Trying to Understand the Complicated Taxonomy in Amaranthus (Amaranthaceae): Insights on Seeds Micromorphology
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Scanning Electron Microscopic Analyses
2.3. Morphometric Analysis
3. Results and Discussion
3.1. Analyses of the Whole Dataset
- (1)
- By classifying the samples using the taxa names [species and below ranks (25 groups); see Table 1], DA predicted two main groups (Figure 5) based on the first two discriminant functions, which explain 71.3% of the total variation [eigenvalues: 52.2% (first function) and 19.1% (second function)]. These two groups (not overlapping each other) correspond to (a) the muricatus/viridis-group and (b) a large group including the remaining taxa (more or less overlapped each other). Concerning the muricatus/viridis-group, the matrix of actual/predicted groups displays high percentages along the diagonal (whose values reveal the matching actual/predicted observations for each group) for Amaranthus viridis (100%). In contrast, 50% of the actual observations for A. muricatus are predicted under A. viridis. Regarding the residual group, just a few taxa have actual/predicted observations matching each other (100%): A. centralis, A. graecizans subsp. sylvestris, A. induratus, A. rajasekharii, A. spinosus, and A. tuberculatus. On the contrary, low or very low percentages characterized the diagonal values of the other taxa, and, for A. caudatus, A. dubius, A. hypochondriacus, A. palmeri, and A. standleyanus, percentages are even zero. The value of correct classification is low (55.8%);
- (2)
- When we classified the samples using the subgenera names (=three groups, i.e., Acnida, Albersia, and Amaranthus), DA do not predict any separate group, and the three groups completely overlapped each other (Figure 6). The first two discriminant functions explain 100% of the total variation [eigenvalues: 86.0% (first function) and 14.0% (second function)]. The matrix of actual/predicted groups reveals that (a) all the actual samples included in the Acnida-group are predicted as included in the other two groups (80% in the Albersia-group, 20% in the Amaranthus-group) and (b) about the 25% of the actual observations of the Albersia- and Amaranthus-groups is predicted under, respectively, the Amaranthus- and Albersia-groups.As a whole, the value of correct classification is low (57.5%);
- (3)
- After running the DA procedure on samples classified using Waselkov’s “molecular clades” [15] (pag. 446, Figure 1A, based on nuclear genes), two groups were predicted (Figure 7) based on the first two discriminant functions, which explain 88.5% of the total variation [eigenvalues: 58.8% (first function) and 29.7% (second function)]. These two groups correspond to (A) the Dioecious/Pumilus-group+ESA-group (=Eurasian/S-African/Australian group [15]) and (B) a larger group including the remaining “molecular clades” sensu Waselkov et al. [15]. Note that the ESA-group partially overlaps the residual-group. The matrix of actual/predicted groups displays high percentages along the diagonal (whose values reveal the matching of actual and predicted observations for each group) for the ESA-group (96%) and Hybridus-group (95.65%), whereas low percentages characterized by the diagonal values of the Galápagos-clade [16.67%, whereas most of the percentage (66.67%) is predicted under the Hybridus-group], the South American-clade [45.45%, whereas the 45.45% is predicted under the Hybridus-group); finally, concerning the Dioecious/Pumilus-group, the 100% of the actual samples are predicted under the ESA-group. As a whole, the value of correct classification is low (64.6%).DA (not shown) performed on Waselkov’s “molecular clades” referred to chloroplast regions reveals the absence of separated groups. In fact, all the samples are intermixed among the various “molecular clades”.
- (1)
- (2)
- Species and below ranks: only one species (Amaranthus tuberculatus) can be clearly distinguished from all the other ones by using the length of the flat border of the seed, which is very small [34–38 µm vs. (40.01–)66.04–214.67(–218.07) µm] (Figure 10);
- (3)
- “Molecular clades” [sensu Waselkov et al. [15] (pag. 446, Figure 1A, based on nuclear genes)]: only the Dioecious/Pumilus-group can be distinguished based on micromorphology of seeds, i.e., by the length of the flat border of the seed and, partially, the length and width of the whole seed (Figure 11).
- (4)
- Box plots (not shown) originated based on Waselkov’s “molecular clades,” which refer to chloroplast regions that do not reveal any separate group.
3.2. Analyses on Subdatasets
- ➢
- Amaranthus centralis: it is an endemic Australian species (Northern Territory, Queensland, South Australia, and Western Australia) macro-morphologically similar to A. induratus. According to Palmer [42], these two species differ from each other based on the shape of the leaves (linear to narrowly oblong or narrowly ovate in A. induratus vs. ovate or elliptic in A. centralis) and tepals (margins with a single or serrated tooth-like projection on each side vs. margins without tooth-like projections). Note that A. centralis and A. induratus are included in the same Waselkov’s “molecular clade,” i.e., the ESA-clade.” (ESA = Eurasian/S-African/Australian group [15]). Moreover, our analyses reveal a micro-morphological similarity between these two taxa as follows:
- >
- In the hierarchical clustering procedure (UPGMA method), the centralis-group and induratus-group are part of the same subgroup of the large “residual-group” (see Figure 3);
- >
- In K-means 10-clustered procedure (not shown), one of the clusters is composed of the samples of A. centralis and A. induratus;
- >
- In DA analysis performed on samples classified using taxa, actual/predicted observations match each other for these two species (100% percentages along the diagonal).
