Effects of Exogenous Auxin on Mesocotyl Elongation of Sorghum
Abstract
:1. Introduction
2. Results
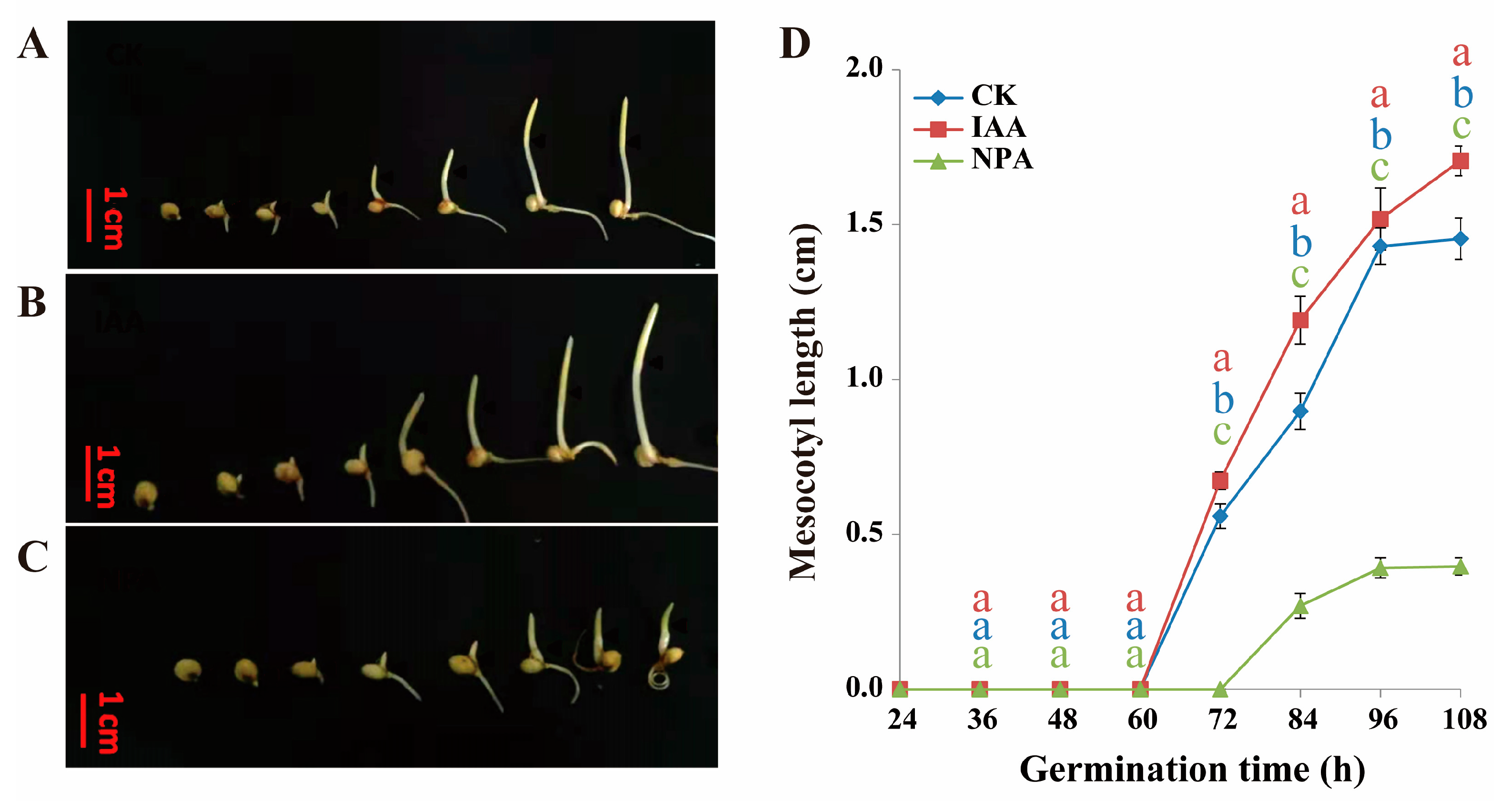
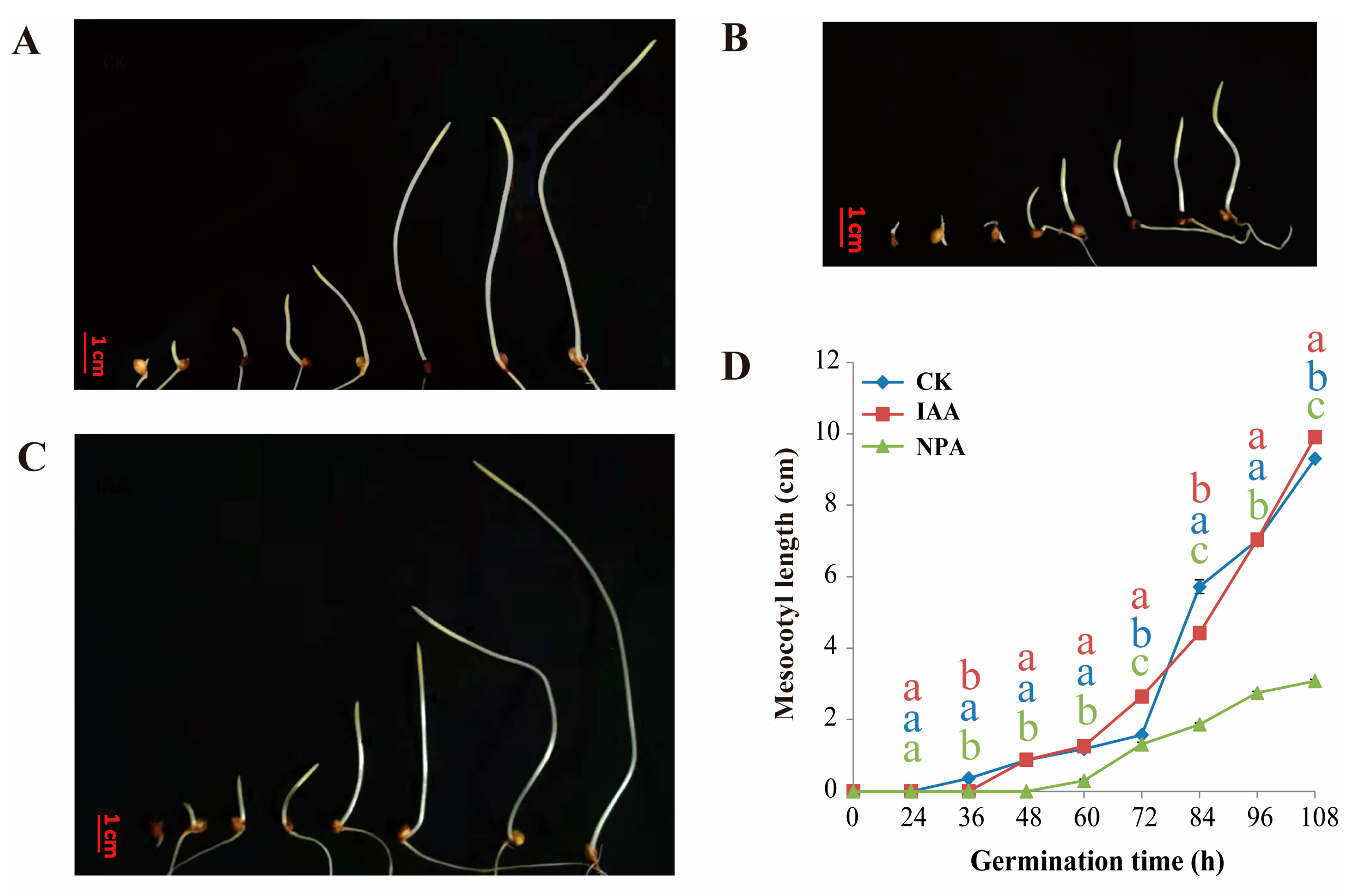
2.1. The Effect of IAA Treatment to Mesocotyl Length of Different Sorghum Lines
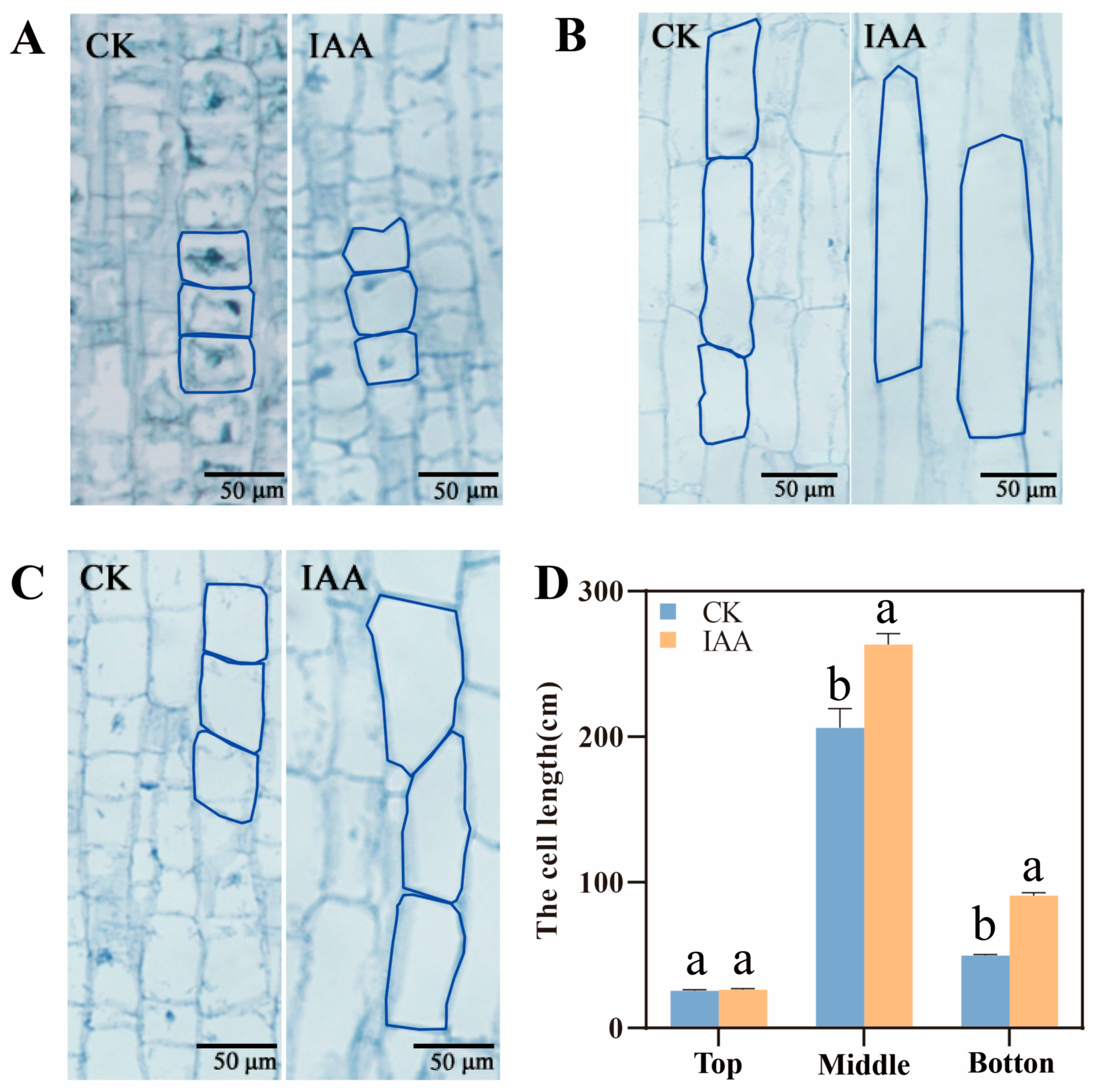
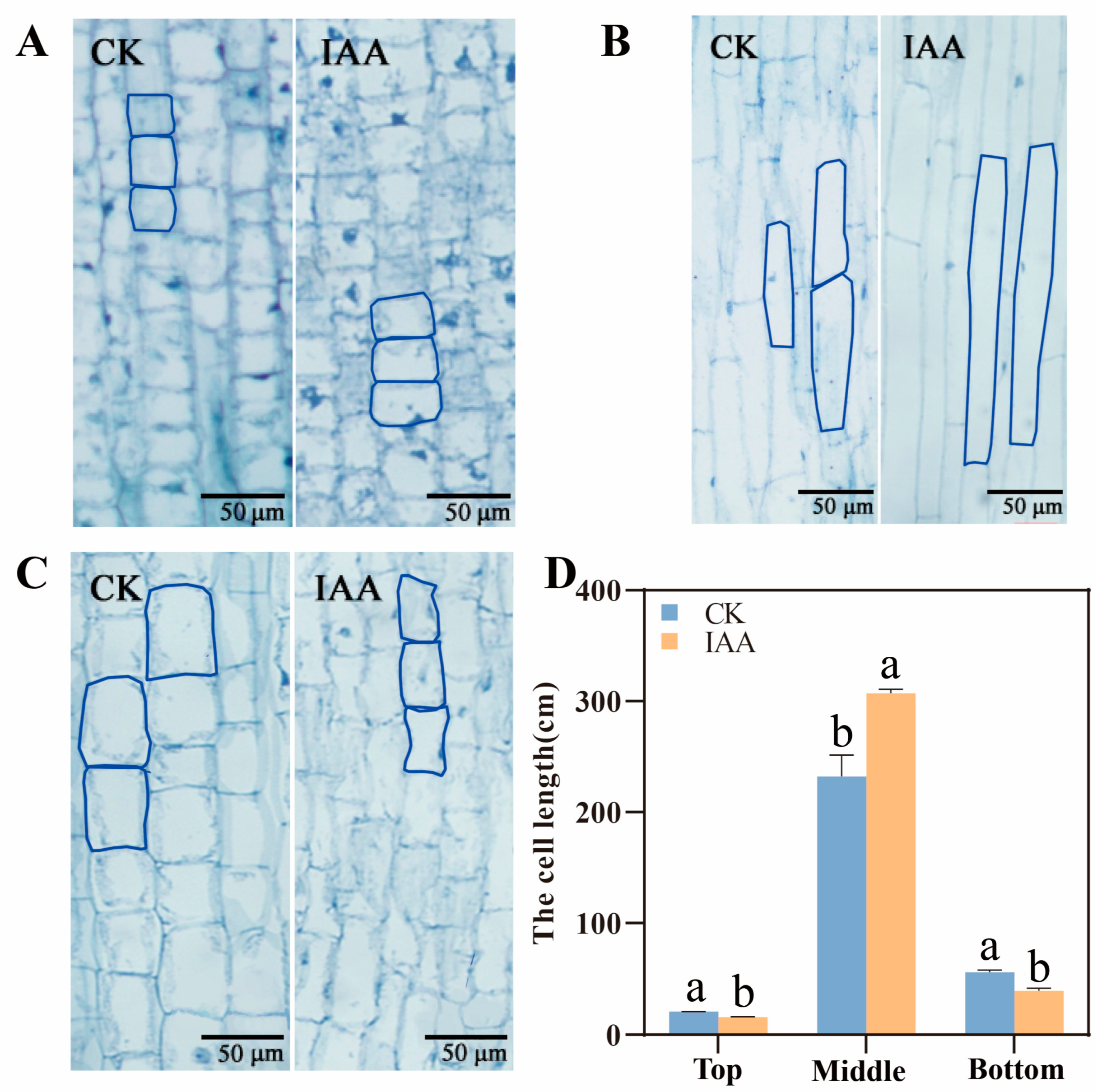
2.2. The Elongation of Mesocotyl by IAA Treatment Depending on the Increase of Mesocotyl Cell Length
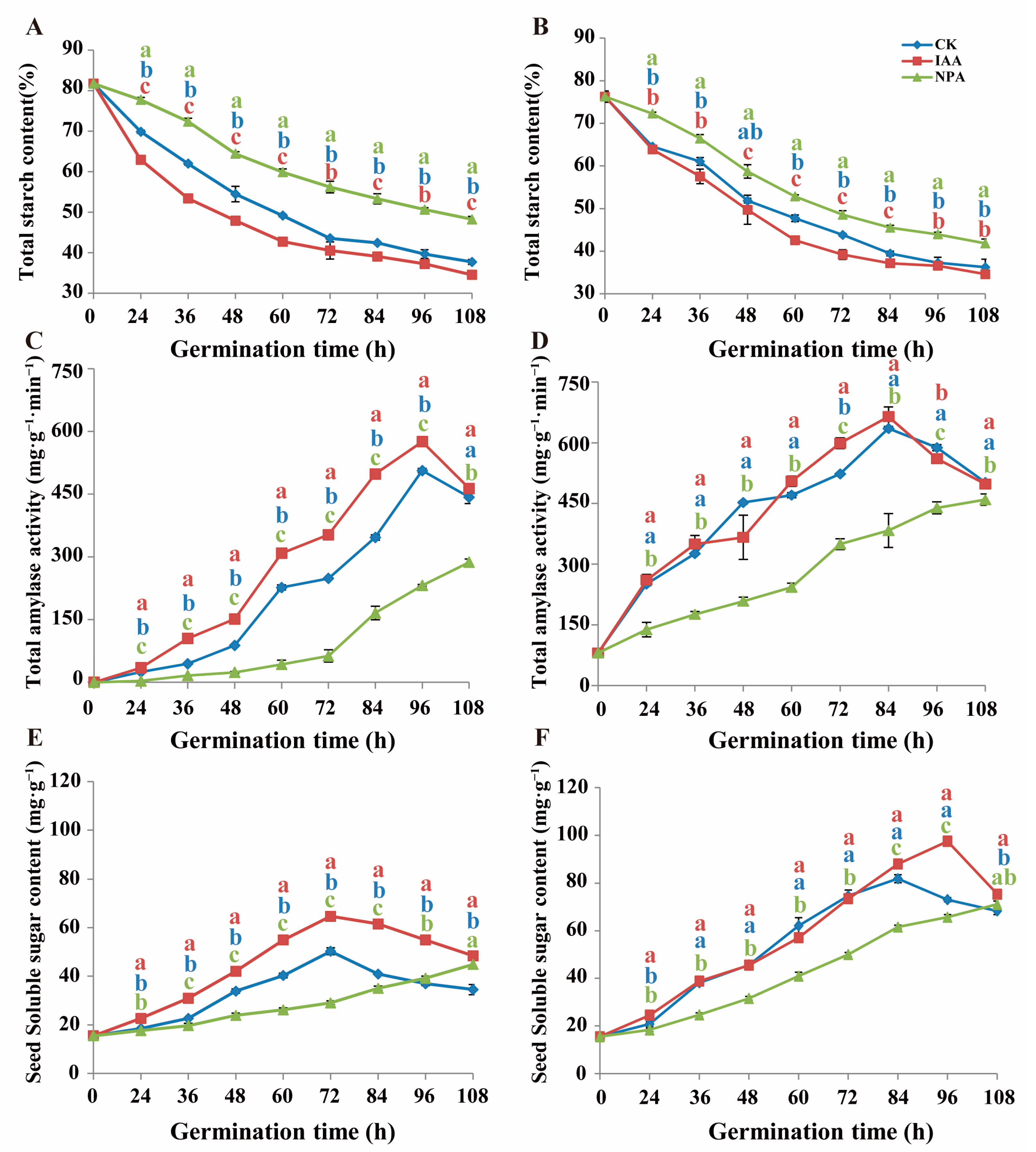
2.3. IAA Application Promote Energy Metabolism Process
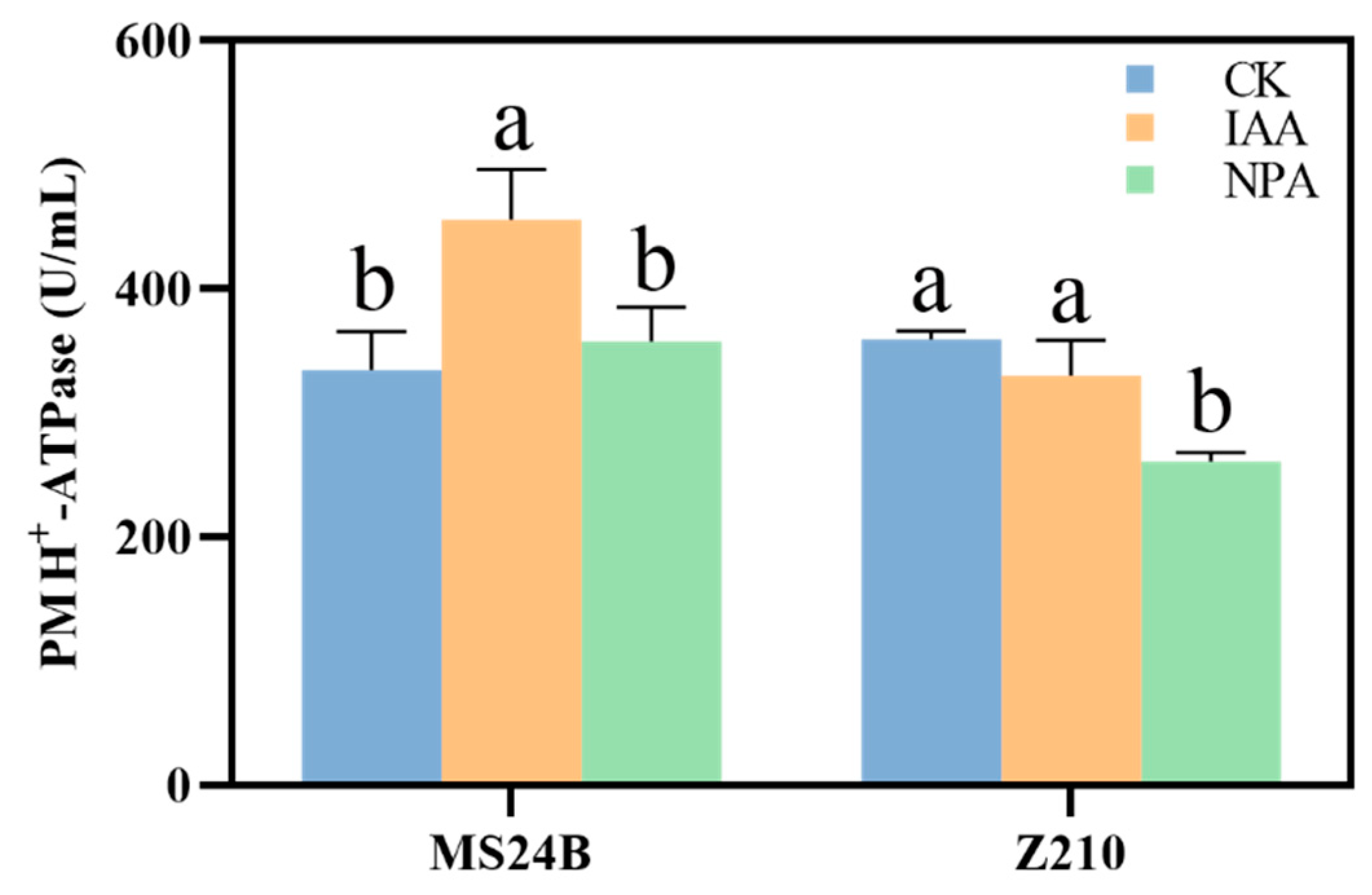
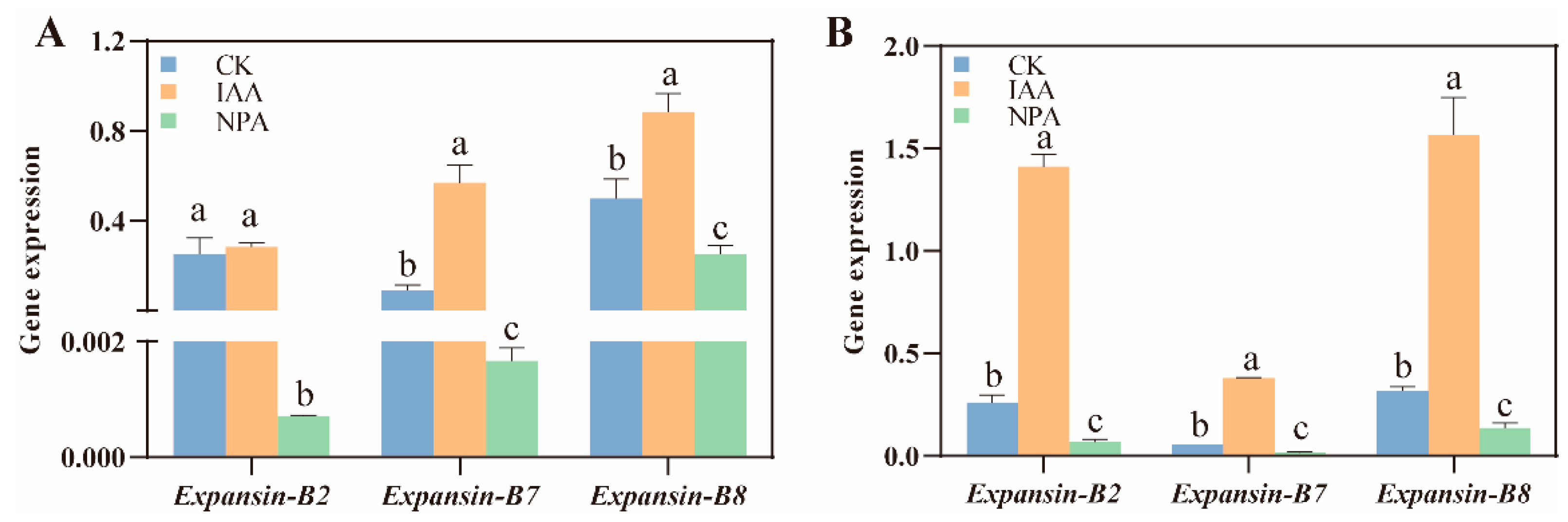
2.4. IAA Application Promote Mesocotyl Length by Membrane Acidification
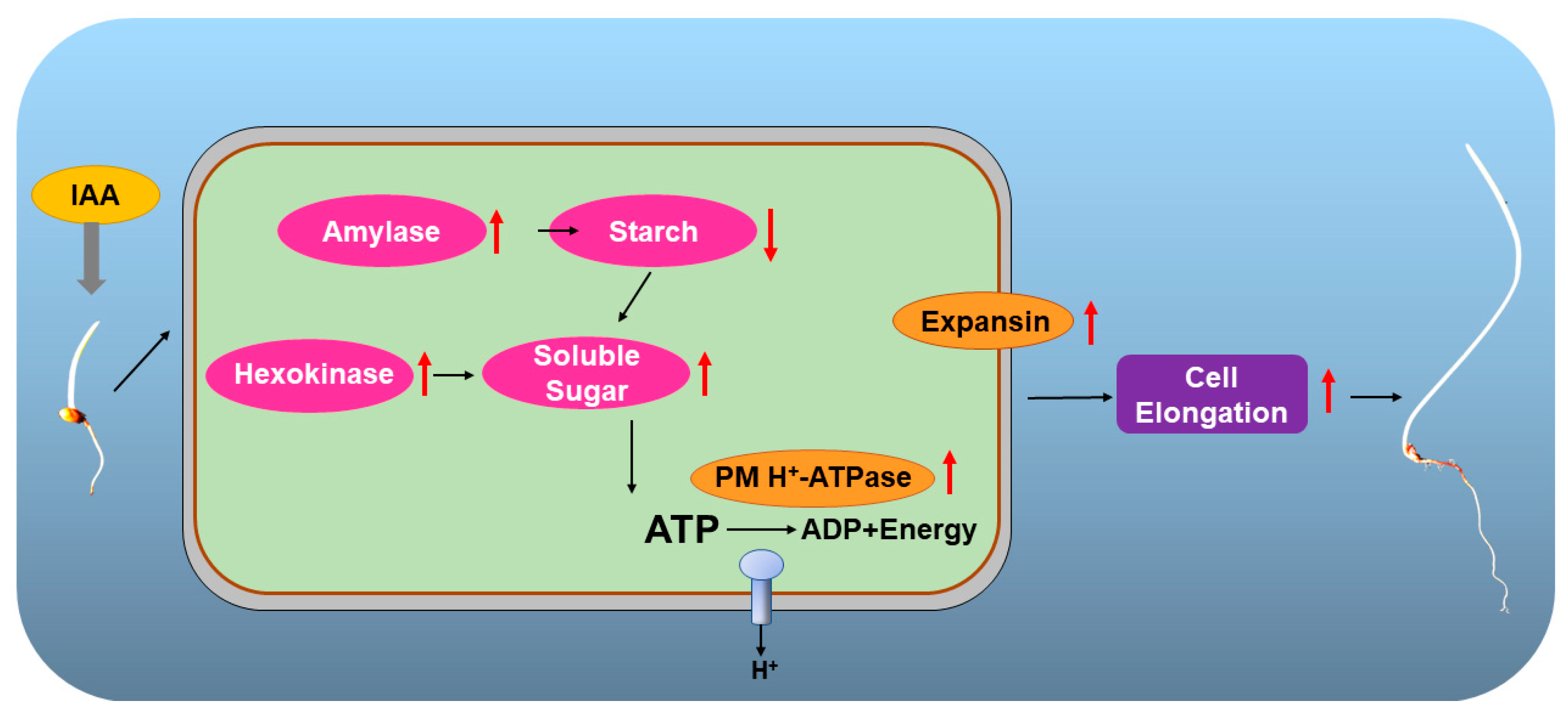
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Observation of Morphological Differences of Each Part of Mesocotyl
4.3. Starch and Soluble Sugar Content Measurement
4.4. Total Amylase Activity Measurement
4.5. Hexokinase and PM H+-ATPase Activity Measurement
4.6. RNA Extraction and Quantitative Real-Time PCR
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, H.S.; Sasaki, K.; Higashitani, A.; Ahn, S.