Calcium Dynamics, WUSCHEL Expression and Callose Deposition during Somatic Embryogenesis in Arabidopsis thaliana Immature Zygotic Embryos
Abstract
1. Introduction
2. Results
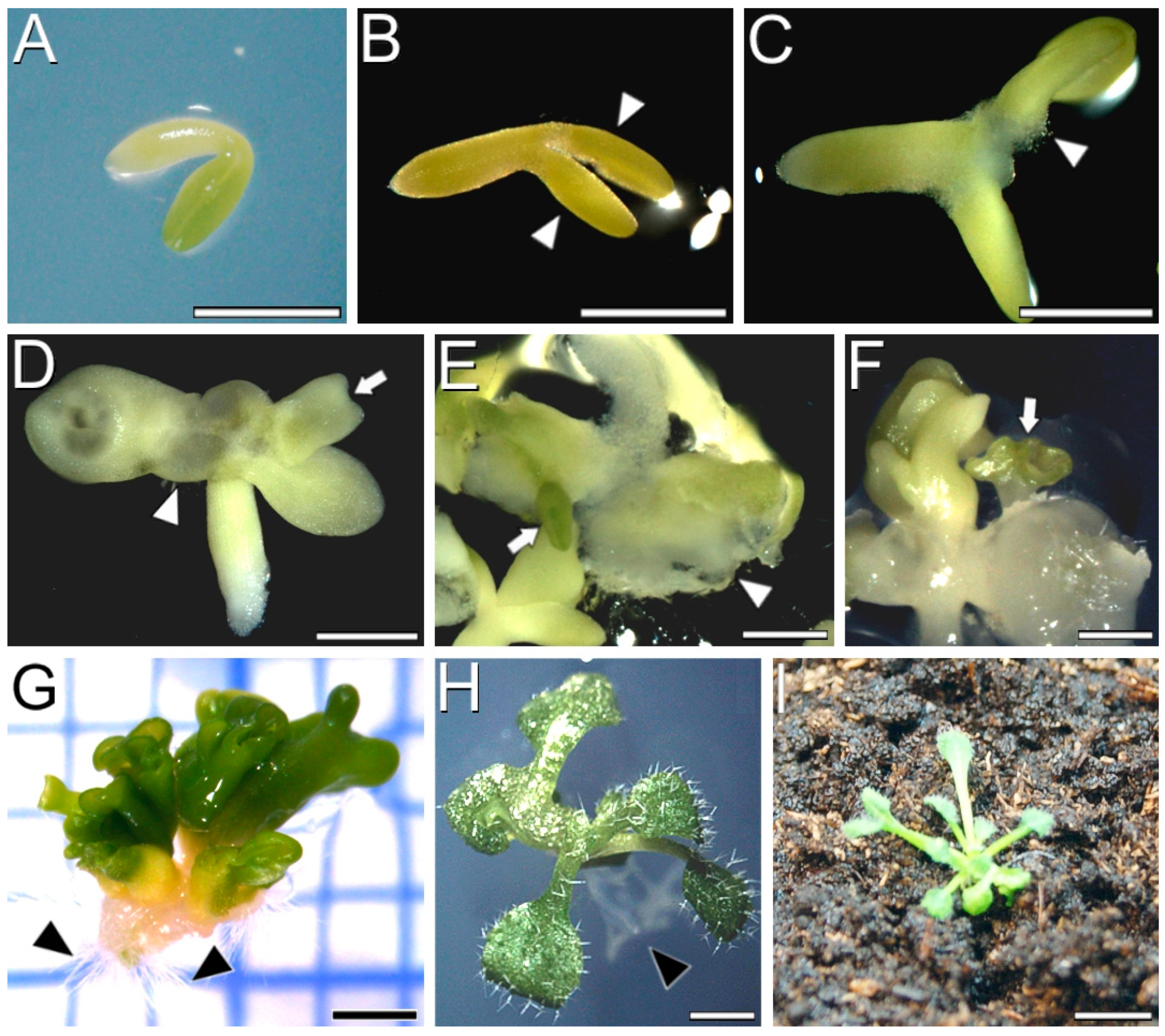
2.1. Induction of Somatic Embryogenesis from IZEs of Arabidopsis Cameleon Lines
2.2. Scanning Electron Microscopy of Somatic Embryogenesis from Arabidopsis IZEs
2.3. Expression of the WUS-Reporter upon Induction of Somatic Embryogenesis in Arabidopsis
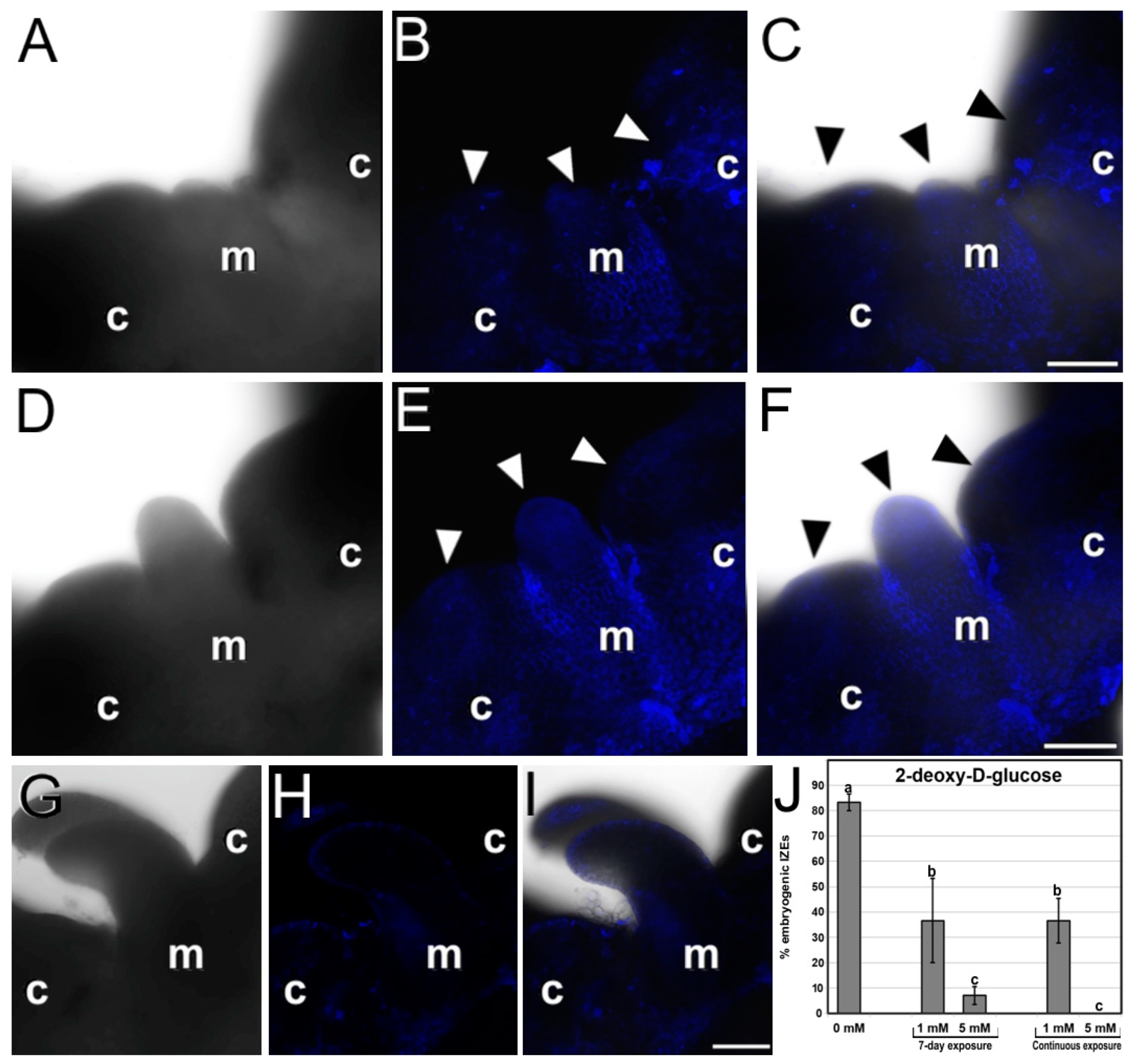
2.4. Callose Staining during Somatic Embryogenesis in Arabidopsis
2.5. FRET Imaging of Ca2+ Distribution during Somatic Embryogenesis in Arabidopsis
2.6. Modulation of Intracellular Ca2+ Levels
3. Discussion
3.1. The Shoot Apical Meristem of IZEs also Produces Somatic Embryos
3.2. High Ca2+ Levels Act as a Trigger of Somatic Embryogenesis, Marking the Onset of the Process
3.3. Ca2+ Homeostasis Cannot Be Altered to Induce Somatic Embryogenesis from IZEs
3.4. Somatic Cells Transition to Embryogenesis First at the Cotyledon Protrusions and then at the Tip of the Shoot Apical Meristem Appendix
3.5. Concluding Remarks
4. Materials and Methods
4.1. Plant Materials
4.2. Induction of Somatic Embryogenesis
4.3. Scanning Electron Microscopy
4.4. Confocal Microscopy and FRET
4.5. Ca2+ Modulators and Callose Inhibitor
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elhiti, M.; Stasolla, C. Transduction of Signals during Somatic Embryogenesis. Plants 2022, 11, 178. [Google Scholar] [CrossRef] [PubMed]
- Jacquier, N.M.A.; Widiez, T. Overview of in vitro and in vivo doubled haploid technologies. In Doubled Haploid Technology, 1st ed.; Seguí-Simarro, J.M., Walker, J.M., Eds.; Methods in Molecular Biology; Springer Science+Business Media, LLC: New York, NY, USA, 2021; Volume 1, General Topics, Alliaceae, Cereals; pp. 3–22. [Google Scholar]
- Steward, F.C.; Mapes, M.O.; Mears, K. Growth and organized development of cultured cells. II. Organization in cultures grown from freely suspended cells. Am. J. Bot. 1958, 45, 705–708. [Google Scholar] [CrossRef]
