclis against Candida species. Fish. Aquat. Sci. 2014, 17, 209–214. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Z.; Bach, S.J.; McAllister, T.A. Sensitivity of Escherichia coli to seaweed (Ascophyllum nodosum) phlorotannins and terrestrial tannins. Asian-Australas. J. Anim. Sci. 2009, 22, 238–245. [Google Scholar] [CrossRef]
- Pradhan, B.; Nayak, R.; Bhuyan, P.P.; Patra, S.; Behera, C.; Sahoo, S.; Ki, J.-S.; Quarta, A.; Ragusa, A.; Jena, M. Algal phlorotannins as novel antibacterial agents with reference to the antioxidant modulation: Current advances and future directions. Mar. Drugs 2022, 20, 403. [Google Scholar] [CrossRef]
- Van Alstyne, K.L. Comparison of three methods for quantifying brown algal polyphenolic compounds. J. Chem. Ecol. 1995, 21, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Ragan, M.A.; Jensen, A. Quantitative studies on brown algal phenols. II. Seasonal variation in polyphenol content of Ascophyllum nodosum (L.) Le Jol. and Fucus vesiculosus (L.). J. Exp. Mar. Biol. Ecol. 1978, 34, 245–258. [Google Scholar] [CrossRef]
- Pavia, H.; Toth, G.B.; Lindgren, A.; Åberg, P. Intraspecific variation in the phlorotannin content of the brown alga Ascophyllum nodosum. Phycologia 2003, 42, 378–383. [Google Scholar] [CrossRef]
- Connan, S.; Goulard, F.; Stiger, V.; Deslandes, E.; Ar Gall, E. Interspecific and temporal variation in phlorotannin levels in an assemblage of brown algae. Bot. Mar. 2004, 47, 410–416. [Google Scholar] [CrossRef]
- Fairhead, V.A.; Amsler, C.D.; McClintock, J.B.; Baker, B.J. Variation in phlorotannin content within two species of brown macroalgae (Desmarestia anceps and D. menziesii) from the Western Antarctic Peninsula. Polar Biol. 2005, 28, 680–686. [Google Scholar] [CrossRef]
- Zubia, M.; Fabre, M.S.; Kerjean, V.; Le Lann, K.; Stiger-Pouvreau, V.; Fauchon, M.; Deslandes, E. Antioxidant and antitumoural activities of some Phaeophyta from Brittany coasts. Food Chem. 2009, 116, 693–701. [Google Scholar] [CrossRef]
- Marambio, J.; Bischof, K. Differential acclimation responses to irradiance and temperature in two co-occurring seaweed species in Arctic fjords. Polar Res. 2021, 40, 5702. [Google Scholar] [CrossRef]
- Pereira, R.C.; Valentin, Y.Y.; Teixeira, V.L.; Kelecom, A. Phlorotannins in Brazilian brown algae: Quantitative study and ecological implications. Planta Med. 1990, 56, 557–558. [Google Scholar] [CrossRef]
- Ye, B.R.; Jang, J.; Kwon, Y.K.; Jeon, S.M.; Jeong, J.Y.; Kang, D.H.; Oh, C.H.; Kim, J.H.; Affan, A.; Hyun, J.H. Antioxidant effect of tropical seaweed Pylaiella littoralis extracts collected from Chuuk lagoon in Federated States of Micronesia. Ocean Polar. Res. 2012, 34, 297–304. [Google Scholar] [CrossRef]
- Magnusson, M.; Yuen, A.K.; Zhang, R.; Wright, J.T.; Taylor, R.B.; Maschmeyer, T.; de Nys, R. A comparative assessment of microwave assisted (MAE) and conventional solid-liquid (SLE) techniques for the extraction of phloroglucinol from brown seaweed. Algal Res. 2017, 23, 28–36. [Google Scholar] [CrossRef]
- Dörschmann, P.; Schmitt, C.; Bittkau, K.S.; Neupane, S.; Synowitz, M.; Roider, J.; Alban, S.; Held-Feindt, J.; Klettner, A. Evaluation of a brown seaweed extract from Dictyosiphon foeniculaceus as a potential therapeutic agent for the treatment of glioblastoma and uveal melanoma. Mar. Drugs 2020, 18, 625. [Google Scholar] [CrossRef] [PubMed]
- Chkhikvishvili, I.D.; Ramazanov, Z.M. Phenolic substances of brown algae and their antioxidant activity. Appl. Biochem. Microbiol. 2000, 36, 289–291. [Google Scholar] [CrossRef]
- Starko, S.; Soto Gomez, M.; Darby, H.; Demes, K.W.; Kawai, H.; Yotsukura, N.; Lindstrom, S.C.; Keeling, P.J.; Graham, S.W.; Martone, P.T. A comprehensive kelp phylogeny sheds light on the evolution of an ecosystem. Mol. Phylogenet. Evol. 2019, 136, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Van Alstyne, K.L.; McCarthy III, J.J.; Hustead, C.L.; Duggins, D.O. Geographic variation in polyphenolic levels of Northeastern Pacific kelps and rockweeds. Mar. Biol. 1999, 133, 371–379. [Google Scholar] [CrossRef]
- Dubois, A.; Iken, K. Seasonal variation in kelp phlorotannins in relation to grazer abundance and environmental variables in the Alaskan sublittoral zone. Algae 2012, 27, 9–19. [Google Scholar] [CrossRef]
- Lee, D.S.; Kang, M.S.; Hwang, H.J.; Eom, S.H.; Yang, J.Y.; Lee, M.S.; Lee, W.J.; Jeon, Y.J.; Choi, J.S.; Kim, Y.M. Synergistic effect between dieckol from Ecklonia stolonifera and β-lactams against methicillin-resistant Staphylococcus aureus. Biotechnol. Bioprocess Eng. 2008, 13, 758–764. [Google Scholar] [CrossRef]
- AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. Available online: https://www.algaebase.org (accessed on 27 October 2022).
