Neuroprotective Effect of Methanolic Ajwa Seed Extract on Lipopolysaccharide-Induced Memory Dysfunction and Neuroinflammation: In Vivo, Molecular Docking and Dynamics Studies
Abstract
:1. Introduction
2. Results
2.1. Quantitate Analysis of Ajwa Extract
2.2. Acute Toxicity Study
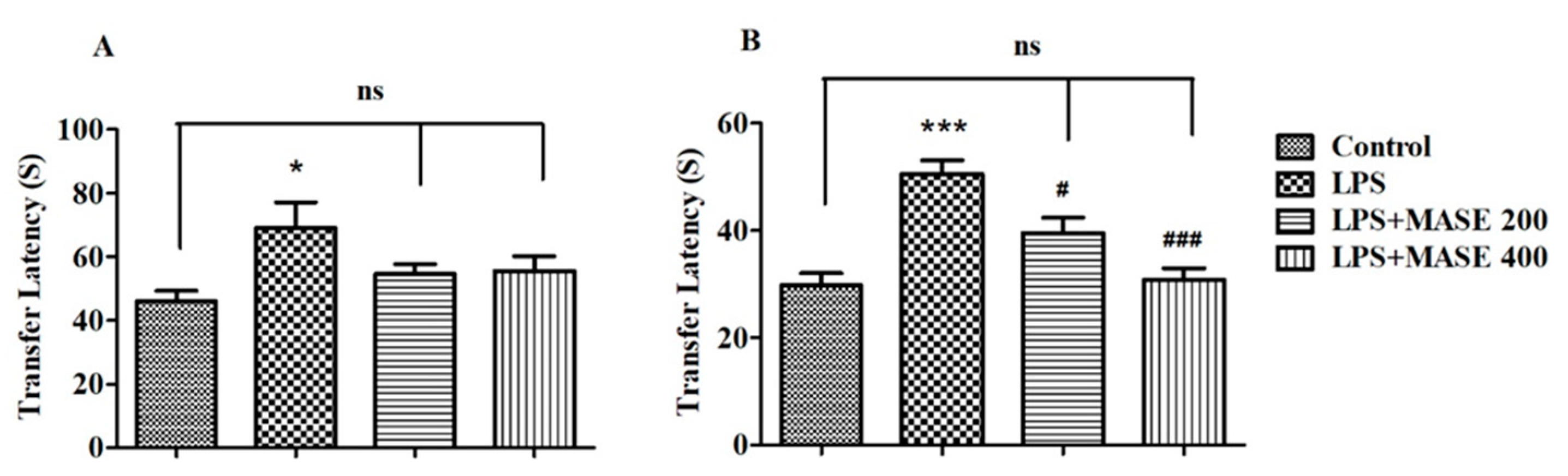
2.3. MASE Improved the Spatial Memory of LPS-Induced Rats in the Elevated Plus-Maze (EPM) Test
2.4. MASE Enhanced the Cognitive Performance of LPS-Induced Rats in the Novel Object Recognition (NOR) Test
2.5. MASE Improved the Cholinergic Functions of LPS-Induced Rats
2.6. MASE Ameliorates the Neuroinflammatory Parameters of LPS-Induced Rats
2.7. Molecular Docking
2.8. Molecular Dynamics
3. Discussion
4. Materials and Methods
4.1. Plant Extraction
4.2. Quantitative Assessment of the Total Polyphenolics, Total Flavonoids, and Total Tannins
4.2.1. Total Phenolic Contents (TPC)
4.2.2. Total Flavonoid Content (TFC)
4.2.3. Total Tannins Contents (TTC)
4.3. Experimental Animals
4.4. Vehicle
4.5. Acute Toxicity Study
4.6. Experimental Design
4.7. Elevated Plus Maze (EPM) Test
4.8. Novel Object Recognition (NOR) Test
4.9. Collection of Brain Samples
4.10. Enzyme-Linked Immunosorbent Assay (ELISA) of Cholinergic and Inflammatory Parameters
4.11. Statistical Analysis
4.12. Molecular Modelling
4.13. Molecular Dynamics (MD) Simulations and MM/GBSA Calculations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhou, R.; Ji, B.; Kong, Y.; Qin, L.; Ren, W.; Guan, Y.; Ni, R. PET Imaging of neuroinflammation in Alzheimer’s disease. Front. Immunol. 2021, 12, 739130. [Google Scholar] [CrossRef]
- Kamdi, S.P.; Raval, A.; Nakhate, K.T. Phloridzin attenuates lipopolysaccharide-induced cognitive impairment via antioxidant, anti-inflammatory and neuromodulatory activities. Cytokine 2021, 139, 155408. [Google Scholar] [CrossRef]
- Mani, V.; Jaafar, S.M.; Azahan, N.S.M.; Ramasamy, K.; Lim, S.M.; Ming, L.C.; Majeed, A.B.A. Ciproxifan improves cholinergic transmission, attenuates neuroinflammation and oxidative stress but does not reduce amyloid level in transgenic mice. Life Sci. 2017, 180, 23–35. [Google Scholar] [CrossRef]
- Gorica, E.; Calderone, V. Arachidonic acid derivatives and neuroinflammation. CNS. Neurol. Disord. Drug Targets 2022, 21, 118–129. [Google Scholar] [CrossRef]
- Skok, M.; Lykhmus, O. The role of α7 nicotinic acetylcholine receptors and α7-specific antibodies in neuroinflammation related to Alzheimer disease. Curr. Pharm. Des. 2016, 22, 2035–2049. [Google Scholar] [CrossRef]
- Mani, V.; Arfeen, M.; Ali, H.M.; Abdel-Moneim, A.H.; Aldubayan, M.; Alhowail, A. Neuroprotective effect of clobenpropit against lipopolysaccharide-induced cognitive deficits via attenuating neuroinflammation and enhancing mitochondrial functions in mice. Brain Sci. 2021, 11, 1617. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Zheng, X.; Fang, T.; Yang, X.; Luo, X.; Guo, A.; Newell, K.A.; Huang, X.F.; Yu, Y. Galantamine improves cognition, hippocampal inflammation, and synaptic plasticity impairments induced by lipopolysaccharide in mice. J. Neuroinflammation 2018, 15, 112. [Google Scholar] [CrossRef] [Green Version]
