Pharmacological Features of 18β-Glycyrrhetinic Acid: A Pentacyclic Triterpenoid of Therapeutic Potential
Abstract
1. Introduction
2. Selection of the Literature
Bioactive Derivatives of 18β-Glycyrrhetinic Acid
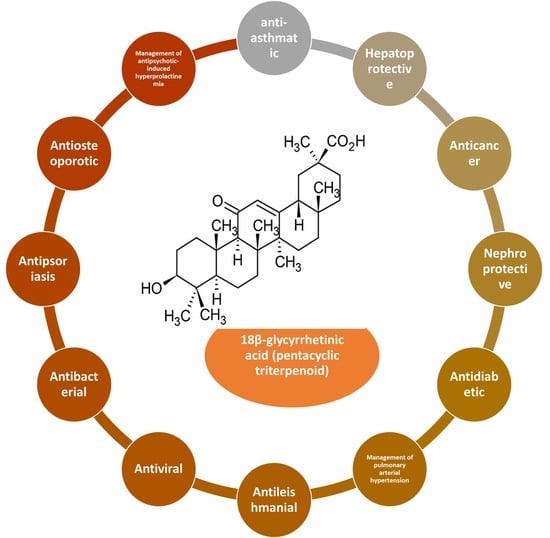
3. Pharmacological Properties of 18β-Glycyrrhetinic Acid
3.1. Anticancer Activity
3.1.1. Breast Cancer
| Assay | Model | Dose/Concentration of 18β-Glycyrrhetinic Acid | Effect | Mechanisms | Reference |
|---|---|---|---|---|---|
| In vivo | Wistar rats | 50 and 100 mg/kg, oral for 7 days | hepatoprotective activity | Downregulation of PPARγ and Nrf2 | [20] |
| In vivo and in vitro | Male Sprague Dawley rats and HEK293T cells | 60 mg/kg intraperitoneally for 7 days; 15, 30, and 60 µM | cholestatic liver injury | Activation of the signaling pathway, including Sirt1 and FXR | [21] |
| In vivo | Male Wistar rats | 25 and 50 mg/kg for 2 weeks | hepatoprotective activity | Nuclear factor kappa B is subsequently suppressed after Nrf2 and PPAR activation | [22] |
| In vivo and in vitro | Male Sprague Dawley rats and c57bl/6 mice and LO2 cells, HEK293T cells | 30, 60, and 120 mg/kg in rats and 40 mg/kg in mice intraperitoneally for 5 days; 30 μM | reduction in acute liver injury | PXR-mediated inhibition of autophagy degradation | [23] |
| In vivo | Wistar rats | 50 and 100 mg/kg, p.o. | hepatoprotective activity | Inhibit oxidative stress and inflammation via activating Nrf2 signaling | [24] |
| In vitro | Human CRC cell lines (LoVo, SW480, and SW620) | 12.5, 25, 50, and 100 µM | antitumor effects against colorectal cancer | p-PI3K, p-AKT, p-STAT3, and p-NF-κB p65 protein levels were reduced | [25] |
| In vitro | Gastric cancer tissues and cell lines | 50, 100, 150, and 100 µM | suppressed gastric tumorigenesis | Potentiating miR-149-3p-Wnt-1 signaling | [26] |
| In vitro | SGC-7901cells | 20, 40, and 60 µM | prevention of gastric cancer metastasis | Prevents invasion and migration via the ROS/PKC-α/ERK signaling pathway | [27] |
| In vitro | Metastatic prostate cancer cell line LNCaP, DU-145, and HUVEC cells | 100 and 200 µM | anti-inflammatory activity on prostate cancer cells | Matrix metallopeptidase-9, NF-κB, and vascular endothelial growth factor (VEGF) expression were all downregulated, whereas NSAID-activated gene-1 expression was elevated | [28] |
| In vitro | LNCaP human prostate cancer cells | 0, 2.5, 5, and 10 μg/mL | human prostate cancer | Suppressed the expression of androgen target genes (TMPRSS2, prostate-specific antigen) | [29] |
| In vitro | Breast cancer cell | 12.5, 25, 50, and 100 μM | inhibits the invasion and metastasis of breast cancer | Reducing p38 MAPK-AP1 signaling axis | [19] |
| In vitro | Human breast cancer cells, MCF-7 | 25, 50, 100, and 200 μM | antitumor properties | Caspase activation and modulation of Akt/forkhead box O3a (FOXO3a) pathway | [30] |
| In vitro | Human ovarian cancer a2780 cells | 50 μM | induces apoptosis in ovarian cancer | A2780 cells expressed more Fas and FasL on their cell surfaces | [31] |
| In vitro | Non-small cell lung cancer (NSCLC) cells A549 and NCI-H460 | 80, 160, and 320 µM | inhibits non-small cell lung cancer | Inhibit extracellular signal-regulated kinase (ERK)1/2 and cyclic adenosine monophosphate response element-binding protein (CREB) | [32] |
| In vitro | A549 lung cancer cells | 10, 20, 30, 40, and 50 μM | treatment of lung cancer | Induced apoptosis and G2/M cell cycle arrest | [33] |
| In vivo and in vitro | Xenograft nude mouse and HepG2 cells | 20, 40, or 80 mg/kg; 5 and 10 μM | antihepatocarcinogenesis | Inhibition of IL-1β-induced activation of the IL-1R1/IκB/IKK/NF-κB signaling pathway | [34] |
| In vitro | Human epithelial ovarian carcinoma cells | 15 and 25 µM | inhibition of epithelial ovarian adenocarcinoma | Hsp90 inhibition-induced apoptosis and activation of caspase-8 | [35] |
| In vivo and in vitro | Immunocompetent C57BL/6 mice and mouse hepatoma cell line Hepa1-6 | 50 mg/kg, once daily (in vivo) for 3 weeks; 20 µg/mL for 3 days (in vitro) | protective role in hepatocellular carcinoma | Reducing T cell apoptosis and regulatory T (Treg) cell expression | [36] |
| in vitro | ΔKPQ Nav1.5 channels | 1, 30, and 100 μmol/L | antiarrhythmic agent | Induces a tonic block of Ina | [37] |
| In vivo and in Vitro | C57BL/J6 mice | 100 mg/kg for 10 days | inhibition of ischemic stroke | Antioxidant and significant decrease in lipid peroxidations | [38] |
| In vivo | Subcutaneous injection of ISO (85 mg/kg/day) in mice | 50 and 100 mg/kg, gavage | cardioprotective effects on acute myocardial infarction | Inhibited PI3K/Akt signaling; reduced cell contractility and Ca2+ concentration | [39] |
3.1.2. Colorectal Cancer
3.1.3. Pituitary Adenomas
3.1.4. Gastric Cancer
3.1.5. Prostate Cancer
3.1.6. Ovarian Cancer
3.1.7. Lung Cancer
3.1.8. Liver Cancer
3.2. Antiarrhythmic Activity
3.3. Cerebral Ischemia
3.4. Hepatoprotective Activity
3.5. Thrombocytopenia
3.6. Anti-Inflammatory Activity
3.7. Antiasthmatic Activity
3.8. Nephroprotective Effects
| Assay | Model | Dose/Concentration of 18β-Glycyrrhetinic Acid | Effect | Mechanisms | Reference |
|---|---|---|---|---|---|
| In vivo | Cisplatin-induced renal injury in male BALB/c mice | 25, 50, and 100 mg/kg | nephroprotective effects | Overexpression of Nrf2 and reduced expression of NF-κB in the kidney | [64] |
| In vitro and in vivo | HK-2 and mTEC cells lines; cisplatin-induced AKI in C57BL/6 mice | 2.5, 5, 10, 20, 30 μM (in vitro); 50, 100, and 200 mg/kg (in vivo) | nephroprotective effects | Enhancing BMP-7 epigenetically through targeting HDAC2 | [65] |
