Comparative Characterization of Fruit Volatiles and Volatile-Related Genes Expression of ‘Benihoppe’ Strawberry and Its Somaclonal Mutant
Abstract
1. Introduction
2. Results
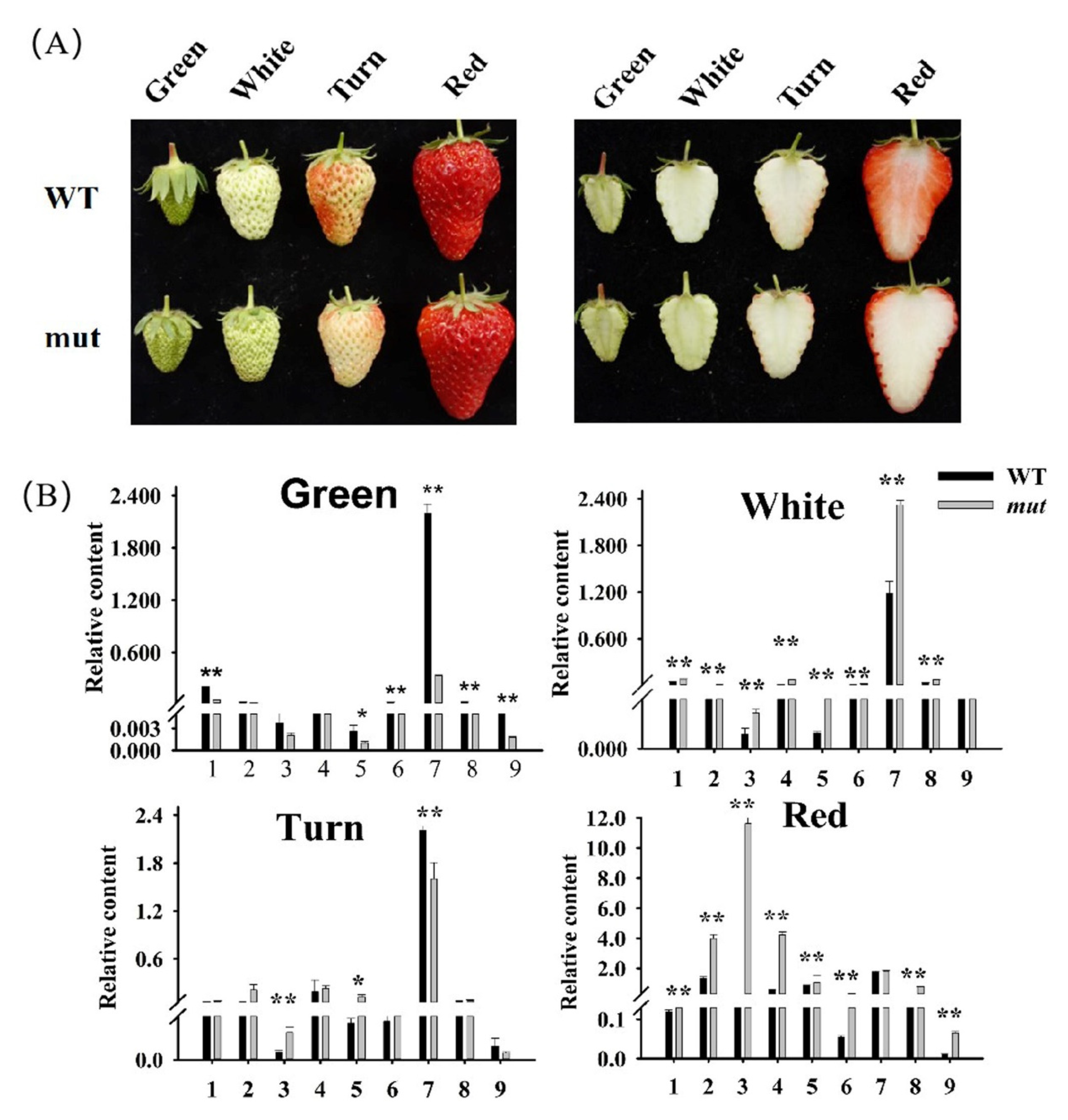
2.1. Comparative Analysis of Phenotypes and Nine Categories of Volatile Compounds in the Fruits of ‘Benihoppe’ and ‘Xiaobai’ at Different Developmental Stages
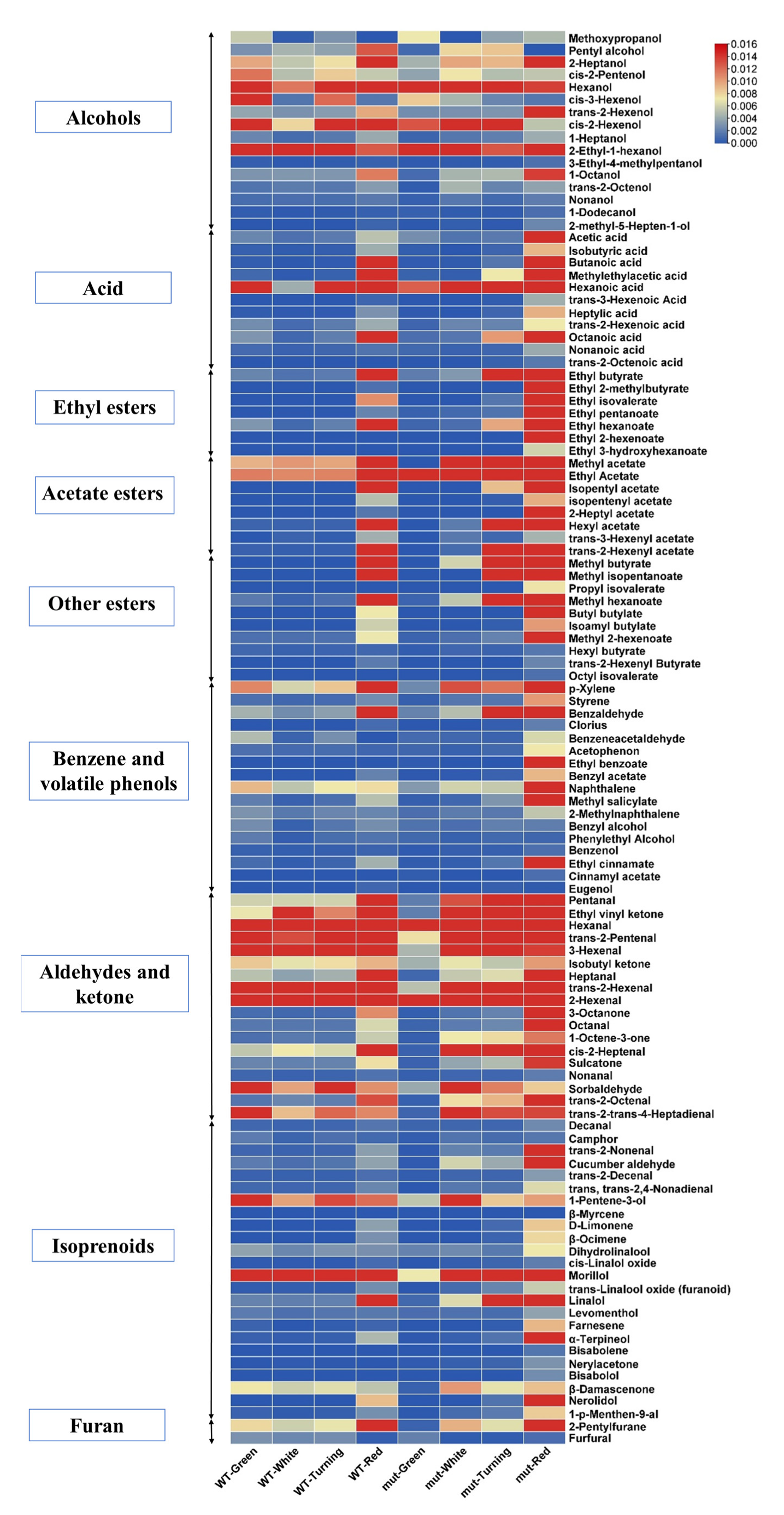
2.2. Comparative Analysis of 113 Volatile Compounds in the Fruits of ‘Benihoppe’ and ‘Xiaobai’ at Different Developmental Stages
2.3. Comparative Analysis of Unique Volatile Compounds in ‘Benihoppe’ and ‘Xiaobai’
2.4. The OAVs of the Main Volatile Compounds in ‘Benihoppe’ and ‘Xiaobai’
2.5. Principal Component Analysis of Volatile Compounds in ‘Benihoppe’ and ‘Xiaobai’
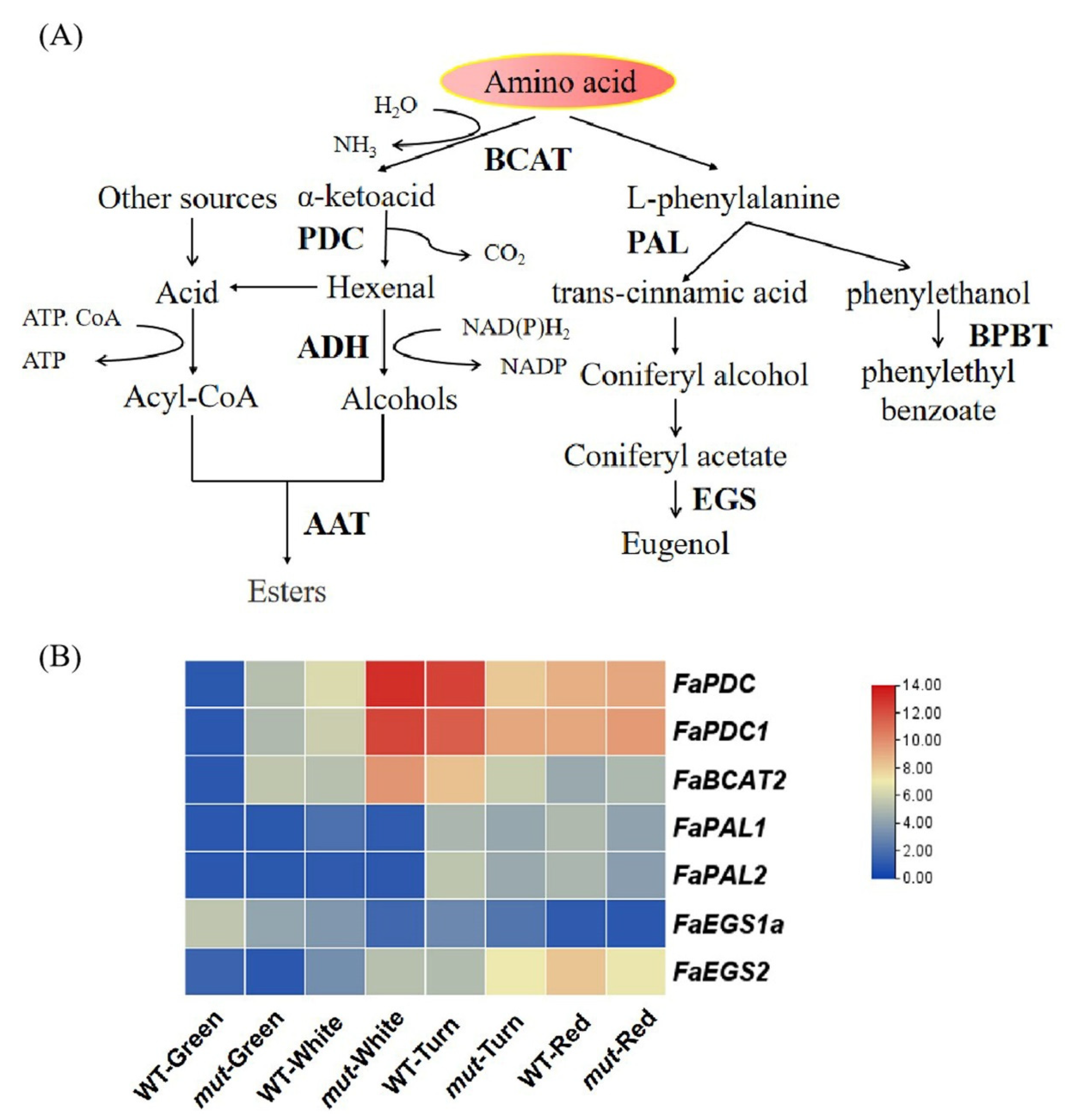
2.6. Expression Analysis of Genes Related to Volatile Compounds Metabolic Pathway
2.6.1. Fatty Acid Pathway
2.6.2. Amino Acid Pathway
2.6.3. Terpenoid Pathway
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Volatile Compounds Extraction
4.3. HS-SPME-GC-MS Analysis
4.4. Quantitative Real-Time PCR
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ariza, M.T.; Forbes-Hernández, T.Y.; Reboredo-Rodríguez, P.; Afrin, S.; Gasparrini, M.; Cervantes, L.; Soria, C.; Martínez-Ferri, E.; Battino, M.; Giampieri, F. Strawberry and achenes hydroalcoholic extracts and their digested fractions efficiently counteract the AAPH-Induced oxidative damage in HepG2 cells. Int. J. Mol. Sci. 2018, 19, 2180. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Song, M.; Rouseff, R. Identification of new strawberry sulfur volatiles and changes during maturation. J. Agric. Food Chem. 2011, 59, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tong, Z.; Zhang, X.; Zhang, X.; Luo, Z.; Shao, W.; Li, L.; Ma, Q.; Zheng, X.; Fang, W. Plant volatile organic compound (E)-2-hexenal facilitates Botrytis cinerea infection of fruits by inducing sulfate assimilation. New Phytol. 2021, 231, 432–446. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef] [PubMed]
- El Hadi, M.A.M.; Zhang, F.J.; Wu, F.F.; Zhou, C.H.; Tao, J. Advances in fruit aroma volatile research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef]
- Yan, J.W.; Ban, Z.J.; Lu, H.Y.; Li, D.; Poverenov, E.; Luo, Z.S.; Li, L. The aroma volatile repertoire in strawberry fruit: A review. J. Sci. Food Agric. 2018, 98, 4395–4402. [Google Scholar] [CrossRef]
