Comprehensive Ecotoxicity Studies on Quaternary Ammonium Salts Synthesized from Vitamin B3 Supported by QSAR Calculations
Abstract
1. Introduction
2. Results
2.1. Synthesis of Ionic Derivatives of Nicotinamide
2.2. Water Solubility
2.3. Octanol-Water Partition Coefficient
2.4. Ecotoxicity
2.4.1. Toxicity toward Terrestrial Plants
2.4.2. Toxicity toward Aquatic Live
2.5. Estimation of the Susceptibility to Biodegradation
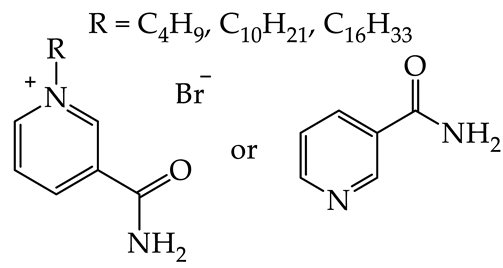
2.6. Comparison of N-hexadecylnicotinamide Bromide with Its Ammonium and Pyridinium Analogs
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of N-alkylnicotinamide Bromides
4.3. Water Solubility
4.4. Octanol-Water Partition Coefficient
4.5. Ecotoxicity
4.5.1. Toxicity toward Terrestrial Plants
4.5.2. Toxicity toward L. minor
4.5.3. Predicted Toxicity towards Other Organisms
4.6. Estimation of Physicochemical Properties and Biodegradation with the BIOWIN Models
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Jacobson, M.K.; Jacobson, E.L. Vitamin B3 in Health and Disease: Toward the Second Century of Discovery BT-ADP-ribosylation and NAD + Utilizing Enzymes: Methods and Protocols. In ADP-Ribosylation and NAD+ Utilizing Enzymes: Methods and Protocols; Chang, P., Ed.; Springer: New York, NY, USA, 2018; pp. 3–8. ISBN 978-1-4939-8588-3. [Google Scholar]
- Chang, T.; Bhalla, S. Vitamin B3, Niacin. In Industrial Biotechnology of Vitamins, Biopigments, and Antioxidants; Vandamme, E.J., Revuelta, J.L., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2016; pp. 41–66. ISBN 9783527337347. [Google Scholar]
- Preedy, V.R. B Vitamins and Folate; Food and Nutritional Components in Focus; The Royal Society of Chemistry: London, UK, 2013; ISBN 978-1-84973-369-4. [Google Scholar]
- Sauve, A.A. NAD+ and vitamin B3: From metabolism to therapies. J. Pharmacol. Exp. Ther. 2008, 324, 883–893. [Google Scholar] [CrossRef]
- Poljsak, B.; Milisav, I. Vitamin B3 forms as precursors to NAD+: Are they safe? Trends Food Sci. Technol. 2018, 79, 198–203. [Google Scholar] [CrossRef]
- Chuck, R. Technology development in nicotinate production. Appl. Catal. A Gen. 2005, 280, 75–82. [Google Scholar] [CrossRef]
- National Institutes of Health. Niacin. Available online: https://ods.od.nih.gov/factsheets/Niacin-HealthProfessional/ (accessed on 10 January 2023).
