Contribution of Sucrose Metabolism in Phloem to Kiwifruit Bacterial Canker Resistance
Abstract
:1. Introduction
2. Results
2.1. Comparison of the Responses of Detached Branches of Five Kiwifruit Cultivars to PSA Infection
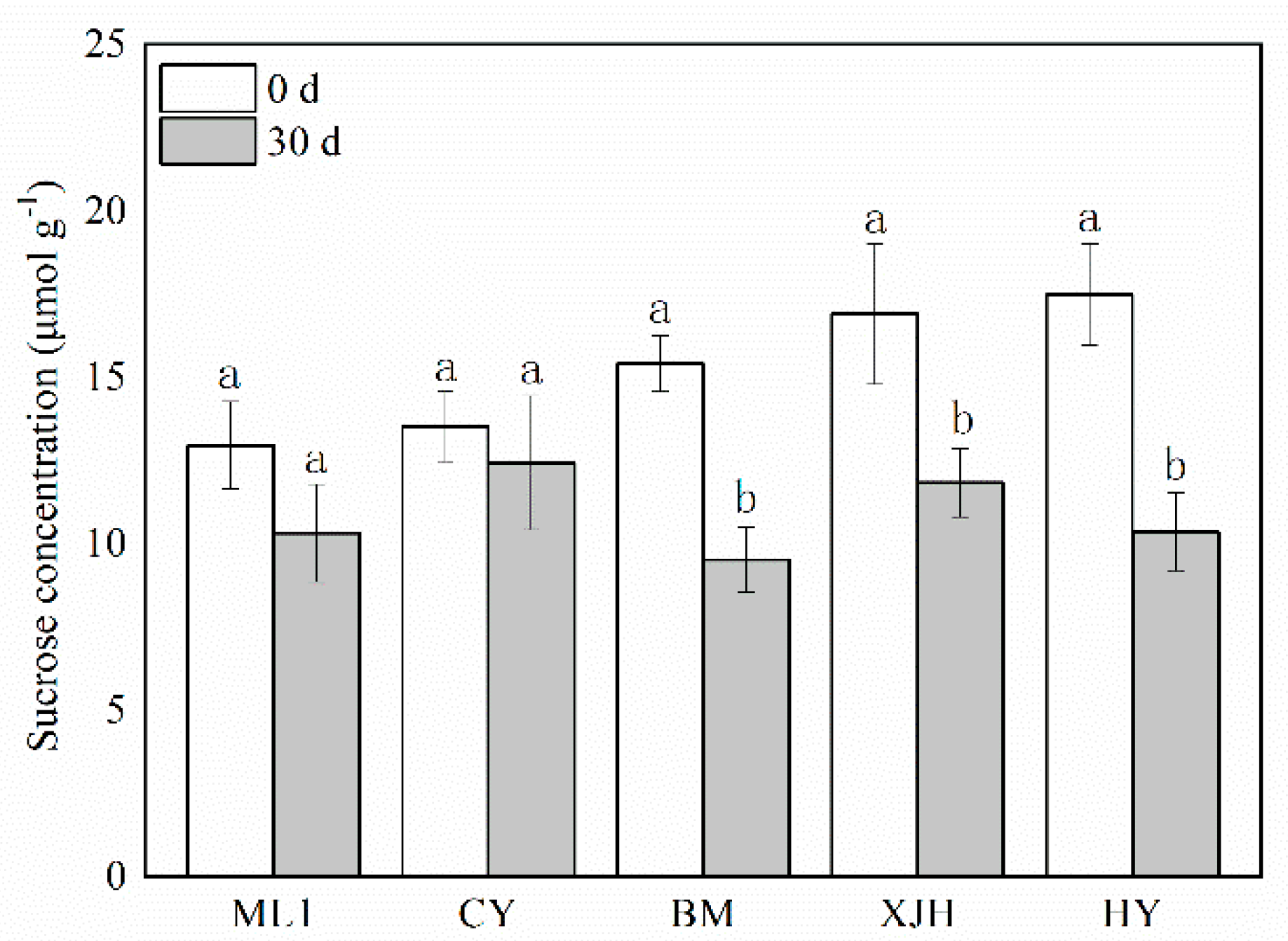
2.2. Effect of Psa Infection on Contents of Sucrose in the Phloem of Kiwifruit Branches
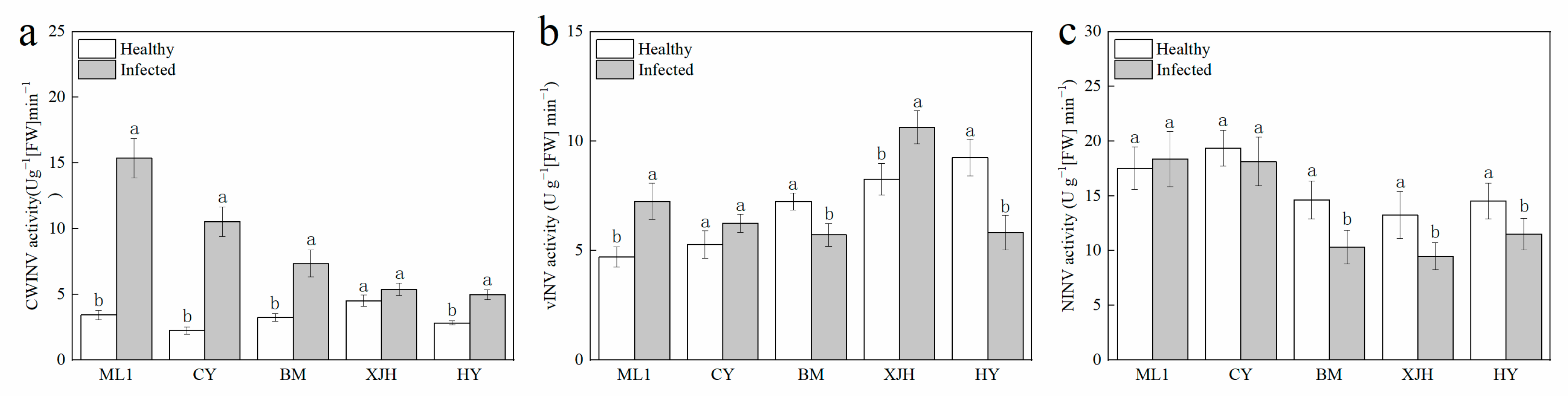
2.3. Effect of Psa Infection on Invertase Activity in the Phloem of Kiwifruit Branches
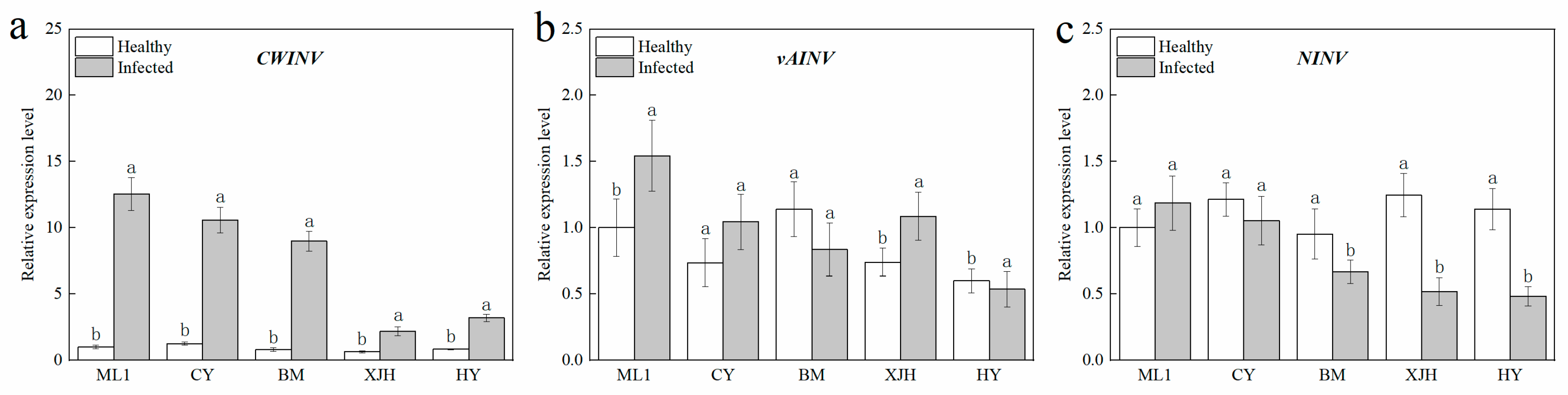
2.4. Response of Sucrose Invertase Biosynthesis Genes Expression to Psa Infection
2.5. Defense Enzyme Activities in Detached Branches of Five Kiwifruit Cultivars Infected with PSA
3. Materials and Methods
3.1. Plant Materials and Growth Conditions
3.2. PSA Activation and Inoculation
3.3. Assessment of Different Cultivars’ Disease Tolerance
3.4. Detection of Sucrose in the Phloem
3.5. Enzyme Activities Analysis
3.6. RNA Extraction and Quantitative RT-PCR
3.7. Statistical Calculations
4. Discussion
5. Conclusion and Indications for the Future
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Zhu, Q.M.; Zhi, T.H.; Fan, R.; Xie, T.; Zhao, Z.B.; Long, Y.H.; Li, Z. Genetic Causes of Non-pathogenic Pseudomonas syringae pv. actinidiae Isolates in Kiwifruit Orchards. Front. Microbiol. 2021, 12, 650099. [Google Scholar] [PubMed]
- Tahir, J.; Hoyte, S.; Bassett, H.; Brendolise, C.; Chatterjee, A.; Templeton, K.; Deng, C.; Crowhurst, R.; Montefiori, M.; Morgan, E.; et al. Multiple Quantitative Trait Loci Contribute to Resistance to Bacterial Canker Incited by Pseudomonas syringae pv. actinidiae in Kiwifruit (Actinidia Chinensis). Hortic. Res. 2019, 6, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donati, I.; Buriani, G.; Cellini, A.; Mauri, S.; Costa, G.; Spinelli, F. New Insights on the Bacterial Canker of Kiwifruit (Pseudomonas syringae pv. actinidiae). J. Berry Res. 2014, 4, 53–67. [Google Scholar] [CrossRef] [Green Version]
