Abstract
Ferula is the third largest genus of the Apiaceae family, its species are utilized as a remedy for diverse ailments all over the world. F. sinkiangensis K. M. Shen (Chou-AWei, Chinese Ferula) is mainly found in Xin-jiang Uygur Autonomous Region, China. Traditionally, it is utilized for treating various illnesses such as digestive disorders, rheumatoid arthritis, wound infection, baldness, bronchitis, ovarian cysts, intestinal worms, diarrhea, malaria, abdominal mass, cold, measles, and bronchitis. It can produce different classes of metabolites such as sesquiterpene coumarins, steroidal esters, lignans, phenylpropanoids, sesquiterpenes, monoterpenes, coumarins, organic acid glycosides, and sulfur-containing compounds with prominent bioactivities. The objective of this work is to point out the reported data on F. sinkiangensis, including traditional uses, phytoconstituents, biosynthesis, and bioactivities. In the current work, 194 metabolites were reported from F. sinkiangensis in the period from 1987 to the end of 2022. Nevertheless, future work should be directed to conduct in vivo, mechanistic, and clinical assessments of this plant`s metabolites to confirm its safe usage.
1. Introduction
People have utilized plants since ancient times for different reasons: food, clothing, shelter, decoration, and construction [1]. Their usage by local and indigenous communities has been vertically and orally transferred among generations [2]. Also, plants are dynamic factories for the production of enormous kinds of metabolites. The plants and/or their metabolites form the backbone for diverse pharmaceutics, perfume, cosmetic, agrochemical, and food industries. Besides, they are traditional remedies for many ailments in various countries particularly the developed ones [3,4].
Ferula is the third largest genus of Apiaceae family that comprises about 180 species. Its species commonly exist in Asian and Mediterranean regions e.g., Iran, Turkey, Algeria, Afghanistan, Saudi Arabia, Pakistan, China, and India [5]. Ferula means “carrier” or “vehicle” in Latin and this genus is distinguished by the existence of oleo-gum-resins (e.g., sagapenum, asafoetida, ammoniacum, and galbanum) [6]. Most of its plants are with a pungent odor and bitter taste due to the existence of disulfides [7]. In Asia, they are utilized as a spice and in pickles, meat sauces, curry, and other foods as flavoring agents [7]. In China, the Ferula resin is employed for treating dysentery, worms, and malaria, and to dissolve phlegm, as well as an insecticide and deodorant [8]. Its plants are utilized as tranquilizers and for treating rheumatism, digestive disorders, headache, dizziness, toothache, and arthritis [9]. Also, they are of great significance in traditional and folk medicine for more than thousand years in treating epilepsy, asthma, stomachache and headache, intestinal parasites, flatulence, influenza, dysentery, and weak digestion in different countries [10]. These plants displayed a myriad of bioactivities: anticancer, anthelmintic, antiepileptic, antioxidant, antiulcer, antimicrobial, antihypertensive, antifungal, antidepressant, antiproliferative, antiprotozoal, antihemolytic, antimycobacterial, anticoagulant, antifertility, antispasmodic, anticonvulsant, relaxant, antinociceptive, hypnotic, memory and digestive enzyme enhancing, antiviral, anxiolytics, antihyperlipidemic, antigenotoxic, anti-inflammatory, antihyperglycemic, antidiabetic, and hepatoprotective [11,12,13]. They also demonstrated aphicidal, phytotoxic, and acaricidal activities [11,12,13]. It was stated that sesquiterpene coumarins, coumarins, aromatic acid lactones, and sesquiterpenes are the prime phytoconstituents of Ferula plants roots [11,14], while sesquiterpenes and monoterpenes and their oxygenated derivatives with diversified structures are the principal metabolites of Ferula species aerial parts oil [15].
It is worth reporting that the improper practice of wild plants is commonly invasive and devasting to the naturally existing medicinal plants which may cause a dangerous menace to these substantial plants and may result in the extinction of some valuable species [16]. Thus, the conservation of land resources and responsible consumption and production are the challenges in sustainable land resources usage [16].
F. sinkiangensis K.M. Shen (Chou-AWei, Chinese Ferula, (Xinjiang’awei)) is an important member of this genus. F. sinkiangensis is a perennial plant endemic in Xinjiang Uygur Autonomous Region, China [17] (Figure 1).
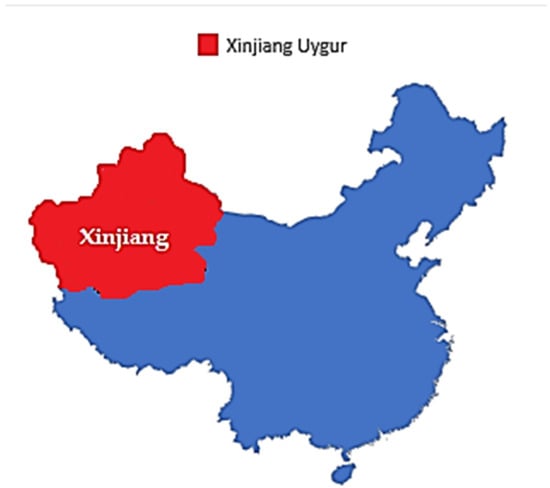
Figure 1.
A map illustrating the geographic location of the Xinjiang-Uigur Autonomous Region in China.
It was reported that this plant is in the menace of evanescence due to irrigation, road building, unconstrained mining, reclamation, climate variation, and original habitat deterioration, leading to annual shrinkage of F. sinkiangensis resources [18]. This plant was included among 2nd class protected wild medicinal species and 3rd class endangered plant in China [19,20]. However, this plant has not yet been included in the IUCN Red List of threatened species, as well as in CITES (Convention on International Trade in Endangered Species of Wild Fauna and Flora) [21,22,23].
Additionally, various metabolites have been identified from its roots, oleo-gum-resin, resin, seeds, and aerial parts such as steroidal esters, phenylpropanoids, sesquiterpene coumarins, aromatic acids, sesquiterpenes, coumarins, monoterpenes, lignans, and sulfanes [11,14,24,25,26,27,28,29,30,31].
The plant and its phytoconstituents revealed various bioactivities such as anti-ulcerative, antibacterial, anti-inflammatory, antioxidant, molluscicidal, anti-schistosome, anti-drug addiction, immunopharmacological, anti-neuroinflammatory, anticancer, antifungal, antiviral, and insecticidal [11,14,25,26,27,28,29,30,31,32]. It is noteworthy that there is no current inclusive review on this plant. Since 1987, many studies were performed revealing new metabolites with diverse structural variation and promising activities from this plant. In this work, the reported studies on this plant, including traditional uses, its metabolites, their structural classes, biosynthesis, and bioactivities are reviewed. Overall, this work intended to give an inclusive introduction to F. sinkiangensis that could help in identifying the future investigations direction and possible implementations of this valuable medicinal plant.
2. Research Methodology
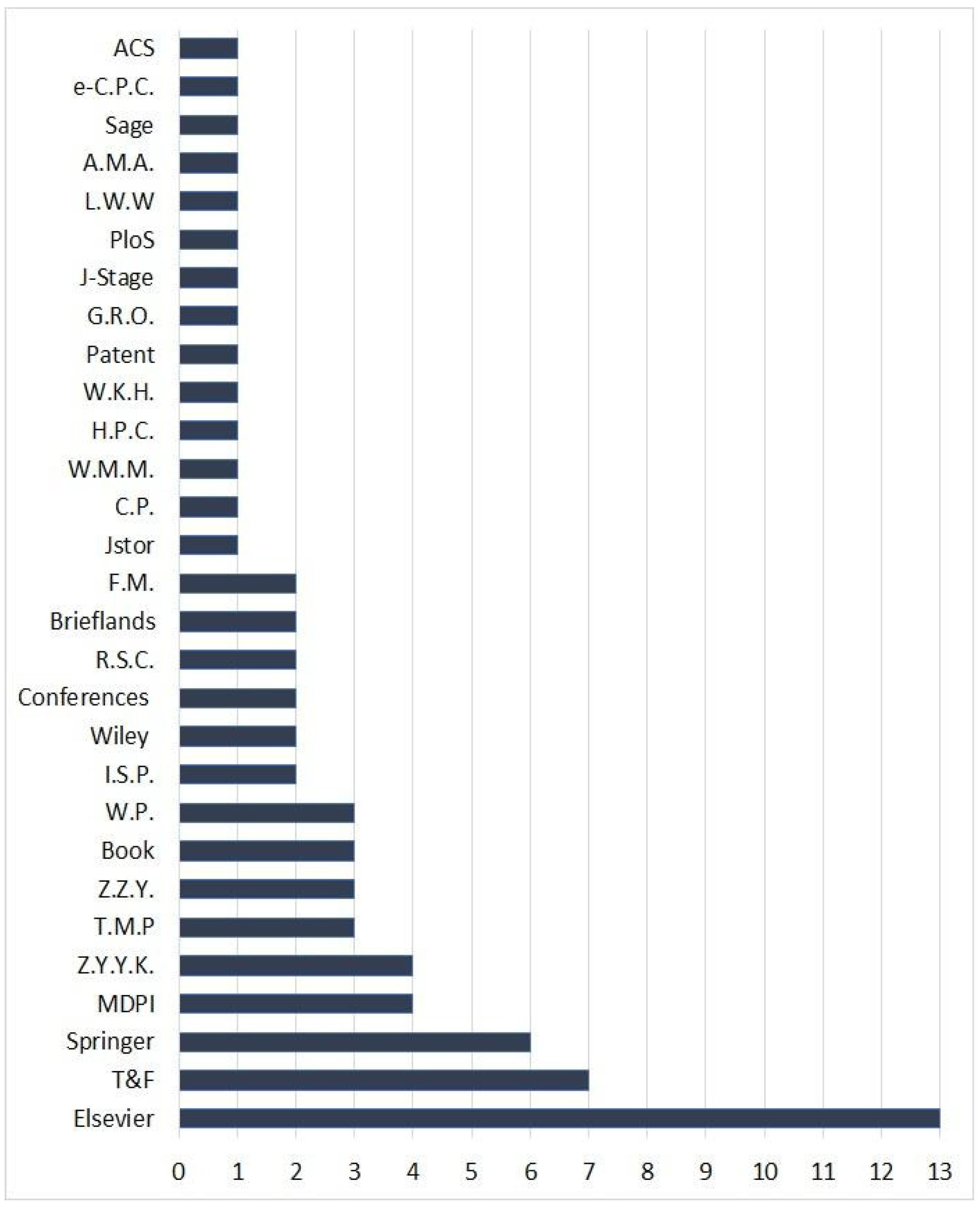
To collect the reported data on F. sinkiangensis, a comprehensive search was carried out on PubMed (37 records) and Google-scholar (529 records) databases, as well as the published articles by various publishers, including Springer, Elsevier, Taylor & Francis, Wiley, MDPI, Thieme, Hindawi, etc. The search keywords were F. sinkiangensis, ethnomedicinal uses, folk uses, bioactive compounds, biosynthesis, phytochemistry, biological activity, and pharmacology.
The selection criteria of the records including in this work were: (1) research articles had to be published in scientific journals (2) studies that reported the traditional uses, metabolites, biosynthesis, and bioactivities of F. sinkiangensis (3) patents, book chapters, and conferences. The covered records in this work included the published articles from various publishers, patents, book chapters, and conferences in the period from 1987 to the end of 2022. For the non-English articles, English abstracts have been utilized. The studies that did not agree with the selection criteria, as well as the whole non-English, non-reviewed, and not journal articles are excluded. In the current work, 72 references have been cited including articles from various publishers, books, conferences, webpages, and patents (Figure 2).
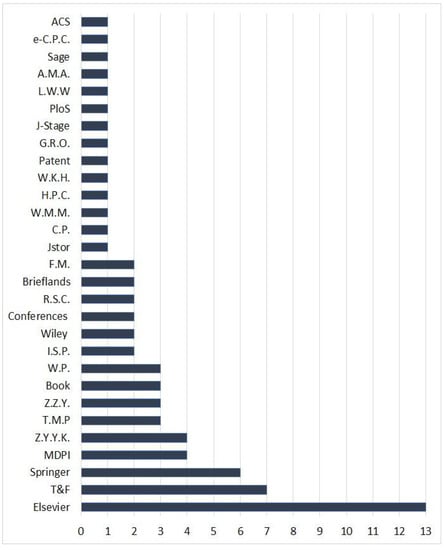
Figure 2.
Sources of the cited references in this work and their numbers. T&F: Taylor and Francis Ltd.; MDPI: Multidisciplinary Digital Publishing Institute; Z.Y.Y.K.: Zhongguo Yufang Yixue Kexueyuan; T.M.P.: Thieme Medical Publishers; Z.Z.Y.: Zhongguo Zhongyi Yanjiuyuan; W.P.: Webpages; I.S.P: Iranian Society of Pharmacognosy; R.S.C.: Royal Society of Chemistry; F.M.: Frontiers Media; C.P.: Cell Press; W.M.M.: Walsh Medical Media; H.P.C.: Hindawi Publishing Corporation; W.K.H.: Wolters Kluwer Health—Pvt.; G.R.O.: Global Research Online; J-Stage: Pharmaceutical Society of Japan; PLOS: Public Library of Science; L.W.W.: Lippincott Williams and Wilkins Ltd.; A.M.A.: American Medical Association; e-C.P.C.: e-Century Publishing Corporation; A.C.S. American Chemical Society.
3. Taxonomy of F. sinkiangensis [32]
| Kingdom | Plantae |
| Division | Magnoliophyta |
| Class | Magnoliopsida |
| Family | Apiaceae |
| Genus | Ferula |
| Species | sinkiangensis |
4. Traditional Uses of F. sinkiangensis
F. sinkiangensis is mainly found in Xinjiang, which is a region with various minorities. The plant has been described in the Chinese Pharmacopoeia and in “Medica of the Tang Dynasty” for a long time as a folk medicine for gastric disorders and rheumatoid arthritis [24].
The resin of the roots or stems of F. sinkiangensis (Ferulae Resina, “AWei” in China) is a folk medicine recorded in Chinese Pharmacopoeia [25]. It is often utilized for reducing the symptoms of lumps, indigestion, joint pain, wound infection, baldness, bronchitis, and ovarian cysts by Uygur people in Xinjiang [25,33,34,35]. It also is efficient in killing intestinal worms, as well as treating parasite-caused malnutrition, abdominal and stomachic swelling pain, diarrhea, malaria, abdominal mass, cold, and measles. However, its powerful odor has restricted its usage [36,37]. The resin is indicated in treating animal stagnation and food accumulation, concertions and conglomerations because of blood stasis, abdominal syndrome, and abdominal pain due to accumulation of worms, also for malaria and dysentery [38] at doses 1–1.5 g in the form of pills or oral powder. The resin should not be decocted with H2O [38]. Its use is prohibited for patients with spleen and stomach weakness, as well as for pregnant women [36,38].
5. Phytoconstituents of F. sinkiangensis
The phytochemical investigation of different parts of F. sinkiangensis, including gum resin, aerial parts, seed, roots, oleo-gum-resin, and resins led to the separation of different classes of phytoconstituents by the mean of diverse chromatographic tools (Table S1). Their structure characterization was performed using various spectral techniques (e.g., UV, NMR, MS), as well as CD, [α]D, and Xray analyses and chemical means. A total of 194 metabolites were separated from F. sinkiangensis (excluding polysaccharides). These metabolites were highlighted below.
5.1. Sesquiterpene Coumarins
The reported studies showed that sesquiterpene coumarins represent the major metabolites produced by this plant. They represent 60 metabolites (Figure 3, Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8) of the total compounds reported from this plant that were mainly separated from gum resin, seed, roots, and resins. It was noted that no sesquiterpene coumarin derivatives were reported from the aerial parts.
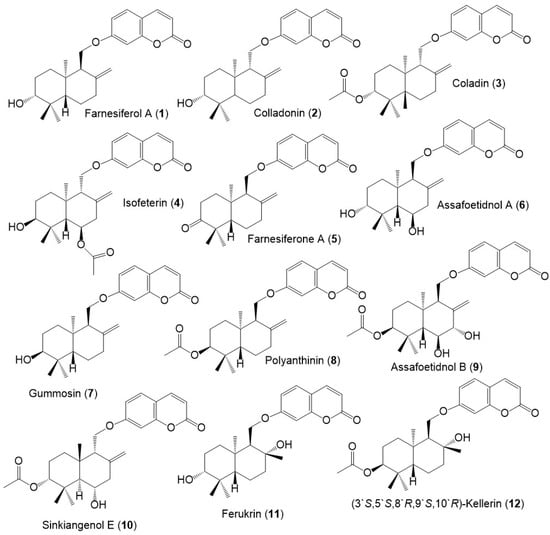
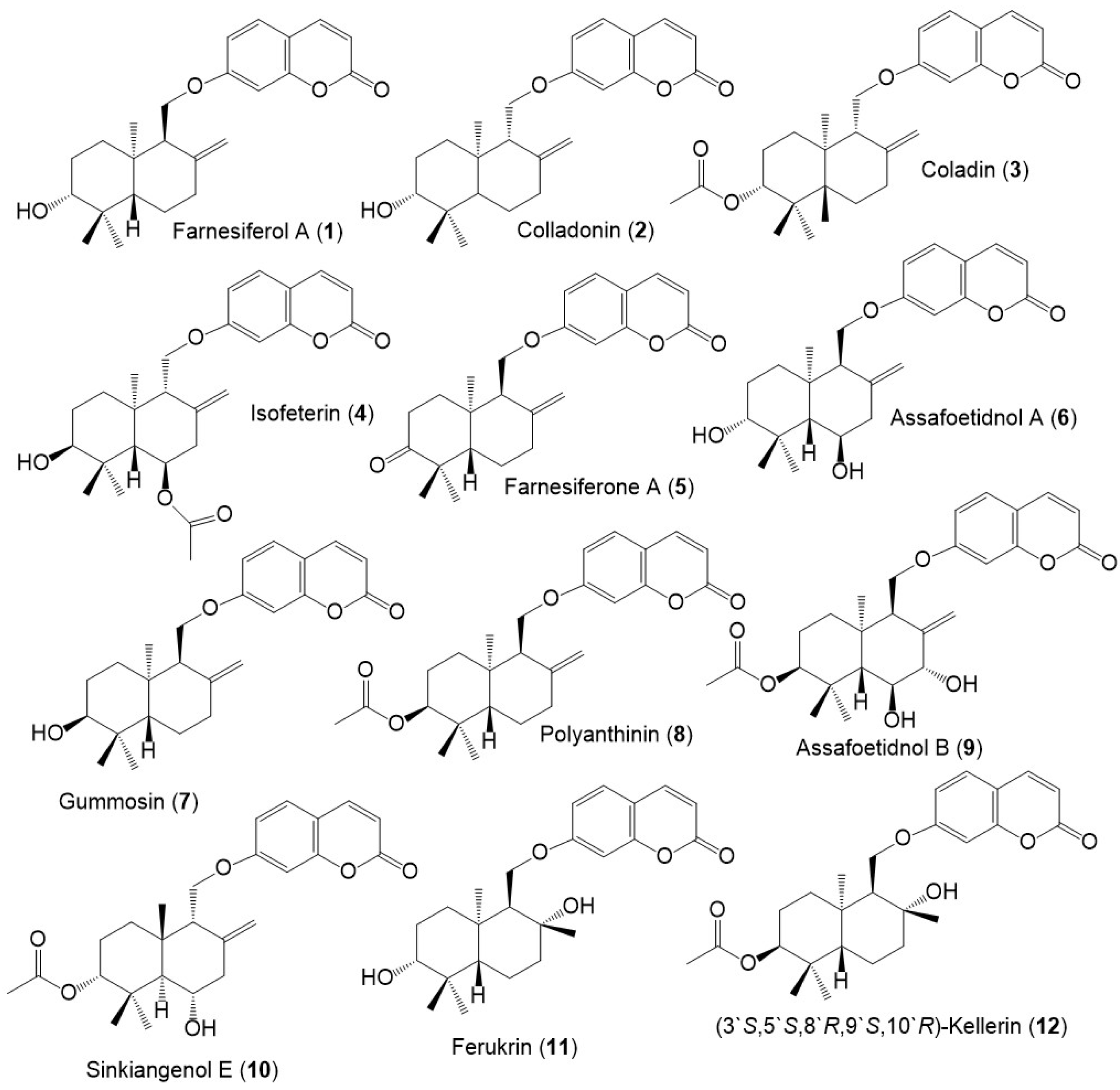
Figure 3.
Structures of sesquiterpene coumarins (1–12) reported from F. sinkiangensis.