- Van Horssen, J.; van Schaik, P.; Witte, M. Inflammation and mitochondrial dysfunction: A vicious circle in neurodegenerative disorders? Neurosci. Lett. 2019, 710, 132931. [Google Scholar] [CrossRef]
- Jin, H.; Kanthasamy, A.; Ghosh, A.; Anantharam, V.; Kalyanaraman, B.; Kanthasamy, A.G. Mitochondria-targeted antioxidants for treatment of Parkinson’s disease: Preclinical and clinical outcomes. Biochim. Biophys. Acta 2014, 1842, 1282–1294. [Google Scholar] [CrossRef] [Green Version]
- Khalid, S.; Khalid, N.; Khan, R.S.; Ahmed, H.; Ahmad, A. A review on chemistry and pharmacology of Ajwa date fruit and pit. Trends Food Sci. Technol. 2017, 63, 60–69. [Google Scholar] [CrossRef]
- Chao, C.T.; Kureger, R.R. The date palm (Phoenix dactylifera L.): Overview of biology, uses, and cultivation. HortScience 2007, 42, 1077–1082. [Google Scholar] [CrossRef] [Green Version]
- Hamad, I.; AbdElgawad, H.; Al Jaouni, S.; Zinta, G.; Asard, H.; Hassan, S.; Hegab, M.; Hagagy, N.; Selim, S. Metabolic analysis of various date palm fruit (Phoenix dactylifera L.) cultivars from Saudi Arabia to assess their nutritional quality. Molecules 2015, 20, 13620–13641. [Google Scholar] [CrossRef]
- Anwar, S.; Raut, R.; Alsahli, M.A.; Almatroudi, A.; Alfheeaid, H.; Alzahrani, F.M.; Khan, A.A.; Allemailem, K.S.; Almatroodi, S.A.; Rahmani, A.H. Role of Ajwa date fruit pulp and seed in the management of diseases through in vitro and in silico analysis. Biology 2022, 11, 78. [Google Scholar] [CrossRef]
- Hasan, M.; Mohieldein, A. In vivo evaluation of anti Diabetic, hypolipidemic, antioxidative activities of Saudi date seed extract on streptozotocin induced diabetic rats. J. Clin. Diagn. Res. 2016, 10, FF06–FF12. [Google Scholar] [CrossRef]
- Khan, T.J.; Kuerban, A.; Razvi, S.S.; Mehanna, M.G.; Khan, K.A.; Almulaiky, Y.Q.; Faidallah, H.M. In vivo evaluation of hypolipidemic and antioxidative effect of ‘Ajwa’ (Phoenix dactylifera L.) date seed-extract in high-fat diet-induced hyperlipidemic rat model. Biomed. Pharmacother. 2018, 107, 675–680. [Google Scholar] [CrossRef]
- Kalantaripour, T.P.; Asadi-Shekaari, M.; Basiri, M.; Gholaamhosseinian Najar, A. Cerebroprotective effect of date seed extract (Phoenix dactylifera) on focal cerebral ischemia in male rats. J. Biol. Sci. 2012, 12, 180–185. Available online: https://scialert.net/abstract/?doi=jbs.2012.180.185 (accessed on 20 May 2022). [CrossRef] [Green Version]
- Subash, S.; Essa, M.M.; Braidy, N.; Awlad-Thani, K.; Vaishnav, R.; Al-Adawi, S.; Al-Asmi, A.; Guillemin, G.J. Diet rich in date palm fruits improves memory, learning and reduces beta amyloid in transgenic mouse model of Alzheimer’s disease. J. Ayurveda. Integr. Med. 2015, 6, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Mani, V.; Arfeen, M.; Sajid, S.; Almogbel, Y. Aqueous Ajwa dates seeds extract improves memory impairment in type-2 diabetes mellitus rats by reducing blood glucose levels and enhancing brain cholinergic transmission. Saudi J. Biol. Sci. 2022, 29, 2738–2748. [Google Scholar] [CrossRef]