- Vanneste, J.L. The Scientific, Economic, and Social Impacts of the New Zealand Outbreak of Bacterial Canker of Kiwifruit (Pseudomonas syringae pv. actinidiae). Annu. Rev. Phytopathol. 2017, 55, 377–399. [Google Scholar] [CrossRef] [PubMed]
- Takikaway, Y.C.; Serizawa, S.; Ichikawa, T.; Tsuyumu, S.; Goto, M. Pseudomonas syringae pv. actinidiae pv. nov. the causal bacterium of canker of kiwifruit in Japan. Ann. Phytopathol. Soc. Jpn. 1989, 55, 437–444. [Google Scholar]
- Balestra, G.M.; Renzi, M.; Mazzaglia, A. First Report of Bacterial Canker of Actinidia Delicosa Caused by Pseudomonas syringae pv. actinidiae in Portugal. New Dis. Rep. 2010, 22, 10. [Google Scholar] [CrossRef] [Green Version]
- Abelleira, A.; López, M.M.; Peñalver, J.; Aguín, O.; Mansilla, J.P.; Picoaga, A.; García, M.J. First Report of Pseudomonas syringae pv. actinidiae on Kiwifruit Plants in Spain. Plant Dis. 2011, 95, 1583. [Google Scholar]
- Vanneste, J.L.; Poliakoff, F.; Audusseau, C.; Cornish, D.A.; Paillard, S.; Rivoal, C.; Yu, J. First Report of Pseudomonas syringae pv. actinidiae, the Causal Agent of Bacterial Canker of Kiwifruit in France. Plant Dis. 2011, 95, 1311–1312. [Google Scholar]
- Sawada, H.; Kondo, K.; Nakaune, R. Novel biovar (biovar 6) of Pseudomonas syringae pv. actinidiae causing bacterial canker of kiwifruit (Actinidia deliciosa) in Japan. Jpn. J. Phytopathol. 2016, 82, 101–115. [Google Scholar]
- Butler, M.I.; Stockwell, P.A.; Black, M.A.; Day, R.C.; Lamont, I.L.; Poulter, R.T. Pseudomonas syringae pv. actinidiae from Recent Outbreaks of Kiwifruit Bacterial Canker Belong to Different Clones that Originated in China. PLoS ONE 2013, 8, e57464. [Google Scholar]