- ➢
- Amaranthus graecizans subsp. sylvestris: it is a taxon native to central and southern Europe and north Africa, and it is (macro-) morphologically characterized by having leaves usually acute, flowers arranged in axillary glomerules, three tepals in pistillate flowers, and fruit as long as or longer than the perianth [3]. This morphological configuration is similar to the forms of the Asian A. tricolor L. without terminal synflorescences [3,7,49], which were originally published by Linnaeus as A. tristis L. [50], A. tricolor [50], and A. polygamus L. [51], but later synonymized with A. tricolor [52]. A. graecizans subsp. sylvestris is included in the ESA-clade by Waselkov et al. [15], where also A. tricolor occurs. A. graecizans subsp. sylvestris and A. tricolor can be distinguished using seed micromorphology by the seed length [(1.09–)1.28–1.34(–1.47) vs. (0.20–)0.90–1.26], the seed width [(1.05–)1.20–1.36] vs. 0.97–1.09(–1.11)], seed shape (circular vs. oval), and seed type (not reticulate vs. reticulate);
- ➢
- Amaranthus retroflexus: it is native to Mexico and alien in all other continents where it is often widely spread [41]. This species can be distinguished by its stem, usually tomentose-pubescent and erect, green synflorescence, and five tepals spathulate with obtuse to emarginate apex [1,3,7,53]. A. retroflexus is macromorphologically similar to A. wrightii S.Watson, which differs mainly by being glabrous or nearly so [1,7]. Other authors e.g., [53] highlighted the similarity between A. retroflexus and A. quitensis (Bolòs & Vigo [54] even proposed to treat A. quitensis as subspecies of A. retroflexus). All these three species (A. quitensis, A. retroflexus, and A. wrightii) belong to the Hybridus-clade sensu Waselkov et al. [15]. Our analyses show that A. retroflexus differs from A. quitensis by the length of seed flat border [(102.53–)160.70–167.86(–180.35) µm vs. (81.8–)96.08–114.05(117–87) µm], the seed length [(1.04–)1.17–1.21 mm vs. (1.04–)1.06–1.11(–1.12) mm], the ratio length/width [(1.09–)1.10–1.14(–1.16) vs. (0.97–)0.99–1.06(–1.09)], and the seed type (not reticulate vs. reticulate);
- ➢
- Amaranthus vulgatissimus: it is a species native to South America (Argentina and Uruguay) that appears well morphologically distinct among the taxa with five tepals and indehiscent fruits [A. cochleitepalus Domin, A. crispus (Lesp. & Thévenau) A.Braun ex J.M.Coult. & S.Watson, A. cuspidifolius Domin, and A. persimilis Hunz.) having tepals oblong with a base 0.3–0.5 mm wide (vs. spathulate with a base up to 0.3 mm wide in the other four mentioned species) [7]. In Waselkov et al. [15], A. vulgatissimus belongs to the SA-clade, sister to A. muricatus. The latter species was part of the muricatus/viridis-group according to our analyses (see Figure 2, Figure 4, Figure 5 and Figure 8), and it differs primarily by the seed coat ornamentation (wrinkled) and shape of the central cell (irregular), whereas A. vulgatissimus has colliculate seed coat and regular central cells. A further difference between these two species refers to the width of the seed [(0.84–)0.85–0.99(–1.07) vs. (1.11–)1.14–1.16(–1.24)].
- ➢
- Amaranthus rajasekharii: a species recently described from India [55] morphologically related to A. dibius Mart. from which differ by the stem (reddish to purple vs. green in A. dubius), bracts (linear and up to 0.1 mm long vs. ovato-deltoid, 1.3–1.7 mm long), tepals shape (ovate to lanceolate vs. oblong-spatulate), gynoecium (whitish vs. green), and pollen grain [with 21–23 pores (vs. 27–30), 3–5 ektexinous bodies (vs. mostly 3), and margin of pores not depressed and without conspicuous ornamentation (vs. clearly depressed and with conspicuous ornamentation)]. A. dubius is included in the Hybridus-clade by Waselkov et al. [15], whereas A. rajasekharii did not appear in their work being published later. Our results reveal that these two species differ from each other by the ratio length/width of seeds [(0.98–)1.03–1.05(–1.11) in A. rajasekharii vs. (1.09–)1.11–1.14(–1.26) in A. dubius];
- ➢
- Amaranthus spinosus: a species native to tropical America (from Mexico to Argentina), which is easy to distinguish from the other ones by its spine-like structure (metamorphosed bracts of the first flower in the first cyme) [1,3,7,53]. Recently, a similar species (A. saradhiana Sindhu Arya, V.S.A.Kumar, W.K.Vishnu & Rajesh Kumar) was described from India. It has, along the stem, only two spines per node, whereas no spines occur in the synflorescence part (4 spines per node along the stem and spines in the synflorescence part in A. spinosus); further differences regard the stem and petiole color (purple vs. green in A. spinosus), the apex of tepals (acute vs. often spathulate), gynoecium (whitish to light green vs. dark green), and the number of pores in pollen grains (26–30 vs. 37–40). A. spinosus is included in the Hybridus-clade by Waselkov et al. [15], whereas A. saradhiana did not appear in their work, being published later. Our results show that these two species differ from each other by the seed length [0.80–0.89(–0.94) vs. 0.98–1.02 in A. spinosus], seed width [(0.80–)0.98–1.04(–1.10) vs. 0.99–1.02], and peripheral cells length [20.00–28.05 vs. (8.39–)11.48–16.31(–17.88)], whereas, concerning the qualitative seed characters, some are partially overlapped [color (mostly reddish brown vs. dark brown), coat ornamentation (mostly punticulate vs. colliculate), type (mostly not reticulate vs. reticulate), shape of peripheral cells (polygonal vs. mostly tetragonal), pleurogram (present vs. sometimes absent)].
