Abstract
Polyphenol has been used in treatment for some health disorders due to their diverse health promoting properties. These compounds can reduce the impacts of oxidation on the human body, prevent the organs and cell structure against deterioration and protect their functional integrity. The health promoting abilities are attributed to their high bioactivity imparting them high antioxidative, antihypertensive, immunomodulatory, antimicrobial, and antiviral activity, as well as anticancer properties. The application of polyphenols such as flavonoids, catechin, tannins, and phenolic acids in the food industry as bio-preservative substances for foods and beverages can exert a superb activity on the inhibition of oxidative stress via different types of mechanisms. In this review, the detailed classification of polyphenolic compunds and their important bioactivity with special focus on human health are addressed. Additionally, their ability to inhibit SARS-CoV-2 could be used as alternative therapy to treat COVID patients. Inclusions of polyphenolic compounds in various foods have demonstrated their ability to extend shelf life and they positive impacts on human health (antioxidative, antihypertensive, immunomodulatory, antimicrobial, anticancer). Additionally, their ability to inhibit the SARS-CoV-2 virus has been reported. Considering their natural occurrence and GRAS status they are highly recommended in food.
1. Introduction
Polyphenols are naturally occurring secondary bioactive compounds derived from plant sources, which show a wide range of bioactivity helping in promoting good health [1,2]. They are basically phenolic rings with attached functional groups. Application of polyphenols has received great attention in all segments from food processing to preservation and the pharmaceutical industry [3,4,5,6,7]. Previous studies have also shown application of several phenols in the formulation of traditional medicine [8,9].
Oxidative stress, hypertension, lowered immunity, microbial infections, and antimicrobial resistance have been reported to cause a large number of deaths globally [10]. The diverse bioactivity possessed by phenols is attributed to their structure (ring) that makes them capable of helping in the treatment of various diseases and disorders [11]. The COVID-19 pandemic has caused the full or partial closure of many countries due to the rapid spread of the virus and higher mortality rate, hampering businesses with an increase in work from home [12]. Polyphenols from natural resources are known to enhance immunity by modulating defence mechanisms [13,14].
Considering the wide availability of the polyphenols, their detailed classification, types, and sources are described. Recent developments relating to bioactivity of polyphenols as well as their antiviral activity against SARS-CoV-2 are also reviewed. Future prospects proposing the direction requiring research are also addressed.
2. Polyphenols
2.1. Classification
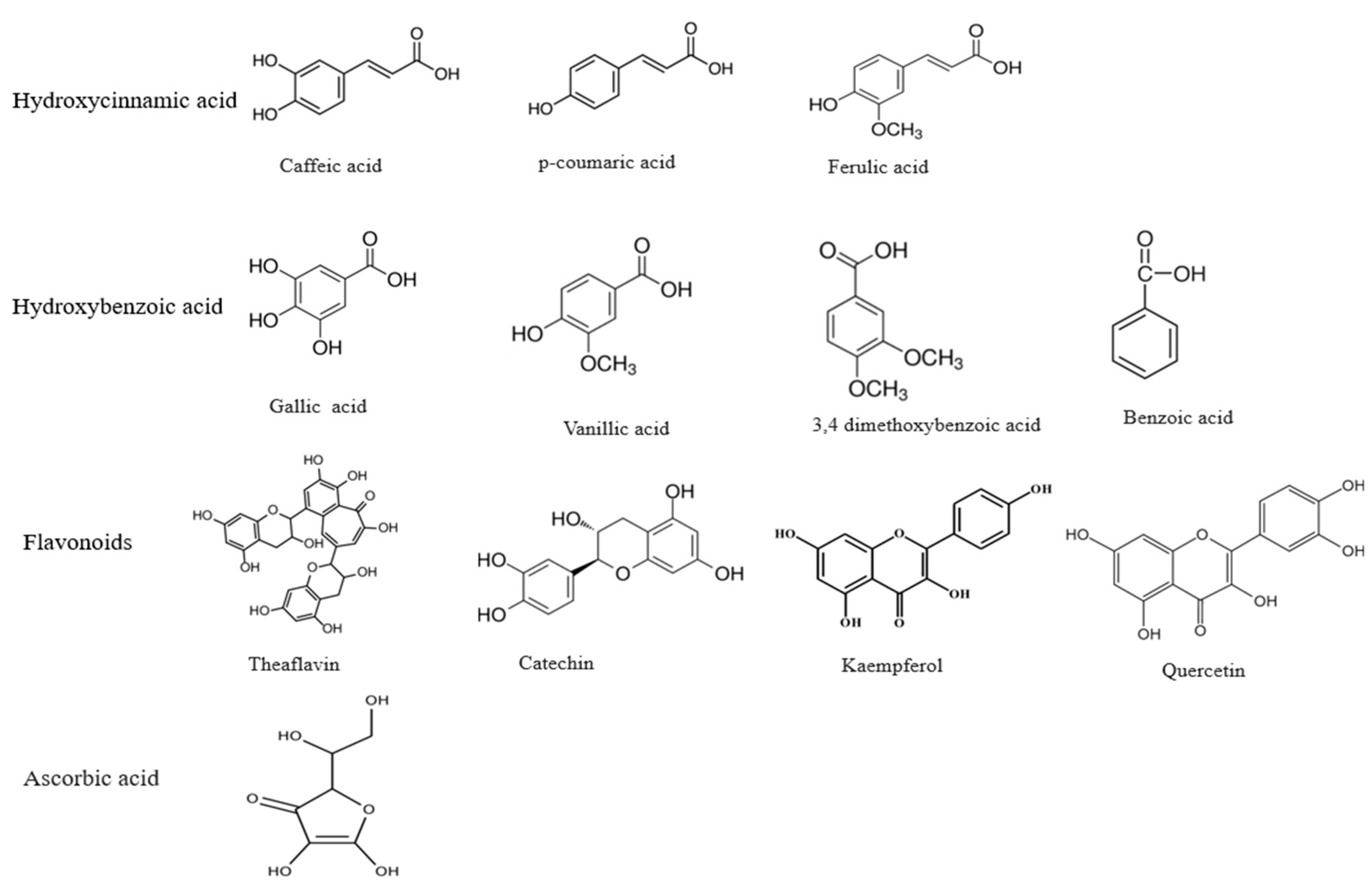
Dietary polyphenols comprise a broad category of natural compounds in the kingdom Plantae with two phenyl rings and one or more hydroxyl (OH) groups [15]. Approximately 8000 polyphenolic compounds are currently known, and more than 4000 belong to flavonoids only [16]. Polyphenols are a heterogeneous group of phenolic compounds [17] with two major classes: flavonoids and phenolic acids. Flavonoids are coloured compounds subdivided into flavones, flavanols, flavanones, flavonols, and isoflavones [18], whereas phenolic acids have two subgroups, namely hydroxycinnamic and hydroxybenzoic acids [19]. They are found in either a non-conjugated (aglycone) or conjugated form with glucose, organic acid, carboxylic acid, amines, lipids, etc. [18,20]. The structures of some important polyphenols are given in Figure 1.
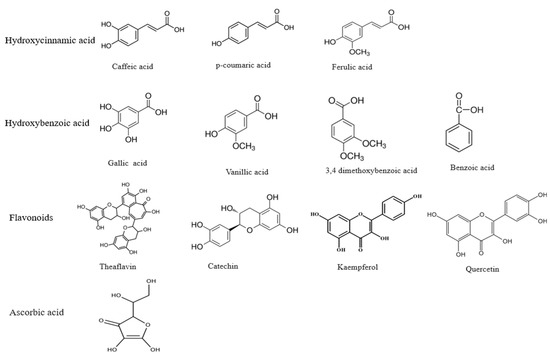
Figure 1.
Some important polyphenolic compounds.
2.2. Types
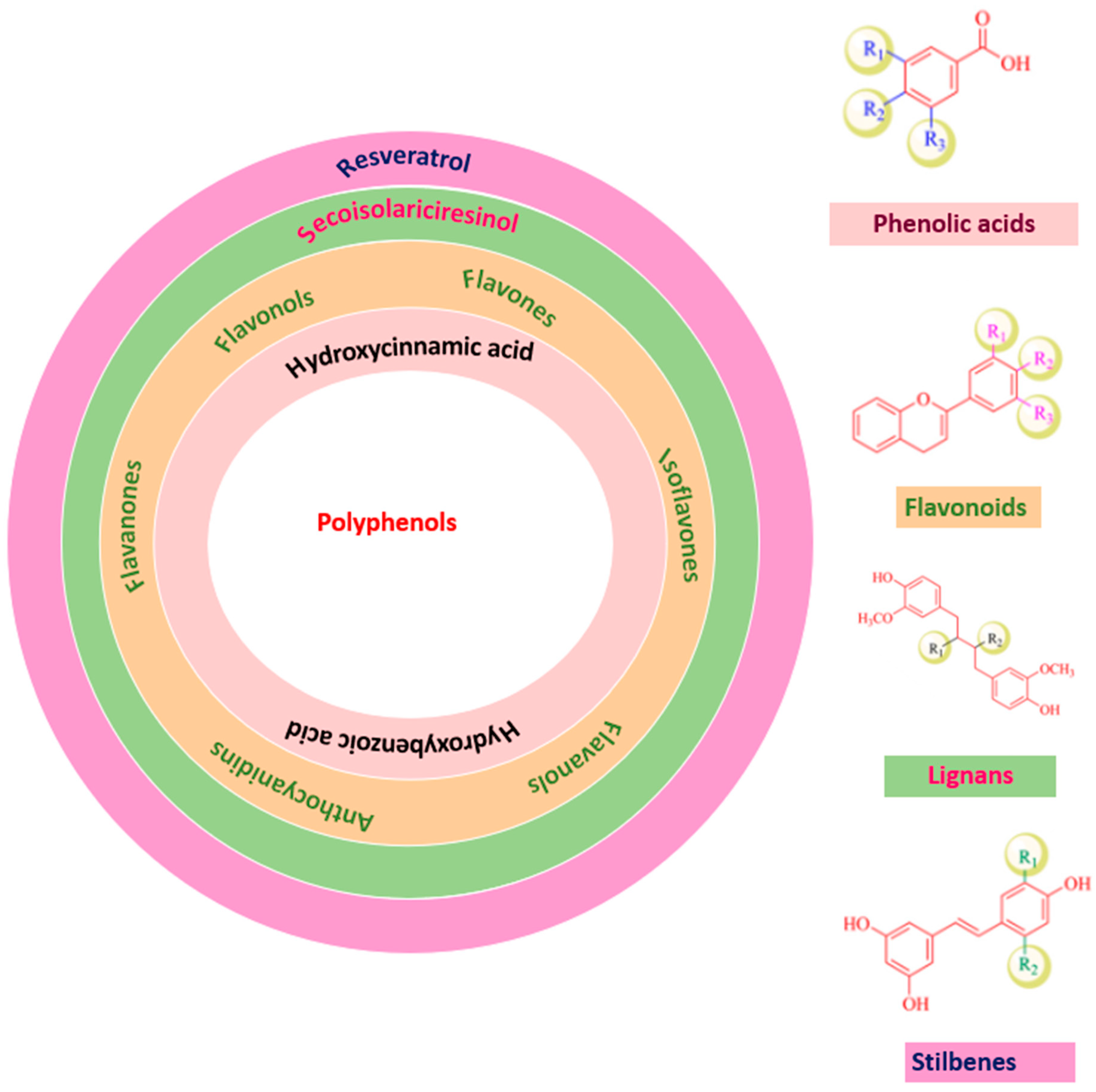
Phenolic compounds have many subclasses based on phenol units in the molecule, substituent groups, and the bond type between phenol units (Figure 2). Depending on their structure variation, polyphenols are categorized into phenolic acids, phenolic aldehyde (vanillin, salicylaldehyde, syringaldehyde, etc.), flavonoids, iso-flavonoids, tannins (hydrolyzable and condensed tannins), lignans, and lignins [21,22].
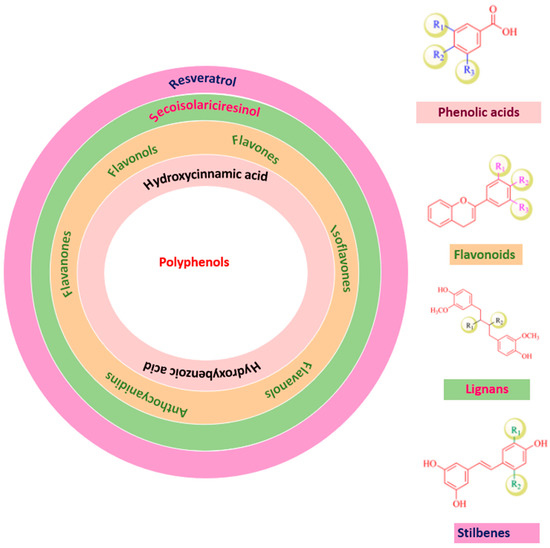
Figure 2.
Polyphenols classification.
2.2.1. Phenolic Acids
Phenolics are categorized as non-flavonoid polyphenols with several OH groups on aromatic rings. Two distinguishing parent skeletons of phenolic acids include benzoic acid containing seven C atoms (C6-C1), and cinnamic acid containing nine carbon atoms (C3-C6) [23]. Hydroxybenzoic acids that contribute to the human diet are rare, thus, not suggested to play a role in human health [19]. Benzoic acid derivatives are p-hydroxybenzoic acid, protocatechuic, salicylic, gallic, and ellagic acid, as well as cinnamic acid derivatives include p-coumaric, caffeic, and ferulic acid [24]. Phenolic acids are essential as human dietary components with tremendous health benefits, including antioxidative, anti-inflammatory, immunoregulatory, anti-allergic, anti-atherogenic, anti-microbial, cardioprotection, anti-cancer, and antidiabetic potential [25].
2.2.2. Flavonoids
Flavonoids are ubiquitous polyphenols that contribute to colourful pigments in fruits, vegetables, herbs, spices, and medicinal plants [26]. They have 15 carbons and two aromatic rings joined by a three-carbon bridge. Depending on the C-ring difference, they are further divided into flavones, flavanones, isoflavones, flavonols, flavan-3-ols, and anthocyanidins [27]. Each subgroup has some differences due to the pattern and degree of hydroxylation, prenylation, glycosylation, or methoxylation. Some examples of flavonoids are quercetin, catechin, naringenin, cyanidin-glycoside, and daidzein [28,29]. They exist as free aglycones and glycosidic conjugates [30,31] as well as various modified forms [32].
Flavone and Flavonols
Flavones comprise a three-ring skeleton with three functional groups; a C4 ketone, a conjugated C2-C3 double bond, and various numbers depending on the flavone of hydroxyl groups [33]. Compared to flavonols, flavones lack an OH group at the three-position. Major flavonols are quercetin, kaempferol, myricetin, and isorhamnetin. The most abundant flavones in plants are luteolin and apigenin [34]. Approximately 450 types of flavonol aglycones have been reported, although quercetin, kaempferol, myricetin, and isorhamnetin are commonly found in fruits [35].
Isoflavones
Isoflavones and their derivatives are eco physiologically active secondary metabolites derived from the phenylpropanoid pathway [36]. They are yellow-pigmented compounds and found in plants mostly as biologically inactive glycosides: 7-O-β-D-glycosides, 6″-O-acetyl-7-O-β-D-glucosides, and 6″-O-malonyl-7-O-β-D-glycosides [37]. Isoflavones exist as phytoestrogens due to their binding affinity for estrogens receptors. Isoflavones differ from other flavonoids in the position of benzene ring B in C3 [38]. Dietary isoflavone has been a good way to study its health benefits, i.e., healthy gut, osteoporosis prevention, anti-inflammatory, anti-cancer, anti-obesity, and anti-diabetic potential [39].
Flavanones
Flavanones are a therapeutically important flavonoids class in all citrus fruits, including oranges, lime, lemons, grapefruit, and grapes [40]. Hesperidin, naringenin, and eriodictyol are some flavanones [41]. Moreover, they are widely distributed in 42 larger plant families, including Compositae, Leguminosae, and Rutaceae. The pharmacological potential of flavanones includes free radical scavenger, anti-inflammatory, anticancer, cardiovascular, and antiviral [42].