- Kumar, S.; Babu, B.V. ChemInform Abstract: Process Intensification of Nicotinic Acid Production via Enzymatic Conversion Using Reactive Extraction. Cheminform 2010, 41, 367–376. [Google Scholar] [CrossRef]
- Shaw, N.M.; Robins, K.T.; Kiener, A. Lonza: 20 Years of Biotransformations. Adv. Synth. Catal. 2003, 345, 425–435. [Google Scholar] [CrossRef]
- Siber, T.; Bušić, V.; Zobundžija, D.; Roca, S.; Vikić-Topić, D.; Vrandečić, K.; Gašo-Sokač, D. An Improved Method for the Quaternization of Nicotinamide and Antifungal Activities of Its Derivatives. Molecules 2019, 24, 1001. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Kang, S.; Luo, L.; Shi, Q.; Ma, J.; Yin, J.; Song, B.; Hu, D.; Yang, S. Synthesis and antifungal activities of novel nicotinamide derivatives containing 1,3,4-oxadiazole. Chem. Cent. J. 2013, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Tot, A.; Vrandečić, K.; Ćosić, J.; Matić, M.; Vraneš, M. Influence of side-chain length on antifungal efficacy of N-alkyl nicotinamide-based compounds. Environ. Sci. Pollut. Res. 2022, 29, 71742–71751. [Google Scholar] [CrossRef]
- Zhang, C.; Cui, F.; Zeng, G.-M.; Jiang, M.; Yang, Z.-Z.; Yu, Z.-G.; Zhu, M.-Y.; Shen, L.-Q. Quaternary ammonium compounds (QACs): A review on occurrence, fate and toxicity in the environment. Sci. Total. Environ. 2015, 518–519, 352–362. [Google Scholar] [CrossRef]
- Di Nica, V.; Gallet, J.; Villa, S.; Mezzanotte, V. Toxicity of Quaternary Ammonium Compounds (QACs) as single compounds and mixtures to aquatic non-target microorganisms: Experimental data and predictive models. Ecotoxicol. Environ. Saf. 2017, 142, 567–577. [Google Scholar] [CrossRef]
- Stachowiak, W.; Smolibowski, M.; Kaczmarek, D.K.; Rzemieniecki, T.; Niemczak, M. Toward revealing the role of the cation in the phytotoxicity of the betaine-based esterquats comprising dicamba herbicide. Sci. Total Environ. 2022, 845, 157181. [Google Scholar] [CrossRef] [PubMed]
- Biczak, R. Quaternary ammonium salts with tetrafluoroborate anion: Phytotoxicity and oxidative stress in terrestrial plants. J. Hazard. Mater. 2016, 304, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Biczak, R.; Pawłowska, B.; Feder-Kubis, J. The phytotoxicity of ionic liquids from natural pool of (−)-menthol with tetrafluoroborate anion. Environ. Sci. Pollut. Res. 2015, 22, 11740–11754. [Google Scholar] [CrossRef] [PubMed]
- Zajac, A.; Kukawka, R.; Pawlowska-Zygarowicz, A.; Stolarska, O.; Smiglak, M. Ionic liquids as bioactive chemical tools for use in agriculture and the preservation of agricultural products. Green Chem. 2018, 20, 4764–4789. [Google Scholar] [CrossRef]
- Stachowiak, W.; Rzemieniecki, T.; Klejdysz, T.; Pernak, J.; Niemczak, M. “Sweet” ionic liquids comprising the acesulfame anion—Synthesis, physicochemical properties and antifeedant activity towards stored product insects. New J. Chem. 2020, 44, 7017–7028. [Google Scholar] [CrossRef]
- Brzęczek-Szafran, A.; Więcek, P.; Guzik, M.; Chrobok, A. Combining amino acids and carbohydrates into readily biodegradable, task specific ionic liquids. RSC Adv. 2020, 10, 18355–18359. [Google Scholar] [CrossRef]
- Stachowiak, W.; Kaczmarek, D.K.; Rzemieniecki, T.; Niemczak, M. Sustainable Design of New Ionic Forms of Vitamin B3 and Their Utilization as Plant Protection Agents. J. Agric. Food Chem. 2022, 70, 8222–8232. [Google Scholar] [CrossRef]
- OECD. OECD Test No. 208: Terrestrial Plant Test: Seedling Emergence and Seedling Growth Test. In OECD Guidelines for the Testing of Chemicals; OECD Publishing: Paris, France, 2006. [Google Scholar]
- Almeida, L.C.; Mattos, A.C.; Dinamarco, C.P.G.; Figueiredo, N.G.; Bila, D.M. Chronic toxicity and environmental risk assessment of antivirals in Ceriodaphnia dubia and Raphidocelis subcapitata. Water Sci. Technol. 2021, 84, 1623–1634. [Google Scholar] [CrossRef]
- Reuschenbach, P.; Silvani, M.; Dammann, M.; Warnecke, D.; Knacker, T. ECOSAR model performance with a large test set of industrial chemicals. Chemosphere 2008, 71, 1986–1995. [Google Scholar] [CrossRef]
- OECD. OECD Test No. 221: Lemna sp. Growth Inhibition Test. In OECD Guidelines for the Testing of Chemicals; OECD Publishing: Paris, France, 2006. [Google Scholar]