- McCann, H.C.; Rikkerink, E.H.; Bertels, F.; Fiers, M.; Lu, A.; Rees-George, J.; Andersen, M.T.; Gleave, A.P.; Haubold, B.; Wohlers, M.W.; et al. Genomic Analysis of the Kiwifruit Pathogen Pseudomonas syringae pv. actinidiae Provides Insight into the Origins of an Emergent Plant Disease. PLoS Pathog. 2013, 9, e1003503. [Google Scholar]
- Wang, F.M.; Mo, Q.H.; Ye, K.Y.; Gong, H.J.; Qi, B.B.; Liu, P.P.; Jiang, Q.S.; Li, J.W. Evaluation of the wild Actinidia germplasm for resistance to Pseudomonas syringae pv. actinidiae. Plant Pathol. 2020, 69, 979–989. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Zeng, Y.; Liu, P. Metabolic Profiling Reveals Local and Systemic Responses of Kiwifruit to Pseudomonas syringae pv. actinidiae. Plant Direct 2020, 4, e00297. [Google Scholar] [CrossRef] [PubMed]
- Petriccione, M.; Mastrobuoni, F.; Zampella, L.; Scortichini, M. Reference Gene Selection for Normalization of Rt-Qpcr Gene Expression Data from Actinidia Deliciosa Leaves Infected with Pseudomonas syringae pv. actinidiae. Sci. Rep. 2015, 19, 16961. [Google Scholar] [CrossRef] [Green Version]
- Nunes da Silva, M.; Machado, G.; Balestra, G.M.; Mazzaglia, M.W.; Vasconcelos, M.W.; Carvalho, S.M.P. Exploring the Expression of Defence-Related Genes in Actinidia Spp. After Infection with Pseudomonas syringae pv. actinidiae and pv. actinidifoliorum: First Steps. Eur. J. Hortic. Sci. 2019, 84, 206–212. [Google Scholar]
- Cameron, A.; Sarojini, V. Pseudomonas syringae pv. actinidiae: Chemical Control, Resistance Mechanisms and Possible Alternatives. Plant Pathol. 2014, 63, 1–11. [Google Scholar]
- Lee, Y.S.; Kim, G.H.; Song, Y.R.; Oh, C.S.; Jung, J.S. Streptomycin Resistant Isolates of Pseudomonas syringae pv. actinidiae in Korea. Res. Plant Dis. 2020, 26, 44–47. [Google Scholar] [CrossRef]