| In vivo | Rats | 50 and 100 mg/kg, oral gavage for 7 days | nephroprotective effects | Upregulating the Nrf2/ARE/heme oxygenase 1 (HO-1) pathway and endogenous antioxidants | [66] |
| In vivo | Single-dose of 50 mg/kg streptozotocin (STZ) intraperitoneally in rats and 20 mg/kg of acrylamide | 50 mg/kg, orally | inhibit reactive oxygen species generation | Decrease serum glucose, cholesterol, creatinine, IL-1β, IL-6, TNF-α | [67] |
| In vitro | High glucose (HG)-induced THP-1 cells | 12.5, 25, and 50 µM | implication to diabetes mellitus | Decrease expressions of ROS, p47s, and iNOS and increase UCP2 levels, promotinga soluble form of RAGE (sRAGE) secretion | [68] |
| In vivo | Streptozotocin-diabetic rats | 50, 100, or 200 mg/kg, oral | antihyperglycemic effect | Increase plasma insulin and lower glycosylated hemoglobin | [69,70] |
| In vivo and in vitro | Sprague Dawley rats and human pulmonary arterial smooth muscle cells | 25, 50, and 100 mg/kg (in vivo); 20, 40, 80, and 160 μM | antiangiogenic effect on pulmonary arterial hypertension | Expression of Rho A, ROCK1, and ROCK2 was decreased, and ROCK activity was inhibited | [71] |
| In vivo | BALB/c mouse model of allergic asthma | 2 and 20 mg/kg, oral | antiasthmatic activity | Inhibition of the RORγt, STAT6, and GATA-3 pathways, as well as activation of the Foxp3 transcription pathway | [60] |
| In vitro | Male SD guinea pigs’ bronchial smooth muscle cells | 50 ng/mL | antiasthmatic activity | Inhibiting the phosphorylation of ERK1/2 | [57] |
| In vitro | Mouse BALB/c macrophage cell line (RAW264.7) | 1 and 5 μM | antiasthmatic activity | Blocking inflammation via PI3K/Akt/GSK3β signaling and dissociating a glucocorticoid receptor-HSP90 complex | [72] |
| In vitro and in vivo | Human bronchial epithelial cell line BEAS2B; C57BL/6 mice | 5, 10, 15, 20, and 25 μM; 50 mg/kg, oral | inhibition of airway; inflammation | Suppression of the mitochondrial ROS/MAPK axis | [62] |
| In vivo | Neonatal rats with hyperoxia exposure | 50 or 100 mg/kg, intragastrically | protected neonatal rats with hyperoxia exposure | Reduced ROS and prevented the activation of NF-κB and the NLRP3 inflammasome | [73] |
3.9. Antidiabetic Activity
3.10. Pulmonary Arterial Hypertension
3.11. Antileishmanial Effect
| Assay | Model | Dose/Concentration of 18β-Glycyrrhetinic acid | Effect | Mechanisms | Reference |
|---|---|---|---|---|---|
| In vitro | Murine macrophage cell line RAW 264.7 | 20 μM for 4 h | antileishmanial effect | Toll-like receptor-dependent canonical and noncanonical p38 activation | [81] |
| In vivo | L. donovani-infected BALB/c mice | 10, 50 and 100 mg/kg i.p. for 3 times | antileishmanial effect | Inhibition of p38 and ERK pathway | [82] |
| In vitro | Rhesus rotavirus strain RRV was propagated in MA104 cell | 25 μg/mL | antiviral activity against rotavirus replication | VP2, VP6, and NSP2 were reduced | [83,84] |
| In vitro | MA104 cells infected with rotavirus | 1, 2, 4, and 8 μg/mL | inhibit cells infected with rotavirus SA11 | Fas/FasL pathway | [23] |
| In vivo | Murine hepatitis virus (MHV) infection model | 10, 100 and 1000 μg/mL | against hepatic inflammation injury in viral hepatitis disease | Inhibition of viral-induced HMGB1-TLR4 immunological regulation axis | [85] |
| In vitro | MRSA strainsaeR and hla | 600 μg | bactericidal to MRSA | Decreasing the expression of saeR, hla, mecA, and sbi | [86] |
| In vitro | Streptococcus mutans and Streptococcus sobrinus | 32, 64, 128 and 256 μg/mL | antibacterial agent | [87] | |
| In vivo | Mongolian gerbils | 0.1% concentration | attenuated H. pylori-infected gastritis | Decrease expression levels of TNF-α, IL-1β, COX-2, and iNOS | [27] |
| In vivo | BALB/c mice | 500 μg/mL | antifungal against Candidal infection | Induced immunological adjuvant activity of Th1 against Candida albicans | [88] |
| In vivo | Male BALB/c mice | 60 and 120 mg/kg for 7 days | prevention of psoriasis | Suppression of mTOR/STAT3 signaling | [89] |
| In vitro and in vivo | Human HaCaT keratinocytes and C57BL6 mice | 10, 20, 40, and 80 µM (in vitro); 50 mg/cm2 cream twice daily for 7 consecutive days (in vivo) | prevention of psoriasis | Inhibition of ROS-mediated PI3K-Akt signaling pathway | [90] |
3.12. Antiviral Action
3.13. Antibacterial Activity
3.14. Antifungal Activity
3.15. Antipsoriasis Effects
3.16. Skin Diseases
3.17. Rheumatoid Arthritis
3.18. Antiosteoporotic Activity
| Assay | Model | Dose/Concentration of 18β-Glycyrrhetinic Acid | Effect | Mechanisms | Reference |
|---|---|---|---|---|---|
| In vivo and in vitro | Bone marrow monocytes (BMMs), RAW264.7 cells, and C57BL/6 female mice | 6.9535, 13.905, and 27.81 μg/mL (in vitro); 50 mg/kg, intraperitoneally | inhibition of postmenopausal osteoporosis | Inhibited osteoclastogenesis by blocking RANKL-mediated RANK-TRAF6 interactions and NF-κB and MAPK signaling pathways | [100] |
| In vivo and in vitro | Chondrocytes and mouse model | 50 mg/kg | inhibition of osteoarthritis | NF-κB activation caused by IL-1 was prevented by activating the Nrf2/HO-1 pathway | [101] |
| In vitro and in vivo | Collagen-induced arthritis mouse model | 100 and 200 μM (In Vitro) 45 mg/kg, p.o. (In Vivo) | inhibit rheumatoid arthritis | Inhibition of MAPK/NF-κB and promotion of FOXO3 signaling | [102] |
| In vitro | Sprague Dawley rats mesenteric artery preparation | 30, 40 μM, 45 min | gap junction blocker | Depolarize the mitochondrial membrane potential by inhibiting IP3-mediated Ca2+ release | [103] |