- United Nations. Globally Harmonized System of Classification and Labelling of Chemicals (GHS); United Nations: New York, NY, USA, 2021. [Google Scholar]
- Çeçen, F.; Gül, G. Biodegradation of five pharmaceuticals: Estimation by predictive models and comparison with activated sludge data. Int. J. Environ. Sci. Technol. 2021, 18, 327–340. [Google Scholar] [CrossRef]
- Kalyanasundaram, K.; Colassis, T.; Humphry-Baker, R.; Savarino, P.; Barni, E.; Pelizzetti, E.; Grätzel, M. Micellar properties of nicotinamide-based surfactants. J. Colloid Interface Sci. 1989, 132, 469–474. [Google Scholar] [CrossRef]
- Maynard, R.L. The Merck Index: 12th edition 1996. Occup. Environ. Med. 1997, 54, 288. [Google Scholar] [CrossRef]
- Van Arnum, S.D. Niacin, Nicotinamide, and Nicotinic Acid. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley Online Library: Hoboken, NJ, USA, 2000; ISBN 9780471238966. [Google Scholar]
- Hansch, C.; Leo, A.; Hoekman, D. Exploring QSAR: Hydrophobic, Electronic, and Steric Constants; American Chemical Society: Washington, DC, USA, 1995. [Google Scholar]
- Pretti, C.; Chiappe, C.; Baldetti, I.; Brunini, S.; Monni, G.; Intorre, L. Acute toxicity of ionic liquids for three freshwater organisms: Pseudokirchneriella subcapitata, Daphnia magna and Danio rerio. Ecotoxicol. Environ. Saf. 2009, 72, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Lee, B.; Ra, J.-S.; Kim, K.-T. Predicting PBT and CMR properties of substances of very high concern (SVHCs) using QSAR models, and application for K-REACH. Toxicol. Rep. 2020, 7, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- European Chemicals Agency. Cetrimonium Bromide. Available online: https://echa.europa.eu/pl/registration-dossier/-/registered-dossier/10369/5/3/3 (accessed on 10 January 2023).
- Couling, D.J.; Bernot, R.J.; Docherty, K.M.; Dixon, J.K.; Maginn, E.J. Assessing the factors responsible for ionic liquid toxicity to aquatic organisms via quantitative structure–property relationship modeling. Green Chem. 2006, 8, 82–90. [Google Scholar] [CrossRef]
- de Sousa Pacheco, E.J. Pacheco Ecotoxicologigal Assessment of Cetylpyridinium Chloride and 1-(2-Hydroxyethyl)-3-Methylimidazolium Chloride. Ph.D. Thesis, Instituto Politecnico do Porto, Porto, Portugal, 2019. [Google Scholar]
- Stachowiak, W.; Wysocki, M.; Niemczak, M. “Bitter” Results: Toward Sustainable Synthesis of the Most Bitter Substances, Denatonium Saccharinate and Denatonium Benzoate, Starting from a Popular Anesthetic, Lidocaine. J. Chem. Educ. 2022, 99, 1604–1611. [Google Scholar] [CrossRef]
- Mesnage, R.; Bernay, B.; Séralini, G.-E. Ethoxylated adjuvants of glyphosate-based herbicides are active principles of human cell toxicity. Toxicology 2013, 313, 122–128. [Google Scholar] [CrossRef]
- Turek, M.; Biczak, R.; Pawłowska, B.; Różycka-Sokołowska, E.; Marciniak, B.; Deska, M.; Skalik, J.; Bałczewski, P. Ammonium haloacetates—An alternative to glyphosate? Chemosphere 2018, 194, 650–656. [Google Scholar] [CrossRef]
- Hong, J.; Kim, H.; Jung, H.; Yang, H.; Kim, D.; Sung, C.; Park, C.-J.; Chang, S. Differential Control Efficacies of Vitamin Treatments against Bacterial Wilt and Grey Mould Diseases in Tomato Plants. Plant Pathol. J. 2016, 32, 469–480. [Google Scholar] [CrossRef]
- Kumari, P.; Pillai, V.V.; Benedetto, A. Mechanisms of action of ionic liquids on living cells: The state of the art. Biophys. Rev. 2020, 12, 1187–1215. [Google Scholar] [CrossRef]
- Woźniak-Karczewska, M.; Parus, A.; Ciesielski, T.; Trzebny, A.; Szumski, R.; Wilms, W.; Homa, J.; Framski, G.; Baranowski, D.; Frankowski, R.; et al. Effect of Cation Sorption on 2,4-D Mobility of Herbicidal Ionic Liquids in Agricultural Soil Combined with Diversity of the Bacterial Community. ACS Sustain. Chem. Eng. 2022, 10, 12559–12568. [Google Scholar] [CrossRef]
- Liu, D.; Liu, H.; Wang, S.; Chen, J.; Xia, Y. The toxicity of ionic liquid 1-decylpyridinium bromide to the algae Scenedesmus obliquus: Growth inhibition, phototoxicity, and oxidative stress. Sci. Total. Environ. 2018, 622–623, 1572–1580. [Google Scholar] [CrossRef]
- European Chemicals Agency. Dodecyltrimethylammonium Chloride. Available online: https://echa.europa.eu/pl/registration-dossier/-/registered-dossier/24215/6/2/4 (accessed on 10 January 2023).
