Deciphering the Genetic Architecture of Color Variation in Whole Grain Rice by Genome-Wide Association
Abstract
:1. Introduction
2. Results
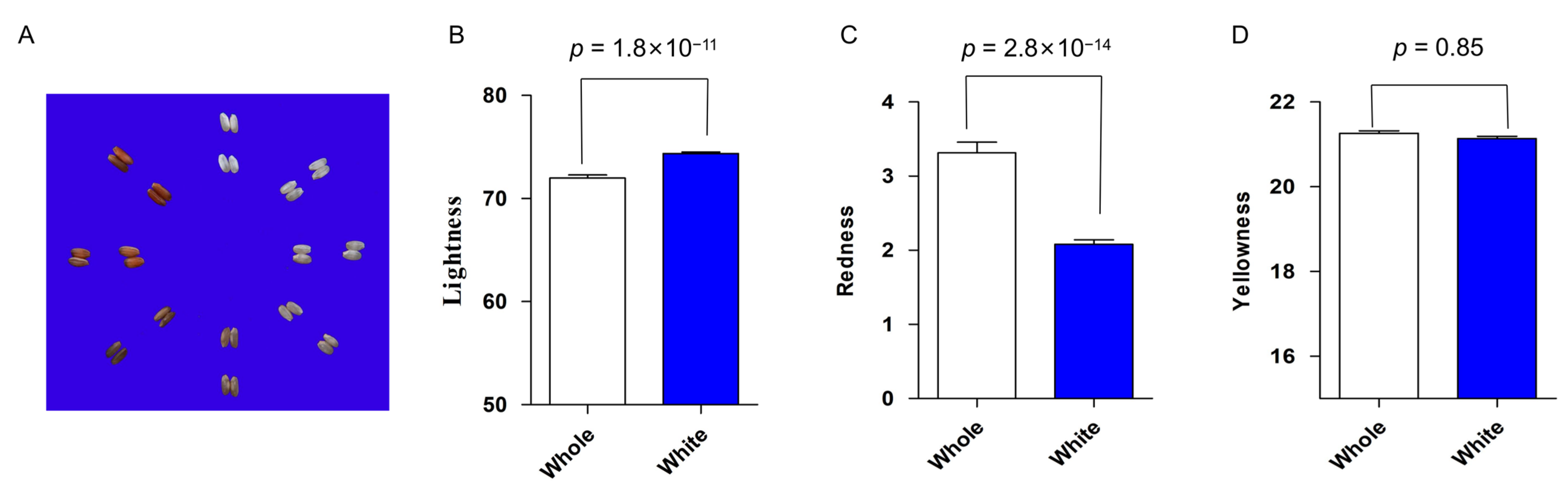
2.1. Large Variation in Rice Grain Color
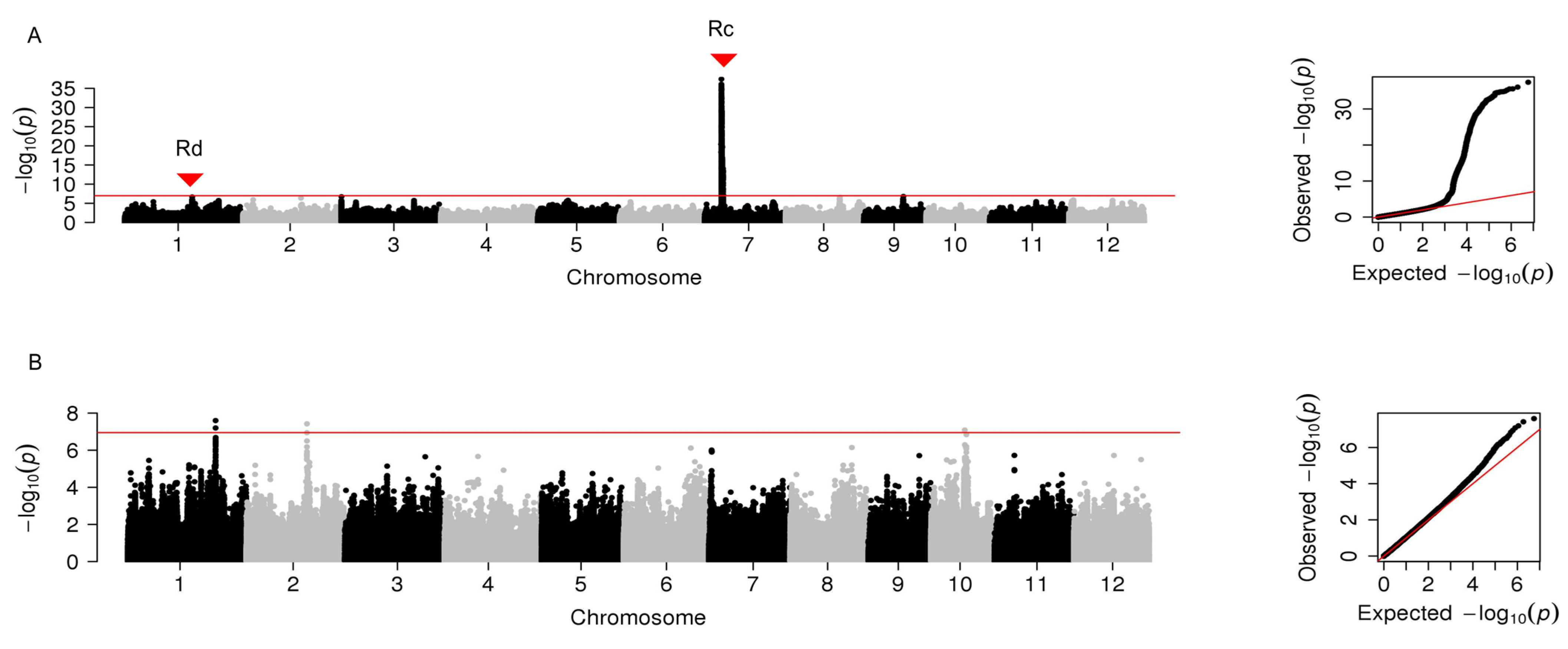
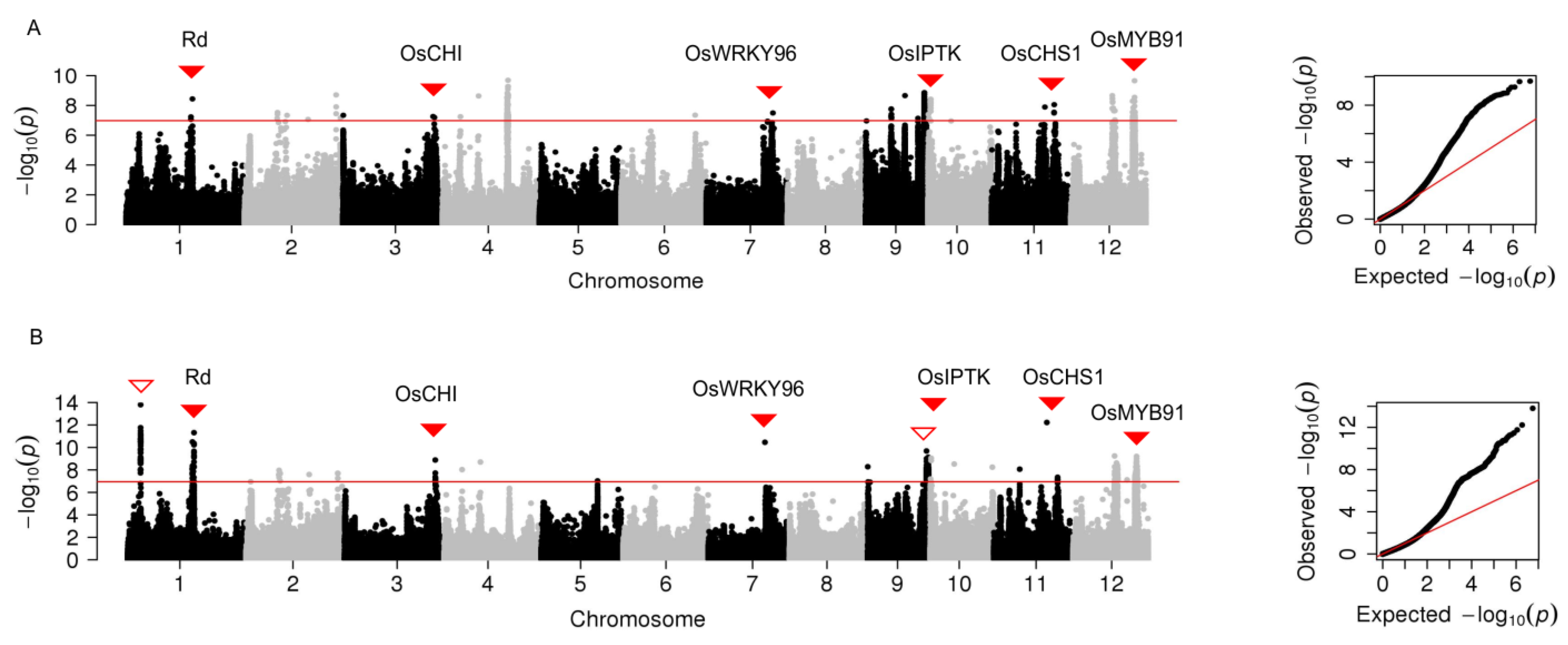
2.2. Genome-Wide Association Analysis of Grain Color
2.3. Validation of the Effect of Lead SNP Regions on Grain Yellowness
2.4. Expression Analysis of Candidate Genes in Two Significant Regions
2.5. Metabolite Analysis of Pooled Samples with Different Grain Colors
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Measurement of Grain Colors
4.3. Genome-Wide Association Analysis
4.4. Expression Profile Analysis
4.5. Metabolite Determination
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shao, Y.; Hu, Z.; Yu, Y.; Mou, R.; Zhu, Z.; Beta, T. Phenolic acids, anthocyanins, proanthocyanidins, antioxidant activity, minerals and their correlations in non-pigmented, red, and black rice. Food Chem. 2018, 239, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Bouis, H.E.; Chassy, B.M.; Ochanda, J.O. Genetically modified food crops and their contribution to human nutrition and food quality. Trends Food Sci. Technol. 2003, 14, 191–209. [Google Scholar] [CrossRef]
- Mbanjo, E.G.N.; Kretzschmar, T.; Jones, H.; Ereful, N.; Blanchard, C.; Boyd, L.A.; Sreenivasulu, N. The Genetic Basis and Nutritional Benefits of Pigmented Rice Grain. Front. Genet. 2020, 11, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mir, S.A.; Shah, M.A.; Bosco, S.J.D.; Sunooj, K.V.; Farooq, S. A review on nutritional properties, shelf life, health aspects, and consumption of brown rice in comparison with white rice. Cereal Chem. 2020, 97, 895–903. [Google Scholar] [CrossRef]
- Brotman, Y.; Llorente-Wiegand, C.; Oyong, G.; Badoni, S.; Misra, G.; Anacleto, R.; Parween, S.; Pasion, E.; Tiozon, R.N.; Anonuevo, J.J.; et al. The genetics underlying metabolic signatures in a brown rice diversity panel and their vital role in human nutrition. Plant J. 2021, 106, 507–525. [Google Scholar] [CrossRef]