-N.; Sato, T. Mapping and characterization of quantitative trait loci for mesocotyl elongation in rice (Oryza sativa L.). Rice 2012, 5, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kutschera, U.; Wang, Z.Y. Growth-limiting proteins in maize coleoptiles and the auxin-brassinosteroid hypothesis of mesocotyl elongation. Protoplasma 2016, 253, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Chung, N.J. Elongation habit of mesocotyls and coleoptiles in weedy rice with high emergence ability in direct-seeding on dry paddy fields. Crop Pasture Sci. 2010, 61, 911–917. [Google Scholar] [CrossRef]
- Simon, A.; Yuri, S.; Hironobu, S.; Kenji, I. Genotypic variation in coleoptile or mesocotyl lengths of upland rice (Oryza sativa L.) and seedling emergence in deep sowing. Afr. J. Agric. Res. 2012, 7, 6239–6248. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Ma, P.; Zhao, Z.; Zhao, G.; Tian, B.; Wang, J.; Wang, G. Mapping QTL controlling maize deep-seeding tolerance-related traits and confirmation of a major QTL for mesocotyl length. Theor. Appl. Genet. 2012, 124, 223–232. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Enzymes and other agents that enhance cell wall extensibility. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 391–417. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Yan, H.; Yang, J.; Yamaguchi, S.; Maekawa, M.; Takamure, I.; Tsutsumi, N.; Kyozuka, J.; Nakazono, M. Strigolactones negatively regulate mesocotyl elongation in rice during germination and growth in darkness. Plant Cell Physiol. 