Anthocyanins
They are naturally occurring pigmented components and the most important subclass of flavonoids [43]. They are glycosylated polyhydroxy and poly methoxy derivatives of two phenylbenzopyrylium (flavylium) salts. Pelargonidin, cyanidin, peonidin, delphinidin, petunidin, and malvidin are some common anthocyanins [44]. They occur predominantly in the outermost layers of berries (raspberries, cranberries, strawberries, blueberries, bilberries, blackberries), black currants, and red and merlot grapes [41]. Anthocyanins can scavenge free radicals by two hypothesized pathways; the first pathway is the attack hydroxyl group/s of the B-ring. The next is the attack of oxonium ion on the C-ring. Some of them are considered among the strongest antioxidants by adopting both pathways [43,45,46]. Additionally, they have potential in food industries as natural dyes and replacers of synthetic dyes [47].
2.2.3. Stilbenes
Another non-flavonoid class, stilbenes, contains two phenyl moieties attached by a 2-C methylene bridge. They contain two aromatic rings, A and B, and exist in isomeric (cis and trans), free and glycosylated forms. At m-position, ring A carries two hydroxyl groups, whereas many positions in ring B are substituted by hydroxy and methoxy groups [48]. Resveratrol (3,5,4′-trihydroxystilbene) is commonly stilbenes, which is produced by berries and grapes as well as nuts [49].
2.2.4. Lignans and Lignin
Two C6-C3 units linked together between positions 8 and 8′ are called lignan, another non-flavonoid compound. The positions of lignan C9 and C9′ are substituted in different patterns, and, thus, they have different structural forms. They are divided into subgroups such as furan, dibenzylbutane, and aryltetralin [48]. Lignans are mainly free form in pulses, seeds, and vegetable oils as the glycosylated structures are not abundant. Lignin is an aromatic biopolymer formed by phenolic oxidative coupling of p-hydroxycinnamoyl alcohol monomers by peroxidase enzymes [50]. The most important alcohols are 4-hydroxycinnamoyl, coniferyl, and sinapoyl.
2.2.5. Tannins
Tannins are a naturally occurring heterogeneous group of water-soluble high molecular weight phenolics. They are subdivided into hydrolyzable and condensed tannins. Hydrolyzable tannins are further categorized into gallotannins (hydrolysis yields sugar and gallic acid) and ellagitannins (hydrolysis yields sugar, gallic and ellagic acid [51]. They exhibit free radical scavenging potential (due to hydroxyl groups), which increases as the number of galloyl groups and molecular weight increase, and in the presence of ano-dihydroxy structure [50].
Recently, phlorotannins, types of tannin found in the sub-cellular structure of brown algae (accounting 25%) are gaining interest in food, feed, and drug industries [52]. They are polyphenolic compounds formed by polymerization of phloroglucinol (1,3,5-trihydroxybenzene) with a wide range of molecular sizes of between 126 and 650,000 Da. Based on linkage, phlorotannins are divided into four categories, including fuhalols (ether linkage), phlorethols (ether linkage), fucophloroethols (ether and phenyl linkage), and eckols (dibenzodioxin linkage) [53]. Phlorotannins have shown a great deal of promise as functional compounds with a number of bioactivities including anti-cancer, anti-diabetic, anti-inflammatory, anti-microbial, and anti-hypertensive [54]. Additionally, these phenolic compounds have significant scavenging potential against superoxide and free radicals so they could be used as antioxidants in the food industries. Beside these functionalities, they also serve as pancreatic lipase inhibitors, which have the potential to control dietary fat digestion. Hence, it can be used for weight control purposes [55].
2.3. Sources
There is growing interest in identifying and exploring polyphenolic sources to prepare functional beverages, extract polyphenolic compounds to fortify functional foods and beverages, or elaborate dietary supplements [56]. They have antioxidant properties that complement the functionality of vitamins and enzymes as a defence against oxidative stress caused by excessive ROS production [23]. Major polyphenolic sources are tabulated in Table 1.
3. Impacts of Polyphenols on Human Health
The polyphenols are known as secondary metabolites widely present in the plant kingdom. Polyphenols have important health-promoting impacts according to their antioxidant, antimicrobial, immunomodulatory, antihypertensive, anticancer, and anti-inflammatory properties.
3.1. Antioxidant Activity
Polyphenols are considered among the phytochemical compounds, which have been recognized as bioactive and functional compounds. They have attracted tremendous attention from food scientists, nutritionists, and consumers owing to their considerable healthful benefits. It has been demonstrated by recent research studies that dietary polyphenols have several important biological properties such as antioxidant activity, which has received great interest. The antioxidant activity of some phenolic compounds is summarized in Table 2.
Generally, oxidative stress appears when there is weak antioxidant protection, or high production of reactive oxygen species (ROS), including hydroxyl radicals (•OH), superoxide anion radicals (O2•−), and hydrogen peroxide (H2O2), during cellular respiration and other different metabolic pathways. Such processes cause chemical reactions and damage to cells, tissues, and several biomolecules, including DNA, proteins, lipids, and carbohydrates [3,57,58]. Consequently, oxidative damage leads to many human chronic diseases including atherosclerosis, inflammation, cancers, diabetes, heart attack, arthritis, liver injury, neurological disorders, cataractogenesis, and retinal damage as well as other several degenerative diseases [3,57,59].

Table 1.
Sources of polyphenols.
Table 1.
Sources of polyphenols.
| Polyphenols | Food Source | Content | Unit | Reference |
|---|---|---|---|---|
| Total phenols | Berry | 85.80–1097.44 | µg GAE/mL) | [56] |
| Oat | 7.6–16.8 | mg GAE/g | [60] | |
| Barley | 2890–3922 | μg FAE/g | [61] | |
| Wheat | 1650–2095 | µg GAE/g | [62] | |
| Wheat | 160 | µmol FAE/100 g | [63] | |
| Rice | 20–47.84 | mg GAE/g | [64] | |
| Rye | 0.984–3.369 | mg GAE/g | [65] | |
| Corn | 451–4899 | mg/kg DW | [66] | |
| Pearl millet | 2394–3137 | µg GAE/g | [67] | |
| Broccoli | 40–100 | mg/L | [68] | |
| Kiwi | 600–1000 | mg/L | [68] | |
| Black carrot | 311.5 | mg/100 g | [69] | |
| Grape | 9.95–146.32 | mg/100 g | [70] | |
| Tea | 152–243 | mg GAE/g | [71] | |
| Tomato | 1422–1564 | mg/100 g | [72] | |
| Onion | 1221–1483 | mg/100 g | [72] | |
| Apple | 905–1030 | mg/100 g | [72] | |
| Phenolic acids | ||||
| Caffeic acid | Grape | 9–138.21 | mg/100 g | [70] |
| p-coumaric acid | Corn flour | 18.69 | μg/g | [73] |
| Rye | 0.343–1.280 | mg/kg | [65] | |
| Barley | 14.61–583.54 | μg/g | [74] | |
| Finger millet | 1.81 | μg/g | [75] | |
| Ferulic acid | Corn flour | 155.69 | μg/g | [73] |
| Wheat | 25.40 | μg/g | [76] | |
| Barley | 5.61–13.88 | μg/g | [74] | |
| Rye | 1.903–6.227 | mg/kg | [65] | |
| Pearl millet | 160 | μg/g | [77] | |
| Coffee | 0.09–0.14 | g/kg | [78] | |
| Broccoli | 1.95 | μg/g | [79] | |
| Banana | 0.49–0.53 | g/kg | [80] | |
| Mango | 0.75 | g/kg | [80] | |
| Catechins | Rice | 0.26–3.98 | mg/100 g | [81] |
| Tea | 3145.04–13,986.41 | mg/100 g | [82] | |
| Theaflavins | Tea | 4.07–1109.78 | mg/100 g | [82] |
| Gallic acid | Rice | 5.43 | mg/100 g | [81] |
| Pearl millet | 120 | μg/g | [77] | |
| Black rice | 1.4 | mg/g | [83] | |
| Barley bran | 405.5 | μg/g | [84] | |
| Vanillic acid | Rye | 1.086–3.130 | mg/kg | [65] |
| Benzoic acid | Barley | 8.81–528.56 | μg/g | [74] |
| 3,4 dimethoxybenzoic acid | Barley | 18.51–110.85 | μg/g | [74] |
| Ascorbic acid | Barley bran | 20.44 | μg/g | [84] |
| Pearl millet | 320 | μg/g | [77] | |
| Dried litchi peel | 225.98 | mg/100 g | [85] | |
| Total flavonoids | Berry | 17.45–67.37 | µg RE/mL | [56] |
| Wheat | 75–121 | µg CE/g | [62] | |
| Barley | 1968–2198 | μg FAE/g | [61] | |
| Rice | 3.35- 7.14 | µg RE/g | [64] | |
| Rye | 0.042–0.203.36 | mg QE/g | [65] | |
| Pearl millet | 1721–2484 | µg CE/g) | [67] | |
| Grape | 20.15–46.27 | mg/100 g | [70] | |
| Flavonoids | ||||
| Kaempferol | Grape | 15.31–43.80 | mg/100 g | [70] |
| Corn flour | 14.58 | μg/g | [73] | |
| Broccoli | 3.42 | μg/g | [79] | |
| Quercetin | Oat | 12.2–51.6 | μg/g | [86] |
| Buckwheat | 3.1–6.71 | μg/g | [86] | |
| Anthocyanins | Berry | 8.08–21.28 | µgC3GE/mL | [56] |
| Black carrot | 837.9 | mg/100 g | [87] | |
| Rice | 0.26–256.5 | mg/100 g | [81] | |
| Corn | 307–321 | mg/kg DW | [66] | |
| Black wheat | 185.8 | mg/kg | [48] | |
| Pigmented maize | 23 to 252 | μg/g | [88] |
In this regard, the use of natural antioxidant compounds derived from plant-based food, such as dietary plant polyphenols, can contribute to the defense of the human body against oxidative stress and its adverse effects. These phytochemical compounds play an important protective role against different diseases and they are considered among the most significant natural antioxidants used in the human diet [89].
The phenolic compounds are considered active antioxidant compounds that can treat and prevent several degenerative diseases through ROS scavenging capacity and the regulation of the activity of various oxidase species in the organism [58]. It has been reported that the ingestion of dietary polyphenols as natural antioxidants can prevent mortality and morbidity caused by the degenerative diseases as well as contribute to the prevention of lipid oxidation in foods, such as fish and seafood [2,90]. Moreover, previous studies have demonstrated that the consumption of some food products enriched with oxidized lipids lead to an increase in the concentration of toxic malonic aldehyde and hydroperoxides in the digestive system, particularly in the stomach. However, the consumption of food products containing a considerable quantity of polyphenol can decrease or inhibit the accumulation of such toxic substances.
The polyphenols are characterised by their capacity to convert the relative free radical products to other non-reactive species in a more stable way [91]. They have also been demonstrated to have the capacity to regulate redox-dependent cellular signalling in living organisms [92]. The considerable antioxidant activity of phenolic compounds is due to the structure of phenolic hydroxyl in which the electrons possess a conjugation impact and the capacity of the hydrogen ion’s binding is reduced [58]. Consequently, there is a neutralization of the free radicals and reactive oxygen species (ROS) [58]. Actually, the antioxidant properties of these bioactive compounds are extremely related to the position and number of the phenolic hydroxyl groups [93].
Therefore, the phenolic compounds can eliminate the production of free radicals. Besides, these phytochemical compounds have a direct radical scavenger activity for the chain of reactions of lipid peroxidation (chain breakers). Through this process, there is a transfer of an electron to the free radicals and then the radicals become more stable by their neutralisation [23]. Thereby, the chain reaction will be stopped [23].
Besides, the polyphenols compounds have also been characterised by their metal chelating activity, which has a considerable role in the protection against the deterioration of DNA in the living cells [94]. The antioxidant mechanism of the phenolic compounds occurs by their ability to entrap the metal ions (for instance Fe2+ and Cu2+), which can be involved in the Fenton reaction in the existence of hydrogen peroxide. Thus, these bioactive compounds reduce and avoid the transition of metal chelation and then can prevent the oxidation by the inhibition of the production of free radicals. Consequently, the polyphenols can obtain stable compounds by their antioxidant activity [94,95].
In addition, in accordance with previous in vitro research studies, it has been demonstrated that these bioactive compounds are characterised by their cancer-preventing ability by the inhibition of the accumulation of reactive oxygen species (ROS) in human organisms [58]. Moreover, previously in-vivo research studies have mentioned that these phytochemical substances can raise the level of superoxide dismutase (SOD), the glutathione peroxidase (GSH-Px), and the serum catalase (CAT), and can then inhibit the generation of malondialdehyde (MDA) [58]. Thus, the polyphenols can lead to the regulation of the oxidoreductase systems, and, hence, they can enhance the antioxidant capacity of the organisms [58].

Table 2.
Antioxidant activity of some phenolic compounds.
Table 2.
Antioxidant activity of some phenolic compounds.
| Sources | Compounds | Concentrations | Assays | Main Findings | Reference |
|---|---|---|---|---|---|
| Vaccinium corymbosum L. (Blueberry fruits) | Anthocyanins Phenolic acids Flavonols | 200 g/day | Ferric reducing antioxidant potential (FRAP). Total radical-trapping antioxidant parameter (TRAP). Total antioxidant capacity (TAC) assays. | The ingestion of blueberry fruits enriched with phenolic compounds contributes to the inducing of an important increase of endogenous plasmatic antioxidant protection. | [96] |
| Thymus lotocephalus | Caffeic acids Rosmarinic acids Apigenin Luteolin |
| Trolox equivalent antioxidant capacity (TEAC) assay. Oxygen radical absorbance capacity (ORAC) assay. Fe2+ chelation assay. Lipid peroxidation assay. | The phenolic compounds found in Thymus lotocephalus are characterized by efficient antioxidant activities. The use of different antioxidant assays (ORAC and TEAC assays) can neutralize free radicals by leading to the production of complexes with Fe2+ and then the protection of mousse brains against lipid peroxidation induced by Fe2+. | [97] |
| Artemisia campestris L. | Condensed tannin Other phenolic compounds |
| DPPH radical scavenging activity. Total antioxidant capacity by phosphomolybdenum. | The Artemisia campestris enriched with phenolic compounds have demonstrated their excellent antioxidant activity, with radical-scavenging activity (85.48%). Further, the polyphenol compounds exhibited a strong total antioxidant capacity (55.75 mg AAE/g DW). | [91] |
| Thymelaea hirsuta L. | Condensed tannin Other phenolic compounds |
| DPPH radical scavenging activity. Total antioxidant capacity by phosphomolybdenum. | The phenolic compounds and condensed tannin found in the plant of Thymelaea hirsuta exhibited an important antioxidant activity, which was demonstrated by their highest DPPH radical-scavenging activity (85.8%) and their excellent total antioxidant capacity (57.54 mg AAE/g DW). | [91] |
| Ipomoea batatas [L.] Lam (leaves harvested at BBCH stage 51 of development). | Phenolic acids:
| 5026.8 mg/100 g−1 DM | Ferric-Reducing/antioxidant power assay (FRAP). ABTS assay. DPPH assay. | The results of this study have demonstrated that important correlations existed between the level of polyphenol compounds and antioxidant properties determined by means of ABTS, DPPH, and FRAP assays. | [98] |
| Ugni molinae | Tannins Flavonoids Phenolic acids | 10 mg/mL | DPPH assay. TBARS assay. TEAC-CUPRAC | The results of this study reported that the plant extract enriched with phenolic compounds was characterized by a considerable in vitro antioxidant activity via the DPPH assay. The consumption of leaves of Ugni molinae contribute to the decrease of TBARS and the increase of plasma antioxidant capacity (TEAC-CUPRAC). | [99] |
However, the flavonoids represent a large class of low-molecular weight polyphenol compounds. This group can inhibit the nitric oxide synthase, which is responsible for the production of nitric oxide components. These latter can be considered as free radicals or react with other free radicals and then produce peroxynitrite species [95]. Furthermore, the flavonoids compounds, including luteolin and quercetin, can inhibit the xanthine oxidases—they intervene in the oxidative injury due to its reaction with molecular oxygen and then liberate the superoxide components. Generally, the phenolic compounds can induce antioxidant enzymes, for instance SOD, catalase, and glutathione peroxidase that break the superoxide anions, hydrogen peroxides, and hydroperoxides and then inhibit the generation of some enzymes, including the xanthine oxidases [23].
Nevertheless, the potential antioxidant activity of phenolic compounds is directly dependant on the category of the plant species used, the harvesting time, the growing conditions, the storage conditions as well as the type of the solvent used in the processes of extraction [91,100].