- Larsen, M.; Poll, L.; Olsen, C.E. Evaluation of the aroma composition of some strawberry (Fragaria × ananassa Duch) cultivars by use of odour threshold values. Z. Lebensm-Unters. Forsch. 1992, 195, 536–539. [Google Scholar] [CrossRef]
- Larsen, M.; Poll, L. Odour thresholds of some important aroma compounds in strawberries. 1992, 195, 120–123. Z. Für Lebensm. Unters. Und Forsch. 1992, 195, 120–123. [Google Scholar] [CrossRef]
- Zabetakis, I.; Holden, M.A. Strawberry flavour: Analysis and biosynthesis. J. Sci. Food Agric. 1997, 74, 421–434. [Google Scholar] [CrossRef]
- Charles, F.F.; Willy, K. The Composition of strawberry aroma as influenced by cultivar, maturity, and storage. HortScience 1997, 35, 1022–1026. [Google Scholar] [CrossRef]
- Leone, A.; Bleve-Zacheo, T.; Gerardi, C.; Melillo, M.T.; Leo, L.; Zacheo, G. Lipoxygenase involvement in ripening strawberry. J. Agric. Food Chem. 2006, 54, 6835–6844. [Google Scholar] [CrossRef]
- Pérez, A.G.; Olías, R.; Luaces, P.; Sanz, C. Biosynthesis of strawberry aroma compounds through amino acid metabolism. J. Agric. Food Chem. 2002, 50, 4037–4042. [Google Scholar] [CrossRef]
- Song, J.; Bangerth, F. Fatty acids as precursors for aroma volatile biosynthesis in pre-climacteric and climacteric apple fruit. Postharvest Biol. Technol. 2003, 30, 113–121. [Google Scholar] [CrossRef]
- Jetti, R.R.; Yang, E.; Kurnianta, A.; Finn, C.; Qian, M.C. Quantification of selected aroma-active compounds in strawberries by headspace solid-phase microextraction gas chromatography and correlation with sensory descriptive analysis. J. Food Sci. 2007, 72, S487–S496. [Google Scholar] [CrossRef]
- Horvat, R.J.; Chapman, G.W., Jr.; Senter, S.D.; Norton, J.D.; Okie, W.R.; Robertson, J.A. Comparison of the volatile compounds from several commercial plum cultivars. J. Sci. Food Agric. 1992, 60, 21–23. [Google Scholar] [CrossRef]
- Cruz-Rus, E.; Sesmero, R.; Angel-Perez, J.A.; Sanchez-Sevilla, J.F.; Ulrich, D.; Amaya, I. Validation of a PCR test to predict the presence of flavor volatiles mesifurane and γ-decalactone in fruits of cultivated strawberry (Fragaria × ananassa). Mol. Breed. 2017, 37, 131. [Google Scholar] [CrossRef]