- Nakajima, M.; Goto, M.; Hibi, T. Similarity between Copper Resistance Genes from Pseudomonas syringae pv. actinidiae and P. syringae pv. tomato. J. Gen. Plant Pathol. 2002, 68, 68–74. [Google Scholar]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar Sensing and Signaling in Plants: Conserved and Novel Mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef] [Green Version]
- Braun, D.M. Phloem Loading and Unloading of Sucrose: What a Long, Strange Trip from Source to Sink. Annu. Rev. Plant Biol. 2022, 73, 553–584. [Google Scholar] [CrossRef]
- Tauzin, A.S.; Giardina, T. Sucrose and Invertases, a part of the Plant Defense Response to the Biotic Stresses. Front Plant Sci. 2014, 5, 293. [Google Scholar] [CrossRef]
- Yoon, J.; Cho, L.H.; Tun, W.; Jeon, J.S.; An, G. Sucrose signaling in higher plants. Plant Sci. 2021, 302, 110703. [Google Scholar] [CrossRef]
- Wind, J.; Smeekens, S.; Hanson, J. Sucrose: Metabolite and Signaling Molecule. Phytochemistry 2010, 71, 1610–1614. [Google Scholar] [CrossRef]
- Wan, H.J.; Wu, L.M.; Yang, Y.J.; Zhou, G.Z.; Ruan, Y.L. Evolution of Sucrose Metabolism: The Dichotomy of Invertases and Beyond. Trends Plant Sci. 2018, 23, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.L.; Jin, Y.; Yang, Y.J.; Li, G.J.; Boyer, J.S. Sugar input, metabolism, and signaling mediated by invertase: Roles in development, yield potential, and response to drought and heat. Mol. Plant 2010, 3, 942–955. [Google Scholar] [CrossRef]
- Satoh, S.; Sturm, A.; Fujii, T.; Chrispeels, M.J. cDNA Cloning of an Extracellular Dermal Glycoprotein of Carrot and Its Expression in Response to Wounding. Planta 1992, 188, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Kocal, N.; Sonnewald, U.; Sonnewald, S. Cell Wall-Bound Invertase Limits Sucrose Export and is Involved in Symptom Development and Inhibition of Photosynthesis during Compatible Interaction between Tomato and Xanthomonas campestris pv vesicatoria. Plant Physiol. 