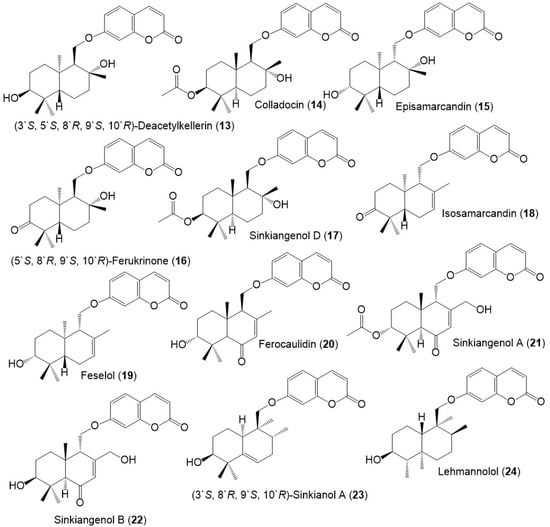
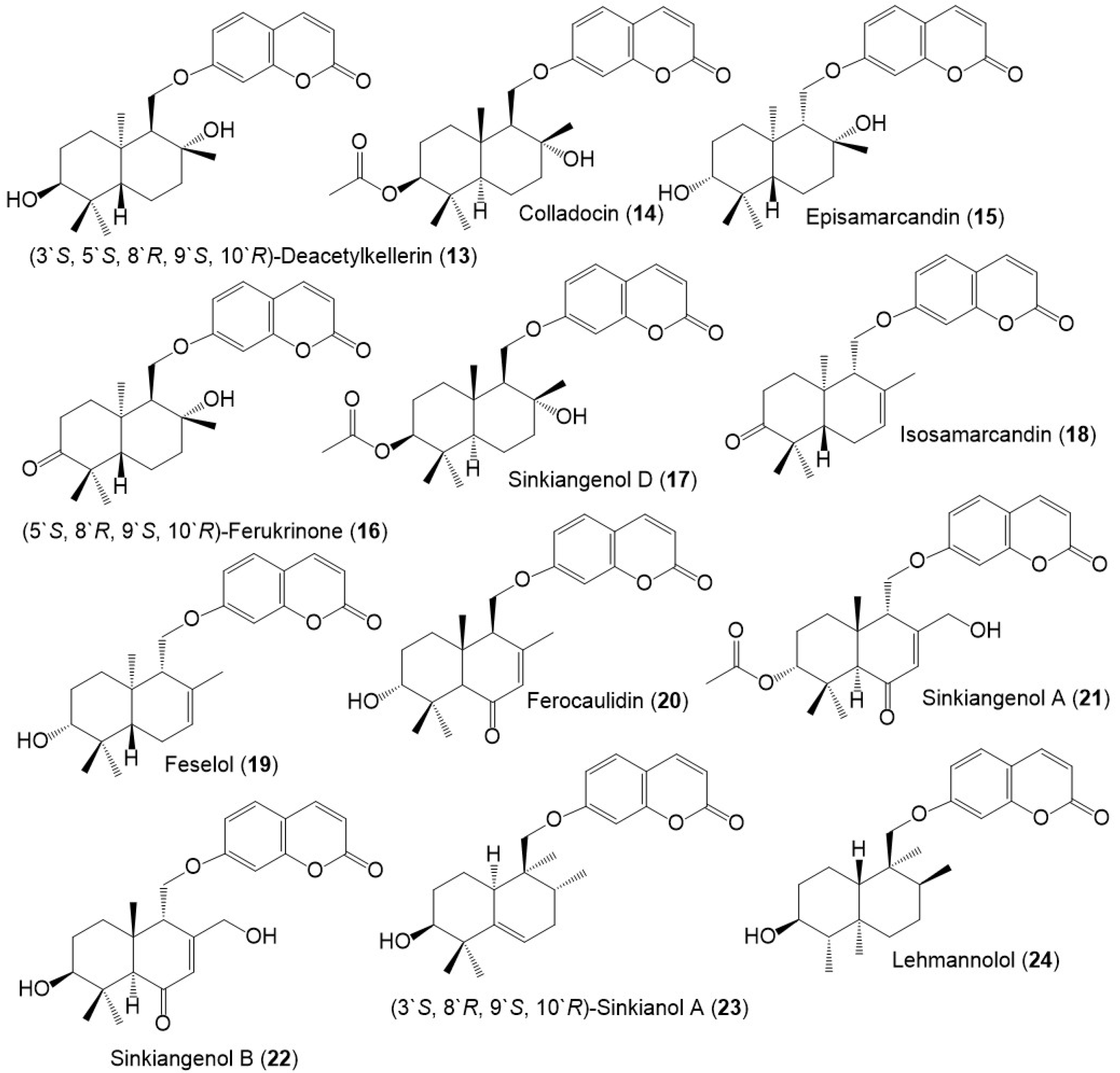
Figure 4.
Structures of sesquiterpene coumarins (13–24) reported from F. sinkiangensis.
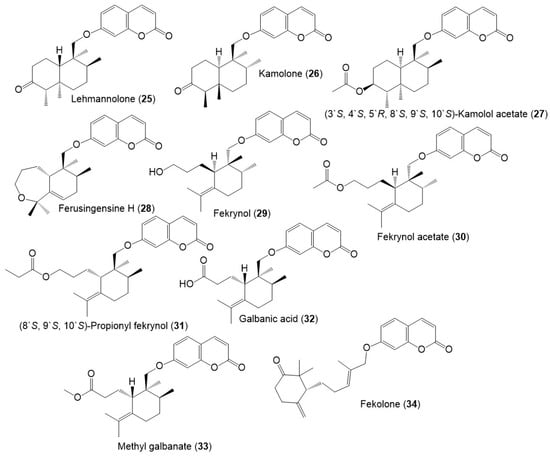
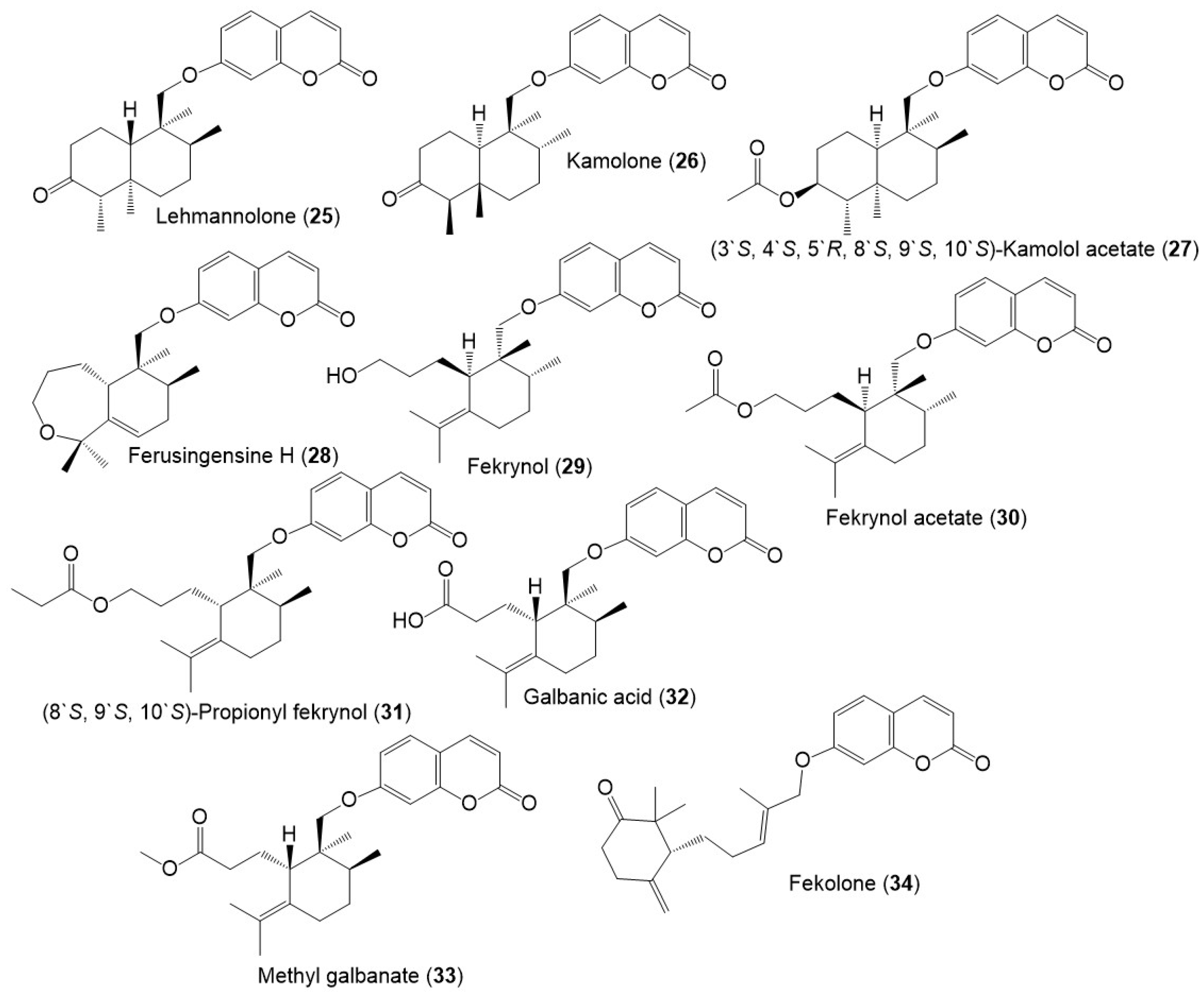
Figure 5.
Structures of sesquiterpene coumarins (25–34) reported from F. sinkiangensis.
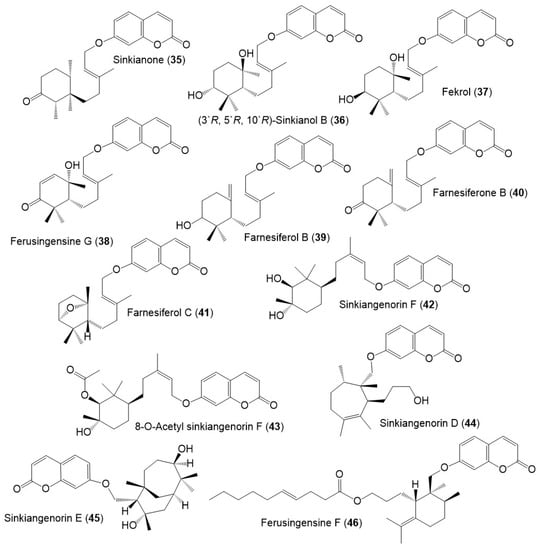
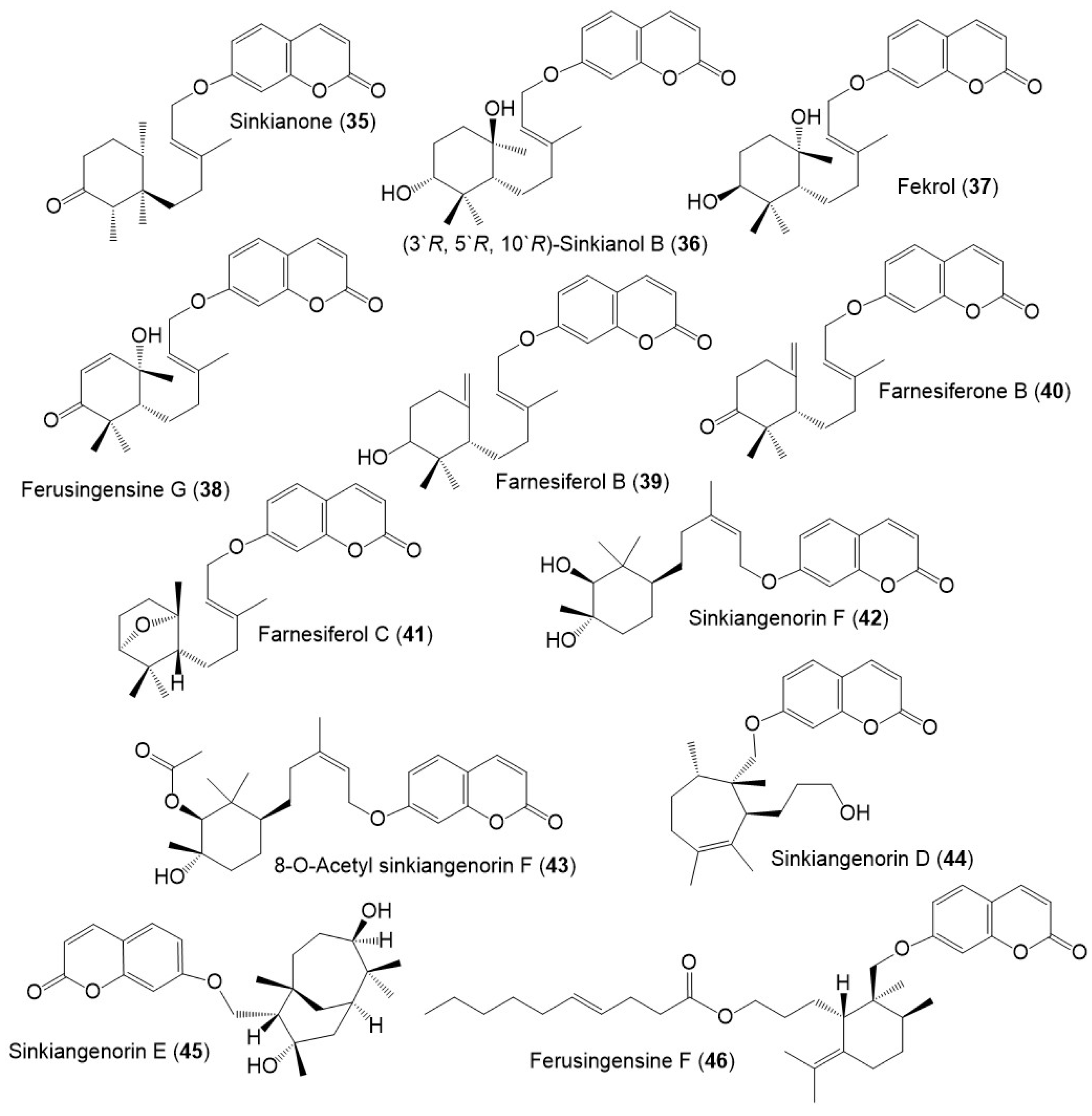
Figure 6.
Structures of sesquiterpene coumarins (35–46) reported from F. sinkiangensis.
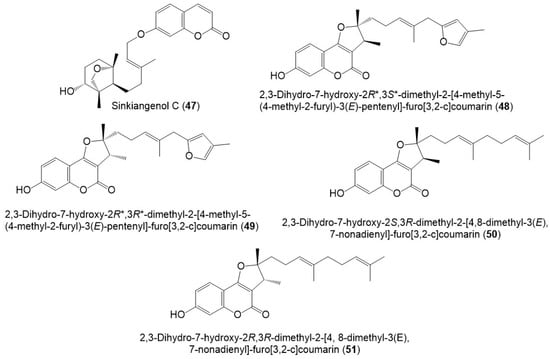
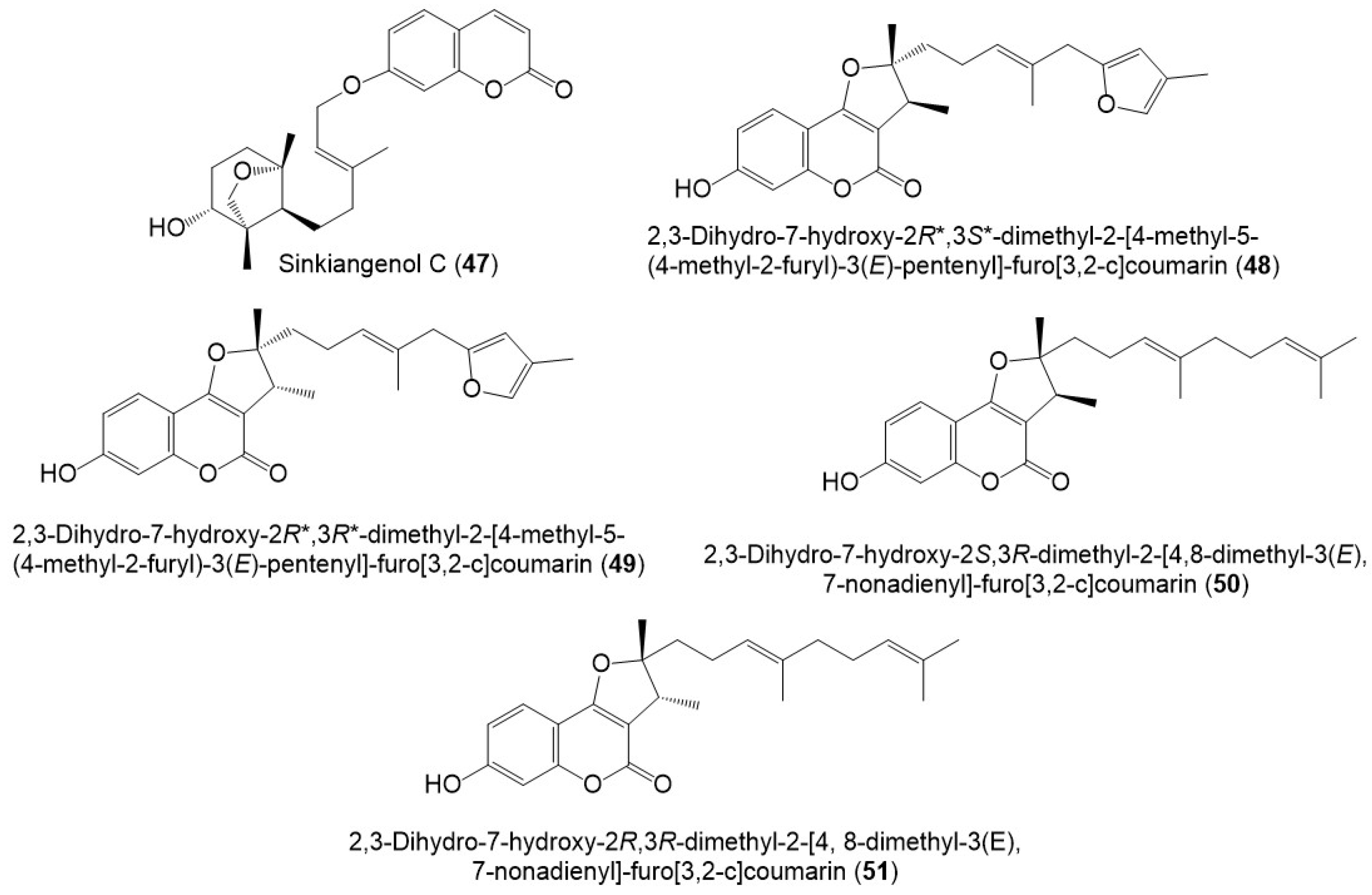
Figure 7.
Structures of sesquiterpene coumarins (47–51) reported from F. sinkiangensis.
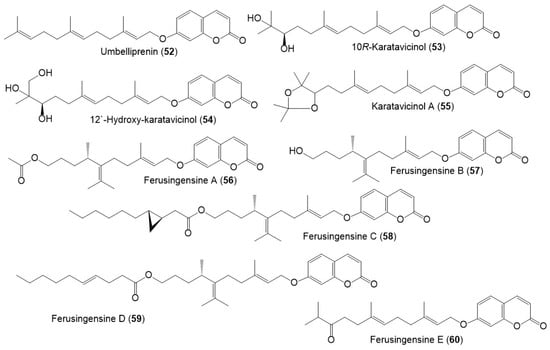
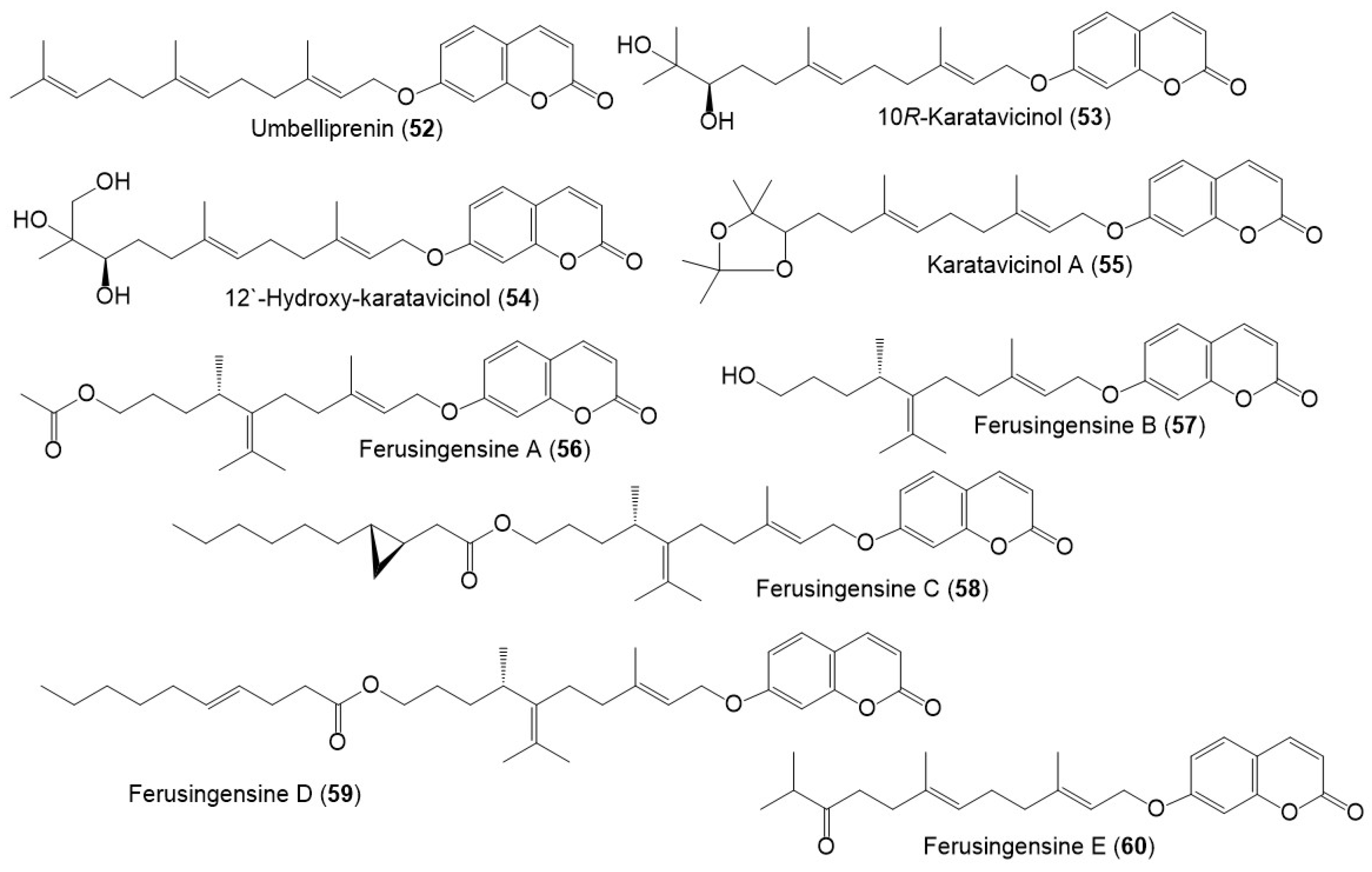
Figure 8.
Structures of sesquiterpene coumarins (52–60) reported from F. sinkiangensis.
These compounds featured linked coumarin and sesquiterpene units through C–O–C ether bridge. These metabolites include monocyclic, bicyclic, or chain derivatives. Also, they could be accountable for many of the stated bioactivities of this plant.
Their separation was performed by different chromatographic techniques, including SiO2/RP-18/Sephadex LH-20/HPLC, whereas the identification and configuration were accomplished using assort spectral tools (e.g., UV, NMR, MS), as well as CD, [α]D and Xray analyses. They had UV absorbance at 320–330 nm and a common fragment at m/z 185 in MS [39].
Among these metabolites, karatavicinol A (55), a new sesquiterpene coumarin along with 32, 39, 41, 52, and 53 were purified from the antiulcer resin CHCl3 extract [14] (Figure 5).
