Abstract
Melatonin (MT) plays a number of key roles in regulating plant growth and secondary metabolite accumulation. Prunella vulgaris is an important traditional Chinese herbal medicinal plant which is used for the treatment of lymph, goiter, and mastitis. However, the effect of MT on the yield and medicinal component content of P. vulgaris remains still unclear. In this research, we have examined the influence of different concentrations of MT (0, 50, 100, 200, 400 μM) on the physiological characteristics, secondary metabolite contents, and yield of P. vulgaris biomass. The results showed that 50–200 μM MT treatment had a positive effect on P. vulgaris. MT treatment at 100 μM greatly increased the activities of superoxide dismutase and peroxidase, the contents of soluble sugar and proline, and obviously decreased the relative electrical conductivity, the contents of malondialdehyde and hydrogen peroxide of leaves. Furthermore, it markedly promoted the growth and development of the root system, increased the content of photosynthetic pigments, improved the performance of photosystems I and II and the coordination of both photosystems, and enhanced the photosynthetic capacity of P. vulgaris. In addition, it significantly increased the dry mass of whole plant and spica and promoted the accumulation of total flavonoids, total phenolics, caffeic acid, ferulic acid, rosmarinic acid, and hyperoside in the spica of P. vulgaris. These findings demonstrated that the application of MT could effectively activate the antioxidant defense system of P. vulgaris, protect the photosynthetic apparatus from photooxidation damage, and improve the photosynthetic capacity and the root absorption capacity, thereby promoting the yield and accumulation of secondary metabolites in P. vulgaris.
1. Introduction
Prunella vulgaris is a perennial herb belonging to the botanical family Labiatae and is widely distributed in temperate and tropical regions such as China, Japan, Korea, Pakistan, Germany, and the United States of America for its diversity of applications [1]. In the south of China, flowers and fresh leaves of this species can be used as ornamental and vegetables, respectively [2]. Importantly, the dried spicas of P. vulgaris, Prunellae Spica, are commonly used as standard herbs for mastitis, pulmonary tuberculosis, goiter, and tuberculosis, and an important raw material for herbal tea. Besides, it is considered as an important raw material for herbal tea, as well as the whole plant can be used as medicine in Europe, Taiwan, and China [3]. In the recent years, it has been observed that there is a growing requirement of raw materials for the pharmaceutical and herbal tea industries and for the production of Chinese patent medicines alone, which would need about 5000 tons of Prunellae Spica every year [4]. Therefore, improving the yield and quality of P. vulgaris to meet the strong demand for uniform and high-quality raw materials has become an important issue to be solved urgently.
Research has shown that P. vulgaris contains a variety of secondary metabolites, rich in phenolic acids and flavonoids such as caffeic acid, ferulic acid, rosmarinic acid, hyperoside, etc. [4]. These phenolic acids and flavonoids are products of secondary metabolic processes and are important medicinal components in P. vulgaris, having antioxidant, anti-inflammatory, antibacterial, antiviral, anti-leukemia, and anticancer activities, as well as neuroprotective and other biological activities; they also play a crucial role in preventing oxidative damage caused by various abiotic stresses [5]. Previous studies have shown that plants could counteract the toxicity of reactive oxygen species (ROS) by activating enzymatic and non-enzymatic systems [6]. ROS can be partially scavenged by antioxidant enzymes such as superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and ascorbate peroxidase (APX) [7]. Meanwhile, non-enzymatic antioxidants including phenols, flavonoids, polysaccharides, carotenoids, α-tocopherols, and other compounds can work in concert with antioxidant enzymes to remove ROS.
It is well documented that the exogenous application of phytohormones can regulate crop growth and development, increase yield, and improve quality [8,9]. Melatonin (N-acetyl-5-methoxytryptamine) is an indoleamine hormone, a pleiotropic biomolecule, and a broad-spectrum antioxidant which is widely found in animals and plants [10]. Studies have shown that MT acts as the first line of defense against oxidative stress in plants, which in plant cells can efficiently remove reactive oxygen (ROS) or reactive nitrogen species (RNS), which are both signal molecules and toxic substances to plant cells [11]. Furthermore, MT not only directly detoxifies ROS and RNS as antioxidants, but also indirectly promotes the activities of the main antioxidative enzymes and inhibits the activity of pro-oxidant enzymes, as well as the content of some non-enzymatic antioxidants such as phenolic compounds [12,13]. A set of studies showed that MT can increase antioxidant enzyme activities to remove ROS and RNS, and protect plants from oxidative damage caused by various abiotic stresses such as drought [14], salt stress [15], chromium toxicity, and so on [16]. The application of MT increased stress enzyme activities and biomass accumulation during callogenesis in P. vulgaris [17]. It also increased the phenolic compounds and antioxidant activities in two Citrus cultivars under drought stress [18]. Other studies have shown that MT can regulate root growth by regulating auxin (IAA) synthesis, transport, and signal transduction in an IAA-dependent manner [19,20]. MT can also be involved in the regulation of Arabidopsis root growth by sensing N-acylserine lactones secreted by rhizosphere bacteria through melatonin receptors (CAND2/PMTR1) [21,22,23,24]. In cotton (Gossypium spp.) and cucumber (Cucumis sativus), exogenous MT could activate the functions of root-related hormones and transcription factor pathways, maintain root cell integrity, increase root vigor, and induce lateral root formation [25,26]. The exogenous application of MT increased the chlorophyll content and photosynthetic capacity in plants [17,27], and enhanced the content of non-structural carbohydrates and nitrogen by upregulating sucrose transporters and nitrogen uptake-related genes, promoting its transport to grains, thereby increasing rice yield under low nitrogen conditions [9]. Under adverse weather conditions, MT treatment improved commercial crop yield and quality traits of sweet cherries [28].
Photosynthesis involves two reaction steps, converting light energy into chemical energy (light reaction) and then converting CO2 into organic metabolites (dark reaction), which is the basis of crop yield [29]. The photochemical reaction is the engine of photosynthesis, which depends on the coordination of photosynthetic pigments, the photosynthetic electron transport chain, and two photosystems (PSI and PSII) [30]. Recent studies have shown that MT plays a positive role in protecting photosynthetic apparatus from damage under various stress conditions [31]. Yin et al. [32] found that exogenous MT reduced the accumulation of ROS by balancing the distribution of photosynthetic electron flux, facilitated the repair of PSII, and improved the electron transfer ability of PSII donor and acceptor sides in tomato plants under salt stress. Wu et al. [33] found that MT (100 μM) enhanced the electron transport capacity between PSII and PSI, and increased the oxygen-evolving complex activity and PSII activity under low temperature stress. In maize seedlings, foliar application of exogenous melatonin improved the photosynthetic electron transfer efficiency between both photosystems and alleviated the damage of the quantum yield of PSI and PSII under drought stress conditions [14]. However, the role of MT on the PSI and PSII activities of P. vulgaris under stress or non-stress conditions remains unclear. Therefore, it is necessary to study the potential role of MT in improving the photosynthetic apparatus.
The identification of MT receptors suggests that MT is a hormone involved in regulating plant hormone crosstalk [11]. MT has been reported to modulate the biosynthesis and signaling of numerous plant hormones, including auxin, gibberellin (GA), salicylic acid (SA), jasmonic acid (JA), ethylene, strigolactones, brassinosteroids, and abscisic acid (ABA) [11,34,35,36]. Especially, MT plays a positive role in the synthesis of secondary metabolites in plants under stress or non-stress conditions [18]. Xu et al. [12] found that MT enhanced the polyphenol content in grape berries, and Coskun et al. [8] found that MT greatly increased the content of secondary metabolites such as rosmarinic acid and caffeic acid in a callus culture of rosemary (Rosmarinus officinalis). Bistgani et al. [37] found that exogenous MT promoted the accumulation of total phenolic compounds in the leaves of garden thyme (Thymus daenensis) to respond to salinity stress.
Many reports have shown that MT can promote rhizogenesis, seedling growth, antioxidant ability, photosynthesis, secondary metabolite accumulation, yield, and biomass production, etc. in plants [8,37,38]. However, the effects of its exogenous application on the accumulation of dry matter and secondary metabolites in medicinal plants such as P. vulgaris have rarely been studied, and little information is available on its effects on photosynthesis by combining PSII and PSI. Therefore, it is necessary to study the effects of MT on antioxidant enzymes, osmotic adjustment substances, root growth, photosynthetic pigments, gas exchange parameters, structure and function of PSII and PSI, yield, and secondary metabolite contents of P. vulgaris. This study will provide a reference to unraveling the response of MT on the growth and development in P. vulgaris, and offer theoretical evidence for improving the yield and quality in practice.
2. Results
2.1. Antioxidant Enzyme Activity and Osmoregulation Substance Content
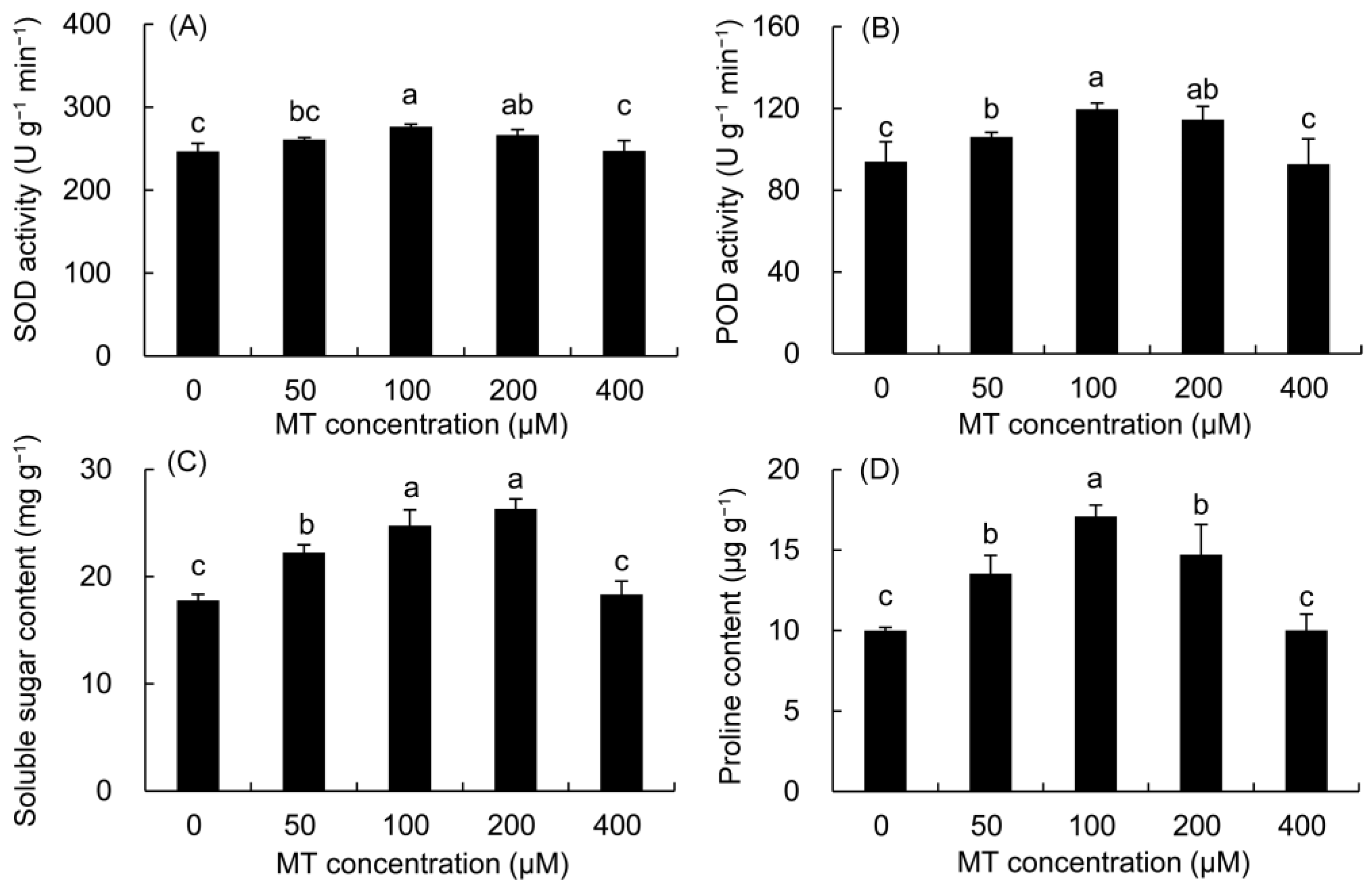
The SOD and POD enzyme activities first increased, but then decreased with the increase in MT concentration (Figure 1). In the 100 μM MT treatment, their activities drastically elevated by 12.08 and 27.35% compared with the control, respectively, reaching the highest value in all MT treatments, followed by the 200 μM treatment.