- Gondal, T.A.; Keast, R.S.J.; Shellie, R.A.; Jadhav, S.R.; Gamlath, S.; Mohebbi, M.; Liem, D.G. Consumer Acceptance of Brown and White Rice Varieties. Foods 2021, 10, 1950. [Google Scholar] [CrossRef]
- Kim, T.; Kim, S.; Park, Y.; Lim, S.-H.; Ha, S.-H.; Park, S.; Lee, B.; Kim, J. Metabolite Profiling Reveals Distinct Modulation of Complex Metabolic Networks in Non-Pigmented, Black, and Red Rice (Oryza sativa L.) Cultivars. Metabolites 2021, 11, 367. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, C.; Li, Q.; Liu, Q. Genetic control of grain appearance quality in rice. Biotechnol. Adv. 2022, 60, 108014. [Google Scholar] [CrossRef]
- Zhuang, H.; Lou, Q.; Liu, H.; Han, H.; Wang, Q.; Tang, Z.; Ma, Y.; Wang, H. Differential Regulation of Anthocyanins in Green and Purple Turnips Revealed by Combined De Novo Transcriptome and Metabolome Analysis. Int. J. Mol. Sci. 2019, 20, 4387. [Google Scholar] [CrossRef] [Green Version]
- Shih, C.H.; Chu, H.; Tang, L.K.; Sakamoto, W.; Maekawa, M.; Chu, I.K.; Wang, M.; Lo, C. Functional characterization of key structural genes in rice flavonoid biosynthesis. Planta 2008, 228, 1043–1054. [Google Scholar] [CrossRef]
- Han, Y.Y.; Wang, J.W.; Han, N.; Liu, Q.J.; Liu, T.M.; Guan, F.M.; Ming, F. Duplication and sequence divergence of rice chalcone synthase genes. Russ. J. Plant Physiol. 2009, 56, 417–422. [Google Scholar] [CrossRef]
- Cheng, A.-X.; Han, X.-J.; Wu, Y.-F.; Lou, H.-X. The Function and Catalysis of 2-Oxoglutarate-Dependent Oxygenases Involved in Plant Flavonoid Biosynthesis. Int. J. Mol. Sci. 2014, 15, 1080–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Zhang, Z.; Chen, C.; Wu, W.; Ren, N.; Jiang, C.; Yu, J.; Zhao, Y.; Zheng, X.; Yang, Q.; et al. The C–S–A gene system regulates hull pigmentation and reveals evolution of anthocyanin biosynthesis pathway in rice. J. Exp. Bot. 2018, 69, 1485–1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lap, B.; Rai, M.; Tyagi, W. Playing with colours: Genetics and regulatory mechanisms for anthocyanin pathway in cereals. Biotechnol. Genet. Eng. Rev. 2021, 37, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shu, Q. Fine mapping and candidate gene analysis of purple pericarp gene Pb in rice (Oryza sativa L.). Chin. Sci. Bull. 2007, 52, 3097–3104. [Google Scholar] [CrossRef]
- Rahman, M.; Lee, K.E.; Lee, E.S.; Matin, M.N.; Lee, D.S.; Yun, J.S.; Kim, J.B.; Kang, S.G. The genetic constitutions of complementary genes Pp and Pb determine the purple color variation in pericarps with cyanidin-3-O-glucoside depositions in black rice. J. Plant Biol. 2013, 56, 24–31. [Google Scholar] [CrossRef]
- Maeda, H.; Yamaguchi, T.; Omoteno, M.; Takarada, T.; Fujita, K.; Murata, K.; Iyama, Y.; Kojima, Y.; Morikawa, M.; Ozaki, H.; et al. Genetic dissection of black grain rice by the development of a near isogenic line. Breed. Sci. 2014, 64, 134–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.; Wu, H.; Zhao, M.; Yang, Z.; Zhou, Z.; Guo, Y.; Lin, Y.; Chen, H. OsMYB3 is a R2R3-MYB gene responsible for anthocyanin biosynthesis in black rice. Mol. Breed. 2021, 41, 51. [Google Scholar] [CrossRef]
- Oikawa, T.; Maeda, H.; Oguchi, T.; Yamaguchi, T.; Tanabe, N.; Ebana, K.; Yano, M.; Ebitani, T.; Izawa, T. The Birth of a Black Rice Gene and Its Local Spread by Introgression. Plant Cell 2015, 27, 2401–2414. [Google Scholar] [CrossRef] [Green Version]
- Sweeney, M.T.; Thomson, M.J.; Pfeil, B.E.; McCouch, S. Caught Red-Handed:RcEncodes a Basic Helix-Loop-Helix Protein Conditioning Red Pericarp in Rice. Plant Cell 2006, 18, 283–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furukawa, T.; Maekawa, M.; Oki, T.; Suda, I.; Iida, S.; Shimada, H.; Takamure, I.; Kadowaki, K.-I. The Rc and Rd genes are involved in proanthocyanidin synthesis in rice pericarp. Plant J. 2006, 49, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Deng, Q.; Li, Y.; Yu, Y.; Liu, X.; Han, Y.; Luo, X.; Wu, X.; Ju, L.; Sun, J.; et al. Transcription Factors Rc and OsVP1 Coordinately Regulate Preharvest Sprouting Tolerance in Red Pericarp Rice. J. Agric. Food Chem. 2020, 68, 14748–14757. [Google Scholar] [CrossRef]