5.2. Sesquiterpene Chromones and Monoterpene Coumarins
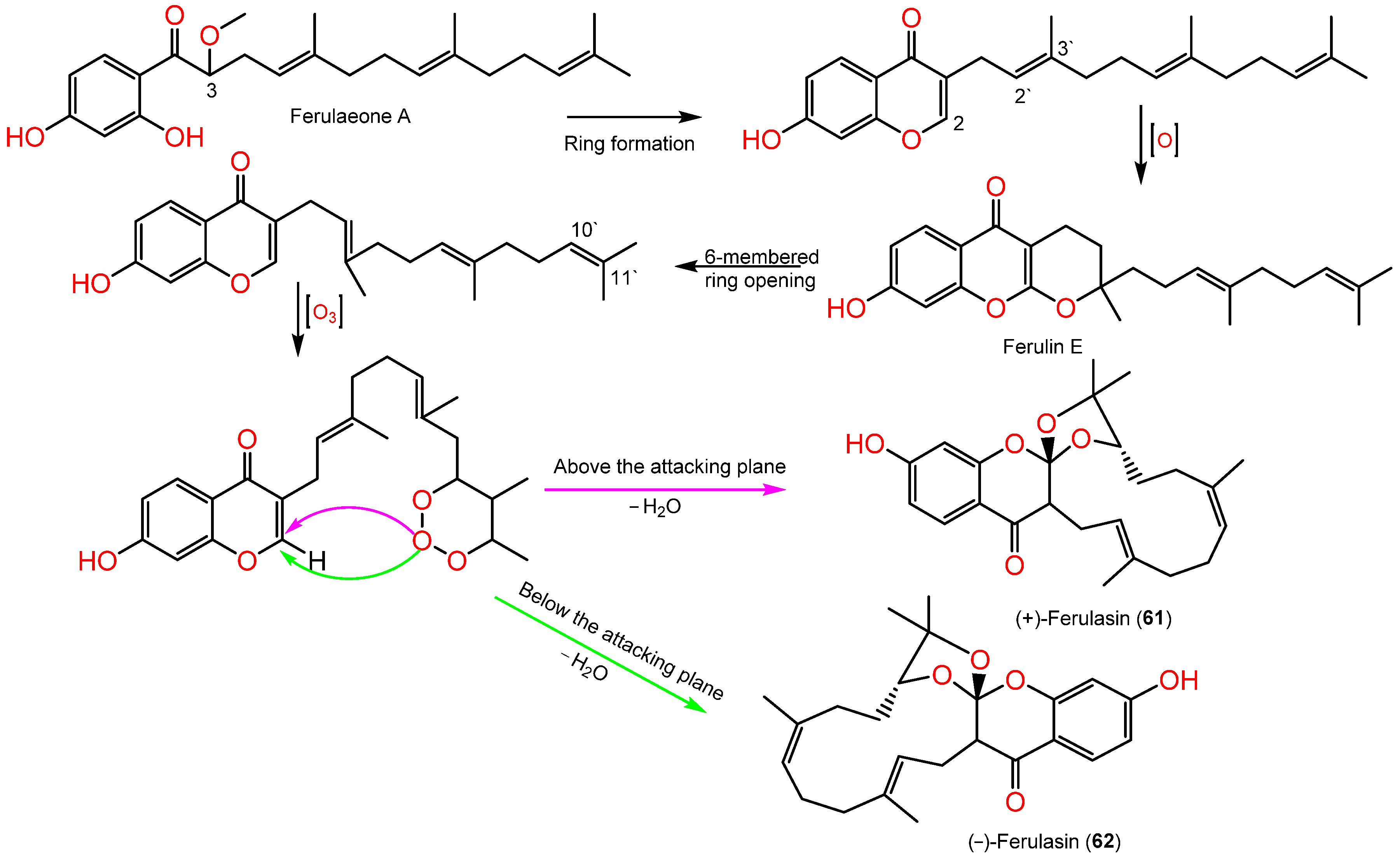
Sesquiterpene chromones possessing a 24-carbon skeleton consisting of sesquiterpene and chromone were reported from F. sinkiangensis roots. In 2022, Wang et al., reported the purification of new derivatives, (±)-ferulasin from the roots MeOH extract that was established by diverse spectral, Xray, and ECD analyses. Ferulasins (61 and 62) showed an unusual oxygen-bearing macrocyclic skeleton with a tri-oxaspiro unit and a new backbone in which the C-10` and C-11` of the sesquiterpene side chain form an oxygen-including 13-membered ring with C-2 of chromone (Figure 9). It was obtained as an enantiomeric mixture that was chiral-separated by HPLC to (+)-61 and (-)-62 with 2R/3R/10`R and 2S/3S/10`S configurations, respectively based on Xray and ECD data [40]. Wang et al., assumed the biosynthesis of 61 and 62 from ferulaeone A (71). The reduction of the 71-side chain C-3 produces 6-membered ring containing oxygen (Scheme 1). After that, the side chain C2`-C3` is oxidized and yields a 6-membered ring having oxygen with C-2 of chromone. After the six-membered ring fission, C10`-C11` bond reacts with ozone. Lastly, attack of oxygen-atoms to C2-C3 the double bond from below or above the plane with removal of H2O a molecule to afford 61 and 62 (Scheme 1).
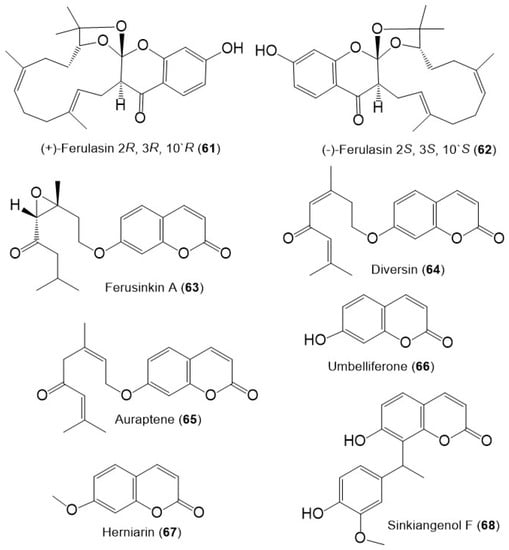
Figure 9.
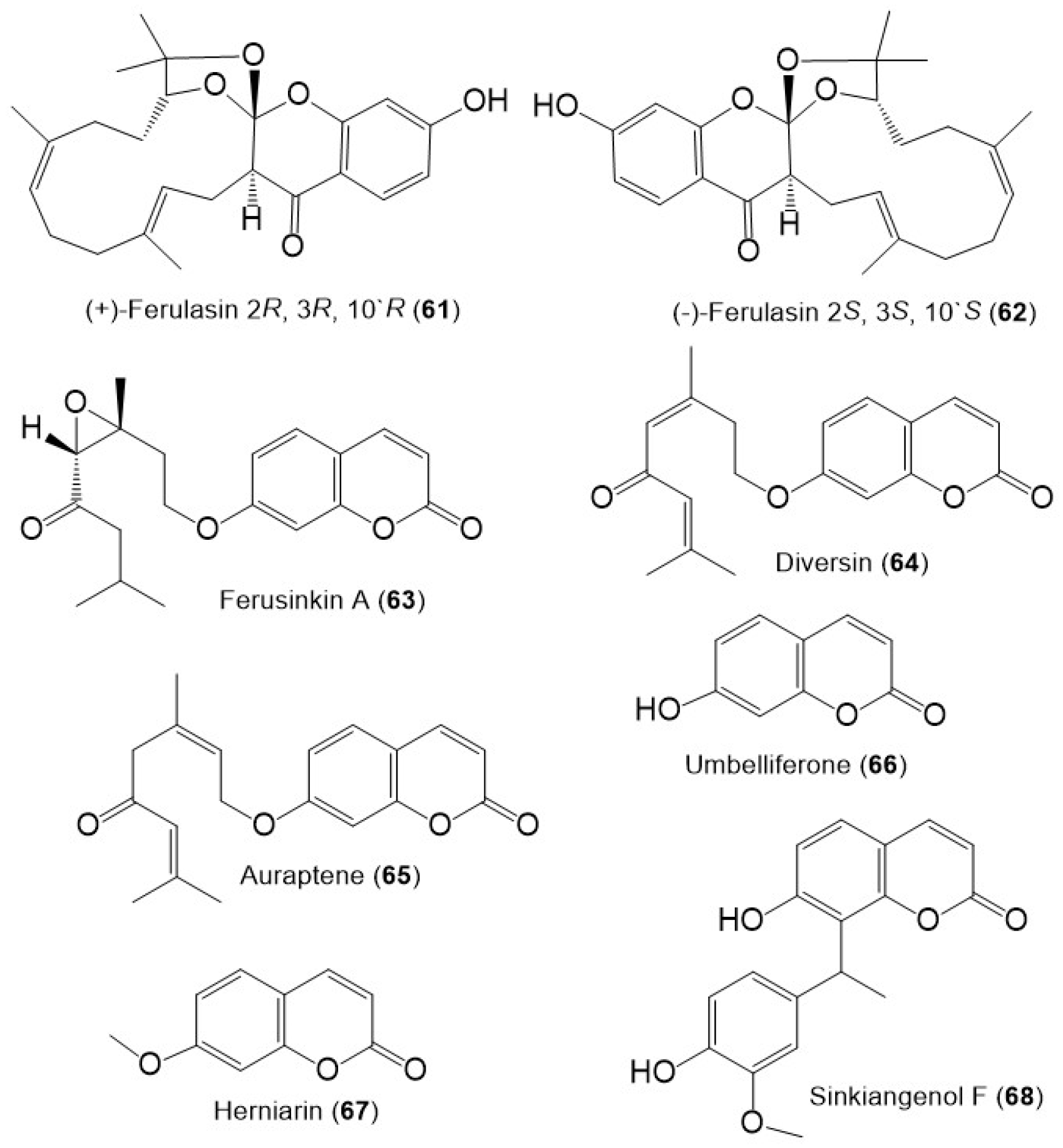
Structures of sesquiterpene chromones (61 and 62), monoterpene coumarins (63–65), and coumarins (66–68) from F. sinkiangensis.
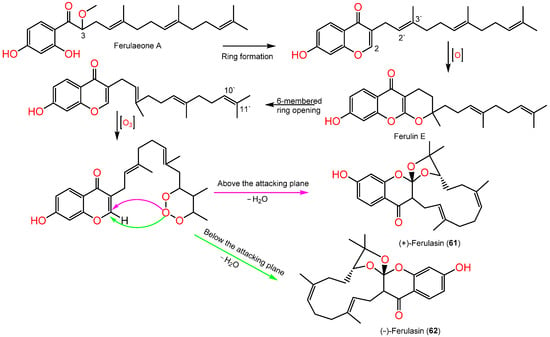
Scheme 1.
Biosynthetic pathway of (±)-ferulasin [40].
Additionally, ferusinkin A (63), a rare new monoterpene coumarin and known analogs 64 and 65 were purified and identified by Liu et al. in 2020 from the aerial parts MeOH extract (Figure 9) [41].
5.3. Coumarins
The coumarins; 66 and 67 were purified from the F. sinkiangensis aerial parts and characterized based on spectral and physical data [41]. Additionally, sinkiangenol F (68) a new coumarin was purified from the resin EtOH extract. This compound is rare coumarin derivative having a coumarin unit connected to phenylethane moiety by C–C linkage at C-8 [39].
5.4. Sesquiterpene Phenylpropanoids
Sesquiterpene phenylpropanoid derivatives were commonly separated from Ferula genus [42]. In 2018, Wang et al., stated the separation of new sesquiterpene phenylpropanoids, sinkiangenones A (69), and B (70), along with 72 from the resin 95% EtOH extract, which were specified utilizing spectral and CD analyses (Figure 10) [29].
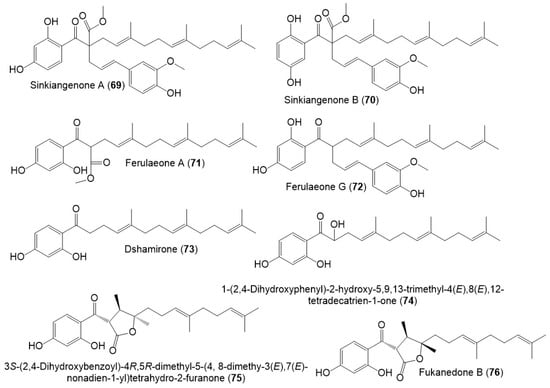
Figure 10.
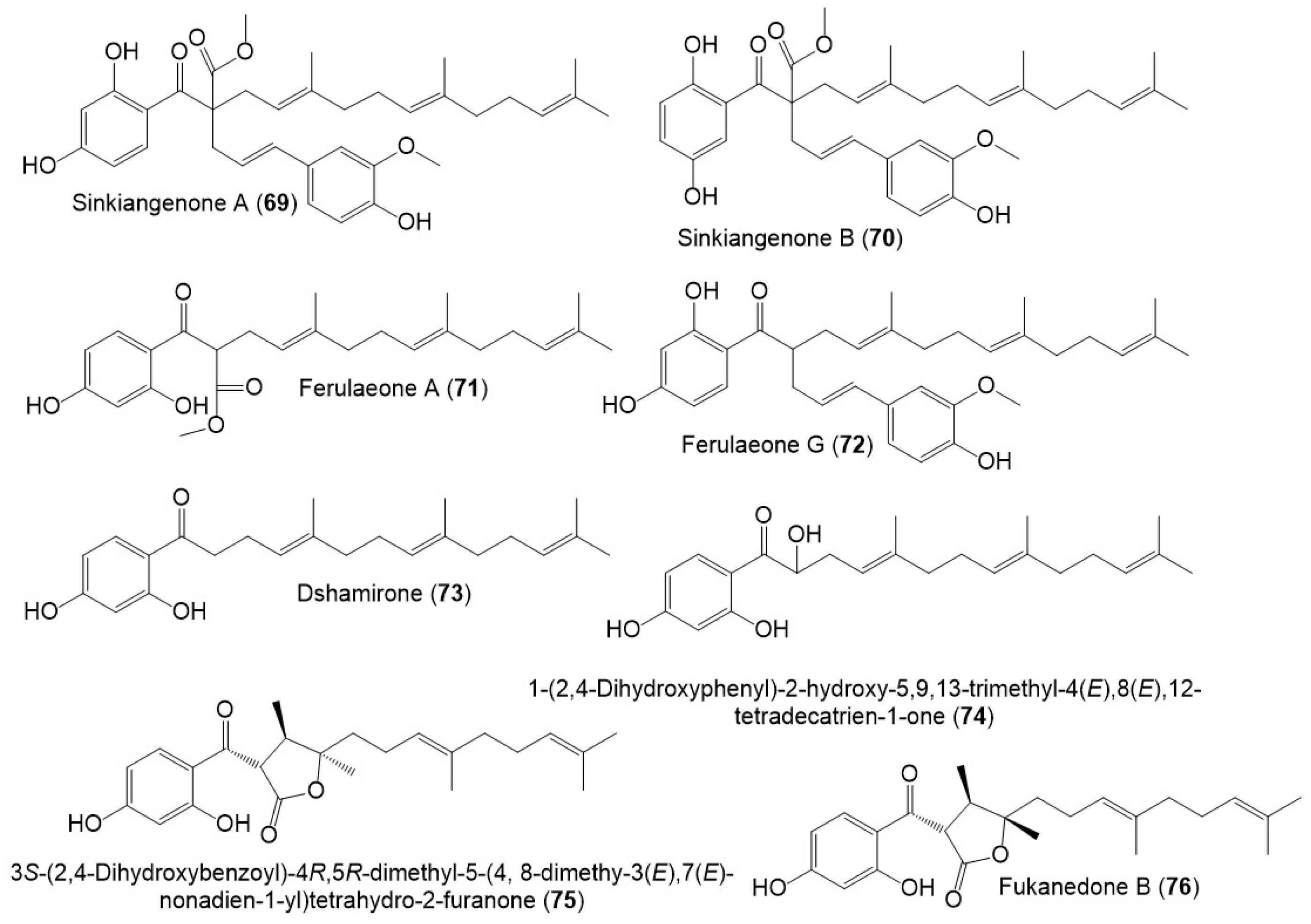
Structures of sesquiterpene phenylpropanoids (69–76) from F. sinkiangensis.
Also, Wang et al., separated sesquiterpene phenylpropanoid derivatives (71 and 73–76) from F. sinkiangensis roots MeOH extract [43]. It is noteworthy that these derivatives were previously reported from other Ferula species: 71 from F. ferulioides roots; 73 from the underground parts of F. heuffelii and roots of F. ferulioides, F. fukanensis, F. dubjanskyi, and F. mongolica; 74 from F. ferulioides roots; 75 from F. heuffelii, F. fukanensis, and F. ferulioides roots, and 76 from F. heuffelii and F. ferulioides roots [42,43], suggesting the close chemotaxonomic relation of F. sinkiangensis and the other Ferula species, therefore, they could share the biosynthetic pathways of these metabolites [43].
5.5. Lignans
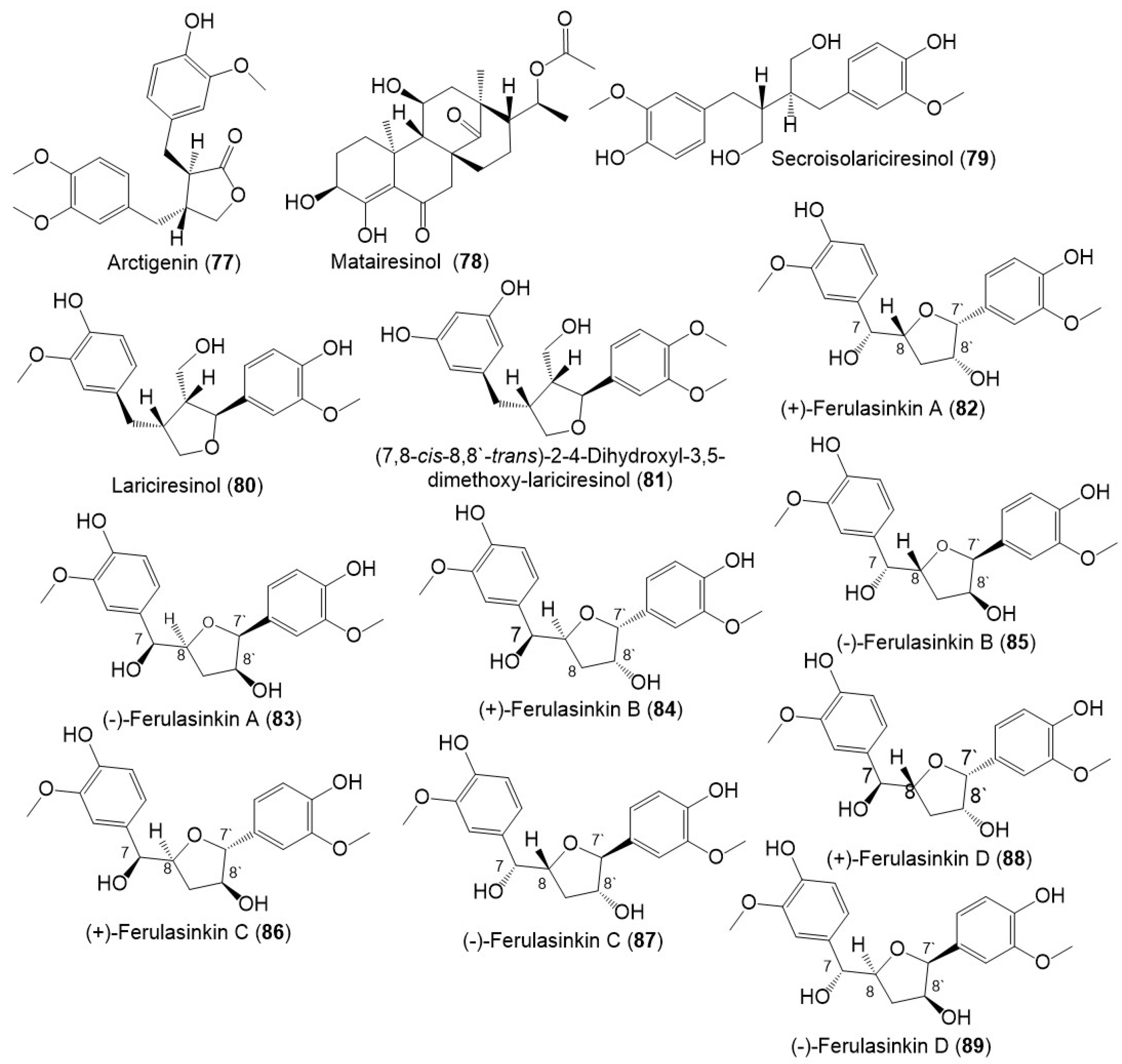
Lignans, norlignans, and sesquilignans were reported mainly from F. sinkiangensis seeds and resins. (±)-Ferulasinkins A–D (82–89) (Figure 11), new norlignans characterized by tetrahydrofuran rings were separated as racemic mixtures from the EtOAc fraction of the resins 95% EtOH extract by MCI gel CHP 20P/RP-18/YMC gel ODS-A-HG/SiO2/Sephadex LH-20/preparative TLC.
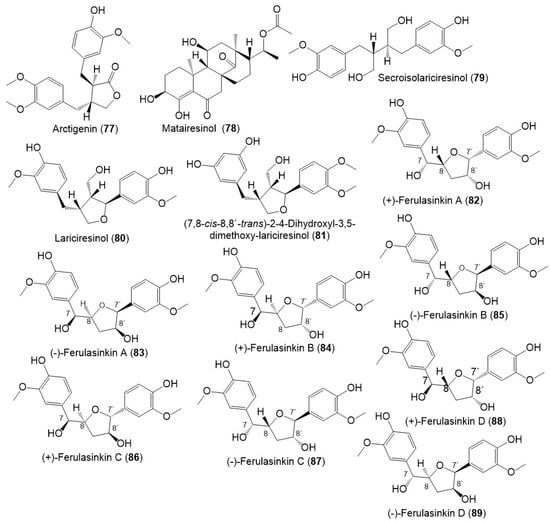
Figure 11.
Structures of lignans (77–89) reported from F. sinkiangensis.
The chiral column HPLC separation afforded their (-)- and (+)-antipodes. Their structures and configurations were specified by spectral tools and computational methods [28].