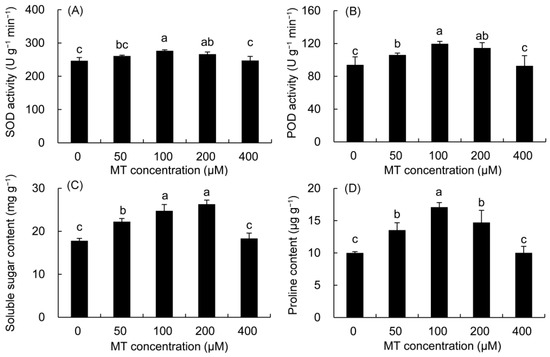
Figure 1.
Effects of different concentrations of MT on the activities of SOD (A) and POD (B) and the contents of soluble sugar (C) and proline (D) in P. vulgaris. MT: melatonin. Data are means ± standard error; different letters indicate significant differences as determined by the Duncan test (p ≤ 0.05).
The contents of soluble sugar and proline were higher in all MT treatments but significantly within the 50–200 μM MT treatments (Figure 1). The soluble sugar content increased by 39.18 and 47.81%, in the 100 and 200 μM MT treatments, respectively compared with the control. The proline contents of the 100 μM and 200 μM MT treatments were 70.88 and 47.16% higher than that in the control, respectively.
2.2. REC and the Contents of MDA and H2O2
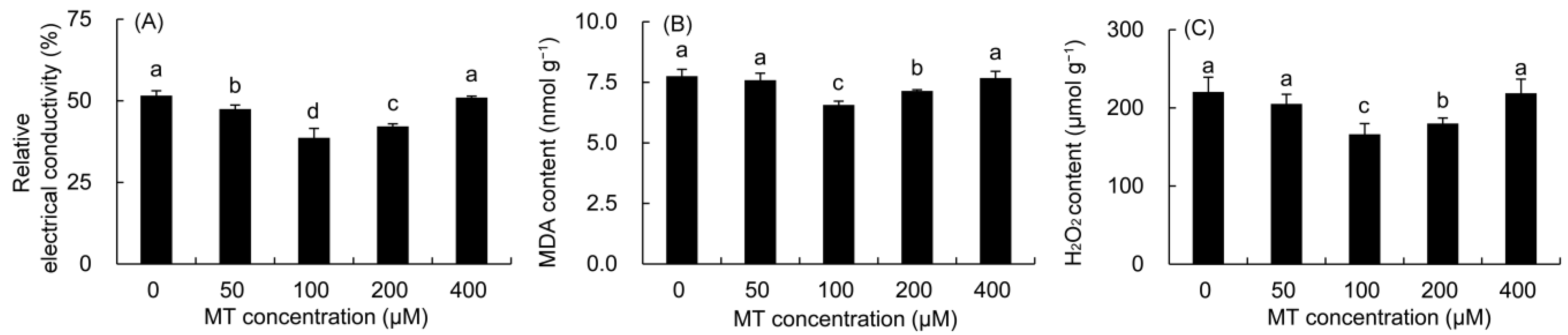
The REC, MDA, and H2O2 contents were first decreased and then elevated with the increase in MT concentration (Figure 2). The REC was greatly lower in the 50–200 μM MT treatments compared with the control, and the minimum value was recorded in the plants sprayed with 100 μM MT, which was 25.14% lower than that in the control. The MDA and H2O2 contents decreased markedly in the 100–200 μM MT treatment. Among these, the contents of MDA and H2O2 decreased the most after the 100 μM MT treatment, which decreased by 15.30 and 24.57% compared to the control, respectively.
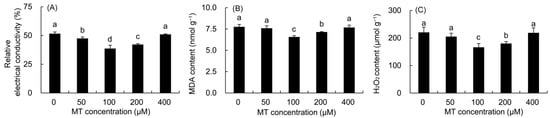
Figure 2.
Effects of different concentrations of MT on REC (A) and the contents of MDA (B) and H2O2 (C) in P. vulgaris. MT: melatonin. Data are means ± standard error; different letters indicate significant differences as determined by the Duncan test (p ≤ 0.05).
2.3. The Contents of Chlorophyll and Carotenoid
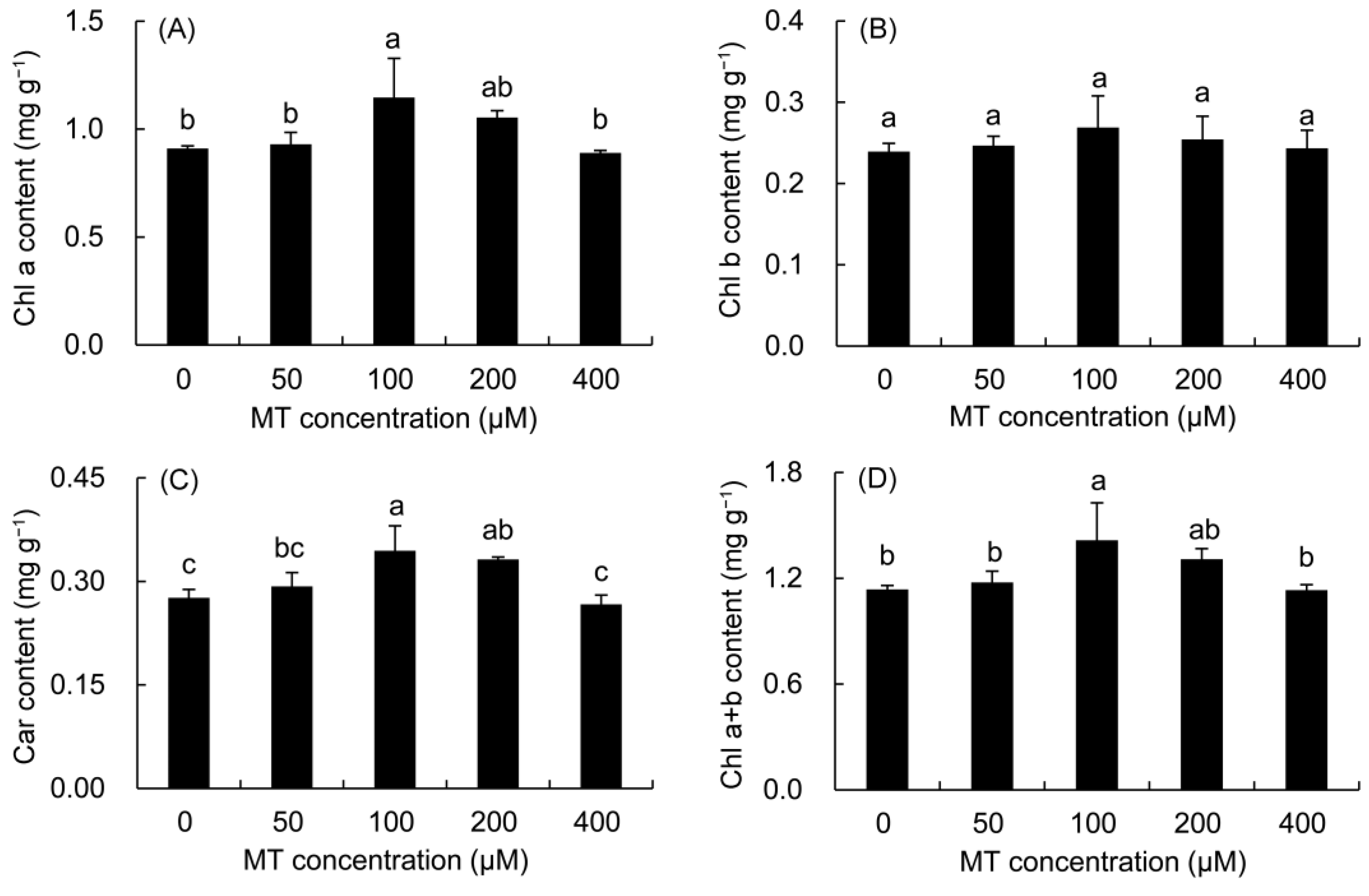
The contents of Chl a, Chl b, Car, and Chl a + b in P. vulgaris showed a trend of first increasing and then decreasing with the increase in MT concentration (Figure 3). The increase in Chl a and Chl a + b contents was the highest in the 100 μM treatment, which was elevated by 23.16% and 21.92%, respectively, compared with those in the control treatment. The Chl b content was higher in all MT treatments than in the control treatment, but did not reach a significant level. All MT treatments augmented the Car content of P. vulgaris leaves. The 100 and 200 μM MT treatments predominantly enhanced the Car content by 24.74 and 20.18% compared with the control treatment, respectively.
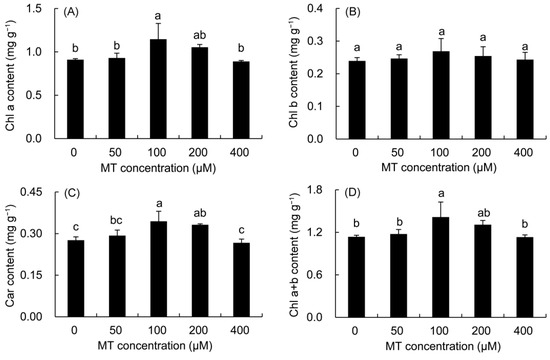
Figure 3.
Effects of different concentrations of MT on photosynthetic pigment content of Chl a (A) and Chl b (B) and Car (C) and Chl a + b (D) in P. vulgaris. MT: melatonin. Data are means ± standard error; different letters indicate significant differences as determined by the Duncan test (p ≤ 0.05).
2.4. Gas Exchange Parameters
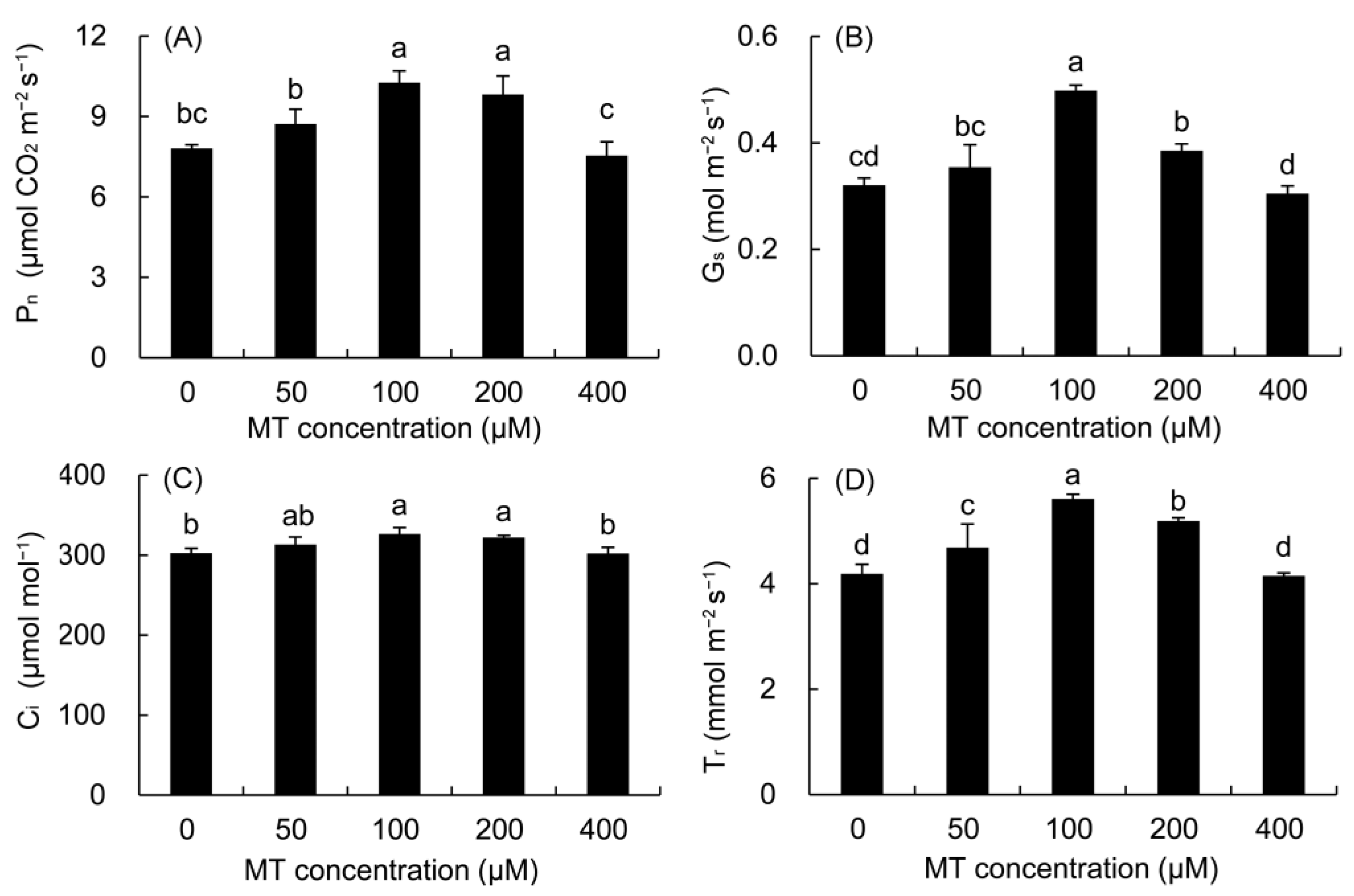
Compared with the control, the Pn was higher in all MT treatments except for the 400 μM MT treatment (Figure 4). The maximum was noted in the 100 μM of MT (31.33%), followed by the 200μM MT treatment. Compared with the control, the Gs, Ci, and Tr of P. vulgaris were higher in the 50–200 μM MT treatments and reached significant levels in the 100 and 200 μM MT treatments.