2008, 148, 1523–1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Yang, D.L.; Kong, Y.; Chen, Y.; Li, X.Z.; Zeng, L.J.; Li, Q.; Wang, E.T.; He, Z.H. Sugar Homeostasis Mediated by Cell Wall Invertase Grain Incomplete Filling 1 (Gif1) Plays a Role in Pre-Existing and Induced Defence in Rice. Mol. Plant Pathol. 2014, 15, 161–173. [Google Scholar] [CrossRef]
- Sonnewald, S.; Priller, J.P.; Schuster, J.; Glickmann, E.; Hajirezaei, M.R.; Siebig, S.; Mudgett, M.B.; Sonnewald, U. Regulation of Cell Wall-Bound Invertase in Pepper Leaves by Xanthomonas campestris pv. vesicatoria Type Three Effectors. PLoS ONE 2012, 7, e51763. [Google Scholar]
- Xiang, L.; Le Roy, K.; Bolouri-Moghaddam, M.R.; Vanhaecke, M.; Lammens, W.; Rolland, F.; Van den Ende, W. Exploring the Neutral Invertase–Oxidative Stress Defence Connection in Arabidopsis Thaliana. J. Exp. Bot. 2011, 62, 3849–3862. [Google Scholar] [CrossRef] [Green Version]
- Essmann, J.; Bones, P.; Weis, E.; Scharte, J. Leaf Carbohydrate Metabolism during Defense Intracellular Sucrose-cleaving Enzymes Do not Compensate Repression of Cell Wall Invertase. Plant Signal. Behav. 2008, 3, 885–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Kim, S.; Choi, E.; Auh, C.K.; Park, J.B.; Kim, D.G.; Chung, Y.J.; Lee, T.K.; Lee, S. Altered Invertase Activities of Symptomatic Tissues on Beet Severe Curly Top Virus (BSCTV) Infected Arabidopsis Thaliana. J. Plant Res. 2013, 126, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.M.; Li, J.W.; Ye, K.Y.; Liu, P.P.; Gong, H.J.; Jiang, Q.S.; Qi, B.B.; Mo, Q.H. An in vitro Actinidia Bioassay to Evaluate the Resistance to Pseudomonas syringae pv. actinidiae. Plant Pathol. J. 2019, 35, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zha, M.; Huang, J.; Li, L.; Imran, M.; Zhang, C. StMYB44 Negatively Regulates Phosphate Transport by Suppressing Expression of PHOSPHATE1 in Potato. J Exp Bot. 2017, 68, 1265–1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, S.S.; Li, Y.L.; Shi, C.X.; Li, E.C.; Zhang, Y.H.; Li, M.L. Relationships between sucrose content and resistance of corn to stalk rot. J. Northwest Sci.-Tech. Univ. Agri. For. 2003, 31, 113–118. [Google Scholar]