- Oh, Y.; Barbey, C.R.; Chandra, S.; Bai, J.; Fan, Z.; Plotto, A.; Pillet, J.; Folta, K.M.; Whitaker, V.M.; Lee, S. Genomic characterization of the fruity aroma gene, FaFAD1, reveals a gene dosage effect on γ-Decalactone production in strawberry (Fragaria × ananassa). Front. Plant Sci. 2021, 12, 639345. [Google Scholar] [CrossRef]
- Fan, Z.; Hasing, T.; Johnson, T.S.; Garner, D.M.; Schwieterman, M.L.; Barbey, C.R.; Colquhoun, T.A.; Sims, C.A.; Resende, M.F.R.; Whitaker, V.M. Strawberry sweetness and consumer preference are enhanced by specific volatile compounds. Hortic. Res. 2021, 8, 66. [Google Scholar] [CrossRef]
- Schreier, P. Quantitative composition of volatile constituents in cultivated strawberries, Fragaria ananassa cv. senga sengana, senga litessa and senga gourmella. J. Sci. Food Agric. 1980, 31, 487–494. [Google Scholar] [CrossRef]
- Dirinck, P.J.; De Pooter, H.L.; Willaert, G.A.; Schamp, N.M. Flavor quality of cultivated strawberries: The role of the sulfur compounds. J. Agric. Food Chem. 1981, 29, 316–321. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Dong, J.; Wang, G. Formation of aroma volatiles in strawberry fruit and aroma breeding. Zhongguo Nongye Kexue 2004, 37, 1039–1044. [Google Scholar]
- Mostafa, S.; Wang, Y.; Zeng, W.; Jin, B. Floral scents and fruit aromas: Functions, compositions, biosynthesis, and regulation. Front. Plant Sci. 2022, 13, 860157. [Google Scholar] [CrossRef] [PubMed]
- Aharoni, A.; Giri, A.P.; Verstappen, F.W.; Bertea, C.M.; Sevenier, R.; Sun, Z.; Jongsma, M.A.; Schwab, W.; Bouwmeester, H.J. Gain and loss of fruit flavor compounds produced by wild and cultivated strawberry species. Plant Cell 2004, 16, 3110–3131. [Google Scholar] [CrossRef]
- Tietel, Z.; Lewinsohn, E.; Fallik, E.; Porat, R. Importance of storage temperatures in maintaining flavor and quality of mandarins. Postharvest Biol. Technol. 2012, 64, 175–182. [Google Scholar] [CrossRef]
- Zhang, B.; Xi, W.P.; Wei, W.W.; Shen, J.Y.; Ferguson, I.; Chen, K.S. Changes in aroma-related volatiles and gene expression during low temperature storage and subsequent shelf-life of peach fruit. Postharvest Biol. Technol. 2011, 60, 7–16. [Google Scholar] [CrossRef]
- Hou, J.; Liang, L.; Wang, Y. Volatile composition changes in navel orange at different growth stages by HS-SPME-GC-MS. Food Res. Int. 2020, 136, 109333. [Google Scholar] [CrossRef]
- Larkin, P.J.; Scowcroft, W.R. Somaclonal variation—A novel source of variability from cell cultures for plant improvement. Theor. Appl. Genet. 1981, 60, 197–214. [Google Scholar] [CrossRef]