| In vivo | Rats | 5, 10, and 20 mg/kg, intragastrically | reduces antipsychotic-induced hyperprolactinemia | Inhibited prolactin hyperactivity and also modulated the expression of 5-HT1A and 5-HT2A receptors | [104] |
| In vivo and in vitro | MOG35–55-immunized mice | 75 mg/kg i.p. daily from day 7 or day 11 (In vivo) and 25 μM and 50 μM (In vitro) | multiple sclerosis | Inhibition of microglia activation and promotion of remyelination through suppression of MAPK signal pathway | [105] |
| In vitro | Irradiated RAW264.7 macrophages | 10 μg/mL | anti-inflammatory actions against radiation-induced skin damage | Inhibition of NADPH oxidase/ROS/p38MAPK and NF-κB pathways | [97] |
| In vitro | RAW 264.7 cells | 20 μM | anti-inflammatory activity | Inhibited the gene expressions of COX-2, iNOS, and NF-κB | [56] |
| In vitro | Kv1.3 channels in Jurkat T cells | 10–100 µM | anti-inflammatory and immunomodulation effects | Blocked Kv1.3 potassium channels and T cell activation in human Jurkat T cells | [106] |
| In vitro | Everted rat gut sac model | 1 mM and 100 μM | increase bioavailability | Inhibition of efflux transport mediated by intestinal P-gp | [107] |
3.19. Antiepileptic Effects
3.20. Antipsychotic-Induced Hyperprolactinemia
3.21. Multiple Sclerosis
3.22. Pseudoaldosteronism
3.23. Increase Bioavailability
4. Pharmacokinetics of Glycyrrhizin and Glycyrrhetinic Acid
5. Drugs in the Clinical Trial of 18βGA and Related Compounds
| NCT Number | Title | Status | Conditions | Interventions | Outcome Measures | Sponsor/Collaborators | Phases | Enrollment | Completion Date |
|---|---|---|---|---|---|---|---|---|---|
| NCT03998982 | Glycyrrhetinic Acid Combined with Dexamethasone in Management of Newly Diagnosed ITP | Recruiting | Immune thrombocytopenia | Drug: glycyrrhetinicacid|Drug: dexamethasone | Sustained response to ITP treatments|Evaluation of platelet response | Shandong University | Phase 4 | 30 | 10 June 2021 |
| NCT00384384 | Glycyrrhetinic Acid-Effect on Serum Potassium and Insulin Resistance in Dialysis Patients | Completed | End-stage renal disease | Drug: oral 18B glycyrrhetinic acid versus placebo | University Hospital Inselspital, Berne | Phase 2 | 24 | 7 April | |
| NCT00759525 | The Role of Mineralocorticoid Receptors in Vascular Function | Completed | Apparent mineralocorticoid excess (AME) | Drug: glycyrrhetic acid|Drug: placebo | Forearm blood flow | Brigham and Women’s Hospital | Phase 2|Phase 3 | 15 | 9 September |
| NCT02939144 | An Investigation into the Effect of Liquorice Ingestion on the Salivary Cortisol to Cortisone Molar Ratio | Completed | Apparent mineralocorticoid excess | dietary supplement: liquorice | Salivary cortisol/cortisone ratio induced by licorice (glycyrrhetinic acid and its metabolites) ingestion | The Royal Wolverhampton Hospitals NHS Trust | Not applicable | 12 | 6 March 2017 |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ekor, M. The Growing Use of Herbal Medicines: Issues Relating to Adverse Reactions and Challenges in Monitoring Safety. Front. Neurol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Sofowora, A.; Ogunbodede, E.; Onayade, A. The Role and Place of Medicinal Plants in the Strategies for Disease Prevention. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 210. [Google Scholar] [CrossRef] [PubMed]
- Plant List The Plant List. Available online: http://theplantlist.org/tpl1.1/search?q=digitalis+%0Ahttp://www.theplantlist.org/tpl1.1/search?q=zantedeschia%0A (accessed on 21 January 2022).
- Batiha, G.E.S.; Beshbishy, A.M.; El-Mleeh, A.; Abdel-Daim, M.M.; Devkota, H.P. Traditional Uses, Bioactive Chemical Constituents, and Pharmacological and Toxicological Activities of Glycyrrhiza glabra L. (Fabaceae). Biomolecules 2020, 10, 352. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.K.; Raj, P. Pharmacological Perspective of Glycyrrhiza glabra Linn.: A Mini-Review. J. Anal. Pharm. Res. 2017, 5, 1–5. [Google Scholar] [CrossRef]
- Chassagne, F.; Samarakoon, T.; Porras, G.; Lyles, J.T.; Dettweiler, M.; Marquez, L.; Salam, A.M.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. A Systematic Review of Plants With Antibacterial Activities: A Taxonomic and Phylogenetic Perspective. Front. Pharmacol. 2021, 11, 2069. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Herrera-Bravo, J.; Belén, L.H.; Kaur, R.; Kregiel, D.; Uprety, Y.; Beyatli, A.; Yeskaliyeva, B.; Kırkın, C.; et al. Glycyrrhiza Genus: Enlightening Phytochemical Components for Pharmacological and Health-Promoting Abilities. Oxid. Med. Cell. Longev. 2021, 2021, 1–20. [Google Scholar] [CrossRef]
- Shah, S.L.; Wahid, F.; Khan, N.; Farooq, U.; Shah, A.J.; Tareen, S.; Ahmad, F.; Khan, T. Inhibitory Effects of Glycyrrhiza glabra and Its Major Constituent Glycyrrhizin on Inflammation-Associated Corneal Neovascularization. Evid. Based Complement. Altern. Med. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Hussain, H.; Green, I.R.; Shamraiz, U.; Saleem, M.; Badshah, A.; Abbas, G.; Rehman, N.U.; Irshad, M. Therapeutic Potential of Glycyrrhetinic Acids: A Patent Review (2010–2017). Expert Opin. Ther. Pat. 2018, 28, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Jitrangsri, K.; Kamata, K.; Akiba, M.; Yajiri, Y.; Ishibashi, M.; Tatsuzaki, J.; Ishikawa, T. Is 18α-Glycyrrhizin a Real Natural Product? Improved Preparation of 18α-Glycyrrhizin from 18β-Glycyrrhizin as a Positive Standard for HPLC Analysis of Licorice Extracts. J. Nat. Med. 2022, 76, 367–378. [Google Scholar] [CrossRef]