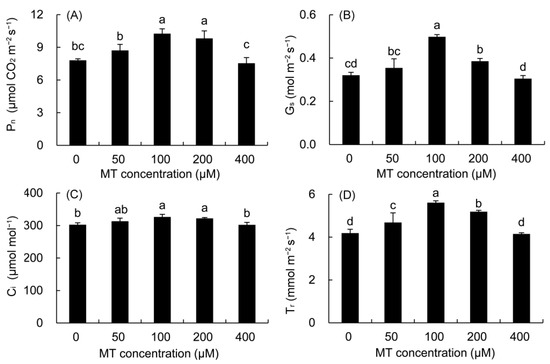
Figure 4.
Effects of different concentrations of MT on Pn (A), Gs (B), Ci (C) and Tr (D) of P. vulgaris. MT: melatonin. Data are means ± standard error; different letters indicate significant differences as determined by the Duncan test (p ≤ 0.05).
2.5. Root Architecture
The root length, total root surface area, and total root volume were higher in the 50–200 μM MT treatments compared with the control (Table 1), and the increase in the 100–200 μM MT treatments all reached a significant level. The root tip number and branch number of P. vulgaris was markedly enhanced in the 50–200 μM MT treatments, and the highest value was found in the 100 μM MT treatment. The root tip and branch numbers were considerably lower in the 400 μM MT treatment than in the control treatment.

Table 1.
Changes in root architecture of P. vulgaris under different MT treatments.
2.6. Chlorophyll Fluorescence OJIP Curve and 820 nm Modulated Reflection
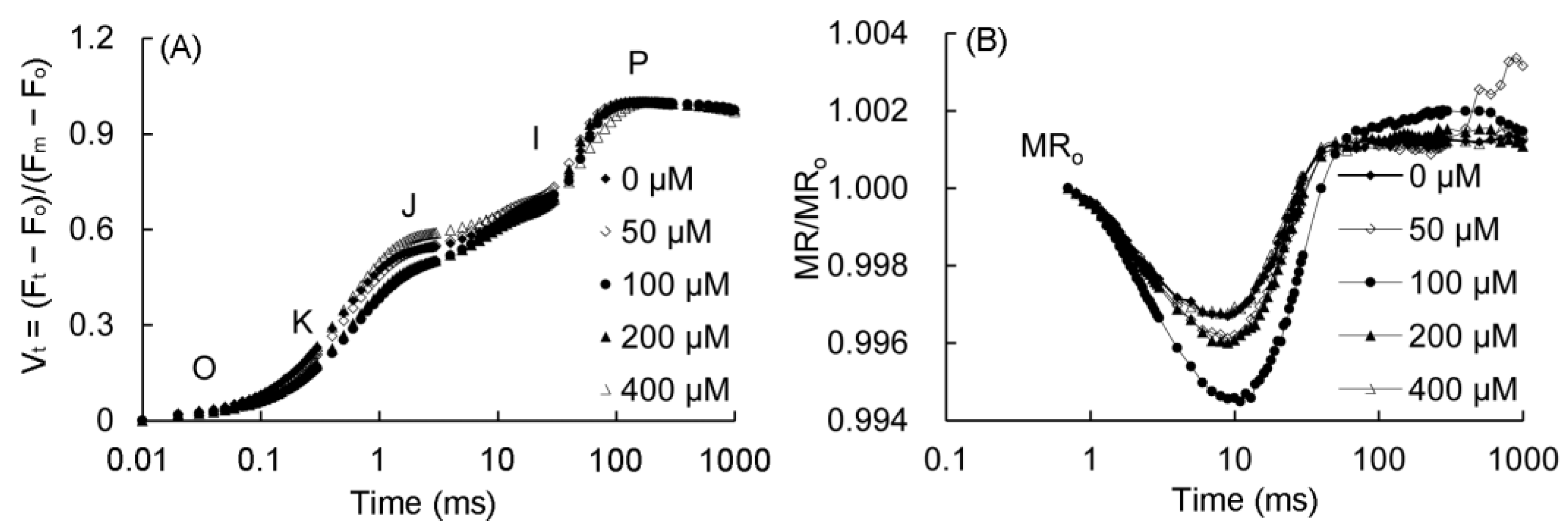
The fast fluorescence OJIP curves and 820 nm modulated reflection curves of P. vulgaris are shown in Figure 5. As can be seen from Figure 5A, the K point (t = 0.3 ms) and J point (t = 2 ms) decreased in the 50–200 μM MT treatment, and the changes were the most obvious in the 100–200 μM treatments. The MR/MR0 of the 820 nm reflection curve of P. vulgaris began to decrease from 0.7 ms (Figure 5B), and reached to the lowest value after about 10 ms, and increased slowly after 200 ms. The lowest point of the MR/MR0 in the 50–200 μM MT treatment decreased, of which the 100 μM group decreased the most, followed by the 200 and 50 μM MT treatments, while the MR/MR0 value in the 400 μM treatment had little change.
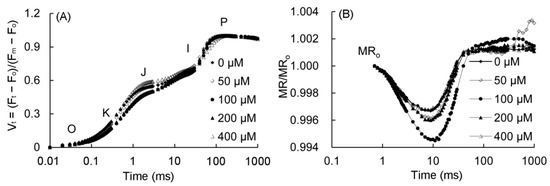
Figure 5.
Fast chlorophyll fluorescence (A) and 820 nm modulated reflection curve (B) of P. vulgaris under different MT treatments. Vt = (Ft − F0)/(Fm − F0): the relative variable fluorescence (Vt) at any time; O, K, J, I, and P represent different phases in the OKJIP curve. MR/MR0: the 820-nm modulated reflection curve; MR is the modulated reflection at different time points; MR0 is the MR value of far-red light irradiated at 0.7 ms. MT: melatonin. Data are means ± standard error; different letters indicate significant differences as determined by the Duncan test (p ≤ 0.05).
2.7. Fast Chlorophyll Fluorescence Parameters of P. vulgaris
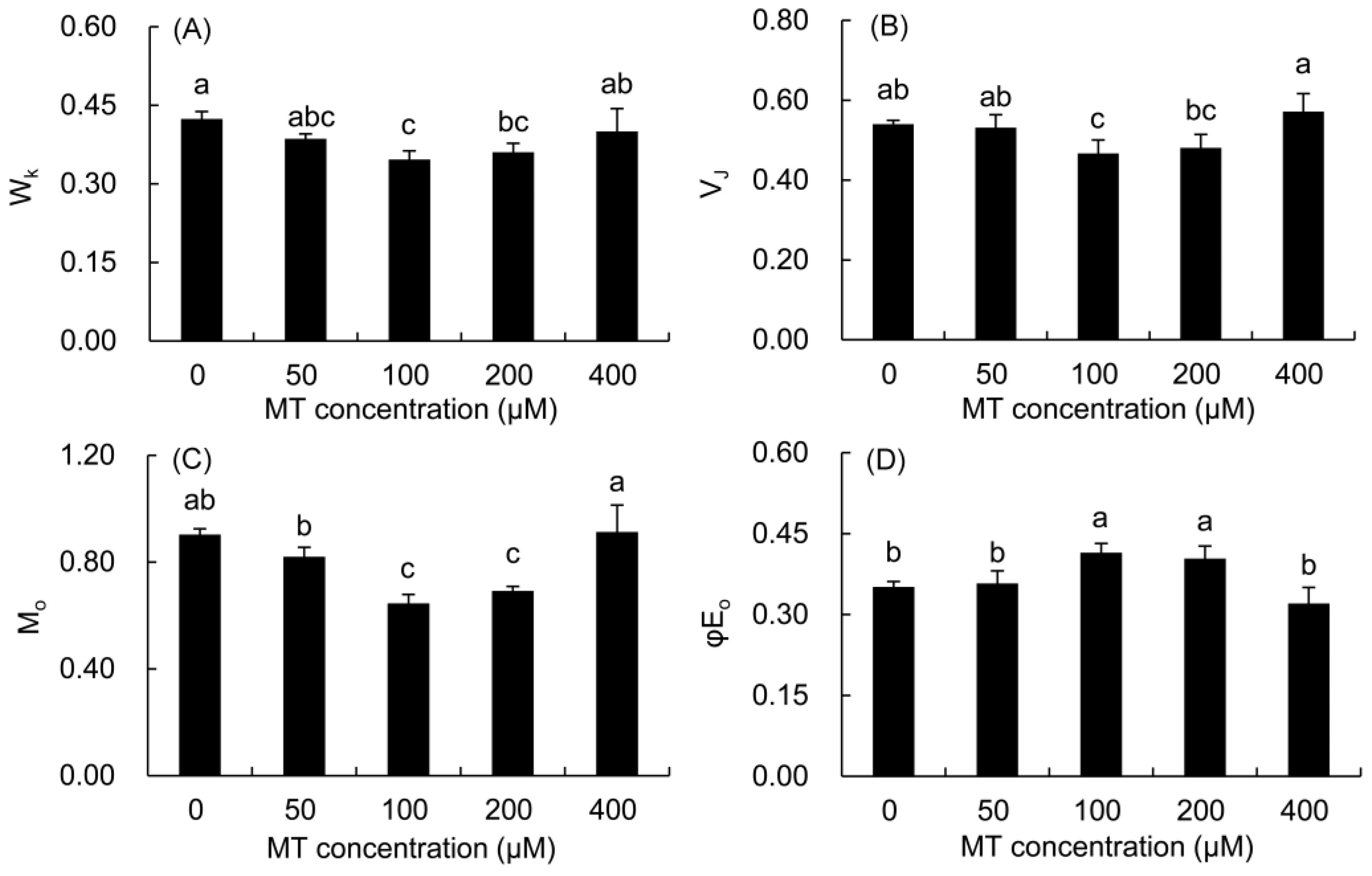
The normalized fluorescence (WK) of the K phase and the relative fluorescence change of the J phase (VJ) in the MT treatment were calculated to quantify the changes in the K and J phases in the fast chlorophyll fluorescence curve (Figure 6). The values of WK, VJ, and M0 first decreased and then elevated with the increase in the MT concentration. The values of the three parameters were significantly lower in the 100 and 200 μM MT groups compared with the control treatment. The value of φE0 first increased and then decreased with the increase in the MT concentration. Only the value of φE0 was markedly higher in the 100 and 200 μM MT treatments compared with the control treatment.