- Reinert, J. Morphogenese und ihre Kontrolle an Gewebekulturen aus Carotten. Naturwissenschaften 1958, 45, 344–345. [Google Scholar] [CrossRef]
- Loyola-Vargas, V.M.; Ochoa-Alejo, N. Somatic Embryogenesis. An Overview. In Somatic Embryogenesis: Fundamental Aspects and Applications; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–8. [Google Scholar]
- Horstman, A.; Bemer, M.; Boutilier, K. A transcriptional view on somatic embryogenesis. Regeneration 2017, 4, 201–216. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, C.M.; Mathias, R.J. Regeneration of Plants from Protoplasts of Arabidopsis thaliana CV. Columbia L. (C24), Via Direct Embryogenesis. In Current Issues in Plant Molecular and Cellular Biology: Proceedings of the VIIIth International Congress on Plant Tissue and Cell Culture, Florence, Italy, 12–17 June 1994; Terzi, M., Cella, R., Falavigna, A., Eds.; Springer: Dordrecht, The Netherlands, 1995; pp. 377–382. [Google Scholar]
- Zuo, J.; Niu, Q.W.; Frugis, G.; Chua, N.H. The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. Plant J. 2002, 30, 349–359. [Google Scholar] [CrossRef]
- Ikeda-Iwai, M.; Umehara, M.; Satoh, S.; Kamada, H. Stress-induced somatic embryogenesis in vegetative tissues of Arabidopsis thaliana. Plant J. 2003, 34, 107–114. [Google Scholar] [CrossRef]
- Kadokura, S.; Sugimoto, K.; Tarr, P.; Suzuki, S.; Matsunaga, S. Characterization of somatic embryogenesis initiated from the Arabidopsis shoot apex. Dev. Biol. 2018, 442, 13–27. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nagayama, Y.; Higashi, K.; Kobayashi, M. Establishment of a tissue culture system for somatic embryogenesis from germinating embryos of Arabidopsis thaliana. Plant Biotechnol. 2010, 27, 359–364. [Google Scholar] [CrossRef]
- Wu, Y.; Haberland, G.; Zhou, C.; Koop, H.-U. Somatic embryogenesis, formation of morphogenetic callus and normal development in zygotic embryos of Arabidopsis thaliana in vitro. Protoplasma 1992, 169, 89–96. [Google Scholar] [CrossRef]
- Kurczynska, E.U.; Gaj, M.D.; Ujczak, A.; Mazur, E. Histological analysis of direct somatic embryogenesis in Arabidopsis thaliana (L.) Heynh. Planta 2007, 226, 619–628. [Google Scholar] [CrossRef]