- Bairu, M.W.; Aremu, A.O.; Van Staden, J. Somaclonal variation in plants: Causes and detection methods. Plant Growth Regul. 2011, 63, 147–173. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, Y.; Tang, X.; Jin, W.; Han, Z. Differences in volatile ester composition between Fragaria × ananassa and F. vesca and implications for strawberry aroma patterns. Sci. Hortic. 2013, 150, 47–53. [Google Scholar] [CrossRef]
- Du, X.; Plotto, A.; Baldwin, E.; Rouseff, R. Evaluation of volatiles from two subtropical strawberry cultivars using GC-olfactometry, GC-MS odor activity values, and sensory analysis. J. Agric. Food Chem. 2011, 59, 12569–12577. [Google Scholar] [CrossRef]
- Wyllie, S.G.; Leach, D.N.; Nonhebel, H.N.; Lusunzi, I. Biochemical pathways for the formation of esters in ripening fruit. In Flavour Science; Recent Developments; Royal Society of Chemistry: Cambridge, UK, 1996; pp. 52–57. [Google Scholar] [CrossRef]
- Yamashita, I.; Iino, K.; Nemoto, Y.; Yoshikawa, S. Studies on flavor development in strawberries. 4. Biosynthesis of volatile alcohol and esters from aldehyde during ripening. J. Agric. Food Chem. 1977, 25, 1165–1168. [Google Scholar] [CrossRef]
- Nogueira, J.M.F.; Fernandes, P.J.P.; Nascimento, A.M.D. Composition of volatiles of banana cultivars from Madeira Island. Phytocheml Anal. 2003, 14, 87–90. [Google Scholar] [CrossRef]
- Pichersky, E.; Noel Joseph, P.; Dudareva, N. Biosynthesis of plant volatiles: Nature’s diversity and ingenuity. Science 2006, 311, 808–811. [Google Scholar] [CrossRef]
- Koutsompogeras, P.; Kyriacou, A.; Zabetakis, I. Characterizing NAD-dependent alcohol dehydrogenase enzymes of methylobacterium extorquens and strawberry (Fragaria × ananassa cv. elsanta). J. Agric. Food Chem. 2006, 54, 235–242. [Google Scholar] [CrossRef]
- Araguez, I.; Osorio, S.; Hoffmann, T.; Rambla, J.L.; Medina-Escobar, N.; Granell, A.; Botella, M.A.; Schwab, W.; Valpuesta, V. Eugenol production in achenes and receptacles of strawberry fruits is catalyzed by synthases exhibiting distinct kinetics. Plant Physiol. 2013, 163, 946–958. [Google Scholar] [CrossRef]
- Du, F.; Wang, T.; Fan, J.M.; Liu, Z.Z.; Zong, J.X.; Fan, W.X.; Han, Y.H.; Grierson, D. Volatile composition and classification of Lilium flower aroma types and identification, polymorphisms, and alternative splicing of their monoterpene synthase genes. Hortic. Res. 2019, 6, 110. [Google Scholar] [CrossRef]