2010, 51, 1136–1142. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Ma, D.R.; Sun, J.; Qian, L.; Cheng, W.F. Observation of mesocotyl cell morphology of weed rice. J. Shenyang Agric. Univ. 2012, 43, 749–753. [Google Scholar]
- Taiz, L. Plant cell expansion: Regulation of cell wall mechanical properties. Annu. Rev. Plant Physiol. 1984, 35, 585–657. [Google Scholar] [CrossRef]
- Janicka-Russak, M. Plant plasma membrane H+-ATPase in adaptation of plants to abiotic stresses. Abiotic Stress Response Plants-Physiol. Biochem. Genet. Perspect. 2011, 1, 197–218. [Google Scholar]
- Janicka-Russak, M.; Kabała, K.; Wdowikowska, A.; Kłobus, G. Modification of plasma membrane proton pumps in cucumber roots as an adaptation mechanism to salt stress. J. Plant Physiol. 2013, 170, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005, 6, 850–861. [Google Scholar] [CrossRef]
- Goh, H.H.; Sloan, J.; Dorca-Fornell, C.; Fleming, A. Inducible repression of multiple expansin genes leads to growth suppression during leaf development. Plant Physiol. 2012, 159, 1759–1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.K.; Ahn, J.H.; Song, S.K.; Choi, Y.D.; Lee, J.S. Expression of an expansin gene is correlated with root elongation in soybean. Plant Physiol. 2003, 131, 985–997. [Google Scholar] [CrossRef] [Green Version]