- National Library of Medicine. PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 10 January 2023).
- Trush, M.; Metelytsia, L.; Semenyuta, I.; Kalashnikova, L.; Papeykin, O.; Venger, I.; Tarasyuk, O.; Bodachivska, L.; Blagodatnyi, V.; Rogalsky, S. Reduced ecotoxicity and improved biodegradability of cationic biocides based on ester-functionalized pyridinium ionic liquids. Environ. Sci. Pollut. Res. 2018, 26, 4878–4889. [Google Scholar] [CrossRef] [PubMed]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef]
- Ventura, S.P.; Gonçalves, A.M.; Gonçalves, F.; Coutinho, J.A. Assessing the toxicity on [C3mim][Tf2N] to aquatic organisms of different trophic levels. Aquat. Toxicol. 2010, 96, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Hafez, N.F.M.; Mutalib, M.I.A.; Bustam, M.A.B.; El-Harbawi, M.; Leveque, J.-M. Ecotoxicity of Pyridinium Based ILs towards Guppy Fish and Four Bacterial Strains. Procedia Eng. 2016, 148, 830–838. [Google Scholar] [CrossRef]
- European Chemicals Agency. Nicotinamide. Available online: https://echa.europa.eu/pl/registration-dossier/-/registered-dossier/14571/5/3/2 (accessed on 10 January 2023).
- Suk, M.; Kümmerer, K. Towards greener and sustainable ionic liquids using naturally occurring and nature-inspired pyridinium structures. Green Chem. 2023, 25, 365–374. [Google Scholar] [CrossRef]
- Neumann, J.; Steudte, S.; Cho, C.-W.; Thöming, J.; Stolte, S. Biodegradability of 27 pyrrolidinium, morpholinium, piperidinium, imidazolium and pyridinium ionic liquid cations under aerobic conditions. Green Chem. 2014, 16, 2174–2184. [Google Scholar] [CrossRef]
- Docherty, K.M.; Dixon, J.K.; Kulpa, C.F., Jr. Biodegradability of imidazolium and pyridinium ionic liquids by an activated sludge microbial community. Biodegradation 2007, 18, 481–493. [Google Scholar] [CrossRef]
- Timmer, N.; Gore, D.; Sanders, D.; Gouin, T.; Droge, S.T. Sorbent-modified biodegradation studies of the biocidal cationic surfactant cetylpyridinium chloride. Ecotoxicol. Environ. Saf. 2019, 182, 109417. [Google Scholar] [CrossRef]
- National Library of Medicine. Cetylpyridinium Bromide. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cetylpyridinium-bromide#section=NSC-Number (accessed on 10 January 2023).
- Harjani, J.R.; Singer, R.D.; Garcia, M.T.; Scammells, P.J. The design and synthesis of biodegradable pyridinium ionic liquids. Green Chem. 2008, 10, 436–438. [Google Scholar] [CrossRef]
- ISO 20079:2005; Water Quality—Determination of the Toxic Effect of Water Constituents and Waste Water on Duckweed (Lemna Minor)—Duckweed Growth Inhibition Test. International Organization for Standardization: Geneva, Switzerland, 2005.