The polyphenols can exhibit antioxidant effects by various mechanism pathways to scavenge and inhibit the production of ROS [101]. Actually, previous studies have reported that polyphenol can react with the ROS by the donation of hydrogen atoms or electrons to the unpaired electrons to free radicals, and then generate the stability of phenolic oxygen radicals [58,101]. Consequently, these bioactive compounds can eliminate the free radical products [58]. Generally, the polyphenols, such as the tocopherols and flavonoids, are considered as considerable primary antioxidant compounds. Their aromatic amines can inhibit the autoxidation by the mechanisms of the electron transfer [101].
Furthermore, the polyphenols that are non-enzymatic antioxidants, for instance phenolic acids, ascorbic acids, simple phenols, and tocopherols, intervene to reduce the pro-oxidative reaction through the elimination of transition metal ions contaminants, scavenge the alkoxy and peroxy radicals, and generate the quenching of the oxygen in the singlet form [101].
3.2. Antihypertensive Activity
Hypertension often has no symptoms even although it is one of the main risk factors for cardiovascular diseases in the world. These disorders are considered among the most severe worldwide public health threats since they are the leading cause of death [102,103]. Although the causes of hypertension are not well known, aging can be a risk factor because most people with hypertension are 60 years old or older, and it is a major risk factor for heart disorders, myocardial infarction [102]. In fact, hypertension can exert a great effect on morbidity and mortality via the generation of various complications. Consequently, the prevention and treatment of hypertension have become essential for inhibiting their devastating complications. Dietary adjustment is considered an important regulation method for the modulation of hypertension. Moreover, the principal efficient non-pharmacological interventions are represented by weight loss, intensification of physical activities, reduction of sodium, diet, and supplementation of potassium [103]. The antihypertensive activities of some phenolics are reported in Table 3.

Table 3.
The antihypertensive properties of some phenolic compounds.
However, in order to treat hypertension, several synthetic and chemical drugs have been used and are recommended by the Word Health Organization (WHO), such as β receptor blockers, angiotensin II receptor antagonists (ARBs), calcium antagonists, angiotensin-transferase inhibitors, and diuretics [109]. In fact, the antihypertensive impact of these drugs is effective; however, they have several contractive and negative effects, such as vertigo, ankle swelling, sodium and water retention, cough as well as elevated blood lipids [109,110]. Moreover, the use of these drugs cannot alleviate the symptoms of hypertension and cannot be utilized for a long time to treat this arterial disease.
The development of novel and non-toxic drugs as an alternative to synthetic ones for the prevention and treatment of hypertension becomes more indispensable. In this context, several research studies regarding the natural and phytochemical compounds have been established to discover promising therapy for hypertension disease. Accordingly, polyphenol compounds as secondary metabolites offer an opportunity to treat this disease. It has been reported that phenolic compounds, including phenolic acids, have several biological potentials and can act as antihypertensive agents.
Furthermore, the polyphenols as bioactive compounds are mainly found in different aromatic and medicinal plants as well as in many foods, such as tea, soybeans, fruits, and vegetables, which are characterized by their preservative impact on the development of hypertension and cardiovascular diseases [111].
Nevertheless, it has been reported that endothelial dysfunction is one of the hallmarks of hypertension disease. Thus, the endothelial dysfunction is characterized by the association of the endothelium-dependent vasodilatation with oxidative damage. In fact, this dysfunction is detected before the modifications in the structure or texture of the arterial walls and consequently this impairment in the function contributes to the beginning and the generation of cardiovascular diseases [112]. Previous studies have demonstrated that phenolic compounds and their derivatives can exert an advantageous impact on the vascular endothelial functions by some mechanisms, such as the normalization of the angiotensin system, the augmentation of the level of nitric oxide (NO), endothelium dependent hyperpolarization (EDH), and the prevention of oxidative stress through the inhibition of the appearance of pro-oxidant enzymes. For instance, the COXs and the NADPH oxidase can contribute to the improvement of endothelial function and the prevention of vascular longevity and, thus, to the protection against hypertension [112].
Furthermore, the polyphenols can induce anti-hypertensive activities via other mechanisms, including the activation of the AMPK pathways through the LKB1, the inhibition of the phosphorylation of the protein mammalian target of rapamycin (mTOR) and p70 ribosomal protein S6 kinase (p70S6k) as well as the increase of SIRT1 activation [113]. Moreover, the phenolic compounds can participate in the decrease of hypertension via the activation of the mTORC2-Rictor survival pathway as well as reduction of the expression of the mTOR signaling proteins [113].
Additionally, the role of polyphenols present in different sources (black currant, beet root, pomegranate, everlasting flower) in the prevention of endothelial dysfunction has been elucidated in several studies [114,115,116,117]. The polyphenols could prevent activation of the angiotensin system, enhance endothelial relaxation by regulating EDH, oxidative stress, and inflammatory response to improve the angiotensin system producing the vasodilation effect [112,114,116,118].
Furthermore, according to several experimental results, it has been reported that the proanthocyanidins as an important group of phenolic compounds have a vasorelaxant effect because of the considerable role of nitric oxide (NO). In fact, the polyphenols can protect the human umbilical vein endothelial cells and then contribute to the prevention of hypertension and its complications [119].
It has been reported in previous in vivo research studies that polyphenols and their derivatives have an important antihypertensive effect because of their contribution to the reduction of systolic blood pressure detected in the spontaneously hypertensive rats [120,121].
In addition, these bioactive compounds, which are found generally in herbal medicine, have been used clinically due to their advantages in the treatment of hypertension and being characterised by the reduction of the prevalence of complications related to metabolic abnormalities [109]. Moreover, it has been proved by scientific research that the flavonoids, which are considered the main active phenolic compounds in several medicinal plants, have considerable effect on the improvement of cardiovascular functions, and the prevention and the treatment of hypertension [122]. Therefore, the principal classes of flavonoids characterised by their antihypertensive impact are luteolin, chrystin, linarin, and apigenin [109]. It has been demonstrated that anthocyanin, as an important class of polyphenols, has a vasodilation activity. Their mechanism has been represented by the regulation of the activity of endothelial nitric oxide synthase (eNOS) in endothelial cells (ECs), inhibition of angiotensin-converting enzyme (ACE) activity, and the thromboxane pathway and the regulation of arterial blood pressure [109]. Moreover, quercetin, as a phenolic compound, can contribute to the reduction of the spontaneous hypertension impact by the enhancement of vasodilatation and blood viscosity [122].
Furthermore, polyphenols have an effective antihypertensive effect through several mechanisms of action. The antihypertensive treatment with polyphenols is manifested by the improvement of endothelial function as well as the relaxation of vascular tissues that appear through ACE inhibition and the nitric oxide-cyclic guanosine monophosphate (NO- cGMP) pathway [102]. Thus, the phenolic compounds can stimulate the endothelium-dependent vasodilation, and inhibit the synthesis of the vasoconstrictorendothelin-1 (ET-1) [102]. It has been shown that polyphenols can also contribute to the reduction of the activity of metalloproteinases, which have an important role in vascular dysfunction and the generation of many categories of cardiovascular diseases, including hypertension [102,123].
3.3. Immunomodulatory Activity
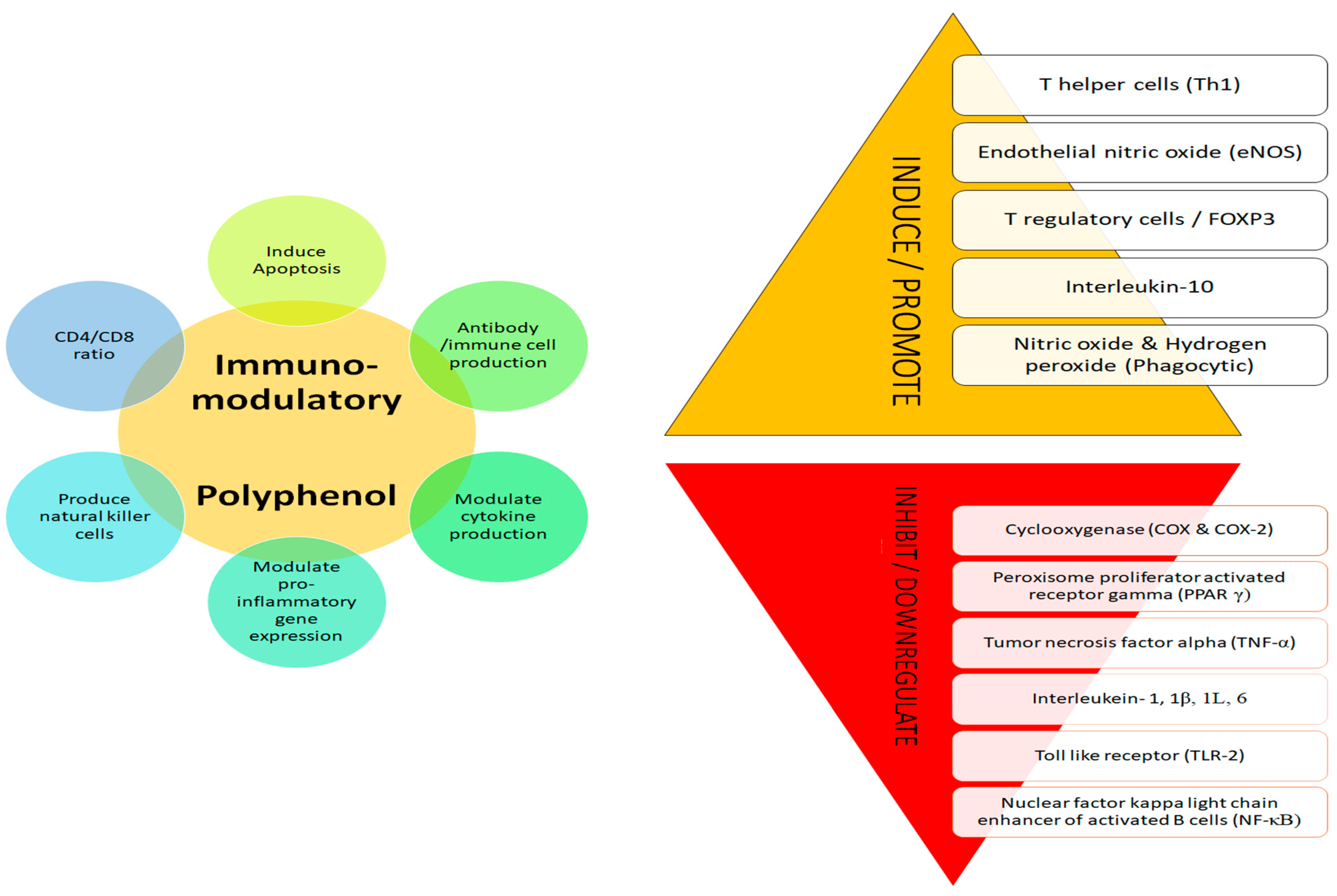
The immune system plays a vital role in well-being by increasing immune response and providing protection [124]. Polyphenols have well demonstrated immunomodulatory effects as they regulate the immune cells, macrophages, cytokines, signalling pathways and influence dendritic cells and lymphocytes (B and T), suppress T cell activation and natural killer cells, and suppress tumour-associated macrophages (Figure 3) [125,126,127,128,129,130]. High immunomodulatory impacts were associated with high antioxidative properties [6]. The negative impacts of synthetic drugs and the quest for natural alternatives for therapy has led to an increased demand for multi target action of phenols for enhancing immunity [127,130].
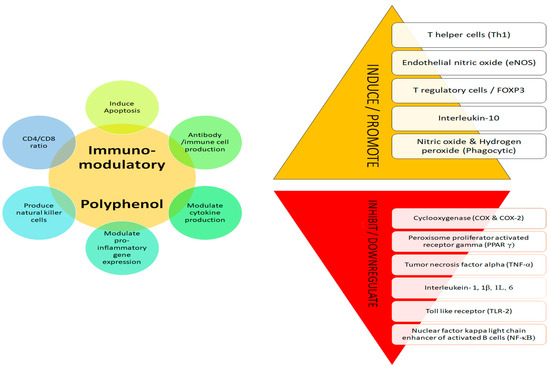
Figure 3.
Immunomodulatory mechanism of polyphenol.
Cytokines and inflammation are the mechanisms used by the body to maintain homeostasis by eliminating harmful stimuli. Cytokine are known to mediate immunity by acting as pro-inflammatory (IL-2, IL-8, TNFα, IL-6, IL-8, IFN-ɣ) and anti-inflammatory (IL-4, IL-10, TGFβ) agents [129]. Moreover, different signalling pathways such as nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB), mitogen-activated protein kinase (MAPK), and arachidonic acid signalling pathway have been reviewed as related to innate and adaptive immunity [129,131]. Polyphenols have been discussed extensively to impact cytokines, managing inflammation and modulating immunity [129,131]. Polyphenols have been reported to exhibit anti-inflammatory activity through different mechanisms such as by inhibiting pro-inflammatory molecules (TNF-α, IL-1β, IL-6, IL-8, iNOS, TLR2, TLR4), downregulating PKC-NFκB pathways inhibiting the production of pro-inflammatory molecules, and inducing production of Nrf2 signalling [132,133,134,135,136]. Furthermore, polyphenols are reviewed by Shakoor and others [137] to modulate the immune system by acting on dendritic cells (initiating immunity), macrophages (maintaining ratio between pro and anti-inflammatory activity), natural killer cells, T and B cells, and by T cell differentiation and regulation of inflammation.
Polyphenol-rich cranberry juice reduced the risk of infections by improved proliferation (Ɣδ-T) of cells, usually taken for reporting the improvement on immune function [138]. Improvements in urinary tract infection for patients using cranberry juice have been reported [11]. Another study showed that resveratrol was abundant in grapes and berries and regulated macrophages known for their ability to activate the immune system (TLR) against infection [139]. Bilberries rich in polyphenols were evaluated for their anti-inflammatory properties [140]. Diet supplementation with 400 g of fresh bilberries decreased the concentration of IL-2, IL-6, LPS, and CRP. The decrease in all cases was characterized by pro-inflammatory activities of bilberries decreasing inflammation.
Resveratrol was also known to irreversibly inhibit the production of cytokine (IFN-ɣ, IL-2, TNF-α) and signalling pathway (NF-κB) [141]. Resveratrol analogues inhibited the inflammatory response by inhibiting enzymes and pathways reducing cytokine mediated inflammation and improving immunity [142]. Curcumin has been reported to modulate inflammatory cytokines, inhibit enzymes (cyclooxygenase and lipoxygenase) responsible for inflammation, and lower the rate of signalling pathways [129]. The ability of curcumin analogue as an anti-inflammatory agent to inhibit NF-κB was evaluated by Olivera et al. [143]. Curcumin analogue (EF31) inhibited NF-κB ability to bind with DNA, nuclear translocation and induction, reducing pro-inflammatory effects.
Tea, a major source of the polyphenolic compound catechins (epicatechin-EC, epicatechin gallate-ECG, epigallocatechin-EGC, and epigallocatechin gallate-EGCG), was evaluated for its ability to produce inflammatory cytokines [144]. EGC and EGCG (10 and 20 µM) inhibited the pro-inflammatory cytokines (IL-1β). On the contrary ECG, EGC, and EGCG (10 µM) increased the production of anti-inflammatory cytokines (IL-10). ECG, EGC, and EGCG have anti-inflammatory action when evaluated as ratio of IL-1β against IL-10. Cocoa polyphenol significantly reduced pro-inflammatory cytokines in activated THP-1 cells. Significant reductions in pro-inflammatory cytokines were also observed [145]. Tea catechin exhibited in vivo cytotoxic activity against natural killer cells [11].
Polyphenols present in pomegranate peel were evaluated for their anti-inflammatory activity through suppression of mitogen activated protein kinases (MAPKs) by Du et al. [146]. Findings highlighted strong anti-inflammatory activity of polyphenols from pomegranate peel and significant inhibition of pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) was observed. The anti-inflammatory impacts were attributed to inhibition of MAPK signalling pathways mediated through inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) expression.
The phenolic compounds can modulate the immune functions via different mechanism pathways, including the inhibition proliferation of mononuclear cells from peripheral blood stimulated by mitogens. In addition, polyphenols can induce an immunomodulatory activity through the reduction of the molecule co-stimulatory, the prevention of the activation of MAPK, and also the translocation of nuclear factor-κB (NF-κB) [147]. These bioactive compounds can participate in the regulation of the activities of various transcription agents, for instance activator protein-1 (AP-1), NF-κB, signal transducer and activator of transcription (STAT) [147].