- Che, J.; Yamaji, N.; Shen, R.F.; Ma, J.F. An Al-inducible expansin gene, OsEXPA10 is involved in root cell elongation of rice. Plant J. 2016, 88, 132–142. [Google Scholar] [CrossRef]
- Zeeman, S.C.; Kossmann, J.; Smith, A.M. Starch: Its metabolism, evolution, and biotechnological modification in plants. Annu. Rev. Plant Biol. 2010, 61, 209–234. [Google Scholar] [CrossRef] [Green Version]
- Mahender, A.; Anandan, A.; Pradhan, S.K. Early seedling vigour, an imperative trait for direct-seeded rice: An overview on physio-morphological parameters and molecular markers. Planta 2015, 241, 1027–1050. [Google Scholar] [CrossRef]
- Nie, L.; Song, S.; Yin, Q.; Zhao, T.; Liu, H.; He, A.; Wang, W. Enhancement in Seed Priming-Induced Starch Degradation of Rice Seed Under Chilling Stress via GA-Mediated α-Amylase Expression. Rice 2022, 15, 19. [Google Scholar] [CrossRef]
- Xiong, M.; Yu, J.; Wang, J.; Gao, Q.; Huang, L.; Chen, C.; Zhang, C.; Fan, X.; Zhao, D.; Liu, Q.-Q.; et al. Brassinosteroids regulate rice seed germination through the BZR1-RAmy3D transcriptional module. Plant Physiol. 2022, 189, 402–418. [Google Scholar] [CrossRef] [PubMed]
