Long-Term Alleviation of the Functional Phenotype in Chlorophyll-Deficient Wheat and Impact on Productivity: A Semi-Field Phenotyping Experiment
Abstract
1. Introduction
2. Results
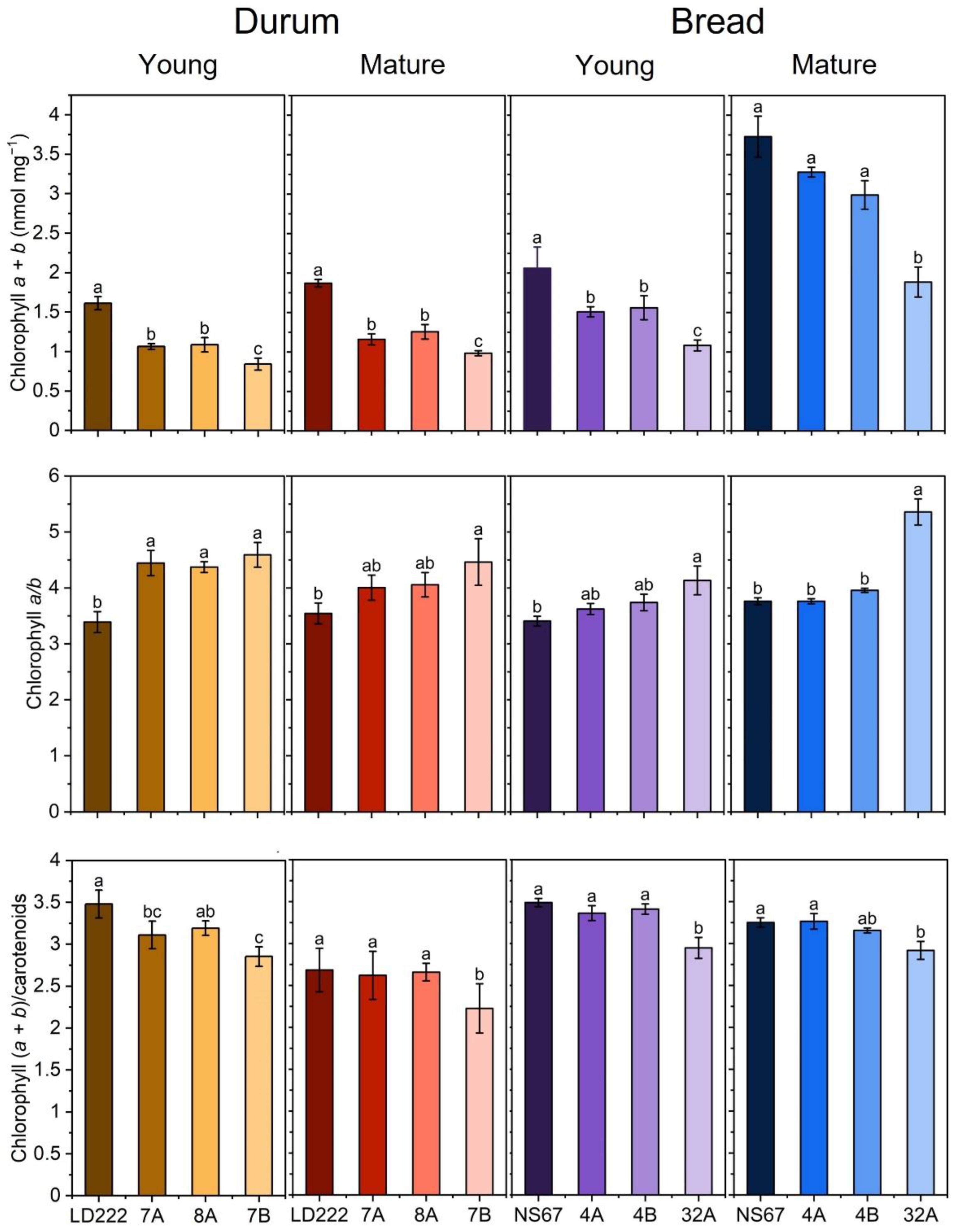
2.1. Pigment Content in Young and Mature Plants
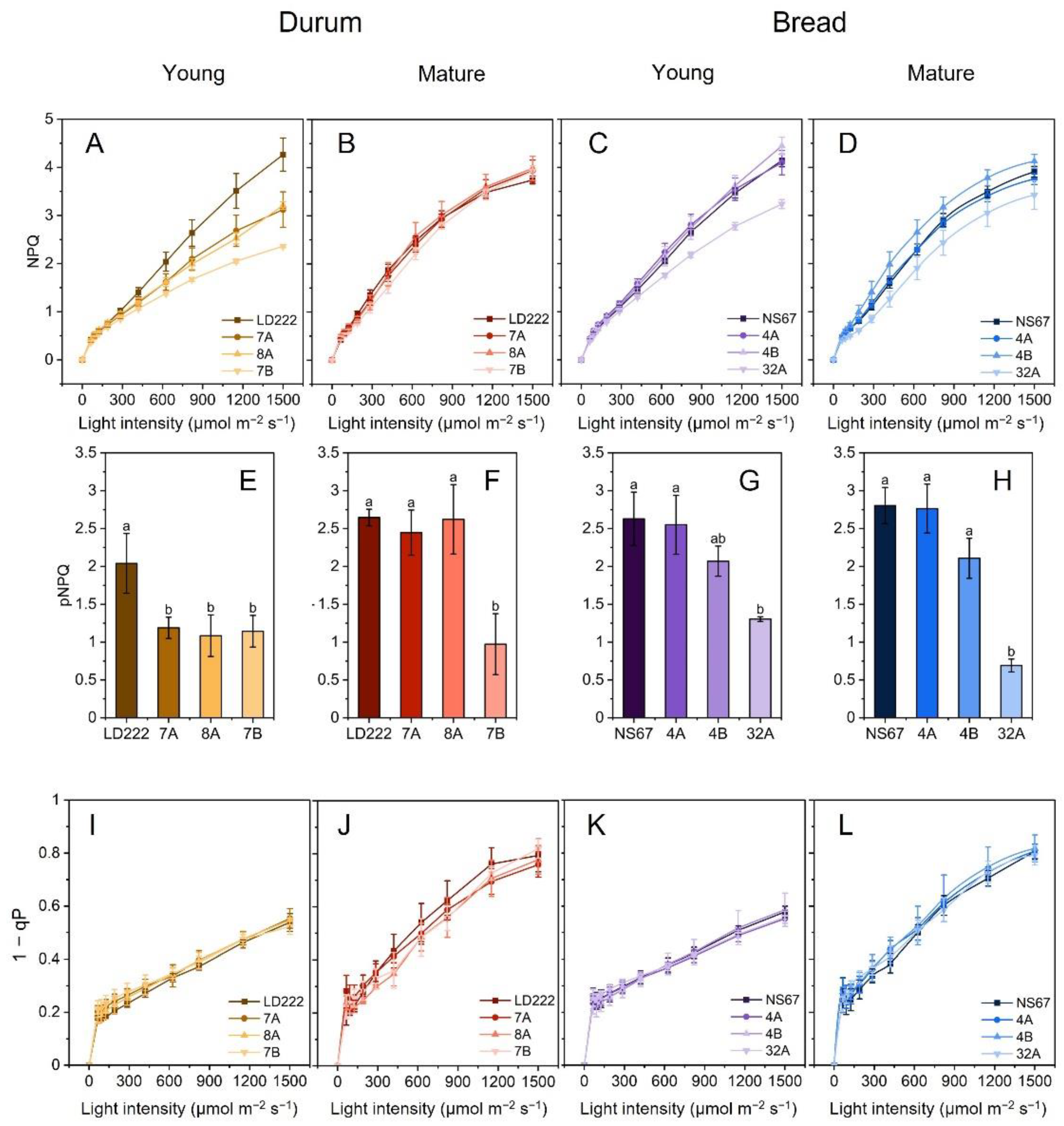
2.2. Irradiance-Dependence of PSII Quantum Yields
2.3. Quenching Analysis and PSII Photoprotection
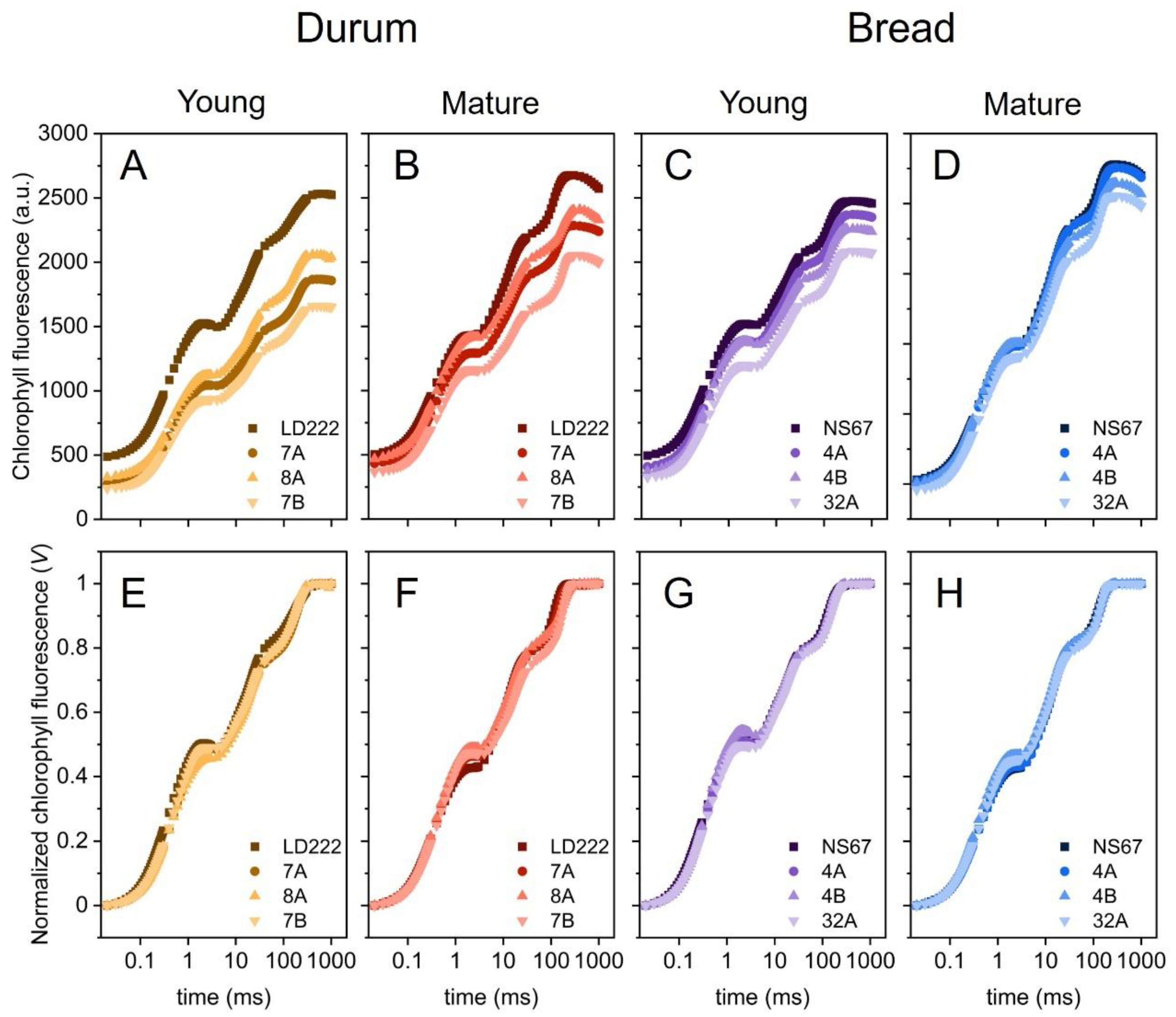
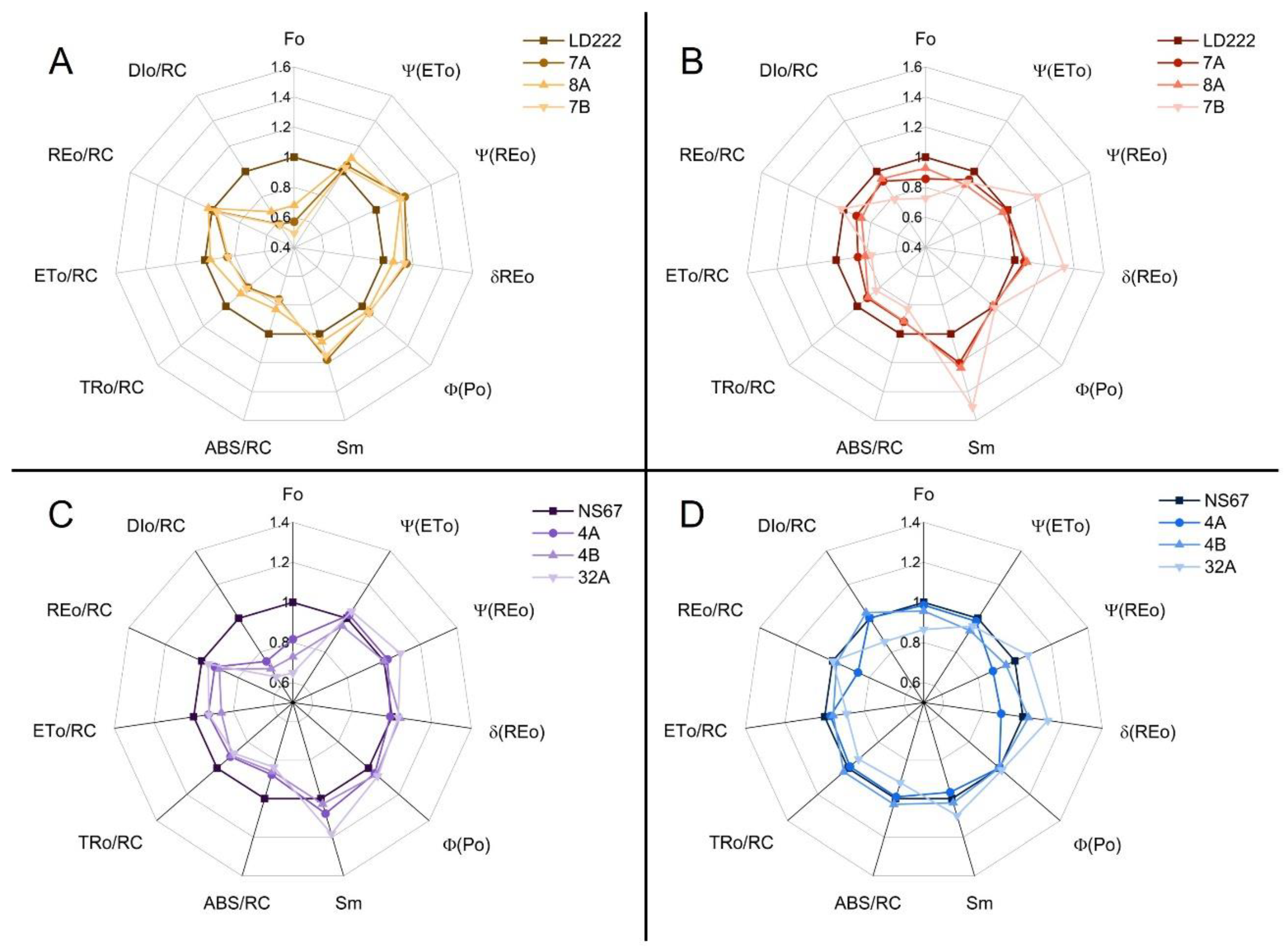
2.4. Fast-Chlorophyll-a-Fluorescence Transient and JIP-Test Parameters
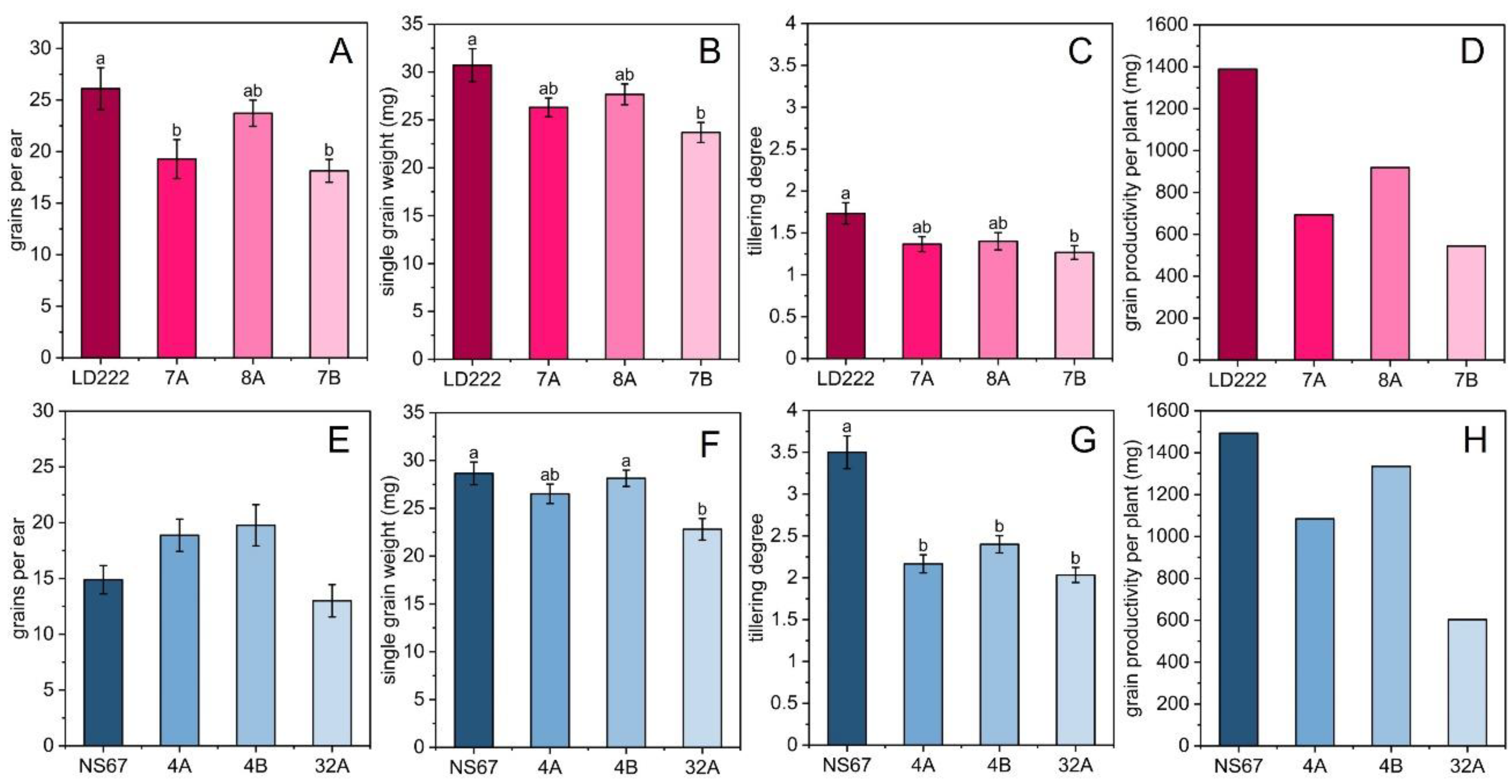
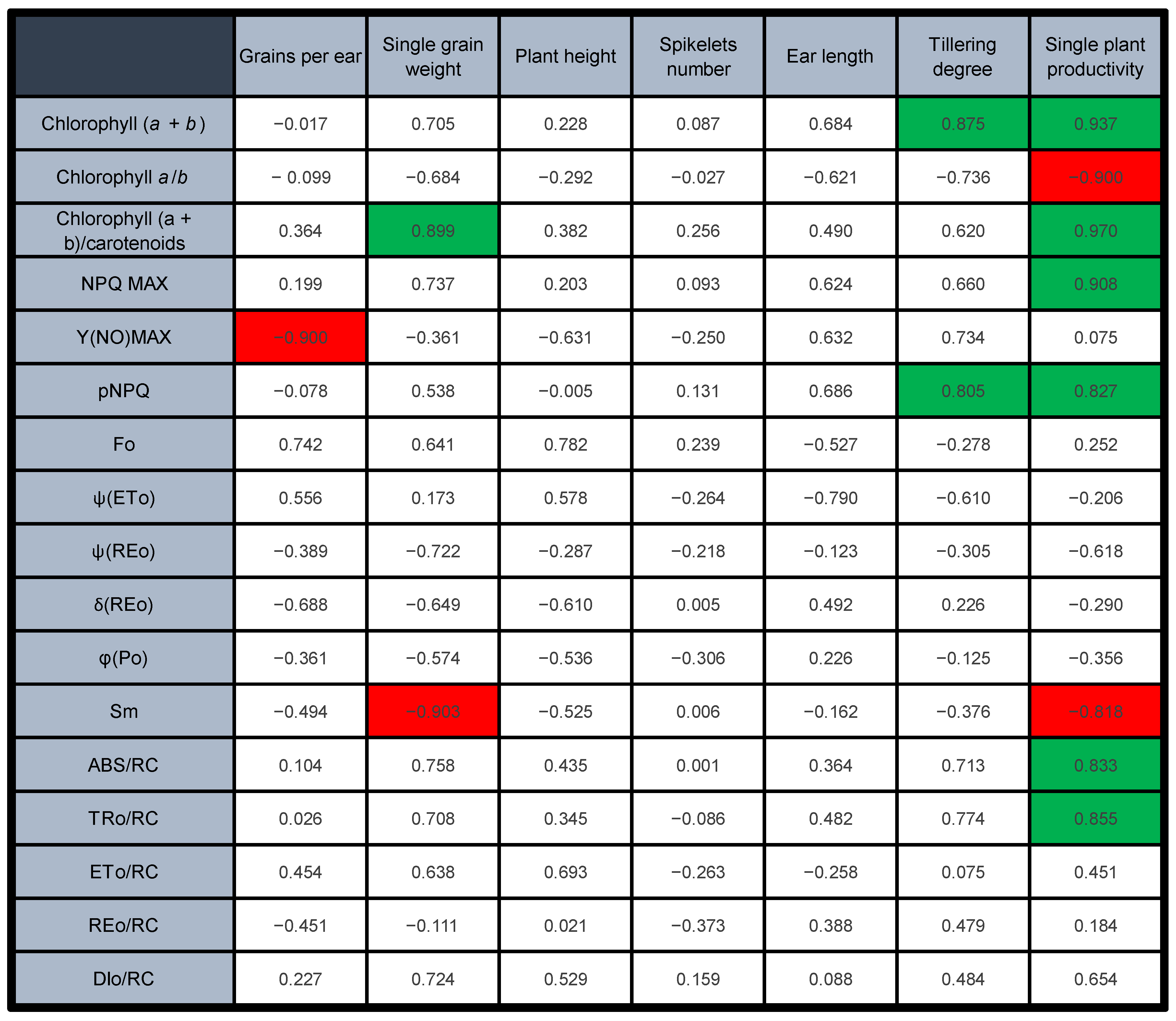
2.5. Productivity Estimation, Morphometric Measurements and Correlation Analysis
3. Discussion
3.1. The Grain Yield per Plant Correlates with the Early Photosynthetic Parameters
3.2. PSII Is Less Photoprotected in the Mutants, but Moderate PSII Photoinhibition Can Help Control the Electron Flow into the Chain
3.3. The Accumulation of Interchain Electron Carriers Can Help Compensate for the Membrane Over-Reduction in Mutants, Particularly of Durum Wheat
4. Conclusions
5. Materials and Methods
5.1. Plant Material
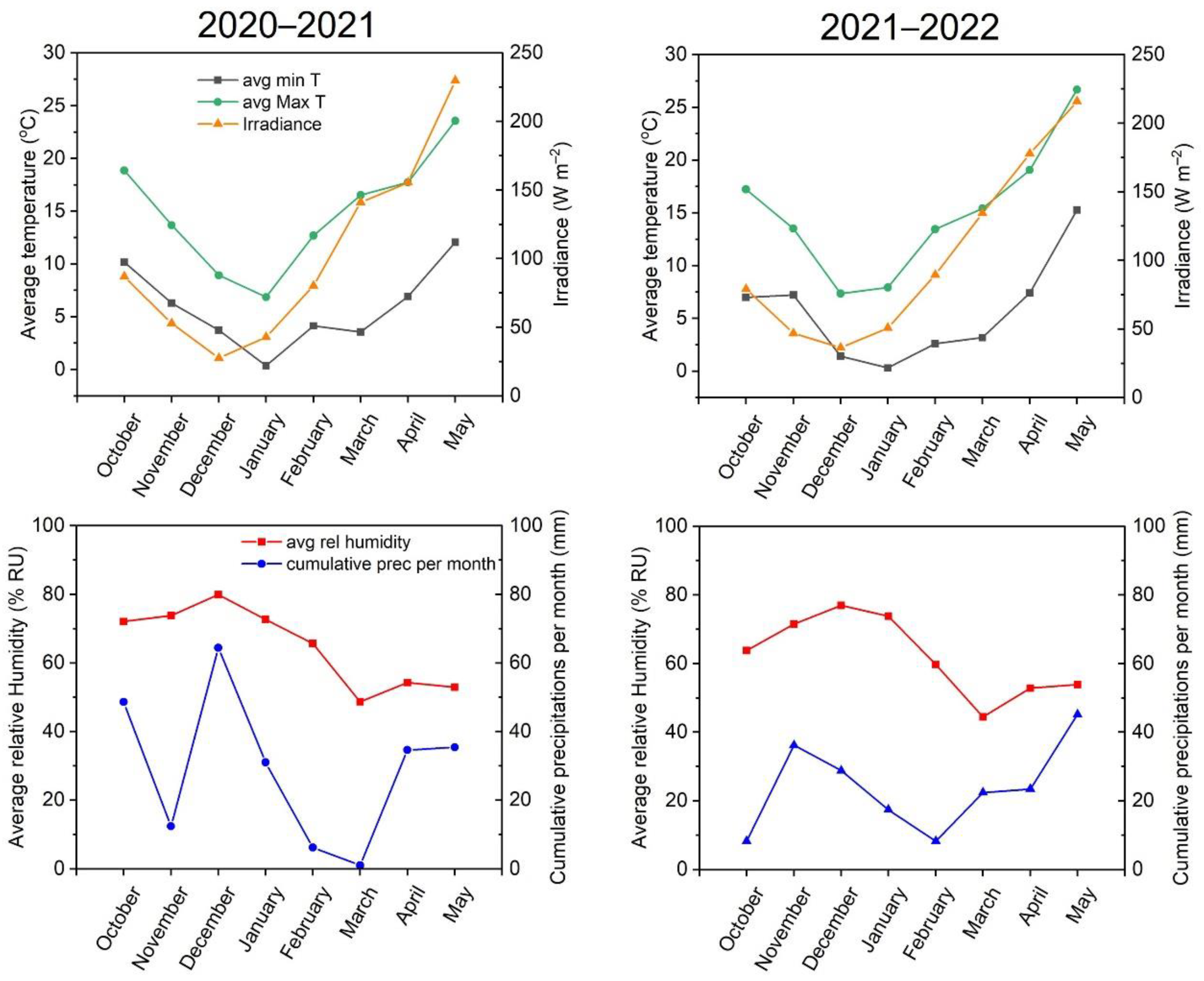
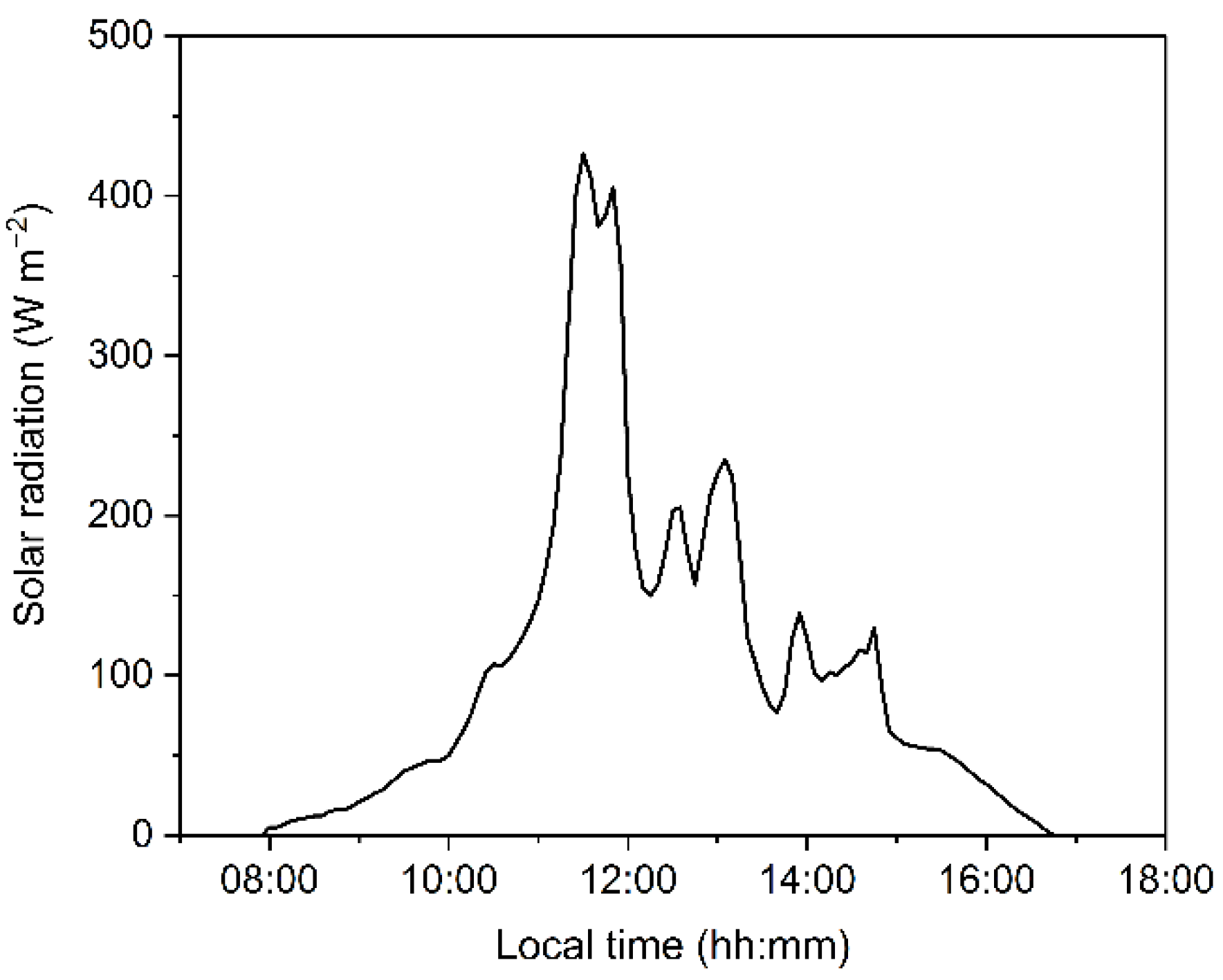
5.2. Study Site and Experiment Design
5.3. Morphometric Measurements and Grain-Productivity Estimation
5.4. Quantification of Photosynthetic Pigments
5.5. Modulated-Chlorophyll-a-Fluorescence Measurements
5.6. Fast-Chlorophyll-a-Fluorescence Measurements
5.7. Data Analysis and Correlation Matrix
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Wheat Line | Mutated Locus | Chlorophyll Level (% Wild Type) 1 | Effect of Fluctuating Light 2 |
|---|---|---|---|
| NS67 | Wild type | - | Slightly negative |
| ANBW-4A | cnA1-d | 10–40% | Positive |
| ANBW-4B | cnB1-d | 0–50% | Very negative |
| ANK-32A | cnA1-a | 30–70% | Insensitive |
| LD222 | Wild type | - | Slightly negative |
| ANDW-7A | cnA1-d | 40–60% | Slightly negative |
| ANDW-7B | cnB1-d | 35–65% | Very negative |
| ANDW-8A | unknown | 10–45% | Insensitive |
Appendix B
| Parameter | Definition 1 |
|---|---|
| Descriptive parameters of the OJIP transient | |
| F0 | Minimum fluorescence value at 20 µs |
| FK | Fluorescence value at 0.3 ms |
| FJ | Fluorescence value at 2 ms |
| FI | Fluorescence value at 30 ms |
| FM | Maximum fluorescence value at plateau |
| FV = FM−F0 | Variable PSII fluorescence |
| Vt = (Ft−F0)/(FM−F0) | Relative variable fluorescence at time t (VJ at 2 ms, VI at 30 ms) |
| Mo = ΔV/Δt | Approximate value of the initial slope (trait 20–300 μs) of the relative variable-fluorescence curve Vt |
| Sm | Normalized area, proportional to the number of electron carriers per electron transport chain |
| Quantum yields and probabilities | |
| φPo = (FM−F0)/FM = FV/FM | Estimate of PSII maximum quantum yield |
