Phenology and Diversity of Weeds in the Agriculture and Horticulture Cropping Systems of Indian Western Himalayas: Understanding Implications for Agro-Ecosystems
Abstract
1. Introduction
2. Results
2.1. Functional Traits Including Flowering Phenology
2.2. Weed Distribution and Their Diversity in Agriculture and Horticulture Systems
2.3. Indicator Species Analysis
3. Discussion
4. Materials and Methods
4.1. Study Area
4.2. Sampling Design and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, M.; Kalra, N.; Ravindranath, N.H. Assessing the response of forests to environmental variables using a dynamic global vegetation model: An Indian perspective. Curr. Sci. 2020, 118, 700–701. [Google Scholar]
- Kumar, M.; Kalra, N.; Khaiter, P.; Ravindranath, N.H.; Singh, V.; Singh, H.; Sharma, S.; Rahnamayan, S. PhenoPine: A simulation model to trace the phenological changes in Pinus roxhburghii in response to ambient temperature rise. Ecol. Modell. 2019, 404, 12–20. [Google Scholar] [CrossRef]
- Kumar, M.; Rawat, S.P.S.; Singh, H.; Ravindranath, N.H.; Kalra, N. Dynamic forest vegetation models for predicting impacts of climate change on forests: An Indian perspective. Indian J. For. 2018, 41, 1–12. [Google Scholar]
- Rawat, A.S.; Kalra, N.; Singh, H.; Kumar, M. Application of vegetation models in India for understanding the forest ecosystem processes. Indian For. 2020, 146, 99–100. [Google Scholar] [CrossRef]
- Richards, C.L.; Bossdorf, O.; Muth, N.Z.; Gurevitch, J.; Pigliucci, M. Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecol. Lett. 2006, 9, 981–993. [Google Scholar] [CrossRef] [PubMed]
- D’antonio, C.; Meyerson, L.A. Exotic plant species as problems and solutions in ecological restoration: A synthesis. Rest. Ecol. 2002, 10, 703–713. [Google Scholar] [CrossRef]
- Holzner, W.; Numata, M. Biology and Ecology of Weeds; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; Volume 2. [Google Scholar]
- Einhellig, F.A. Interactions involving allelopathy in cropping systems. Agron. J. 1996, 88, 886–893. [Google Scholar] [CrossRef]
- Weston, L.A.; Duke, S.O. Weed and crop allelopathy. Crit. Rev. Plant Sci. 2003, 22, 367–389. [Google Scholar] [CrossRef]
- Zimdahl, R.L. Fundamentals of Weed Science; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Sinden, J.; Jones, R.; Hester, S.; Odom, D.; Kalisch, C.; James, R.; Cacho, O.; Griffith, G. The Economic Impact of Weeds in Australia; Technical Series 8; CRC for Australian Weed Management: Glen Osmond, Australia, 2004.
- Derpsch, R.; Friedrich, T.; Kassam, A.; Li, H. Current status of adoption of no-till farming in the world and some of its main benefits. Int. J. Biol. Eng. 2010, 3, 1–25. [Google Scholar]
- Chmielewski, F.M. Phenology in agriculture and horticulture. In Phenology: An Integrative Environmental Science; Springer: Berlin/Heidelberg, Germany, 2013; pp. 539–561. [Google Scholar]
- Kumar, M.; Padalia, H.; Nandy, S.; Singh, H.; Khaiter, P.; Kalra, N. Does spatial heterogeneity of landscape explain the process of plant invasion? A case study of Hyptis suaveolens from Indian Western Himalaya. Environ. Monit. Assess. 2019, 191, 794. [Google Scholar] [CrossRef]
- Norsworthy, J.K.; Bond, J.; Scott, R.C. Weed management practices and needs in Arkansas and Mississippi rice. Weed Technol. 2013, 27, 623–630. [Google Scholar] [CrossRef]
- Haq, S.M.; Hamid, M.; Lone, F.A.; Singh, B. Himalayan hotspot with Alien Weeds: A case study of biological spectrum, phenology, and diversity of weedy plants of high altitude mountains in District Kupwara of J&K Himalaya, India. Proc. Natl. Acad. Sci. India Sect. B Boil. Sci. 2021, 91, 139–152. [Google Scholar]
