Changes in Metal-Chelating Metabolites Induced by Drought and a Root Microbiome in Wheat
Abstract
:1. Introduction
2. Results
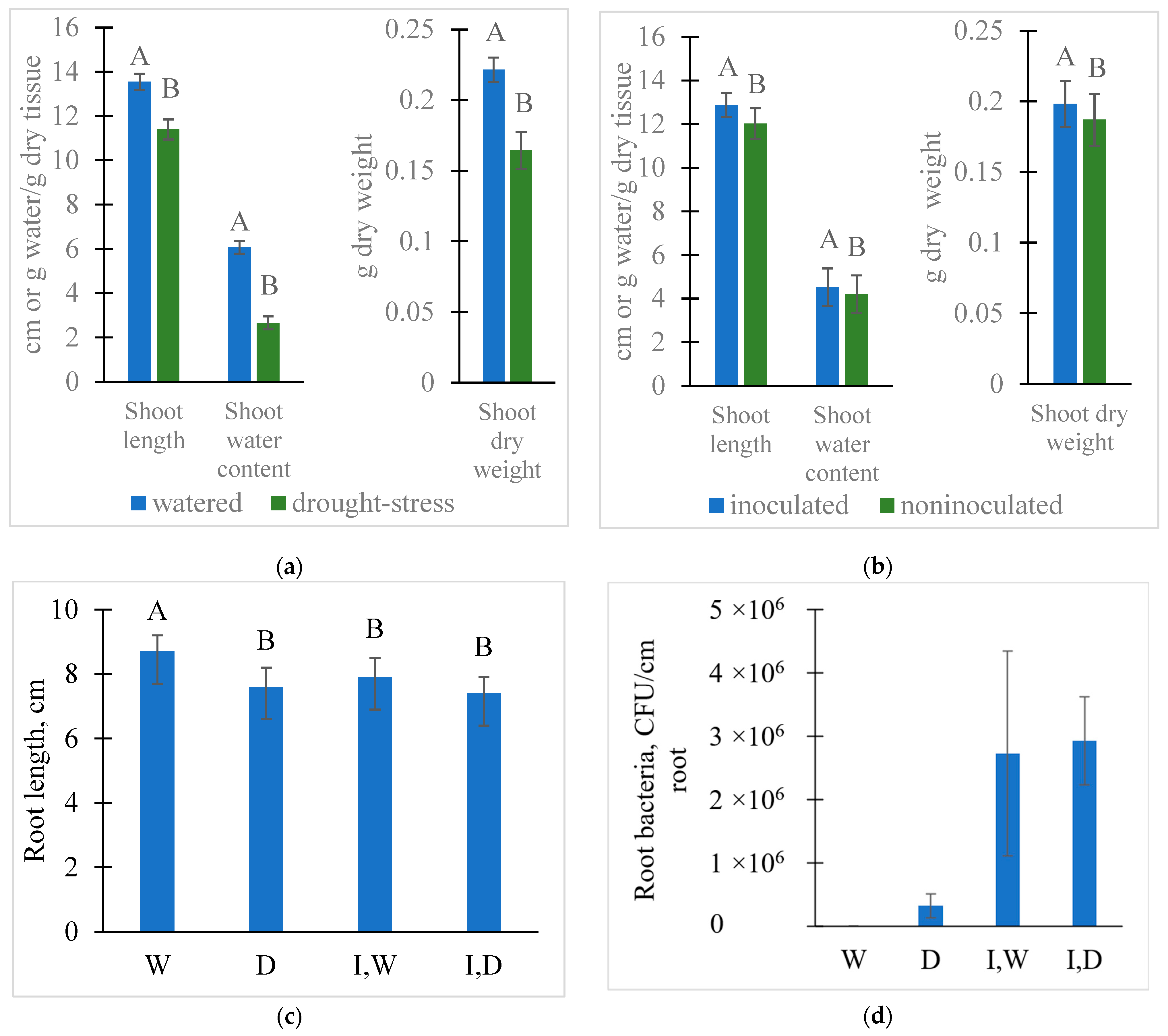
2.1. Treatment Effects on Seedling Growth, Microbial Colonization, and Rhizosphere Solution Properties
2.2. Metabolite Changes in Shoots with Drought and PcO6 Root Colonization
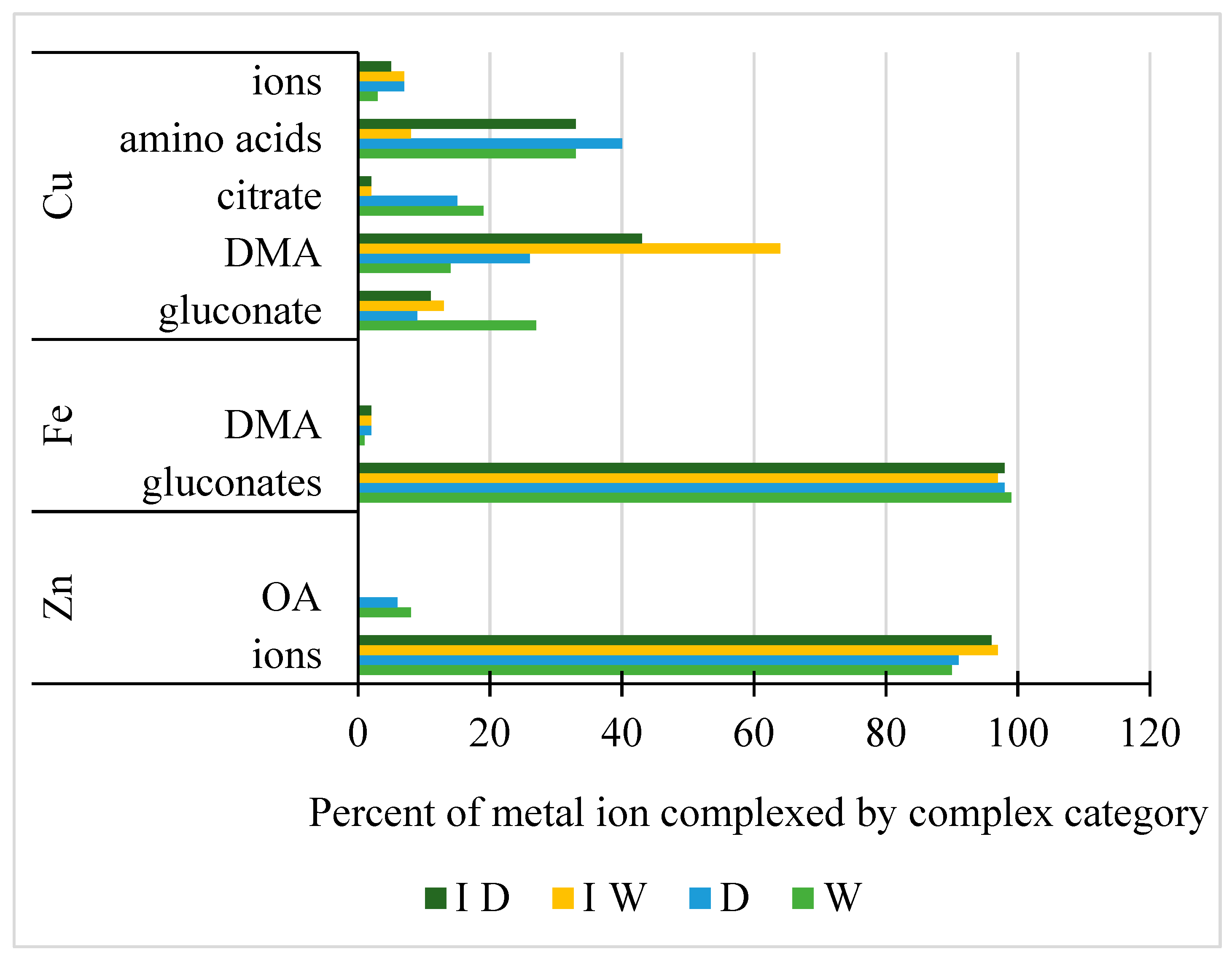
2.3. Changes in Metabolites in the Rhizosphere Solutions by Drought and PcO6 Colonization
2.4. Geochemical Modeling of Metal Chelation in the Rhizosphere Solutions
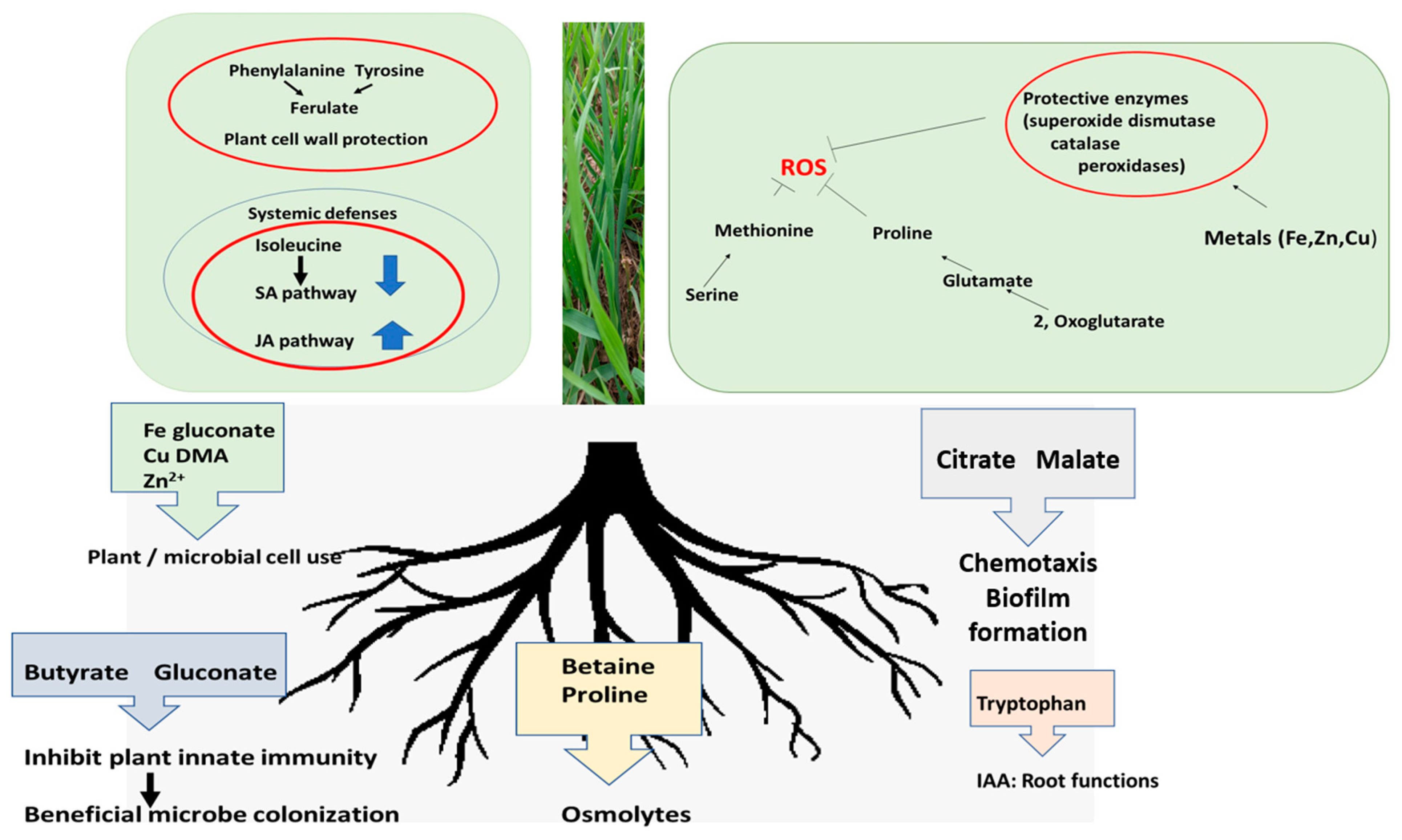
3. Discussion
4. Materials and Methods
4.1. Wheat Growth with and without a Drought Stress and Colonization by PcO6
4.2. Determination of Plant Growth and Colonization of Plant Roots
4.3. Shoot Extraction and Analysis
4.4. Extraction of Rhizosphere Solutions and Assay of Properties
4.5. Data Transformations, Statistical Analysis, and QC
4.6. Geochemical Modeling
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Vries, F.T.; Griffiths, R.I.; Knight, C.G.; Nicolitch, O.; Williams, A. Harnessing rhizosphere microbiomes for drought-resilient crop production. Science 2020, 368, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.K.; Lundberg, D.; Torres, M.A.; Matthews, R.; Akimoto-Tomiyama, C.; Farmer, L.; Dangl, J.L.; Grant, S.R. The Pseudomonas syringae type III effector HopAM1 enhances virulence on water-stressed plants. Mol. Plant-Microbe Interact. 2008, 21, 361–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolozsváriné, N.J.; Schwarczinger, I.; Király, L.; Bacsó, R.; Ádám, A.L.; Künstler, A. Near-isogenic barley lines show enhanced susceptibility to powdery mildew infection following high-temperature stress. Plants 2022, 11, 903. [Google Scholar] [CrossRef]
- Yu, J.; Hennessy, D.A.; Tack, J.; Wu, F. Climate change will increase aflatoxin presence in US corn. Environ. Res. Lett. 2022, 17, 054017. [Google Scholar] [CrossRef]
- Zhang, J.; Kirkham, M.B. Drought-stress-induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species. Plant Cell Physiol. 1994, 35, 785–791. [Google Scholar] [CrossRef]
- Huseynova, I.M.; Aliyeva, D.R.; Aliyev, J.A. Subcellular localization and responses of. superoxide dismutase isoforms in local wheat varieties subjected to continuous soil drought. Plant Physiol. Biochem. 2014, 81, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Bown, J.B.; Erwin, T.A.; Juttner, J.; Schnurbusch, T.; Langridge, P.; Bacic, A.; Roessner, U. Drought responses of leaf tissues from wheat cultivars of differing drought tolerance at the metabolite level. Mol. Plant 2012, 5, 418–429. [Google Scholar] [CrossRef] [Green Version]
- Guo, R.; Shi, L.; Jiao, Y.; Li, M.; Zhong, X.; Gu, F.; Liu, Q.; Xia, X.; Li, H. Metabolic responses to drought stress in the tissues of drought-tolerant and drought-sensitive wheat genotype seedlings. AoB Plants 2018, 10, ply016. [Google Scholar] [CrossRef] [Green Version]
- Rai, S.; Singh, P.K.; Mankotia, S.; Swain, J.; Satbhai, S.B. Iron homeostasis in plants and its crosstalk with copper, zinc, and manganese. Plant Stress 2021, 1, 100008. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Singh, U.; Bindraban, P.S.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Zinc oxide nanoparticles alleviate drought-induced alterations in sorghum performance, nutrient acquisition, and grain fortification. Sci. Total Environ. 2019, 688, 926–934. [Google Scholar] [CrossRef]