- Pavan, M.; Worth, A.P. Review of Estimation Models for Biodegradation. QSAR Comb. Sci. 2008, 27, 32–40. [Google Scholar] [CrossRef]
- Boethling, R.S.; Sommer, E.; DiFiore, D. Designing Small Molecules for Biodegradability. Chem. Rev. 2007, 107, 2207–2227. [Google Scholar] [CrossRef] [PubMed]
- Hermens, J.L. Electrophiles and Acute Toxicity to Fish. Environ. Health Perspect. 1990, 87, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Hodges, G.; Eadsforth, C.; Bossuyt, B.; Bouvy, A.; Enrici, M.-H.; Geurts, M.; Kotthoff, M.; Michie, E.; Miller, D.; Müller, J.; et al. A Comparison of Log Kow (n-Octanol–Water Partition Coefficient) Values for Non-Ionic, Anionic, Cationic and Amphoteric Surfactants Determined Using Predictions and Experimental Methods. Environ. Sci. Eur. 2019, 31, 1. [Google Scholar] [CrossRef]
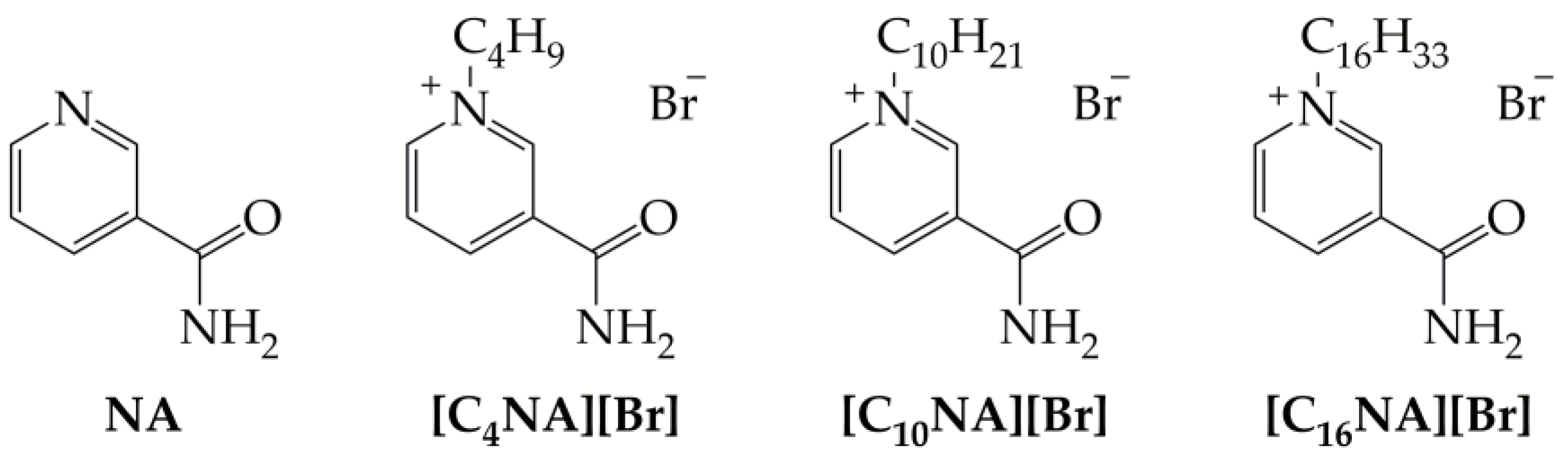
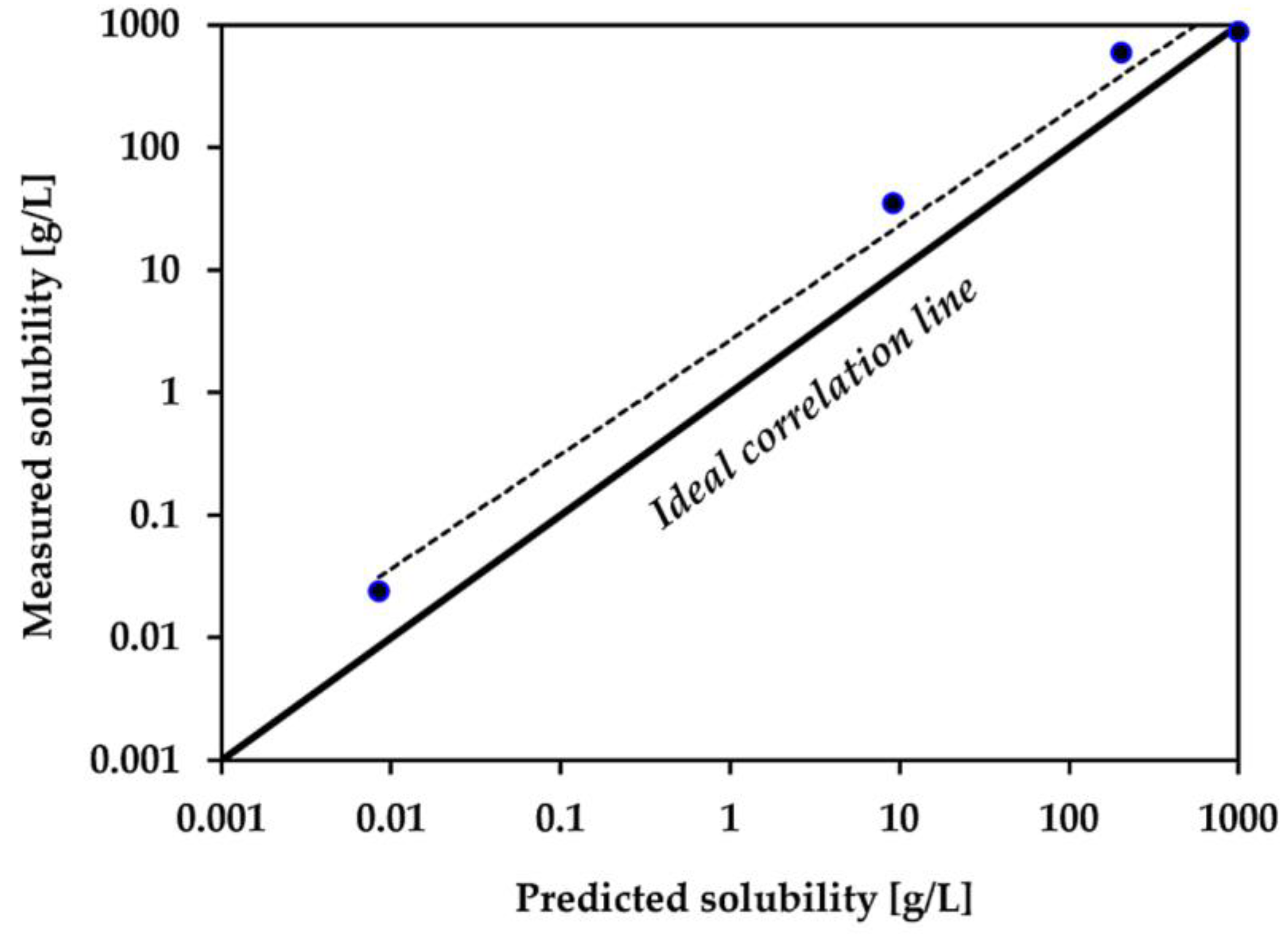
| Compound | Measured Solubility (g/L) | Predicted Solubility (g/L) |
|---|---|---|
| NA a | 592 | 204 |
| [C4NA][Br] | 873 | 1000 |
| [C10NA][Br] | 34.9 | 9.14 |
| [C16NA][Br] | 0.024 | 0.0085 |
| Compound | Measured Log KOW | Predicted Log KOW |
|---|---|---|
| NA a | −0.66 | −0.45 |
| [C4NA][Br] | −1.41 | −3.10 |
| [C10NA][Br] | −0.18 | −0.15 |
| [C16NA][Br] | 1.70 | 2.80 |
| NA | [C4NA][Br] | [C10NA][Br] | [C16NA][Br] | |||
|---|---|---|---|---|---|---|
| Organism a | Duration | Endpoint | Concentration [mg/L] | |||
| Fish | 96 h | LC50 | 3900 | 34,000 | 54 | 1.90 |
| Daphnid | 48 h | LC50 | 5700 | 56,000 | 5.9 | 1.60 |
| Green Algae | 96 h | EC50 | 180 | 1200 | --- b | --- |