- Haga, K.; Iino, M. Auxin-growth relationships in maize coleoptiles and pea internodes and control by auxin of the tissue sensitivity to auxin. Plant Physiol. 1998, 117, 1473–1486. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, K.; Nakamura, Y.; Asami, T.; Yoshida, S.; Matsuo, T.; Okamoto, S. Physiological roles of brassinosteroids in early growth of Arabidopsis: Brassinosteroids have a synergistic relationship with gibberellin as well as auxin in light-grown hypocotyl elongation. J. Plant Growth Regul. 2003, 22, 259–271. [Google Scholar] [CrossRef]
- Cao, L.Y. Effect of different hormones on mesocotyl length in Oryza sativa L. Acta Agronom. Sin. 2005, 31, 1098–1100. [Google Scholar]
- Feng, F.; Mei, H.; Fan, P.; Li, Y.; Xu, X.; Wei, H.; Yan, M.; Luo, L. Dynamic transcriptome and phytohormone profiling along the time of light exposure in the mesocotyl of rice seedling. Sci. Rep. 2017, 7, 11961. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.W.; Wang, J.H. Effect of auxin on mesocotyl elongation of dark-grown maize under different seeding depths. Russ. J. Plant Physiol. 2010, 57, 79–86. [Google Scholar] [CrossRef]
- Miransari, M.; Smith, D.L. Plant hormones and seed germination. Environ. Exp. Bot. 2014, 99, 110–121. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Loosening of plant cell walls by expansins. Nature 2000, 407, 321–326. [Google Scholar] [CrossRef]