- Tan, Y.F.; Sun, M.; Xing, Y.Z.; Hua, J.P.; Sun, X.L.; Zhang, Q.F.; Corke, H. Mapping quantitative trait loci for milling quality, protein content and color characteristics of rice using a recombinant inbred line population derived from an elite rice hybrid. Theor. Appl. Genet. 2001, 103, 1037–1045. [Google Scholar] [CrossRef]
- Jin, L.; Xiao, P.; Lu, Y.; Shao, Y.; Shen, Y.; Bao, J. Quantitative Trait Loci for Brown Rice Color, Phenolics, Flavonoid Contents, and Antioxidant Capacity in Rice Grain. Cereal Chem. 2009, 86, 609–615. [Google Scholar] [CrossRef]
- Shao, Y.; Jin, L.; Zhang, G.; Lu, Y.; Shen, Y.; Bao, J. Association mapping of grain color, phenolic content, flavonoid content and antioxidant capacity in dehulled rice. Theor. Appl. Genet. 2010, 122, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Liu, S.; Zhao, G.; Zhang, C.; Peng, Z.; Wang, Z.; Yang, J.; Li, Y. Genetic Diversity Relationship Between Grain Quality and Appearance in Rice. Front. Plant Sci. 2021, 12, 708996. [Google Scholar] [CrossRef]
- Yang, W.; Chen, L.; Zhao, J.; Wang, J.; Li, W.; Yang, T.; Dong, J.; Ma, Y.; Zhou, L.; Chen, J.; et al. Genome-Wide Association Study of Pericarp Color in Rice Using Different Germplasm and Phenotyping Methods Reveals Different Genetic Architectures. Front. Plant Sci. 2022, 13, 841191. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Sreenivasulu, N.; Hamilton, R.S.; Kohli, A. Brown Rice, a Diet Rich in Health Promoting Properties. J. Nutr. Sci. Vitaminol. 2019, 65, S26–S28. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.-H.; Lee, Y.-J.; Byeon, E.-J.; Kang, B.-C.; Kyeoung, D.-S.; Kim, C.-K. Whole-genome resequencing and transcriptomic analysis of genes regulating anthocyanin biosynthesis in black rice plants. 3 Biotech 2018, 8, 115. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Gao, Y.; Xie, W.; Gong, L.; Lu, K.; Wang, W.; Li, Y.; Liu, X.; Zhang, H.; Dong, H.; et al. Genome-wide association analyses provide genetic and biochemical insights into natural variation in rice metabolism. Nat. Genet. 2014, 46, 714–721. [Google Scholar] [CrossRef]