- Gómez-Ariza, J.; Campo, S.; Rufat, M.; Estopà, M.; Messeguer, J.; Segundo, B.S.; Coca, M. Sucrose-Mediated Priming of Plant Defense Responses and Broad-Spectrum Disease Resistance by Overexpression of the Maize Pathogenesis-Related PRms Protein in Rice Plants. Mol. Plant Microbe Interact. 2007, 20, 832–842. [Google Scholar] [CrossRef] [Green Version]
- Siemens, J.; González, M.C.; Wolf, S.; Hofmann, C.; Greiner, S.; Du, Y.; Rausch, T.; Roitsch, T.; Ludwig-Müller, J. Extracellular Invertase Is Involved in the Regulation of Clubroot Disease in Arabidopsis Thaliana. Mol. Plant Pathol. 2011, 12, 247–262. [Google Scholar] [CrossRef]
- Santi, S.; Grisan, S.; Pierasco, A.; DE Marco, F.; Musetti, R. Laser Microdissection of Grapevine Leaf Phloem Infected by Stolbur Reveals Site-Specific Gene Responses Associated to Sucrose Transport and Metabolism. Plant Cell Environ. 2013, 36, 343–355. [Google Scholar] [CrossRef]
- Boudet, A.M. Evolution and Current Status of Research in Phenolic Compounds. Phytochemistry 2007, 68, 2722–2735. [Google Scholar] [CrossRef]
- Sturm, A.; Chrispeels, M.J. cDNA Cloning of Carrot Extracellular Beta-Fructosidase and Its Expression in Response to Wounding and Bacterial Infection. Plant Cell 1990, 2, 1107–1119. [Google Scholar] [CrossRef] [Green Version]
- Barratt, D.H.; Derbyshire, P.; Findlay, K.; Pike, M.; Wellner, N.; Lunn, J.; Feil, R.; Simpson, C.; Maule, A.J.; Smith, A.M. Normal Growth of Arabidopsis Requires Cytosolic Invertase but Not Sucrose Synthase. Proc. Natl. Acad. Sci. USA 2009, 106, 13124–13129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotchoni, S.O.; Gachomo, E.W. The Reactive Oxygen Species Network Pathways: An Essential Prerequisite for Perception of Pathogen Attack and the Acquired Disease Resistance in Plants. J. Biosci. 