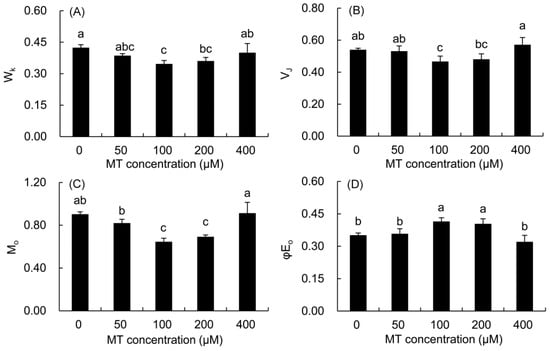
Figure 6.
Effects of different concentrations of MT on WK (A), VJ (B), Mo (C) and φE0 (D) of P. vulgaris. MT: melatonin. Data are means ± standard error; different letters indicate significant differences as determined by the Duncan test (p ≤ 0.05).
The ABS/RC, DI0/RC, and TR0/RC parameters initially decreased and then increased with the increase in the MT concentration (Table 2). Except for the 50 and 400 μM MT treatments, the three parameters were predominantly lower in the 100 and 200 μM MT treatments compared with the control, and decreased the most in the 100 μM MT treatment. Compared with control treatments, the ET0/RC of all MT treatments did not reach a significant level. The four parameters RC/CSm, ABS/CSm, TR0/CSm, and ET0/CSm initially increased and then decreased with the increase in the MT concentration. The above four parameters were all drastically higher in the 100–200 μM MT treatments compared with the control treatment, and the increase was the highest in the 100 μM MT treatment. The DI0/CSm in all MT treatments did not reach a significant level compared with the control.

Table 2.
The changes in energy fluxes in P. vulgaris leaves under different MT treatments (μM).
2.8. The Function and Coordination of PSII and PSI
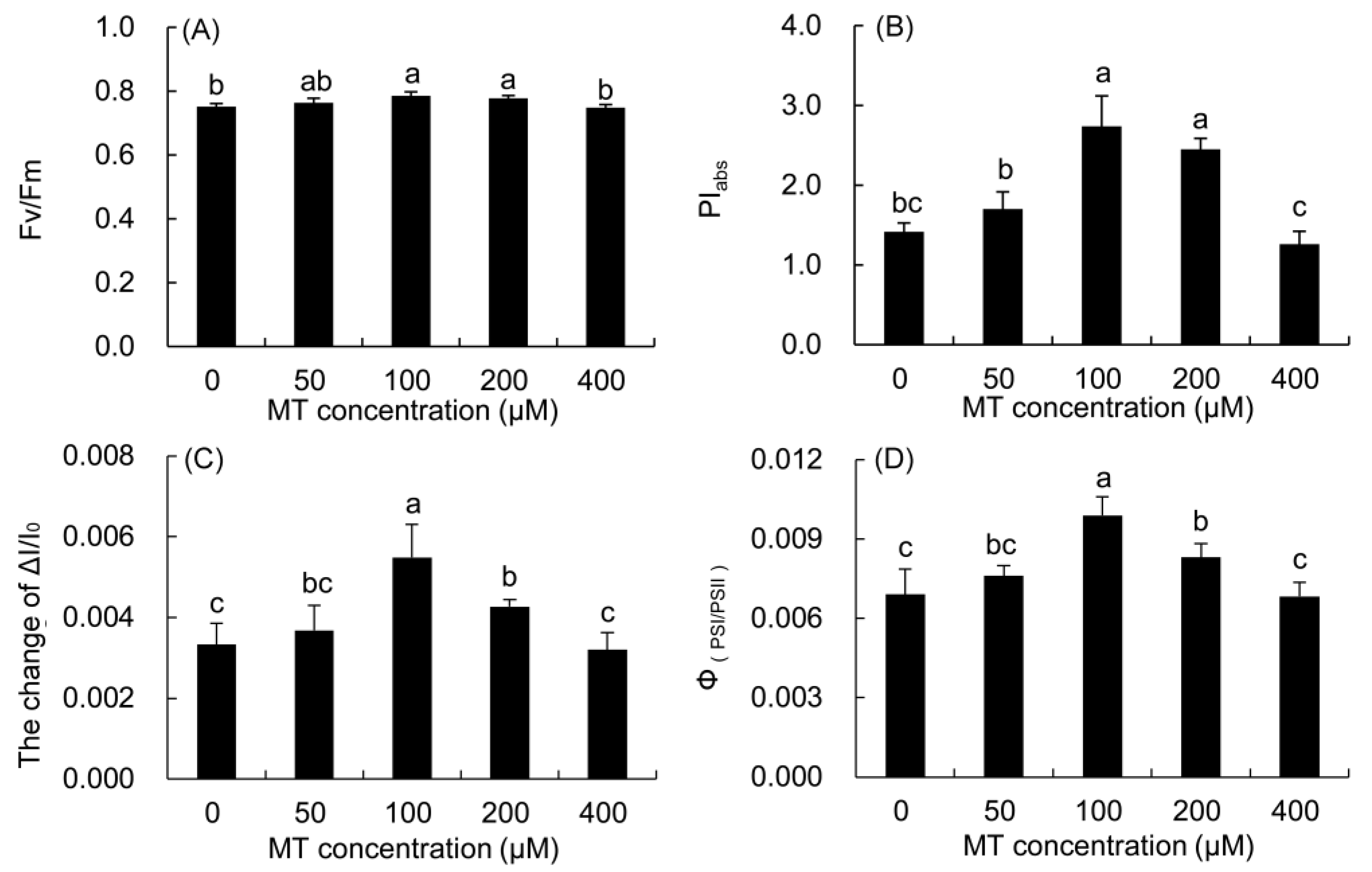
The Fv/Fm and PIabs of P. vulgaris leaves treated with 100 and 200 μM MT were considerably higher than those in the control (Figure 7), and the increase in the 100 μM treatment was the highest. These two parameters were higher in the 50 μM MT treatment, but lower in the 400 μM MT treatment than the control.
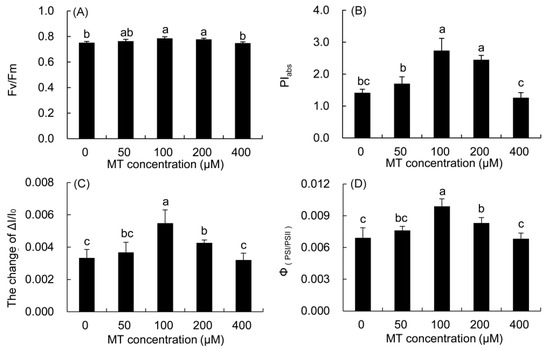
Figure 7.
Effects of different concentrations of MT on the function and coordination of PSII and PSI of P. vulgaris. Fv/Fm, (A); PIabs, (B); ΔI/I0, (C) and ΦPSI/PSII, (D); MT: melatonin. Data are means ± standard error; different letters indicate significant differences as determined by the Duncan test (p ≤ 0.05).
The maximum redox activity (ΔI/I0) of PSI and the coordination of PSII and PSI (ΦPSI/PSII) in the 50–200 μM MT treatments increased, but decreased in the 400 μM MT treatment compared with the control. The two parameters were markedly higher in the 100 μM MT treatment than those in the control, and the increase was the largest.
2.9. Dry Mass of Whole Plant and Spica
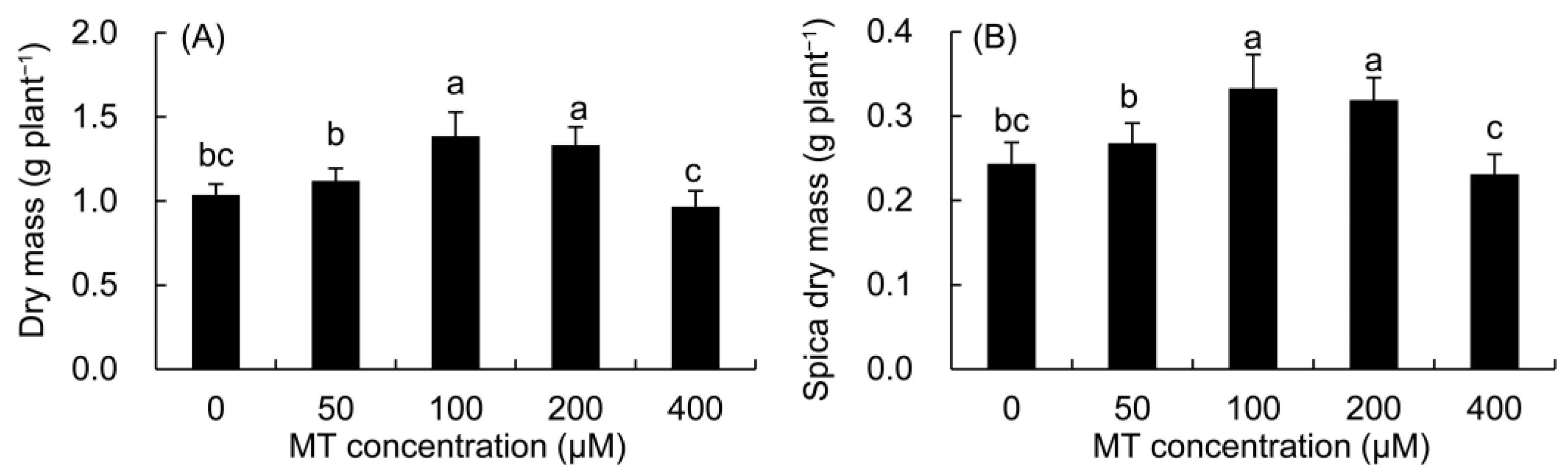
Compared with the control (Figure 8), the dry mass of the whole plant and spica of P. vulgaris increased in the 50–200 μM MT treatments, but decreased in the 400 μM MT treatment, and the maximum value was found in the 100 μM MT treatment.
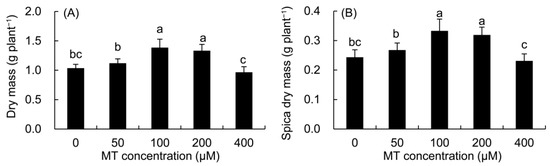
Figure 8.
Effects of different concentrations of MT on the dry mass of whole plant (A) and spica (B) of P. vulgaris. MT: melatonin. Data are means ± standard error; different letters indicate significant differences as determined by the Duncan test (p ≤ 0.05).
2.10. Secondary Metabolite Contents
The contents of total flavonoids and phenolics were drastically higher in all MT treatments compared with the control treatment (Table 3). The contents of total flavonoids and phenolics increased the most in the 100 μM MT treatment. The contents of caffeic acid, ferulic acid, rosmarinic acid, and hyperoside were all higher after the 50–200 μM MT treatments, with the largest increase in the 100 μM MT treatment.

Table 3.
Effects of different concentrations of MT on the contents of total flavonoids, total phenolics, caffeic acid, ferulic acid, rosmarinic acid, and hyperoside in P. vulgaris (mg/g).
3. Discussion
ROS are considered toxic by-products in aerobic metabolism, often produced during chlorophyll biosynthesis and the electron transfer reaction of photosynthesis [39,40]. The SOD enzyme can convert O2·− into H2O2 and O2, and the POD enzyme catalyzes H2O2 to H2O and O2 [41]. In the present study, pretreatment with MT (100 μM) markedly reduced ROS levels in P. vulgaris leaves, which may be due to the fact that MT itself is an antioxidant compound stronger than ascorbic acid and hence might directly react with ROS to stop lipid oxidation. On the other hand, MT treatments improved the activities of SOD and POD, which contributed to the further scavenging of ROS [42,43]; this was also confirmed by the significant decrease in the REC, MDA, and H2O2 content in P. vulgaris. These results were in agreement with previous studies on Brassica juncea [44], Limonium bicolor [45], and Momordica charantia [46] treated with MT. Studies have shown that plants can co-accumulate biochemical solutes such as soluble sugars and proline to regulate the osmotic potential of cells and stabilize the structure of biomolecules to adapt to oxidative stress [4,31]. In particular, soluble sugars and prolines are also involved in the detoxification of ROS in different organelles against abiotic stress [16,47]. In this study, MT treatments increased the content of soluble sugar, soluble protein and proline in P. vulgaris, which helped to maintain the osmotic balance in cells, and reduced the damage to the plasma membrane and the content of ROS in coordination with antioxidant enzymes. Similar results were also found in maize (Zea mays) [15] and sugar beet (Beta vulgaris) [48].