- Alabdallah, N.M.; Hasan, M.M.; Hammami, I.; Alghamdi, A.I.; Alshehri, D.; Alatawi, H.A. Green synthesized metal oxide nanoparticles mediate growth regulation and physiology of crop plants under drought stress. Plants 2021, 10, 1730. [Google Scholar] [CrossRef]
- Potter, M.; Deakin, J.; Cartwright, A.; Hortin, J.; Sparks, D.; Anderson, A.J.; McLean, J.E.; Jacobson, A.; Britt, D.W. Absence of nanoparticle-induced drought tolerance in nutrient sufficient wheat seedlings. Environ. Sci. Technol. 2021, 55, 13541–13550. [Google Scholar] [CrossRef]
- Lurthy, T.; Cantat, C.; Jeudy, C.; Declerck, P.; Gallardo, K.; Barraud, C.; Leroy, F.; Ourry, A.; Lemanceau, P.; Salon, C.; et al. Impact of bacterial siderophores on iron status and ionome in pea. Front. Plant Sci. 2020, 11, 730. [Google Scholar] [CrossRef]
- Sammauria, R.; Kumawat, S.; Kumawat, P.; Singh, J.; Jatwa, T.K. Microbial inoculants: Potential tool for sustainability of agricultural production systems. Arch. Microbiol. 2020, 202, 677–693. [Google Scholar] [CrossRef] [PubMed]
- Mitter, E.K.; Tosi, M.; Obregón, D.; Dunfield, K.E.; Germida, J.J. Rethinking crop nutrition in times of modern microbiology: Innovative biofertilizer technologies. Front. Sustain. Food Syst. 2021, 5, 606815. [Google Scholar] [CrossRef]
- McManus, P.; Hortin, J.; Anderson, A.J.; Jacobson, A.R.; Britt, D.W.; Stewart, J.; McLean, J.E. Rhizosphere interactions between copper oxide nanoparticles and wheat root exudates in a sand matrix: Influences on copper bioavailability and uptake. Environ. Toxicol. Chem. 2018, 37, 2619–2632. [Google Scholar] [CrossRef]
- Williams, A.; de Vries, F.T. Plant root exudation under drought: Implications for ecosystem functioning. New Phytol. 2020, 225, 1899–1905. [Google Scholar] [CrossRef] [Green Version]
- Palta, J.A.; Gregory, P.J. Drought affects the fluxes of carbon to roots and soil in 13C pulse-labelled plants of wheat. Soil Biol. Biochem. 1997, 29, 1395–1403. [Google Scholar] [CrossRef]
- Henry, A.; Doucette, W.; Norton, J.; Bugbee, B.J. Changes in crested wheatgrass root exudation caused by flood, drought, and nutrient stress. Environ. Qual. 2007, 36, 904–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, F.; Han, X.; Zhu, X.; Herbert, S.J. Response to water stress of soil enzymes and root exudates from drought and non-drought tolerant corn hybrids at different growth stages. Can. J. Soil Sci. 2012, 92, 501–507. [Google Scholar] [CrossRef]
- Canarini, A.; Merchant, A.; Dijkstra, F.A. Drought effects on Helianthus annuus and Glycine max metabolites: From phloem to root exudate. Rhizosphere 2016, 2, 85–97. [Google Scholar] [CrossRef]
- Warren, C. Response of organic N monomers in a sub-alpine soil to a dry wet cycle. Soil Biol. Biochem. 2014, 77, 233–242. [Google Scholar] [CrossRef]
- Naylor, D.; Coleman-Derr, D. Drought stress and root-associated bacterial communities. Front Plant Sci. 2018, 9, 2223. [Google Scholar] [CrossRef]