- Yi, D.; Zhang, H.; Lai, B.; Liu, L.; Pan, X.; Ma, Z.; Wang, Y.; Xie, J.; Shi, S.; Wei, Y. Integrative Analysis of the Coloring Mechanism of Red Longan Pericarp through Metabolome and Transcriptome Analyses. J. Agric. Food Chem. 2020, 69, 1806–1815. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Huhman, D.; Shadle, G.; He, X.-Z.; Sumner, L.W.; Tang, Y.; Dixon, R.A. MATE2 Mediates Vacuolar Sequestration of Flavonoid Glycosides and Glycoside Malonates in Medicago truncatula. Plant Cell 2011, 23, 1536–1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Zhou, G.; Chen, J.; Liu, X.; Lu, X.; Chen, H.; Tian, Y. Integrated Metabolome and Transcriptome Analysis Unveils Novel Pathway Involved in the Formation of Yellow Peel in Cucumber. Int. J. Mol. Sci. 2021, 22, 1494. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Zhou, H.; Wang, Y.; Li, P.; Fu, P.; Wu, B.; He, Y. How rice organs are colored: The genetic basis of anthocyanin biosynthesis in rice. Crop J. 2021, 9, 598–608. [Google Scholar] [CrossRef]
- Ham, T.-H.; Kwon, S.W.; Ryu, S.-N.; Koh, H.-J. Correlation Analysis between Grain Color and Cyanidin-3-glucoside Content of Rice Grain in Segregate Population. Plant Breed. Biotechnol. 2015, 3, 160–166. [Google Scholar] [CrossRef] [Green Version]
- Min, B.; Gu, L.; McClung, A.M.; Bergman, C.J.; Chen, M.-H. Free and bound total phenolic concentrations, antioxidant capacities, and profiles of proanthocyanidins and anthocyanins in whole grain rice (Oryza sativa L.) of different bran colours. Food Chem. 2012, 133, 715–722. [Google Scholar] [CrossRef]
- Yu, S.; Ali, J.; Zhou, S.; Ren, G.; Xie, H.; Xu, J.; Yu, X.; Zhou, F.; Peng, S.; Ma, L.; et al. From Green Super Rice to green agriculture: Reaping the promise of functional genomics research. Mol. Plant 2021, 15, 9–26. [Google Scholar] [CrossRef]