3.3. Seed Types and Taxonomic Key
- 1. Seed coat ornamentation pebble stoned ................................. crassipes-type (A. crassipes)
- 1. Seed coat ornamentation never pebble stoned .................................................................... 2
- 2. Seed coat ornamentation wrinkled ..................................................................... viridis-type
- 2a. Seed black, (0.89–)0.95–1.04(–1.06) mm long, (0.82–)0.87–0.95(–1.04) mm wide, pleurogram often absent .................................................................................... A. viridis
- 2b. Seed dark-brown, (1.24–)1.26–1.33 mm long, (1.11–)1.14-1.16(–1.24) mm wide, pleurogram present ...................................................................................... A. muricatus
- 2. Seed coat ornamentation never wrinkled ............................................................................. 3
- 3. Flat border of the seed 34.14–38.04 µm long ............... tuberculatus-type (A. tuberculatus)
- 3. Flat border of the seed longer ................................................................................................. 4
- 4. Seed coat ornamentation mostly puncticulate ..................................................................... 5
- 4. Seed coat ornamentation mostly colliculate ....................................................................... 10
- 5. Peripheral cells polygonal, seed mostly reddish-brown ............................... A. sarahdiana
- 5. Peripheral cells tetragonal, seed dark-brown ...................................................................... 6
- 6. Seed ratio length/width 1.17–1.33 ........................................................................................... 7
- 6. Seed ratio length/width smaller ............................................................................................. 8
- 7. Seed circular, 0.99–1.12(–1.18) mm long, ratio length/width (1.17–)1.20–1.22; flat border of the seed (92.82–)96.36–112.90(–124.65) µm long ....................................... A. stadleyanus
- 7. Seed mostly oval, (1.18–)1.21–1.32(–1.39) mm long, ratio length/width 1.31–1.33; flat border of the seed (129.28–)141.30–176.98(–180.30) µm long ........................... A. centralis
- 8. Seed mostly not reticulate ......................................................................................................... 9
- 9. Seed (1.09–)1.28–1.34(–1.47) mm long, (1.05–)1.20–1.24(–1.36) mm wide........................................................................................... A. graecizans subsp. sylvestris
- 9. Seed (0.84–)0.86–0.89(–0.98) mm long, (0.83–)0.94–0.95(–0.98) mm wide................................................................................................................................ A. albus
- 8. Seed reticulate ......................................................................................................... blitum-type
- 8a. Seed (0.95–)1.02–1.16(–1.21) mm long .......................................... A. blitum var. blitum
- 8b. Seed 0.84–1.02 mm long ...................................... A. emarginatus subsp. pseudogracilis
- 10. Peripheral cells polygonal ................................................................................... A. rajasekarii
- 10. Peripheral cells tetragonal .................................................................................................... 11
- 11. Seed ratio length/width 1.17–1.33 ........................................................................................ 12
- 11. Seed ratio length/width smaller ........................................................................................... 17
- 12. Seed (1.36–)1.39–1.48(–1.77) mm long ................................................................ A. induratus
- 12. Seed shorter ............................................................................................................................ 13
- 13. Seed 1.25–1.39 mm long ......................................................................................................... 14
- 13. Seed shorter ............................................................................................................................ 15
- 14. Peripheral cells 16.38–16.94 mm long, central cells 13.61–15.86 mm wide............................................................................................................................. A. palmeri
- 14. Peripheral cells (15.44–)19.04–25.71(–33.15) mm long, central cells (15.25–)16.88–20.83(–22.71) mm wide ..................................................... A. hypochondriacus
- 15. Seed not reticulate ................................................................................................ A. retroflexus
- 15. Seed reticulate ........................................................................................................................ 16
- 16. Seed black ................................................................................................................. A. hybridus
- 16. Seed mostly dark-brown ................................................................................... deflexus-type
- 17. Seed 0.87–1.12 mm long ........................................................................................................ 18
- 17. Seed longer .............................................................................................................................. 19
- 18. Seed 0.87–0.91(–1.04) mm long, (0.77–)0.79–0.87(–0.94) mm wide ................... A. spinosus
- 18. Seed (1.02–)1.04–1.11(–1.12) mm long, 0.99–1.07 mm wide .............................. A. quitensis
- 19. Seed (1.13–)1.41-1.58(–1.64) mm long ................................................................... A. blitoides
- 19. Seed (0.99–)1.16-1.30 mm long .............................................................................. A. caudatus
- (1)
- Blitum-type: seed (0.83)0.94–1.37(–1.42) × (0.80–)0.83–0.99(1.35) mm (length × width), circular, dark-brown, puncticulate, reticulate; ratio length/width (0.95–)1.00–1.04(–1.17); flat border (75.76–)80.93–120.97(–139.58) µm long; peripheral cells (3.58–)12.48–26.55(–29.11) × (7.61–)13.20–19.99(–20.95) µm on average, tetragonal; central cells (27.58–)27.99–38.59(–54.77) × (12.33–)13.36–25.97(–35.88) µm on average, mostly regular; pleurogram mostly present.Species included: Amaranthus blitum, A. emarginatus subsp. pseudogracilis.