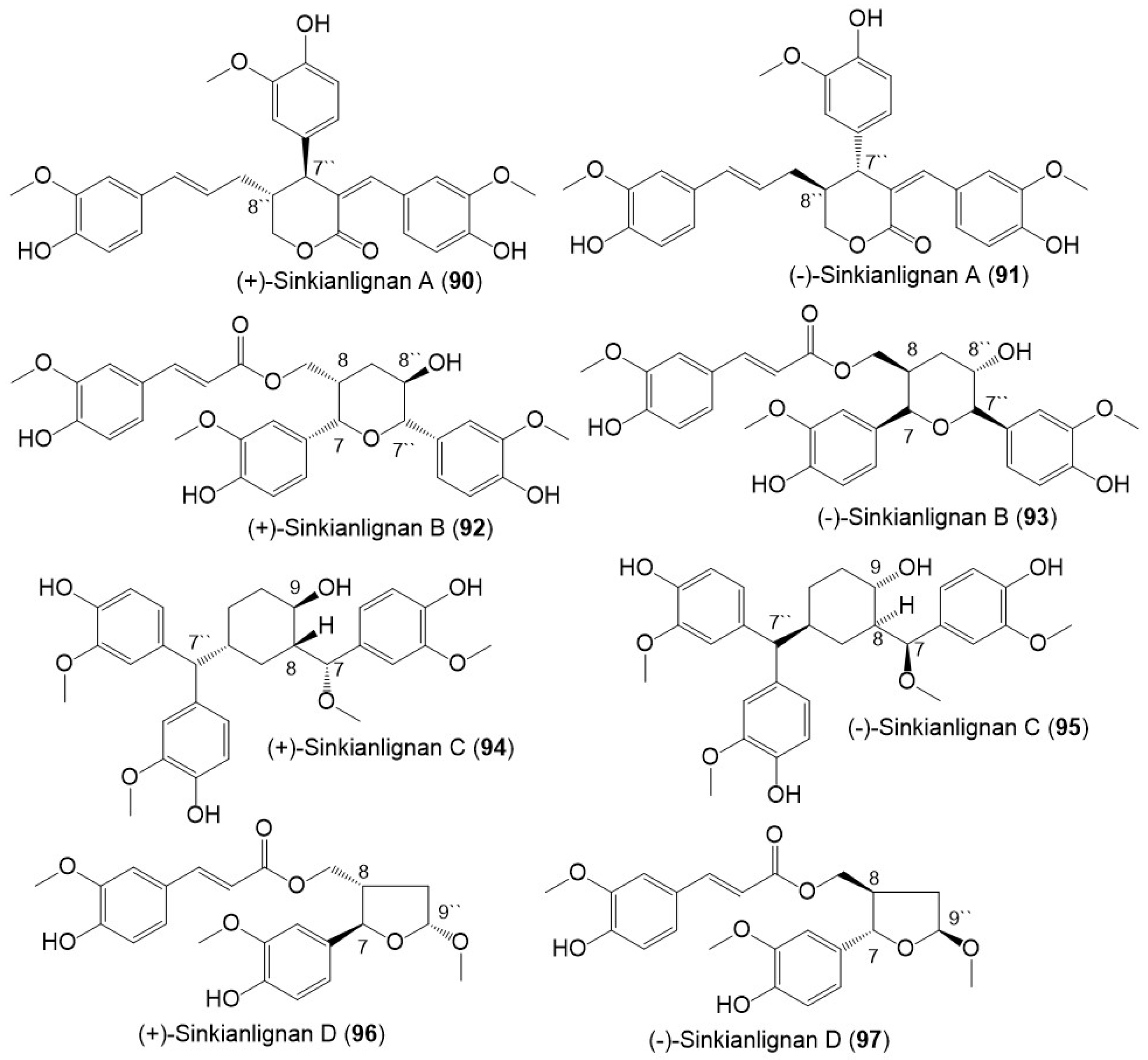
Additionally, Li et al., purified new racemic sesquilignans; sinkianlignans (±)-A–D (90–97) characterized by a rare α-γ′, β-γ′, and γ-γ′ linkage pattern, and new lignans; sinkianlignans (±)-E–F (98–101) from the resin 95% EtOH extract using SiO2/RP-18/MCI gel CHP 20P/YMC gel ODS-A-HG/Sephadex LH-20 CC/preparative TLC and chiral HPLC and elucidated by spectral and computational tools (Figure 12 and Figure 13) [44].
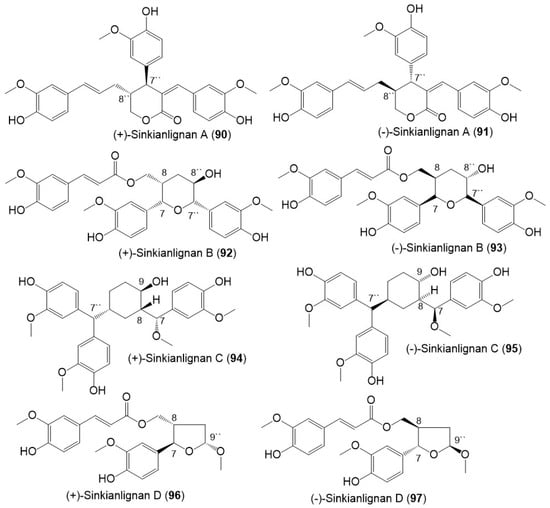
Figure 12.
Structures of lignans (90–97) reported from F. sinkiangensis.
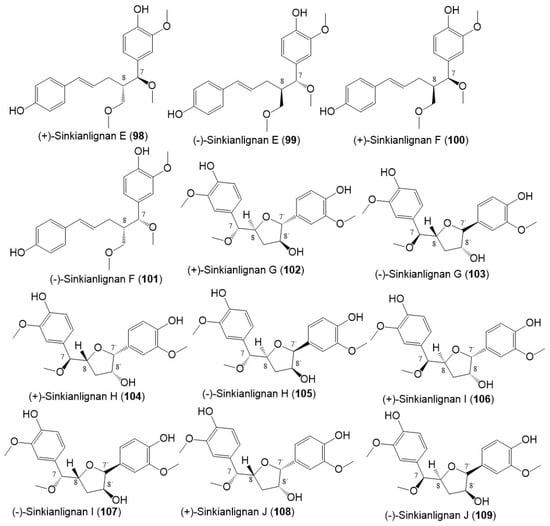
Figure 13.
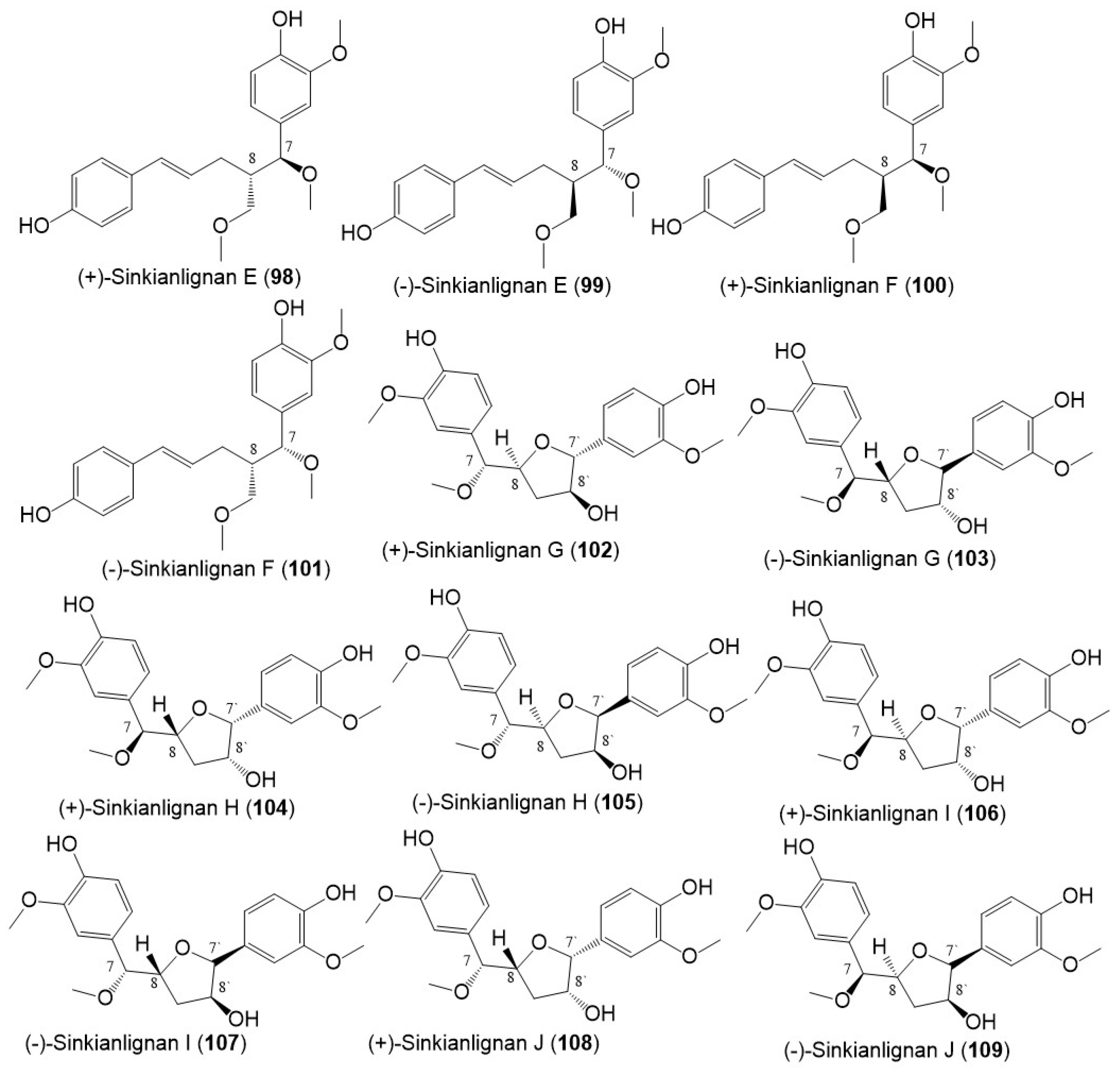
Structures of lignans (98–109) reported from F. sinkiangensis.
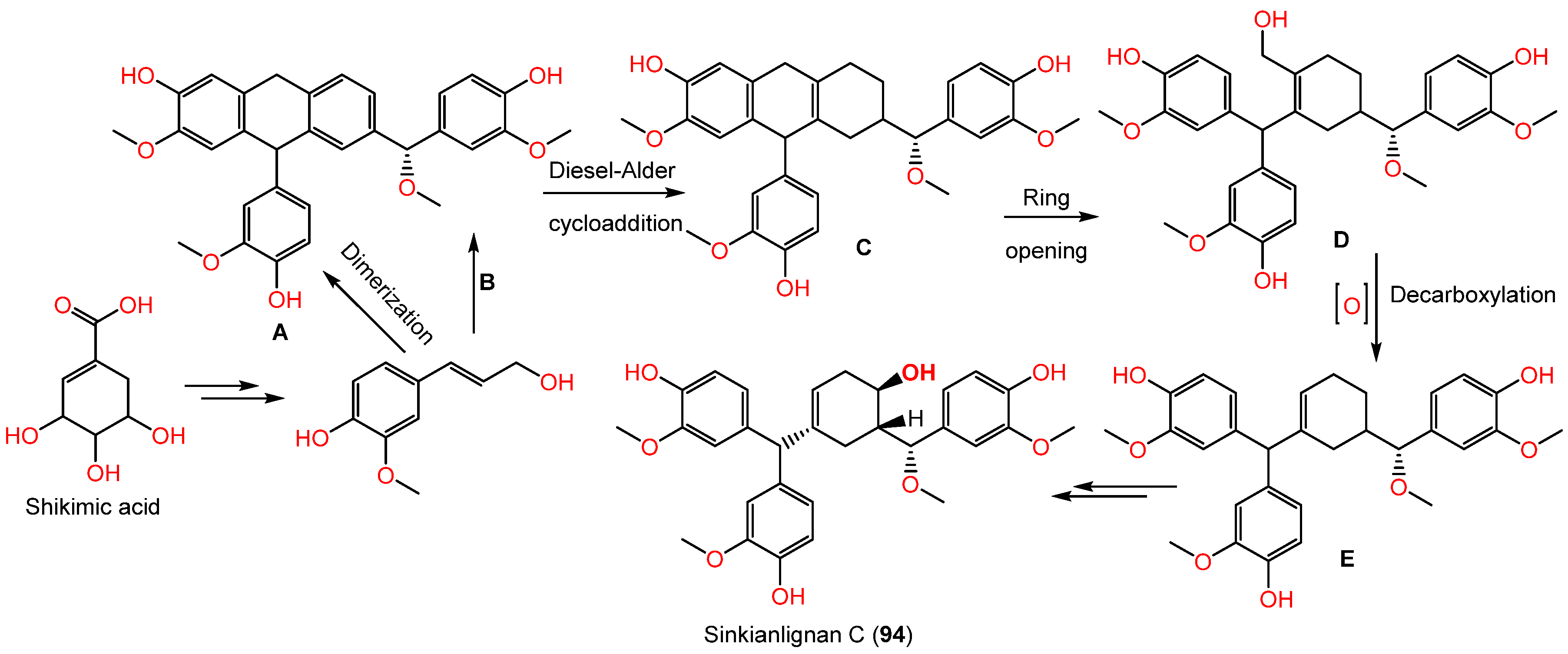
Sesquilignans are type of lignans that consist of 3 phenylpropanoid units. Compound 94 was assumed to be biosynthesized by the shikimate pathway (Scheme 2). First, phenylpropanoid is formed by a shikimic acid pathway that undergoes polymerization to produce intermediate A (aryltetralin lignan). In addition, intermediate C with a new six-membered ring skeleton is yielded from the intermediates A and B by the Diesel-Alder cycloaddition reaction. Moreover, C produces D by opening the ring at C1−C7. Subsequent oxidization and decarboxylation of D yields E. After a set of redox reactions, intermediate E gives 94 [44].
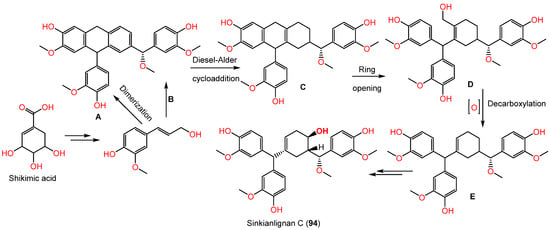
Scheme 2.
Biosynthesis of sinkianlignan C (94) via shikimate pathway [44].
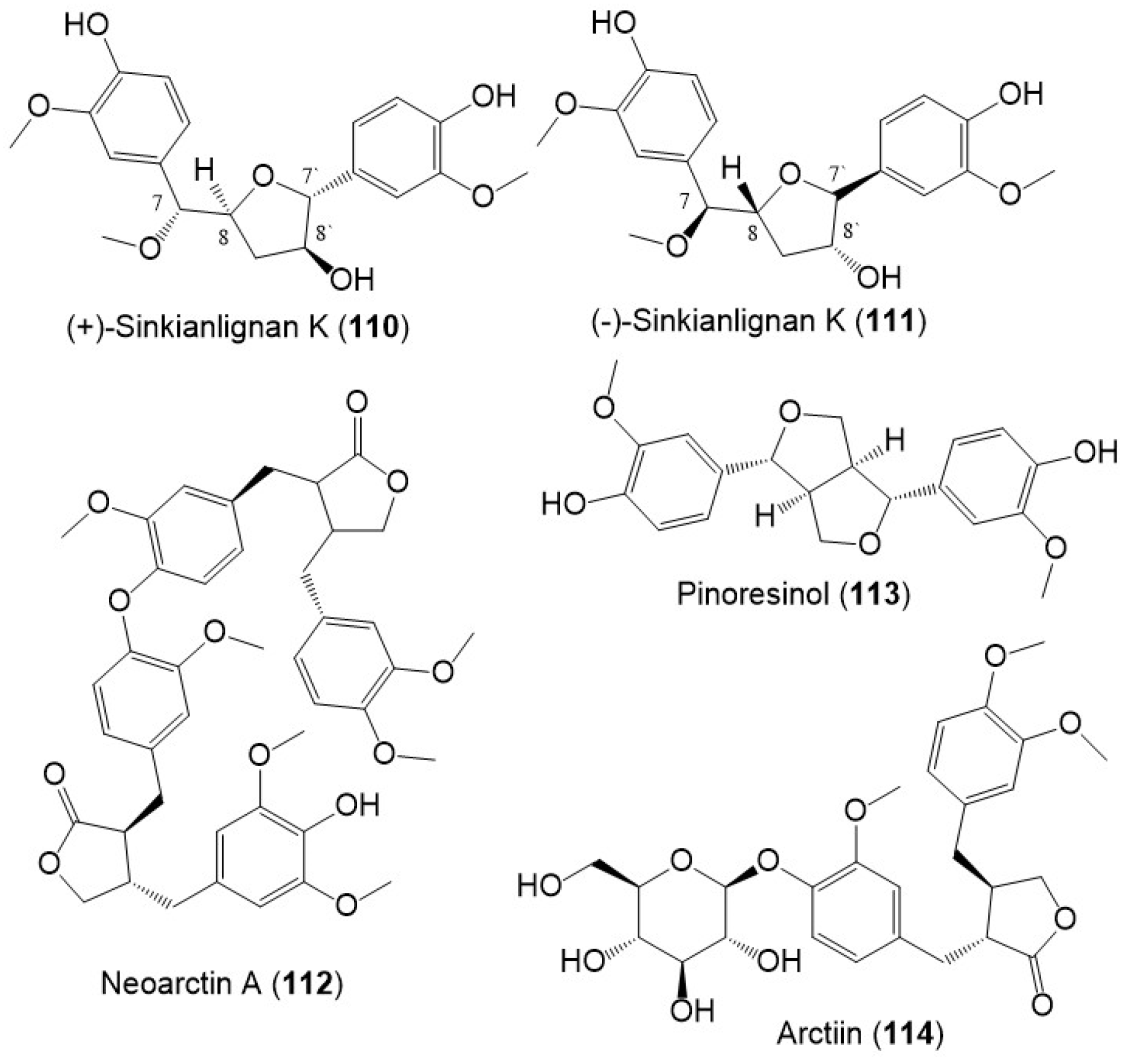
Sinkianlignans G–K (102–111) new norneolignans were purified from 95% resin EtOH extract utilizing SiO2/RP-18/MCI gel CHP 20P/Sephadex LH-20/preparative TLC (Figure 14). Compounds 102–111 were obtained as racemic mixtures that were separated by chiral HPLC and characterized by spectral and computational means [18].
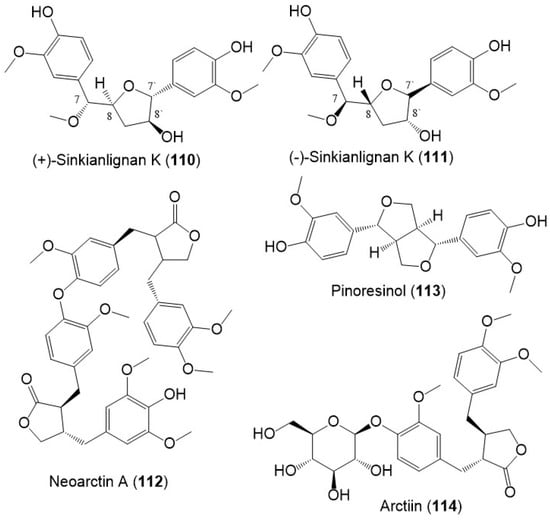
Figure 14.
Structures of lignans (110–114) reported from F. sinkiangensis.
5.6. Sesquiterpenes and Monoterpenes
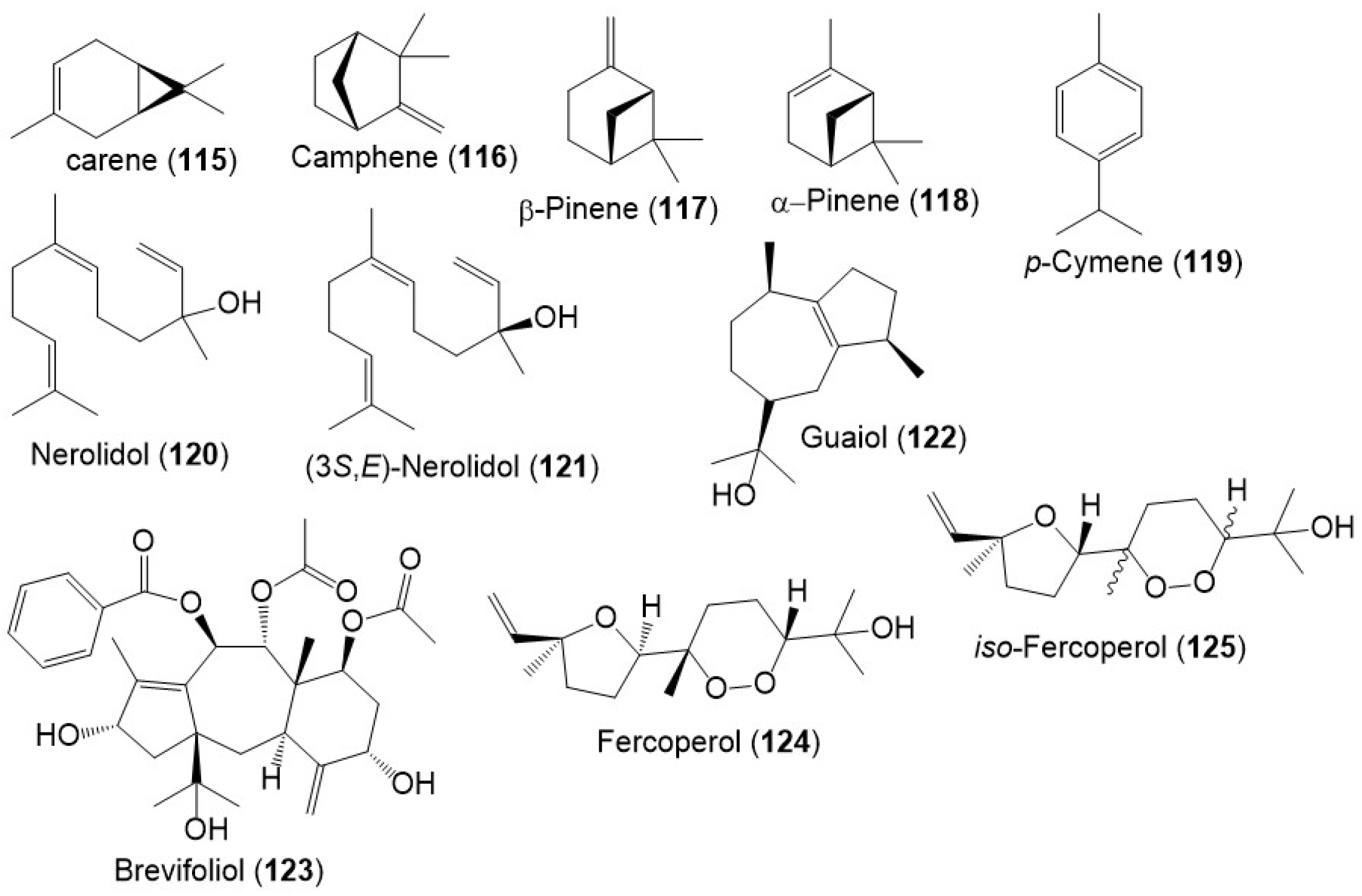
Besides, monoterpenes (e.g., carene (115), camphene (116), β-pinene (117), α- pinene (118), and p-cymene (119)) were encountered in the F. sinkiangensis oleo-gum resins` volatile oil (Figure 15) [8]. Further, Wang et al., purified a new sesquiterpenoid 125, along with 120, 122, and 124 from F. sinkiangensis roots. Compounds 124 and 125 were isomeric cyclic-endoperoxy-nerlildol sesquiterpene derivatives, whereas 120 and 122 were chain and guaiane-type sesquiterpenoid, respectively [40].