- Choi, J.-S.; Lee, K.; Lee, B.-B.; Kim, Y.-C.; Kim, Y.D.; Hong, Y.-K.; Cho, K.K.; Choi, I.S. Antibacterial activity of the phlorotannins dieckol and phlorofucofuroeckol-A from Ecklonia cava against Propionibacterium acnes. Bot. Sci. 2014, 92, 425–431. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Kim, Y.-M.; Kim, E.; Lee, M.-S. Synergistic antibacterial activity of Ecklonia cava extract against antibiotic resistant Enterococcus faecalis. Fish. Aquat. Sci. 2015, 48, 51–57. [Google Scholar] [CrossRef]
- Lee, M.H.; Lee, K.B.; Oh, S.M.; Lee, B.H.; Chee, H.Y. Antifungal activities of dieckol isolated from the marine brown alga Ecklonia cava against Trichophyton rubrum. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 504–507. [Google Scholar] [CrossRef]
- Kim, K.H.; Yu, D.; Eom, S.H.; Kim, H.J.; Kim, D.H.; Song, H.S.; Kim, D.M.; Kim, Y.M. Fucofuroeckol-A from edible marine alga Eisenia bicyclis to restore antifungal activity of fluconazole against fluconazole-resistant Candida albicans. J. Appl. Phycol. 2018, 30, 605–609. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, Q.; Xu, C.; Yu, J.; Zhao, L.; Guo, Q. Damage to the membrane permeability and cell death of Vibrio parahaemolyticus caused by phlorotannins with low molecular weight from Sargassum thunbergii. J. Aquat. Food Prod. Technol. 2016, 25, 323–333. [Google Scholar] [CrossRef]
- Iken, K.; Amsler, C.D.; Amsler, M.O.; McClintock, J.B.; Baker, B.J. Field studies on deterrent properties of phlorotannins in Antarctic brown algae. Bot. Mar. 2009, 52, 547–557. [Google Scholar] [CrossRef]
- Draisma, S.G.A.; Prud’homme van Reine, W.F.; Stam, W.T.; Olsen, J.L. A reassessment of phylogenetic relationships within the Phaeophyceae based on RUBISCO large subunit and ribosomal DNA sequences. J. Phycol. 2001, 37, 586–603. [Google Scholar] [CrossRef]
- McInnes, A.G.; Ragan, M.A.; Smith, D.G.; Walter, J.A. High-molecular-weight phloroglucinol-based tannins from brown algae: Structural variants. Hydrobiologia 1984, 116, 597–602. [Google Scholar] [CrossRef]
- Haslam, E. Polyphenol-protein interactions. Biochem. J. 1974, 139, 285–288. [Google Scholar] [CrossRef]
- Schofield, P.; Mbugua, D.M.; Pell, A.N. Analysis of condensed tannins: A review. Anim. Feed Sci. Technol. 2001, 91, 21–40. [Google Scholar] [CrossRef]
- Pohl, A.; Lübke-Becker, A.; Heuwieser, W. Minimum inhibitory concentrations of frequently used antibiotics against Escherichia coli and Trueperella pyogenes isolated from uteri of postpartum dairy cows. J. Dairy Sci. 2018, 101, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Kidsley, A.K.; Abraham, S.; Bell, J.M.; O’Dea, M.; Laird, T.J.; Jordan, D.; Mitchell, P.; McDevitt, C.A.; Trott, D.J. Antimicrobial susceptibility of Escherichia coli and Salmonella spp. isolates from healthy pigs in Australia: Results of a pilot national survey. Front. Microbiol. 2018, 9, 1207. [Google Scholar] [CrossRef]
- Borman, A.M.; Muller, J.; Walsh-Quantick, J.; Szekely, A.; Patterson, Z.; Palmer, M.D.; Fraser, M.; Johnson, E.M. MIC distributions for amphotericin B, fluconazole, itraconazole, voriconazole, flucytosine and anidulafungin and 35 uncommon pathogenic yeast species from the UK determined using the CLSI broth microdilution method. J. Antimicrob. Chemother. 2020, 75, 1194–1205. [Google Scholar] [CrossRef]
- Paterson, D.M.; Wright, S.J.L. Diffusion gradient plates for herbicide toxicity tests on micro-algae and cyanobacteria. Lett. Appl. Microbiol. 1988, 7, 87–90. [Google Scholar] [CrossRef]
- Cérantola, S.; Breton, F.; Ar Gall, E.; Deslandes, E. Co-occurrence and antioxidant activities of fucol and fucophlorethol classes of polymeric phenols in Fucus spiralis. Bot. Mar. 2006, 49, 347–351. [Google Scholar] [CrossRef]