- Cleland, R.E. Auxin-induced growth of Avena coleoptiles involves two mechanisms with different pH optima. Plant Physiol. 1992, 99, 1556–1561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Liang, Q.; Wang, C.; Cheng, W.F. Effect of auxin on weedy rice mesocotyl cell wall oxidase. Appl. Mech. Mater. 2014, 448, 69–73. [Google Scholar] [CrossRef]
- Rober-Kleber, N.; Albrechtová, J.T.P.; Fleig, S.; Huck, N.; Michalke, W.; Wagner, E.; Speth, V.; Neuhaus, G.; Fischer-Iglesias, C. Plasma membrane H+-ATPase is involved in auxin-mediated cell elongation during wheat embryo development. Plant Physiol. 2003, 131, 1302–1312. [Google Scholar] [CrossRef] [Green Version]
- Arsuffi, G.; Braybrook, S.A. Acid growth: An ongoing trip. J. Exp. Bot. 2018, 69, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Hayashi, K.; Kinoshita, T. Auxin activates the plasma membrane H+-ATPase by phosphorylation during hypocotyl elongation in Arabidopsis. Plant Physiol. 2012, 159, 632–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marowa, P.; Ding, A.; Kong, Y. Expansins: Roles in plant growth and potential applications in crop improvement. Plant Cell Rep. 2016, 35, 949–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, X.; Cao, Y.; Huang, L.; Zhao, J.; Xu, C.; Li, X.; Wang, S. Activation of the indole-3-acetic acid–amido synthetase