| ψETo = ΔVJ = 1−VJ | Estimate of the oxidized-plastoquinone size, or the probability of which a PSII-trapped electron is transferred from reduced QA to QB |
| ψREo = ΔVI = 1−VI | Estimate of the relative size of the pool of PSI end-electron acceptors, or the probability of which a PSII trapped electron is transferred from reduced QA to PSI final acceptors (ferredoxin, ferredoxin-NADP+ oxidoreductase) |
| δREo = VI/VJ | Pool of PSII electron acceptors relative to the pool of plastoquinone, or the probability of which an electron is transferred from reduced QB to PSI final acceptors |
| Specific energy fluxes per active PSII reaction centre | |
| ABS/RC = Mo/(VJ · φPo) | Flux of light absorption per PSII, or apparent antenna size of an active PSII |
| TRo/RC = (Mo/VJ) | Maximum trapped-exciton flux per PSII |
| ETo/RC = (Mo/VJ) · ψETo | Electron-transport flux from QA to QB per PSII |
| REo/RC = (Mo/VJ) · ψREo | Electron-transport flux from QA to final PSI acceptors per PSII |
| DIo/RC = (Mo/VJ) · FM/F0 | Flux of energy dissipation per active PSII |
Appendix C
| Wheat Line | Plant Height (cm) | Ear Length (cm) | Spikelet Number Per Ear |
|---|---|---|---|
| LD222 | 162.2 ± 9.7 (a) | 6.81 ± 1.16 | 19.8 ± 3.2 |
| 7A | 156.9 ± 13.1 (a) | 6.79 ± 0.55 | 21.7 ± 1.9 |
| 8A | 143.7 ± 8.5 (b) | 6.52 ± 1.17 | 19.1 ± 1.8 |
| 7B | 160.9 ± 5.8 (a) | 6.79 ± 1.01 | 20.3 ± 1.4 |
| NS67 | 151.3 ± 5.0 (a) | 9.37 ± 0.45 (a) | 20.2 ± 2.7 |
| 4A | 141.5 ± 4.6 (b) | 8.12 ± 1.19 (ab) | 20.8 ± 1.5 |
| 4B | 146.5 ± 4.5 (ab) | 9.39 ± 1.15 (a) | 20.5 ± 1.4 |
| 32A | 144.2 ± 3.4 (b) | 7.80 ± 1.34 (b) | 18.8 ± 2.3 |
Appendix D
References
- Song, P.; Wang, J.; Guo, X.; Yang, W.; Zhao, C. High-throughput phenotyping: Breaking through the bottleneck in future crop breeding. Crop. J. 2021, 9, 633–645. [Google Scholar] [CrossRef]
- Yang, W.; Feng, H.; Zhang, X.; Zhang, J.; Doonan, J.H.; Batchelor, W.D.; Xiong, L.; Yan, J. Crop phenomics and high-throughput phenotyping: Past decades, current challenges, and future perspectives. Mol. Plant 2020, 13, 187–214. [Google Scholar] [CrossRef]
- Pratap, A.; Gupta, S.; Nair, R.M.; Gupta, S.K.; Schafleitner, R.; Basu, P.S.; Singh, C.M.; Prajapati, U.; Gupta, A.K.; Nayyar, H.; et al. Using plant phenomics to exploit the gains of genomics. Agronomy 2019, 9, 126. [Google Scholar] [CrossRef]
- Gielen, R.; Põldmaa, K.; Tammaru, T. In search of ecological determinants of fungal infections: A semi-field experiment with folivorous moths. Ecol. Evol. 2022, 12, e8926. [Google Scholar] [CrossRef]
- Van Bezouw, R.F.; Keurentjes, J.J.; Harbinson, J.; Aarts, M.G. Converging phenomics and genomics to study natural variation in plant photosynthetic efficiency. Plant J. 2019, 97, 112–133. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Brestič, M.; Bussotti, F.; Calatayud, A.; Ferroni, L.; Goltsev, V.; Guidi, L.; Jajoo, A.; Li, P.; et al. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth. Res. 2017, 132, 13–66. [Google Scholar] [CrossRef]