Compared with the control treatment, MT treatments elevated the chlorophyll content in P. vulgaris leaves. In B. juncea [44], Camellia sinensis [49], and Triticum aestivum, the application of MT enhanced the chlorophyll content [50], and these results supported our findings. The increase in chlorophyll by MT may be achieved by up-regulating chlorophyll biosynthesis genes to promote the accumulation of chlorophyll content, and by inhibiting the expression of chlorophyll degradation enzymes to delay the degradation of chlorophyll [49,51]. Moreover, carotenoids can not only absorb and transform light energy, but also quench ROS to reduce the intensity of oxidative stress and protect chlorophyll from photooxidation damage [52,53]. So, the increase in the Car content in P. vulgaris might be one of the reasons for the higher chlorophyll content after MT treatment.
MT application (100 μM) greatly promoted the increase in the stomatal opening alone with Gs, resulting in more CO2 production in intercellular spaces, and thus improving CO2 assimilation efficiency [44]. In this study, Pn, Gs, Ci, and Tr all increased after MT treatments, indicating that MT could improve stomatal function and increase the accumulation of intracellular CO2, thus increasing the photosynthetic capacity of P. vulgaris leaves [45]. The application of MT in B. juncea [44] and C. sinensis [49] also produced similar results. Water use efficiency is closely related to the short-term regulation of stomatal aperture [54]. In transgenic apples, the overexpression of MdASMT9 increased MT biosynthesis, and improved water use efficiency by increasing the photosynthetic rate and stomatal opening [55], which was also observed in our study (data not shown).
The characteristics of root architecture and physiology are closely related to the absorption of fertilizer and water by plants. The root length reflects the extension range of the root system, the vertical extension of the root system is conducive to the absorption of water and nutrients in the deep soil by plants, and the total root volume reflects the robustness of the plant anchoring [56]. The root surface area reflects the contact area between plants and soil, which is closely related to the absorption of water and nutrients by roots, and the number of root tips and branches reflects the absorption capacity of roots [57]. In this study, exogenous applications of MT obviously improved the root architecture of P. vulgaris, which would help to promote the absorption of water and nutrients by roots, and promoted the synthesis of carbohydrates and the accumulation of dry matter [58]. Studies showed that genes and networks related to the IAA pathway were regulated by MT at the transcription and post-transcription level [19,20], and a MT receptor (CAND2/PMTR1) was proved to be involved in the regulation of root growth by bacterial N-acyl-homoserine lactones [21,22,23,24]. These studies suggested that MT could regulate multiple signal pathways to regulate the root growth of P. vulgaris. In this study, the low concentrations of 50–200 μM MT promoted root growth, while the high concentration of 400 μm MT inhibited the root growth of P. vulgaris. Similar results were also found in B. juncea [59] and Cyamopsis tetragonoloba [60], indicating that MT affects root growth and development in a dose-dependent manner.
The relative variable fluorescence (WK) at the K point reflects the inhibition of the oxygen-evolving complex (OEC) on the donor side of the PSII reaction center. The increase in variable fluorescence at the J point reflects the electron blockage between the quinone acceptor QA and QB. M0 refers to the rate at which the primary quinone acceptor (QA) is reduced and reflects the maximum rate of closure of the PSII reaction center, and φE0 reflects the quantum of light energy absorbed by the reaction center for electron transfer production [61]. The study showed that MT application (100 μM) decreased the variable fluorescence at the K point (WK) and J point (VJ) in the OJIP curve, and M0 decreased while φE0 augmented. These indicated that the application of MT improved the activity of OEC, reduced the maximum closing rate of the PSII reaction center, and elevated the electron transfer capacity of the PSII donor side and receptor side in P. vulgaris [14]. The blockage of electron transport resulted in electron leakage during electron transfer, which would attack O2 to generate ROS and cause membrane lipid peroxidation [62]. Conversely, the improvement of the electron transport capacity of PSII after 100 μM MT treatment inevitably reduced the levels of ROS and membrane lipid peroxidation in P. vulgaris leaves and protected the stability of the cell membrane.
In this study, the energy absorption (ABS/CSm), capture (TR0/CSm), electron transfer (ET0/CSm), and heat dissipation (DI0/CSm) per cross section in the 100 μM MT treatment were all greatly higher than those in the control treatment. Except for DI0/CSm, which was slightly higher in the 100 μM MT treatment than in the control, the other three parameters in the treated leaves were much higher than those of the control. However, the absorption (ABS/RC), capture (TR0/RC), electron transfer (ET0/RC), and heat dissipation (DI0/RC) per reaction center in the 100 μM MT treatment were all decreased compared to those in the control treatment. Furthermore, the 100 μM MT treatment significantly elevated the number of active reaction centers per cross section (RC/CSm) of PSII. These showed that the 100 μM MT treatment could greatly decrease the energy charge pressure of PSII reaction to reduce the occurrence of photoinhibition, and improve the energy conversion and utilization efficiency by increasing the RC/CSm of PSII [63,64].
Fv/Fm refers to the maximum photochemical efficiency of PSII; PIabs represents the performance index on absorption basis, and includes three aspects of light energy absorption, capture, and electron transfer, which can comprehensively reflect the performance of PSII [65]. In this study, the parameters of RC/CSm, Fv/Fm and PIabs by 100 μM MT were enhanced in P. vulgaris. Similar results were also found in Vigna angularis [66] and Chara australis [64] under the appropriate MT treatments. The increase in Fv/Fm and PIabs indicated that 100 μM MT could provide maximum light energy for the photosynthetic electron transport chain, and increased the maximum photochemical efficiency of PSII and the photosynthetic activity of PSII in P. vulgaris, which was also confirmed by the increase in the ABS/CSm, Tr0/CSm, ET0/CSm, and Pn of leaves after the 100 μM MT treatment. P. vulgaris had higher root absorption and photosynthetic capacities under the suitable MT treatments, which explained the higher dry mass of the whole plant and spica under these treatments.
The 820-nm modulated reflectance curve represents the redox activity of PSI; the lower the minimum MR/MR0, the stronger the ability of the PSI reaction center to reduce the terminal electron acceptor [63]. The variable ΔI/I0 reflects the maximum redox capacity of the PSI reaction center P700 and is used to comprehensively evaluate the performance of PSI; ΦPSI/PSII (ΔI/I0/ψ0) is used to characterize the coordination between PSII and PSI [67]. The minimum value of MR/MR0 greatly decreased, and the values of ΔI/I0 and ΦPSI/PSII markedly enhanced after 100 μM MT treatment. These indicated that 100 μM MT greatly increased the activity of the PSI reaction center, elevated the mobility of electrons from PSII to PSI, and improved the coordination between both the photosystems.
Many studies have shown that flavonoids and phenolic components are secondary metabolites with antioxidant capabilities in plants and are also important components of non-enzymatic antioxidants in plants, which are another line of defense in the scavenging process of ROS [4,12,68]. In the present study, 100 μM MT significantly enhanced the antioxidant capacity via promoting the accumulation of total flavonoids and phenolic components in P. vulgaris. Caffeic acid is a naturally occurring hydroxycinnamic acid phenol in many plants, such as coffee and tea, and has been proven to have antioxidant, anti-inflammatory, cardiovascular protective, and hypoglycemic activities [69]. Ferulic acid is a phenolic acid with antithrombotic, anti-inflammatory, anticancer, and antioxidant activities [70]. As an important phenolic component, rosmarinic acid is used as a standard to measure the quality of P. vulgaris in Chinese Pharmacopoeia; it also has good scavenging ability against ROS [4]. Hyperoside is also a ROS scavenger; it has been shown to increase the activity of glutathione peroxidase and inhibit the H2O2-induced apoptosis of Chinese hamster lung fibroblasts [4]. Therefore, the increase in the above four compounds might contribute to improving the antioxidant capacity of P. vulgaris. In this study, the increase in the contents of total flavonoids, total phenolics, caffeic acid, ferulic acid, and rosmarinic acid might be due to the signal function of MT, which stimulates the biosynthesis of secondary metabolites by inducing various physiological and metabolic pathways of plant hormones [18]. Previous studies have also shown that MT can induce the expression of flavonoid biosynthesis genes and related transcription factors [68], promote the upregulation of genes related to phenolic acid synthesis such as PAL, STS, C4H, and CHS [12], and enhance the expression of genes related to rosmarinic acid synthesis such as PAL and RAS genes [71]. In line with these results obtained in the present investigation, the application of MT has also been shown to increase the contents of phenolic compounds, caffeic acid, and rosmarinic acid in the callus of R. officinalis [8], and the contents of phenolics and rosmarinic acid in the callus of O. basilicum [72].
4. Materials and Methods
4.1. Plant Materials
The seeds of P. vulgaris were disinfected with 2% sodium hypochlorite, washed with clean water three times, and then planted in the experimental field in October 2021. In April 2022, seedlings of the same growth were transplanted into pots (26 cm × 22 cm, height × diameter) filled with soil and peat soil (v/v = 3:1), and four seedlings were planted in each pot. The seedlings were cultured in a growth room with a light intensity of 900 μmol·m−2·s−1, photoperiod of 12 h, temperature of 26 /20 °C (day/night), relative humidity of 70% ± 5%, under normal water management.
4.2. Experimental Design
Pot culture was carried out to see the effect of exogenous MT on P. vulgaris. MT was first dissolved in ethanol and then diluted to five concentrations such as 0 (CK), 50, 100, 200, and 400 μM, respectively, where CK was the control containing no MT but deionized water. For each treatment level of MT concentration, 10 replicate pots were used. So, a total of 50 pots were prepared with P. vulgaris for the experiment. Besides MT, a 0.2% KH2PO4 solution was also sprayed. In May 2022, the treatment was started at the flowering stage of P. vulgaris and continued for six consecutive days. Starting with the first day of the experiment, the respective concentration of MT was sprayed to each of the four groups of treatment plants and continued for each one-day interval. The plants of the control group were always sprayed with the same amount of deionized water. While starting from the second day, the experimental plants from all experimental groups received spraying with the 0.2% KH2PO4 solution and continued at each one-day interval for foliar fertilization of all treatment groups. So, the experimental plants had received an alternating MT or 0.2% KH2PO4 solution for a total of three days, respectively, out of a six-day treatment duration. Foliar spraying with MT and 0.2% KH2PO4 solution was carried out around 6:00 P.M. on each occasion, subject to the leaves being covered with droplets. After the treatment was complete, the experimental plants were allowed to grow for 2 weeks. Thereafter, photosynthetic and fluorescence indexes were determined, and the biochemical indexes of P. vulgaris were measured. P. vulgaris seedlings were harvested in late June, and dried to a constant weight in an oven at 50 °C to determine the dry mass. The dried spicas were powdered and passed through a 60-mesh sieve. Then, the bioactive components were determined using high-performance liquid chromatography (HPLC).
4.3. Determination of Antioxidant Enzyme Activity
Fresh leaves (0.20 g) of P. vulgaris were ground into powder with liquid nitrogen, and then 0.05 mM phosphate-buffered saline (PBS, pH 7.8) was added to the ice bath to prepare the homogenate. The homogenate was centrifuged at 3000 rpm for 10 min at 4 °C; the supernatant was used for determining superoxide dismutase (SOD) and peroxidase (POD) enzyme activities, and the results were expressed as U g−1 min−1 FW. The SOD enzyme activity was determined as described by Li et al. [73]. The 200 μL extract was mixed with 0.05 mM PBS, ethylenediaminetetraacetic acid disodium salt (100 mM), L-methionine (130 mM), nitroblue tetrazolium (NBT) (750 μM), and riboflavin (20 μM) to form a 3 mL reaction solution. The absorbance of the mixture at 560 nm was measured using a spectrophotometer (752N, INESA, Shanghai, China) after irradiation under 50 μmol m−2 s−1 for 20 min, and the inhibition of NBT photoreduction by 50% was taken as the unit of SOD enzyme activity. The POD activity was measured as described by Li et al. [74]. The reaction solution mixture comprised 50 mM PBS (pH 6.0), 30% H2O2 (v/v), 100% guaiacol solution, and 1 mL enzyme extract. The POD activity was calculated by measuring the changes in absorbance at 470 nm.