- (2)
- Crassipes-type: seed 1.04–12.0 × 0.92–1.04 mm (length × width), circular, dark-brown, pebble stone-like, reticulate; ratio length/width 1.13–1.23; flat border 112.71–123.36 µm long; peripheral cells 8.84–20.31 × 12.70–23.71 µm on average, tetragonal; central cells 25.40–28.18 × 16.23–24.19 µm on average, regular; pleurogram not present.Species included: Amaranthus crassipes.
- (3)
- Deflexus-type: seed (0.87–)0.92–1.18(–1.24) × (0.75–)0.91–0.97(1.10) mm (length × width), oval or circular, mostly dark-brown, puncticulate or colliculate, reticulate; ratio length/width (0.98–)1.09–1.34(–1.41); flat border (49.66–)55.66–144.49(–164.43) µm long; peripheral cells (8.39–)10.51–39.16(–45.41) × (12.02–)12.90–24.10(–25.85) µm on average, tetragonal; central cells (13.85–)14.41–45.39(–51.24) × (10.58–)11.01–27.89(–28.43) µm, regular; pleurogram mostly present.Species included: Amaranthus deflexus, A. vulgatissimus, A. cacciatoi, A. spinosus, A. dubius, and A. stadleyanus.
- (4)
- Tuberculatus-type: seed 0.78–0.86 × 0.78–0.87 mm (length × width), circular, dark-brown, puncticulate, mostly not reticulate; ratio length/width 1.01–1.10; flat border 34.14–38.04 µm long; peripheral cells 10.86–16.05 × 15.35–24.53 µm on average, tetragonal; central cells 19.14–31.47 × 15.04–21.79 µm on average, regular; pleurogram present.Species included: Amaranthus tuberculatus.
- (5)
- Viridis-type: seed (0.89–)0.95–1.31(–1.33) × (0.82–)0.87–1.16(–1.24) mm (length × width), circular, dark-brown or black, wrinkled, reticulate; flat border (69.20)73.76–135.88(–151.69) µm long; peripheral cells (8.56–)8.84–19.87(–23.16) × (9.63–)11.00–20.75(–20.85) µm on average, tetragonal; central cells (11.16–)14.38–22.01(–38.95) × (8.04–)12.33–25.78(–30.49) µm on average, usually irregular; pleurogram present or not.Species included: Amaranthus muricatus, A. viridis.
4. Conclusions


Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. List of Specimens Considered in the Study
References
- Mosyakin, S.L.; Robertson, K.R. Amaranthus L. In Flora of North America North of Mexico (Magnoliophyta: Caryophyllidae, Part 1); Flora of North America Editorial Committee, Ed.; Oxford University Press: Oxford, UK, 2003; Volume 4, pp. 410–435. [Google Scholar] [CrossRef]
- Hernández-Ledesma, P.; Berendsohn, W.G.; Borsch, T.; Von Mering, S.; Akhani, H.; Arias, S.; Castañeda-Noa, I.; Eggli, U.; Eriksson, R.; Flores-Olvera, H.; et al. A taxonomic backbone for the global sunthesis of species diversity in the angiosperm orden Caryophyllales. Wildenowia 2015, 45, 281–383. [Google Scholar] [CrossRef]
- Iamonico, D. Taxonomic revision of the genus Amaranthus (Amaranthaceae) in Italy. Phytotaxa 2015, 199, 1–84. [Google Scholar] [CrossRef]
- Iamonico, D.; El Mokni, R. First record of Amaranthus crassipes subsp. warnockii (Amaranthaceae) out of Americas, with nomenclatural notes. Bothalia 2022, 53, a2. [Google Scholar] [CrossRef]
- Costea, M.; Sanders, A.; Waines, G. Preliminary results towards a revision of the Amaranthus hybridus complex (Amaranthaceae). Sida 2001, 19, 931–974. [Google Scholar] [CrossRef][Green Version]
- Das, S. Amaranthus: A Promising Crop of the Future; Springer Nature: Singapore, 2016. [Google Scholar] [CrossRef]
- Bayón, N.D. Revisión taxonómica de las especies monoicas de Amaranthus (Amaranthaceae): Amaranthus subg. Amaranthus and Amaranthus subg. Albersia. Ann. Mo. Bot. Gard. 2015, 101, 261–383. [Google Scholar] [CrossRef]
- Iamonico, D. Nomenclature survey of the genus Amaranthus (Amaranthaceae). 3. Names linked to the Italian flora. Plant Biosyst. 2016, 150, 519–531. [Google Scholar] [CrossRef]