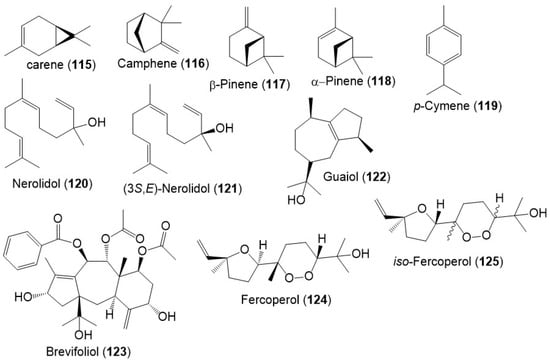
Figure 15.
Structures of monoterpenes (115–119) and sesquiterpenes (120–125) reported from F. sinkiangensis.
5.7. Sulfanes
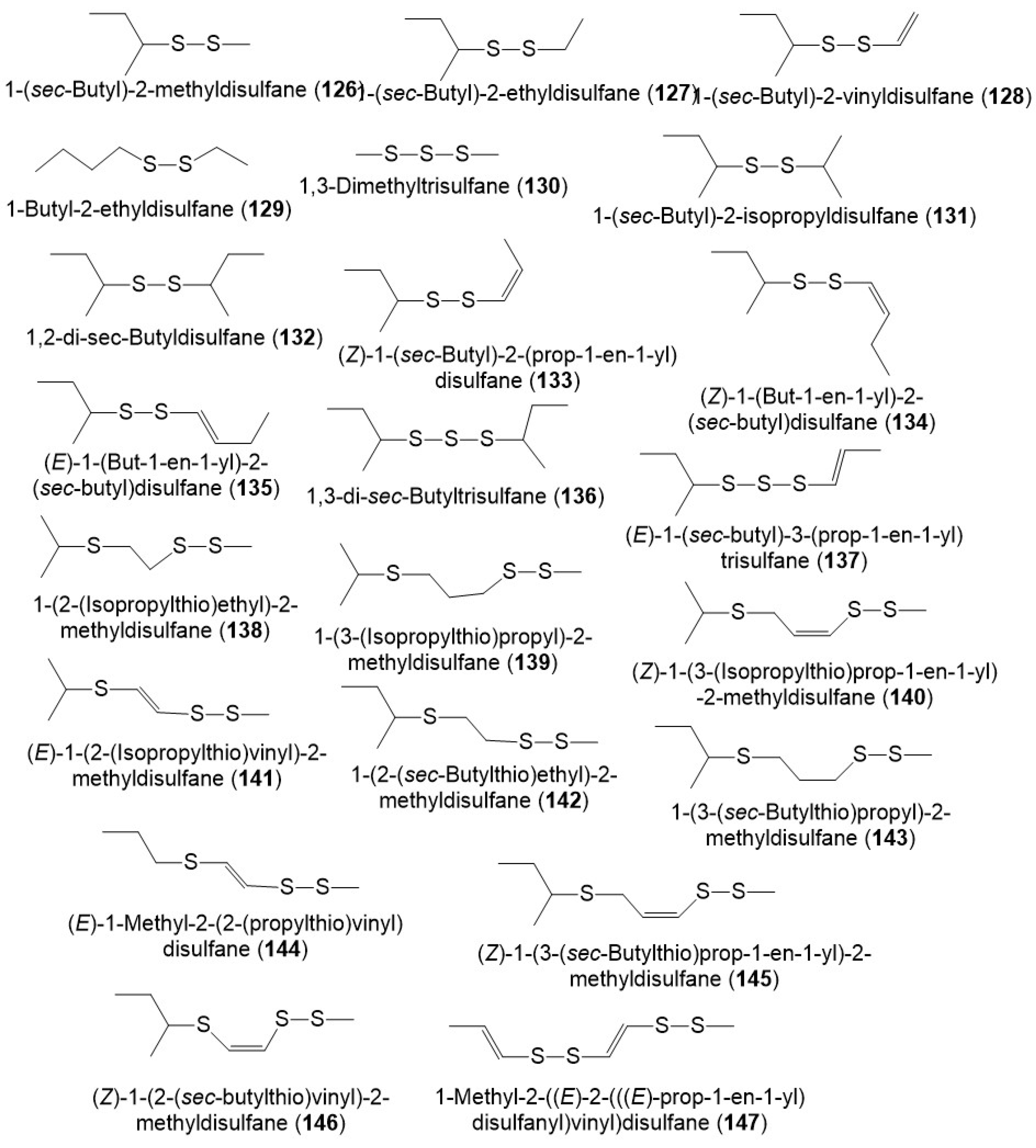
It was reported that polysulfides including disulfanes, trisulfanes, di-disulfanes, and thio-disulfanes are the predominant constituents of the F. sinkiangensis volatile oil oleo-gum resins. The oil content was 16.7% of which 64.1% were sulfur compounds. The disulfanes were the prime components: 126–130, 134, and 135 (Figure 16) [8]. Further, the GC-MS analysis of essential oil (3.8% yield) of F. sikiangensis seeds obtained from Xinjiang, China that was prepared by hydro-distillation method revealed the existence of 26 metabolites, comprising 99.001% of total oil [33].
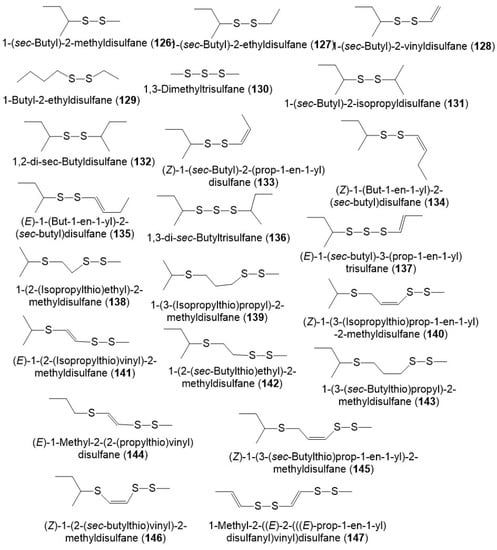
Figure 16.
Structures of sulfanes (126–147) reported from F. sinkiangensis.
5.8. Sterols
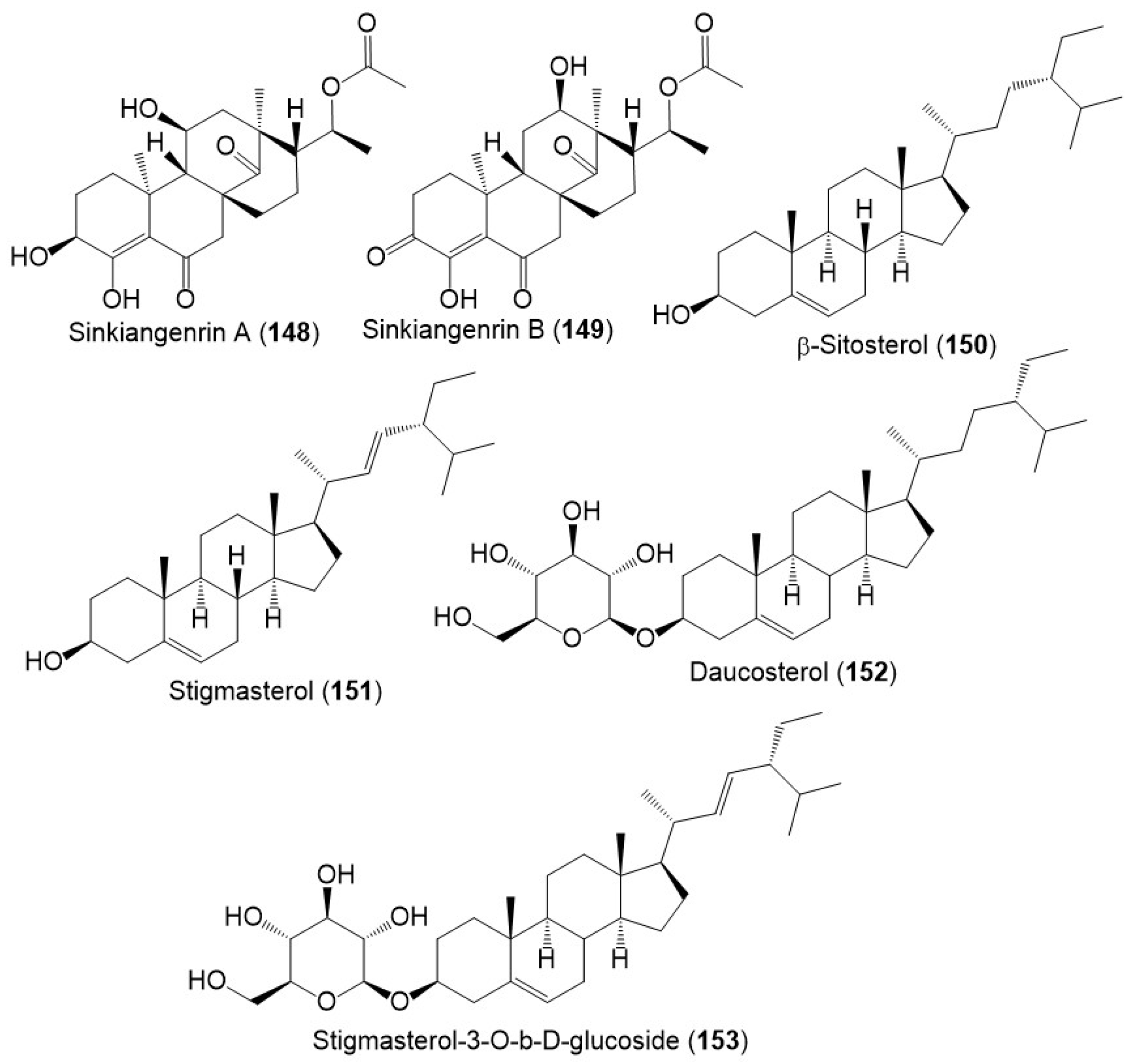
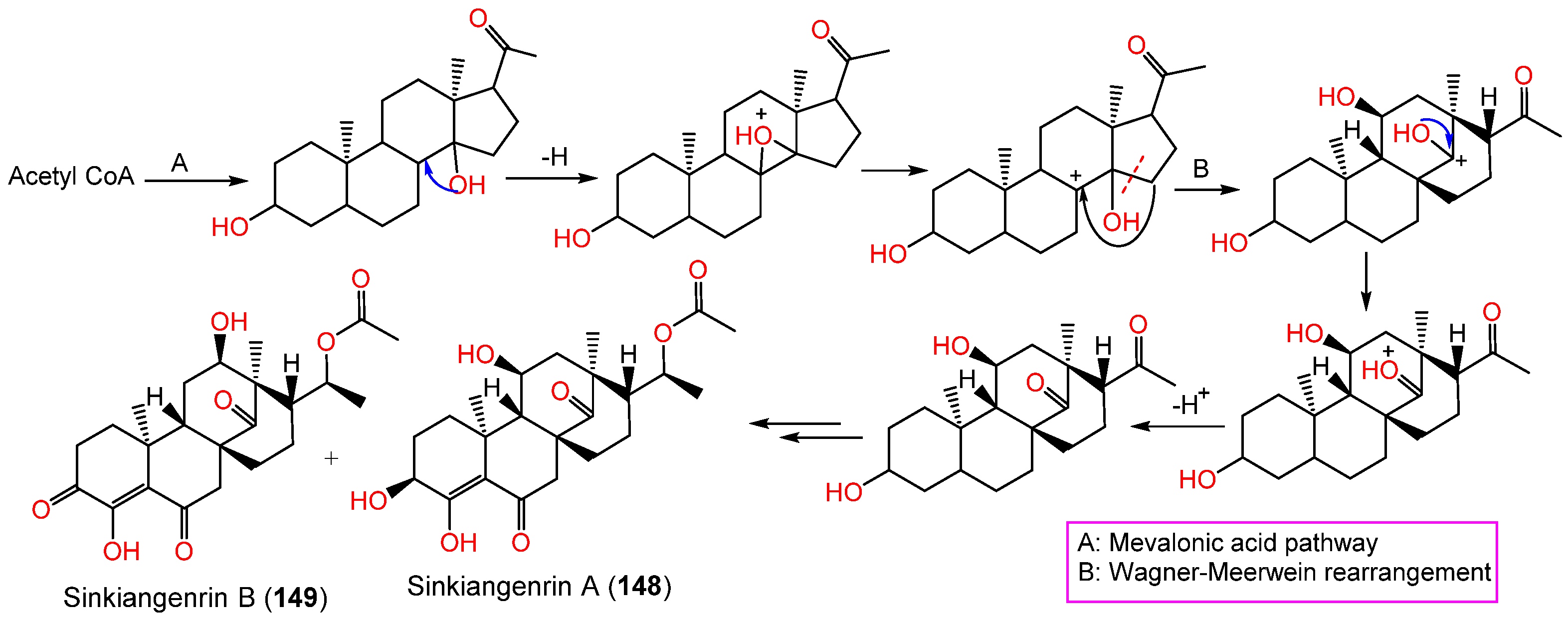
Chromatography separation of F. sinkiangensis 95% EtOH seed extract using SiO2, Sephadex LH-20, and HPLC yielded new steroidal esters: sinkiangenrins A (148) and B (149) that were characterized by NMR and Xray analyses [17]. These compounds are related to oleagenin-cardenolide with different C-13/C-10/C-9/C-8 configuration, having 3S/8R/9S/10S/11S/13S/17R/18R and 8S/9S/10S/12R/13R/17R/18R-configurations, respectively (Figure 17).
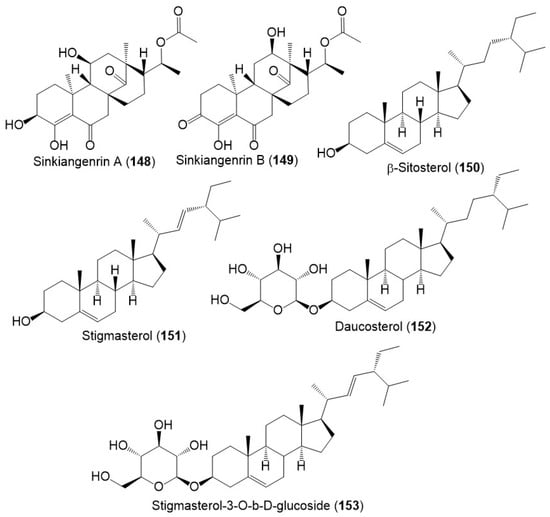
Figure 17.
Structures of sterols (148–153) reported from F. sinkiangensis.
These metabolites have an unparallel carbon framework that originates from C21-steroids (Scheme 3). Firstly, the initiation of D-ring rearrangement by C8–C14 pregnane epoxide formation, then C-8 carbocation formation. After that, Wagner–Meerwein rearrangement results in a C14–15 bond migration to C-8 and the creation of a C-14 protonated carbonyl that is deprotonated [45]. Following that set of enzyme-catalyzed reactions produce 148 and 149 [17].
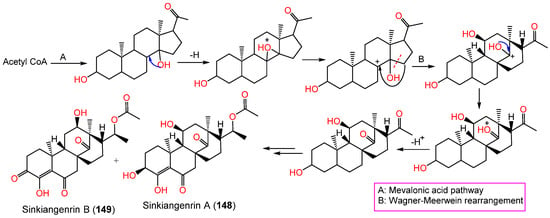
Scheme 3.
Biosynthesis of sinkiangenrins A (148) and B (149) [17].
5.9. Phenolic Compounds and Other Metabolites
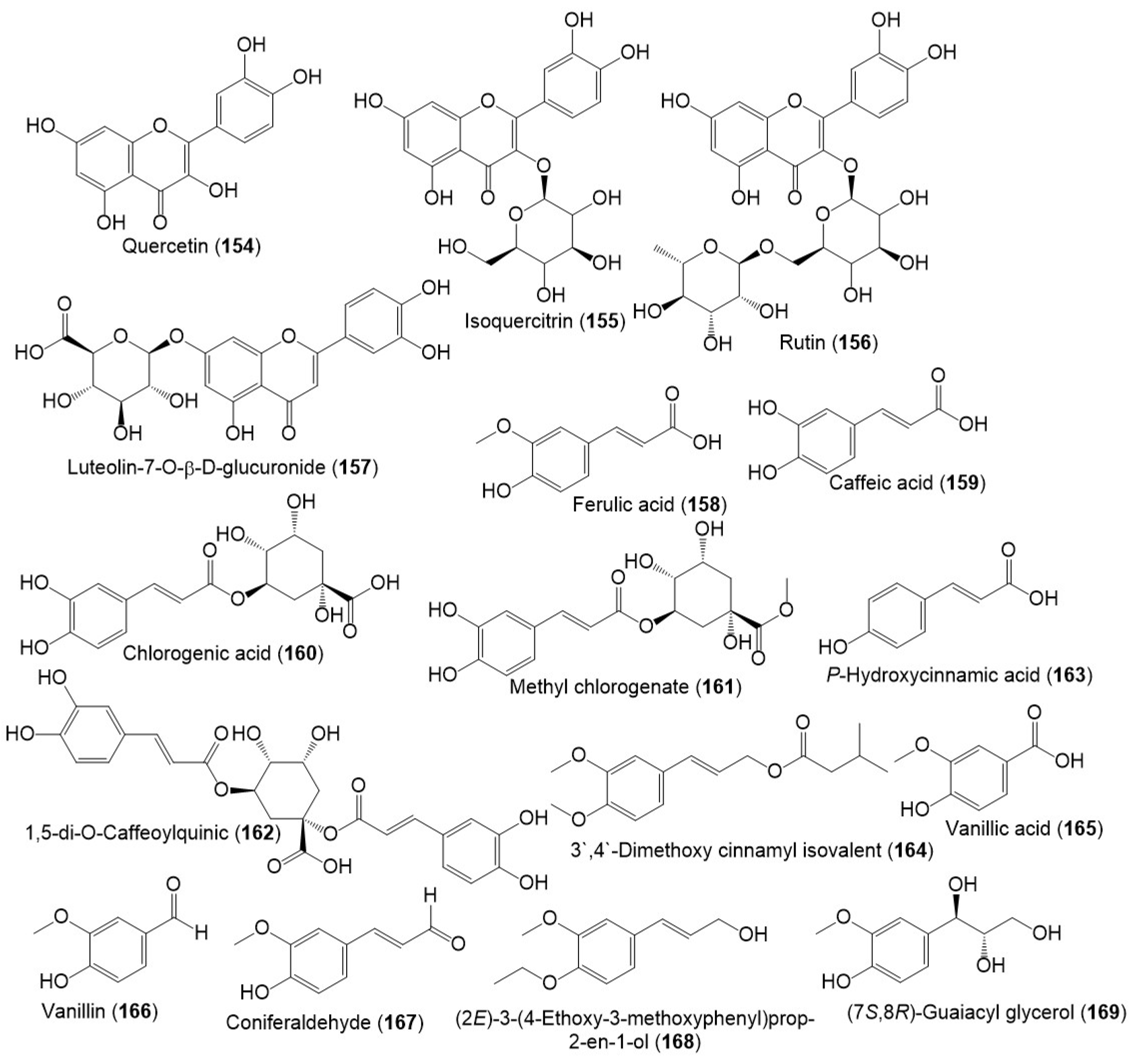
Several studies reported the separation of phenolics metabolites such as flavonoids, phenylpropanoids, and acids from resin and seed extracts (Figure 18 and Figure 19) [18,24,27,29].
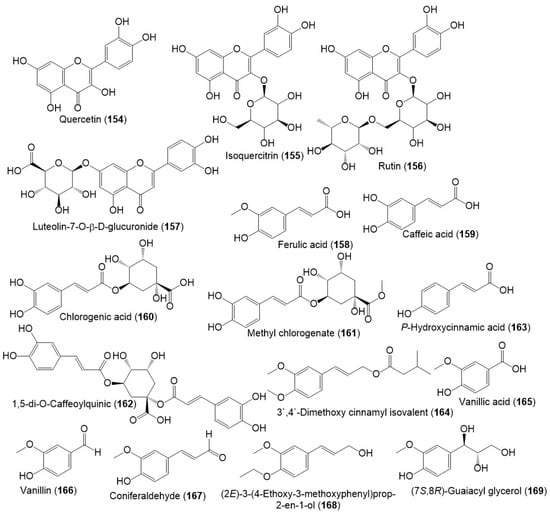
Figure 18.
Structures of phenolic compounds (154–169) reported from F. sinkiangensis.