4.4. Determination of Soluble Sugar Content
The fresh leaves from the different treatments were immersed in a test tube containing 8 mL of distilled water. The samples were extracted twice in boiling water for 30 min and then filtered into a volumetric flask. Then, 0.5 mL of the extract was mixed with 1.5 mL of deionized water, 0.5 mL of ethyl anthracene acetate, and 5 mL of concentrated sulfuric acid. The mixture was boiled for 1 min. According to the method of Dong et al. [75], the absorbance of the extract at 630 nm (X) was measured using a spectrophotometer. The soluble sugar content (Y) was calculated according to the standard curve of glucose (Y = (X − 0.0057)/0.0047).
4.5. Determination of Photosynthetic Pigment
The contents of chlorophyll a (Chl a, Ca), Chl b (Cb) and carotenoid (Car, Ccar) were measured by the method proposed by Lichtenthaler [76]. The leaf samples (0.20 g) were suspended in 10 mL of 80% acetone in the dark for 48 h, and the absorbance of the extract at 470, 646, and 663 nm was determined using a spectrophotometer, the photosynthetic pigments were calculated according to the following formulae:
Ca (mg/g FW) = (12.21 × A663 − 2.81 × A646) × V/W,
Cb (mg/g FW) = (20.13 × A646 − 5.03 × A663) × V/W,
Ccar (mg/g FW) = ((1000 × A470 − 3.27 × Ca − 104 × Cb)/229) × V/W.
4.6. Determination of Proline Content
The proline content was determined as proposed by Bates et al. [77]. At first, 0.25 g leaves were homogenized in 5 mL of 3% aqueous sulfosalicylic acid and boiled for 10 min. The supernatant (2 mL) was reacted with 2 mL of acetic acid and 2 mL of acid ninhydrin in a test tube for 30 min at 100 °C. Afterwards, the reaction mixture was cooled, and 4 mL of toluene was added to complete extraction. The absorbance of the toluene layer was measured at 520 nm (X) using a spectrophotometer. The content of proline (Y) in the sample was calculated according to the standard curve (Y = (X − 0.0011)/0.0525), and the results were expressed as µg g−1 FW.
4.7. Measurement of Malondialdehyde Content
The malondialdehyde (MDA) content was measured as described by Cai et al. [78]. Fresh leaves (0.25 g) were ground to make powder with liquid nitrogen, and 5 mL of trichloroacetic acid (5%, w/v) was added to prepare a homogenate, which was centrifuged at 3000 rpm for 10 min to collect the supernatant. Then, 2 mL of the supernatant was mixed with 2-thiobarbituric acid (0.67%, w/v) and incubated at 100 °C for 30 min. The absorbance of the supernatant at 450, 532, and 600 nm was recorded using a spectrophotometer. The content of MDA was calculated according to the formula (C (µmol g−1) = 6.45 × (A532 − A600) − 0.56 × A450).
4.8. Determination of Relative Electrical Conductivity
The relative electrical conductivity (REC) of the leaves was determined as described by Zhang et al. [67]. Fresh leaves (0.10 g) were cut into strips, rinsed with deionized water, and placed in test tubes with 5 mL of deionized water. The samples were placed at room temperature in the dark for 24 h, and their initial electrical conductivity (C1) was measured. Then, the test tubes were placed in boiling water for 30 min to obtain the maximum electrical conductivity (C2) after cooling. The REC was calculated based on the percentage of C1/C2 × 100%.
4.9. Determination of Hydrogen Peroxide Content
The hydrogen peroxide (H2O2) content was measured according to the method described by Alexieva et al. [79]. Fresh leaf samples (0.5 g) were quickly ground into a powder with liquid nitrogen. The homogenate was obtained by grinding in an ice bath after adding 5 mL of trichloroacetic acid (1% w/v), and then centrifuged at 12,000 rpm at 4 °C for 20 min. The supernatant (0.7 mL) was mixed with 0.7 mL of 10 mM phosphate buffer (pH 7.0) and 1.4 mL of 1 M potassium iodide. After 1 h of reaction in the dark, the absorbance of the supernatant at 390 nm (X) was measured using a spectrophotometer. The H2O2 content (Y) was calculated according to the standard curve (Y = (X + 0.0187)/0.0136), and the results were represented as µmol g−1 FW.
4.10. Root Morphology
The roots were cut from the seedlings and washed with deionized water, and then the root morphology after different treatments was examined using a scanner (Epson Expression 12000XL, Seiko Epson Co., Ltd. Tokyo, Japan). The total root length, -surface area, -volume, -tip number, and -branch number were measured using the WinRHIZO Root Analysis System (Regent Instruments, Sainte Foy, QC, Canada). A total of 10 seedlings were measured for each treatment.
4.11. Determination of Dry Mass
Ten plants per biological replicate were randomly selected for each assay. The plants were dried at 50 °C for 7 days for the biomass measurements, including the dry mass of the whole plant and spica.
4.12. Determination of Gas Exchange Parameters
Gas exchange parameters including net photosynthetic rate (Pn), intercellular CO2 concentration (Ci), stomatal conductance (Gs), and transpiration rate (Tr) were measured using an LI-6400 portable photosynthetic system (LI-COR, Lincoln, NE, USA) from 9:00–10:00 A.M. on sunny days. An internal light-emitting diode light source was used to adjust the saturated photosynthetic photon flux density to 1000 μmol m−2 s−1. The leaf chamber temperature was set to 25 °C. An air flow rate of 500 μmol s−1 was used to obtain CO2 from the environmental source, and the CO2 concentration was maintained at 380 μmol mol−1 using soda lime. All treatments were conducted for five biological repeats, and three leaves were selected for each biological repeat.
4.13. Fast Chlorophyll Fluorescence and 820 nm Modulated Reflection
M-PEA (Hansatech, Norfolk, UK), a multifunctional plant efficiency analyzer, was used simultaneously to measure the fast fluorescence OJIP curve and 820 nm modulated reflection curve of P. vulgaris leaves [65]. Before the measurement, the leaves were dark-adapted for at least 30 min and then exposed to saturated red light (3000 μmol m−2 s−1) for 1 s. The chlorophyll fluorescence signal was continuously recorded to obtain the fast fluorescence OJIP curve. The modulated reflection curve at 820 nm was measured by simultaneous exposure to 250 μmol m−2 s−1 far-red light as described by Strasser et al. [65]. The following parameters were calculated according to the analysis of the JIP test (Table 4). The relative variable fluorescence (Vt) at any time was calculated as: Vt = (Ft − F0)/(Fm − F0), based on the method proposed by Li et al. [29]. The 820-nm modulated reflection curve was plotted according to its relative value (MR/MR0), where MR is the modulated reflection at different time points, and MR0 is the MR value of far-red light irradiated at 0.7 ms [65]. The maximum redox activity of PSI and the coordination between PSII and PSI were calculated according to the 820 nm modulated reflection curve: ΔI/I0 = (I0 − Im)/Im, Φ(PSII/PSII) = (ΔI/I0)/ψ0 [67,80]. Ten replicates were measured for each treatment.

Table 4.
Parameters in JIP test analysis.
4.14. Determination of Total Phenolic Content
The total phenolic content of P. vulgaris spicas was determined according to the method of Zhu et al. [81]. Dried spica powder (0.2 g) was added to 10 mL of 30% ethanol and extracted by ultrasound at 50 °C for 30 min. Then the extract (0.5 mL) was mixed with 2.5 mL of 10% Folin Ciocalteu’s reagent and 2 mL of 7.5% (w/v) Na2CO3. After the mixture was shaken well, the absorption value was determined at 765 nm (X) using a spectrophotometer after 60 min in the dark at room temperature. The content of phenolic compounds (Y) was calculated by the standard correction curve (Y = (X − 0.214)/0.0798) drawn by gallic acid.
4.15. Determination of Total Flavonoids Content
The content of total flavonoids was determined using the method proposed by Chen et al. [4]. The dried spica powder (0.2 g) was mixed with 10 mL of a 35% ethanol solution and extracted three times in a water bath at 86 °C for 3.5 h. Then, 1 mL of the extract was mixed with 0.3 mL of 5% NaNO2 and reacted for 6 min. Next, 0.3 mL of 10% Al(NO3)3 solution was mixed with the reaction solution for 6 min. Finally, 4.0 mL of 4% NaOH was added and mixed, and the absorbance was measured at 510 nm (X) after 15 min using a spectrophotometer. The content of total flavonoids (Y) was calculated by the standard correction curve (Y = (X + 0.0301)/11.688) generated by rutin.
4.16. Caffeic Acid, Ferulic Acid, Rosmarinic Acid, and Hyperoside Contents
The contents of caffeic acid, ferulic acid, rosmarinic acid, and hyperoside were determined using a previously described method with minor modifications [4]. Dried spica powder (0.2 g) was extracted ultrasonically by adding 20 mL of 80% methanol (containing 1% formic acid) for 30 min at 50 °C, and the extract was centrifuged at 10,000 rpm for 10 min. The supernatant was filtered with a 0.22 μm organic membrane filter for HPLC analysis. The extracts were quantified using a HPLC instrument (Agilent 1260, Agilent Technology Co., Ltd., Santa Clara, CA, USA) equipped with a diode array detector and Waters C18 column (250 mm × 4.6 mm).
Methanol (A) and sodium dihydrogen phosphate solution (0.2%, B) were used as the mobile phase for gradient elution. The elution program was as follows: 0–20 min, 20–40% A; 20–35 min, 40–70% A; 35–45 min, 70–90% A; 45–60 min, 90–20% A; injection volume 20 μL; flow rate 0.8 mL min−1; column temperature 30 °C. Caffeic acid, ferulic acid, rosmarinic acid, and hyperoside were determined at 325 nm. Each peak was identified by comparing the relative retention time according to the signal spectrum of the standard. The contents of caffeic acid (Y = (X − 72.87)/4458.00), ferulic acid (Y = X + 67.67)/4704.1), rosmarinic acid (Y = (X − 242.04)/3398.70) and hyperoside (Y = (X − 23.24)/1944.90) were calculated according to the standard curve of the reference substances; Y was the concentration of each substance, and X was the peak area of the sample. The results were represented as mg g−1.
4.17. Statistical Analysis
A one-way analysis of variance and Duncan test (p < 0.05) were performed using SPSS 13.0 software (SPSS, New York, IL, USA). The data were expressed as mean ± standard deviation. At least three biological replicates were used to determine the biochemical parameters.
5. Conclusions
In this study, the 100 μM treatment of MT greatly improved the antioxidant capacity by increasing the activities of antioxidant enzymes and the contents of osmoregulation substances, carotenoids, flavonoids and polyphenols, markedly reduced the content of ROS, and enhanced the stability of cell membrane in P. vulgaris. MT significantly stimulated the development of the root system and improved its absorption capacity. The photosynthetic capacity of P. vulgaris leaves considerably improved through the increase in the activities and coordination of PSII and PSI, as well as the photosynthetic pigment contents. Furthermore, the dry mass and secondary metabolite contents of P. vulgaris were drastically improved. Our results demonstrated that MT increased the yield and medicinal quality, through regulating antioxidant metabolism, root growth and photosynthetic capacity. The concentration of 100 μM proved to be the best among the various concentrations tested, which can be considered for the production of P. vulgaris.