- Meeran, M.F.N.; Goyal, S.N.; Suchal, K.; Sharma, C.; Patil, C.R.; Ojha, S.K. Pharmacological Properties, Molecular Mechanisms, and Pharmaceutical Development of Asiatic Acid: A Pentacyclic Triterpenoid of Therapeutic Promise. Front. Pharmacol. 2018, 9, 892. [Google Scholar] [CrossRef]
- Kao, T.C.; Wu, C.H.; Yen, G.C. Glycyrrhizic Acid and 18β-Glycyrrhetinic Acid Recover Glucocorticoid Resistance via PI3K-Induced AP1, CRE and NFAT Activation. Phytomedicine 2013, 20, 295–302. [Google Scholar] [CrossRef]
- Mohammed, E.A.H.; Peng, Y.; Wang, Z.; Qiang, X.; Zhao, Q. Synthesis, Antiviral, and Antibacterial Activity of the Glycyrrhizic Acid and Glycyrrhetinic Acid Derivatives. Russ. J. Bioorganic Chem. 2022, 48, 906–918. [Google Scholar] [CrossRef]
- Xu, B.; Wu, G.R.; Zhang, X.Y.; Yan, M.M.; Zhao, R.; Xue, N.N.; Fang, K.; Wang, H.; Chen, M.; Guo, W.B.; et al. An Overview of Structurally Modified Glycyrrhetinic Acid Derivatives as Antitumor Agents. Molecules 2017, 22, 924. [Google Scholar] [CrossRef]
- Hussain, H.; Ali, I.; Wang, D.; Hakkim, F.L.; Westermann, B.; Ahmed, I.; Ashour, A.M.; Khan, A.; Hussain, A.; Green, I.R.; et al. Glycyrrhetinic Acid: A Promising Scaffold for the Discovery of Anticancer Agents. Expert Opin. Drug Discov. 2021, 16, 1497–1516. [Google Scholar] [CrossRef]
- Sun, J.; Liu, H.Y.; Lv, C.Z.; Qin, J.; Wu, Y.F. Modification, Antitumor Activity, and Targeted PPARγ Study of 18β-Glycyrrhetinic Acid, an Important Active Ingredient of Licorice. J. Agric. Food Chem. 2019, 67, 9643–9651. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Gnant, M. Breast Cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Li, Y.; Liu, X.; Wu, Z.; Li, J.; Ma, Z. Roles of Plant-Derived Bioactive Compounds and Related MicroRNAs in Cancer Therapy. Phyther. Res. 2021, 35, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Zhou, Q.M.; Lu, Y.Y.; Zhang, H.; Huang, S.; Su, S.B. Glycyrrhetinic Acid Potently Suppresses Breast Cancer Invasion and Metastasis by Impairing the P38 MAPK-AP1 Signaling Axis. Expert Opin. Ther. Targets 2015, 19, 577–587. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hussein, O.E.; Hozayen, W.G.; Abd El-Twab, S.M. Methotrexate Hepatotoxicity Is Associated with Oxidative Stress, and down-Regulation of PPARγ and Nrf2: Protective Effect of 18β-Glycyrrhetinic Acid. Chem. Biol. Interact. 2017, 270, 59–72. [Google Scholar] [CrossRef]
- Wu, S.Y.; Cui, S.C.; Wang, L.; Zhang, Y.T.; Yan, X.X.; Lu, H.L.; Xing, G.Z.; Ren, J.; Gong, L.K. 18β-Glycyrrhetinic Acid Protects against Alpha-Naphthylisothiocyanate-Induced Cholestasis through Activation of the Sirt1/FXR Signaling Pathway. Acta Pharmacol. Sin. 2018, 39, 1865–1873. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Al Dera, H.S. 18β-Glycyrrhetinic Acid Exerts Protective Effects against Cyclophosphamide-Induced Hepatotoxicity: Potential Role of PPARγ and Nrf2 Upregulation. Genes Nutr. 2015, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Lu, H.; Wang, W.; Song, L.; Liu, M.; Cao, Y.; Qi, X.; Sun, J.; Gong, L. Prevention of D-GalN/LPS-Induced ALI by 18β-Glycyrrhetinic Acid through PXR-Mediated Inhibition of Autophagy Degradation. Cell Death Dis. 2021, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wang, L.; Yu, X.; Huang, Y.; Qu, C.; Zhang, Z.; Luo, D.; Lin, J.; Zhou, L.; Su, Z.; et al. Protective Effect of 18 β -Glycyrrhetinic Acid against Triptolide-Induced Hepatotoxicity in Rats. Evidence-based Complement. Altern. Med. 2017, 2017, 1–12. [Google Scholar] [CrossRef]
- Wang, S.; Shen, Y.; Qiu, R.; Chen, Z.; Chen, Z.; Chen, W. 18 Β-Glycyrrhetinic Acid Exhibits Potent Antitumor Effects Against Colorectal Cancer Via Inhibition of Cell Proliferation and Migration. Int. J. Oncol. 2017, 51, 615–624. [Google Scholar] [CrossRef]
- Cao, D.; Jia, Z.; You, L.; Wu, Y.; Hou, Z.; Suo, Y.; Zhang, H.; Wen, S.; Tsukamoto, T.; Oshima, M.; et al. 18β-Glycyrrhetinic Acid Suppresses Gastric Cancer by Activation of MiR-149-3p-Wnt-1 Signaling. Oncotarget 2016, 7, 71960–71973. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Jiang, J.; You, L.; Jia, Z.; Tsukamoto, T.; Cai, H.; Wang, S.; Hou, Z.; Suo, Y.E.; Cao, X. The Protective Effects of 18β-Glycyrrhetinic Acid on Helicobacter Pylori -Infected Gastric Mucosa in Mongolian Gerbils. BioMed Res. Int. 2016, 2016, 4943793. [Google Scholar] [CrossRef]
- Shetty, A.V.; Thirugnanam, S.; Dakshinamoorthy, G.; Samykutty, A.; Zheng, G.; Chen, A.; Bosland, M.C.; Kajdacsy-Balla, A.; Gnanasekar, M. 18A-Glycyrrhetinic Acid Targets Prostate Cancer Cells By Down-Regulating Inflammation-Related Genes. Int. J. Oncol. 2011, 39, 635–640. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, M.; Park, P.H.; Song, K. Transcriptional Suppression of Androgen Receptor by 18β-Glycyrrhetinic Acid in LNCaP Human Prostate Cancer Cells. Arch. Pharm. Res. 2020, 43, 433–448. [Google Scholar] [CrossRef]
- Sharma, G.; Kar, S.; Palit, S.; Das, P.K. 18β-Glycyrrhetinic Acid (Concur) Induces Apoptosis through Modulation of Akt/FOXO3a/Bim Pathway in Human Breast Cancer MCF-7 Cells. J. Cell. Physiol. 2012, 227, 1923–1931. [Google Scholar] [CrossRef]
- Haghshenas, V.; Fakhari, S.; Mirzaie, S.; Rahmani, M.; Farhadifar, F.; Pirzadeh, S.; Jalili, A. Glycyrrhetinic Acid Inhibits Cell Growth and Induces Apoptosis in Ovarian Cancer A2780 Cells. Adv. Pharm. Bull. 2014, 4, 437–441. [Google Scholar] [CrossRef]
- Huang, R.Y.; Chu, Y.L.; Huang, Q.C.; Chen, X.M.; Jiang, Z.B.; Zhang, X.; Zeng, X. 18Β-Glycyrrhetinic Acid Suppresses Cell Proliferation through Inhibiting Thromboxane Synthase in Non-Small Cell Lung Cancer. PLoS ONE 2014, 9, e93690. [Google Scholar] [CrossRef]