- Mani, V.; Arfeen, M.; Sajid, S.; Almogbel, Y. Neuroprotective effect of aqueous extract of Ajwa seeds via anti-inflammatory pathways in type-2 diabetic-induced rats. Int. J. Pharmacol. 2022, 18, 299–306. Available online: https://scialert.net/abstract/?doi=ijp.2022.299.306 (accessed on 1 February 2022). [CrossRef]
- Mani, V.; Arfeen, M.; Mohammed, H.A.; Elsisi, H.A.; Sajid, S.; Almogbel, Y.; Aldubayan, M.; Dhanasekaran, M.; Alhowail, A. Sukkari dates seed improves type-2 diabetes mellitus-induced memory impairment by reducing blood glucose levels and enhancing brain cholinergic transmission: In vivo and molecular modeling studies. Saudi Pharm. J. 2022, 30, 750–763. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Al-Omar, M.S.; Mohammed, H.A.; Mohammed, S.; Abd-Elmoniem, E.; Kandil, Y.I.; Eldeeb, H.M.; Chigurupati, S.; Sulaiman, G.M.; Al-Khurayyif, H.K.; Almansour, B.S.; et al. Anti-microbial, anti-oxidant, and α-amylase inhibitory activity of traditionally-used medicinal herbs: A comparative analyses of pharmacology, and phytoconstituents of regional halophytic plants’ diaspora. Molecules 2020, 25, 5457. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Ali, H.M.; Qureshi, K.A.; Alsharidah, M.; Kandil, Y.I.; Said, R.; Mohammed, S.; Al-Omar, M.S.; Rugaie, O.A.; Abdellatif, A.; et al. Comparative phytochemical profile and biological activity of four major medicinal halophytes from Qassim flora. Plants 2021, 10, 2208. [Google Scholar] [CrossRef]
- Borowiec, K.; Michalak, A. Flavonoids from edible fruits as therapeutic agents in neuroinflammation-A comprehensive review and update. Crit. Rev. Food Sci. Nutr. 2022, 62, 6742–6760. [Google Scholar] [CrossRef]
- Rahman, I.; Biswas, S.K.; Kirkham, P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006, 72, 1439–1452. [Google Scholar] [CrossRef]
- Mohammadi, M.; Soltani, M.; Siahpoosh, A.; Shekarabi, S.P.H.; Mehrgan, M.S.; Lymbery, A. Effect of date palm (Phoenix dactylifera) seed extract as a dietary supplementation on growth performance immunological haematological biochemical parameters of common carp. Aquac. Res. 2018, 49, 2903–2912. [Google Scholar] [CrossRef]
- Zhang, Y.; Kua, J.; McCammon, J.A. Role of the catalytic triad and oxyanion hole in acetylcholinesterase catalysis: an ab initio QM/MM study. J. Am. Chem. Soc. 2002, 124, 10572–10577. [Google Scholar] [CrossRef]
- Mani, V.; Arfeen, M.; Rabbani, S.I.; Shariq, A.; Amirthalingam, P. Levetiracetam ameliorates doxorubicin-induced chemobrain by enhancing cholinergic transmission and reducing neuroinflammation using an experimental rat model and molecular docking study. Molecules 2022, 27, 7364. [Google Scholar] [CrossRef]