Author Contributions
Conceptualization, Q.C. and L.Z.; methodology, L.L. and X.H.; validation, S.C., X.W., J.W. and Y.M.; formal analysis, writing—original draft preparation, Q.C.; writing—review and editing, M.G.; visualization, L.Z., Y.Z. and Y.S.; funding acquisition, X.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Project for the Technical System of Traditional Chinese Medicinal Material Industry in Henan Province (YuCaiKe [2022] No.24), the National Natural Science Foundation of China (U1804233, 31870093, 31800096, 31872092, 31872157), the Natural Science Foundation of Henan Province (182300410082), the Key Technology Research and Development Program of Henan Province (212102110475), the Henan Province Science and Technology Research and Development Plan Joint Fund (222103810057), the National Key Research and Development Program of China (2018YFD1000800), the Innovative Research Team (in Science and Technology) of the University of Henan Province (23IRTSTHN024) and Student research training program (SRTP) project of Henan University of Science and Technology (2022509, 2022475).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, Y.H.; Yu, M.M.; Zhu, Z.B.; Zhang, L.X.; Guo, Q.S. Optimisation of potassium chloride nutrition for proper growth, physiological development and bioactive component production in Prunella vulgaris L. PLoS ONE 2013, 8, e66259. [Google Scholar]
- Chen, Y.H.; Liu, L.; Guo, Q.S.; Zhu, Z.B.; Zhang, L.X. Effects of different water management options and fertilizer supply on photosynthesis, fluorescence parameters and water use efficiency of Prunella vulgaris seedlings. Biol. Res. 2016, 49, 12. [Google Scholar] [CrossRef]
- Bai, Y.B.; Xia, B.H.; Xie, W.J.; Zhou, Y.M.; Xie, J.C.; Li, H.Q.; Liao, D.F.; Lin, L.M.; Li, C. Phytochemistry and pharmacological activities of the genus Prunella. Food Chem. 2016, 204, 483–496. [Google Scholar]
- Chen, Y.H.; Zhang, X.R.; Guo, Q.S.; Cao, L.P.; Qin, Q.; Li, C.; Zhao, M.; Wang, W.M. Plant morphology, physiological characteristics, accumulation of secondary metabolites and antioxidant activities of Prunella vulgaris L. under UV solar exclusion. Biol. Res. 2019, 52, 17. [Google Scholar] [PubMed]
- Lv, L. Protective Effect and Mechanism of Rosmarinik Acid on Acute Liver Injury Induced by APAP in Mice. Bachelor’s Thesis, Guangxi University, Nanning, China, 2020. [Google Scholar]
- Zhang, L.X.; Chang, Q.S.; Hou, X.G.; Wang, J.Z.; Chen, S.D.; Zhang, Q.M.; Wang, Z.; Yin, Y.; Liu, J.K. The effect of high-temperature stress on the physiological indexes, chloroplast ultrastructure, and photosystems of two herbaceous peony cultivars. J. Plant Growth Regul. 2022. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Coskun, Y.; Duran, R.E.; Kilic, S. Striking effects of melatonin on secondary metabolites produced by callus culture of rosemary (Rosmarinus officinalis L.). Plant Cell Tissue Org. 2019, 138, 89–95. [Google Scholar] [CrossRef]
- Qin, B.; Zou, J.N.; Cao, L.; Wang, M.Y.; Zhang, Y.X. Melatonin regulates material transport to reduce carbon emissions and increase yield under different nitrogen in rice. Agric. Ecosyst. Environ. 2023, 342, 108235. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Mora, P.F.; Arnao, M.B.; Naz, S.; Anwar, M.; Altaf, M.M.; Shahid, S.; Shakoor, A.; et al. Phytomelatonin: An overview of the importance and mediating functions of melatonin against environmental stresses. Physiol. Plantarum. 2021, 172, 820–846. [Google Scholar] [CrossRef]
- Sun, C.L.; Liu, L.J.; Wang, L.X.; Li, B.H.; Jin, C.W.; Lin, X.Y. Melatonin: A master regulator of plant development and stress responses. J. Integr. Plant Biol. 2021, 63, 126–145. [Google Scholar] [CrossRef]
- Xu, L.L.; Yue, Q.Y.; Bian, F.E.; Sun, H.; Zhai, H.; Yao, Y.X. Melatonin enhances phenolics accumulation partially via ethylene signaling and resulted in high antioxidant capacity in grape berries. Front. Plant Sci. 2017, 8, 1426. [Google Scholar]
- Iqbal, R.; Khan, T. Application of exogenous melatonin in vitro and in planta: A review of its effects and mechanisms of action. Biotechnol. Lett. 2022, 44, 933–950. [Google Scholar]
- Guo, Y.Y.; Li, H.J.; Liu, J.; Bai, Y.W.; Xue, J.Q.; Zhang, R.H. Melatonin alleviates drought-induced damage of photosynthetic apparatus in maize seedlings. Russ. J. Plant Physiol. 2020, 67, 312–322. [Google Scholar] [CrossRef]
- Ahmad, S.; Cui, W.; Kamran, M.; Ahmad, I.; Meng, X.P.; Wu, X.R.; Su, W.; Javed, T.; El-Serehy, H.A.; Jia, Z.K.; et al. Exogenous application of melatonin induces tolerance to salt stress by improving the photosynthetic efficiency and antioxidant defense system of maize seedling. J. Plant Growth Regul. 2020, 40, 1270–1283. [Google Scholar] [CrossRef]
- Malik, Z.; Afzal, S.; Dawood, M.; Abbasi, G.H.; Khan, M.I.; Kamran, M.; Zhran, M.; Hayat, M.T.; Aslam, M.N.; Rafay, M. Exogenous melatonin mitigates chromium toxicity in maize seedlings by modulating antioxidant system and suppresses chromium uptake and oxidative stress. Environ. Geochem. Health 2021, 44, 1451–1469. [Google Scholar] [CrossRef]
- Fazal, H.; Abbasi, B.H.; Ahmad, N.; Ali, M. Exogenous melatonin trigger biomass accumulation and production of stress enzymes during callogenesis in medicinally important Prunella vulgaris L. (Selfheal). Physiol. Mol. Biol. Plants 2018, 24, 1307–1315. [Google Scholar] [CrossRef]
- Jafari, M.; Shahsavar, A. The effect of foliar application of melatonin on changes in secondary metabolite contents in two citrus species under drought stress conditions. Front. Plant Sci. 2021, 12, 692735. [Google Scholar]
- Yang, L.; You, J.; Li, J.Z.; Wang, Y.P.; Chan, Z.L.; Sunkar, R. Melatonin promotes Arabidopsis primary root growth in an IAA-dependent manner. J. Exp. Bot. 2021, 72, 5599–5611. [Google Scholar]
- Wang, Q.N.; An, B.; Wei, Y.X.; Reiter, R.J.; Shi, H.T.; Luo, H.L.; He, C.Z. Melatonin regulates root meristem by repressing auxin synthesis and polar auxin transport in Arabidopsis. Front. Plant Sci. 2016, 7, 1882. [Google Scholar] [CrossRef]
- Yang, X.X.; Chen, J.; Ma, Y.; Huang, M.H.; Qiu, T.; Bian, H.W.; Han, N.; Wang, J.H. Function, mechanism, and application of plant melatonin: An update with a focus on the cereal crop, barley (Hordeum vulgare L.). Antioxidants 2022, 11, 634. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Li, D.X.; Zhang, J.R.; Shan, C.; Rengel, Z.; Song, Z.B.; Chen, Q. Phytomelatonin receptor PMTR1-mediated signaling regulates stomatal closure in Arabidopsis thaliana. J. Pineal Res. 2018, 65, e12500. [Google Scholar] [CrossRef]
- Wang, L.; Li, T.; Zhang, Y.; Guo, J.; Lu, K.; Liu, W. CAND2/PMTR1 Is Required for Melatonin-Conferred Osmotic Stress Tolerance in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 4014. [Google Scholar] [CrossRef]
- Jin, G.P.; Liu, F.; Ma, H.; Hao, S.Y.; Zhao, Q.; Bian, Z.R.; Jia, Z.H.; Song, S.S. Two G-protein-coupled-receptor candidates, Cand2 and Cand7, are involved in Arabidopsis root growth mediated by the bacterial quorum-sensing signals N-acyl-homoserine lactones. Biochem. Biophys. Res. Commun. 2012, 417, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.J.; Lu, B.; Liu, L.T.; Meng, Y.J.; Ma, X.Y.; Li, J.; Zhang, K.; Sun, H.C.; Zhang, Y.J.; Dong, H.Z.; et al. Effects of exogenous melatonin on root physiology, transcriptome and metabolome of cotton seedlings under salt stress. Int. J. Mol. Sci. 2022, 23, 9456. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, H.J.; Zhao, B.; Sun, Q.Q.; Cao, Y.Y.; Li, R.; Wu, X.X.; Weeda, S.; Li, L.; Ren, S.; et al. The RNA-seq approach to discriminate gene expression profiles in response to melatonin on cucumber lateral root formation. J. Pineal Res. 2014, 56, 39–50. [Google Scholar]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Khan, L.U.; Altaf, M.M.; Jahan, M.S.; Nawaz, M.A.; Naz, S.; Shahid, S.; Lal, M.K.; et al. Protective mechanisms of melatonin against vanadium phytotoxicity in tomato seedlings: Insights into nutritional status, photosynthesis, root architecture system, and antioxidant machinery. J. Plant Growth Regul. 2022, 41, 3300–3316. [Google Scholar] [CrossRef]
- Xie, J.; Qin, Z.Y.; Pan, J.L.; Li, J.; Li, X.; Khoo, H.E.; Dong, X.H. Melatonin treatment improves postharvest quality and regulates reactive oxygen species metabolism in “Feizixiao” litchi based on principal component analysis. Front. Plant Sci. 2022, 13, 965345. [Google Scholar] [PubMed]
- Li, Y.T.; Xu, W.W.; Ren, B.Z.; Zhao, B.; Zhang, J.; Liu, P.; Zhang, Z.S. High temperature reduces photosynthesis in maize leaves by damaging chloroplast ultrastructure and photosystem II. J. Agron. Crop Sci. 2020, 206, 548–564. [Google Scholar] [CrossRef]
- Cruz, J.A.; Avenson, T.J. Photosynthesis: A multiscopic view. J. Plant Res. 2021, 134, 665–682. [Google Scholar]
- Varghese, N.; Alyammahi, O.; Nasreddine, S.; Alhassani, A.; Gururani, M.A. Melatonin positively influences the photosynthetic machinery and antioxidant system of avena sativa during salinity stress. Plants 2019, 8, 610. [Google Scholar] [CrossRef]
- Yin, Z.P.; Lu, J.Z.; Meng, S.D.; Liu, Y.L.; Mostafa, I.; Qi, M.F.; Li, T.L. Exogenous melatonin improves salt tolerance in tomato by regulating photosynthetic electron flux and the ascorbate-glutathione cycle. J. Plant Interact. 2019, 14, 453–463. [Google Scholar] [CrossRef]
- Wu, P.; Ma, Y.D.; Ahammed, G.J.; Hao, B.Y.; Chen, J.Y.; Wan, W.L.; Zhao, Y.H.; Cui, H.M.; Xu, W.; Cui, J.X.; et al. Insights into melatonin-induced photosynthetic electron transport under low-temperature stress in cucumber. Front. Plant Sci. 2022, 13, 1029854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shi, Y.; Zhang, X.Z.; Du, H.M.; Xu, B.; Huang, B.R. Melatonin suppression of heat-induced leaf senescence involves changes in abscisic acid and cytokinin biosynthesis and signaling pathways in perennial ryegrass (Lolium perenne L.). Environ. Exp. Bot. 2017, 138, 36–45. [Google Scholar] [CrossRef]
- Weeda, S.; Zhang, N.; Zhao, X.; Ndip, G.; Guo, Y.; Buck, G.A.; Fu, C.; Ren, S. Arabidopsis transcriptome analysis reveals key roles of melatonin in plant defense systems. PLoS ONE 2014, 9, e93462. [Google Scholar] [CrossRef]
- Hwang, O.J.; Back, K. Melatonin is involved in skotomorphogenesis by regulating brassinosteroid biosynthesis in rice plants. J. Pineal Res. 2018, 65, e12495. [Google Scholar] [CrossRef]