- Ort, D.; Merchant, S.S.; Alric, J.; Barkan, A.; Blankenship, R.E.; Bock, R.; Croce, R.; Hanson, M.R.; Hibberd, J.M.; Long, S.P.; et al. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc. Natl. Acad. Sci. USA 2015, 112, 8529–8536. [Google Scholar] [CrossRef]
- Genesio, L.; Bassi, R.; Miglietta, R. Plants with less chlorophyll: A global change perspective. Glob. Chang. Biol. 2021, 27, 959–967. [Google Scholar] [CrossRef]
- Terao, T.; Katoh, S. Antenna sizes of photosystem I and photosystem II in chlorophyll b-deficient mutants of rice. Evidence for an antenna function of photosystem II centers that are inactive in electron transport. Plant Cell Physiol. 1996, 37, 307–312. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, W.; Zheng, W.; Zhu, J.; Liu, Z.; Qin, J.; Li, H. Physiological and transcriptomic analyses of a yellow-green mutant with high photosynthetic efficiency in wheat (Triticum aestivum L.). Funct. Integr. Genom. 2018, 18, 175–194. [Google Scholar] [CrossRef]
- Wang, G.; Zeng, F.; Song, P.; Sun, B.; Wang, Q.; Wang, J. Effects of reduced chlorophyll content on photosystem functions and photosynthetic electron transport rate in rice leaves. J. Plant Physiol. 2022, 272, 153669. [Google Scholar] [CrossRef] [PubMed]
- Friedland, N.; Negi, S.; Vinogradova-Shah, T.; Wu, G.; Ma, L.; Flynn, S.; Kumssa, T.; Lee, C.H.; Sayre, R.T. Fine-tuning the photosynthetic light harvesting apparatus for improved photosynthetic efficiency and biomass yield. Sci. Rep. 2019, 9, 13028. [Google Scholar] [CrossRef]
- Li, Y.T.; Li, Y.; Song, J.M.; Guo, Q.H.; Yang, C.; Zhao, W.J.; Wang, J.Y.; Luo, J.; Xu, Y.N.; Zhang, Q.; et al. Has breeding altered the light environment, photosynthetic apparatus, and photosynthetic capacity of wheat leaves? J. Exp. Bot. 2022, 73, 3205–3220. [Google Scholar] [CrossRef]
- Živčak, M.; Brestič, M.; Botyanszka, L.; Chen, Y.E.; Allakhverdiev, S.I. Phenotyping of isogenic chlorophyll-less bread and durum wheat mutant lines in relation to photoprotection and photosynthetic capacity. Photosynth. Res. 2019, 139, 239–251. [Google Scholar] [CrossRef]
- Ferroni, L.; Živčak, M.; Sytar, O.; Kovar, M.; Watanabe, N.; Pancaldi, S.; Baldisserotto, C.; Brestič, M. Chlorophyll-depleted wheat mutants are disturbed in photosynthetic electron flow regulation but can retain an acclimation ability to a fluctuating light regime. Environ. Exp. Bot. 2020, 178, 104156. [Google Scholar] [CrossRef]