- Xu, Y.; Charles, M.T.; Luo, Z.; Mimee, B.; Tong, Z.; Véronneau, P.Y.; Roussel, D.; Rolland, D. Ultraviolet-C priming of strawberry leaves against subsequent Mycosphaerella fragariae infection involves the action of reactive oxygen species, plant hormones, and terpenes. Plant Cell Environ. 2019, 42, 815–831. [Google Scholar] [CrossRef]
- Vranová, E.; Coman, D.; Gruissem, W. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Gershenzon, J. The chemical diversity of floral scent. In Biology of Floral Scent, 1st ed.; CRC Press: Boca Raton, FL, USA, 2006; p. 26. [Google Scholar]
- Kaushal, K.; Nath, A.K.; Kaundal, P.; Sharma, D.R. Studies on somaclonal variation in strawberry (Fragaria × ananassa Duch.) cultivars. Acta Hortic. 2005, 662, 269–276. [Google Scholar] [CrossRef]
- Chambers, A.H.; Pillet, J.; Plotto, A.; Bai, J.; Whitaker, V.M.; Folta, K.M. Identification of a strawberry flavor gene candidate using an integrated genetic-genomic-analytical chemistry approach. BMC Genom. 2014, 15, 217. [Google Scholar] [CrossRef]
- Ulrich, D.; Komes, D.; Olbricht, K.; Hoberg, E. Diversity of aroma patterns in wild and cultivated Fragaria accessions. Genet. Resour. Crop. Evol. 2007, 54, 1185–1196. [Google Scholar] [CrossRef]
- Van de Poel, B.; Vandendriessche, T.; Hertog, M.L.A.T.M.; Nicolai, B.M.; Geeraerd, A. Detached ripening of non-climacteric strawberry impairs aroma profile and fruit quality. Postharvest Biol. Tec. 2014, 95, 70–80. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Hou, H.; Ma, X.; Sun, S.; Wang, H.; Kong, L. Metabolomics and gene expression analysis reveal the accumulation patterns of phenylpropanoids and flavonoids in different colored-grain wheats (Triticum aestivum L.). Food Res. Int 2020, 138 Pt A, 109711. [Google Scholar] [CrossRef]
- González, M.; Gaete-Eastman, C.; Valdenegro, M.; Figueroa, C.R.; Fuentes, L.; Herrera, R.; Moya-León, M.A. Aroma development during ripening of Fragaria chiloensis fruit and participation of an alcohol acyltransferase (FcAAT1) gene. J. Agric. Food Chem. 2009, 57, 9123–9132. [Google Scholar] [CrossRef] [PubMed]
- Cumplido-Laso, G.; Medina-Puche, L.; Moyano, E.; Hoffmann, T.; Sinz, Q.; Ring, L.; Studart-Wittkowski, C.; Caballero, J.L.; Schwab, W.; Muñoz-Blanco, J.; et al. The fruit ripening-related gene FaAAT2 encodes an acyl transferase involved in strawberry aroma biogenesis. J. Exp. Bot. 2012, 63, 4275–4290. [Google Scholar] [CrossRef] [PubMed]