| L. minorc | 72 h | EC50 | >1000 | >1000 | 100–1000 | 10–24 |
| Potential to bioaccumulation d | No | No | No | No | ||
| GHS short-term toxicity category e | None | None | Acute II | Acute II | ||
| Compound | NA | [C4NA][Br] | [C10NA][Br] | [C16NA][Br] |
|---|---|---|---|---|
| BIOWIN 1 | 0.7450 | 0.9428 | 0.9027 | 0.8626 |
| BIOWIN 2 | 0.9111 | 0.9794 | 0.9352 | 0.8136 |
| BIOWIN 3 | 2.6609 | 2.8706 | 2.6846 | 2.4986 |
| BIOWIN 4 | 3.8582 | 3.9483 | 3.8269 | 3.7055 |
| BIOWIN 5 | 0.3843 | 0.2936 | 0.3140 | 0.3345 |
| BIOWIN 6 | 0.3606 | 0.1540 | 0.1478 | 0.1417 |
| BIOWIN 7 | 0.5276 | −0.4328 | −0.2769 | −0.1210 |
| [C16TMA][Br] | [C16PIR][Br] | [C16NA][Br] | |||
|---|---|---|---|---|---|
| Organism | Duration | Endpoint | Concentration [mg/L] | ||
| Fish | 96 h | LC50 | 0.1–0.28 | 36.5; 43.5 | 1.90 a |
| Daphnid | 48 h | LC50 | 0.026 | 0.012 | 1.60 a |
| Green Algae | 96 h | EC50 | 0.00411 | 11 | --- |
| L. minor | 72 h | EC50 | --- | --- | 10–24 |
| Potential to bioaccumulation | No | No | No | ||
| GHS short-term toxicity category | Acute I | Acute I | Acute II | ||
| GHS long-term toxicity category | Chronic I | Chronic I | --- | ||
| Phytotoxicity | |||
|---|---|---|---|
| Structure of Compound | Monocotyledonous Plant | Dicotyledonous Plant | Conclusions |
 | Sorghum | White mustard | Non-phytotoxic towards mono- and dicotyledonous plants. |
 | No data available | Cornflower Oilseed rape | Enhanced herbicidal effect toward dicotyledonous plants compared to original herbicides (2,4-D, MCPA). Utilized cations most likely play the role of non-phytotoxic adjuvants [21]. |