- Gaj, M.D. Somatic Embryogenesis and Plant Regeneration in the Culture of Arabidopsis thaliana (L.) Heynh. Immature Zygotic Embryos. In Plant Embryo Culture: Methods and Protocols; Thorpe, T.A., Yeung, E.C., Eds.; Humana Press: Totowa, NJ, USA, 2011; pp. 257–265. [Google Scholar]
- Godel-Jedrychowska, K.; Kulinska-Lukaszek, K.; Horstman, A.; Soriano, M.; Li, M.; Malota, K.; Boutilier, K.; Kurczynska, E.U. Symplasmic isolation marks cell fate changes during somatic embryogenesis. J. Exp. Bot. 2020, 71, 2612–2628. [Google Scholar] [CrossRef] [PubMed]
- de Silva, K.K.; Dunwell, J.M.; Wickramasuriya, A.M. Weighted Gene Correlation Network Analysis (WGCNA) of Arabidopsis Somatic Embryogenesis (SE) and Identification of Key Gene Modules to Uncover SE-Associated Hub Genes. Int. J. Genom. 2022, 4, 7471063. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi-Darvari, F.; Noor, N.M.; Ismanizan, I. Epigenetic regulation and gene markers as signals of early somatic embryogenesis. Plant Cell Tissue Organ Cult. 2015, 120, 407–422. [Google Scholar] [CrossRef]
- Jha, P.; Ochatt, S.J.; Kumar, V. WUSCHEL: A master regulator in plant growth signaling. Plant Cell Rep. 2020, 39, 431–444. [Google Scholar] [CrossRef]
- Su, Y.H.; Zhao, X.Y.; Liu, Y.B.; Zhang, C.L.; O’Neill, S.D.; Zhang, X.S. Auxin-induced WUS expression is essential for embryonic stem cell renewal during somatic embryogenesis in Arabidopsis. Plant J. 2009, 59, 448–460. [Google Scholar] [CrossRef]
- Kulinska-Lukaszek, K.; Tobojka, M.; Adamiok, A.; Kurczynska, E.U. Expression of the BBM gene during somatic embryogenesis of Arabidopsis thaliana. Biol. Plant. 2012, 56, 389–394. [Google Scholar] [CrossRef]
- Schmidt, E.D.; Guzzo, F.; Toonen, M.A.; de Vries, S.C. A leucine-rich repeat containing receptor-like kinase marks somatic plant cells competent to form embryos. Development 1997, 124, 2049–2062. [Google Scholar] [CrossRef]
- Mohanta, T.K.; Yadav, D.; Khan, A.L.; Hashem, A.; Abd_Allah, E.F.; Al-Harrasi, A. Molecular Players of EF-hand Containing Calcium Signaling Event in Plants. Int. J. Mol. Sci. 2019, 20, 1476. [Google Scholar] [CrossRef]