3.4. Antimicrobial Activity
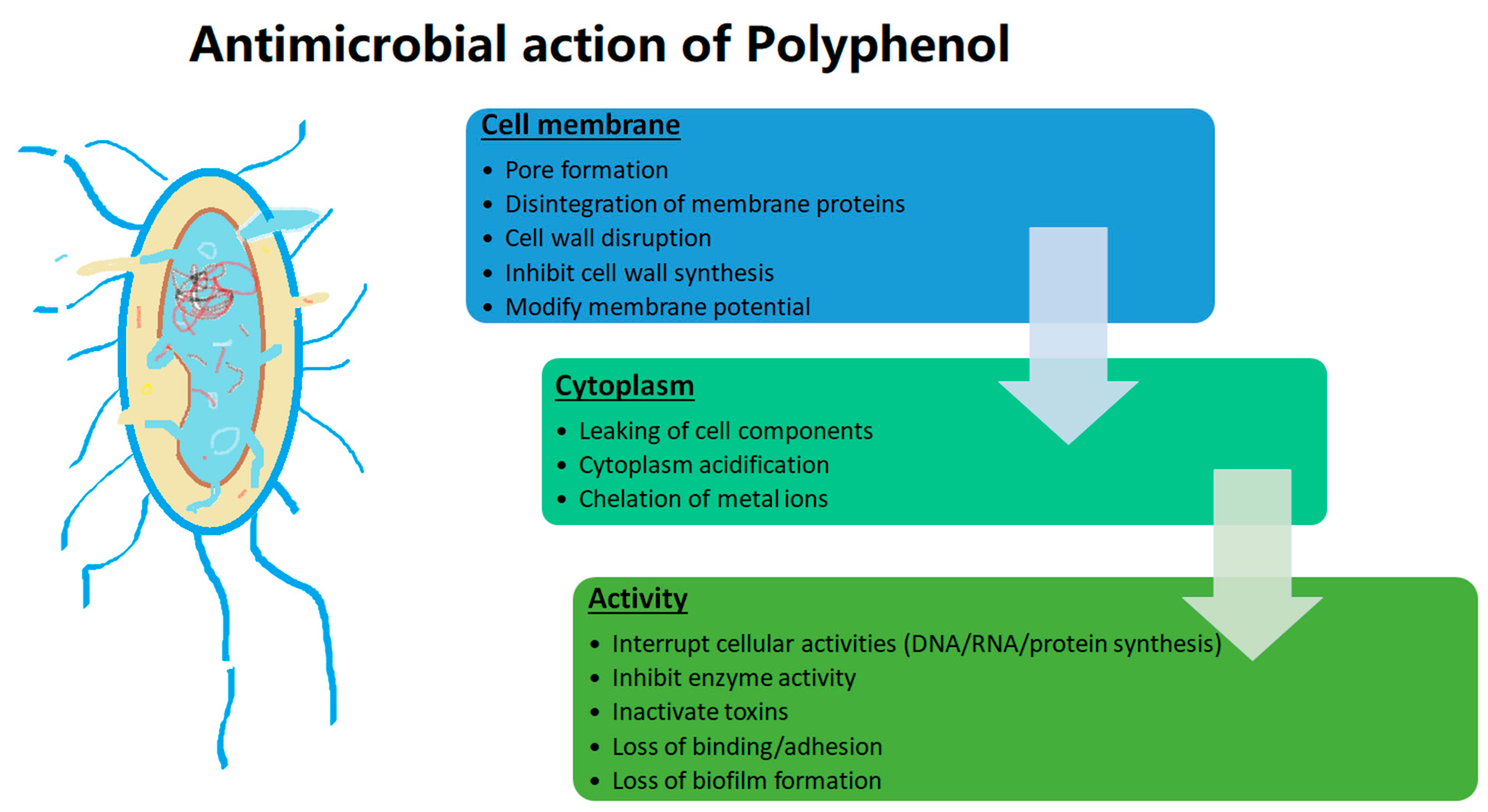
Microorganisms are of critical concern for humans, as they are responsible for causing several infections and are accountable for several deaths in severe cases [148]. However, the usage of antimicrobials from synthetic sources and their excess usage have led to development of resistance against antimicrobials responsible for increased incidence and virulent strains [148,149]. Hence, the quest for alternate naturally occurring compounds showing antimicrobial activity has gained immense importance [150]. Polyphenols have gained specific importance due to their diverse mode of action against a large microbial populations, which is why they have an antimicrobial effect, and at the same time they benefit favourable microorganisms [149,151]. The antimicrobial activity of some polyphenols is tabulated in Table 4 and illustrated in Figure 4.
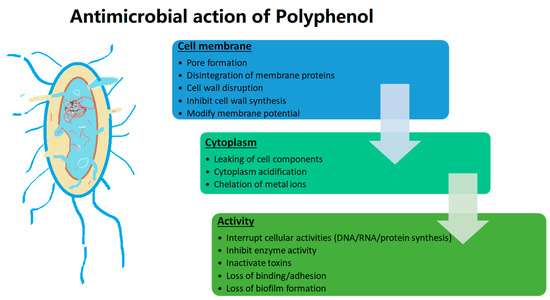
Figure 4.
Antimicrobial activity of polyphenol.
Polyphenols are known to exhibit antimicrobial action by disturbing the lipid membrane, altering the structure leaking cellular constituents and disturbing intracellular functioning, inhibiting/inactivating enzymes, impacting the degree of hydroxylation as well as level of oxidation [148,149,152]. Novel extraction and encapsulation technologies have been reviewed to enhance the antimicrobial activity of polyphenols [92,153,154].
Furthermore, polyphenols can generate antimicrobial activity so these bioactive compounds provoke an alteration of the permeability of the microbial cells, leading to the destruction of the cellular composition [101]. Moreover, the hydroxyl group (OH−) that characterises the phenolic compounds has a considerable role in the death of bacterial cells. Hence, the interaction of bacterial cell wall with “OH−” of polyphenols can produce the destabilization of proton interchange, reduce the gradient through the cytoplasmic membrane of bacterial cells, and decrease the ATP pool, and, thus, generate the death of microbial cells [101].
Phenolics from Japanese apricot (umeboshi) were evaluated for antimicrobial property against Enterobacteria [155]. Phenolics exhibited higher antimicrobial activity against evaluated strains but at relatively higher concentration (1250–5000 µg/mL). The chemical analysis revealed the presence of hydroxycinnamic acids and chlorogenic acid derivatives. Salvia leriifolia extracts were evaluated for antimicrobial activity [156]. The higher phenolic composition is related to high antimicrobial activity. The highest inhibition was observed for Pseudomonas aeruginosa, Staphylococcus aureus, and Escherichia coli (MIC-80, 110 and 120 mg/mL), respectively. Higher phenol present (TPC- 178 mg GAE/100 g) in Pistacia atlantica subsp. kurdica hulls essential oil (178 mg GAE/100 g) was attributed to the antimicrobial activity of essential oils derived from Pistacia atlantica subsp. kurdica hulls [157]. Higher control was observed in Gram positive bacteria over Gram negative bacteria due to their low susceptibility to polyphenols.

Table 4.
Antimicrobial activity of phenolic compounds.
Table 4.
Antimicrobial activity of phenolic compounds.
| Source | Compound | Main Findings | Reference |
|---|---|---|---|
| Turmeric powder | Polyphenol | Grinding, i.e., reducing the particle size increased the antimicrobial activity. Polyphenol content also improved efficiency. | [158] |
| Matricariaaurea | Phenols | Gram positive bacteria were inhibited (MIC—0.4–12.5 mg/mL) and gram negative bacteria were found resistant (MIC—25–50 mg/mL). | [159] |
| Grape seed extract and pine bark extract | Gallic acid, vanillic acid, caffeic acid, ferullic acid | Inhibited E. coli, Salmonella, L. monocytogenes, and A. hydrophila. | [160] |
| Grapefruit seed extract | Naringin | Effective inhibition of pathogenic indicator organism was observed at lower concentration in comparison with positive control. | [161] |
| Rumextingitanus leaves extract | Total phenolics and flavonoids | Ethyl acetate extract inhibited gram positive (MIC—0.312–10 mg/mL) and pathogenic microorganisms. | [162] |
| American cranberry (Vacciniummacrocarpon) fruit pomace | Polyphenols (34%)––catechins, procatechuic acid, chlorogenic acid, epicatechin, trans-cinnamic acid | Extract (2–8 mg/mL) exhibited significant inhibition of 12 strains of Listeria strains. In meat, a model protein rich matrix had impact on antibacterial activity. | [163] |
| Arugula (Erucasativa) seeds extract | Flavonoids | Methanol extract inhibited S. aureus and B. Cereus (MIC- 80 µg/mL). | [164] |
| Olive leaf extract | Luteolin-7-o-Glucoside, Luteolin-4-o-Glucoside, Oleuropein, and Vabascoside | Complete inhibition of L. monocytogenes and S. entertidis and E. coli (95%) was obtained using 62.5 mg/mL extract. Biofilm formation of L. monocytogenes and S. entertidis was also inhibited. | [165] |
| Clove essential oil | Phenols | Encapsulation masked the strong odor of clove limiting application. In vitro inhibition of S. aureus, E. coli, and S. Typhimurium. High total phenolic composition (9.07 GAE mg/g). | [154] |
Wine industry by-products (skins, stems, seeds, and skins) were evaluated for their antimicrobial potential due to the abundance in phenolic content (TPC- skin-360.2 µg/mg, seeds-363 µg/mg, stem-226.8 µg/mg) [166]. Results highlighted a direct relation between phenols and antimicrobial activity, and a significant reduction in the antibiotic resistant strains was reported. The highest antimicrobial activity was observed against L. monocytogenes (seeds), E. faecium (seeds), and K. Pneumonia (skins). Different plant parts of Moringa oleifera (leaf, root, flower, bark, and seed) extract (methanolic, ethanolic, ethyl acetate, water, and acetone) were evaluated for their antimicrobial potential [167]. Amongst the plant parts evaluated, moringa leaf (112 mg/g) and extract of ethyl acetate (200 mg/g) and aqueous (69 mg/g) extract had the highest levels of total phenolics and total flavonoid content, respectively. Myricetin was found in highest concentration, followed by quercetin, responsible for the antimicrobial activity. Ethanolic extract from leaves exhibited the highest inhibition of P. aeruginosa and E. carotovora. Similarly, a recent study highlighted the antimicrobial activity of ethanolic extracts from Centellaasiatica on six evaluated indicator organisms [168]. MIC ranged from 62.5–125 (25% ethanolic extract) to 7.81–125 (50% ethanolic extract). The results were related to higher total phenolic (69.54 mg GAE/g) and flavonoid (13.90 mg QE/g) composition present in the ethanolic extract.
Phenolic extracts (flavonoid) from Lavandula stoechas were evaluated against pathogenic microorganisms [169]. The phenolic extract exhibited antimicrobial activity against all evaluated strains (E. coli, K. pneumonia, S. aureus, E. cloacae, A. baumanii, P. aeruginosa). The minimum inhibitory concentration ranged from 10 to 40 mg/mL.
Furthermore, due to high antimicrobial activity and diverse mechanisms, polyphenols are under wide application to treat several infectious diseases. A recent study by De Angelis and others in 2021 [170] reported the antiviral impact of polydatin (precursor of resveratrol) against influenza virus. The application significantly lowered MTT assay on vero E6 cells at a concentration of 80 and 100 µg/mL. Similarly, application inhibited replication of the virus for the 24 h duration observed and viral titre from supernatant of infected treated cells [170]. Moreover, several reviews focused on the specialized ability of polyphenols to protect against human diseases from noncommunicable, viral diseases to oral microorganisms are recently reported [171,172,173,174,175,176].
3.5. Anticancer Activity
Nowadays, cancer is one of the major chronic diseases occurring in the modern world [177]. Cancer generation is mainly associated with the uncontrollable development of tumor cells. Thus, this disease has received considerable attentions worldwide. The WHO has reported that many people around the world are affected by this disease every year [178]. In fact, global statistics indicate that there were about 14.1 million new cases of cancer and about 8.2 million deaths worldwide in 2012 [177]. As a result, cancer has remained the disease most frequently responsible for the deaths of many people around the world.
The results of epidemiologic studies have suggested that the etiology of cancer is principally attributed to the variations in lifestyles, ever-increasing urbanization, the predominant diet, and successive changes in environmental conditions [177,178].
Although the traditional treatment of cancer, such as chemotherapy, radiotherapy, immunotherapy, and surgery have a considerable impact on the therapy of this chronic disorder, the generation of cases of toxicities, the apparition of drug resistances, and the high costs of therapy represent the main problems to treat cancer patients [179]. Therefore, it is important to develop effective and non-toxic antitumor drugs derived from natural product resources, which have become an area of research. Anticancer activity of some polyphenols is tabulated in Table 5.
Plant polyphenols are one of the most widespread bioactive compounds. They are characterised by high structural varieties which, in turn, generate several ranges of biological properties, including anticancer effectiveness [177]. Recently, polyphenols have received considerable attention for cancer treatments because they have fewer side effects and low toxicity. In this context, epidemiological, preclinical, and clinical research have shown that the daily consumption of polyphenols has a strong correlation with the prevention of different types of cancer [179]. Several data indicate that polyphenols have numerous target actions, and they can transmit multiple cell signaling pathways to exert their anticancer effects against different types of cancers. In fact, many investigations have revealed that polyphenols can exercise their anticancer impacts by regulating numerous cell signaling pathways.
Moreover, these compounds regulate the activity of certain enzymes and other useful proteins [180]. The phenolic compounds can, thus, affect the carcinogenesis process through several mechanisms, making their use appropriate to treat different varieties of cancer [177]. Nevertheless, the principal obstacles to successful treatment based on these bioactive compounds are their metabolic modifications, weak membrane permeability, low systemic bioavailability, physiological fluctuation, and oxidative damage [181,182]. Therefore, the bioavailability of polyphenol compounds has been identified as the proportions of the substance reaching the circular system and distributed in numerous tissues [4]. In fact, several studies on enhancement of polyphenol bioavailability are ongoing to overcome difficulties in reaching their therapeutic concentrations in target tissues [177]. Moreover, the bioavailability and biotransformation appear to be the two causal characteristics that affect the effectiveness of polyphenols. Based on the dimensions, polyphenols can be absorbed by small and/or unimpeded intestinal interfaces [4]. Thus, it has been suggested that polyphenols are common in conjugated form in plasma. However, it has been demonstrated that conjugation with proteins in the oral cavity and the acid pH of the stomach does not alter the stability and biological activity of polyphenols.
However, the anti-cancer characteristics demonstrated are primarily attributed to their anti-inflammatory, cell cycle-stopping, anti-metastatic, anti-angiogenic, autophagic, anti-proliferative, and apoptotic effects [178]. Nevertheless, the regular consumption and utilization of dietary phytochemicals, which are generally present in plant-based foods (such as vegetables, fruits, tea, and cereals) and used as food additives, may be a promising significant approach in the prevention of cancer disease [178,183,184]. Thus, many research studies have demonstrated that the dietary polyphenols have an important role in the prevention of cancer.
Resveratrol (stilbenes), anthocyanins, curcumin, and epigallocatechin-3-gallate (EGCG) are considered as polyphenols compounds, which are secreted and produced in response to environmental stimulators such as stress. These compounds are characterised by their protective efficiencies in some cancer models by various signaling pathways [42,185,186].
Moreover, phenolic molecules are considered the main health-protective bio-compounds, such as proanthocyanidins, flavonoids, and hydroxycinnamates, which possess anticancer properties and, thus, reveal their potential therapeutic and chemo-preventive effects [187].
Furthermore, several preclinical studies have investigated the anticancer effects of resveratrol and have demonstrated that this compound has a considerable prevention impacts against different types of cancers including digestive tract, breast, skin, lung, and prostate cancers [4]. Moreover, previous research has reported that the synergic impacts of the polyphenol compounds of catechin, resveratrol, and quercetin are more important than their individual effect on the inhibition of breast cancer cell progression, cell cycle proliferation, and primary mammary tumors [188].

Table 5.
The anticancer properties of some phenolic compounds.
Table 5.