2006, 31, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, A.; Queval, G.; Chaouch, S.; Vanderauwera, S.; Van Breusegem, F.; Noctor, G. Catalase Function in Plants: A Focus on Arabidopsis Mutants as Stress-Mimic Models. J. Exp. Bot. 2010, 61, 4197–4220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
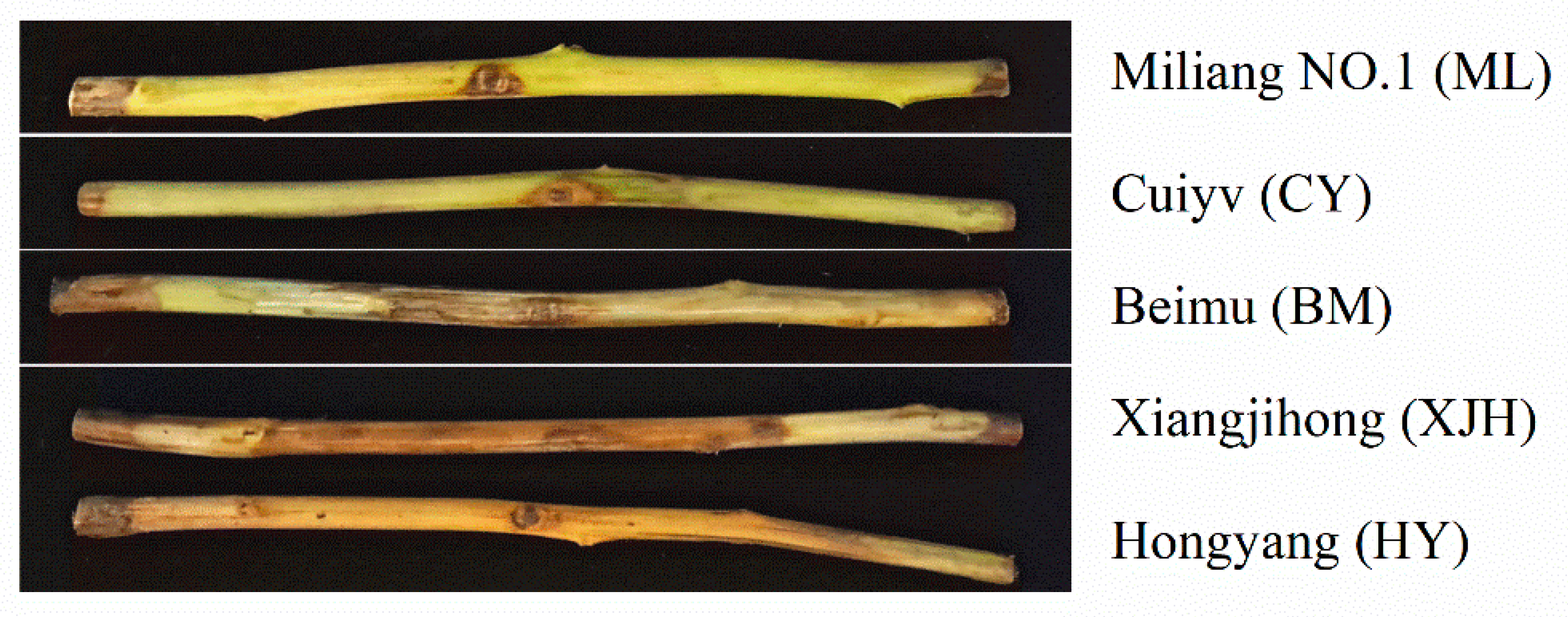

| Cultivars | PSA Invasion into Xylem | ALLT (Mean ± SD) | Response to Psa |
|---|---|---|---|
| Miliang NO.1 | None | 1.72 ± 0.22 a | T |
| Cuiyv | None | 3.17 ± 0.41 b | T |
| Beimu | Partly | 7.68 ± 1.43 c | S |
| Xiangjihong | Partly | 21.51 ± 4.69 d | HS |
| hongyang | Whole | 30.00 ± 0.00 e | HS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Tan, Z.; Zhen, X.; Liang, Y.; Gao, J.; Zhao, Y.; Liu, S.; Zha, M. Contribution of Sucrose Metabolism in Phloem to Kiwifruit Bacterial Canker Resistance. Plants 2023, 12, 918. https://doi.org/10.3390/plants12040918
Wang Y, Tan Z, Zhen X, Liang Y, Gao J, Zhao Y, Liu S, Zha M. Contribution of Sucrose Metabolism in Phloem to Kiwifruit Bacterial Canker Resistance. Plants. 2023; 12(4):918. https://doi.org/10.3390/plants12040918
Chicago/Turabian StyleWang, Yan, Zecheng Tan, Xi Zhen, Yuanyuan Liang, Jianyou Gao, Yanhui Zhao, Shibiao Liu, and Manrong Zha. 2023. "Contribution of Sucrose Metabolism in Phloem to Kiwifruit Bacterial Canker Resistance" Plants 12, no. 4: 918. https://doi.org/10.3390/plants12040918
APA StyleWang, Y., Tan, Z., Zhen, X., Liang, Y., Gao, J., Zhao, Y., Liu, S., & Zha, M. (2023). Contribution of Sucrose Metabolism in Phloem to Kiwifruit Bacterial Canker Resistance. Plants, 12(4), 918. https://doi.org/10.3390/plants12040918