- Pirayesh, N.; Giridhar, M.; Ben Khedher, A.; Vothknecht, U.C.; Chigri, F. Organellar calcium signaling in plants: An update. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2021, 1868, 118948. [Google Scholar] [CrossRef]
- Stael, S.; Wurzinger, B.; Mair, A.; Mehlmer, N.; Vothknecht, U.C.; Teige, M. Plant organellar calcium signalling: An emerging field. J. Exp. Bot. 2011, 63, 1525–1542. [Google Scholar] [CrossRef]
- Tian, W.; Wang, C.; Gao, Q.; Li, L.; Luan, S. Calcium spikes, waves and oscillations in plant development and biotic interactions. Nat. Plants 2020, 6, 750–759. [Google Scholar] [CrossRef]
- Marimuthu, K.; Subbaraya, U.; Suthanthiram, B.; Marimuthu, S.S. Molecular analysis of somatic embryogenesis through proteomic approach and optimization of protocol in recalcitrant Musa spp. Physiol. Plant. 2019, 167, 282–301. [Google Scholar] [CrossRef]
- Rivera-Solís, G.; Sáenz-Carbonell, L.; Narváez, M.; Rodríguez, G.; Oropeza, C. Addition of ionophore A23187 increases the efficiency of Cocos nucifera somatic embryogenesis. 3 Biotech 2018, 8, 366. [Google Scholar] [CrossRef]
- Etienne, H.; Lartaud, M.; Carron, M.P.; Michaux-Ferriere, N. Use of calcium to optimize long-term proliferation of friable embryogenic calluses and plant regeneration in Hevea brasiliensis (Mull Arg). J. Exp. Bot. 1997, 48, 129–137. [Google Scholar] [CrossRef]
- Takeda, T.; Inose, H.; Matsuoka, H. Stimulation of somatic embryogenesis in carrot cells by the addition of calcium. Biochem. Eng. J. 2003, 14, 143–148. [Google Scholar] [CrossRef]
- Overvoorde, P.J.; Grimes, H.D. The role of calcium and calmodulin in carrot somatic embryogenesis. Plant Cell Physiol. 1994, 35, 135–144. [Google Scholar]
- Anil, V.S.; Rao, K.S. Calcium-mediated signaling during sandalwood somatic embryogenesis. Role for exogenous calcium as second messenger. Plant Physiol. 2000, 123, 1301–1312. [Google Scholar] [CrossRef]