- Ward, S.M.; Cousens, R.D.; Bagavathiannan, M.V.; Barney, J.N.; Beckie, H.J.; Busi, R.; Davis, A.S.; Dukes, J.S.; Forcella, F.; Freckleton, R.P.; et al. Agricultural weed research: A critique and two proposals. Weed Sci. 2014, 62, 672–678. [Google Scholar] [CrossRef]
- Nunez, M.A.; Pauchard, A. Biological invasions in developing and developed countries: Does one model fit all? Biol. Invas. 2010, 12, 707–714. [Google Scholar] [CrossRef]
- Monaco, T.J.; Weller, S.C.; Ashton, F.M. Weed Science: Principles and Practices; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Forbes, J.C.; Forbes, J.C.; Watson, D. Plants in Agriculture; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Khan, M.; Hussain, F.; Musharaf, S. Floristic composition and biological characteristics of the vegetation of Sheikh Maltoon Town District Mardan, Pakistan. Annu. Res. Rev. Biol. 2013, 31, 31–41. [Google Scholar]
- Ul Haq, Z.; Gul, B.; Shah, S.M.; Razaq, A.; Raza, H. Ecological characteristics of weeds of onion crop of University of Peshawar Botanical Garden, District Nowshera, Pakistan. Pak. J. Weed Sci. Res. 2016, 22, 263–267. [Google Scholar]
- Ali, A.; Badshah, L.; Hussain, F.; Shinwari, Z.K. Floristic composition and ecological characteristics of plants of chail valley, district Swat, Pakistan. Pak. J. Bot. 2016, 48, 1013–1026. [Google Scholar]
- Zeb, U.; Ali, S.; Li, Z.H.; Khan, H.; Shahzad, K.; Shuaib, M.; Ihsan, M. Floristic diversity and ecological characteristics of weeds at Atto Khel Mohmand Agency, KPK, Pakistan. Acta Ecol. Sin. 2017, 37, 363–367. [Google Scholar] [CrossRef]
- Iqbal, M.; Khan, S.M.; Khan, M.A.; Ahmad, Z.; Ahmad, H. A novel approach to phytosociological classification of weeds flora of an agro-ecological system through Cluster, Two Way Cluster and Indicator Species Analyses. Ecol. Indic. 2018, 84, 590–606. [Google Scholar] [CrossRef]
- Nafeesa, Z.; Haq, S.M.; Bashir, F.; Gaus, G.; Mazher, M.; Anjum, M.; Rasool, A.; Rashid, N. Observations on the floristic, life-form, leaf-size spectra and habitat diversity of vegetation in the Bhimber hills of Kashmir Himalayas. Acta Ecol. Sin. 2021, 41, 228–234. [Google Scholar] [CrossRef]
- Chhetri, N.B.K.; Shrestha, K.K. Floristic diversity and important value indices of tree species in lower Kanchenjunga Singhalila ridge Eastern Nepal. Am. J. Plant Sci. 2019, 10, 248–263. [Google Scholar] [CrossRef]
- Haq, S.M.; Malik, A.H.; Khuroo, A.A.; Rashid, I. Floristic composition and biological spectrum of Keran-a remote valley of northwestern Himalaya. Acta Ecol. Sin. 2019, 39, 372–379. [Google Scholar] [CrossRef]
- Pichi-Sermolli, R.E. An index for establishing the degree of maturity in plant communities. J. Ecol. 1948, 36, 85–90. [Google Scholar] [CrossRef]
- Saxena, A.K.; Pandey, P.; Singh, J.S. Biological Spectrum and other structural functional attributes of the vegetation of Kumaun Himalaya. Vegetatio 1982, 49, 111–119. [Google Scholar] [CrossRef]
- Sahu, S.C.; Dhal, N.K.; Datt, B. Environmental implications of Biological spectrum vis-a-vis tree species diversity in two protected forests (PFs) of Gandhamardan hill ranges, Eastern Ghats, India. Environmentalist 2012, 32, 420–432. [Google Scholar] [CrossRef]
- Waheed, M.; Haq, S.M.; Arshad, F.; Bussmann, R.W.; Iqbal, M.; Bukhari, N.A.; Hatamleh, A.A. Grasses in Semi-Arid Lowlands—Community Composition and Spatial Dynamics with Special Regard to the Influence of Edaphic Factors. Sustainability 2022, 14, 14964. [Google Scholar] [CrossRef]
- Mamariani, F.; Joharchi, M.R.; Ejtehadi, H.; Emadzade, K. Contributions to the flora and vegetation of Binalood mountain range, NE Iran: Floristic and chorological studies in Fereizi region. JCMR 2009, 1, 1–18. [Google Scholar]