- Luo, Y.H.; Wang, C.; Xu, W.T.; Zhang, Y.; Zhang, T.; Xue, H.; Li, Y.N.; Fu, Z.R.; Wang, Y.; Jin, C.H. 18β-Glycyrrhetinic Acid Has Anti-Cancer Effects via Inducing Apoptosis and G2/M Cell Cycle Arrest, and Inhibiting Migration of A549 Lung Cancer Cells. OncoTargets Ther. 2021, 14, 5131–5144. [Google Scholar] [CrossRef]
- Wang, X.; Tan, Y.; Zhang, Y.; Xu, Z.; Xu, B.; Lei, H.; Ding, C.; Cheng, S.; Wang, X.; Wei, P.; et al. The Novel Glycyrrhetinic Acid–Tetramethylpyrazine Conjugate TOGA Induces Anti-Hepatocarcinogenesis by Inhibiting the Effects of Tumor-Associated Macrophages on Tumor Cells. Pharmacol. Res. 2020, 161, 105233. [Google Scholar] [CrossRef]
- Yang, J.C.; Myung, S.C.; Kim, W.; Lee, C.S. 18β-Glycyrrhetinic Acid Potentiates Hsp90 Inhibition-Induced Apoptosis in Human Epithelial Ovarian Carcinoma Cells via Activation of Death Receptor and Mitochondrial Pathway. Mol. Cell. Biochem. 2012, 370, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Kuang, P.; Zhao, W.; Su, W.; Zhang, Z.; Zhang, L.; Liu, J.; Ren, G.; Yin, Z.; Wang, X. 18Β-Glycyrrhetinic Acid Inhibits Hepatocellular Carcinoma Development By Reversing Hepatic Stellate Cell-Mediated Immunosuppression in Mice. Int. J. Cancer 2013, 132, 1831–1841. [Google Scholar] [CrossRef]
- Du, Y.M.; Xia, C.K.; Zhao, N.; Dong, Q.; Lei, M.; Xia, J.H. 18β-Glycyrrhetinic Acid Preferentially Blocks Late Na Current Generated by ΔKPQ Nav1.5 Channels. Acta Pharmacol. Sin. 2012, 33, 752–760. [Google Scholar] [CrossRef]
- Oztanir, M.N.; Ciftci, O.; Cetin, A.; Durak, M.A.; Basak, N.; Akyuva, Y. The Beneficial Effects of 18β-Glycyrrhetinic Acid Following Oxidative and Neuronal Damage in Brain Tissue Caused by Global Cerebral Ischemia/Reperfusion in a C57BL/J6 Mouse Model. Neurol. Sci. 2014, 35, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Wang, W.; Zhang, N.; Liu, T.; Li, J.; Chu, X.; Zuo, S.; Ma, Z.; Ma, D.; Chu, L. Protective Effects of 18β-Glycyrrhetinic Acid against Myocardial Infarction: Involvement of PI3K/Akt Pathway Activation and Inhibiting Ca2+ Influx via L-Type Ca2+ Channels. Food Sci. Nutr. 2021, 9, 6831–6843. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Xiao, J.; Zhang, K.; Li, Q.; Chen, H.; Liu, F. 18 Beta-Glycyrrhetinic Acid Ameliorates the Cognitive Functions and Decreases the Recurrence Rate of Pituitary Adenomas Patients. EXCLI J. 2018, 17, 753–761. [Google Scholar] [CrossRef]
- Cai, H.; Chen, X.; Zhang, J.; Wang, J. 18β-Glycyrrhetinic Acid Inhibits Migration and Invasion of Human Gastric Cancer Cells via the ROS/PKC-α/ERK Pathway. J. Nat. Med. 2018, 72, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Rebello, R.J.; Oing, C.; Knudsen, K.E.; Loeb, S.; Johnson, D.C.; Reiter, R.E.; Gillessen, S.; Van der Kwast, T.; Bristow, R.G. Prostate Cancer. Nat. Rev. Dis. Prim. 2021, 7, 1–27. [Google Scholar] [CrossRef]
- Hallas-Potts, A.; Dawson, J.C.; Herrington, C.S. Ovarian Cancer Cell Lines Derived from Non-Serous Carcinomas Migrate and Invade More Aggressively than Those Derived from High-Grade Serous Carcinomas. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.E.M.M.; Raghavendra, N.M.; Penido, C. Natural Heat Shock Protein 90 Inhibitors in Cancer and Inflammation. Eur. J. Med. Chem. 2020, 189, 112063. [Google Scholar] [CrossRef]
- Anderson, R.; Blidner, A.G.; Rapoport, B.L. Frontiers in Pharmacology: Review Manuscript Targeting of the Neutrophil as an Adjunctive Strategy in Non-Small Cell Lung Cancer. Front. Pharmacol. 2021, 12, 1338. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular Carcinoma. Nat. Rev. Dis. Prim. 2021, 7, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, L.; Yin, Z.; Su, W.; Ren, G.; Zhou, C.; You, J.; Fan, J.; Wang, X. Activated Hepatic Stellate Cells Promote Hepatocellular Carcinoma Development in Immunocompetent Mice. Int. J. Cancer 2011, 129, 2651–2661. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Shou, W. Cardiac Sodium Channel Nav1.5 Mutations and Cardiac Arrhythmia. Pediatr. Cardiol. 2012, 33, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.V.; De Silva, D.A.; Macleod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic Stroke. Nat. Rev. Dis. Prim. 2019, 5, 1–22. [Google Scholar] [CrossRef]
- Malayeri, A.; Badparva, R.; Mombeini, M.A.; Khorsandi, L.; Goudarzi, M. Naringenin: A Potential Natural Remedy against Methotrexate-Induced Hepatotoxicity in Rats. Drug Chem. Toxicol. 2020, 45, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Jiang, Y.; Zhang, W.; Wang, J.; Wang, R.; Wang, L.; Wei, S.; Wen, J.; Li, H.; Zhao, Y. Natural Products for the Prevention and Treatment of Cholestasis: A Review. Phyther. Res. 2020, 34, 1291–1309. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.H.; Wang, Y.Y.; Lin, S.Y.; Liao, S.L.; Chen, Y.F.; Huang, W.C.; Chen, C.J.; Chen, W.Y. 18β-Glycyrrhetinic Acid Protects against Cholestatic Liver Injury in Bile Duct-Ligated Rats. Antioxidants 2022, 11, 961. [Google Scholar] [CrossRef]
- Hasan, S.K.; Khan, R.; Ali, N.; Khan, A.Q.; Rehman, M.U.; Tahir, M.; Lateef, A.; Nafees, S.; Mehdi, S.J.; Rashid, S.; et al. 18-β Glycyrrhetinic Acid Alleviates 2-Acetylaminofluorene-Induced Hepatotoxicity in Wistar Rats: Role in Hyperproliferation, Inflammation and Oxidative Stress. Hum. Exp. Toxicol. 2015, 34, 628–641. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, J.; He, Y.; Miu, K.K.; Yao, S.; Tang, C.; Ye, Y.; Lin, G. Nrf2-Mediated Liver Protection by 18β-Glycyrrhetinic Acid against Pyrrolizidine Alkaloid-Induced Toxicity through PI3K/Akt/GSK3β Pathway. Phytomedicine 2022, 102, 154162. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; An, N.; Han, P.; Yunqi, S.; Hou, M. 18β-Glycyrrhetic Acid Modulates Th1/Th17/Th22/Regulatory T Cells Homeostasis Via HMGB1/NF-ΚB Signaling Pathway in Immune Thrombocytopenia. Blood 2018, 132, 1144. [Google Scholar] [CrossRef]