- Iamonico, D. Nomenclature survey of the genus Amaranthus (Amaranthaceae). 4. Detailed questions arising around the name Amaranthus gracilis. Bot. Serbica 2016, 40, 61–68. [Google Scholar] [CrossRef]
- Iamonico, D. Nomenclature survey of the genus Amaranthus (Amaranthaceae). 5. Moquin-Tandon’s names. Phytotaxa 2016, 273, 81–114. [Google Scholar] [CrossRef]
- Iamonico, D. Nomenclature survey of the genus Amaranthus (Amaranthaceae s.s.). 8. About Amaranthus polygonoides s.l. and A. anderssonii, two related taxa described from the tropical regions of America with notes on their taxonomy. Acta Bot. Mex. 2020, 127, e1687. [Google Scholar] [CrossRef]
- Iamonico, D. A nomenclature survey of the genus Amaranthus (Amaranthaceae). 7. Wildenow’s names. Willdenowia 2020, 50, 147–155. [Google Scholar] [CrossRef]
- Iamonico, D.; Palmer, J. Nomenclature survey of the genus Amaranthus (Amaranthaceae). 6. Names linked to the Australian flora. Aust. Syst. Bot. 2020, 33, 169–173. [Google Scholar] [CrossRef]
- Mosyakin, S.L.; Robertson, K.R. New infrageneric taxa and combinations in Amaranthus (Amaranthaceae). Ann. Bot. Fenn. 1996, 33, 275–281. [Google Scholar]
- Waselkov, K.E.; Boleda, A.S.; Olsen, K.M. A phylogeny of the genus Amaranthus (Amaranthaceae) based on several low-copy nuclear loci and chloroplast regions. Syst. Bot. 2018, 43, 439–458. [Google Scholar] [CrossRef]
- Iamonico, D. Amaranthus gangeticus (Amaranthaceae), a name incertae sedis. Phytotaxa 2014, 162, 299–300. [Google Scholar] [CrossRef][Green Version]
- Hassan, N.M.S.; Meve, U.; Liede-Schumann, S. Seed coat morphology of Aizoaceae–Sesuvioideae, Gisekiaceae and Molluginaceae and its systematic significance. Bot. J. Linn. Soc. 2005, 148, 189–206. [Google Scholar] [CrossRef]
- Sukhorukov, A.P.; Zhang, M. Fruit and seed anatomy of Chenopodium and related genera (Chenopodioideae, Chenopodiaceae/Amaranthaceae): Implications for evolution and taxonomy. PLoS ONE 2013, 8, e61906. [Google Scholar] [CrossRef]
- Lemus-Barrios, H.; Barrios, D.; García-Beltrán, J.A.; Arias, S.; Majure, L.C. Taxonomic implications of seed morphology in Melocactus (Cactaceae) from Cuba. Willdenowia 2021, 51, 91–113. [Google Scholar] [CrossRef]
- Arroyo-Cosultchi, G.; Terrazas, T.; Arias, S.; Arreola-Nava, H.J. The Systematic Significance of Seed Morphology in Stenocereus (Cactaceae). Taxon 2006, 55, 983–992. [Google Scholar] [CrossRef]
- Sadeghian, S.; Zarre, S.; Heubl, G. Systematic implication of seed micromorphology in Arenaria (Caryophyllaceae) and allied genera. Flora 2014, 209, 513–529. [Google Scholar] [CrossRef]
- Amini, E.; Zarre, S.; Assadi, M. Seed micro-morphology and its systematic significance in Gypsophila (Caryophyllaceae) and allied genera. Nord. J. Bot. 2011, 29, 660–669. [Google Scholar] [CrossRef]
- Minuto, L.; Fior, S.; Roccotiello, E.; Casazza, G. Seed morphology in Moehringia L. and its taxonomic significance in comparative studies within the Caryophyllaceae. Plant Syst. Evol. 2006, 262, 189–208. [Google Scholar] [CrossRef]
- Hong, S.P.; Han, M.J.; Kim, K.J. Systematic significance of seed coat morphology in Silene L. s. str. (Sileneae-Caryophyllaceae) from Korea. J. Plant Biol. Korea 1999, 42, 146–150. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Boughalleb, F.; Pellegrino, G.; Abdellaoui, R.; Nasri, N. Flower, seed, and fruit development in three Tunisian species of Polygonum: Implications for their taxonomy and evolution of distyly in Polygonaceae. PLoS ONE 2020, 15, e0227099. [Google Scholar] [CrossRef]
- Soliman, M.A. Cytogenetical studies on Aerva javanica (Amaranthaceae). Flora Mediterr 2006, 16, 333–339. [Google Scholar]