- Malabadi, R.B.; van Staden, J. Cold-enhanced somatic embryogenesis in Pinus patula is mediated by calcium. S. Afr. J. Bot. 2006, 72, 613–618. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Giridhar, P.; Ravishankar, G.A. Calcium and calcium ionophore A23187 induce high-frequency somatic embryogenesis in cultured tissues of Coffea canephora P ex Fr. In Vitro Cell. Dev. Biol. Plant 2011, 47, 667–673. [Google Scholar] [CrossRef]
- Krebs, M.; Held, K.; Binder, A.; Hashimoto, K.; Den Herder, G.; Parniske, M.; Kudla, J.; Schumacher, K. FRET-based genetically encoded sensors allow high-resolution live cell imaging of Ca2+ dynamics. Plant J. 2012, 69, 181–192. [Google Scholar] [CrossRef]
- Ge, L.L.; Tian, H.Q.; Russell, S.D. Calcium function and distribution during fertilization in angiosperms. Am. J. Bot. 2007, 94, 1046–1060. [Google Scholar] [CrossRef]
- Winnicki, K. The Winner Takes It All: Auxin—The Main Player during Plant Embryogenesis. Cells 2020, 9, 606. [Google Scholar] [CrossRef]
- Denninger, P.; Bleckmann, A.; Lausser, A.; Vogler, F.; Ott, T.; Ehrhardt, D.W.; Frommer, W.B.; Sprunck, S.; Dresselhaus, T.; Grossmann, G. Male-female communication triggers calcium signatures during fertilization in Arabidopsis. Nat. Commun. 2014, 5, 4645. [Google Scholar] [CrossRef] [PubMed]
- Mordhorst, A.P.; Hartog, M.V.; El Tamer, M.K.; Laux, T.; de Vries, S.C. Somatic embryogenesis from Arabidopsis shoot apical meristem mutants. Planta 2002, 214, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Pullman, G.S.; Montello, P.; Cairney, J.; Xu, N.F.; Feng, X.R. Loblolly pine (Pinus taeda L.) somatic embryogenesis: Maturation improvements by metal analyses of zygotic and somatic embryos. Plant Sci. 2003, 164, 955–969. [Google Scholar] [CrossRef]
- Mayer, K.F.X.; Schoof, H.; Haecker, A.; Lenhard, M.; Jurgens, G.; Laux, T. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 1998, 95, 805–815. [Google Scholar] [CrossRef]
- Rivas-Sendra, A.; Corral-Martínez, P.; Porcel, R.; Camacho-Fernández, C.; Calabuig-Serna, A.; Seguí-Simarro, J.M. Embryogenic competence of microspores is associated with their ability to form a callosic, osmoprotective subintinal layer. J. Exp. Bot. 2019, 70, 1267–1281. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–479. [Google Scholar] [CrossRef]