- Zhou, J.-X.; Wink, M. Evidence for Anti-Inflammatory Activity of Isoliquiritigenin, 18β Glycyrrhetinic Acid, Ursolic Acid, and the Traditional Chinese Medicine Plants Glycyrrhiza glabra and Eriobotrya Japonica, at the Molecular Level. Medicines 2019, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-L.; Li, B.; Zhang, X.; Zhu, J.; Xie, Y.; Shen, T.; Tang, W.; Zhang, J. 18β-Glycyrrhetinic Acid Monoglucuronide (GAMG) Alleviates Single-Walled Carbon Nanotubes (SWCNT)-Induced Lung Inflammation and Fibrosis in Mice through PI3K/AKT/NF-ΚB Signaling Pathway. Ecotoxicol. Environ. Saf. 2022, 242, 113858. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, Y.; Yan, M.; Yu, Y.; Guo, Y. 18β-Glycyrrhetinic Acid Suppresses Allergic Airway Inflammation through NF-ΚB and Nrf2/HO-1 Signaling Pathways in Asthma Mice. Sci. Rep. 2022, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Cellular and Molecular Mechanisms of Asthma and COPD. Clin. Sci. 2017, 131, 1541–1558. [Google Scholar] [CrossRef]
- Kim, S.H.; Hong, J.H.; Lee, J.E.; Lee, Y.C. 18β-Glycyrrhetinic Acid, the Major Bioactive Component of Glycyrrhizae Radix, Attenuates Airway Inflammation by Modulating Th2 Cytokines, GATA-3, STAT6, and Foxp3 Transcription Factors in an Asthmatic Mouse Model. Environ. Toxicol. Pharmacol. 2017, 52, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liao, J.Y.; Yu, L.; Liu, G.S. Regulating Effect of Glycyrrhetinic Acid on Bronchial Asthma Smooth Muscle Proliferation and Apoptosis as Well as Inflammatory Factor Expression through ERK1/2 Signaling Pathway. Asian Pac. J. Trop. Med. 2017, 10, 1172–1176. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, D.E.; Lee, S.H. Effects of 18β-Glycyrrhetinic Acid on Fungal Protease-Induced Airway Inflammatory Responses. Mediators Inflamm. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Lou, D.; Zhou, L.; Wang, J.; Yang, B.; He, Q.; Wang, J.; Weng, Q. Natural Products: Potential Treatments for Cisplatin-Induced Nephrotoxicity. Acta Pharmacol. Sin. 2021, 2021, 1–19. [Google Scholar] [CrossRef]
- Wu, C.-H.; Chen, A.-Z.; Yen, G.-C. Protective Effects of Glycyrrhizic Acid and 18β-Glycyrrhetinic Acid against Cisplatin-Induced Nephrotoxicity in BALB/c Mice. J. Agric. Food Chem. 2015, 63, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Huang, C.; Meng, X.; Li, X.; Zhang, Y.; Ji, S.; Li, J.; Ye, M.; Liang, H. A Potential Adjuvant Chemotherapeutics, 18β-Glycyrrhetinic Acid, Inhibits Renal Tubular Epithelial Cells Apoptosis via Enhancing BMP-7 Epigenetically through Targeting HDAC2. Sci. Rep. 2016, 6, 25396. [Google Scholar] [CrossRef]
- Abd El-Twab, S.M.; Hozayen, W.G.; Hussein, O.E.; Mahmoud, A.M. 18β-Glycyrrhetinic Acid Protects against Methotrexate-Induced Kidney Injury by up-Regulating the Nrf2/ARE/HO-1 Pathway and Endogenous Antioxidants. Ren. Fail. 2016, 38, 1516–1527. [Google Scholar] [CrossRef]
- Alanazi, I.S.; Emam, M.; Elsabagh, M.; Alkahtani, S.; Abdel-Daim, M.M. The Protective Effects of 18β-Glycyrrhetinic Acid against Acrylamide-Induced Cellular Damage in Diabetic Rats. Environ. Sci. Pollut. Res. 2021, 28, 58322–58330. [Google Scholar] [CrossRef]
- Li, Z.Y.; Tung, Y.T.; Chen, S.Y.; Yen, G.C. Novel Findings of 18β-Glycyrrhetinic Acid on SRAGE Secretion through Inhibition of Transient Receptor Potential Canonical Channels in High-Glucose Environment. BioFactors 2019, 45, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Kalaiarasi, P.; Pugalendi, K.V. Antihyperglycemic Effect of 18β-Glycyrrhetinic Acid, Aglycone of Glycyrrhizin, on Streptozotocin-Diabetic Rats. Eur. J. Pharmacol. 2009, 606, 269–273. [Google Scholar] [CrossRef]
- Kalaiarasi, P.; Kaviarasan, K.; Pugalendi, K.V. Hypolipidemic Activity of 18β-Glycyrrhetinic Acid on Streptozotocin-Induced Diabetic Rats. Eur. J. Pharmacol. 2009, 612, 93–97. [Google Scholar] [CrossRef]
- Zhang, M.; Chang, Z.; Zhang, P.; Jing, Z.; Yan, L.; Feng, J.; Hu, Z.; Xu, Q.; Zhou, W.; Ma, P.; et al. Protective Effects of 18β-Glycyrrhetinic Acid on Pulmonary Arterial Hypertension via Regulation of Rho A/Rho Kinsase Pathway. Chem. Biol. Interact. 2019, 311, 108749. [Google Scholar] [CrossRef]
- Kao, T.C.; Shyu, M.H.; Yen, G.C. Glycyrrhizic Acid and 18β-Glycyrrhetinic Acid Inhibit Inflammation via PI3K/Akt/GSK3β Signaling and Glucocorticoid Receptor Activation. J. Agric. Food Chem. 2010, 58, 8623–8629. [Google Scholar] [CrossRef]
- Qing, C.; Ziyun, L.; Xuefei, Y.; Xinyi, Z.; Xindong, X.; Jianhua, F. Protective Effects of 18β-Glycyrrhetinic Acid on Neonatal Rats with Hyperoxia Exposure. Inflammation 2022, 45, 1224–1238. [Google Scholar] [CrossRef]
- Caglayan, C.; Kandemir, F.M.; Ayna, A.; Gür, C.; Küçükler, S.; Darendelioğlu, E. Neuroprotective Effects of 18β-Glycyrrhetinic Acid against Bisphenol A-Induced Neurotoxicity in Rats: Involvement of Neuronal Apoptosis, Endoplasmic Reticulum Stress and JAK1/STAT1 Signaling Pathway. Metab. Brain Dis. 2022, 37, 1931–1940. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global Aetiology and Epidemiology of Type 2 Diabetes Mellitus and Its Complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Jugran, A.K.; Rawat, S.; Devkota, H.P.; Bhatt, I.D.; Rawal, R.S. Diabetes and Plant-Derived Natural Products: From Ethnopharmacological Approaches to Their Potential for Modern Drug Discovery and Development. Phyther. Res. 2021, 35, 223–245. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, M.; Liu, Q.; Xu, T.; Huang, T.; Yao, D.; Wong, C.W.; Liu, J.; Guan, M. 18β-Glycyrrhetinic Acid Acts through Hepatocyte Nuclear Factor 4 Alpha to Modulate Lipid and Carbohydrate Metabolism. Pharmacol. Res. 2020, 157, 104840. [Google Scholar] [CrossRef]