- Ferroni, L.; Živčak, M.; Kovar, M.; Colpo, A.; Pancaldi, S.; Allakhverdiev, S.I.; Brestič, M. Fast chlorophyll a fluorescence induction (OJIP) phenotyping of chlorophyll-deficient wheat suggests that an enlarged acceptor pool size of Photosystem I helps compensate for a deregulated photosynthetic electron flow. J. Photochem. Photobiol. B Biol. 2022, 234, 112549. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.R.; Fryer, M.J.; Baker, N.R. Consequences of LHC II deficiency for photosynthetic regulation in chlorina mutants of barley. Photosynth. Res. 1995, 44, 81–91. [Google Scholar] [CrossRef]
- Terao, T.; Sonoike, K.; Yamazaki, J.Y.; Kamimura, Y.; Katoh, S. Stoichiometries of photosystem I and photosystem II in rice mutants differently deficient in chlorophyll b. Plant Cell Physiol. 1996, 37, 299–306. [Google Scholar] [CrossRef]
- Bielczynski, L.W.; Schansker, G.; Croce, R. Consequences of the reduction of the Photosystem II antenna size on the light acclimation capacity of Arabidopsis thaliana. Plant Cell Environ. 2020, 43, 866–879. [Google Scholar] [CrossRef]
- Brestič, M.; Živčak, M.; Kunderlikova, K.; Sytar, O.; Shao, H.; Kalaji, H.M.; Allakhverdiev, S.I. Low PSI content limits the photoprotection of PSI and PSII in early growth stages of chlorophyll b-deficient wheat mutant lines. Photosynth. Res. 2015, 125, 151–166. [Google Scholar] [CrossRef]
- Furutani, R.; Wada, S.; Ifuku, K.; Maekawa, S.; Miyake, C. Higher Reduced State of Fe/S-Signals, with the Suppressed Oxidation of P700, Causes PSI Inactivation in Arabidopsis thaliana. Antioxidants 2023, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Yamori, W. Photosynthetic response to fluctuating environments and photoprotective strategies under abiotic stress. J. Plant Res. 2016, 129, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Tikkanen, M.; Rantala, S.; Grieco, M.; Aro, E.M. Comparative analysis of mutant plants impaired in the main regulatory mechanisms of photosynthetic light reactions-From biophysical measurements to molecular mechanisms. Plant Physiol. Biochem. 2017, 112, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, S.B.; Cao, K.F. Stimulation of cyclic electron flow during recovery after chilling-induced photoinhibition of PSII. Plant Cell Physiol. 2010, 51, 1922–1928. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yang, Y.J.; Zhang, S.B.; Liu, T. Cyclic electron flow around photosystem I promotes ATP synthesis possibly helping the rapid repair of photodamaged photosystem II at low light. Front. Plant Sci. 2018, 9, 239. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, S.B.; Wang, J.H.; Huang, W. Pre-illumination at high light significantly alleviates the over-reduction of photosystem I under fluctuating light. Plant Sci. 2021, 312, 111053. [Google Scholar] [CrossRef]
- Tan, S.L.; Liu, T.; Zhang, S.B.; Huang, W. Balancing light use efficiency and photoprotection in tobacco leaves grown at different light regimes. Environ. Exp. Bot. 2020, 175, 104046. [Google Scholar] [CrossRef]