- De Vries, F.T.; Williams, A.; Stringer, F.; Willcocks, R.; McEwing, R.; Langridge, H.; Straathof, A.L. Changes in root-exudate-induced respiration reveal a novel mechanism through which drought affects ecosystem carbon cycling. New Phytol. 2019, 224, 132–145. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Dong, Z.; Chiniquy, D.; Pierroz, G.; Deng, S.; Gao, C.; Diamond, S.; Simmons, T.; Wipf, H.M.-L.; Caddell, D.; et al. Genome-resolved metagenomics reveals role of iron metabolism in drought-induced rhizosphere microbiome dynamics. Nat. Commun. 2021, 12, 3209. [Google Scholar] [CrossRef]
- Mark, G.L.; Dow, J.M.; Kiely, P.D.; Higgins, H.; Haynes, J.; Baysse, C.; Abbas, A.; Foley, T.; Franks, A.; Morrissey, J.; et al. Transcriptome profiling of bacterial responses to root exudates identifies genes involved in microbe-plant interactions. Proc. Natl. Acad. Sci. USA 2005, 102, 17454–17459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, K.; Pieterse, C.M.J.; Bakker, P.A.H.M.; Berendsen, R.L. Beneficial microbes going underground of root immunity. Plant Cell Environ. 2019, 42, 2860–2870. [Google Scholar] [CrossRef] [Green Version]
- Hartman, K.; Tringe, S.G. Interactions between plants and soil shaping the root microbiome under abiotic stress. Biochem. J. 2019, 476, 2705–2724. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Yao, Z.; Sun, Y.; Wang, E.; Tian, C.; Sun, Y.; Liu, J.; Sun, C.; Tian, L. Current studies of the effects of drought stress on root exudates and rhizosphere microbiomes of crop plant species. Int. J. Mol. Sci. 2022, 23, 2374. [Google Scholar] [CrossRef]
- Seitz, V.A.; McGivern, B.B.; Daly, R.A.; Chaparro, J.M.; Borton, M.A.; Sheflin, A.M.; Kresovich, S.; Shields, L.; Schipanski, M.E.; Wrighton, K.C.; et al. Variation in root exudate composition influences soil microbiome membership and function. Appl. Environ. Microbiol. 2022, 88, e00226-22. [Google Scholar] [CrossRef]
- Feng, H.; Fu, R.; Hou, X.; Ly, Y.; Zhang, N.; Liu, Y.; Xu, Z.; Miao, Y.; Krell, T.; Shen, Q.; et al. Chemotaxis of beneficia rhizobacteria to root exudates: The first step towards root–microbe rhizosphere interactions. Int. J. Mol. Sci. 2021, 22, 6655. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, D.; Liu, Y.; Li, S.; Shen, Q.; Zhang, R. Effects of different plant root exudates and their organic acid components on chemotaxis, biofilm formation and colonization by beneficial rhizosphere-associated bacterial strains. Plant Soil 2014, 374, 689–700. [Google Scholar] [CrossRef]
- Stringlis, I.A.; Yu, K.; Feussner, K.; de Jonge, R.; Van Bentum, S.; Van Verk, M.C.; Berendsen, R.L.; Bakker, P.A.H.M.; Feussner, I.; Pieterse, C.M.J. MYB72-dependent coumarin exudation shapes root microbiome assembly to promote plant health. Proc. Natl. Acad. Sci. USA 2018, 115, E5213–E5222. [Google Scholar] [CrossRef] [Green Version]
- Lombardi, N.; Vitale, S.; Turrà, D.; Reverberi, M.; Fanelli, C.; Vinale, F.; Marra, R.; Ruocco, M.; Pascale, A.; d’Errico, G.; et al. Root exudates of stressed plants stimulate and attract Trichoderma soil fungi. Mol. Plant-Microbe Interact. 2018, 31, 982–994. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Zhao, J.; Wen, T.; Zhao, M.; Li, R.; Goossens, P.; Huang, Q.; Bai, Y.; Vivanco, J.M.; Kowalchuk, G.A.; et al. Root exudates drive the soil-borne legacy of aboveground pathogen infection. Microbiome 2018, 6, 156. [Google Scholar] [CrossRef] [Green Version]