- Cielo, P.; Kate, M.; Graeme, S.; Rohan, D.; Larry, A.; Marjorie, M.; Diann, V.M.; Kathy, A.; James, M.C. Acaricidal activity of eugenol based compounds against scabies mites. PLoS ONE 2010, 5, e12079. [Google Scholar] [CrossRef]
- Iijima, Y.; Davidovich-Rikanati, R.; Fridman, E.; Gang, D.R.; Bar, E.; Pichersky, L.E. The biochemical and molecular basis for the divergent patterns in the biosynthesis of terpenes and phenylpropenes in the peltate glands of three cultivars of basil. Plant Physiol. 2004, 136, 3724–3736. [Google Scholar] [CrossRef]
- Zorrilla-Fontanesi, Y.; Rambla, J.L.; Cabeza, A.; Medina, J.J.; Sánchez-Sevilla, J.; Valpuesta, V.; Botella, M.A.; Amaya, G.I. Genetic analysis of strawberry fruit aroma and identification of O-methyltransferase FaOMT as the locus controlling natural variation in mesifurane content. Plant Physiol. 2012, 159, 851–870. [Google Scholar] [CrossRef]
- Baldi, P.; Orsucci, S.; Moser, M.; Brilli, M.; Giongo, L.; Si-Ammour, A. Gene expression and metabolite accumulation during strawberry (Fragaria × ananassa) fruit development and ripening. Planta 2018, 248, 1143–1157. [Google Scholar] [CrossRef]
- Zhao, F.; Song, P.; Zhang, X.; Li, G.; Hu, P.; Aslam, A.; Zhao, X.; Zhou, H. Identification of candidate genes influencing anthocyanin biosynthesis during the development and ripening of red and white strawberry fruits via comparative transcriptome analysis. PeerJ 2021, 9, e10739. [Google Scholar] [CrossRef]
- Rodríguez-Concepción, M.; Boronat, A. Elucidation of the methylerythritol phosphate pathway for isoprenoid biosynthesis in bacteria and plastids. A metabolic milestone achieved through genomics. Plant Physiol. 2002, 130, 1079–1089. [Google Scholar] [CrossRef]
- Bian, R.; Yu, S.; Song, X.; Yao, J.; Zhang, J.; Zhang, Z. An Integrated Metabolomic and Gene Expression Analysis of ‘Sachinoka’ Strawberry and Its Somaclonal Mutant Reveals Fruit Color and Volatiles Differences. Plants 2022, 12, 82. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Dou, Y.; Li, W.; Wang, S.; Shi, W.; Sun, Y.; Zhang, Z. Single nucleotide mutation in FvMYB10 may lead to the yellow fruit in Fragaria vesca. Mol. Breed. 2017, 37, 1–10. [Google Scholar] [CrossRef]