- Vakhlamova, T.; Rusterholz, H.P.; Kanibolotskaya, Y.; Baur, B. Effects of road type and urbanization on the diversity and abundance of alien species in roadside verges in Western Siberia. Plant Ecol. 2016, 217, 241–252. [Google Scholar] [CrossRef]
- Rashid, I.; Haq, S.M.; Lembrechts, J.J.; Khuroo, A.A.; Pauchard, A.; Dukes, J.S. Railways redistribute plant species in mountain landscapes. J. Appl. Ecol. 2021, 58, 967–1980. [Google Scholar] [CrossRef]
- Asim, Z.I.; Haq, F.; Iqbal, A. Phenology, life form and leaf spectra of the vegetation of kokarai valley, district swat. J. Biol. Environ. Sci. 2016, 9, 23–31. [Google Scholar]
- Rahman, I.U.; Afzal, A.; Iqbal, Z.; Ijaz, F.; Ali, N.; Asif, M.; Alam, J.; Majid, A.; Hart, R.; Bussmann, R.W. First insights into the floristic diversity, biological spectra and phenology of Manoor Valley, Pakistan. Pak. J. Bot. 2018, 50, 1113–1124. [Google Scholar]
- Rahman, I.U.; Afzal, A.; Iqbal, Z.; Abd Allah, E.F.; Alqarawi, A.A.; Calixto, E.S.; Ali, N.; Ijaz, F.; Kausar, R.; Alsubeie, M.S.; et al. Role of multivariate approaches in floristic diversity of Manoor Valley (Himalayan Region), Pakistan. Appl. Ecol. Environ. Res. 2019, 17, 1475–1498. [Google Scholar] [CrossRef]
- Joly, M.; Bertrand, P.; Gbangou, R.Y.; White, M.C.; Dubé, J.; Lavoie, C. Paving the way for invasive species: Road type and the spread of common ragweed (Ambrosia artemisiifolia). Environ. Manag. 2011, 48, 514–522. [Google Scholar] [CrossRef]
- Khan, W.; Khan, S.M.; Ahmad, H.; Alqarawi, A.A.; Shah, G.M.; Hussain, M.; Abd_Allah, E.F. Life forms, leaf size spectra, regeneration capacity and diversity of plant species grown in the Thandiani forests, district Abbottabad, Khyber Pakhtunkhwa, Pakistan. Saud. J. Biol. Sci. 2018, 25, 94–100. [Google Scholar] [CrossRef]
- Hussain, F.; Shah, S.M.; Badshah, L.; Durrani, M.J. Diversity and ecological characteristics of flora of Mastuj valley, district Chitral, Hindukush range, Pakistan. Pak. J. Bot. 2015, 47, 495–510. [Google Scholar]
- Rosenzweig, M.L. A theory of habitat selection. Ecology 1981, 62, 327–335. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Schwilk, D.W.; Ackerly, D.D. A trait-based test for habitat filtering: Convex hull volume. Ecology 2006, 87, 1465–1471. [Google Scholar] [CrossRef]
- Olokeogun, O.S.; Kumar, M. An indicator based approach for assessing the vulnerability of riparian ecosystem under the influence of urbanization in the Indian Himalayan city, Dehradun. Ecol. Indic. 2020, 119, 106796. [Google Scholar] [CrossRef]
- Andrabi, S.M.; Reshi, Z.A.; Shah, M.A.; Qureshi, S. Studying the patterns of alien and native floras of some habitats in Srinagar city, Kashmir, India. Ecol. Processes 2015, 4, 2. [Google Scholar] [CrossRef]
- Haq, S.M.; Khuroo, A.A.; Malik, A.H.; Rashid, I.; Ahmad, R.; Hamid, M.; Dar, G.H. Forest ecosystems of Jammu and Kashmir state. In Biodiversity of the Himalaya: Jammu and Kashmir State; Springer: Singapore, 2020; pp. 191–208. [Google Scholar]
- Curtis, J.T.; Mcintosh, R.P. The interrelations of certain analytic and synthetic phytosociological characters. Ecology 1950, 31, 434–455. [Google Scholar] [CrossRef]
- Raunkiaer, C. The Life Forms of Plants and Statistical Plant Geography; Being the Collected Papers of C. Raunkiaer; Clarendon Press: Oxford, UK, 1934. [Google Scholar]
- Perez-Harguindeguy, N.; Diaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Pielou, E.C. Ecological Diversity; No. 574.524018 P5; Wiley: Hoboken, NJ, USA, 1975. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. vegan: Community Ecology Package, R package Version 2.5-2; 2018. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 8 June 2022).
- Team, R.D.C. A Language and Environment for Statistical Computing. 2009. Available online: https://www.R-project.org (accessed on 8 June 2022).