- Yamamoto, H.; Shikanai, T. PGR5-dependent cyclic electron flow protects photosystem I under fluctuating light at donor and acceptor sides. Plant Physiol. 2019, 179, 588–600. [Google Scholar] [CrossRef]
- Tikkanen, M.; Rantala, S.; Aro, E.M. Electron flow from PSII to PSI under high light is controlled by PGR5 but not by PSBS. Front. Plant Sci. 2015, 6, 521. [Google Scholar] [CrossRef]
- Hendrickson, L.; Furbank, R.T.; Chow, W.S. A simple alternative approach to assessing the fate of absorbed light energy using chlorophyll fluorescence. Photosynth. Res. 2004, 82, 73–81. [Google Scholar] [CrossRef]
- Bilger, W.; Björkman, O. Role of the xanthophylls cycle, fluorescence and photosynthesis in Hedera canariensis. Photosynth. Res. 1990, 25, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestič, M.; Bussotti, F.; Calatayud, A.; Dąbrowski, P.; et al. Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, L.; Angeleri, M.; Pantaleoni, L.; Pagliano, C.; Longoni, P.; Marsano, F.; Aro, E.-M.; Suorsa, M.; Baldisserotto, C.; Giovanrdi, M.; et al. Light-dependent reversible phosphorylation of the minor photosystem II antenna Lhcb6 (CP 24) occurs in lycophytes. Plant J. 2014, 77, 893–905. [Google Scholar] [CrossRef]
- Lazár, D. Parameters of photosynthetic energy partitioning. J. Plant Physiol. 2015, 175, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Adams, W.W., III; Heber, U.; Neimanis, S.; Winter, K.; Krüger, A.; Czygan, F.C.; Bilger, W.; Bjorkman, O. Inhibition of zeaxanthin formation and of rapid changes in radiationless energy dissipation by dithiothreitol in spinach leaves and chloroplasts. Plant Physiol. 1990, 92, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Adams, W.W., III. Photoprotection in an ecological context: The remarkable complexity of thermal energy dissipation. New Phytol. 2006, 172, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Long, S.P.; Taylor, S.H.; Burgess, S.J.; Carmo-Silva, E.; Lawson, T.; De Souza, A.P.; Leonelli, L.; Wang, Y. Into the shadows and back into sunlight: Photosynthesis in fluctuating light. Annu. Rev. Plant Biol. 2022, 73, 617–648. [Google Scholar] [CrossRef]
- Ruban, A.V. Nonphotochemical chlorophyll fluorescence quenching: Mechanism and effectiveness in protecting plants from photodamage. Plant Physiol. 2016, 170, 1903–1916. [Google Scholar] [CrossRef]
- Ruban, A.V.; Murchie, E.H. Assessing the photoprotective effectiveness of non-photochemical chlorophyll fluorescence quenching: A new approach. Biochim. Biophys. Acta BBA-Bioenerg. 2012, 1817, 977–982. [Google Scholar] [CrossRef]
- Björkman, O.; Demmig-Adams, B. Regulation of photosynthetic light energy capture, conversion, and dissipation in leaves of higher plants. In Ecophysiology of Photosynthesis; Schulze, E.D., Caldwell, M.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 17–47. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis. Advances in Photosynthesis and Respiration; Papageorgiou, G., Govindjee, Eds.; Springer: Dordrecht, Germany, 2004; pp. 321–362. [Google Scholar]