- Edger, P.P.; Poorten, T.J.; Vanburen, R.; Hardigan, M.A.; Colle, M.; McKain, M.R.; Smith, R.D.; Teresi, S.J.; Nelson, A.D.L.; Wai, C.M. Origin and evolution of the octoploid strawberry genome. Nat. Genet. 2019, 51, 541–547. [Google Scholar] [CrossRef]
- Liu, T.; Li, M.; Liu, Z.; Ai, X.; Li, Y. Reannotation of the cultivated strawberry genome and establishment of a strawberry genome database. Hortic. Res. 2021, 8, 9. [Google Scholar] [CrossRef]
- Lei, Y.; Sun, Y.; Wang, B.; Yu, S.; Dai, H.; Li, H.; Zhang, Z.; Zhang, J. Woodland strawberry WRKY71 acts as a promoter of flowering via a transcriptional regulatory cascade. Hortic. Res. 2020, 7, 137. [Google Scholar] [CrossRef]
- Dong, X.; Guan, Y.; Zhang, Z.; Li, H. miR390-tasiRNA3-ARF4 pathway is involved in regulating flowering time in woodland strawberry. Plant Cell Rep. 2022, 41, 921–934. [Google Scholar] [CrossRef]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2ˆ(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013, 3, 71–85. [Google Scholar]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]



| Aroma Components | Threshold (mg·kg−1) | WT-Green | WT-White | WT-Turning | WT-Red | mut-Green | mut-White | mut-Turning | mut-Red |
|---|---|---|---|---|---|---|---|---|---|
| Ethyl butyrate | 0.001 bde | 1.50 | 0.90 | 3.60 | 127.80 | 1.30 | 2.20 | 10.00 | 4421.60 |
| Ethyl isovalerate | 0.002 e | 0.00 | 0.00 | 0.00 | 3.70 | 0.00 | 0.00 | 0.55 | 542.15 |
| Ethyl pentanoate | 0.0015 c | 0.00 | 0.00 | 0.00 | 1.00 | 0.27 | 0.40 | 0.67 | 24.87 |
| Ethyl hexanoate | 0.0003 ad | 7.00 | 2.00 | 6.00 | 226.33 | 1.67 | 2.67 | 22.67 | 19,548.33 |
| Ethyl Acetate | 1 bc | 0.01 | 0.01 | 0.01 | 0.03 | 0.02 | 0.02 | 0.02 | 3.75 |
| Hexyl acetate | 0.002 ade | 0.20 | 0.20 | 4.85 | 34.50 | 0.00 | 0.60 | 7.50 | 52.10 |
| Methyl butyrate | 0.01 bc | 0.00 | 0.00 | 1.76 | 45.72 | 0.00 | 0.42 | 6.07 | 42.76 |
| Methyl hexanoate | 0.087 ad | 0.01 | 0.00 | 0.04 | 4.20 | 0.00 | 0.04 | 0.55 | 6.07 |
| Hexanal | 0.1 b | 5.50 | 3.55 | 7.41 | 6.23 | 0.92 | 6.82 | 4.62 | 6.94 |
| Octanal | 0.001 e | 1.10 | 1.10 | 1.80 | 4.30 | 0.50 | 1.20 | 1.70 | 10.00 |
| Nonanal | 0.001 e | 0.50 | 0.40 | 0.50 | 0.90 | 0.30 | 0.50 | 0.50 | 1.30 |
| Linalool | 0.001 bcd | 1.50 | 1.30 | 20.70 | 146.90 | 0.60 | 4.50 | 36.30 | 528.00 |
| Nerolidol | 0.1 b | 0.00 | 0.00 | 0.00 | 0.06 | 0.00 | 0.00 | 0.01 | 1.47 |
| Hexanol | 0.1 b | 0.94 | 0.08 | 0.13 | 0.37 | 0.12 | 0.19 | 0.15 | 0.10 |