- Sindhu, A.; Iamonico, D.; Suresh, V.; Venugopalan Nair Saradamma, A.K. First molecular and morphometric data for the genus Allmania (Amaranthaceae), with the description of a new species from India. Phytotaxa 2021, 559, 221–237. [Google Scholar] [CrossRef]
- Parveen, M.; Mitra, M.; Tah, J.; Chattopahyay, N.C. Study of intraspecies variation in seed coat micro-morphology of Amaranthus hybridus by scanning electron microscope. Int. J. Plant Breed. Genet. 2015, 9, 198–205. [Google Scholar] [CrossRef]
- Costea, M.; Weaver, S.E.; Tardif, F.J. The Biology of Invasive Alien Plants in Canada. 3. Amaranthus tuberculatus (Moq.) Sauer var. rudis (Sauer) Costea & Tardif. Can. J. Plant Sci. 2005, 85, 507–522. [Google Scholar] [CrossRef]
- Kamble, S.R.; Gaikwad, D.K. Seed Morphology of Some Species of The Genus Amaranthus from India. Sci. Technol. Dev. 2022, XI, 170–181. [Google Scholar]
- Falatoury, A.N.; Hatami, S.; Torabi, H.; Ghezeli, F.; Sarani, M. Taxonomic significance of inflorescence and seed characteristics in the genus Amaranthus in Iran. Rostaniha 2021, 22, 43–55. [Google Scholar] [CrossRef]
- Irving, D.W.; Beckler, R. Seed Structure and Composition of Potential New Crops. Food Struct. 1985, 4, 43–53. [Google Scholar]
- Iamonico, D. Contributo alla conoscenza del genere Amaranthus, L. (Amaranthaceae) nel Lazio. Proposta per una chiave analitica. Inform. Bot. Ital. 2009, 41, 25–28. [Google Scholar]
- Iamonico, D. On the presence of Amaranthus polygonoides L. (Amaranthaceae) in Europe. Phyton 2011, 50, 205–219. [Google Scholar]
- Iamonico, D. Amaranthus × romanus (Amaranthaceae), hybr. nov. Phytotaxa 2017, 295, 89–91. [Google Scholar] [CrossRef]
- Iamonico, D.; El Mokni, R. Amaranthus palmeri (Amaranthaceae) in Tunisia, a second record for the continental African flora and nomenclatural notes on A. sonoriensis nom. nov. pro A. palmeri var. glomeratus. Bothalia 2017, 47, a2100. [Google Scholar] [CrossRef]
- Iamonico, D.; El Mokni, R. A new addition to the alien flora of Tunisia, Amaranthus spinosus L. (Amaranthaceae s.l.), with notes on A. diacanthus Raf. Acta Bot. Croat. 2019, 78, 91–94. [Google Scholar] [CrossRef]
- Iamonico, D.; Fortini, P.; Noor Hussain, A. On the occurrence and naturalization of Amaranthus hypochondriacus (Amaranthaceae) in some european countries, with notes on its climatic features. Hacquetia 2022, 21, 211–222. [Google Scholar] [CrossRef]
- Sindhu, A.; Iamonico, D.; Venugopalan Nair Saradamma, A.K. Amaranthus powellii (Amaranthaceae), a new addition for the flora of India and a preliminary list of the indian Amaranthus species. Hacquetia 2021, 20, 257–262. [Google Scholar] [CrossRef]
- Thiers, B. [Continuously Updated]. “Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff. New York Botanical Garden’s Virtual Herbarium”. Available online: http://sweetgum.nybg.org/ih/ (accessed on 19 September 2022).
- POWO. (2023 [Continuously Updated]) Plant of The World Online. Amaranthus L. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:327362-2 (accessed on 1 January 2023).
- Palmer, J. A conspectus of the genus Amaranthus L. (Amaranthaceae) in Australia. Nuytsia 2009, 19, 107–128. [Google Scholar]
- Iamonico, D.; Das, S. Amaranthus bengalense (Amaranthaceae) a new species from India, with taxonomical notes on A. blitum aggregate. Phytotaxa 2014, 181, 293–300. [Google Scholar] [CrossRef]
- Cox, D.R. The analysis of multivariate binary data. Journal of the Royal Statistical Society. Series C. Appl. Stat. 1972, 2, 113–120. [Google Scholar] [CrossRef]