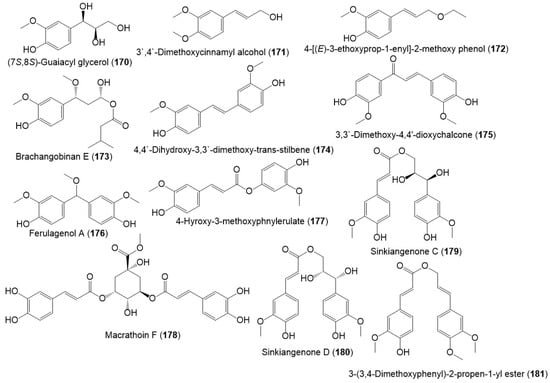
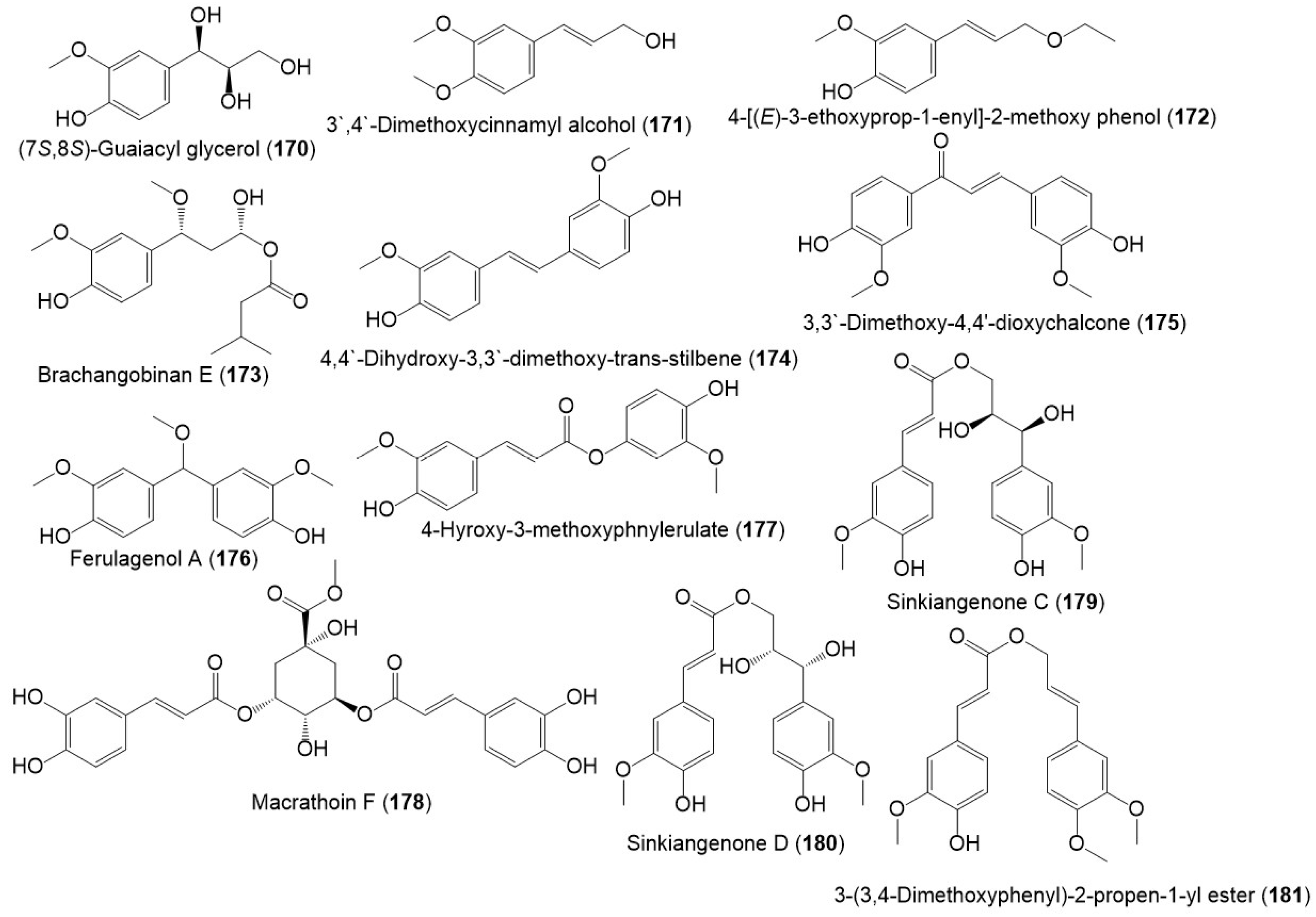
Figure 19.
Structures of phenolic compounds (170–181) reported from F. sinkiangensis.
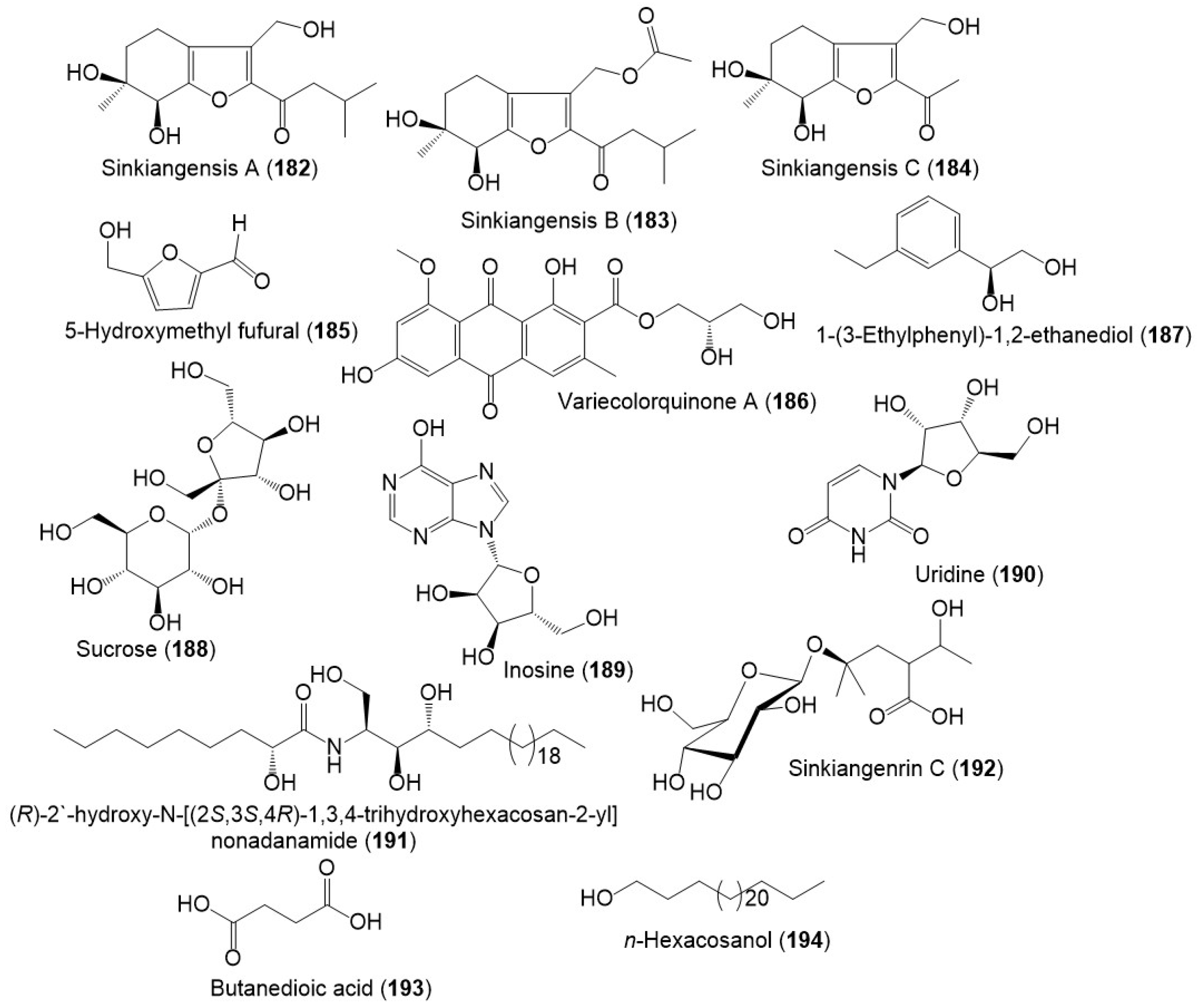
From the resin extract, new tetrahydrobenzofuran derivatives: sinkiangensis A–C (182–184) were purified using SiO2 CC/HPLC and elucidated by spectral and ECD analyses (Figure 20) [46]. Besides, sinkiangenrin C (192), a new organic acid glycoside was purified from seeds 95%EtOH extract. It is a 2-(1-hydroxyethyl)-4-methyl pentanoic acid 4-O-β-D-glucopyranoside [17].
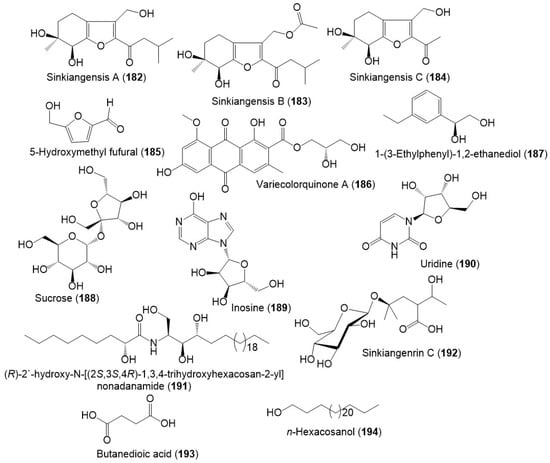
Figure 20.
Structures of other metabolites (182–194) reported from F. sinkiangensis.
5.10. Polysaccharides
Ghulameden et al., reported the separation of water-soluble polysaccharide from F. sinkiangensis roots gathered from Yili, Xinjiang Region, China using DEAE-cellulose-52 column (distilled H2O, as eluent) that was assigned by IR and HPLC analyses. The FSPs (crude polysaccharides) had ribose/arabinose/glucose/fucose/galactose (ratios 8.9/3.3/2.1/1.5/0.3), while FSPs-n (neutral polysaccharides) and FSPs-a (acidic polysaccharides) contained glucose/xylose/arabinose/galactose/mannose (ratios 3.9/4.0/1.8/1.4/0.8) and glucose/xylose/mannose/arabinose (ratios 6.5/4.0/1.7/1.0), respectively [34].
In another study, the sequential extraction of F. sinkiangensis roots yielded 28.86 wt% total polysaccharides. The polysaccharide fractions are heteropolysaccharides, containing galacturonic and glucuronic acids, galactose, xylose, rhamnose, fructose, and arabinose [47].
6. Biological Activities of F. sinkiangensis Extracts and Metabolites
6.1. Anti-Inflammatory and Anti-Neuroinflammatory Activity
From the Chinese medicine Awei (F. sinkiangensis gum resin) CHCl3 extract, new metabolites; 23 and 36, in addition to formerly reported 1, 5, 7, 8, 11–13, 16, 32, 33, 39, 41, 52, and 57 (Table 1)were assessed for their anti-neuroinflammatory activity against LPS (lipopolysaccharide)-stimulated NO production in BV-2 microglial cells using the nitrite and MTT (3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazoliumbromide) assays. It was noted that 7, 12, 32, and 33 possessed marked activity (IC50s 6.93, 4.96, 10.5, and 5.95 µM, respectively) compared to minocycline (IC50 37.04 µM), whereas 5, 8, 11, 13, 39, 41, and 57 greatly lessened NO production (IC50s ranged 19.88–47.43 µM). Compound 12 (Conc. 1–10 µM) remarkably suppressed IL-6, TNF-α, and IL-1β expression, as well as COX-2 (Conc. 3–10 µM) caused by LPS in BV2 cells. Thus, this plant might have the potential as anti-Alzheimer′s disease therapy [48]. Structure-activity relationship revealed that the substitution at C-3` in the bicyclic derivatives that possess 8`R-CH3 and 8`R-OH had a substantial role in the activity. The capability of C-3`-substitutents to boost the effect followed this order: acetoxy, α-OH, β-OH, and C=O, however, this order varied in bicyclic derivatives with C-8` terminal olefinic bond. In the mono-cyclic derivatives, the rings` breaking position of sesquiterpene moiety could influence the efficacy e.g., 32 and 33 with broken A-ring were more active than 39 and 41 with broken B-ring. Besides, the O-bridge in ring A in monocyclic derivatives improved the efficacy. On the other hand, the chain derivatives (e.g., 57 and 52) had weak activity [48]. In 2020, Zhang et al., evaluated the potential of 12 on ischemic stroke utilizing BCCAO (bilateral common carotid artery occlusion) and LPS-invigorated microglia models. It was found that 12 relieved cognitive weakness, lowered neuronal forfeiture, repressed microglial stimulation, and converted microglia from the proinflammation M1 type to the anti-inflammation M2 type in the BCCAO-mice model. Moreover, it organized microglial polarization and suppressed the MAPK (mitogen-activated protein kinase) and NLRP3 signaling pathways subsequent to LPS-treatment in vitro. These findings highlighted the possible activity of 12 for treating ischemic stroke [49].

Table 1.
Biological activity of reported metabolites from Ferula sinkiangensis.
Further, in 2021, Mi et al., explored the potential of 12 on cerebral ischemia utilizing MCAO (middle-cerebral-artery occlusion) and LPS-boosted microglia models. In the MCAO model, 12 amended neurological outcomes and decreased infarct size and edema of the brain in rats. It also mitigated neuron injury and restrained microglial activation. Moreover, 12 guarded neuronal cells against damage by repressing microglial activation in LPS-invigorated BV2 cells. It also diminished the proinflammatory cytokines levels, NADPH oxidase activity, and ROS generation, along with the NF-κB signaling pathway repression [28].
Chemical investigation of F. sinkiangensis resin 95% EtOH extract resulted in new sesquiterpene coumarins: 27, 28, 31, 38, 46, and 56–60, along with early reported 3, 5, 8, 23, 25, 29, 30, 40, 41, and 52. They were characterized spectral/ECD/[α]D analyses. Their inhibitory potential on NO production induced by LPS using nitrite and MTT assays in BV2 cells. Compounds 3, 5, 8, 25, 28–31, 38, 40, 41, 46, and 56 possessed noticeable inhibition of NO production in over-activated BV2 cells, (IC50s 1.2–93.8 μM) compared to minocycline (IC50 65.5 μM), while 27, 23, 52, and 57–59 had weak inhibitory capacity (IC50 > 00 μM). It was noted that 28 (IC50 1.2 μM) revealed the potent in vitro anti-neuroinflammatory that was confirmed by docking to TLR4/MD-2. Structure-activity relation showed that chain sesquiterpene coumarins with C-10` acetoxy group (e.g., 56) had powerful activity than the ones with C-10`-OH, an unsaturated fatty chain or 4-decylenic acid ester (e.g., 57, 58, and 59, respectively). A-ring substitution pattern affected the potential of monocyclic derivatives with opened B-ring. The α,β-unsaturated ketone (e.g., 38) increased the effect, while the seven-membered ring-A resulted from C-4′-O–C–3` linkage (e.g., 28) remarkably repressed NO producing relative to six-membered ring-A derivatives. In the compounds with opened A-ring (e.g., 29–31 and 46), increase the length of oxyacyl side chain at C-3` weakened the anti-neuroinflammatory activity [25].
Among the lignan derivatives, 90–101, 92 and 93 possessed anti-inflammatory potential via inhibiting TNF-α and IL-6 production mediated by LPS in RAW264.7 cells without affecting the RAW264.7 viability. Besides 92 and 93 notably suppressed LPS-produced iNOS and COX-2 expression in RAW264.7 cells [44], whereas 102–111 had COX-2 inhibition capacity (IC50s 4.47–21.96 μM) [18]. This evidence supported the role of F. sinkiangensis in treating inflammation [18,44].
In 2022, ferulagenol A (176) a new phenolic metabolite, in addition to 158, 171–173, 175, and 177 were reported by Yan et al., Compounds 176 and 177 possessed notable COX-2 inhibitory capacity (IC50s 3.63 and 3.0 μM, respectively) [18].
On the other hand, F. sinkiangensis gum resin CHCl3 extract considerably prohibited production of NO in LPS-boosted BV-2 microglial cells (IC50 1.66 µg/mL) [48].
6.2. Anticancer Activity
New sesquiterpene coumarins, 10, 17, 21, and 22 and related analogs 1, 5–9, 11–14, 20, 24, 26, 29, 30, 32, 37, 39, 41, 52, 54, and 57 were reported from the 95% EtOH extract of the resin [39]. These compounds displayed moderate to weak anticancer potential against AGS, HeLa, and MGC-803 cell lines. Compounds 5, 8, and 29 demonstrated anticancer potential against AGS, HeLa, and MGC-803 cell lines (IC50s 20.0–49.0 μM), compared to taxol (IC50 1.8, 7.5, and 3.4, respectively). Compound 10 had (IC5016.0 μM) selective activity against HeLa cells. The mechanistic study demonstrated that 10 caused G0/G1 cell cycle arrest and apoptosis in HeLa cells. It induced its effect by affecting the expression of cell cycle regulation- and apoptosis-related proteins by stimulating the MAPK pathway [39]. Additional work by Li et al., reported the separation of another new sesquiterpene coumarins, sinkiangenorin F (42) and its 8-acetyl derivative, and 8-O-acetyl sinkiangenorin F (43) from the seeds EtOH extract. They feature ether-linked coumarin and sesquiterpene moieties with 6`S/8`S/9`S. They exhibited anticancer potential (IC50s 27.1 and 62.7 µM, respectively) against AGS cell lines in the MTT assay [51].
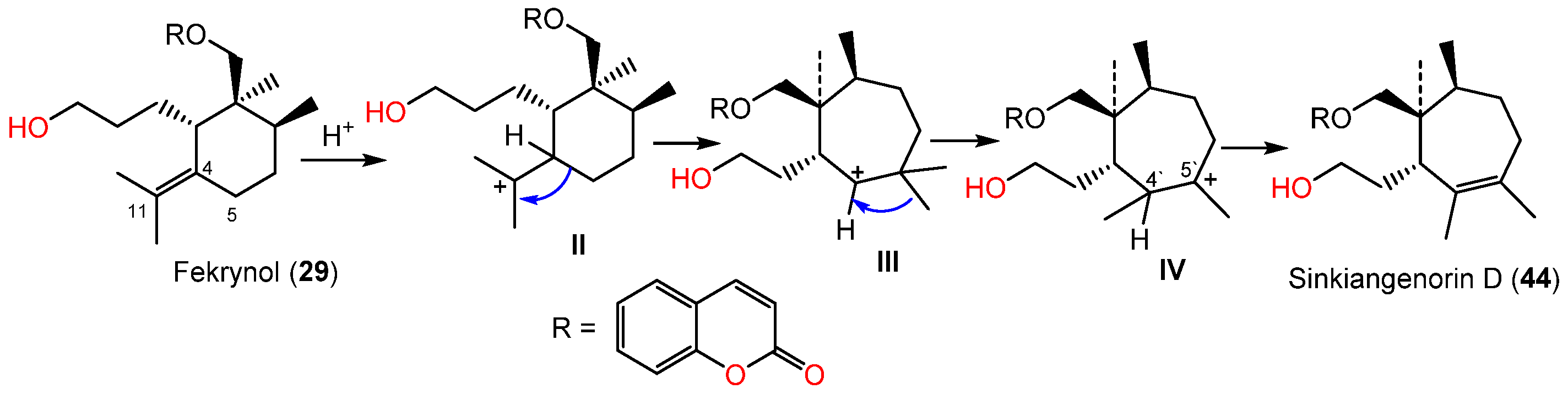
Li et al. in 2015 purified and characterized sinkiangenorin D (44), a novel sesquiterpene coumarin having fekrynol- sesquiterpene skeleton, along with 2, 15, 19, 24, 25, 29, 34, 35, 41, and 52 from the seeds’ EtOH extracts. Compound 44 is sesquiterpene coumarin involving a monocyclic cycloheptene sesquiterpene unit. These metabolites (IC50s 12.7–226.6 μM) demonstrated anticancer capacity on HeLa, AGS, and K562 in the MTT assay. Compounds 24, 25, 29, 35, 41, and 44 had activity on HeLa cells (IC50s 20.4–226.2 μM), whereas 2, 15, 24, 34, 41, 44, and 52 were active against AGS cells (IC50s 12.7–104.8 μM) compared to taxol (IC50s 3.5–8.5 μM) [50]. Sinkiangenorin D (44) was proposed to be biosynthesized from fekrynol-kind sesquiterpene [50]. Firstly, the formation of II is accomplished by C-4 protonation and the olefinic bond electron-transport reaction. Thereafter, the C4–C5 electrons would transmit to C11, resulting in a seven-member ring formation. The C-5′ methyl transmission and successive loss of proton form C4′–C5′ double bond result in this novel framework formation [50] (Scheme 4).
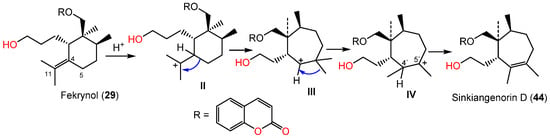
Scheme 4.
Biosynthetic pathway of sinkiangenorin D (44) [52].
A novel metabolite (45) having unrivaled bicyclo[4.3.1]decane-type sesquiterpene skeleton was purified from the 95% EtOH extract of the seeds. Its 2′S/3′R/5′R/7′R/10′R configuration was determined based on NMR and CD analyses. It had moderate and weak anticancer activity against AGS (IC50 12.7 μM) and Hela (IC50 82.9 μM) cells, respectively compared to taxol (IC50 3.5 and 5.6 μM, respectively) [52].