- Satpute, G.; Long, H.; Seguí-Simarro, J.M.; Risueño, M.C.; Testillano, P.S. Cell architecture during gametophytic and embryogenic microspore development in Brassica napus. Acta Physiol. Plant. 2005, 27, 665–674. [Google Scholar] [CrossRef]
- Rizza, A.; Walia, A.; Tang, B.; Jones, A.M. Visualizing cellular gibberellin levels using the nlsGPS1 Förster resonance energy transfer (FRET) biosensor. J. Vis. Exp. 2019, 143, e58739. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Fernández, C.; Hervás, D.; Rivas-Sendra, A.; Marín, M.P.; Seguí-Simarro, J.M. Comparison of six different methods to calculate cell densities. Plant Methods 2018, 14, 30. [Google Scholar] [CrossRef] [PubMed]






| Induction | Germination | |
|---|---|---|
| GB5 (g/L) | 3.16 | |
| MS (g/L) | 4.6 | |
| Sucrose (%) | 2 | 2 |
| 2,4-D (mg/L) | 1.1 | |
| Plant agar (%) | 0.8 | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabuig-Serna, A.; Mir, R.; Seguí-Simarro, J.M. Calcium Dynamics, WUSCHEL Expression and Callose Deposition during Somatic Embryogenesis in Arabidopsis thaliana Immature Zygotic Embryos. Plants 2023, 12, 1021. https://doi.org/10.3390/plants12051021
Calabuig-Serna A, Mir R, Seguí-Simarro JM. Calcium Dynamics, WUSCHEL Expression and Callose Deposition during Somatic Embryogenesis in Arabidopsis thaliana Immature Zygotic Embryos. Plants. 2023; 12(5):1021. https://doi.org/10.3390/plants12051021
Chicago/Turabian StyleCalabuig-Serna, Antonio, Ricardo Mir, and Jose M. Seguí-Simarro. 2023. "Calcium Dynamics, WUSCHEL Expression and Callose Deposition during Somatic Embryogenesis in Arabidopsis thaliana Immature Zygotic Embryos" Plants 12, no. 5: 1021. https://doi.org/10.3390/plants12051021
APA StyleCalabuig-Serna, A., Mir, R., & Seguí-Simarro, J. M. (2023). Calcium Dynamics, WUSCHEL Expression and Callose Deposition during Somatic Embryogenesis in Arabidopsis thaliana Immature Zygotic Embryos. Plants, 12(5), 1021. https://doi.org/10.3390/plants12051021