- Macdonald, I.R.; Martin, E.; Rosenberry, T.L.; Darvesh, S. Probing the peripheral site of human butyrylcholinesterase. Biochemistry 2012, 51, 7046–7053. [Google Scholar] [CrossRef]
- Kiefer, J.R.; Pawlitz, J.L.; Moreland, K.T.; Stegeman, R.A.; Hood, W.F.; Gierse, J.K.; Stevens, A.M.; Goodwin, D.C.; Rowlinson, S.W.; Marnett, L.J.; et al. Structural insights into the stereochemistry of the cyclooxygenase reaction. Nature 2000, 405, 97–101. [Google Scholar] [CrossRef]
- Mani, V.; Arfeen, M.; Ali, H.M.; Hafez Abdel-Moneim, A.M.; Aldubayan, M.; Dhanasekaran, M.; Alhowail, A. Ciproxifan attenuates the memory impairment induced by lipopolysaccharide through modulation of cholinergic transmission in the mouse brain. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 1897–1905. [Google Scholar] [CrossRef]
- Marefati, N.; Beheshti, F.; Memarpour, S.; Bayat, R.; Naser Shafei, M.; Sadeghnia, H.R.; Ghazavi, H.; Hosseini, M. The effects of acetyl-11-keto-β-boswellic acid on brain cytokines and memory impairment induced by lipopolysaccharide in rats. Cytokine 2020, 131, 155107. [Google Scholar] [CrossRef]
- Vasconcelos, A.R.; Yshii, L.M.; Viel, T.A.; Buck, H.S.; Mattson, M.P.; Scavone, C.; Kawamoto, E.M. Intermittent fasting attenuates lipopolysaccharide-induced neuroinflammation and memory impairment. J. Neuroinflammation 2014, 11, 85. [Google Scholar] [CrossRef] [Green Version]
- Sadraie, S.; Kiasalari, Z.; Razavian, M.; Azimi, S.; Sedighnejad, L.; Afshin-Majd, S.; Baluchnejadmojarad, T.; Roghani, M. Berberine ameliorates lipopolysaccharide-induced learning and memory deficit in the rat: Insights into underlying molecular mechanisms. Metab. Brain Dis. 2019, 34, 245–255. [Google Scholar] [CrossRef]
- Silvers, J.M.; Harrod, S.B.; Mactutus, C.F.; Booze, R.M. Automation of the novel object recognition task for use in adolescent rats. J. Neurosci. Methods 2007, 166, 99–103. [Google Scholar] [CrossRef] [Green Version]
- Ennaceur, A. One-trial object recognition in rats and mice: Methodological and theoretical issues. Behav. Brain Res. 2010, 215, 244–254. [Google Scholar] [CrossRef]
- Dehghanian, F.; Kalantaripour, T.P.; Esmaeilpour, K.; Elyasi, L.; Oloumi, H.; Pour, F.M.; Asadi-Shekaari, M. Date seed extract ameliorates β-amyloid-induced impairments in hippocampus of male rats. Biomed. Pharmacother. 2017, 89, 221–226. [Google Scholar] [CrossRef]
- Stancampiano, R.; Cocco, S.; Cugusi, C.; Sarais, L.; Fadda, F. Serotonin and acetylcholine release response in the rat hippocampus during a spatial memory task. Neuroscience 1999, 89, 1135–1143. [Google Scholar] [CrossRef]
- Ming, Z.; Wotton, C.A.; Appleton, R.T.; Ching, J.C.; Loewen, M.E.; Sawicki, G.; Bekar, L.K. Systemic lipopolysaccharide-mediated alteration of cortical neuromodulation involves increases in monoamine oxidase-A and acetylcholinesterase activity. J. Neuroinflammation 2015, 12, 37. [Google Scholar] [CrossRef] [Green Version]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef]