The anticancer properties of some phenolic compounds.
| Sources | Compounds | Assays | Main Findings | Reference |
|---|---|---|---|---|
|
|
| In vitro: EGCG can inhibit the migration of B16-F3m cells as well as their invasion. Moreover, there is inhibition by the EGCG of the homotypic cell aggregation and also the activity of MMP-9 (matrix metalloproteinase-9) as well as the tyrosine phosphorylation of focal adhesion kinase (FAK). In vivo: EGCG can decrease lung metastases in mice bearing B16-F3m melanomas but it can increase the survival rate of melanoma-bearing mice. | [189] |
|
|
| In vitro: the use of AIF can contribute to the inhibition of the migration, invasion, and proliferation of tumors. In vivo: the AIF participates in the inhibition of mRNA expressions of MMP-2, MMP-9 as well as the inhibition of lung metastasis. | [190] |
|
|
| In vitro: the use of phenolic compounds, apigenin and/or quercetin, can inhibit the TNF-α-induced VCAM-1 expression and decrease the adhesion of melanoma cells to lung sections. In vivo: inhibition of lung metastasis and the melanoma cell adhesion to vascular lung endothelium. | [191] |
|
|
| In vitro: the treatment with curcumin can increase the tumor-suppressor genes tissue inhibitor metalloproteinase (TIMP-2) as well as the expression of E-cadherin and nonmetastatic gene 23 (Nm23). Moreover, this bioactive compound can contribute to the decrease of the binding of the treated cells to 4 extracellular matrix (ECM) proteins. Furthermore, there is a reduction of the binding to vitronectin, fibronectin, and collagen IV. Moreover, decrease in the expression of α5β1 and α (v) β3 integrin receptors. In vivo: the curcumin can contribute to the decrease of lung metastasis. | [192] |
|
|
|
| [193] |
In contrast, it has been revealed that polyphenol compounds can contribute to the treatment of cancer disease by various biological processes. Therefore, the cancer prevention and regulation by these bioactive compounds have targeted the expression of genes, cell cycle proliferation, development, migration, and progression of cells. In addition, the cytoprotective and anticancer properties of the polyphenol substances can be generally attributed to their pro-oxidant and antioxidant attributes [4,194]. In this regard, the anticancer activity of polyphenols can be manifested by various mechanisms. Thus, these bioactive compounds have demonstrated important anticancer properties due to their role in the scavenging of ROS and other free radicals, which is manifested by the transfer of the electrons to the oxidants. Consequently, the strong antioxidant activities of polyphenols lead to a decrease of the mutation and damage of DNA, which can cause genetic diseases and cancer. Thus, the polyphenols lead to the generation of suppression of the cell cycle, apoptosis, down-regulation of proliferation of cells via different modulations of many signaling pathways, such as phosphatidylinositide 3-kinases/protein kinase B (PI3K/Akt), epidermal development factor receptor/mitogen activated protein kinase (EGFR/MAPK), anti-inflammatory factors, and nuclear factor kappa-light-chain-enhancer of stimulated B cells (NF-kB) [4].
Polyphenols can exhibit anti-cancer effects via other different mechanism pathways, for instance the perforin-granzyme apoptotic pathway, mitochondrial-mediated apoptosis by over generation of ROS, and the death receptor pathway [195,196,197]. Moreover, the phenolic compounds can induce the regulation of metabolism, development of the cell cycle, and the inhibition of tumor expression through the p53 mechanism pathway [186]. Furthermore, these compounds can stop the replication of DNA, the transcription of RNA, and repair the damage of DNA of the cancer cells [198,199,200].
5. Conclusions and Future Prospect
The toxicity and the undesirable side impacts of synthetic and chemical molecules and the resistance of some microbial species to chemical drugs lead several scientific researchers to discover other alternative sources of bioactive compounds. Thus, there is an increasing demand for products of natural origin such as the polyphenol compounds, which constitute a single group of phytochemicals present in vegetables, fruits, herbs, and other natural sources.
The current review has shown that the phenolic compounds constitute a highly multifunctional and diversified group of bioactive compounds with numerous beneficial impacts in many areas. In fact, these compounds have been associated with human health. In this regard, there is a growing interest in their potential application in food industries as well as in therapeutic and pharmacological sectors. Their activity is supported by their functional groups, which are capable of accepting the negative load of a free radical. This review has also summarized the positives effects and biological activities of polyphenols and their significance for human health due to antioxidant, antimicrobial, anti-hypertensive, anticancer, immunomodulatory, and antiviral properties. Therefore, the application of polyphenols, such as flavonoids, catechin, tannins, phenolic acids, etc., in the food industry as bio-preservative substances for foods and beverages can lead to a superb activity on the inhibition of oxidative stress by different types of mechanisms. Additionally, these compounds can reduce the lipid oxidation in the human body, prevent the organs and cell structure against deterioration, and protect their functional integrity, since the polyphenols have strong antioxidant properties.
Moreover, the antioxidant features of polyphenols can contribute to the preservation and protection of human health against diverse diseases, in which oxidative damages are involved as a contributing and casual factor. There are a several investigation studies regarding the health benefits of polyphenol on hypertensive and cardiovascular diseases. In this context, these investigations have included lipoprotein oxidation, which is related to hypertension through endothelial dysfunction and then contribute to cardiovascular disease production. Furthermore, the antioxidant properties of polyphenols can decrease the generation of ROS and the reduction of inducible nitric oxide synthase (iNOS) expression. Consequently, these properties contribute to the inhibition of DNA damage, metastasis blockage, inhibition of cell cycle progression, and stimulation of apoptosis of malignant cells. All of these make polyphenols promising compounds that can be used for the treatment and prevention of cancer diseases. Thus, polyphenols have superb anticancer and antitumor properties because of their immunomodulatory effects on different type of cancer cells. Thus, they can regulate the production of chemokine and cytokine as well as activate the immune cells.
Additionally, this review has reported that polyphenol compounds have important antimicrobial properties and are characterised by their potential to substitute synthetic chemical antibiotics. Further, bioactive compounds derived from polyphenols can be applied as a good antiviral agent.
Despite the data relating to the properties of polyphenols, further efforts are needed to discover their exact mechanism of action, antagonism, synergism, and other interrelations among them, and how their appropriateness can be applied. Furthermore, clinical trials of these compounds are essential in order to develop efficient and safe alternatives to protect human health.
Author Contributions
Conceptualization, N.B.R. and F.O.; writing—original draft preparation, N.B.R.; N.E. and S.P.; writing—review and editing, N.B.R.; F.O.; S.-K.K. and J.M.R.; supervision, F.O.; S.-K.K. and J.M.R.; project administration, F.O.; S.-K.K. and J.M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rathod, N.B.; Kulawik, P.; Ozogul, F.; Regenstein, J.M.; Ozogul, Y. Biological Activity of Plant-Based Carvacrol and Thymol and Their Impact on Human Health and Food Quality. Trends Food Sci. Technol. 2021, 116, 733–748. [Google Scholar] [CrossRef]
- Rathod, N.B.; Ranveer, R.C.; Benjakul, S.; Kim, S.-K.; Pagarkar, A.U.; Patange, S.; Ozogul, F. Recent Developments of Natural Antimicrobials and Antioxidants on Fish and Fishery Food Products. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4182–4210. [Google Scholar] [CrossRef]
- Inanli, A.G.; Tümerkan, E.T.A.; El Abed, N.; Regenstein, J.M.; Özogul, F. The Impact of Chitosan on Seafood Quality and Human Health: A Review. Trends Food Sci. Technol. 2020, 97, 404–416. [Google Scholar] [CrossRef]
- Sajadimajd, S.; Bahramsoltani, R.; Iranpanah, A.; Patra, J.K.; Das, G.; Gouda, S.; Rahimi, R.; Rezaeiamiri, E.; Cao, H.; Giampieri, F.; et al. Advances on Natural Polyphenols as Anticancer Agents for Skin Cancer. Pharmacol. Res. 2020, 151, 104584. [Google Scholar] [CrossRef]
- Cutrim, C.S.; Cortez, M.A.S. A Review on Polyphenols: Classification, Beneficial Effects and Their Application in Dairy Products. Int. J. Dairy Technol. 2018, 71, 564–578. [Google Scholar] [CrossRef]
- Erukainure, O.L.; Mesaik, A.M.; Muhammad, A.; Chukwuma, C.I.; Manhas, N.; Singh, P.; Aremu, O.S.; Islam, M.S. Flowers of Clerodendrum Volubile Exacerbate Immunomodulation by Suppressing Phagocytic Oxidative Burst and Modulation of COX-2 Activity. Biomed. Pharmacother. 2016, 83, 1478–1484. [Google Scholar] [CrossRef]
- Kammerer, D.R.; Kammerer, J.; Valet, R.; Carle, R. Recovery of Polyphenols from the By-Products of Plant Food Processing and Application as Valuable Food Ingredients. Food Res. Int. 2014, 65, 2–12. [Google Scholar] [CrossRef]
- Liu, J.; Henkel, T. Traditional Chinese Medicine (TCM): Are Polyphenols and Saponins the Key Ingredients Triggering Biological Activities? Curr. Med. Chem. 2002, 9, 1483–1485. [Google Scholar] [CrossRef]
- Haslam, E.; Lilley, T.; Cai, Y.; Martin, R.; Mangnolato, D. Traditional Herbal Medicines-the Role of Polyphenols. Planta Med. 1989, 55, 1–8. [Google Scholar] [CrossRef]
- Gupta, S.C.; Tyagi, A.K.; Deshmukh-Taskar, P.; Hinojosa, M.; Prasad, S.; Aggarwal, B.B. Downregulation of Tumor Necrosis Factor and Other Proinflammatory Biomarkers by Polyphenols. Arch. Biochem. Biophys. 2014, 559, 91–99. [Google Scholar] [CrossRef]
- Kim, Y.H.; Won, Y.-S.; Yang, X.; Kumazoe, M.; Yamashita, S.; Hara, A.; Takagaki, A.; Goto, K.; Nanjo, F.; Tachibana, H. Green Tea Catechin Metabolites Exert Immunoregulatory Effects on CD4+ T Cell and Natural Killer Cell Activities. J. Agric. Food Chem. 2016, 64, 3591–3597. [Google Scholar] [CrossRef]
- Hamadani, J.D.; Hasan, M.I.; Baldi, A.J.; Hossain, S.J.; Shiraji, S.; Bhuiyan, M.S.A.; Mehrin, S.F.; Fisher, J.; Tofail, F.; Tipu, S.M.U.; et al. Immediate Impact of Stay-at-Home Orders to Control COVID-19 Transmission on Socioeconomic Conditions, Food Insecurity, Mental Health, and Intimate Partner Violence in Bangladeshi Women and Their Families: An Interrupted Time Series. Lancet Glob. Health 2020, 8, e1380–e1389. [Google Scholar] [CrossRef]
- Adem, Ş.; Eyupoglu, V.; Sarfraz, I.; Rasul, A.; Zahoor, A.F.; Ali, M.; Abdalla, M.; Ibrahim, I.M.; Elfiky, A.A. Caffeic Acid Derivatives (CAFDs) as Inhibitors of SARS-CoV-2: CAFDs-Based Functional Foods as a Potential Alternative Approach to Combat COVID-19. Phytomedicine 2021, 85, 153310. [Google Scholar] [CrossRef]
- Mu, C.; Sheng, Y.; Wang, Q.; Amin, A.; Li, X.; Xie, Y. Potential Compound from Herbal Food of Rhizoma Polygonati for Treatment of COVID-19 Analyzed by Network Pharmacology: Viral and Cancer Signaling Mechanisms. J. Funct. Foods 2021, 77, 104149. [Google Scholar] [CrossRef]
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Impact of Dietary Polyphenols on Carbohydrate Metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef]
- Cheynier, V. Polyphenols in Foods Are More Complex than Often Thought. Am. J. Clin. Nutr. 2005, 81, 223S–229S. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Dias, R.; Pereira, C.B.; Pérez-Gregorio, R.; Mateus, N.; Freitas, V. Recent Advances on Dietary Polyphenol’s Potential Roles in Celiac Disease. Trends Food Sci. Technol. 2021, 107, 213–225. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural Polyphenols: An Overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Ziegler, V.; Ferreira, C.D.; Hoffmann, J.F.; Chaves, F.C.; Vanier, N.L.; de Oliveira, M.; Elias, M.C. Cooking Quality Properties and Free and Bound Phenolics Content of Brown, Black, and Red Rice Grains Stored at Different Temperatures for Six Months. Food Chem. 2018, 242, 427–434. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, V.S.; Junior, M.R.M. Phenolic Compounds: Structure, Classification, and Antioxidant Power. In Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 33–50. [Google Scholar]
- Tsimogiannis, D.; Oreopoulou, V. Classification of Phenolic Compounds in Plants. In Polyphenols in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 263–284. [Google Scholar]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Padmanabhan, P.; Correa-Betanzo, J.; Paliyath, G. Berries and Related Fruits. In Encyclopedia of food and health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 364–371. [Google Scholar]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Kanwal, Q.; Hussain, I.; Siddiqui, L.H.; Javaid, A. Antimicrobial Activity Screening of Isolated Flavonoids from Azadirachta Indica Leaves. J. Serb. Chem. Soc. 2011, 76, 375–384. [Google Scholar] [CrossRef]
- Wang, T.; Li, Q.; Bi, K. Bioactive Flavonoids in Medicinal Plants: Structure, Activity and Biological Fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Archivio, M.D.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, Dietary Sources and Bioavailability. Ann.-Ist. Super. Sanita 2007, 43, 348. [Google Scholar]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Stobiecki, M.; Malosse, C.; Kerhoas, L.; Wojlaszek, P.; Einhorn, J. Detection of Isoflavonoids and Their Glycosides by Liquid Chromatography/Electrospray Ionization Mass Spectrometry in Root Extracts of Lupin (Lupinus albus). Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 1999, 10, 198–207. [Google Scholar] [CrossRef]