| cis-3-Hexenol | 0.03 c | 0.53 | 0.03 | 0.03 | 0.04 | 0.19 | 0.11 | 0.05 | 0.04 |
| trans-2-Hexenol | 1 b | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.02 |
| 1-Octanol | 0.11 e | 0.02 | 0.02 | 0.04 | 0.07 | 0.01 | 0.03 | 0.03 | 0.09 |
| Acetic acid | 100 b | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Butanoic acid | 1 b | 0.00 | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 | 0.00 | 0.04 |
| Hexanoic acid | 10 b | 0.00 | 0.00 | 0.01 | 0.11 | 0.00 | 0.00 | 0.02 | 0.34 |
| Heptylic acid | 0.64 c | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 |
| Octanoic acid | 0.91 c | 0.00 | 0.00 | 0.00 | 0.12 | 0.00 | 0.00 | 0.01 | 0.37 |
| Benzaldehyde | 0.35 e | 0.01 | 0.01 | 0.02 | 0.04 | 0.00 | 0.01 | 0.03 | 0.38 |
| Benzyl acetate | 0.75 e | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 |
| Methyl salicylate | 0.04 c | 0.03 | 0.01 | 0.02 | 0.09 | 0.01 | 0.01 | 0.05 | 0.26 |
| Benzyl alcohol | 0.62 c | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| trans-3-Hexenyl acetate | 0.016 c | 0.00 | 0.03 | 0.03 | 0.19 | 0.00 | 0.07 | 0.03 | 0.19 |
| trans-2-Hexenyl acetate | 0.21 c | 0.00 | 0.00 | 0.05 | 0.52 | 0.00 | 0.00 | 0.11 | 0.33 |
| Butyl butylate | 0.11 c | 0.00 | 0.00 | 0.00 | 0.05 | 0.00 | 0.00 | 0.00 | 0.14 |
| Hexyl butyrate | 0.25 ae | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Yu, S.; Zhang, Z.; Zhang, J.; Li, H. Comparative Characterization of Fruit Volatiles and Volatile-Related Genes Expression of ‘Benihoppe’ Strawberry and Its Somaclonal Mutant. Plants 2023, 12, 1109. https://doi.org/10.3390/plants12051109
Zhang Z, Yu S, Zhang Z, Zhang J, Li H. Comparative Characterization of Fruit Volatiles and Volatile-Related Genes Expression of ‘Benihoppe’ Strawberry and Its Somaclonal Mutant. Plants. 2023; 12(5):1109. https://doi.org/10.3390/plants12051109
Chicago/Turabian StyleZhang, Zhuo, Shuang Yu, Zhihong Zhang, Junxiang Zhang, and He Li. 2023. "Comparative Characterization of Fruit Volatiles and Volatile-Related Genes Expression of ‘Benihoppe’ Strawberry and Its Somaclonal Mutant" Plants 12, no. 5: 1109. https://doi.org/10.3390/plants12051109
APA StyleZhang, Z., Yu, S., Zhang, Z., Zhang, J., & Li, H. (2023). Comparative Characterization of Fruit Volatiles and Volatile-Related Genes Expression of ‘Benihoppe’ Strawberry and Its Somaclonal Mutant. Plants, 12(5), 1109. https://doi.org/10.3390/plants12051109