- Thenappan, T.; Ormiston, M.L.; Ryan, J.J.; Archer, S.L. Pulmonary Arterial Hypertension: Pathogenesis and Clinical Management. BMJ 2018, 360, j5492. [Google Scholar] [CrossRef]
- Wang, L.J.; Ma, K.T.; Shi, W.Y.; Wang, Y.Z.; Zhao, L.; Chen, X.Y.; Li, X.Z.; Jiang, X.W.; Zhang, Z.S.; Li, L.; et al. Enhanced Gap Junctional Channel Activity between Vascular Smooth Muscle Cells in Cerebral Artery of Spontaneously Hypertensive Rats. Clin. Exp. Hypertens. 2017, 39, 295–305. [Google Scholar] [CrossRef]
- Ashwin, H.; Sadlova, J.; Vojtkova, B.; Becvar, T.; Lypaczewski, P.; Schwartz, E.; Greensted, E.; Van Bocxlaer, K.; Pasin, M.; Lipinski, K.S.; et al. Characterization of a New Leishmania Major Strain for Use in a Controlled Human Infection Model. Nat. Commun. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Gupta, P.; Das, P.K.; Ukil, A. Antileishmanial Effect of 18β-Glycyrrhetinic Acid Is Mediated by Toll-like Receptor-Dependent Canonical and Noncanonical P38 Activation. Antimicrob. Agents Chemother. 2015, 59, 2531–2539. [Google Scholar] [CrossRef]
- Ukil, A.; Kar, S.; Srivastav, S.; Ghosh, K.; Das, P.K. Curative Effect of 18β-Glycyrrhetinic Acid in Experimental Visceral Leishmaniasis Depends on Phosphatase-Dependent Modulation of Cellular MAP Kinases. PLoS ONE 2011, 6. [Google Scholar] [CrossRef]
- Hardy, M.E.; Hendricks, J.M.; Paulson, J.M.; Faunce, N.R. 18-Glycyrrhetinic Acid Inhibits Rotavirus Replication in Culture. Virol. J. 2012, 9, 96. [Google Scholar] [CrossRef]
- Wang, X.; Xie, F.; Zhou, X.; Chen, T.; Xue, Y.; Wang, W. 18β-Glycyrrhetinic Acid Inhibits the Apoptosis of Cells Infected with Rotavirus SA11 via the Fas/FasL Pathway. Pharm. Biol. 2021, 59, 1098–1105. [Google Scholar] [CrossRef]
- Shi, X.; Yu, L.; Zhang, Y.; Liu, Z.; Zhang, H.; Zhang, Y.; Liu, P.; Du, P. Glycyrrhetinic Acid Alleviates Hepatic Inflammation Injury in Viral Hepatitis Disease via a HMGB1-TLR4 Signaling Pathway. Int. Immunopharmacol. 2020, 84, 106578. [Google Scholar] [CrossRef]
- Long, D.R.; Mead, J.; Hendricks, J.M.; Hardy, M.E.; Voyich, J.M. 18β-Glycyrrhetinic Acid Inhibits Methicillin-Resistant Staphylococcus Aureus Survival and Attenuates Virulence Gene Expression. Antimicrob. Agents Chemother. 2013, 57, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Dewake, N.; Ma, X.; Sato, K.; Nakatsu, S.; Yoshimura, K.; Eshita, Y.; Fujinaka, H.; Yano, Y.; Yoshinari, N.; Yoshida, A. Β-Glycyrrhetinic Acid Inhibits the Bacterial Growth and Biofilm Formation by Supragingival Plaque Commensals. Microbiol. Immunol. 2021, 65, 343–351. [Google Scholar] [CrossRef]
- Kim, J.; Joo, I.; Kim, H.; Han, Y. 18β-Glycyrrhetinic Acid Induces Immunological Adjuvant Activity of Th1 against Candida Albicans Surface Mannan Extract. Phytomedicine 2013, 20, 951–955. [Google Scholar] [CrossRef]
- Chen, H.; Liu, H.; Tang, B.; Chen, Y.; Han, L.; Yu, J.; Yan, Y.; Lu, C. The Protective Effects of 18 β-Glycyrrhetinic Acid on Imiquimod-Induced Psoriasis in Mice via Suppression of MTOR/STAT3 Signaling. J. Immunol. Res. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Gao, J.; Guo, J.; Nong, Y.; Mo, W.; Fang, H.; Mi, J.; Qi, Q.; Yang, M. 18β-Glycyrrhetinic Acid Induces Human HaCaT Keratinocytes Apoptosis through ROS-Mediated PI3K-Akt Signaling Pathway and Ameliorates IMQ-Induced Psoriasis-like Skin Lesions in Mice. BMC Pharmacol. Toxicol. 2020, 21, 1–11. [Google Scholar] [CrossRef]
- Crawford, S.E.; Ramani, S.; Tate, J.E.; Parashar, U.D.; Svensson, L.; Hagbom, M.; Franco, M.A.; Greenberg, H.B.; O’Ryan, M.; Kang, G.; et al. Rotavirus Infection. Nat. Rev. Dis. Prim. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G. Methicillin-Resistant Staphylococcus Aureus: An Overview of Basic and Clinical Research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Krausse, R.; Bielenberg, J.; Blaschek, W.; Ullmann, U. In Vitro Anti-Helicobacter Pylori Activity of Extractum Liquiritiae, Glycyrrhizin and Its Metabolites. J. Antimicrob. Chemother. 2004, 54, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Weaver, A.J.; Borgogna, T.R.; O’Shea-Stone, G.; Peters, T.R.; Copié, V.; Voyich, J.; Teintze, M. 18β-Glycyrrhetinic Acid Induces Metabolic Changes and Reduces Staphylococcus Aureus Bacterial Cell-to-Cell Interactions. Antibiotics 2022, 11, 781. [Google Scholar] [CrossRef] [PubMed]
- Rendon, A.; Schäkel, K. Psoriasis Pathogenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; He, Y.Y. Ultraviolet Radiation-Induced Non-Melanoma Skin Cancer: Regulation of DNA Damage Repair and Inflammation. Genes Dis. 2014, 1, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Wang, Z.; Huang, F.; Lan, R.; Chen, X.; Han, D.; Zhang, L.; Zhang, W.; Hong, J. 18β-Glycyrrhetinic Acid Mitigates Radiation-Induced Skin Damage via NADPH Oxidase/ROS/P38MAPK and NF-ΚB Pathways. Environ. Toxicol. Pharmacol. 2018, 60, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Puchner, A.; Hayer, S.; Niederreiter, B.; Hladik, A.; Blueml, S.; Bonelli, M.; Scheinecker, C.; Smolen, J.; Redlich, K. Effects of 18β-Glycyrrhetinic Acid in HTNFtg Mice—A Model of Rheumatoid Arthritis. Wien. Klin. Wochenschr. 2012, 124, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.H.; Park, P.S.U.; Park-Min, K.H. The M-CSF Receptor in Osteoclasts and Beyond. Exp. Mol. Med. 2020, 52, 1239–1254. [Google Scholar] [CrossRef]