- He, X.-G. On-Line Identification of Phytochemical Constituents in Botanical Extracts by Combined High-Performance Liquid Chromatographic–Diode Array Detection–Mass Spectrometric Techniques. J. Chromatogr. A 2000, 880, 203–232. [Google Scholar] [CrossRef]
- Cuyckens, F.; Claeys, M. Mass Spectrometry in the Structural Analysis of Flavonoids. J. Mass Spectrom. 2004, 39, 1–15. [Google Scholar] [CrossRef]
- Jiang, N.; Doseff, A.I.; Grotewold, E. Flavones: From Biosynthesis to Health Benefits. Plants 2016, 5, 27. [Google Scholar] [CrossRef]
- Hollman, P.C.H.; Arts, I.C.W. Flavonols, Flavones and Flavanols–Nature, Occurrence and Dietary Burden. J. Sci. Food Agric. 2000, 80, 1081–1093. [Google Scholar] [CrossRef]
- Robards, K.; Li, X.; Antolovich, M.; Boyd, S. Characterisation of Citrus by Chromatographic Analysis of Flavonoids. J. Sci. Food Agric. 1997, 75, 87–101. [Google Scholar] [CrossRef]
- Malla, A.; Ramalingam, S. Health Perspectives of an Isoflavonoid Genistein and Its Quantification in Economically Important Plants. In Role of Materials Science in Food Bioengineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 353–379. [Google Scholar]
- Bolca, S. Bioavailability of Soy-Derived Isoflavones and Human Breast Cancer. In Polyphenols in Human Health and Disease; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1241–1256. [Google Scholar]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of Phenolic Compounds: A Review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Hsiao, Y.-H.; Ho, C.-T.; Pan, M.-H. Bioavailability and Health Benefits of Major Isoflavone Aglycones and Their Metabolites. J. Funct. Foods 2020, 74, 104164. [Google Scholar] [CrossRef]
- Das, A.B.; Goud, V.V.; Das, C. Phenolic Compounds as Functional Ingredients in Beverages. In Value-Added Ingredients and Enrichments of Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 285–323. [Google Scholar]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Khan, M.K.; Dangles, O. A Comprehensive Review on Flavanones, the Major Citrus Polyphenols. J. Food Compos. Anal. 2014, 33, 85–104. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Corradini, E.; Foglia, P.; Giansanti, P.; Gubbiotti, R.; Samperi, R.; Lagana, A. Flavonoids: Chemical Properties and Analytical Methodologies of Identification and Quantitation in Foods and Plants. Nat. Prod. Res. 2011, 25, 469–495. [Google Scholar] [CrossRef]
- de Gaulejac, N.S.-C.; Glories, Y.; Vivas, N. Free Radical Scavenging Effect of Anthocyanins in Red Wines. Food Res. Int. 1999, 32, 327–333. [Google Scholar] [CrossRef]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The Case for Anthocyanin Consumption to Promote Human Health: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508. [Google Scholar] [CrossRef]
- Lao, F.; Giusti, M.M. Extraction of Purple Corn (Zea mays L.) Cob Pigments and Phenolic Compounds Using Food-Friendly Solvents. J. Cereal Sci. 2018, 80, 87–93. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- Han, X.; Shen, T.; Lou, H. Dietary Polyphenols and Their Biological Significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar] [CrossRef]
- Pereira, D.M.; Valentão, P.; Pereira, J.A.; Andrade, P.B. Phenolics: From Chemistry to Biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Lamy, E.; Pinheiro, C.; Rodrigues, L.; Capela-Silva, F.; Lopes, O.; Tavares, S.; Gaspar, R. Determinants of Tannin-Rich Food and Beverage Consumption: Oral Perception vs. Psychosocial Aspects; Nova Publishers: New York, NY, USA, 2016. [Google Scholar]
- Jude, S.; Gopi, S. Multitarget Approach for Natural Products in Inflammation. In Inflammation and Natural Products; Elsevier: Amsterdam, The Netherlands, 2021; pp. 83–111. [Google Scholar]
- Li, Y.-X.; Wijesekara, I.; Li, Y.; Kim, S.-K. Phlorotannins as Bioactive Agents from Brown Algae. Process Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- Charoensiddhi, S.; Abraham, R.E.; Su, P.; Zhang, W. Seaweed and Seaweed-Derived Metabolites as Prebiotics. Adv. Food Nutr. Res. 2020, 91, 97–156. [Google Scholar]
- Qin, Y. Applications of Bioactive Seaweed Substances in Functional Food Products. In Bioactive Seaweeds for Food Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 111–134. [Google Scholar]
- Reynoso-Camacho, R.; Sotelo-González, A.M.; Patiño-Ortiz, P.; Rocha-Guzmán, N.E.; Pérez-Ramírez, I.F. Berry By-Products Obtained from a Decoction Process Are a Rich Source of Low-and High-Molecular Weight Extractable and Non-Extractable Polyphenols. Food Bioprod. Process. 2021, 127, 371–387. [Google Scholar] [CrossRef]
- Kumarappan, C.; Thilagam, E.; Mandal, S.C. Antioxidant Activity of Polyphenolic Extracts of Ichnocarpus Frutescens. Saudi J. Biol. Sci. 2012, 19, 349–355. [Google Scholar] [CrossRef]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant Mechanism of Tea Polyphenols and Its Impact on Health Benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef]
- Lee, C.Y.; Nanah, C.N.; Held, R.A.; Clark, A.R.; Huynh, U.G.; Maraskine, M.C.; Uzarski, R.L.; McCracken, J.; Sharma, A. Effect of Electron Donating Groups on Polyphenol-Based Antioxidant Dendrimers. Biochimie 2015, 111, 125–134. [Google Scholar] [CrossRef]
- Singh, S.; Kaur, M.; Sogi, D.S.; Purewal, S.S. A Comparative Study of Phytochemicals, Antioxidant Potential and in-Vitro DNA Damage Protection Activity of Different Oat (Avena sativa) Cultivars from India. J. Food Meas. Charact. 2019, 13, 347–356. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Punia, S. Enhancement of Bioactive Compounds in Barley Cultivars by Solid Substrate Fermentation. J. Food Meas. Charact. 2017, 11, 1355–1361. [Google Scholar] [CrossRef]
- Punia, S.; Sandhu, K.S.; Siroha, A.K. Difference in Protein Content of Wheat (Triticum aestivum L.): Effect on Functional, Pasting, Color and Antioxidant Properties. J. Saudi Soc. Agric. Sci. 2019, 18, 378–384. [Google Scholar] [CrossRef]
- Ma, D.; Li, Y.; Zhang, J.; Wang, C.; Qin, H.; Ding, H.; Xie, Y.; Guo, T. Accumulation of Phenolic Compounds and Expression Profiles of Phenolic Acid Biosynthesis-Related Genes in Developing Grains of White, Purple, and Red Wheat. Front. Plant Sci. 2016, 7, 528. [Google Scholar] [CrossRef]
- Abd Latiff, N.; Alam, S.A.Z.; Hanapi, S.Z.; Sarmidi, M.R. Quantification of Polyphenol Content, Antioxidant Properties and LC-MS/MS Analysis in Malaysian Indigenous Rice Cultivars (Oryza sativa L.). Agric. Nat. Resour. 2019, 53, 402–409. [Google Scholar]
- Kulichová, K.; Sokol, J.; Nemeček, P.; Maliarová, M.; Maliar, T.; Havrlentová, M.; Kraic, J. Phenolic Compounds and Biological Activities of Rye (Secale cereale L.) Grains. Open Chem. 2019, 17, 988–999. [Google Scholar] [CrossRef]
- Del Pozo-Insfran, D.; Brenes, C.H.; Saldivar, S.O.S.; Talcott, S.T. Polyphenolic and Antioxidant Content of White and Blue Corn (Zea mays L.) Products. Food Res. Int. 2006, 39, 696–703. [Google Scholar] [CrossRef]
- Siroha, A.K.; Sandhu, K.S.; Kaur, M. Physicochemical, Functional and Antioxidant Properties of Flour from Pearl Millet Varieties Grown in India. J. Food Meas. Charact. 2016, 10, 311–318. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Leja, M.; Kamińska, I.; Kramer, M.; Maksylewicz-Kaul, A.; Kammerer, D.; Carle, R.; Baranski, R. The Content of Phenolic Compounds and Radical Scavenging Activity Varies with Carrot Origin and Root Color. Plant Foods Hum. Nutr. 2013, 68, 163–170. [Google Scholar] [CrossRef]
- Nile, S.H.; Kim, S.; Ko, E.Y.; Park, S.W. Polyphenolic Contents and Antioxidant Properties of Different Grape (V. Vinifera, V. Labrusca, and V. Hybrid) Cultivars. BioMed Res. Int. 2013, 2013, 718065. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Zhang, J.-R.; Li, H.-B.; Wu, D.-T.; Geng, F.; Corke, H.; Wei, X.-L.; Gan, R.-Y. Green Extraction of Antioxidant Polyphenols from Green Tea (Camellia sinensis). Antioxidants 2020, 9, 785. [Google Scholar] [CrossRef]
- Cieślik, E.; Gręda, A.; Adamus, W. Contents of Polyphenols in Fruit and Vegetables. Food Chem. 2006, 94, 135–142. [Google Scholar] [CrossRef]
- Gong, K.; Chen, L.; Li, X.; Sun, L.; Liu, K. Effects of Germination Combined with Extrusion on the Nutritional Composition, Functional Properties and Polyphenol Profile and Related in Vitro Hypoglycemic Effect of Whole Grain Corn. J. Cereal Sci. 2018, 83, 1–8. [Google Scholar] [CrossRef]
- Yang, X.-J.; Dang, B.; Fan, M.-T. Free and Bound Phenolic Compound Content and Antioxidant Activity of Different Cultivated Blue Highland Barley Varieties from the Qinghai-Tibet Plateau. Molecules 2018, 23, 879. [Google Scholar] [CrossRef]
- Hithamani, G.; Srinivasan, K. Effect of Domestic Processing on the Polyphenol Content and Bioaccessibility in Finger Millet (Eleusine coracana) and Pearl Millet (Pennisetum glaucum). Food Chem. 2014, 164, 55–62. [Google Scholar] [CrossRef]
- Martín-Diana, A.B.; García-Casas, M.J.; Martínez-Villaluenga, C.; Frías, J.; Peñas, E.; Rico, D. Wheat and Oat Brans as Sources of Polyphenol Compounds for Development of Antioxidant Nutraceutical Ingredients. Foods 2021, 10, 115. [Google Scholar] [CrossRef]
- Salar, R.K.; Purewal, S.S.; Sandhu, K.S. Fermented Pearl Millet (Pennisetum glaucum) with in Vitro DNA Damage Protection Activity, Bioactive Compounds and Antioxidant Potential. Food Res. Int. 2017, 100, 204–210. [Google Scholar] [CrossRef]
- Bagchi, D.; Moriyama, H.; Swaroop, A. Green Coffee Bean Extract in Human Health; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Li, Z.; Lee, H.W.; Liang, X.; Liang, D.; Wang, Q.; Huang, D.; Ong, C.N. Profiling of Phenolic Compounds and Antioxidant Activity of 12 Cruciferous Vegetables. Molecules 2018, 23, 1139. [Google Scholar] [CrossRef]
- Siriamornpun, S.; Kaewseejan, N. Quality, Bioactive Compounds and Antioxidant Capacity of Selected Climacteric Fruits with Relation to Their Maturity. Sci. Hortic. 2017, 221, 33–42. [Google Scholar] [CrossRef]
- Goufo, P.; Trindade, H. Rice Antioxidants: Phenolic Acids, Flavonoids, Anthocyanins, Proanthocyanidins, Tocopherols, Tocotrienols, γ-Oryzanol, and Phytic Acid. Food Sci. Nutr. 2014, 2, 75–104. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Liu, Y.-J.; Kang, J.; Zhang, C.-M.; Kang, S.-G. Antioxidant Activity and Main Chemical Components of a Novel Fermented Tea. Molecules 2019, 24, 2917. [Google Scholar] [CrossRef]
- Limtrakul, P.; Semmarath, W.; Mapoung, S. Anthocyanins and Proanthocyanidins in Natural Pigmented Rice and Their Bioactivities. Phytochem. Hum. Health 2019, 1, 1–24. [Google Scholar]
- Bangar, S.P.; Sandhu, K.S.; Purewal, S.S.; Kaur, M.; Kaur, P.; Siroha, A.K.; Kumari, K.; Singh, M.; Kumar, M. Fermented Barley Bran: An Improvement in Phenolic Compounds and Antioxidant Properties. J. Food Process. Preserv. 2022, 46, e15543. [Google Scholar] [CrossRef]
- Queiroz, E.D.R.; Abreu, C.M.P.D.; Oliveira, K.D.S.; Ramos, V.D.O.; Fráguas, R.M. Bioactive Phytochemicals and Antioxidant Activity in Fresh and Dried Lychee Fractions. Rev. Cienc. Agronómica 2015, 46, 163–169. [Google Scholar] [CrossRef]
- Kerienė, I.; Mankevičienė, A.; Bliznikas, S.; Jablonskytė-Raščė, D.; Maikštėnienė, S.; Česnulevičienė, R. Biologically Active Phenolic Compounds in Buckwheat, Oats and Winter Spelt Wheat. Zemdirb.-Agric. 2015, 102, 289–296. [Google Scholar] [CrossRef]
- Witrowa-Rajchert, D.; Bawoł, A.; Czapski, J.; Kidoń, M. Studies on Drying of Purple Carrot Roots. Dry. Technol. 2009, 27, 1325–1331. [Google Scholar] [CrossRef]
- Paulsmeyer, M.; Chatham, L.; Becker, T.; West, M.; West, L.; Juvik, J. Survey of Anthocyanin Composition and Concentration in Diverse Maize Germplasms. J. Agric. Food Chem. 2017, 65, 4341–4350. [Google Scholar] [CrossRef]
- Mihanfar, A.; Nouri, M.; Roshangar, L.; Khadem-Ansari, M.H. Polyphenols: Natural Compounds with Promising Potential in Treating Polycystic Ovary Syndrome. Reprod. Biol. 2021, 21, 100500. [Google Scholar] [CrossRef]
- Saeed, N.; Khan, M.R.; Shabbir, M. Antioxidant Activity, Total Phenolic and Total Flavonoid Contents of Whole Plant Extracts Torilis leptophylla L. BMC Complement. Altern. Med. 2012, 12, 221. [Google Scholar] [CrossRef]
- El Abed, N.; Guesmi, F.; Mejri, M.; Marzouki, M.; Ben Hadj Ahmed, S. Phytochemical Screening and Assessment of Antioxidant, Antibacterial and Cytotoxicity Activities of Five Tunisian Medicinal Plants. Int. J. Pharm. Res. Biosci. 2014, 3, 770–789. [Google Scholar]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The Effects of Ultrasound Assisted Extraction on Yield, Antioxidant, Anticancer and Antimicrobial Activity of Polyphenol Extracts: A Review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Li, Q.; Li, J.; Duan, M.; Liu, L.; Fu, Y.; McClements, D.J.; Zhao, T.; Lin, H.; Shi, J.; Chen, X. Impact of Food Additive Titanium Dioxide on the Polyphenol Content and Antioxidant Activity of the Apple Juice. LWT 2022, 154, 112574. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Hou, H.; Zhuang, Y.; Sun, L. Metal Chelating, Inhibitory DNA Damage, and Anti-Inflammatory Activities of Phenolics from Rambutan (Nephelium lappaceum) Peel and the Quantifications of Geraniin and Corilagin. Molecules 2018, 23, 2263. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Toscano, M. The Molecular Basis of Working Mechanism of Natural Polyphenolic Antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Serafini, M.; Testa, M.F.; Villaño, D.; Pecorari, M.; van Wieren, K.; Azzini, E.; Brambilla, A.; Maiani, G. Antioxidant Activity of Blueberry Fruit Is Impaired by Association with Milk. Free Radic. Biol. Med. 2009, 46, 769–774. [Google Scholar] [CrossRef]
- Costa, P.; Gonçalves, S.; Valentão, P.; Andrade, P.B.; Coelho, N.; Romano, A. Thymus Lotocephalus Wild Plants and in Vitro Cultures Produce Different Profiles of Phenolic Compounds with Antioxidant Activity. Food Chem. 2012, 135, 1253–1260. [Google Scholar] [CrossRef]
- Krochmal-Marczak, B.; Cebulak, T.; Kapusta, I.; Oszmiański, J.; Kaszuba, J.; Żurek, N. The Content of Phenolic Acids and Flavonols in the Leaves of Nine Varieties of Sweet Potatoes (Ipomoea batatas L.) Depending on Their Development, Grown in Central Europe. Molecules 2020, 25, 3473. [Google Scholar] [CrossRef]