 | Spring barley | Common radish | The toxicity toward mono- and dicotyledonous plants decreases 6-fold with increase in alkyl chain length from C1 to C11 [17]. |
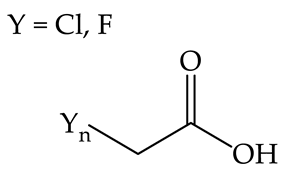 | Spring barley | Common radish | Toxicity of the tested solutions increases with the number of halo-substituents and is equally high for mono- and dicotyledonous plants [39]. |
 | No data available | Tomato | Vitamin B3 in a form of sodium nicotinate applied to tomato plants can reduce the bacterial wilt of plants, while not causing phytotoxic effects [40]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowacka, A.; Olejniczak, A.; Stachowiak, W.; Niemczak, M. Comprehensive Ecotoxicity Studies on Quaternary Ammonium Salts Synthesized from Vitamin B3 Supported by QSAR Calculations. Plants 2023, 12, 914. https://doi.org/10.3390/plants12040914
Nowacka A, Olejniczak A, Stachowiak W, Niemczak M. Comprehensive Ecotoxicity Studies on Quaternary Ammonium Salts Synthesized from Vitamin B3 Supported by QSAR Calculations. Plants. 2023; 12(4):914. https://doi.org/10.3390/plants12040914
Chicago/Turabian StyleNowacka, Aleksandra, Adriana Olejniczak, Witold Stachowiak, and Michał Niemczak. 2023. "Comprehensive Ecotoxicity Studies on Quaternary Ammonium Salts Synthesized from Vitamin B3 Supported by QSAR Calculations" Plants 12, no. 4: 914. https://doi.org/10.3390/plants12040914
APA StyleNowacka, A., Olejniczak, A., Stachowiak, W., & Niemczak, M. (2023). Comprehensive Ecotoxicity Studies on Quaternary Ammonium Salts Synthesized from Vitamin B3 Supported by QSAR Calculations. Plants, 12(4), 914. https://doi.org/10.3390/plants12040914