- Stirbet, A.; Govindjee. On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and photosystem II: Basics and applications of the OJIP fluorescence transient. J. Photochem. Photobiol. B Biol. 2011, 104, 236–257. [Google Scholar] [CrossRef]
- Živčak, M.; Brestič, M.; Kunderlikova, K.; Olsovska, K.; Allakhverdiev, S.I. Effect of photosystem I inactivation on chlorophyll a fluorescence induction in wheat leaves: Does activity of photosystem I play any role in OJIP rise? J. Photochem. Photobiol. B Biol. 2015, 152, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Tóth, S.Z.; Schansker, G.; Strasser, R.J. A non-invasive assay of the plastoquinone pool redox state based on the OJIP-transient. Photosynth. Res. 2007, 93, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Falbel, T.G.; Meehl, J.B.; Staehelin, L.A. Severity of mutant phenotype in a series of chlorophyll-deficient wheat mutants depends on light intensity and the severity of the block in chlorophyll synthesis. Plant Physiol. 1996, 112, 821–832. [Google Scholar] [CrossRef]
- Jiang, H.B.; Wang, N.; Jian, J.T.; Wang, C.S.; Xie, Y.Z. Rapid mapping of a chlorina mutant gene cn-A1 in hexaploid wheat by bulked segregant analysis and single nucleotide polymorphism genotyping arrays. Crop. Pasture Sci. 2019, 70, 827. [Google Scholar] [CrossRef]
- Wu, H.; Shi, N.; An, X.; Liu, C.; Fu, H.; Cao, L.; Feng, Y.; Sun, D.; Zhang, L. Candidate genes for yellow leaf color in common wheat (Triticum aestivum L.) and major related metabolic pathways according to transcriptome profiling. Int. J. Mol. Sci. 2018, 19, 1594. [Google Scholar] [CrossRef]
- Brestič, M.; Živčak, M.; Kunderlikova, K.; Allakhverdiev, S.I. High temperature specifically affects the photoprotective responses of chlorophyll b-deficient wheat mutant lines. Photosynth. Res. 2016, 130, 251–266. [Google Scholar] [CrossRef]
- Li, N.; Jia, J.; Xia, C.; Liu, X.; Kong, X. Characterization and mapping of novel chlorophyll deficient mutant genes in durum wheat. Breed. Sci. 2013, 63, 169–175. [Google Scholar] [CrossRef]
- Evers, J.B.; Vos, J.; Andrieu, B.; Struik, P.C. Cessation of tillering in spring wheat in relation to light interception and red:far-red ratio. Ann. Bot. 2006, 97, 649–658. [Google Scholar] [CrossRef]
- Moeller, C.; Evers, J.B.; Rebetzk, G. Canopy architectural and physiological characterization of near-isogenic wheat lines differing in the tiller inhibition gene tin. Front. Plant Sci. 2014, 5, 617. [Google Scholar] [CrossRef] [PubMed]
- Malnoë, A. Photoinhibition or photoprotection of photosynthesis? Update on the (newly termed) sustained quenching component qH. Environ. Exp. Bot. 2018, 154, 123–133. [Google Scholar] [CrossRef]