- Chen, X.; Zhi, X.; Yin, Z.; Li, X.; Qin, L.; Qiu, Z.; Su, J. 18β-Glycyrrhetinic Acid Inhibits Osteoclastogenesis in Vivo and In Vitro by Blocking RANKL-Mediated RANK-TRAF6 Interactions and NF-ΚB and MAPK Signaling Pathways. Front. Pharmacol. 2018, 9, 647. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhu, D.; Xie, C.; Shi, Y.; Ni, L.; Zhang, H.; Li, S.; Lu, J.; Xiao, J.; Xia, W.; et al. 18β-Glycyrrhetinic Acid Inhibits IL-1β-Induced Inflammatory Response in Mouse Chondrocytes and Prevents Osteoarthritic Progression by Activating Nrf2. Food Funct. 2021, 12, 8399–8410. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Mei, L.; Wang, M.; Huang, Q.; Huang, R. Anti-Inflammatory and Pro-Apoptotic Effects of 18beta-Glycyrrhetinic Acid In Vitro and In Vivo Models of Rheumatoid Arthritis. Front. Pharmacol. 2021, 12, 681525. [Google Scholar] [CrossRef]
- Buckley, C.; Zhang, X.; Wilson, C.; McCarron, J.G. Carbenoxolone and 18β-Glycyrrhetinic Acid Inhibit Inositol 1,4,5-Trisphosphate-Mediated Endothelial Cell Calcium Signalling and Depolarise Mitochondria. Br. J. Pharmacol. 2021, 178, 896–912. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Wang, C.; Jia, D.; Cai, G.; Lu, J.; Zhang, Z.J. 18β-Glycyrrhetinic Acid, a Novel Naturally Derived Agent, Suppresses Prolactin Hyperactivity and Reduces Antipsychotic-Induced Hyperprolactinemia in In Vitro and In Vivo Models. Neurochem. Res. 2016, 41, 2233–2242. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Cai, W.; Jin, M.; Xu, J.; Wang, Y.; Xiao, Y.; Hao, L.; Wang, B.; Zhang, Y.; Han, J.; et al. 18Β-Glycyrrhetinic Acid Suppresses Experimental Autoimmune Encephalomyelitis Through Inhibition of Microglia Activation and Promotion of Remyelination. Sci. Rep. 2015, 5, 13713. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.X.; Du, L.L.; Zhao, N.; Dong, Q.; Liao, Y.H.; Du, Y.M. 18β-Glycyrrhetinic Acid Potently Inhibits Kv1.3 Potassium Channels and T Cell Activation in Human Jurkat T Cells. J. Ethnopharmacol. 2013, 148, 647–654. [Google Scholar] [CrossRef]
- He, R.; Xu, Y.; Peng, J.; Ma, T.; Li, J.; Gong, M. The Effects of 18β-Glycyrrhetinic Acid and Glycyrrhizin on Intestinal Absorption of Paeoniflorin Using the Everted Rat Gut Sac Model. J. Nat. Med. 2017, 71, 198–207. [Google Scholar] [CrossRef]
- Manjarrez-Marmolejo, J.; Franco-Pérez, J. Gap Junction Blockers: An Overview of Their Effects on Induced Seizures in Animal Models. Curr. Neuropharmacol. 2016, 14, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Petruzzelli, M.G.; Margari, M.; Peschechera, A.; de Giambattista, C.; De Giacomo, A.; Matera, E.; Margari, F. Hyperprolactinemia and Insulin Resistance in Drug Naive Patients with Early Onset First Episode Psychosis. BMC Psychiatry 2018, 18, 246. [Google Scholar] [CrossRef]
- Filippi, M.; Bar-Or, A.; Piehl, F.; Preziosa, P.; Solari, A.; Vukusic, S.; Rocca, M.A. Multiple Sclerosis. Nat. Rev. Dis. Prim. 2018, 4, 1–27. [Google Scholar] [CrossRef]
- Guo, Y.X.; Zhang, Y.; Gao, Y.H.; Deng, S.Y.; Wang, L.M.; Li, C.Q.; Li, X. Role of Plant-Derived Natural Compounds in Experimental Autoimmune Encephalomyelitis: A Review of the Treatment Potential and Development Strategy. Front. Pharmacol. 2021, 12, 639651. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Zou, C.; Qin, M.; Li, Y.; Huang, J. A Simple Method for Evaluation Pharmacokinetics of Glycyrrhetinic Acid and Potential Drug-Drug Interaction between Herbal Ingredients. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Zuo, J.; Yang, J.-G.; Wu, C.; Yang, Y.; Tang, W.; Zhu, L. Physiology-Based Pharmacokinetic Study on 18β-Glycyrrhetic Acid Mono-Glucuronide (GAMG) Prior to Glycyrrhizin in Rats. Molecules 2022, 27, 4657. [Google Scholar] [CrossRef] [PubMed]
- Ravanfar, P.; Namazi, G.; Atigh, M.; Zafarmand, S.; Hamedi, A.; Salehi, A.; Izadi, S.; Borhani-Haghighi, A. Efficacy of Whole Extract of Licorice in Neurological Improvement of Patients after Acute Ischemic Stroke. J. Herb. Med. 2016, 6, 12–17. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinu, P.; Gupta, G.L.; Sharma, M.; Khan, S.; Goyal, M.; Nair, A.B.; Kumar, M.; Soliman, W.E.; Rahman, A.; Attimarad, M.; et al. Pharmacological Features of 18β-Glycyrrhetinic Acid: A Pentacyclic Triterpenoid of Therapeutic Potential. Plants 2023, 12, 1086. https://doi.org/10.3390/plants12051086
Shinu P, Gupta GL, Sharma M, Khan S, Goyal M, Nair AB, Kumar M, Soliman WE, Rahman A, Attimarad M, et al. Pharmacological Features of 18β-Glycyrrhetinic Acid: A Pentacyclic Triterpenoid of Therapeutic Potential. Plants. 2023; 12(5):1086. https://doi.org/10.3390/plants12051086
Chicago/Turabian StyleShinu, Pottathil, Girdhari Lal Gupta, Manu Sharma, Shahzad Khan, Manoj Goyal, Anroop B. Nair, Manish Kumar, Wafaa E. Soliman, Aminur Rahman, Mahesh Attimarad, and et al. 2023. "Pharmacological Features of 18β-Glycyrrhetinic Acid: A Pentacyclic Triterpenoid of Therapeutic Potential" Plants 12, no. 5: 1086. https://doi.org/10.3390/plants12051086
APA StyleShinu, P., Gupta, G. L., Sharma, M., Khan, S., Goyal, M., Nair, A. B., Kumar, M., Soliman, W. E., Rahman, A., Attimarad, M., Venugopala, K. N., & Altaweel, A. A. A. (2023). Pharmacological Features of 18β-Glycyrrhetinic Acid: A Pentacyclic Triterpenoid of Therapeutic Potential. Plants, 12(5), 1086. https://doi.org/10.3390/plants12051086