- Topliss, J.G.; Costello, R.J. Chance correlations in structure-activity studies using multiple regression analysis. J. Med. Chem. 1972, 15, 1066–1068. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.H. Confronting multicollinearity in ecological multiple regression. Ecology 2003, 81, 2809–2815. [Google Scholar] [CrossRef]
- Stewart, S.; Ivy, M.I.; Anslyn, E.V. The use of principal component analysis and discriminant analysis in differential sensing routines. Chem. Soc. Rev. 2013, 43, 70–84. [Google Scholar] [CrossRef]
- Aristidis, L.; Vlassis, N.; Verbeek, J.J. The global k-means clustering algorithm. Pattern Recognit. 2003, 36, 451–461. [Google Scholar] [CrossRef]
- Townsend, C.C. Amaranthaceae Juss. In Flora of West Pakistan; Nasir, E., Ali, S.I., Eds.; Fakhri Press: Rawalpindi, Pakistan, 1974; Volume 71, pp. 1–49. [Google Scholar]
- Linnaeus, C. Species Plantarum; Laurentius Salvius: Stockholm, Sweden, 1753; Volume 2. [Google Scholar] [CrossRef]
- Linnaeus, C. Centuria I. Plantarum; Reg. Acad. Typogr. Upps. Finl.: Stockholmiae, Sweden, 1755. [Google Scholar] [CrossRef]
- Iamonico, D. Lectotypification of Linnaean names in the genus Amaranthus L. (Amaranthaceae). Taxon 2014, 63, 146–150. [Google Scholar] [CrossRef]
- Akeroyd, J.R. Amaranthus L. In Flora Europaea, 2nd ed.; Tutin, T.G., Burges, N.A., Chater, A.O., Edmondson, J.R., Heywood, V.H., Moore, D.M., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1993; Volume 1, pp. 130–132. [Google Scholar]
- Bolòs, O.; De Vigo, J. Notes sobre taxonomía i nomenclatura de plantes, I. Butletí Inst. Catalana DHist. Nat. Barc. Secc. Bot. 1974, 38, 61–89. [Google Scholar]
- Sindhu, A.; Venugopalan Nair Saradamma, A.K.; Walsan Kalarikkal, V.; Iamonico, D. Amaranthus rajasekharii (Amaranthaceae), a new species from Kerala (SW-India). Phytotaxa 2020, 433, 153–160. [Google Scholar] [CrossRef]
- Iamonico, D. Amaranthus graecizans s.l. (Amaranthacee) in Italia: Note tassonomiche e distributive. Inform. Bot. Ital. 2014, 46, 39–46. [Google Scholar]
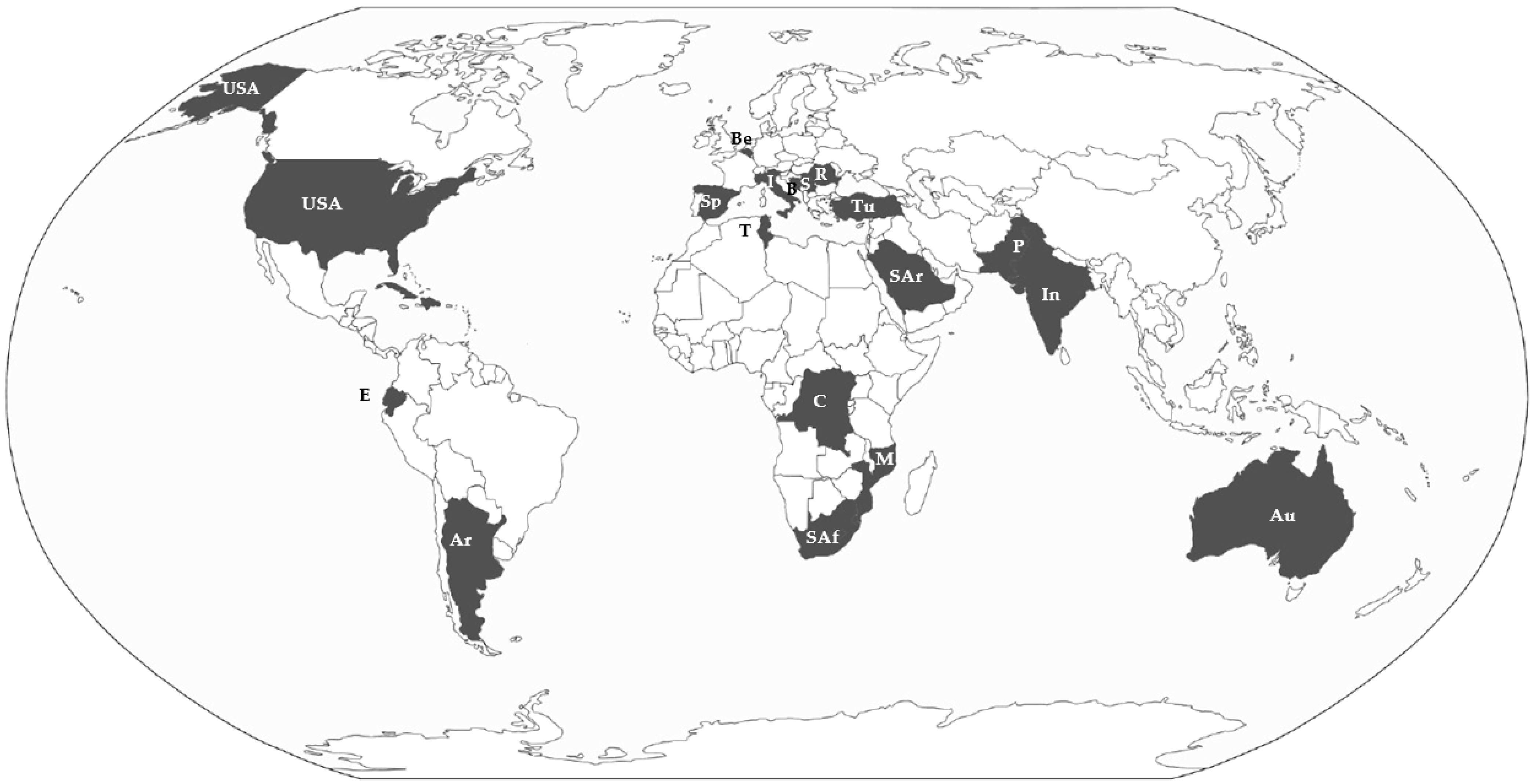










| Code | Taxon | Native Distribution Area |
|---|---|---|
| al | Amaranthus albus L. | S-U.S.A. to NE-Mexico |
| bo | Amaranthus blitoides S.Watson | CE-U.S.A. |
| bu | Amaranthus blitum L. | Mediterranean area and C-Europe |
| cc | Amaranthus cacciotoi (Aellen ex Cacciato) Iamonico | C-Italy |
| ce | Amaranthus caudatus L. | Ecuador to NW-Argentina |
| cr | Amaranthus centralis J.Palmer & Mowatt. | Australia |
| cu | Amaranthus crassipes Schltdl. | U.S.A. to N-Mexico, Caribbean, S-America |
| de | Amaranthus deflexus L. | S-America |
| du | Amaranthus dubius Mart. ex Thell. | S-America |
| hb | Amaranthus emarginatus Salzm. ex Uline & Bray | Brazil to N-Argentina |
| hp | Amaranthus graecizans subsp. sylvestris L. | CS-Europe and N-Africa |
| in | Amaranthus hybridus L. | NC-America |
| mu | Amaranthus hypochondriacus L. | C-U.S.A. to Mexico |
| pa | Amaranthus induratus J.Palmer & Mowatt. | N-Australia |
| ps | Amaranthus muricatus (Gillies ex Moq.) Hieron. | S-America |
| qu | Amaranthus palmeri S.Watson. | S-California to Texas and Mexico |
| ra | Amaranthus quitensis Kunth. | S-America |
| re | Amaranthus rajasekharii Sindu Arya et al. | India |
| sa | Amaranthus retroflexus L. | N-America |
| sp | Amaranthus saradhiana Sindu Arya et al. | India |
| st | Amaranthus spinosus L. | Mexico to Tropical America |
| sy | Amaranthus standleyanus Parodi ex Covas. | S-America |
| tu | Amaranthus tuberculatus (Moq) J.D.Sauer | EC-U.S.A. |
| vi | Amaranthus viridis L. | Mexico to Tropical America |
| vu | Amaranthus vulgatissimus Speg. | S-America |
| 1. Seed length (mm) |
| 2. Seed width (mm) |
| 3. Length of flat border opposite to hilum (µm) |