Umbelliprenin (52) possessed dose-dependent and time-dependent apoptosis in CLL (chronic lymphocytic leukemia) that was more sensitive to 52 than PBMCs. It is noteworthy that IL-4 could not decline 52-caused apoptosis in CLL (Figure 7). Thus, 52 oral administration as foods or folk medicines, might stimulate the protection against CLL development with few side effects, however, additional clinical investigations are needed [53]. Gholami et al., reported that 52 potentiated apoptosis intrinsic/extrinsic pathways in Jurkat cells by activating caspase-9 and -8, as well as Bcl-2 prohibition [54]. Another study by Zhang et al., revealed that 52 had a notable anticancer activity (IC50s 13.67 and 20.82 µM, respectively) against AGS cells with less anticancer to GES-1 (normal human gastric epithelial cell line). It boosted AGS cells apoptosis with elevated Bax/Bcl-2 ratios, ROS generation, lessened mitochondrial-membrane potential, and PARP and caspase 3 activation resulting in mitochondrial apoptosis pathway activation. It also arrested the G0/G1 phase of the cell cycle, increased P27, P53, and P16, expression, and diminished cyclin E, cyclin D, Cdk2, and Cdk4 expression in cancer cells. Therefore, it could be developed into anti-cancer therapy [24]. In 2019, Zhang et al., also reported that 52 also demonstrated anticancer capacity against BGC-823 and AGS, with less toxic influence on the normal GES-1 gastric cells. It was proven to prevent gastric cancer cell invasion, growth, and migration by disconcerting the Wnt signaling pathway. Additionally, it exhibited no harm in the in vivo BGC-823 xenograft model as evidenced by no observed abnormality in daily diet, body weight, liver function, and histological features of the spleen, liver, lung, kidney, and heart tissue. This further supported the previous evidence of its promising potential for treating gastric cancer [55].
The anticancer investigation against SW1990, CFPAC-1, Capan-2, and PANC-1 revealed that (+)-61 and (-)-62 had marked proliferation inhibition capacity on PANC-1 cells (IC50s 2.24 and 0.92 μM, respectively) and moderate activity against CFPAC-1 cells (IC50s 6.12 and 19.13 μM, respectively) compared to taxol (IC50s 0.22 and 0.38 μM, respectively). It was noted that the anticancer potential of (+)-61 was more powerful than (-)-62 [40]. Also, 63–65 had anticancer activity (EC50s 22.78–65.38 μM) against IOZCAS-Spex-II, where 65 was the most potent (EC50 22.78 μM) relative to camptothecin (EC50 51.27 μM) [41].
Wang et al., examined the activity of 69 and 70 against AGS, MGC-803, and GES-1 using MTT assay. Only 70 possessed the notable anticancer potential against MGC-803 and AGS (IC50s 18.89 and 16.15, respectively) with less toxicity against GES-1 (IC50 36.73 μM) comparing with taxol (IC50 3.35, 1.82, and 2.67 μM, respectively), whereas the other metabolites had no notable potential. The mechanistic study revealed that 70 was found to elevate Bax/Bcl-2 ratios, as well as RB, P16, and P27 expression and decrease cyclins (D1 and E), Cdk4, P53, and Cdk2, resulting in apoptosis and G0/G1 cell cycle arrest in AGS cells. This compound could be a potential candidate for gastric cancer therapy [29].
F. sinkiangensis seeds afforded 77–80 that displayed weak to moderate anticancer activity on AGS and Hela cells in the MTT assay [17] (Figure 10), whereas 77, 80, and 81 had a weak anticancer capacity against AGS, HeLa, A549, and PC3 cell lines in the MTT assay (IC50s 54.4–167.3 µM) [24].
In the CCK-8 assay, 84 and 86 were found to significantly prohibit the migration and invasion of TNBC) cell lines. On the other hand, 88 and 89 promoted the HUVECs) proliferation which was more remarkable than bFGF (basic-fibroblast-growth factor, positive control) in the wound-healing assay [35].
New phenylpropanoid derivative; sinkiangenones C (179) and D (180), along with 158, 163, 164, 166–170, 174, and 181 were separated from the resin 95% EtOH extract and specified by spectral and CD analyses. In the MTT assay against AGS, MGC-803, and GES-1, they had no or weak potential (IC50s 18.89 to 182.46 μM) [29].
Sinkiangensis A–C (182–184) possessed anticancer activity on the AGS cell line (IC50s 87.1, 72.7, and 15.6 μM, respectively), whereas 184 was the most active in comparison to taxol (IC50 4.69 μM) and induced AGS cell apoptosis in the MTT assay. Unfortunately, they (IC50 ˃100 μM) had no effect against HeLa and K562 cells [46]. Whilst 192 exhibited anticancer activity on AGS cells (IC50 36.9 μM) [17].
The petroleum ether, EtOAc, n-BuOH, and MeOH fraction possessed of F. sinkiangensis resin anticancer activity against Caco-2, HC-T116, MFC, and HepG2 cells in the SRB assay. EtOAc fraction was found to have the potent anti-proliferative and apoptotic effects against all tested cell lines. This was correlated to its content of sesquiterpene coumarins [31].
6.3. Antiviral, Insecticidal, and Antimicrobial Activities
Besides, 45 prohibited (IC50 4.0 μM) BJ09/H1N1 (influenza A/Beijing/7/2009 H1N1) infection in MDCK cells [52]. The monoterpene coumarins; 63–65 and the coumarins: 66 and 67 displayed insecticidal potential (Conc. 10 μg/larva, 24 h) against S. exigua 3rd instar larvae (%mortality ranging from 26.67–52.22%) compared to camptothecin (18.89% mortality) [41]. From the aerial parts, sesquiterpenes; nerolidol (120) and guaiol (122) (58.89 and 41.11% mortality, respectively) possessed toxic potential on the S. exigua insect 3rd instar larvae [41]. Additionally, 120 exhibited antifungal effect against plant pathogens: inhibitory effects on Pyricularia grisea, Alternaria alternata, and Botrytis cinereal (MICs 16, 32, and 32 μg/mL, respectively) compared to carbendazim (MICs 32, 16, and 32 μg/mL, respectively) [41].
6.4. Anti-Drug Addiction Activity
Drug addiction is a prime health concern that influences a growing number of persons and gives rise to severe economic and social burdens to economy society [56,57]. Despite the fact that diverse remedial strategies for drug addiction and abuse are developed, including psychological, sociological, and pharmacological interventions, their activity is yet restricted [58,59].
A mixture of 133 and 135 was obtained from the F. sinkiangensis crude essential oil. A mixture of 133 and 135 (1:3 ratio, doses: 20, 40, and 60 mg/kg, ip) significantly repressed the morphine abstinence syndrome and physiological addiction in rats and mice [60]. At the same doses, this mixture (1:3 ratio, i.p.) reduced morphine-induced bodyweight loss. While the mixture declined the abdominal writhing movements number and automatic activity (doses 10.73, 21.45, and 43.55 mg/kg, i.p.) revealing its analgesic and sedative potential. Its acute toxicity evaluation showed the LD50 values of its iv and ip injections were 1.42 g/kg and 1.66 g/kg, respectively [60].
In 2006, Wang reported in his patent that the resin extract in the form of capsules, powder, injection, drop pills, granules, tablets, or oral liquid) ameliorated the influences of serious and moderate long-time drug addictions in addicted patients, indicating its potential for treating subjects addicted to morphine, opioid, diamorphine, and marijuana [36,38]. It is noteworthy that 0.1–20 g/kg was found to be the therapeutically effective amount of the extract for producing an effect of abstinence of morphine, whereas the preferable dose was 1–3 g/kg [36].
6.5. Protein-Tyrosine Phosphatase 1B Inhibition Activity
FSPs-a (acidic polysaccharides) fraction of F. sinkiangensis roots water-soluble polysaccharides revealed in vitro PTP1B (protein-tyrosine phosphatase 1B) competitive inhibition (IC50 0.29 µg/mL, % inhibition 91.23%) [34]. In another study, the PTP1B inhibitory potential of the different polysaccharides fractions was estimated. It was noted that the inhibitory capacity of the tested fractions elevated with raising their galactose content, therefore, galactose may be a ligand for blocking PTP1B catalytic site [47].
6.6. Antiulcer and Antioxidant Activities
In the in vivo antiulcer assay, different F. sinkiangensis resin extracts possessed antiulcer capacity, whereas CHCl3 extract (%inhibition 48.43) had comparatively better antiulcer potential than the n-BuOH and EtOAC (%inhibition 37.07 and 46.06%, respectively) extracts comparing to famotidine (%inhibition 45.37) [14]. In the DPPH assay, the F. sinkiangensis resin n-BuOH, EtOAc, and MeOH fractions significantly scavenged DPPH, whereas the EtOAc fraction was the most effective and the petroleum ether fraction was weakly active in the DPPH assay [31].
6.7. Feed Attraction Activity
In 2020, Xu et al., reported that feeding Lateolabrax japonicus (commercial fish) with F. sinkiangensis was found to notably promote L. japonicus foraging and better digestive enzyme activity and fish growth performance, where 10 g/kg was appropriate in the fish diet. Thus, it had an efficient role in L. japonicus farming and could have potential in the aquaculture industry as aquafeed formulation [61].
7. Traditional Ethnomedicinal Uses in Asian Countries
Medicinal plants are fundamental to humans and utilized for thousands of years in various cultures to treat or prevent diseases or promote health and well-being [62]. Many communities continue to depend on plants as the main tool for healing various illnesses and have established their medical systems on the basis of their unique beliefs, experiences, and theories worldwide [63]. Traditional and indigenous medical systems are especially widespread throughout communities in Asia that are accountable for a remarkable proportion of the healthcare provided in these countries [64,65]. Ayurveda, Jamu, traditional Philippines, traditional Malay, Sowa Rigpa, Tibetan, Kampo, Siddha, Thai medicine, Unani, and traditional Chinese systems are important sources of livelihood and health for millions of Asian people [62,66]. Generally, the region’s traditional medicine systems are greatly affected by those practiced in the neighboring areas especially of South and East Asia, mainly that of India and China [62]. In China, different sociolinguistic groups have their own traditional systems and medicinal plant usages that vary based on associated ecology and geography [67]. For example, Southwest China (kingdom of plants) is renowned for its large variety of ethnic groups with featured traditional cultures. Populations from 33 ethnicities are using plants as a traditional remedy for thousands of years, including Bai, Achang, Bulang, Tibetan, Buyi, Dai, Dong, De’ang, Hani, Dulong, Hui, Han, Jinuo, Lahu, Jingpo, Lisu, Maonan, Luoba, Menba, Molao, Miao, Naxi, Pumi, Nu, Qiang, Shui, She, Tujia, Yao, Wa, Yi, Zhuang, and Gelao people [67,68].
8. Conclusions
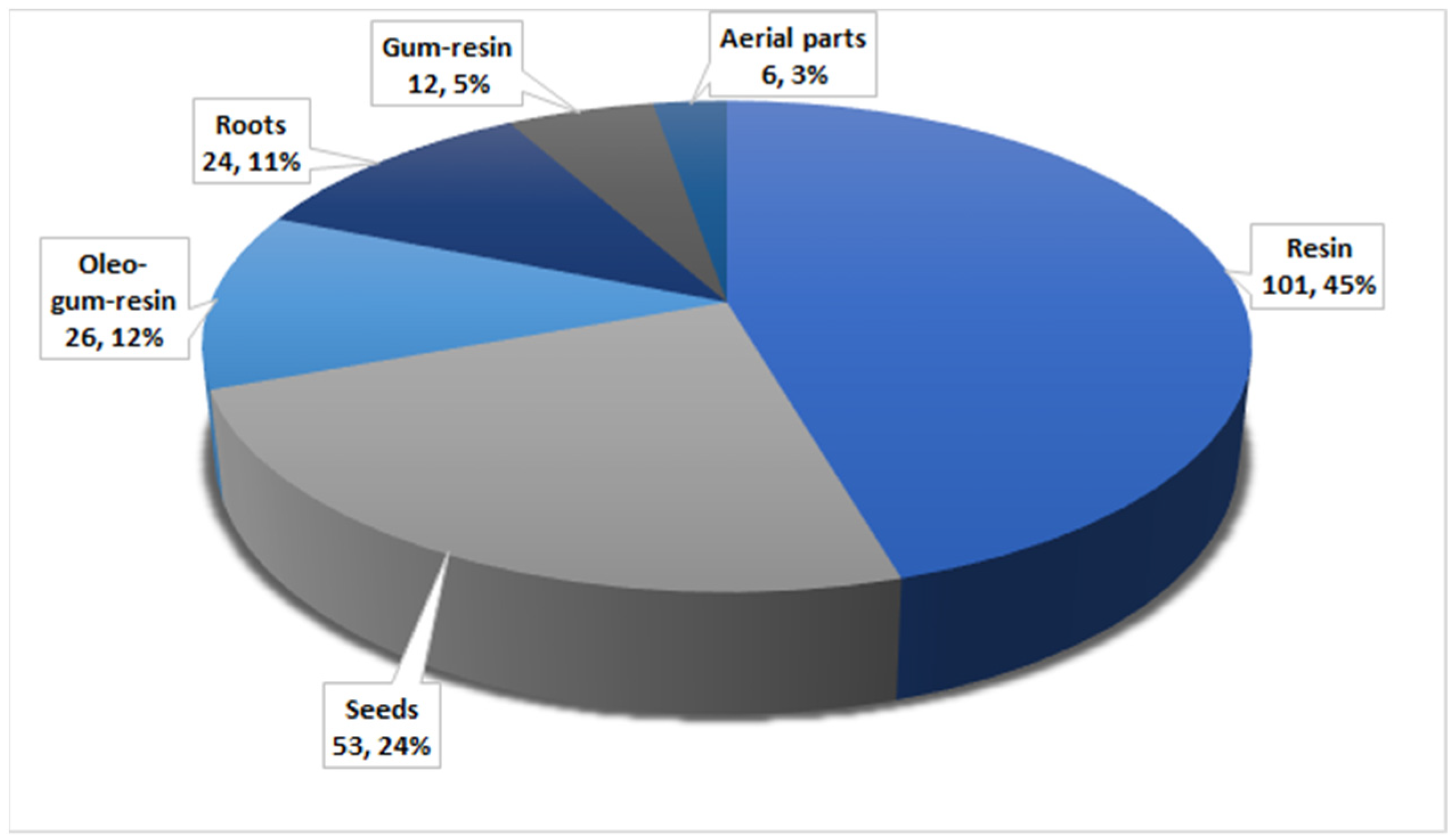
Herbal medicines have been utilized for thousands of years as principal therapeutic agents for treating various human illnesses in many countries. Recently, a growing number of studies have been carried out to prove the efficacy of these medicines against the assigned disorders. F. sinkiangensis is among the most valuable species of the genus Ferula that possess various traditional applications in treating various disorders such as bronchitis, diarrhea, malaria, gastric disorders, and rheumatoid arthritis. In this work, 194 metabolites have been characterized from various parts of this plant, including aerial parts, seed, roots, gum resin, oleo-gum resin, and resins (Figure 21).
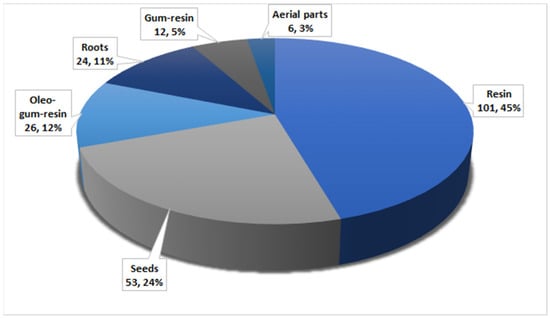
Figure 21.
Number of compounds and their percentage reported from various parts of F. sinkiangensis.
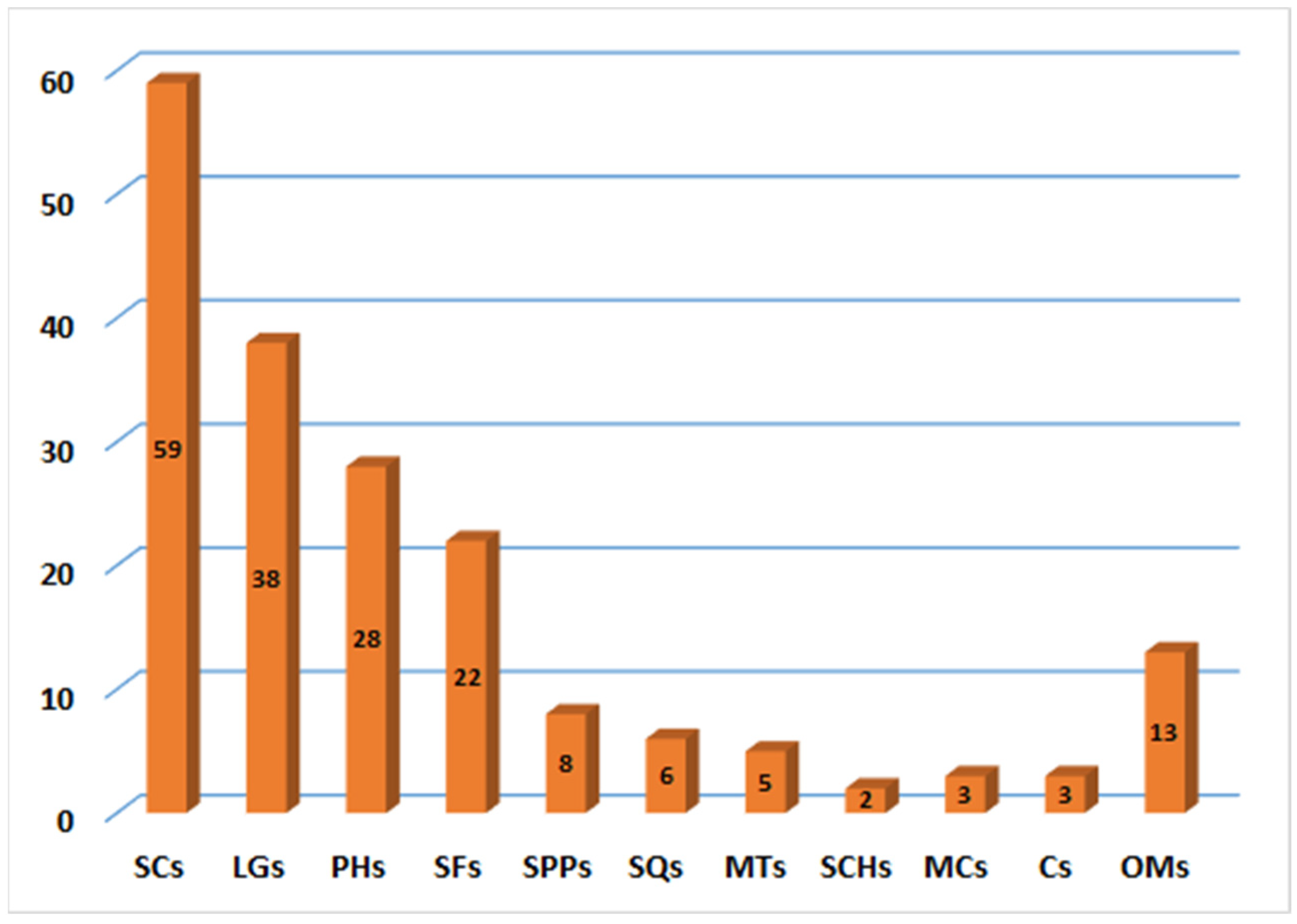
It was obvious that the majority of metabolites have been distinguished from resin extract. These metabolites belong to various chemical classes. Sesquiterpene coumarins with their structural diversity and contents represent the main and substantial metabolites of this plant (Figure 22).
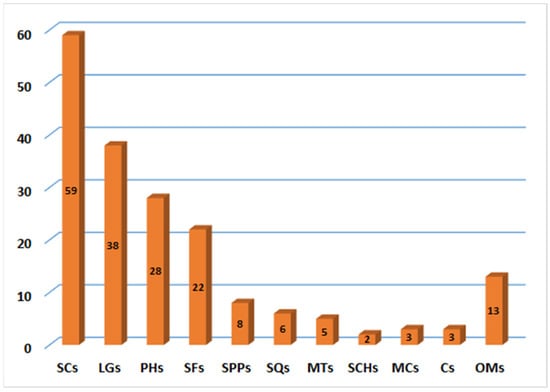
Figure 22.
Number of compounds reported from various classes of F. sinkiangensis. SCs: Sesquiterpene coumarins; LGs: Lignans; PHs: Phenolics; SFs: Sulfanes; SPPs: Sesquiterpene phenylpropanoids; SQs: Sesquiterpenes; MTs: monoterpenes; SCHs: Sesquiterpene chromones; MCs: Monoterpene coumarins; Cs: coumarins; OMs: Other metabolites.