- Radtke, C.; Cook, W.S.; Anderson, A. Factors affecting antagonism of the growth of Phanerochaete chrysosporium by bacteria isolated from soils. Appl. Microbiol. Biotechnol. 1994, 41, 274–280. [Google Scholar] [CrossRef]
- Cho, S.M.; Kang, B.R.; Han, S.H.; Anderson, A.J.; Park, J.Y.; Lee, Y.H.; Cho, B.H.; Yang, K.Y.; Ryu, C.M.; Kim, Y.C. 2R, 3R-butanediol, a bacterial volatile produced by Pseudomonas chlororaphis O6, is involved in induction of systemic tolerance to drought in Arabidopsis thaliana. Mol. Plant-Microbe Interact. 2008, 21, 1067–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.M.; Kim, Y.H.; Anderson, A.J.; Kim, Y.C. Nitric oxide and hydrogen peroxide production are involved in systemic drought tolerance induced by 2R, 3R-butanediol in Arabidopsis thaliana. Plant Path. J. 2013, 29, 427. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, A.; Doxey, S.; Potter, M.; Adams, J.; Britt, D.; McManus, P.; McLean, J.; Anderson, A. Interactions between a plant probiotic and nanoparticles on plant responses related to drought tolerance. Ind. Biotechnol. 2018, 14, 148–156. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.Y.; Doxey, S.; McLean, J.E.; Britt, D.; Watson, A.; Al Qassy, D.; Jacobson, A.; Anderson, A.J. Remodeling of root morphology by CuO and ZnO nanoparticles: Effects on drought tolerance for plants colonized by a beneficial pseudomonad. Botany 2018, 96, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Cho, S.M.; Kang, E.Y.; Im, Y.J.; Hwangbo, H.; Kim, Y.C.; Ryu, C.M.; Yang, K.Y.; Chung, G.C.; Cho, B.H. Galactinol is a signaling component of the induced systemic resistance caused by Pseudomonas chlororaphis O6 root colonization. Mol. Plant-Microbe Interact. 2008, 21, 1643–1653. [Google Scholar] [CrossRef] [Green Version]
- Hortin, J.M.; Anderson, A.J.; Britt, D.W.; Jacobson, A.R.; McLean, J.E. Soil-derived fulvic acid and root exudates, modified by soil bacteria, alter CuO nanoparticle-induced root stunting of wheat via Cu complexation. Environ. Sci. Nano 2019, 6, 3638–3652. [Google Scholar] [CrossRef]
- Hortin, J.M.; Anderson, A.J.; Britt, D.W.; Jacobson, A.R.; McLean, J.E. Copper oxide nanoparticle dissolution at alkaline pH is controlled by dissolved organic matter: Influence of soil-derived organic matter, wheat, bacteria, and nanoparticle coating. Environ. Sci. Nano 2020, 7, 2618–2631. [Google Scholar] [CrossRef]
- Waditee, R.; Bhuiyan, N.H.; Hirata, E.; Hibino, T.; Tanaka, Y.; Shikata, M.; Takabe, T.J. Metabolic engineering for betaine accumulation in microbes and plants. J. Biol. Chem. 2007, 282, 34185–34193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furlan, A.L.; Bianucci, E.; Giordano, W.; Castro, S.; Becker, D.F. Proline metabolic dynamics and implications in drought tolerance of peanut plants. Plant Physiol. Biochem. 2020, 151, 566–578. [Google Scholar] [CrossRef]
- Basirat, M.; Mousavi, S.; Abbaszadeh, S.; Ebrahimi, M.; Zarebanadkouki, M. The rhizosheath: A potential root trait helping plants to tolerate drought stress. Plant Soil 2019, 45, 565–575. [Google Scholar] [CrossRef]