- Bistgani, Z.E.; Hashemi, M.; Dacosta, M.; Craker, L.; Maggi, F.; Morshedloo, M.R. Effect of salinity stress on the physiological characteristics, phenolic compounds and antioxidant activity of Thymus vulgaris L. and Thymus daenensis Celak. Ind. Crop. Prod. 2019, 135, 311–320. [Google Scholar] [CrossRef]
- Asif, M.; Pervez, A.; Ahmad, R. Role of melatonin and plant-growth-promoting rhizobacteria in the growth and development of plants. Clean-Soil Air Water 2019, 47, 1800459. [Google Scholar] [CrossRef]
- Qi, J.S.; Song, C.P.; Wang, B.S.; Zhou, J.M.; Kangasjarvi, J.; Zhu, J.K.; Gong, Z.Z. Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J. Integr. Plant Biol. 2018, 60, 805–826. [Google Scholar] [CrossRef]
- Lee, M.W.; Huffaker, A.; Crippen, D.; Robbins, R.T.; Goggin, F.L. Plant elicitor peptides promote plant defences against nematodes in soybean. Mol. Plant Pathol. 2018, 19, 858–869. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Debnath, B.; Islam, W.; Li, M.; Sun, Y.; Lu, X.; Mitra, S.; Hussain, M.; Liu, S.; Qiu, D. Melatonin mediates enhancement of stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 1040. [Google Scholar] [CrossRef]
- Elsayed, A.I.; Rafudeen, M.S.; Gomaa, A.M.; Hasanuzzaman, M. Exogenous melatonin enhances the reactive oxygen species metabolism, antioxidant defense-related gene expression, and photosynthetic capacity of Phaseolus vulgaris L. to confer salt stress tolerance. Physiol. Plant. 2021, 173, 1369–1381. [Google Scholar] [CrossRef]
- Mir, A.R.; Siddiqui, H.; Alam, P.; Hayat, S. Melatonin modulates photosynthesis, redox status, and elemental composition to promote growth of Brassica juncea-a dose-dependent effect. Protoplasma 2020, 257, 1685–1700. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Y.; Zhang, M.; Xu, H.; Ning, K.; Wang, B.; Chen, M. Melatonin increases growth and salt tolerance of Limonium bicolor by improving photosynthetic and antioxidant capacity. BMC Plant Biol. 2022, 22, 16. [Google Scholar] [CrossRef]
- Sheikhalipour, M.; Mohammadi, S.A.; Esmaielpour, B.; Zareei, E.; Kulak, M.; Ali, S.; Nouraein, M.; Bahrami, M.K.; Gohari, G.; Fotopoulos, V. Exogenous melatonin increases salt tolerance in bitter melon by regulating ionic balance, antioxidant system and secondary metabolism-related genes. BMC Plant Biol. 2022, 22, 380. [Google Scholar] [CrossRef] [PubMed]
- Keunen, E.; Peshev, D.; Vangronsveld, J.; Van Den Ende, W.; Cuypers, A. Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Z.Y.; Gai, Z.J.; Wang, Y.B.; Wang, B.; Zhang, P.F.; Liu, X.Y.; Chen, J.T.; Zhang, S.Y.; Liu, D.; et al. Exogenous application of melatonin improves salt tolerance of sugar beet (Beta vulgaris L.) seedlings. Acta Physiol. Plant. 2022, 44, 1–15. [Google Scholar] [CrossRef]
- Yang, N.; Han, M.H.; Teng, R.M.; Yang, Y.Z.; Wang, Y.H.; Xiong, A.S.; Zhuang, J. Exogenous melatonin enhances photosynthetic capacity and related gene expression in a dose-dependent manner in the tea plant (Camellia sinensis (L.) Kuntze). Int. J. Mol. Sci. 2022, 23, 6694. [Google Scholar] [CrossRef]
- Kurt-Celebi, A.; Colak, N.; Torun, H.; Dosedelova, V.; Tarkowski, P.; Ayaz, F.A. Exogenous melatonin ameliorates ionizing radiation-induced damage by modulating growth, osmotic adjustment and photosynthetic capacity in wheat seedlings. Plant Physiol. Biochem. 2022, 187, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.H.; Huang, B.; Ding, C.B.; Zhang, Z.W.; Chen, Y.E.; Hu, C.; Zhou, L.J.; Huang, Y.; Liao, J.Q.; Yuan, S.; et al. Effects of melatonin on anti-oxidative systems and photosystem II in cold-stressed rice seedlings. Front. Plant Sci. 2017, 8, 785. [Google Scholar] [CrossRef]
- Dogru, A. Effects of heat stress on photosystem II activity and antioxidant enzymes in two maize cultivars. Planta 2021, 253, 85. [Google Scholar] [CrossRef] [PubMed]
- Danilova, E.D.; Efimova, M.V.; Kolomeichuk, L.V.; Kuznetsov, V.V. Melatonin supports photochemical activity of assimilation apparatus and delays senescence of leaves of monocotyledonous plants. Dokl. Biochem. Biophys. 2020, 495, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Harrison, E.L.; Arce Cubas, L.; Gray, J.E.; Hepworth, C. The influence of stomatal morphology and distribution on photosynthetic gas exchange. Plant J. 2020, 101, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Li, Y.T.; Hu, L.Y.; Zhang, J.Y.; Yue, H.; Yang, S.L.; Liu, Y.; Gong, X.Q.; Ma, F.W. Overexpression of MdASMT9, an N-acetylserotonin methyltransferase gene, increases melatonin biosynthesis and improves water-use efficiency in transgenic apple. Tree Physiol. 2022, 42, 1114–1126. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; York, L.M. Maize with fewer nodal roots allocates mass to more lateral and deep roots that improve nitrogen uptake and shoot growth. J. Exp. Bot. 2019, 70, 5299–5309. [Google Scholar] [CrossRef]
- Ma, Z.; Baskin, T.I.; Brown, K.M.; Lynch, J.P. Regulation of root elongation under phosphorus stress involves changes in ethylene responsiveness. Plant Physiol. 2003, 131, 1381–1390. [Google Scholar] [CrossRef]
- Sarropoulou, V.; Dimassi-Theriou, K.; Therios, I.; Koukourikou-Petridou, M. Melatonin enhances root regeneration, photosynthetic pigments, biomass, total carbohydrates and proline content in the cherry rootstock PHL-C (Prunus avium × Prunus cerasus). Plant Physiol. Biochem. 2012, 61, 162–168. [Google Scholar] [CrossRef]
- Mir, A.R.; Faizan, M.; Bajguz, A.; Sami, F.; Siddiqui, H.; Hayat, S. Occurrence and biosynthesis of melatonin and its exogenous effect on plants. Acta Soc. Bot. Pol. 2020, 89, 1–23. [Google Scholar] [CrossRef]
- Meftahizadeh, H.; Baath, G.S.; Saini, R.K.; Falakian, M.; Hatami, M. Melatonin-mediated alleviation of soil salinity stress by modulation of redox reactions and phytochemical status in guar (Cyamopsis tetragonoloba L.). J. Plant Growth Regul. 2022. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Liu, M.J.; Scheibe, R.; Selinski, J.; Zhang, L.T.; Yang, C.; Meng, X.L.; Gao, H.Y. Contribution of the alternative respiratory pathway to psii photoprotection in C3 and C4 plants. Mol. Plant. 2017, 10, 131–142. [Google Scholar] [CrossRef]
- Chalanika De Silva, H.C.; Asaeda, T. Effects of heat stress on growth, photosynthetic pigments, oxidative damage and competitive capacity of three submerged macrophytes. J. Plant Interact. 2017, 12, 228–236. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, J.T.; Zhang, H.W.; Xu, Y.; An, Y.Y.; Wang, L.J. Effect of 5-Aminolevulinic acid (5-ALA) on leaf chlorophyll fast fluorescence characteristics and mineral element content of Buxus megistophylla grown along urban roadsides. Horticulturae 2021, 7, 95. [Google Scholar] [CrossRef]
- Lazar, D.; Murch, S.J.; Beilby, M.J.; Al Khazaaly, S. Exogenous melatonin affects photosynthesis in characeae Chara australis. Plant Signal. Behav. 2013, 8, e23279. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.J.; Tsimilli-Michael, M.; Qiang, S.; Goltsev, V. Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. BBA-Bioenergetics 2010, 1797, 1313–1326. [Google Scholar] [CrossRef]
- Chen, Z.C.; Jin, X.J.; Li, H.; Zhou, W.X.; Qiang, B.B.; Liu, J.; Zhang, Y.X. Effects of exogenous melatonin on growth, photosynthetic fluorescence characteristics and yield components of adzuki bean. Crops 2021, 37, 88–94. [Google Scholar]
- Zhang, L.X.; Chang, Q.S.; Hou, X.G.; Chen, S.D.; Zhang, Q.M.; Wang, J.Z.; Liu, S.S.; Li, S. Biochemical and photosystem characteristics of wild-type and Chl b-deficient mutant in tree peony (Paeonia suffruticosa). Photosynthetica 2021, 59, 256–265. [Google Scholar] [CrossRef]
- Liang, D.; Shen, Y.Q.; Ni, Z.Y.; Wang, Q.; Lei, Z.; Xu, N.Q.; Deng, Q.X.; Lin, L.J.; Wang, J.; Lv, X.L.; et al. Exogenous melatonin application delays senescence of kiwifruit leaves by regulating the antioxidant capacity and biosynthesis of flavonoids. Front. Plant Sci. 2018, 9, 426. [Google Scholar] [CrossRef]
- Wang, X.M.; Liao, W.N.; Chen, J.; Wu, Y.D.; Liu, C.N.; Chen, S.L.; Xu, Y.; Wang, S.; Su, Y.P.; Du, C.H.; et al. Caffeic acid attenuates irradiation-induced hematopoietic stem cell apoptosis through inhibiting mitochondrial damage. Exp. Cell Res. 2021, 409, 112934. [Google Scholar] [CrossRef]
- Zhang, L.B.; Lv, J.L. A new ferulic acid derivative and other anticoagulant compounds from Angelica sinensis. Chem. Nat. Compd. 2018, 54, 13–17. [Google Scholar] [CrossRef]
- Vafadar, F.; Amooaghaie, R.; Ehsanzadeh, P.; Ghanadian, M.; Talebi, M.; Ghanati, F. Melatonin and calcium modulate the production of rosmarinic acid, luteolin, and apigenin in Dracocephalum kotschyi under salinity stress. Phytochemistry 2020, 177, 112422. [Google Scholar] [CrossRef]
- Duran, R.E.; Kilic, S.; Coskun, Y. Melatonin influence on in vitro callus induction and phenolic compound production in sweet basil (Ocimum basilicum L.). In Vitro Cell Dev.-An. 2019, 55, 468–475. [Google Scholar] [CrossRef]
- Li, X.N.; Brestic, M.; Tan, D.X.; Zivcak, M.; Zhu, X.C.; Liu, S.Q.; Song, F.B.; Reiter, R.J.; Liu, F.L. Melatonin alleviates low PS I-limited carbon assimilation under elevated CO2 and enhances the cold tolerance of offspring in chlorophyll b-deficient mutant wheat. J. Pineal Res. 2018, 64, e12453. [Google Scholar] [CrossRef]
- Li, Y.; Liang, W.; Zhao, B. Physiological and microstructural responses of two Rhododendron cultivars to high temperature and low light. Hortic. Environ. Biotechnol. 2020, 61, 445–458. [Google Scholar] [CrossRef]
- Dong, F.; Wang, C.Z.; Sun, X.D.; Bao, Z.L.; Dong, C.; Sun, C.H.; Ren, Y.Q.; Liu, S.Q. Sugar metabolic changes in protein expression associated with different light quality combinations in tomato fruit. Plant Growth Regul. 2019, 88, 267–282. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Method Enzymol. 1987, 148, 350–382. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Cai, H.L.; Xie, P.F.; Zeng, W.A.; Zhai, Z.G.; Zhou, W.; Tang, Z. Root-specific expression of rice OsHMA3 reduces shoot cadmium accumulation in transgenic tobacco. Mol. Breed. 2019, 39, 49. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Jia, Y.J.; Gao, H.Y.; Zhang, L.T.; Li, H.D.; Meng, Q.W. Characterization of PSI recovery after chilling-induced photoinhibition in cucumber (Cucumis Sativus L.). Planta 2011, 234, 883–889. [Google Scholar] [CrossRef]
- Zhu, M.; Zhan, J.L.; Gao, Z.Y.; Fan, J.F.; Zhao, E.L. Study on ultrasonic-assisted extraction and antioxidant activities of polyphone from Prunella vulgaris L. China Food Addit. 2018, 12, 136–142. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).