Many studies surmised that these compounds may have a substantial contribution in many of the reported activities of F. sinkiangensis. F. sinkiangensis had metabolites with marked antifungal and insecticidal capacity that can be valuable in agriculture for insect and plant pathogens control, however, additional field assessment is requested. Its metabolites; 61, 62, 65, 70, and 120 had notable anticancer potential against different cancer cell lines. These findings would provide evidence for the application of this and its fractions in treating cancers. Compound 12 had marked anti-inflammatory and anti-neuroinflammatory potential, revealing its potential as a lead metabolite for therapeutic intervention in various illnesses such as ischemic stroke, Alzheimer`s disease, and cerebral ischemia. Many studies proved the anticancer and apoptotic potential of 52 against different cancer cell lines particularly the gastric cancer cells with no toxic effect on the normal cells and no observed abnormality in daily diet, body weight, liver function, and histological features of the spleen, liver, lung, kidney, and heart tissue. This further supported its promising potential for treating gastric cancer as foods or folk medicine, however, additional clinical investigations are needed. To find out more metabolites with unique structures and bioactivity, more phytochemical investigations are demanded and indispensable. Also, new technologies such as metabolomics, transcriptomics, genomics, and proteomics can be applied for discovering more metabolites from this valuable medicinal plant. The plant`s mechanism in treating gastric disorders and rheumatoid arthritis is insufficiently explored. Additionally, in-depth in-vivo and in vitro studies of the other bio-activities mechanisms are required. Meanwhile, toxicological, pharmacokinetic, preclinical, quality control, and clinical studies are insistent to estimate the safety and rationale usage of this plant. Finally, the integration of traditional knowledge into ecology-based research for the endangered medicinal plant protection must be promoted.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12040902/s1, Table S1: Secondary metabolites reported from Ferula sinkiangensis (Chemical class, molecular weight and formulae, plant part, and origin). References [8,13,14,17,18,19,24,25,27,29,30,35,39,40,41,43,44,46,48,50,51,52,60] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, S.R.M.I. and G.A.M.; methodology, S.R.M.I., G.A.M., M.T.K., M.A. and K.F.G.; software, M.T.K., M.A. and K.F.G.; M.T.K., M.A. and K.F.G.; writing—original draft preparation, S.R.M.I. and G.A.M.; writing—review and editing, S S.R.M.I., G.A.M., M.T.K., M.A. and K.F.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| 1D NMR | One-dimensional nuclear magnetic resonance |
| 2D NMR | Two-dimensional nuclear magnetic resonance |
| A549 | Human lung adenocarcinoma epithelial cell line |
| AGS | Human gastric carcinoma cell line |
| Bax/Bcl-2 | B-cell lymphoma protein 2 (Bcl-2)-associated X (Bax) |
| BCCAO | Bilateral common carotid artery occlusion |
| bFGF | Basic fibroblast growth factor |
| BGC-823 | Human gastric carcinoma cell line |
| n-BuOH | n-Butanol |
| BV-2 | Microglia cells |
| Caco-2 | Human colon adenocarcinoma cell line |
| Capan-2 | Human pancreatic cancer cell line |
| CCK-8 | Cell counting kit-8 |
| CD | Circular dichroism |
| Cdk2 | Cyclin dependent kinase 2 |
| CFPAC-1 | Human pancreatic cancer cell line |
| CHCl3 | Chloroform |
| CLL | Chronic lymphocytic leukemia |
| COX-2 | Cyclooxygenase-2 |
| CITES | Convention on International Trade in Endangered Species of Wild Fauna and Flora |
| DCFH-DA | 2′, 7′-Dichlorofluorescein diacetate |
| DEAE-Cellulose 52 | Diethylaminoethyl cellulose-52 |
| DPPH | 1,1-Diphenyl-2-picrylhydrazyl |
| EC50 | Half maximal effective concentration |
| ECD | Electronic circular dichroism |
| ELISA | Enzyme-linked immunosorbent assay |
| EtOH | Ethanol |
| EtOAc | Ethyl acetate |
| HR-ESIMS | High resolution electrospray ionization mass spectrometry |
| GES-1 | Human normal gastric epithelial cell line |
| GSK-3β | Glycogen synthase kinase-3β |
| H2O | Water |
| HCT-116 | Human colon cancer cell line |
| HepG2 | Human hepatocellular liver carcinoma cell line |
| HeLa | Human cervical epitheloid carcinoma cell line |
| HPLC | High-performance liquid chromatography |
| HUVECs | Human umbilical vein endothelial cell line |
| IC50 | Half-maximal inhibitory concentration |
| LD50 | Lethal dose 50, |
| IL-6 | Interleukin-6 |
| IL-1β | Interleukin-1beta |
| IR | Infrared |
| IOZCAS-Spex-II | A cell strain cloned from Spodoptera exigua cell line |
| IUCN | International Union for Conservation of Nature |
| K562 | Human erythroleukemic cell line |
| IR | Infrared |
| iNOS | Inducible nitric oxide synthase |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| MCAO | Middle cerebral artery occlusion |
| MDCK | Madin-Darby Canine Kidney |
| MeOH | Methanol |
| MFC | Mouse forestomach cancer cell line |
| MIC | Minimum inhibitory concentrations |
| MGC-803 | Human gastric cancer cell line |
| MMP2 and MMP9 | matrix metalloproteinases |
| MS | Mass spectrometry |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NBT | Nitrotetrazolium blue chloride |
| NLRP3 | NLR family pyrin domain containing 3 |
| NO | Nitric oxide |
| P16 | Protein regulating the cell circle |
| P27 | A key protein that regulator of cell proliferation |
| P53 | Tumor suppressor protein |
| PANC-1 | Human pancreas ductal carcinoma cell line |
| PARP | Poly (ADP-ribose) polymerase |
| PC-3 | Human prostatic-testosterone-independent cell line |
| PCR | Polymerase chain reaction |
| PTP1B | Protein tyrosine phosphatase 1B |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| RAW264.7 | Mouse macrophage cell line |
| ROS | Reactive oxygen species |
| RP-18 | Reversed-phase-18 |
| SCs | Sesquiterpene coumarins |
| SRB | Sulforhodamine B |
| SiO2 CC | Silica gel column chromatography |
| SW1990 | Human pancreatic cancer cell line |
| TCF/LEF | T-cell factor/lymphoid enhancer factor |
| TLC | Thin layer chromatography |
| TLR4 | Toll-like receptor 4 |
| TNBC | Triple-negative breast cancer |
| TNF-α | Tumor necrosis factor alpha |
| TTC | 2, 3, 5-Triphenyltetrazoliumchloride |
| UV | Ultraviolet |
| Wnt | Wingless-related integration site |
References
- Balick, M.J. Transforming Ethnobotany for the New Millennium. Ann. Mo. Bot. Gard. 1996, 83, 58–66. [Google Scholar] [CrossRef]
- Garnatje, T.; Peñuelas, J.; Vallès, J. Reaffirming ‘ethnobotanical Convergence’. Trends Plant Sci. 2017, 22, 640–641. [Google Scholar] [CrossRef] [PubMed]
- Abdul, R.B. Medicinal plants (Importants and uses). Pharm. Anal. Acta 2012, 3, e139. [Google Scholar]
- Salmerón-Manzano, E.; Garrido-Cardenas, J.A.; Manzano-Agugliaro, F. Worldwide research trends on medicinal plants. Int. J. Environ. Res. Public Health 2020, 17, 3376. [Google Scholar] [CrossRef]
- Sahebkar, A.; Iranshahi, M. Volatile constituents of the genus Ferula (Apiaceae): A review. J. Essent. Oil-Bear. Plants 2011, 14, 504–531. [Google Scholar] [CrossRef]
- Ahmadi Koulaei, S.; Hadjiakhoondi, A.; Delnavazi, M.R.; Tofighi, Z.; Ajani, Y.; Kiashi, F. Chemical composition and biological activity of Ferula aucheri essential oil. Res. J. Pharmacogn. 2020, 7, 21–31. [Google Scholar]
- Wilson, L. Spices and Flavoring Crops: Tubers and Roots. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 93–97. [Google Scholar]
- Min, Z.; Mai, Q.; Mizuno, M.; Tanaka, T.; Iinuma, M. Polysulfanes in the volatile oils of Ferula species. Planta Med. 1987, 53, 300–302. [Google Scholar]
- Gonzalez, A.G.; Barrera, J.B. Chemistry and sources of mono-and bicyclic sesquiterpenes from Ferula species. In Fortschritte Der Chemie Organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products; Anonymous; Springer: Berlin/Heidelberg, Germany, 1995; pp. 1–92. [Google Scholar]
- Fan, C.; Li, X.; Zhu, J.; Song, J.; Yao, H. Endangered Uyghur medicinal plant Ferula identification through the second internal transcribed spacer. Evid. Based Complement. Altern. Med. 2015, 2015, 479879. [Google Scholar] [CrossRef]
- Nazari, Z.E.; Iranshahi, M. Biologically active sesquiterpene coumarins from Ferula species. Phytother. Res. 2011, 25, 315–323. [Google Scholar] [CrossRef]
- Iranshahi, M.; Rezaee, R.; Najafi, M.N.; Haghbin, A.; Kasaian, J. Cytotoxic Activity of the genus Ferula (Apiaceae) and its bioactive constituents. Avicenna J. Phytomed. 2018, 8, 296. [Google Scholar]
- Sattar, Z.; Iranshahi, M. Phytochemistry and pharmacology of Ferula hermonis Boiss.—A review. Drug Res. 2017, 67, 437–446. [Google Scholar] [CrossRef]
- Teng, L.; Ma, G.Z.; Li, L.; Ma, L.Y.; Xu, X.Q. Karatavicinol a, a new anti-ulcer sesquiterpene coumarin from Ferula Sinkiangensis. Chem. Nat. Compd. 2013, 49, 606–609. [Google Scholar] [CrossRef]
- Mohammadhosseini, M.; Mahdavi, B.; Shahnama, M. Chemical composition of essential oils from aerial parts of Ferula Gummosa (Apiaceae) in Jajarm Region, Iran using traditional hydrodistillation and solvent-free microwave extraction methods: A comparative approach. J. Essent. Oil-Bear. Plants 2015, 18, 1321–1328. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S.; Ramchiary, N.; Singh, P. Role of traditional ethnobotanical knowledge and indigenous communities in achieving sustainable development goals. Sustainability 2021, 13, 3062. [Google Scholar] [CrossRef]
- Li, G.; Li, X.; Cao, L.; Shen, L.; Zhu, J.; Zhang, J.; Wang, J.; Zhang, L.; Si, J. Steroidal esters from Ferula sinkiangensis. Fitoterapia 2014, 97, 247–252. [Google Scholar] [CrossRef]
- Yan, Y.; Bao, X.; Li, Y.; Li, Q.; Cheng, Y.; Jiao, Y.; Zhang, H. Racemic Norneolignans from the resin of Ferula sinkiangensis and their Cox-2 inhibitory activity. Fitoterapia 2023, 164, 105341. [Google Scholar] [CrossRef]
- Yang, J.; Jing, S.; Li, Z.; Qin, H. Chemical constituents from roots of Ferula sinkiangensis. Zhongguo Zhong Yao Za Zhi 2007, 32, 2382–2384. [Google Scholar]
- Fan, C.; Wang, G.; Qiu, Y.; Zhao, Y.; Zhang, J.; Song, J.; Li, X. The complete chloroplast genome sequence of Ferula sinkiangensis K.M. Shen, a precious and endangered traditional Chinese medicine. Mitochondrial DNA Part B 2021, 6, 1670–1672. [Google Scholar] [CrossRef]
- IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org (accessed on 25 January 2023).
- Convention on International Trade in Endangered Species of Wild Fauna and Flora. Available online: https://www.dcceew.gov.au/environment/wildlife-trade/cites (accessed on 25 January 2023).
- Li, L.P.; Cui, W.H.; Wang, T.; Tian, S.; Xing, W.J.; Yin, L.K.; Abdusalih, N.; Jiang, Y.M. Plant Conservation Priorities of Xinjiang Region, China. IOP Conf. Ser. Earth Environ. Sci. 2017, 57, 012034. [Google Scholar] [CrossRef]
- Zhang, L.; Si, J.; Li, G.; Li, X.; Zhang, L.; Gao, L.; Huo, X.; Liu, D.; Sun, X.; Cao, L. Umbelliprenin and lariciresinol isolated from a long-term-used herb medicine Ferula sinkiangensis induce apoptosis and G0/G1 arresting in gastric cancer cells. RSC Adv. 2015, 5, 91006–91017. [Google Scholar] [CrossRef]
- Guo, T.; Zhou, D.; Yang, Y.; Zhang, X.; Chen, G.; Lin, B.; Sun, Y.; Ni, H.; Liu, J.; Hou, Y. Bioactive sesquiterpene coumarins from the resin of Ferula sinkiangensis targeted on over-activation of microglia. Bioorg. Chem. 2020, 104, 104338. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Zhao, H. Effects of Ferulic sesquiterpene on intrahepatic egg granuloma in ducklings infected with Trichobilharzia. J. Tradit. Chin. Vet. Med. 2013, 2, 013. [Google Scholar]
- Li, G. Chemical constituents from seeds of Ferula sinkiangensis. Chin. Tradit. Herb. Drugs. 2015, 24, 1730–1736. [Google Scholar]
- Mi, Y.; Jiao, K.; Xu, J.; Wei, K.; Liu, J.; Meng, Q.; Guo, T.; Zhang, X.; Zhou, D.; Qing, D. Kellerin from Ferula sinkiangensis exerts neuroprotective effects after focal cerebral ischemia in rats by inhibiting microglia-mediated inflammatory responses. J. Ethnopharmacol. 2021, 269, 113718. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, Y.; Wang, H.; Chen, L.; Cao, L.; Xu, J.; Li, X.; Zhao, Y.; Zhu, J.; Si, J. Apoptosis induction and cell cycle arrest induced by sinkiangenone B, a novel phenylpropanoid derivative from the resin of Ferula sinkiangensis KM Shen. RSC Adv. 2018, 8, 4093–4103. [Google Scholar] [CrossRef]
- Yang, J.; An, Z.; Li, Z.; Jing, S.; Qina, H. Sesquiterpene coumarins from the roots of Ferula sinkiangensis and Ferula teterrima. Chem. Pharm. Bull. 2006, 54, 1595–1598. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, J.; Zhou, L.; Jiang, L.; Zhou, M. Antioxidant and antitumor effects of Ferula sinkiangensis KM Shen. Int. J. Clin. Exp. Med. 2015, 8, 20845. [Google Scholar]
- Choudhary, S.; Walia, B.; Chaudhary, G. Ferula asafetida (Hing): A review based upon its Ayurvedic and pharmacological properties. Int. J. Pharm. Sci. Rev. Res. 2021, 68, 31–39. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Zhu, J.; Xiao, Q. Essential oil composition analysis of three cultivars seeds of Resina Ferulae from Xinjiang, China. Pharmacogn. Mag. 2011, 7, 116. [Google Scholar] [CrossRef]
- Ghulameden, S.; Yili, A.; Zhao, H.Q.; Gao, Y.H.; Aisa, H.A. Polysaccharides from Ferula sinkiangensis and potent inhibition of protein tyrosine phosphatase 1B. Chem. Nat. Compd. 2014, 50, 515–517. [Google Scholar] [CrossRef]
- Li, Y.; Yang, B.; Zheng, S.; Cheng, Y.; Cui, H. Racemic norlignans as diastereoisomers from Ferula sinkiangensis resins with antitumor and wound-healing promotion activities. Molecules 2022, 27, 3907. [Google Scholar] [CrossRef]
- Wang, Z. Asafetida Extract as Medicine for Abstinence of Drugs. U.S. Patent 7,288,269, 30 October 2007. [Google Scholar]
- Zhu, W.; Zhang, Y.; Huang, Y.; Lu, L. Chinese Herbal Medicine for The Treatment of Drug Addiction. Int. Rev. Neurobiol. 2017, 135, 279–295. [Google Scholar]
- Xi, S.; Gong, Y. Essentials of Chinese Materia Medica and Medical Formulas: New Century Traditional Chinese Medicine; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Wang, J.; Wang, H.; Zhang, M.; Li, X.; Zhao, Y.; Chen, G.; Si, J.; Jiang, L. Sesquiterpene coumarins from Ferula sinkiangensis KM Shen and their cytotoxic activities. Phytochemistry 2020, 180, 112531. [Google Scholar] [CrossRef]
- Wang, J.; Meng, X.; Zheng, Y.; Sang, C.; Wang, W.; Ma, J.; Zhao, Y.; Yang, J. (±)-Ferulasin, unusual sesquiterpene chromones from Ferula sinkiangensis. Tetrahedron 2022, 122, 132953. [Google Scholar]
- Liu, T.; Wang, L.; Zhang, L.; Jiang, H.; Zhang, Y.; Mao, L. Insecticidal, cytotoxic and anti-phytopathogenic fungal activities of chemical constituents from the aerial parts of Ferula sinkiangensis. Nat. Prod. Res. 2020, 34, 1430–1436. [Google Scholar] [CrossRef]
- Mohammadhosseini, M.; Venditti, A.; Sarker, S.D.; Nahar, L.; Akbarzadeh, A. The genus Ferula: Ethnobotany, phytochemistry and bioactivities—A review. Indust. Crops Prod. 2019, 129, 350–394. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Qiang, Y. Chemical constituents from Ferula sinkiangensis and their chemotaxonomic significance. Biochem. Syst. Ecol. 2022, 105, 104519. [Google Scholar]
- Li, Q.; Li, J.; Bao, X.; Zhang, S.; Luo, Q.; Li, K.; Jiao, Y.; Cheng, Y.; Yan, Y. Unusual sesquilignans with anti-inflammatory activities from the resin of Ferula sinkiangensis. Bioorg. Chem. 2022, 127, 105986. [Google Scholar]
- Zhang, M.; Zhu, Y.; Zhan, G.; Shu, P.; Sa, R.; Lei, L.; Xiang, M.; Xue, Y.; Luo, Z.; Wan, Q.; et al. Micranthanone A, a new diterpene with an unprecedented carbon skeleton from Rhododendron micranthum. Org. Lett. 2013, 15, 3094–3097. [Google Scholar] [CrossRef]
- Yi, X.; Li, Z.; Zheng, Q.; Sang, R.; Li, H.; Gao, G.; Qin, Q.; Zhu, N. Three new tetrahydrobenzofuran derivatives from Ferula sinkiangensis KM shen and their cytotoxic activities. Nat. Prod. Res. 2022, 1–5. [Google Scholar] [CrossRef]
- Wulamu, S.; Yimamu, H.; Abuduwaili, A.; Mutailifu, P.; Maksimov, V.V.; Gao, Y.H.; Yili, A.; Aisa, H.A. Determination of the inhibitory activity of Ferula sinkiangensis polysaccharides for protein tyrosine phosphatase 1B. Chem. Nat. Compd. 2019, 55, 235–238. [Google Scholar] [CrossRef]
- Xing, Y.; Li, N.; Zhou, D.; Chen, G.; Jiao, K.; Wang, W.; Si, Y.; Hou, Y. Sesquiterpene coumarins from Ferula sinkiangensis act as neuroinflammation inhibitors. Planta Med. 2017, 83, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Mi, Y.; Jiao, K.; Xu, J.; Guo, T.; Zhou, D.; Zhang, X.; Ni, H.; Sun, Y.; Wei, K. Kellerin alleviates cognitive impairment in mice after ischemic stroke by multiple mechanisms. Phytother. Res. 2020, 34, 2258–2274. [Google Scholar] [CrossRef]
- Li, G.; Wang, J.; Li, X.; Cao, L.; Gao, L.; Lv, N.; Si, J. An unusual sesquiterpene coumarin from the seeds of Ferula sinkiangensis. J. Asian Nat. Prod. Res. 2016, 18, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, X.; Cao, L.; Zhang, L.; Shen, L.; Zhu, J.; Wang, J.; Si, J. Sesquiterpene coumarins from seeds of Ferula sinkiangensis. Fitoterapia 2015, 103, 222–226. [Google Scholar] [CrossRef]
- Li, G.; Wang, J.; Li, X.; Cao, L.; Lv, N.; Chen, G.; Zhu, J.; Si, J. Two New sesquiterpene coumarins from the seeds of Ferula sinkiangensis. Phytochem. Lett. 2015, 13, 123–126. [Google Scholar] [CrossRef]
- Ziai, S.A.; Gholami, O.; Iranshahi, M.; Zamani, A.H.; Jeddi-Tehrani, M. Umbelliprenin induces apoptosis in cll cell lines. Iran. J. Pharm. Res. 2012, 11, 653. [Google Scholar]
- Gholami, O.; Jeddi-Tehrani, M.; Iranshahi, M.; Zarnani, A.H.; Ziai, S.A. Umbelliprenin from Ferula szowitsiana activates both intrinsic and extrinsic pathways of apoptosis in jurkat T-CLL cell line. Iran. J. Pharm. Res. 2013, 12, 371. [Google Scholar]
- Zhang, L.; Sun, X.; Si, J.; Li, G.; Cao, L. Umbelliprenin isolated from Ferula sinkiangensis inhibits tumor growth and migration through the disturbance of Wnt signaling pathway in gastric cancer. PLoS ONE 2019, 14, e0207169. [Google Scholar] [CrossRef]
- Florence, C.; Luo, F.; Xu, L.; Zhou, C. The economic burden of prescription opioid overdose, abuse and dependence in the United States, 2013. Med. Care 2016, 54, 901. [Google Scholar] [CrossRef]
- Kenna, G.A.; Nielsen, D.M.; Mello, P.; Schiesl, A.; Swift, R.M. Pharmacotherapy of dual substance abuse and dependence. CNS Drugs 2007, 21, 213–237. [Google Scholar] [CrossRef]
- Murphy, S.A.; Lynch, K.G.; Oslin, D.; McKay, J.R.; TenHave, T. Developing adaptive treatment strategies in substance abuse research. Drug Alcohol Depend. 2007, 88, S24–S30. [Google Scholar] [CrossRef]
- Volkow, N.D.; Jones, E.B.; Einstein, E.B.; Wargo, E.M. Prevention and treatment of opioid misuse and addiction: A review. JAMA Psychiatry 2019, 76, 208–216. [Google Scholar] [CrossRef]
- Ye, B.; Wang, S.; Zhang, L. Studies on the detoxification effects and acute toxicity of a mixture of cis-sec-butyl-1-propoenyl disulphide and trans-sec-butyl-1-propoenyl disulphide isolated from crude essential oil of Ferula sinkiangensis KM Shen, a Chinese traditional herbal medicine. Nat. Prod. Res. 2011, 25, 1161–1170. [Google Scholar]
- Xu, A.; Shang-Guan, J.; Li, Z.; Gao, Z.; Huang, Y.C.; Chen, Q. Effects of dietary asafoetida (Ferula sinkiangensis KM Shen) levels on feeding attraction activity, growth performance, healthiness, and digestive enzyme activity in juvenile Lateolabrax japonicus. Fish Physiol. Biochem. 2020, 46, 1991–2003. [Google Scholar] [CrossRef]
- Refaz, A.D.; Mohd, S.; Parvaiz, H.Q. Overview of medicinal plants spread and their uses in Asia. J. Phytopharmacol. 2017, 6, 349–351. [Google Scholar]
- Traditional Medicine: Definition; World Health Organization: Geneva, Switzerland, 2013; Available online: http://www.who.int/medicines/areas/traditional/definitions/en/ (accessed on 26 January 2023).
- Zhu, Z.; Wang, T.; Fu, D.; Gui, Y.; Wang, J.; Cui, T. Innovative development path of ethnomedicines: An overview of ethnomedicines in China. Front. Med. 2016, 10, 166–177. [Google Scholar] [CrossRef]
- Gu, S.; Pei, J. Innovating Chinese herbal medicine: From traditional health practice to scientific drug discovery. Front. Pharmacol. 2017, 8, 381. [Google Scholar] [CrossRef]
- Astutik, S.; Pretzsch, J.; Ndzifon Kimengsi, J. Asian medicinal plants’ production and utilization potentials: A review. Sustainability 2019, 11, 5483. [Google Scholar] [CrossRef]
- Liu, B.; Guo, Z.; Bussmann, R.; Li, F.; Li, J.; Hong, L.; Long, C. Ethnobotanical approaches of traditional medicine studies in Southwest China: A literature review. J. Ethnopharmacol. 2016, 186, 343–350. [Google Scholar] [CrossRef]
- Xiong, Y.; Sui, X.; Ahmed, S.; Wang, Z.; Long, C. Ethnobotany and diversity of medicinal plants used by the Buyi in Eastern Yunnan, China. Plant Divers. 2020, 42, 401–414. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).