- Bonebrake, M.; Anderson, K.; Valiente, J.; Jacobson, A.; McLean, J.E.; Anderson, A.; Britt, D.W. Biofilms benefiting plants exposed to ZnO and CuO nanoparticles studied with a root-mimetic hollow fiber membrane. Agric. Food Chem. 2018, 66, 6619–6627. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Carroll, A.J.; Estavillo, G.M.; Rebetzke, G.J.; Pogson, B.J. Wheat drought tolerance in the field is predicted by amino acid responses to glasshouse-imposed drought. J. Exp. Bot. 2019, 70, 4931–4948. [Google Scholar] [CrossRef] [PubMed]
- Marček, T.; Hamow, K.Á.; Végh, B.; Janda, T.; Darko, E. Metabolic response to drought in six winter wheat genotypes. PLoS ONE 2019, 14, e0212411. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Morris, C.F.; Bettge, A.D. Genotype and environment variation for arabinoxylans in hard winter and spring wheats of the U.S. Pacific Northwest. Cereal Chem. 2009, 86, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Peremarti, A.; Marè, C.; Aprile, A.; Roncaglia, E.; Cattivelli, L.; Villegas, D.; Royo, C. Transcriptomic and proteomic analyses of a pale-green durum wheat mutant shows variations in photosystem components and metabolic deficiencies under drought stress. BMC Genom. 2014, 15, 125. [Google Scholar] [CrossRef] [PubMed]
- Ebeed, H.T.; Stevenson, S.R.; Cuming, A.C.; Baker, A. Conserved and differential transcriptional responses of peroxisome associated pathways to drought, dehydration and ABA. J. Exp. Bot. 2018, 69, 4971–4985. [Google Scholar] [CrossRef] [PubMed]
- Gardestrom, P.; Edwards, G.E.; Henricson, D.; Ericson, I. The localization of serine hydroxymethyltransferase in leaves of C3 and C4 species. Physiol. Plant. 1985, 54, 29–33. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Bykova, N.V.; Kleckowski, L.A. Origins and metabolism of formate in higher plants. Plant Physiol. Biochem. 1999, 37, 503–513. [Google Scholar] [CrossRef]
- Biehler, K.; Fock, H. Evidence for the contribution of the Mehler-peroxidase reaction in dissipating excess electrons in drought-stressed wheat. Plant Physiol. 1996, 112, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Rasheed, S.; Bashir, K.; Kim, J.M.; Ando, M.; Tanaka, M.; Seki, M. The modulation of acetic acid pathway genes in Arabidopsis improves survival under drought stress. Sci. Rep. 2018, 8, 7831. [Google Scholar] [CrossRef]
- Hura, T.; Hura, K.; Grzesiak, S.J. Possible contribution of cell-wall-bound ferulic acid in drought resistance and recovery in triticale seedlings. Plant Physiol. 2009, 166, 1720–1733. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, A.; Klöppner, U.; Derst, C.; Röhm, K.H. Utilization of acidic amino acids and their amides by pseudomonads: Role of periplasmic glutaminase-asparaginase. Arch. Microbiol. 2003, 179, 151–159. [Google Scholar] [CrossRef]
- Molina, L.; Rosa, R.; Nogales, J.; Rojo, F. Pseudomonas putida KT2440 metabolism undergoes sequential modifications during exponential growth in a complete medium as compounds are gradually consumed. Environ. Microbiol. 2019, 21, 2375–2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, J.; Wright, M.; Wagner, H.; Valiente, J.; Britt, D.; Anderson, A. Cu from dissolution of CuO nanoparticles signals changes in root morphology. Plant Phys. Biochem. 2017, 110, 108–117. [Google Scholar] [CrossRef] [Green Version]