GH3-8 suppresses expansin expression and promotes salicylate-and jasmonate-independent basal immunity in rice. Plant Cell 2008, 20, 228–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, D.; Lee, Y.; Cho, H.T.; Kende, H. Regulation of expansin gene expression affects growth and development in transgenic rice plants. Plant Cell 2003, 15, 1386–1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornell, W.C.; Morgan, C.J.; Koyama, L.; Sakhtah, H.; Mansfield, J.H.; Dietrich, L.E. Paraffin embedding and thin sectioning of microbial colony biofilms for microscopic analysis. JoVE J. Vis. Exp. 2018, 133, e57196. [Google Scholar]
- Li, W.; Meng, R.; Liu, Y.; Chen, S.; Jiang, J.; Wang, L.; Zhao, S.; Wang, Z.; Fang, W.; Chen, F.; et al. Hetero-grafted chrysanthemums enhance salt stress tolerance by integrating the ROS, soluble sugar, and proline. Hortic. Res. 2022, 9, uhac073. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508. [Google Scholar] [CrossRef] [Green Version]
- Tárrago, J.F.; Nicolás, G. Starch degradation in the cotyledons of germinating lentils. Plant Physiol. 1976, 58, 618–621. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Q.S.; Su, X.F. The influence of extracellular-side Ca2+ on the activity of the plasma membrane H+-ATPase from wheat roots. Funct. Plant Biol. 1998, 25, 923–928. [Google Scholar] [CrossRef]