| 4. Average peripheral cells length (µm) |
| 5. Average peripheral cells width (µm) |
| 6. Average central cells length (µm) |
| 7. Average central cells width (µm) |
| 8. Seed color (reddish-brown, dark-brown, black) * |
| 9. Seed shape (circular, not circular) * |
| 10. Seed coat ornamentation (puncticulate, colliculate, pebble stone-like, wrinkled) * |
| 11. Seed reticulation (reticulate, not reticulate) * |
| 12. Peripheral cells shape (tetragonal, polygonal) * |
| 13. Central cells shape (regular, irregular) * |
| 14. Pleurogram (present, not present) * |
| Seed Coat Ornamentation | Seed Shape | Seed Reticulation | ||||
|---|---|---|---|---|---|---|
Puncticulate: a surface that has fine and widely spaced punctures | Circular: ratio length/width ≈ 1 | Reticulate: a surface that have a network of lines | ||||
Colliculate: a surface that has low, rounded elevations | Not circular: ratio length/width > 1 | Not reticulate: a surface without a network or network unclear  | ||||
Pebble stone-like: a surface that has ± rounded like-stones structures | Peripheral cells shape | Central cells shape | ||||
Tetragonal: cells with four sides | Regular: cells with distinct boundaries | |||||
Wrinkled: a surface that has slight folds or creases and crumples | Polygonal: cells with > 5 sides | Irregular: cells without clear boundaries | ||||
| Pleurogram | Present | Not present | ||||
| No. of Clusters | Component | Between Mean Square | Within Mean Square | F-Ratio |
|---|---|---|---|---|
| 2 | 1 | 73.46075 | 0.396 | 185.31 |
| 2 | 25.385695 | 0.816 | 31.1 | |
| 3 | 0.1758203 | 1.008 | 0.17 | |
| 4 | 11.79855 | 0.988 | 11.94 | |
| 5 | 0.8752245 | 1.069 | 0.82 | |
| 6 | 1.435734 | 2.422 | 0.59 | |
| 3 | 1 | 74.58856 | 0.3783803 | 197.13 |
| 2 | 31.96614 | 0.6963916 | 45.9 | |
| 3 | 2.013769 | 0.9821099 | 2.05 | |
| 4 | 16.71954 | 0.9021953 | 18.53 | |
| 5 | 26.66274 | 0.5785692 | 46.08 | |
| 6 | 4.862731 | 2.378553 | 2.04 | |
| 4 | 1 | 49.88319 | 0.377458 | 132.16 |
| 2 | 21.86316 | 0.6869719 | 31.83 | |
| 3 | 18.39766 | 0.4901165 | 37.54 | |
| 4 | 20.30244 | 0.6417438 | 31.64 | |
| 5 | 16.79983 | 0.6129277 | 27.41 | |
| 6 | 1.237907 | 2.460811 | 0.5 | |
| 5 | 1 | 37.54745 | 0.3758467 | 99.9 |
| 2 | 17.43485 | 0.6526853 | 26.71 | |
| 3 | 14.16077 | 0.4806115 | 29.46 | |
| 4 | 15.74832 | 0.6274446 | 25.1 | |
| 5 | 12.90233 | 0.6070178 | 21.26 | |
| 6 | 47.03387 | 0.6592174 | 71.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iamonico, D.; Hussain, A.N.; Sindhu, A.; Saradamma Anil Kumar, V.n.; Shaheen, S.; Munir, M.; Fortini, P. Trying to Understand the Complicated Taxonomy in Amaranthus (Amaranthaceae): Insights on Seeds Micromorphology. Plants 2023, 12, 987. https://doi.org/10.3390/plants12050987
Iamonico D, Hussain AN, Sindhu A, Saradamma Anil Kumar Vn, Shaheen S, Munir M, Fortini P. Trying to Understand the Complicated Taxonomy in Amaranthus (Amaranthaceae): Insights on Seeds Micromorphology. Plants. 2023; 12(5):987. https://doi.org/10.3390/plants12050987
Chicago/Turabian StyleIamonico, Duilio, Amara Noor Hussain, Arya Sindhu, Venugopalan nair Saradamma Anil Kumar, Shabnum Shaheen, Mamoona Munir, and Paola Fortini. 2023. "Trying to Understand the Complicated Taxonomy in Amaranthus (Amaranthaceae): Insights on Seeds Micromorphology" Plants 12, no. 5: 987. https://doi.org/10.3390/plants12050987
APA StyleIamonico, D., Hussain, A. N., Sindhu, A., Saradamma Anil Kumar, V. n., Shaheen, S., Munir, M., & Fortini, P. (2023). Trying to Understand the Complicated Taxonomy in Amaranthus (Amaranthaceae): Insights on Seeds Micromorphology. Plants, 12(5), 987. https://doi.org/10.3390/plants12050987