- Bienert, M.D.; Werner, L.M.; Wimmer, M.A.; Bienert, G.P. Root hairs: The villi of plants. Biochem. Soc. Trans. 2021, 49, 1133–1146. [Google Scholar] [CrossRef]
- Scribani Rossi, C.; Barrientos-Moreno, L.; Paone, A.; Cutruzzolà, F.; Paiardini, A.; Espinosa-Urgel, M.; Rinaldo, S. Nutrient sensing and biofilm modulation: The example of L-arginine in Pseudomonas. Int. J. Mol. Sci. 2022, 23, 4386. [Google Scholar] [CrossRef]
- Alharby, H.F.; Al-Zahrani, H.S.; Alzahrani, Y.M.; Alsamadany, H.; Hakeem, K.R.; Rady, M.M. Maize grain extract enriched with polyamines alleviates drought stress in Triticum aestivum through up-regulation of the ascorbate–glutathione cycle, glyoxalase system, and polyamine gene expression. Agronomy 2021, 11, 949. [Google Scholar] [CrossRef]
- Chen, C.; Beattie, G.A. Pseudomonas syringae BetT is a low-affinity choline transporter that is responsible for superior osmoprotection by choline over glycine betaine. J. Bacteriol. 2008, 190, 2717–2725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Beskrovnaya, P.; Melnyk, R.A.; Hossain, S.S.; Khorasani, S.; O’Sullivan, L.R.; Wiesmann, C.L.; Bush, J.; Richard, J.D.; Haney, C.H. A genome-wide screen identifies genes in rhizosphere-associated Pseudomonas required to evade plant defenses. mBio 2018, 9, e00433-18. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.; Liu, Y.; Tichelaar, R.; Savant, N.; Lagendijk, E.; van Kuijk, S.J.L.; Stringlis, I.A.; van Dijken, A.J.H.; Pieterse, C.M.J.; Bakker, P.A.H.M.; et al. Rhizosphere-associated Pseudomonas suppress local root immune responses by gluconic acid-mediated lowering of environmental pH. Curr. Biol. 2019, 29, 3913–3920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vital, M.; Howe, A.C.; Tiedje, J.M. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. mBio 2014, 5, e00889-14. [Google Scholar] [CrossRef] [Green Version]
- Crawford, L.A.; Bown, A.W.; Breitkreuz, K.E.; Cuine, F.C. The synthesis of [gamma]-aminobutyric acid in response to treatments reducing cytosolic pH. Plant Physiol. 1994, 104, 865–871. [Google Scholar] [CrossRef] [Green Version]
- Pagano, A.; de Sousa Araújo, S.; Macovei, A.; Dondi, D.; Lazzaroni, S.; Balestrazzi, A. Metabolic and gene expression hallmarks of seed germination uncovered by sodium butyrate in Medicago truncatula. Plant Cell Environ. 2019, 42, 259–269. [Google Scholar] [CrossRef] [Green Version]
- Bie, X.M.; Dong, L.; Li, X.H.; Wang, H.; Gao, X.-P.; Li, X.G. Trichostatin A and sodium butyrate promotes plant regeneration in common wheat. Plant Signal. Behav. 2020, 15, 1820681. [Google Scholar] [CrossRef] [PubMed]
- Blackmon, R.H.; Bell, M.T.; McDaniel, M.A.; Harmon, G.L. Sodium butyrate induces superoxide dismutase in rapid-cycling Brassica rapa. Int. J. New Technol. Sci. Eng. 2016, 3, 1–13. [Google Scholar]
- Huang, X.X.; Zhu, G.Q.; Liu, Q.; Chen, L.; Li, Y.J.; Hou, B.K. Modulation of plant salicylic acid-associated immune responses via glycosylation of dihydroxybenzoic acids. Plant Physiol. 2018, 176, 3103–3119. [Google Scholar] [CrossRef]