- Rojo, L.E.; Fernández, J.A.; Maccioni, A.A.; Jimenez, J.M.; Maccioni, R.B. Neuroinflammation: Implications for the pathogenesis and molecular diagnosis of Alzheimer’s disease. Arch. Med. Res. 2008, 39, 1–16. [Google Scholar] [CrossRef]
- Rubio-Perez, J.M.; Morillas-Ruiz, J.M. A review: Inflammatory process in Alzheimer’s disease, role of cytokines. Sci. World J. 2012, 2012, 756357. [Google Scholar] [CrossRef]
- Consilvio, C.; Vincent, A.M.; Feldman, E.L. Neuroinflammation, COX-2, and ALS--a dual role? Exp. Neurol. 2004, 187, 1–10. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, R.; Tong, Y.; Chen, P.; Shen, Y.; Miao, S.; Liu, X. Neuroprotection by dihydrotestosterone in LPS-induced neuroinflammation. Neurobiol. Dis. 2020, 140, 104814. [Google Scholar] [CrossRef]
- Lee, J.; Chan, S.L.; Mattson, M.P. Adverse effect of a presenilin-1 mutation in microglia results in enhanced nitric oxide and inflammatory cytokine responses to immune challenge in the brain. Neuromolecular Med. 2002, 2, 29–45. [Google Scholar] [CrossRef]
- Joshi, R.; Garabadu, D.; Teja, G.R.; Krishnamurthy, S. Silibinin ameliorates LPS-induced memory deficits in experimental animals. Neurobiol. Learn. Mem. 2014, 116, 117–131. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, L.J.; Huang, T.Q.; Kim, J.; Gu, M.Y.; Yang, H.O. Narciclasine inhibits LPS-induced neuroinflammation by modulating the Akt/IKK/NF-κB and JNK signaling pathways. Phytomedicine 2021, 85, 153540. [Google Scholar] [CrossRef]
- Brochu, M.E.; Girard, S.; Lavoie, K.; Sébire, G. Developmental regulation of the neuroinflammatory responses to LPS and/or hypoxia-ischemia between preterm and term neonates: An experimental study. J. Neuroinflammation 2011, 8, 55. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, W.; O’Garra, A. IL-10 family cytokines IL-10 and IL-22: From basic science to clinical translation. Immunity 2019, 50, 871–891. [Google Scholar] [CrossRef]
- Chen, J.H.; Ke, K.F.; Lu, J.H.; Qiu, Y.H.; Peng, Y.P. Protection of TGF-β1 against neuroinflammation and neurodegeneration in Aβ1-42-induced Alzheimer’s disease model rats. PLoS ONE 2015, 10, e0116549. [Google Scholar] [CrossRef] [Green Version]
- Salam, H.S.; Tawfik, M.M.; Elnagar, M.R.; Mohammed, H.A.; Zarka, M.A.; Awad, N.S. Potential apoptotic activities of Hylocereus undatus peel and pulp extracts in MCF-7 and Caco-2 cancer cell lines. Plants 2022, 11, 2192. [Google Scholar] [CrossRef]
- Banc, R.; Rusu, M.E.; Filip, L.; Popa, D.S. The impact of ellagitannins and their metabolites through gut microbiome on the gut health and brain wellness within the gut-brain axis. Foods 2023, 12, 270. [Google Scholar] [CrossRef]
- OECD Test No. 423: Acute Oral toxicity—Acute Toxic Class Method. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2002; Available online: https://www.oecd-ilibrary.org/environment/test-no-423-acute-oraltoxicity-acute-toxic-class-method_9789264071001-en (accessed on 19 April 2022).