- Avello, M.A.; Pastene, E.R.; Bustos, E.D.; Bittner, M.L.; Becerra, J.A. Variation in Phenolic Compounds of Ugni Molinae Populations and Their Potential Use as Antioxidant Supplement. Rev. Bras. Farmacogn. 2013, 23, 44–50. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Ferreira, I.C. In Vivo Antioxidant Activity of Phenolic Compounds: Facts and Gaps. Trends Food Sci. Technol. 2016, 48, 1–12. [Google Scholar] [CrossRef]
- Bensid, A.; El Abed, N.; Houicher, A.; Regenstein, J.M.; Özogul, F. Antioxidant and Antimicrobial Preservatives: Properties, Mechanism of Action and Applications in Food–a Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2985–3001. [Google Scholar] [CrossRef] [PubMed]
- Hügel, H.M.; Jackson, N.; May, B.; Zhang, A.L.; Xue, C.C. Polyphenol Protection and Treatment of Hypertension. Phytomedicine 2016, 23, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Micucci, M.; Bolchi, C.; Budriesi, R.; Cevenini, M.; Maroni, L.; Capozza, S.; Chiarini, A.; Pallavicini, M.; Angeletti, A. Antihypertensive Phytocomplexes of Proven Efficacy and Well-Established Use: Mode of Action and Individual Characterization of the Active Constituents. Phytochemistry 2020, 170, 112222. [Google Scholar] [CrossRef] [PubMed]
- Paredes, M.D.; Romecín, P.; Atucha, N.M.; O’Valle, F.; Castillo, J.; Ortiz, M.C.; García-Estañ, J. Beneficial Effects of Different Flavonoids on Vascular and Renal Function in L-NAME Hypertensive Rats. Nutrients 2018, 10, 484. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.M.; França-Silva, M.S.; Alves, N.F.; Porpino, S.K.; Braga, V.A. Quercetin Improves Baroreflex Sensitivity in Spontaneously Hypertensive Rats. Molecules 2012, 17, 12997–13008. [Google Scholar] [CrossRef] [PubMed]
- Padilla, E.; Ruiz, E.; Redondo, S.; Gordillo-Moscoso, A.; Slowing, K.; Tejerina, T. Relationship between Vasodilation Capacity and Phenolic Content of Spanish Wines. Eur. J. Pharmacol. 2005, 517, 84–91. [Google Scholar] [CrossRef]
- Kaur, S.; Muthuraman, A. Therapeutic Evaluation of Rutin in Two-Kidney One-Clip Model of Renovascular Hypertension in Rat. Life Sci. 2016, 150, 89–94. [Google Scholar] [CrossRef]
- Jain, P.K.; Jain, S.; Sharma, S.; Paliwal, S.; Singh, G. Evaluation of Anti-Diabetic and Antihypertensive Activity of Phoenix Sylvestris (L.) Roxb Leaves Extract and Quantification of Biomarker Quercetin by HPTLC. Phytomedicine Plus 2021, 1, 100136. [Google Scholar] [CrossRef]
- Cao, Y.; Xie, L.; Liu, K.; Liang, Y.; Dai, X.; Wang, X.; Lu, J.; Zhang, X.; Li, X. The Antihypertensive Potential of Flavonoids from Chinese Herbal Medicine: A Review. Pharmacol. Res. 2021, 174, 105919. [Google Scholar] [CrossRef]
- Lamb, S.A.; Al Hamarneh, Y.N.; Houle, S.K.; Leung, A.A.; Tsuyuki, R.T. Hypertension Canada’s 2017 Guidelines for Diagnosis, Risk Assessment, Prevention and Treatment of Hypertension in Adults for Pharmacists: An Update. Can. Pharm. J./Rev. Pharm. Can. 2018, 151, 33–42. [Google Scholar] [CrossRef]
- Yamagata, K.; Tagami, M.; Yamori, Y. Dietary Polyphenols Regulate Endothelial Function and Prevent Cardiovascular Disease. Nutrition 2015, 31, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Oak, M.-H.; Auger, C.; Belcastro, E.; Park, S.-H.; Lee, H.-H.; Schini-Kerth, V.B. Potential Mechanisms Underlying Cardiovascular Protection by Polyphenols: Role of the Endothelium. Free Radic. Biol. Med. 2018, 122, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Sanches-Silva, A.; Testai, L.; Nabavi, S.F.; Battino, M.; Devi, K.P.; Tejada, S.; Sureda, A.; Xu, S.; Yousefi, B.; Majidinia, M.; et al. Therapeutic Potential of Polyphenols in Cardiovascular Diseases: Regulation of MTOR Signaling Pathway. Pharmacol. Res. 2020, 152, 104626. [Google Scholar] [CrossRef]
- Valero, M.S.; Nuñez, S.; Les, F.; Castro, M.; Gómez-Rincón, C.; Arruebo, M.P.; Plaza, M.Á.; Köhler, R.; López, V. The Potential Role of Everlasting Flower (Helichrysum Stoechas Moench) as an Antihypertensive Agent: Vasorelaxant Effects in the Rat Aorta. Antioxidants 2022, 11, 1092. [Google Scholar] [CrossRef]
- Rashid, S.; Idris-Khodja, N.; Auger, C.; Kevers, C.; Pincemail, J.; Alhosin, M.; Boehm, N.; Oswald-Mammosser, M.; Schini-Kerth, V.B. Polyphenol-Rich Blackcurrant Juice Prevents Endothelial Dysfunction in the Mesenteric Artery of Cirrhotic Rats with Portal Hypertension: Role of Oxidative Stress and the Angiotensin System. J. Med. Food 2018, 21, 390–399. [Google Scholar] [CrossRef]
- Tropea, T.; Renshall, L.J.; Nihlen, C.; Weitzberg, E.; Lundberg, J.O.; David, A.L.; Tsatsaris, V.; Stuckey, D.J.; Wareing, M.; Greenwood, S.L.; et al. Beetroot Juice Lowers Blood Pressure and Improves Endothelial Function in Pregnant ENOS −/− Mice: Importance of Nitrate-independent Effects. J. Physiol. 2020, 598, 4079–4092. [Google Scholar] [CrossRef]
- Delgado, N.T.B.; Rouver, W.N.; dos Santos, R.L. Protective Effects of Pomegranate in Endothelial Dysfunction. Curr. Pharm. Des. 2020, 26, 3684–3699. [Google Scholar] [CrossRef] [PubMed]
- An, P.; Wan, S.; Luo, Y.; Luo, J.; Zhang, X.; Zhou, S.; Xu, T.; He, J.; Mechanick, J.I.; Wu, W.-C.; et al. Micronutrient Supplementation to Reduce Cardiovascular Risk. J. Am. Coll. Cardiol. 2022, 80, 2269–2285. [Google Scholar] [CrossRef] [PubMed]
- Kolodziej, H. Beneficial Vascular Responses to Proanthocyanidins: Critical Assessment of Plant-Based Test Materials and Insight into the Signaling Pathways. In Recent Advances in Polyphenol Research; John Wiley & Sons Ltd.: London, UK, 2017; pp. 226–258. [Google Scholar]
- López-Sepúlveda, R.; Jiménez, R.; Romero, M.; Zarzuelo, M.J.; Sánchez, M.; Gómez-Guzmán, M.; Vargas, F.; O’Valle, F.; Zarzuelo, A.; Pérez-Vizcaíno, F.; et al. Wine Polyphenols Improve Endothelial Function in Large Vessels of Female Spontaneously Hypertensive Rats. Hypertension 2008, 51, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.-L.; Capdeville-Atkinson, C.; Atkinson, J. Red Wine Polyphenols Improve Endothelium-Dependent Dilation in Rat Cerebral Arterioles. J. Cardiovasc. Pharmacol. 2008, 51, 553–558. [Google Scholar] [CrossRef]
- Maaliki, D.; Shaito, A.A.; Pintus, G.; El-Yazbi, A.; Eid, A.H. Flavonoids in Hypertension: A Brief Review of the Underlying Mechanisms. Curr. Opin. Pharmacol. 2019, 45, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Botden, I.P.; Langendonk, J.G.; Meima, M.E.; Boomsma, F.; Seynhaeve, A.L.; Hagen, T.L.; Danser, A.J.; Sijbrands, E.J. Daily Red Wine Consumption Improves Vascular Function by a Soluble Guanylyl Cyclase-Dependent Pathway. Am. J. Hypertens. 2011, 24, 162–168. [Google Scholar] [PubMed]
- Jayaraman, S.; Variyar, J. Plant Metabolites as Immunomodulators. In Plant Metabolites: Methods, Applications and Prospects; Springer: Singapore, 2020; pp. 441–464. [Google Scholar]
- Marefati, N.; Ghorani, V.; Shakeri, F.; Boskabady, M.; Kianian, F.; Rezaee, R.; Boskabady, M.H. A Review of Anti-Inflammatory, Antioxidant, and Immunomodulatory Effects of Allium Cepa and Its Main Constituents. Pharm. Biol. 2021, 59, 285–300. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, J.T.G.; Baldivia, D.D.S.; de Castro, D.T.H.; Dos Santos, H.F.; Dos Santos, C.M.; Oliveira, A.S.; Alfredo, T.M.; Vilharva, K.N.; de Picoli Souza, K.; Dos Santos, E.L. The Immunoregulatory Function of Polyphenols: Implications in Cancer Immunity. J. Nutr. Biochem. 2020, 85, 108428. [Google Scholar] [CrossRef]
- Mohammadian Haftcheshmeh, S.; Khosrojerdi, A.; Aliabadi, A.; Lotfi, S.; Mohammadi, A.; Momtazi-Borojeni, A.A. Immunomodulatory Effects of Curcumin in Rheumatoid Arthritis: Evidence from Molecular Mechanisms to Clinical Outcomes. In Reviews of Physiology, Biochemistry and Pharmacology; Springer: Cham, Switzerland, 2021; pp. 1–29. [Google Scholar]
- Malaguarnera, L. Influence of Resveratrol on the Immune Response. Nutrients 2019, 11, 946. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Grigore, A. Plant Phenolic Compounds as Immunomodulatory Agents. In Phenolic Compounds-Biological Activity; InTech Open: Norderstedt, Germany, 2017. [Google Scholar]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar]
- Singh, A.; Yau, Y.F.; Leung, K.S.; El-Nezami, H.; Lee, J.C.-Y. Interaction of Polyphenols as Antioxidant and Anti-Inflammatory Compounds in Brain–Liver–Gut Axis. Antioxidants 2020, 9, 669. [Google Scholar] [CrossRef]
- Bucciantini, M.; Leri, M.; Nardiello, P.; Casamenti, F.; Stefani, M. Olive Polyphenols: Antioxidant and Anti-Inflammatory Properties. Antioxidants 2021, 10, 1044. [Google Scholar] [CrossRef]
- Nani, A.; Murtaza, B.; Sayed Khan, A.; Khan, N.A.; Hichami, A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef]
- Pap, N.; Fidelis, M.; Azevedo, L.; do Carmo, M.A.V.; Wang, D.; Mocan, A.; Pereira, E.P.R.; Xavier-Santos, D.; Sant’Ana, A.S.; Yang, B.; et al. Berry Polyphenols and Human Health: Evidence of Antioxidant, Anti-Inflammatory, Microbiota Modulation, and Cell-Protecting Effects. Curr. Opin. Food Sci. 2021, 42, 167–186. [Google Scholar] [CrossRef]
- Magrone, T.; Magrone, M.; Russo, M.A.; Jirillo, E. Recent Advances on the Anti-Inflammatory and Antioxidant Properties of Red Grape Polyphenols: In Vitro and In Vivo Studies. Antioxidants 2019, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, H.; Feehan, J.; Apostolopoulos, V.; Platat, C.; Al Dhaheri, A.S.; Ali, H.I.; Ismail, L.C.; Bosevski, M.; Stojanovska, L. Immunomodulatory Effects of Dietary Polyphenols. Nutrients 2021, 13, 728. [Google Scholar] [CrossRef] [PubMed]
- Nantz, M.P.; Rowe, C.A.; Muller, C.; Creasy, R.; Colee, J.; Khoo, C.; Percival, S.S. Consumption of Cranberry Polyphenols Enhances Human Γδ-T Cell Proliferation and Reduces the Number of Symptoms Associated with Colds and Influenza: A Randomized, Placebo-Controlled Intervention Study. Nutr. J. 2013, 12, 161. [Google Scholar] [CrossRef]
- Yang, Y.; Li, S.; Yang, Q.; Shi, Y.; Zheng, M.; Liu, Y.; Chen, F.; Song, G.; Xu, H.; Wan, T.; et al. Resveratrol Reduces the Proinflammatory Effects and Lipopolysaccharide-Induced Expression of HMGB1 and TLR4 in RAW264. 7 Cells. Cell. Physiol. Biochem. 2014, 33, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Kolehmainen, M.; Mykkänen, O.; Kirjavainen, P.V.; Leppänen, T.; Moilanen, E.; Adriaens, M.; Laaksonen, D.E.; Hallikainen, M.; Puupponen-Pimiä, R.; Pulkkinen, L.; et al. Bilberries Reduce Low-Grade Inflammation in Individuals with Features of Metabolic Syndrome. Mol. Nutr. Food Res. 2012, 56, 1501–1510. [Google Scholar] [CrossRef]
- Gao, X.; Xu, Y.X.; Janakiraman, N.; Chapman, R.A.; Gautam, S.C. Immunomodulatory Activity of Resveratrol: Suppression of Lymphocyte Proliferation, Development of Cell-Mediated Cytotoxicity, and Cytokine Production. Biochem. Pharmacol. 2001, 62, 1299–1308. [Google Scholar] [CrossRef]
- Capiralla, H.; Vingtdeux, V.; Venkatesh, J.; Dreses-Werringloer, U.; Zhao, H.; Davies, P.; Marambaud, P. Identification of Potent Small-Molecule Inhibitors of STAT 3 with Anti-Inflammatory Properties in RAW 264.7 Macrophages. FEBS J. 2012, 279, 3791–3799. [Google Scholar] [CrossRef]
- Olivera, A.; Moore, T.W.; Hu, F.; Brown, A.P.; Sun, A.; Liotta, D.C.; Snyder, J.P.; Yoon, Y.; Shim, H.; Marcus, A.I.; et al. Inhibition of the NF-ΚB Signaling Pathway by the Curcumin Analog, 3,5-Bis(2-Pyridinylmethylidene)-4-Piperidone (EF31): Anti-Inflammatory and Anti-Cancer Properties. Int. Immunopharmacol. 2012, 12, 368–377. [Google Scholar] [CrossRef]
- Crouvezier, S.; Powell, B.; Keir, D.; Yaqoob, P. The Effects of Phenolic Components of Tea on the Production of Pro-and Anti-Inflammatory Cytokines by Human Leukocytes in Vitro. Cytokine 2001, 13, 280–286. [Google Scholar] [CrossRef]
- Dugo, L.; Belluomo, M.G.; Fanali, C.; Russo, M.; Cacciola, F.; Maccarrone, M.; Sardanelli, A.M. Effect of Cocoa Polyphenolic Extract on Macrophage Polarization from Proinflammatory M1 to Anti-Inflammatory M2 State. Oxid. Med. Cell. Longev. 2017, 2017, 6293740. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Li, J.; Zhang, X.; Wang, L.; Zhang, W. Pomegranate Peel Polyphenols Inhibits Inflammation in LPS-Induced RAW264. 7 Macrophages via the Suppression of MAPKs Activation. J. Funct. Foods 2018, 43, 62–69. [Google Scholar] [CrossRef]
- Mohammadi, A.; Blesso, C.N.; Barreto, G.E.; Banach, M.; Majeed, M.; Sahebkar, A. Macrophage Plasticity, Polarization and Function in Response to Curcumin, a Diet-Derived Polyphenol, as an Immunomodulatory Agent. J. Nutr. Biochem. 2019, 66, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zamuz, S.; Munekata, P.E.; Dzuvor, C.K.; Zhang, W.; Sant’Ana, A.S.; Lorenzo, J.M. The Role of Phenolic Compounds against Listeria Monocytogenes in Food. A Review. Trends Food Sci. Technol. 2021, 110, 385–392. [Google Scholar] [CrossRef]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M. Mechanisms of Action, Resistance, and Stress Adaptation. In Antimicrobials in Food; CRC Press: Boca Raton, FL, USA, 2020; pp. 735–784. [Google Scholar]
- López-Malo, A.; Alzamora, S.M.; Paris, M.J.; Lastra-Vargas, L.; Coronel, M.B.; Gómez, P.L.; Palou, E. Naturally Occurring Compounds–Plant Sources. In Antimicrobials in Food; CRC Press: Boca Raton, FL, USA, 2020; pp. 527–594. [Google Scholar]
- Freitas, I.R.; Cattelan, M.G. Antimicrobial and Antioxidant Properties of Essential Oils in Food Systems—An Overview. In Microbial Contamination and Food Degradation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 443–470. [Google Scholar]
- Ferrentino, G.; Haman, N.; Morozova, K.; Tonon, G.; Scampicchio, M. Phenolic Compounds Extracted from Spruce (Picea abies) by Supercritical Carbon Dioxide as Antimicrobial Agents against Gram-Positive Bacteria Assessed by Isothermal Calorimetry. J. Therm. Anal. Calorim. 2021, 145, 3093–3103. [Google Scholar] [CrossRef]
- Saini, A.; Panwar, D.; Panesar, P.S.; Bera, M.B. Encapsulation of Functional Ingredients in Lipidic Nanocarriers and Antimicrobial Applications: A Review. Environ. Chem. Lett. 2021, 19, 1107–1134. [Google Scholar] [CrossRef]
- Mitani, T.; Ota, K.; Inaba, N.; Kishida, K.; Koyama, H.A. Antimicrobial Activity of the Phenolic Compounds of Prunus Mume against Enterobacteria. Biol. Pharm. Bull. 2018, 41, 208–212. [Google Scholar] [CrossRef]
- Baghaenezhad, M.; Mollania, N.; Kazemi-Noreini, S. Antioxidant Capacities, Antimicrobial Activity, Phenolic Contents and α-Amylase Inhibitory of Salvia leriifolia Extracts from Sabzevar. Iran. J. Sci. Technol. Trans. Sci. 2021, 45, 1–9. [Google Scholar] [CrossRef]