- Maksym, R.P.; Ghirardo, A.; Zhang, W.; von Saint Paul, V.; Lange, B.; Geist, B.; Hajirezaei, M.-R.; Schnitzler, J.-P.; Schäffner, A.R. The defense-related isoleucic acid differentially accumulates in Arabidopsis among branched-chain amino acid-related 2-hydroxy carboxylic acids. Front. Plant Sci. 2018, 9, 766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Han, X.; Feng, D.; Yuan, D.; Huang, L.J. Signaling crosstalk between salicylic acid and ethylene/jasmonate in plant defense: Do we understand what they are whispering? Int. J. Mol. Sci. 2019, 20, 671. [Google Scholar] [CrossRef] [Green Version]
- Spencer, M.; Ryu, C.-M.; Yang, K.Y.; Kim, Y.C.; Kloepper, J.W.; Anderson, A.J. Induced defence in tobacco by Pseudomonas chlororaphis O6 involves at least the ethylene pathway. Physiol. Mol. Plant. Pathol. 2003, 63, 27–34. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Xie, J.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Jiang, D. Abscisic acid and jasmonic acid are involved in drought priming-induced tolerance to drought in wheat. Crop J. 2021, 9, 120–132. [Google Scholar] [CrossRef]
- Kim, D.R.; Jeon, C.W.; Cho, G.; Thomashow, L.S.; Weller, D.M.; Paik, M.J.; Lee, Y.B.; Kwak, Y.S. Glutamic acid reshapes the plant microbiota to protect plants against pathogens. Microbiome 2021, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lucena, P.; Hernández-Apaolaza, L.; Lucena, J.J. Comparison of iron chelates and complexes supplied as foliar sprays and in nutrient solution to correct iron chlorosis of soybean. Plant Nutr. Soil Sci. 2010, 173, 120–126. [Google Scholar] [CrossRef]
- Martín-Fernández, C.; Solti, Á.; Czech, V.; Kovács, K.; Fodor, F.; Gárate, A.; Hernández-Apaolaza, L.; Lucena, J.J. Response of soybean plants to the application of synthetic and biodegradable Fe chelates and Fe complexes. Plant Physiol. Biochem. 2017, 118, 579–588. [Google Scholar] [CrossRef] [Green Version]
- Hacisalihoglu, G.; Hart, J.J.; Kochian, L.V. High- and low-affinity zinc transport systems and their possible role in zinc efficiency in bread wheat. Plant Physiol. 2001, 125, 456–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alloway, B.J. Soil factors associated with zinc deficiency in crops and humans. Environ. Geochem. Health 2009, 31, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Moridi, A.; Zarei, M.; Moosavi, A.; Ronaghi, A. Influence of PGPR-enriched liquid organic fertilizers on the growth and nutrients uptake of maize under drought condition in calcareous soil. J. Plant Nutr. 2019, 42, 2745–2756. [Google Scholar] [CrossRef]
- Tadayyon, A.; Pejman Nikneshan, P.; Pessarakli, M. Effects of drought stress on concentration of macro- and micro-nutrients in Castor (Ricinus communis L.) plant. J. Plant Nutr. 2018, 41, 304–310. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Calder, A.; Britt, D.W.; McLean, J.E.; Anderson, A.J. Responses of a soil bacterium, Pseudomonas chlororaphis O6 to commercial metal oxide nanoparticles compared with responses to metal ions. Environ. Pollut. 2011, 159, 1749–1756. [Google Scholar] [CrossRef]
- Zörb, C.; Steinfurth, D.; Gödde, V.; Niehaus, K.; Mühling, K.H. Metabolite profiling of wheat flag leaf and grains during grain filling phase as affected by sulfure fertilization. Funct. Plant Biol. 2012, 39, 156–166. [Google Scholar] [CrossRef]