- Audibert, L.; Fauchon, M.; Blanc, N.; Hauchard, D.; Gall, E.A. Phenolic compounds in the brown seaweed Ascophyllum nodosum: Distribution and radical-scavenging activities. Phytochem. Anal. 2010, 21, 399–405. [Google Scholar] [CrossRef]
- Lalegerie, F.; Stengel, D.B. Concise review of the macroalgal species Pelvetia canaliculata (Linnaeus) Decaisne & Thuret. J. Appl. Phycol. 2022, 34, 2807–2825. [Google Scholar] [CrossRef]
- Ikigai, H.; Nakae, T.; Hara, Y.; Shimamura, T. Bactericidal catechins damage the lipid bilayer. Biochim. Biophys. Acta 1993, 1147, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Koebnik, R.; Locher, K.P.; Van Gelder, P. Structure and function of bacterial outer membrane proteins: Barrels in a nutshell. Mol. Microbiol. 2000, 37, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, W.; Wang, X. Insights into the structure of Escherichia coli outer membrane as the target for engineering microbial cell factories. Microb. Cell Fact. 2021, 20, 73. [Google Scholar] [CrossRef]
- Osumi, M. The ultrastructure of yeast: Cell wall structure and formation. Micron 1998, 29, 207–233. [Google Scholar] [CrossRef]
- Lesage, G.; Bussey, H. Cell wall assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2006, 70, 317–343. [Google Scholar] [CrossRef]
- Guadalupe, Z.; Ayestarán, B. Effect of commercial mannoprotein addition on polysaccharide, polyphenolic, and color composition in red wines. J. Agric. Food Chem. 2008, 56, 9022–9029. [Google Scholar] [CrossRef]
- Dunker, S.; Wilhelm, C. Cell wall structure of coccoid green algae as an important trade-off between biotic interference mechanisms and multidimensional cell growth. Front. Microbiol. 2018, 9, 719. [Google Scholar] [CrossRef] [PubMed]
- Blumreisinger, M.; Meindl, D.; Loos, E. Cell wall composition of chlorococcal algae. Phytochemistry 1983, 22, 1603–1604. [Google Scholar] [CrossRef]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A reproducible, rapid and inexpensive Folin–Ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Schaaper, R.M.; Danforth, B.N.; Glickman, B.W. Rapid repeated cloning of mutant lac repressor genes. Gene 1985, 39, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Stepchenkova, E.I.; Tarakhovskaya, E.R.; Siebler, H.M.; Pavlov, Y.I. Defect of Fe-S cluster binding by DNA polymerase delta in yeast suppresses UV-induced mutagenesis but enhances DNA polymerase zeta-dependent spontaneous mutagenesis. DNA Repair 2017, 49, 60–69. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001; Volume 1, 999p. [Google Scholar]
- Vogel, H.J.; Bonner, D.M. Acetylornithinase of Escherichia coli: Partial purification and some properties. J. Biol. Chem. 1956, 218, 97–106. [Google Scholar] [CrossRef]
- Kaiser, C.; Michaelis, S.; Mitchel, A. Methods in Yeast Genetics, a Cold Spring Harbor Laboratory Course Manual, 1994 ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1994; 202p. [Google Scholar]
- Cramer, M.; Myers, J. Growth and photosynthetic characteristics of Euglena gracilis. Arch. Mikrobiol. 1952, 17, 384–402. [Google Scholar] [CrossRef]
- Gorman, D.S.; Levine, R.P. Cytochrome f and plastocyanin: Their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardii. Proc. Natl. Acad. Sci. USA 1965, 54, 1665–1669. [Google Scholar] [CrossRef]
- Andersen, R.A.; Berges, J.A.; Harrison, P.J.; Watanabe, M.M. Recipes for freshwater and seawater media. In Algal Culture Techniques, 1st ed.; Andersen, R.A., Ed.; Elsevier Academic Press: London, UK, 2005; pp. 429–538. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48, 5–16. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).