- Hasheminya, S.-M.; Dehghannya, J. Composition, Phenolic Content, Antioxidant and Antimicrobial Activity of Pistacia atlantica Subsp. Kurdica Hulls’ Essential Oil. Food Biosci. 2020, 34, 100510. [Google Scholar] [CrossRef]
- Alnashi, B.; Abdel Fattah, A. Antimicrobial Activity of Raw and Nano Turmeric Powder Extracts. Middle East J. Appl. Sci. 2016, 6, 787–796. [Google Scholar]
- Rizwana, H.; Alwhibi, M.S.; Soliman, D.A. Research Article Antimicrobial Activity and Chemical Composition of Flowers of Matricaria Aurea a Native Herb of Saudi Arabia. Int. J. Pharmacol. 2016, 12, 576–586. [Google Scholar] [CrossRef]
- Ahn, J.; Grün, I.U.; Mustapha, A. Effects of Plant Extracts on Microbial Growth, Color Change, and Lipid Oxidation in Cooked Beef. Food Microbiol. 2007, 24, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-S.; Lee, Y.-R.; Ha, Y.-M.; Seo, H.J.; Kim, Y.H.; Park, S.-M.; Sohn, J.H. Antibacterial Effect of Grapefruit Seed Extract (Gse) on Makgeolli-Brewing Microorganisms and Its Application in the Preservation of Fresh Makgeolli. J. Food Sci. 2014, 79, M1159–M1167. [Google Scholar] [CrossRef]
- Mhalla, D.; Bouaziz, A.; Ennouri, K.; Chawech, R.; Smaoui, S.; Jarraya, R.; Tounsi, S.; Trigui, M. Antimicrobial Activity and Bioguided Fractionation of Rumex Tingitanus Extracts for Meat Preservation. Meat Sci. 2017, 125, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Diarra, M.S.; Hassan, Y.I.; Block, G.S.; Drover, J.C.; Delaquis, P.; Oomah, B.D. Antibacterial Activities of a Polyphenolic-Rich Extract Prepared from American Cranberry (Vaccinium macrocarpon) Fruit Pomace against Listeria Spp. LWT 2020, 123, 109056. [Google Scholar] [CrossRef]
- Malik, S.N. Antibacterial Activity of Olive (Olea europaea) Leaves and Arugula (Eruca sativa) Seeds Extract. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 307–310. [Google Scholar]
- Liu, Y.; McKeever, L.C.; Malik, N.S. Assessment of the Antimicrobial Activity of Olive Leaf Extract against Foodborne Bacterial Pathogens. Front. Microbiol. 2017, 8, 113. [Google Scholar] [CrossRef]
- Silva, V.; Igrejas, G.; Falco, V.; Santos, T.P.; Torres, C.; Oliveira, A.M.; Pereira, J.E.; Amaral, J.S.; Poeta, P. Chemical Composition, Antioxidant and Antimicrobial Activity of Phenolic Compounds Extracted from Wine Industry by-Products. Food Control 2018, 92, 516–522. [Google Scholar] [CrossRef]
- Prabakaran, M.; Kim, S.-H.; Sasireka, A.; Chandrasekaran, M.; Chung, I.-M. Polyphenol Composition and Antimicrobial Activity of Various Solvent Extracts from Different Plant Parts of Moringa Oleifera. Food Biosci. 2018, 26, 23–29. [Google Scholar] [CrossRef]
- Wong, J.X.; Ramli, S. Antimicrobial Activity of Different Types of Centella Asiatica Extracts against Foodborne Pathogens and Food Spoilage Microorganisms. LWT 2021, 142, 111026. [Google Scholar] [CrossRef]
- Ez zoubi, Y.; Farah, A.; Zaroual, H.; El Ouali Lalami, A. Antimicrobial Activity of Lavandula Stoechas Phenolic Extracts against Pathogenic Bacteria Isolated from a Hospital in Morocco. Vegetos 2020, 33, 703–711. [Google Scholar] [CrossRef]
- De Angelis, M.; Della-Morte, D.; Buttinelli, G.; Di Martino, A.; Pacifici, F.; Checconi, P.; Ambrosio, L.; Stefanelli, P.; Palamara, A.T.; Garaci, E.; et al. Protective Role of Combined Polyphenols and Micronutrients against Influenza A Virus and SARS-CoV-2 Infection In Vitro. Biomedicines 2021, 9, 1721. [Google Scholar] [CrossRef] [PubMed]
- Montenegro-Landívar, M.F.; Tapia-Quirós, P.; Vecino, X.; Reig, M.; Valderrama, C.; Granados, M.; Cortina, J.L.; Saurina, J. Polyphenols and Their Potential Role to Fight Viral Diseases: An Overview. Sci. Total Environ. 2021, 801, 149719. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, X.; Hao, J.; Zhang, Y.; Huang, R. Tea Polyphenols: Application in the Control of Oral Microorganism Infectious Diseases. Arch. Oral Biol. 2019, 102, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights Into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Margină, D.; Ungurianu, A.; Purdel, C.; Nițulescu, G.M.; Tsoukalas, D.; Sarandi, E.; Thanasoula, M.; Burykina, T.I.; Tekos, F.; Buha, A.; et al. Analysis of the Intricate Effects of Polyunsaturated Fatty Acids and Polyphenols on Inflammatory Pathways in Health and Disease. Food Chem. Toxicol. 2020, 143, 111558. [Google Scholar] [CrossRef]
- Abenavoli, L.; Larussa, T.; Corea, A.; Procopio, A.C.; Boccuto, L.; Dallio, M.; Federico, A.; Luzza, F. Dietary Polyphenols and Non-Alcoholic Fatty Liver Disease. Nutrients 2021, 13, 494. [Google Scholar] [CrossRef]
- Koch, W. Dietary Polyphenols—Important Non-Nutrients in the Prevention of Chronic Noncommunicable Diseases. A Systematic Review. Nutrients 2019, 11, 1039. [Google Scholar] [CrossRef]
- Lewandowska, U.; Fichna, J.; Gorlach, S. Enhancement of Anticancer Potential of Polyphenols by Covalent Modifications. Biochem. Pharmacol. 2016, 109, 1–13. [Google Scholar] [CrossRef]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Das, S.; Patra, S.K.; Efferth, T.; Jena, M.; Bhutia, S.K. Dietary Polyphenols in Chemoprevention and Synergistic Effect in Cancer: Clinical Evidences and Molecular Mechanisms of Action. Phytomedicine 2021, 90, 153554. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Li, S.; Wang, C.; Cao, N.; Qu, H.; Cheng, C.; Wang, Z.; Wang, L.; Zhou, L. Potential Applications of Polyphenols on Main NcRNAs Regulations as Novel Therapeutic Strategy for Cancer. Biomed. Pharmacother. 2019, 113, 108703. [Google Scholar] [CrossRef] [PubMed]
- Symonds, E.L.; Konczak, I.; Fenech, M. The Australian Fruit Illawarra Plum (Podocarpus Elatus Endl., Podocarpaceae) Inhibits Telomerase, Increases Histone Deacetylase Activity and Decreases Proliferation of Colon Cancer Cells. Br. J. Nutr. 2013, 109, 2117–2125. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, S.; Rudloff, S.; Asseburg, H.; Borsch, C.; Fröhling, B.; Unger, F.; Dold, S.; Spengler, B.; Römpp, A.; Kunz, C. Uptake and Bioavailability of Anthocyanins and Phenolic Acids from Grape/Blueberry Juice and Smoothie in Vitro and in Vivo. Br. J. Nutr. 2015, 113, 1044–1055. [Google Scholar] [CrossRef]
- Pandareesh, M.; Mythri, R.; Bharath, M.S. Bioavailability of Dietary Polyphenols: Factors Contributing to Their Clinical Application in CNS Diseases. Neurochem. Int. 2015, 89, 198–208. [Google Scholar] [CrossRef]
- Law, B.Y.K.; Mok, S.W.F.; Wu, A.G.; Lam, C.W.K.; Yu, M.X.Y.; Wong, V.K.W. New Potential Pharmacological Functions of Chinese Herbal Medicines via Regulation of Autophagy. Molecules 2016, 21, 359. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2020, 10, 1614. [Google Scholar] [CrossRef]
- Curti, V.; Di Lorenzo, A.; Dacrema, M.; Xiao, J.; Nabavi, S.M.; Daglia, M. In Vitro Polyphenol Effects on Apoptosis: An Update of Literature Data. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 46, pp. 119–131. [Google Scholar]
- Khan, S.; Siddique, R.; Shereen, M.A. The Emergence of a Novel Coronavirus (SARS-CoV-2), Their Biology and Therapeutic Options. [Published Online Ahead of Print, 2020 Mar 11]. J. Clin. Microbiol. 2020, 58, e00187-20. [Google Scholar]
- Salehi, B.; Vlaisavljevic, S.; Adetunji, C.O.; Adetunji, J.B.; Kregiel, D.; Antolak, H.; Pawlikowska, E.; Uprety, Y.; Mileski, K.S.; Devkota, H.P.; et al. Plants of the Genus Vitis: Phenolic Compounds, Anticancer Properties and Clinical Relevance. Trends Food Sci. Technol. 2019, 91, 362–379. [Google Scholar] [CrossRef]
- Schlachterman, A.; Valle, F.; Wall, K.M.; Azios, N.G.; Castillo, L.; Morell, L.; Washington, A.V.; Cubano, L.A.; Dharmawardhane, S.F. Combined Resveratrol, Quercetin, and Catechin Treatment Reduces Breast Tumor Growth in a Nude Mouse Model. Transl. Oncol. 2008, 1, 19–27. [Google Scholar] [CrossRef]
- Liu, J.-D.; Chen, S.-H.; Lin, C.-L.; Tsai, S.-H.; Liang, Y.-C. Inhibition of Melanoma Growth and Metastasis by Combination with (-)-Epigallocatechin-3-Gallate and Dacarbazine in Mice. J. Cell. Biochem. 2001, 83, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Chang, Y.; Wang, X.; Ban, C.; Zhang, F. Reduction of COX-2 through Modulating MiR-124/SPHK1 Axis Contributes to the Antimetastatic Effect of Alpinumisoflavone in Melanoma. Am. J. Transl. Res. 2017, 9, 986. [Google Scholar] [PubMed]
- Piantelli, M.; Rossi, C.; Iezzi, M.; La Sorda, R.; Iacobelli, S.; Alberti, S.; Natali, P.G. Flavonoids Inhibit Melanoma Lung Metastasis by Impairing Tumor Cells Endothelium Interactions. J. Cell. Physiol. 2006, 207, 23–29. [Google Scholar] [CrossRef]
- Chatterjee, A.; Mitra, A.; Ray, S.; Chattopadhyay, N.; Siddiqi, M. Curcumin Exhibits Antimetastatic Properties by Modulating Integrin Receptors, Collagenase Activity, and Expression of Nm23 and E-Cadherin. J. Environ. Pathol. Toxicol. Oncol. 2003, 22, 49–58. [Google Scholar] [CrossRef]
- Di Leo, N.; Battaglini, M.; Berger, L.; Giannaccini, M.; Dente, L.; Hampel, S.; Vittorio, O.; Cirillo, G.; Raffa, V. A Catechin Nanoformulation Inhibits WM266 Melanoma Cell Proliferation, Migration and Associated Neo-Angiogenesis. Eur. J. Pharm. Biopharm. 2017, 114, 1–10. [Google Scholar] [CrossRef]
- Amawi, H.; Ashby, C.R., Jr.; Samuel, T.; Peraman, R.; Tiwari, A.K. Polyphenolic Nutrients in Cancer Chemoprevention and Metastasis: Role of the Epithelial-to-Mesenchymal (EMT) Pathway. Nutrients 2017, 9, 911. [Google Scholar] [CrossRef]
- Gupta, N.; Singh, S.; Chauhan, D.; Srivastava, R.; Singh, V.K. Exploring the Anticancer Potentials of Polyphenols: A ComprehensiveReview of Patents in the Last Five Years. Recent Pat. Anticancer. Drug Discov. 2023, 18, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Gálvez, M.Á.; González-Sarrías, A.; Martínez-Díaz, F.; Abellán, B.; Martínez-Torrano, A.J.; Fernández-López, A.J.; Giménez-Bastida, J.A.; Espín, J.C. Disposition of Dietary Polyphenols in Breast Cancer Patients’ Tumors, and Their Associated Anticancer Activity: The Particular Case of Curcumin. Mol. Nutr. Food Res. 2021, 65, 2100163. [Google Scholar] [CrossRef]
- Bhosale, P.B.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Kim, S.M.; Kim, G.S. Functions of Polyphenols and Its Anticancer Properties in Biomedical Research: A Narrative Review. Transl. Cancer Res. 2020, 9, 7619–7631. [Google Scholar] [CrossRef]
- Mancini, M.; Cerny, M.E.V.; Cardoso, N.S.; Verissimo, G.; Maluf, S.W. Grape Seed Components as Protectors of Inflammation, DNA Damage, and Cancer. Curr. Nutr. Rep. 2023, 12, 141–150. [Google Scholar] [CrossRef]
- Li, F.; Qasim, S.; Li, D.; Dou, Q.P. Updated Review on Green Tea Polyphenol Epigallocatechin-3-Gallate as a Cancer Epigenetic Regulator. Semin. Cancer Biol. 2022, 83, 335–352. [Google Scholar] [CrossRef]
- Majidinia, M.; Bishayee, A.; Yousefi, B. Polyphenols: Major Regulators of Key Components of DNA Damage Response in Cancer. DNA Repair 2019, 82, 102679. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Organization (WHO) Coronavirus (COVID-19) Dashboard. 2022. Available online: https://covid19.who.int/ (accessed on 28 February 2022).
- Brief, T.A. Implications of the Emergence and Spread of the SARS-CoV-2 B. 1.1. 529 Variant of Concern (Omicron) for the EU/EEA. Eur. Cent. Dis. Prev. Control. 2021, 1, 529. [Google Scholar]
- Paraiso, I.L.; Revel, J.S.; Stevens, J.F. Potential Use of Polyphenols in the Battle against COVID-19. Curr. Opin. Food Sci. 2020, 32, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Mehany, T.; Khalifa, I.; Barakat, H.; Althwab, S.A.; Alharbi, Y.M.; El-Sohaimy, S. Polyphenols as Promising Biologically Active Substances for Preventing SARS-CoV-2: A Review with Research Evidence and Underlying Mechanisms. Food Biosci. 2021, 40, 100891. [Google Scholar] [CrossRef] [PubMed]
- Dejani, N.N.; Elshabrawy, H.A.; Bezerra Filho, C.D.S.M.; de Sousa, D.P. Anticoronavirus and Immunomodulatory Phenolic Compounds: Opportunities and Pharmacotherapeutic Perspectives. Biomolecules 2021, 11, 1254. [Google Scholar] [CrossRef]
- Bahun, M.; Jukić, M.; Oblak, D.; Kranjc, L.; Bajc, G.; Butala, M.; Bozovičar, K.; Bratkovič, T.; Podlipnik, Č.; Ulrih, N.P. Inhibition of the SARS-CoV-2 3CLpro Main Protease by Plant Polyphenols. Food Chem. 2022, 373, 131594. [Google Scholar] [CrossRef]
- Besednova, N.N.; Andryukov, B.G.; Zaporozhets, T.S.; Kryzhanovsky, S.P.; Fedyanina, L.N.; Kuznetsova, T.A.; Zvyagintseva, T.N.; Shchelkanov, M.Y. Antiviral Effects of Polyphenols from Marine Algae. Biomedicines 2021, 9, 200. [Google Scholar] [CrossRef]
- Horne, J.R.; Vohl, M.-C. Biological Plausibility for Interactions between Dietary Fat, Resveratrol, ACE2, and SARS-CoV Illness Severity. Am. J. Physiol.-Endocrinol. Metab. 2020, 318, E830–E833. [Google Scholar] [CrossRef]
- Khalifa, I.; Nawaz, A.; Sobhy, R.; Althwab, S.A.; Barakat, H. Polyacylated Anthocyanins Constructively Network with Catalytic Dyad Residues of 3CLpro of 2019-NCoV than Monomeric Anthocyanins: A Structural-Relationship Activity Study with 10 Anthocyanins Using in-Silico Approaches. J. Mol. Graph. Model. 2020, 100, 107690. [Google Scholar] [CrossRef]
- Utomo, R.Y.; Ikawati, M.; Meiyanto, E. Revealing the Potency of Citrus and Galangal Constituents to Halt SARS-CoV-2 Infection. Med. Pharmacol. 2020, Preprints. [Google Scholar]
- Xiao, T.; Cui, M.; Zheng, C.; Wang, M.; Sun, R.; Gao, D.; Bao, J.; Ren, S.; Yang, B.; Lin, J.; et al. Myricetin Inhibits SARS-CoV-2 Viral Replication by Targeting Mpro and Ameliorates Pulmonary Inflammation. Front. Pharmacol. 2021, 12, 669642. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wei, J.; Huang, T.; Lei, L.; Shen, C.; Lai, J.; Yang, M.; Liu, L.; Yang, Y.; Liu, G.; et al. Resveratrol Inhibits the Replication of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Cultured Vero Cells. Phytother. Res. 2021, 35, 1127. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Rane, J.S.; Chatterjee, A.; Kumar, A.; Khan, R.; Prakash, A.; Ray, S. Targeting SARS-CoV-2 Spike Protein of COVID-19 with Naturally Occurring Phytochemicals: An in Silico Study for Drug Development. J. Biomol. Struct. Dyn. 2021, 39, 6306–6316. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).