- Le, T.T.; Shafaei, A.; Genoni, A.; Christophersen, C.T.; Devine, A.; Lo, J.; Wall, P.L.; Boyce, M.C. Development and validation of a simple LC-MS/MS method for the simultaneous quantitative determination of trimethylamine-N-oxide and branched chain amino acids in human serum. Anal. Bioanal. Chem. 2018, 411, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tu, M.J.; Zhang, C.; Jilek, J.L.; Zhang, Q.Y.; Yu, A.M. A reliable LC-MS/MS method for the quantification of natural amino acids in mouse plasma: Method validation and application to a study on amino acid dynamics during hepatocellular carcinoma progression. J. Chromatogr. B 2019, 1124, 72–81. [Google Scholar] [CrossRef]
- Boiteau, R.M.; Shaw, J.B.; Pasa-Tolić, L.; Koppenaal, D.W.; Jansson, J.K. Micronutrient metal speciation is controlled by competitive organic chelation in grassland soils. Soil Biol. Biochem. 2018, 120, 283–291. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater; American Public Health Assocation: Washington, DC, USA, 2012. [Google Scholar]
- United States Environmental Protection Agency. EPA Publication SW-846, Test Methods for Evaluating Solid Waste, Physical/Chemical Methods, Final Updates I (1993), II (1995), IIA (1994), IIB (1995), III (1997), IIIA (1999), IIIB (2005), IV (2008), and V (2015), 3rd ed.; Environmental Protection Agency: Washington, DC, USA, 2015.
- Cohen, A.C. Simplified estimators for the normal distribution when samples are singly censored or truncated. Technometrics 1959, 1, 217–237. [Google Scholar] [CrossRef]
- Gustafsson, J.P. Visual MINTEQ, ver. 3.1; KTH: Stockholm, Sweden, 2013. [Google Scholar]
- Igamberdiev, A.U.; Eprinstev, A.T. Organic acids: The pools of fixed carbon involved in redox regulation and energy balance in higher plants. Front. Plant Sci. 2016, 7, 1042. [Google Scholar] [CrossRef] [Green Version]
- Dupont, F.M. Metabolic pathways of the wheat (Triticum aestivum) endosperm amyloplast revealed by proteomics. BMC Plant Biol. 2008, 8, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Growth Condition | Normalized % Cu | Normalized % Zn | Normalized % Fe |
|---|---|---|---|
| Non-inoculated, watered | 100 | 100 | 100 |
| Non-inoculated, drought-stressed | 137 | 105 | 56 |
| PcO6-colonized, watered | 54 | 91 | 39 |
| PcO6-colonized, drought-stressed | 77 | 98 | 45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, A.J.; Hortin, J.M.; Jacobson, A.R.; Britt, D.W.; McLean, J.E. Changes in Metal-Chelating Metabolites Induced by Drought and a Root Microbiome in Wheat. Plants 2023, 12, 1209. https://doi.org/10.3390/plants12061209
Anderson AJ, Hortin JM, Jacobson AR, Britt DW, McLean JE. Changes in Metal-Chelating Metabolites Induced by Drought and a Root Microbiome in Wheat. Plants. 2023; 12(6):1209. https://doi.org/10.3390/plants12061209
Chicago/Turabian StyleAnderson, Anne J., Joshua M. Hortin, Astrid R. Jacobson, David W. Britt, and Joan E. McLean. 2023. "Changes in Metal-Chelating Metabolites Induced by Drought and a Root Microbiome in Wheat" Plants 12, no. 6: 1209. https://doi.org/10.3390/plants12061209
APA StyleAnderson, A. J., Hortin, J. M., Jacobson, A. R., Britt, D. W., & McLean, J. E. (2023). Changes in Metal-Chelating Metabolites Induced by Drought and a Root Microbiome in Wheat. Plants, 12(6), 1209. https://doi.org/10.3390/plants12061209