- Ferroni, L.; Colpo, A.; Baldisserotto, C.; Pancaldi, S. In an ancient vascular plant the intermediate relaxing component of NPQ depends on a reduced stroma: Evidence from dithiothreitol treatment. J. Photochem. Photobiol. B Biol. 2021, 215, 112114. [Google Scholar] [CrossRef]
- Nilkens, M.; Kress, E.; Lambrev, P.; Miloslavina, Y.; Müller, M.; Holzwarth, A.R.; Jahns, P. Identification of a slowly inducible zeaxanthin-dependent component of nonphotochemical quenching of chlorophyll fluorescence generated under steady stateconditions in Arabidopsis. Biochim. Biophys. Acta 2010, 1797, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Aro, E.-M.; Virgin, I.; Andersson, B. Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim. Biophys. Acta 1993, 1143, 113–134. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Mamedov, F.; Grieco, M.; Suorsa, M.; Jajoo, A.; Styring, S.; Tikkanen, M.; Aro, E.-M. Photodamage of iron-sulphur clusters in photosystem I induces nonphotochemical energy dissipation. Nat. Plants 2016, 2, 16035. [Google Scholar] [CrossRef]
- Nawrocki, W.J.; Liu, X.; Raber, B.; Hu, C.; de Vitry, C.; Bennett, D.I.G.; Croce, R. Molecular origins of induction and loss of photoinhibition-related energy dissipation qI. Sci. Adv. 2021, 7, eabj0055. [Google Scholar] [CrossRef]
- Lambrev, P.H.; Miloslavina, Y.; Jahns, P.; Holzwarth, A.R. On the relationship between non-photochemical quenching and photoprotection of photosystem II. Biochim. Biophys. Acta BBA-Bioenerg. 2012, 1817, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Colpo, A.; Baldisserotto, C.; Pancaldi, S.; Sabia, A.; Ferroni, L. Photosystem II photoinhibition and photoprotection in a lycophyte, Selaginella martensii. Physiol. Plant. 2022, 174, e13604. [Google Scholar] [CrossRef]
- Huang, W.; Yang, Y.J.; Hu, H.; Zhang, S.B. Moderate photoinhibition of photosystem II protects photosystem I from photodamage at chilling stress in tobacco leaves. Front. Plant Sci. 2016, 7, 182. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, S.B.; Liu, T.; Huang, W. Decreased photosystem II activity facilitates acclimation to fluctuating light in the understory plant Paris polyphylla. Biochim. Biophys. Acta BBA-Bioenerg. 2020, 1861, 148135. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.E.; Lodeyro, A.; Poli, H.O.; Zurbriggen, M.; Peisker, M.; Palatnik, J.F.; Tognetti, V.B.; Tschiersch, H.; Hajirezaei, M.R.; Valle, E.M.; et al. Transgenic tobacco plants overexpressing chloroplastic ferredoxin-NADP(H) reductase display normal rates of photosynthesis and increased tolerance to oxidative stress. Plant Physiol. 2007, 143, 639–649. [Google Scholar] [CrossRef]