- Ohnishi, T.; Gall, R.S.; Mayer, M.L. An improved assay of inorganic phosphate in the presence of extralabile phosphate compounds: Application to the ATPase assay in the presence of phosphocreatine. Anal. Biochem. 1975, 69, 261–267. [Google Scholar] [CrossRef]


| Gene | PCR Primer (5′-3′) |
|---|---|
| SbActin | ATGGCTGACGCCGAGGATATCCA GAGCCACACGGAGCTCGTTGTAG |
| SbEXPB2 | GCGAGGTGAAGACGGTGATGATC GTGCCGGAGCTTGTCGTTGAG |
| SbEXPB7 | CCTCCACCAGCCGTCGTCTAC CACCACCACCACTGCCGTTG |
| SbEXPB8 | TAGGTAGTTGGATCGGAGCAGAGC CAGGCAGGAGAGCGAGGACAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Yao, Z.; Jiang, B.; Yu, W.; Wang, Y.; Dong, W.; Li, Y.; Shi, X.; Liu, C.; Zhou, Y. Effects of Exogenous Auxin on Mesocotyl Elongation of Sorghum. Plants 2023, 12, 944. https://doi.org/10.3390/plants12040944
Liu C, Yao Z, Jiang B, Yu W, Wang Y, Dong W, Li Y, Shi X, Liu C, Zhou Y. Effects of Exogenous Auxin on Mesocotyl Elongation of Sorghum. Plants. 2023; 12(4):944. https://doi.org/10.3390/plants12040944
Chicago/Turabian StyleLiu, Chang, Ziqing Yao, Bing Jiang, Wenbo Yu, Yu Wang, Wenhui Dong, Yutong Li, Xiaolong Shi, Chunjuan Liu, and Yufei Zhou. 2023. "Effects of Exogenous Auxin on Mesocotyl Elongation of Sorghum" Plants 12, no. 4: 944. https://doi.org/10.3390/plants12040944