- Sun, W.Q.; Zhou, Q.L.; Yao, Y.; Qiu, X.J.; Xie, K.; Yu, S.B. Identification of genomic regions and the isoamylase gene for reduced grain chalkiness in rice. PLoS ONE 2015, 10, e0122013. [Google Scholar] [CrossRef]
- Wang, C.; Yu, H.; Huang, J.; Wang, W.; Faruquee, M.; Zhang, F.; Zhao, X.; Fu, B.; Chen, K.; Zhang, H.; et al. Towards a deeper haplotype mining of complex traits in rice with RFGB v2.0. Plant Biotechnol. J. 2019, 18, 14–16. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Zhang, F.; Liu, D.; Chen, K.; Du, B.; Qiu, X.; Xu, J.; Xing, D. Distribution characteristics of selenium, cadmium and arsenic in rice grains and their genetic dissection by genome-wide association study. Front. Genet. 2022, 13, 1007896. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Yang, J.; Zhang, F.; Niu, Y.; Zhao, X.; Shen, C.; Chen, K.; Teng, S.; Xu, J. Genetic dissection of rice appearance quality and cooked rice elongation by genome-wide association study. Crop J. 2021, 9, 1470–1480. [Google Scholar] [CrossRef]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luo, J. A Novel Integrated Method for Large-Scale Detection, Identification, and Quantification of Widely Targeted Metabolites: Application in the Study of Rice Metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Qiu, X.; Wang, Z.; Xie, T.; Sun, W.; Xu, J.; Zhang, F.; Yu, S. Deciphering the Genetic Architecture of Color Variation in Whole Grain Rice by Genome-Wide Association. Plants 2023, 12, 927. https://doi.org/10.3390/plants12040927
Wang W, Qiu X, Wang Z, Xie T, Sun W, Xu J, Zhang F, Yu S. Deciphering the Genetic Architecture of Color Variation in Whole Grain Rice by Genome-Wide Association. Plants. 2023; 12(4):927. https://doi.org/10.3390/plants12040927
Chicago/Turabian StyleWang, Wenjun, Xianjin Qiu, Ziqi Wang, Tianyi Xie, Wenqiang Sun, Jianlong Xu, Fan Zhang, and Sibin Yu. 2023. "Deciphering the Genetic Architecture of Color Variation in Whole Grain Rice by Genome-Wide Association" Plants 12, no. 4: 927. https://doi.org/10.3390/plants12040927