- Rodriguez-Heredia, M.; Saccon, F.; Wilson, S.; Finazzi, G.; Ruban, A.V.; Hanke, G.T. Protection of photosystem I during sudden light stress depends on ferredoxin:NADP(H) reductase abundance and interactions. Plant Physiol. 2022, 188, 1028–1042. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Grodzinski, B.; Han, J.; Marie, T.; Zhang, Y.-J.; Song, Y.C.; Sun, Y. Granal thylakoid structure and function: Explaining an enduring mystery of higher plants. New Phytol. 2022, 236, 319–329. [Google Scholar] [CrossRef]
- Chovancek, E.; Živčak, M.; Brestič, M.; Hussain, S.; Allahkverdiev, S.I. The different patterns of post-heat stress responses in wheat genotypes: The role of the transthylakoid proton gradient in efficient recovery of leaf photosynthetic capacity. Photosynth. Res. 2021, 150, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Force, L.; Critchley, C.; Van Rensen, J.J. New fluorescence parameters for monitoring photosynthesis in plants. Photosynth. Res. 2003, 78, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, R.J. Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth. Res. 2006, 89, 27–41. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Oxborough, K.; Baker, N.R. Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components–calculation of qP and Fv/Fm; without measuring Fo. Photosynth. Res. 1997, 54, 135–142. [Google Scholar] [CrossRef]
- Ware, M.A.; Belgio, E.; Ruban, A.V. Photoprotective capacity of non-photochemical quenching in plants acclimated to different light intensities. Photosynth. Res. 2015, 126, 261–274. [Google Scholar] [CrossRef]
- Tsimilli-Michael, M. Revisiting JIP-test: An educative review on concepts, assumptions, approximations, definitions and terminology. Photosynthetica 2020, 58, 275–292. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colpo, A.; Demaria, S.; Baldisserotto, C.; Pancaldi, S.; Brestič, M.; Živčak, M.; Ferroni, L. Long-Term Alleviation of the Functional Phenotype in Chlorophyll-Deficient Wheat and Impact on Productivity: A Semi-Field Phenotyping Experiment. Plants 2023, 12, 822. https://doi.org/10.3390/plants12040822
Colpo A, Demaria S, Baldisserotto C, Pancaldi S, Brestič M, Živčak M, Ferroni L. Long-Term Alleviation of the Functional Phenotype in Chlorophyll-Deficient Wheat and Impact on Productivity: A Semi-Field Phenotyping Experiment. Plants. 2023; 12(4):822. https://doi.org/10.3390/plants12040822
Chicago/Turabian StyleColpo, Andrea, Sara Demaria, Costanza Baldisserotto, Simonetta Pancaldi, Marian Brestič, Marek Živčak, and Lorenzo Ferroni. 2023. "Long-Term Alleviation of the Functional Phenotype in Chlorophyll-Deficient Wheat and Impact on Productivity: A Semi-Field Phenotyping Experiment" Plants 12, no. 4: 822. https://doi.org/10.3390/plants12040822
APA StyleColpo, A., Demaria, S., Baldisserotto, C., Pancaldi, S., Brestič, M., Živčak, M., & Ferroni, L. (2023). Long-Term Alleviation of the Functional Phenotype in Chlorophyll-Deficient Wheat and Impact on Productivity: A Semi-Field Phenotyping Experiment. Plants, 12(4), 822. https://doi.org/10.3390/plants12040822