- Malik, J.; Kumar, M.; Deshmukh, R.; Kumar, P. Ameliorating effect of lyophilized extract of Butea frondosa leaves on scopolamine-induced amnesia in rats. Pharm. Biol. 2013, 51, 233–239. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Bowers, K.J.; Chow, E.; Xu, H.; Dror, R.O.; Eastwood, M.P.; Gregersen, B.A.; Klepeis, J.L.; Kolossvary, I.; Moraes, M.A.; Sacerdoti, F.D.; et al. Scalable algorithms for molecular dynamics simulations on commodity clusters. In Proceedings of the ACM/IEEE Conference on Supercomputing (SC06), Tampa, FL, USA, 11–17 November 2006; Available online: https://ieeexplore.ieee.org/document/4090217 (accessed on 10 November 2022).
- Sastry, G.M.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aid. Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef]









| TPC | TFC | TTC |
|---|---|---|
| mg/gm of the dried plant extract | ||
| 76.06 ± 0.94 | 8.66 ± 0.02 | 11.48 ± 1.19 |
| Sr. No. | Ligands | AChE | COX-2 |
|---|---|---|---|
| 1 | Co L | −10.5 | −9.1 |
| 2 | Ellagic acid | −10.2 | −7.5 |
| 3 | Epicatechin | −9.6 | −7 |
| 4 | Catechin | −9.3 | −7.4 |
| 5 | Pyragallol | −5.4 | −5.4 |
| 6 | Syringic acid | −6.3 | −6 |
| 7 | Vanillic acid | −6.1 | −6.1 |
| 8 | Benzoic acid | −6.4 | −5.5 |
| 9 | Catechol | −5.4 | −5.2 |
| 10 | Cinnamic acid | −7 | −6.1 |
| 11 | Gallic acid | −6.3 | −5.9 |
| 12 | Hesperidin | −11.2 | −6.6 |
| 13 | Hesperetin | −10.3 | −5.5 |
| 14 | Kaempferol | −9.9 | −7.8 |
| 15 | Narengin | −11.1 | −3.5 |
| 16 | Quercetin | −9.6 | −7.9 |
| 17 | Rutin | −10.9 | −4.7 |
| 18 | Apigenin | −9.9 | −8.3 |
| Sr. No. | Ligands | Residue-Wise Interaction (Hydrogen and Hydrophobic) |
|---|---|---|
| 1 | Ellagic acid | Asp72, Trp84, Gly117, Tyr130, Glu199, Phe330, His440, |
| 2 | Epicatechin | Trp84, Asn85, Tyr121, Ser122, Glu199, |
| 3 | Catechin | Trp84, Gly117, Gly118,Tyr121, Ser122, Tyr130, Phe330, Phe331, |
| 4 | Pyragallol | Trp84, Tyr130, Glu199, |
| 5 | Syringic acid | Asp72, Trp84, Phe330, Phe331, Tyr334, His440 |
| 6 | Vanillic acid | Tyr121, Ser122, Glu199, Ser200, His440 |
| 7 | Benzoic acid | Phe330, Phe331 Tyr334 |
| 8 | Catechol | Asp72, Phe330, Phe331, Tyr334 |
| 9 | Cinnamic acid | Gly119, Ser200, Phe330, Phe331, Tyr334, His440 |
| 10 | Gallic acid | Trp84, Ser122, Tyr130, Glu199 |
| 11 | Hesperidin | Tyr70, Asp72, Trp84, Asn85, Gly118, Trp279, Ile287, Phe330, Phe331, Tyr334, His440 |
| 12 | Hesperetin | Asp72, Trp84, Tyr121, Ser122, Ile287, Phe330, Phe331 |
| 13 | Kaempferol | Trp84, Tyr121, Ser122, Tyr130, Tyr334 |
| 14 | Narengin | Trp84, Asn85, Gly118, Ser122, Glu199, Ser200, Trp279, Phe331, Tyr334, His440 |
| 15 | Quercetin | Trp84, Gly117, Gly118, Tyr121, Ser122, Tyr130, Phe330, |
| 16 | Rutin | Tyr70, Tyr121, Ser122, Ser286, Arg289, Trp279, Phe290 |
| 17 | Apigenin | Tyr70, Trp84, Asn85, Tyr121, Ser122 |
| Sr. No. | Ligands | Residue-Wise Interaction (Hydrogen and Hydrophobic) |
|---|---|---|
| 1 | Ellagic acid | Ser530, Val349, Leu352, Ala527, Gly526, Val523 |
| 2 | Epicatechin | Val116, Val349, Leu352, Tyr355, Leu359, Tyr385, Gly526, Val523, Ala527, Leu531 |
| 3 | Catechin | Val116, Arg120, Val349, Leu352, Ala527, Leu531 |
| 4 | Pyragallol | Leu352, Val523, Ala527 |
| 5 | Syringic acid | Val349, Tyr385, Trp387, Leu352, Phe518, Met522, Val523, Ala527, Leu531 |
| 6 | Vanillic acid | Val349, Leu352, Val523, Gly526, Ala527, Leu531, |
| 7 | Benzoic acid | Tyr385, Leu352, Phe518, Val523, Ala527 Ser530, |
| 8 | Catechol | Val349, Leu352, Met522, Val523, Ala527 |
| 9 | Cinnamic acid | Arg120, Val349, Leu352, Ser353, Tyr355, Ala527 |
| 10 | Gallic acid | Leu352, Tyr385, Gly526, Ala527 Ser530, |
| 11 | Hesperidin | Tyr115, Val116, Ser119, Arg120, Tyr355, Phe381, Leu384, Tyr385, Trp387, Val349, Leu352, Ala527, Met522, Val523, Ala527, Leu531 |
| 12 | Hesperetin | Val116, Arg120, Val349, Leu352, Leu359, Tyr385, Trp387, Ala527, Ala527, Leu531 |
| 13 | Kaempferol | Arg120, Val349, Leu352, Phe518, Val523, Gly526, Ala527, Ser530, Leu531, |
| 14 | Narengin | VAL89, VAL116, ARG120, VAL349, LEU352, TYR355, VAL523, GLU524, GLY526, ALA527 |
| 15 | Quercetin | Val349, Leu352, Phe518, Val523, Gly526, Ala527, Ser530, Leu531, Ala527, |
| 16 | Rutin | Pro86, Val89, Leu93, Met113, Val116, Arg120, Val349, Leu352, Tyr355, Leu359, Tyr385, Ala527, Leu531 |
| 17 | Apigenin | Val116, Arg120, Val349, Leu352, Tyr355, Ala527, Leu531, Leu359, Ala527 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mani, V.; Arfeen, M.; Dhaked, D.K.; Mohammed, H.A.; Amirthalingam, P.; Elsisi, H.A. Neuroprotective Effect of Methanolic Ajwa Seed Extract on Lipopolysaccharide-Induced Memory Dysfunction and Neuroinflammation: In Vivo, Molecular Docking and Dynamics Studies. Plants 2023, 12, 934. https://doi.org/10.3390/plants12040934
Mani V, Arfeen M, Dhaked DK, Mohammed HA, Amirthalingam P, Elsisi HA. Neuroprotective Effect of Methanolic Ajwa Seed Extract on Lipopolysaccharide-Induced Memory Dysfunction and Neuroinflammation: In Vivo, Molecular Docking and Dynamics Studies. Plants. 2023; 12(4):934. https://doi.org/10.3390/plants12040934
Chicago/Turabian StyleMani, Vasudevan, Minhajul Arfeen, Devendra Kumar Dhaked, Hamdoon A. Mohammed, Palanisamy Amirthalingam, and Hossam A. Elsisi. 2023. "Neuroprotective Effect of Methanolic Ajwa Seed Extract on Lipopolysaccharide-Induced Memory Dysfunction and Neuroinflammation: In Vivo, Molecular Docking and Dynamics Studies" Plants 12, no. 4: 934. https://doi.org/10.3390/plants12040934
APA StyleMani, V., Arfeen, M., Dhaked, D. K., Mohammed, H. A., Amirthalingam, P., & Elsisi, H. A. (2023). Neuroprotective Effect of Methanolic Ajwa Seed Extract on Lipopolysaccharide-Induced Memory Dysfunction and Neuroinflammation: In Vivo, Molecular Docking and Dynamics Studies. Plants, 12(4), 934. https://doi.org/10.3390/plants12040934