- Baselga, A.; Orme, D.; Villeger, S.; De Bortoli, J.; Leprieur, F.; Baselga, M.A. Package ‘betapart’. Partitioning Beta Diversity into Turnover and Nestedness Components, Version 1.5.6; 2018. Available online: https://cran.r-project.org/web/packages/betapart/betapart.pdf (accessed on 8 June 2022).
- Waheed, M.; Haq, S.M.; Fatima, K.; Arshad, F.; Bussmann, R.W.; Masood, F.R.; Alataway, A.Z.; Dewidar, A.F.; Almutairi, K.; Elansary, H.O.; et al. Ecological Distribution Patterns and Indicator Species Analysis of Climber Plants in Changa Manga Forest Plantation. Diversity 2022, 14, 988. [Google Scholar] [CrossRef]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Haq, S.M.; Amjad, M.S.; Waheed, M.; Bussmann, R.W.; Proćków, J. The floristic quality assessment index as ecological health indicator for forest vegetation: A case study from Zabarwan Mountain Range, Himalayas. Ecol. Indic. 2022, 145, 109670. [Google Scholar] [CrossRef]
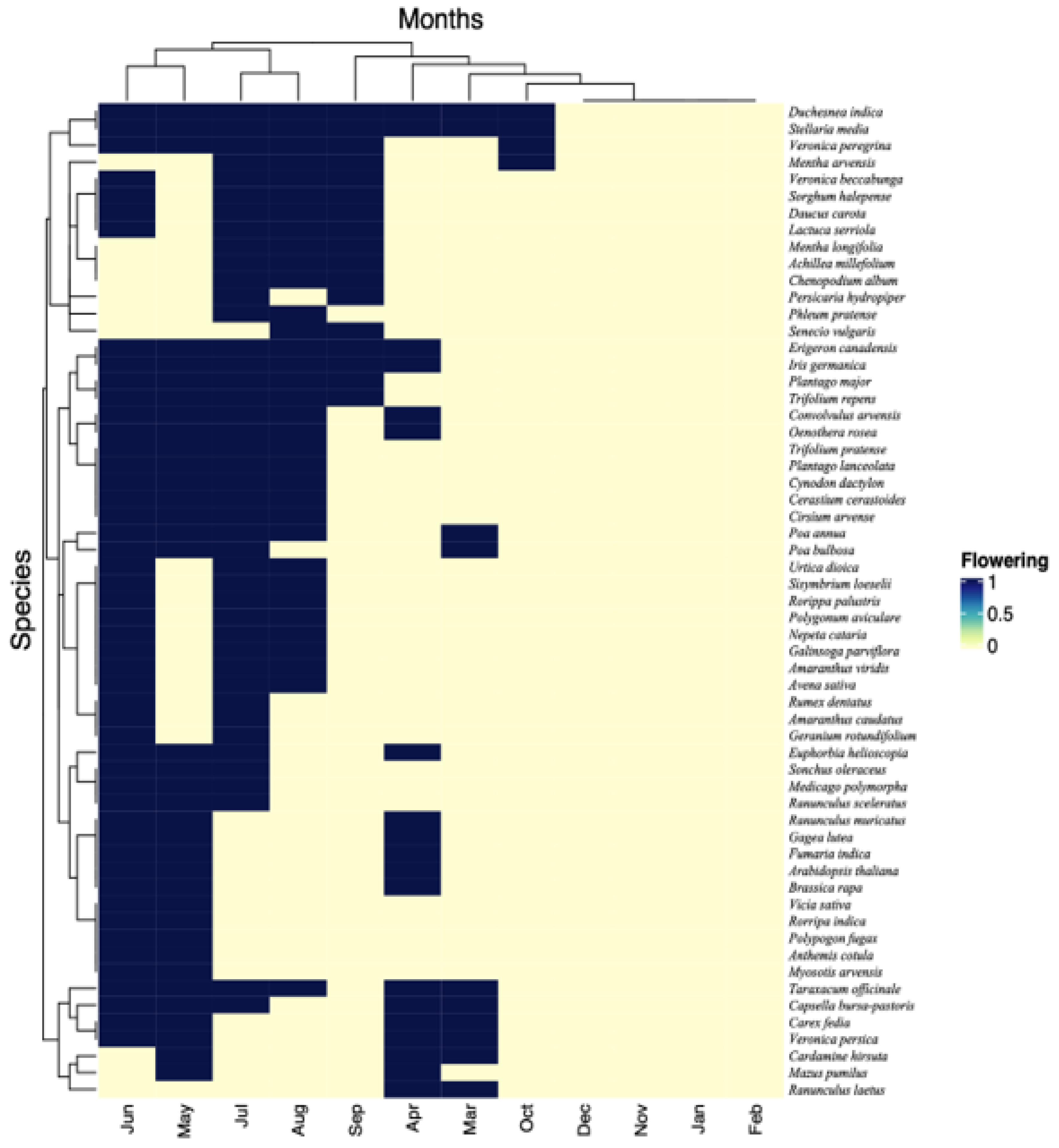

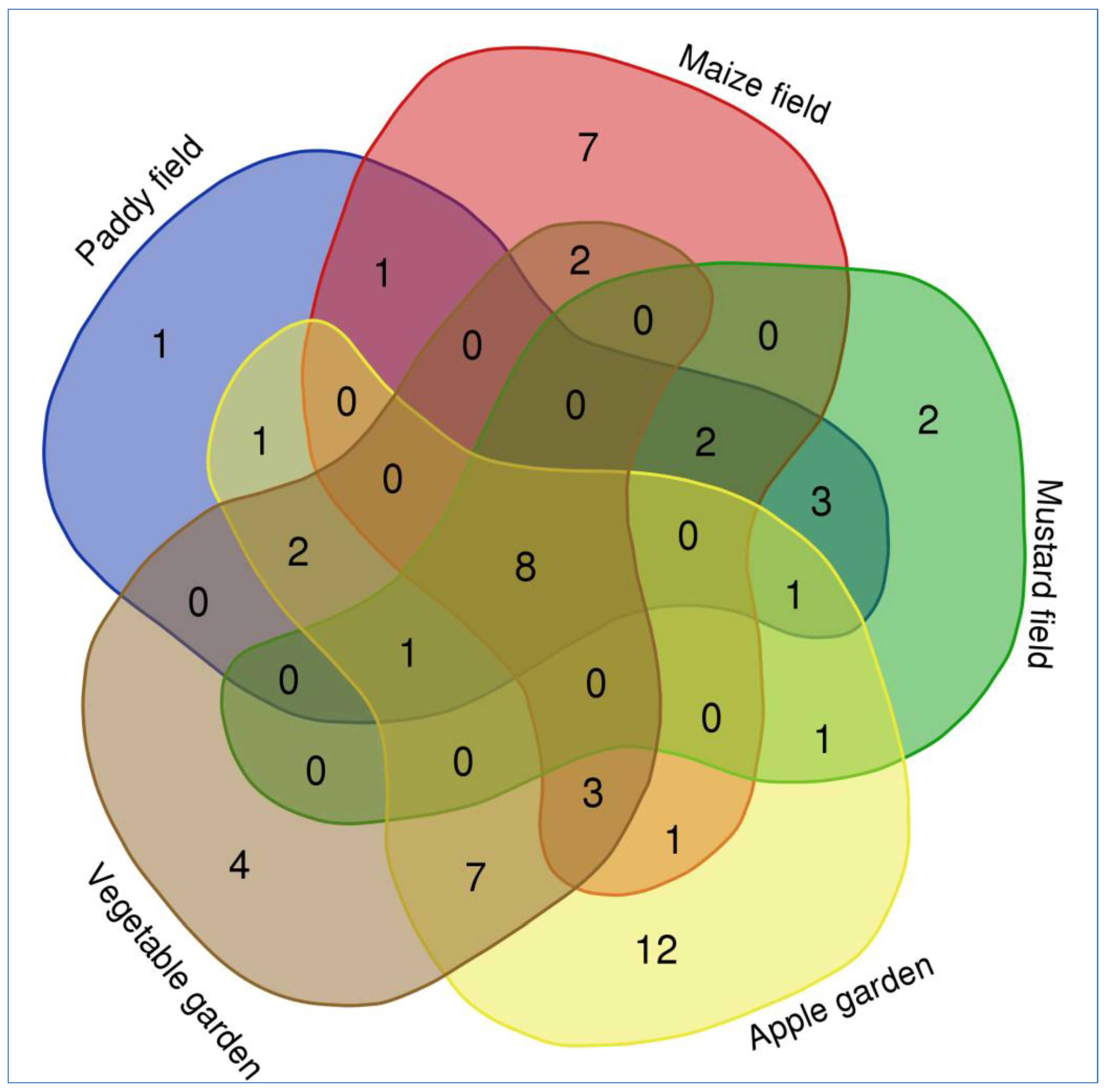
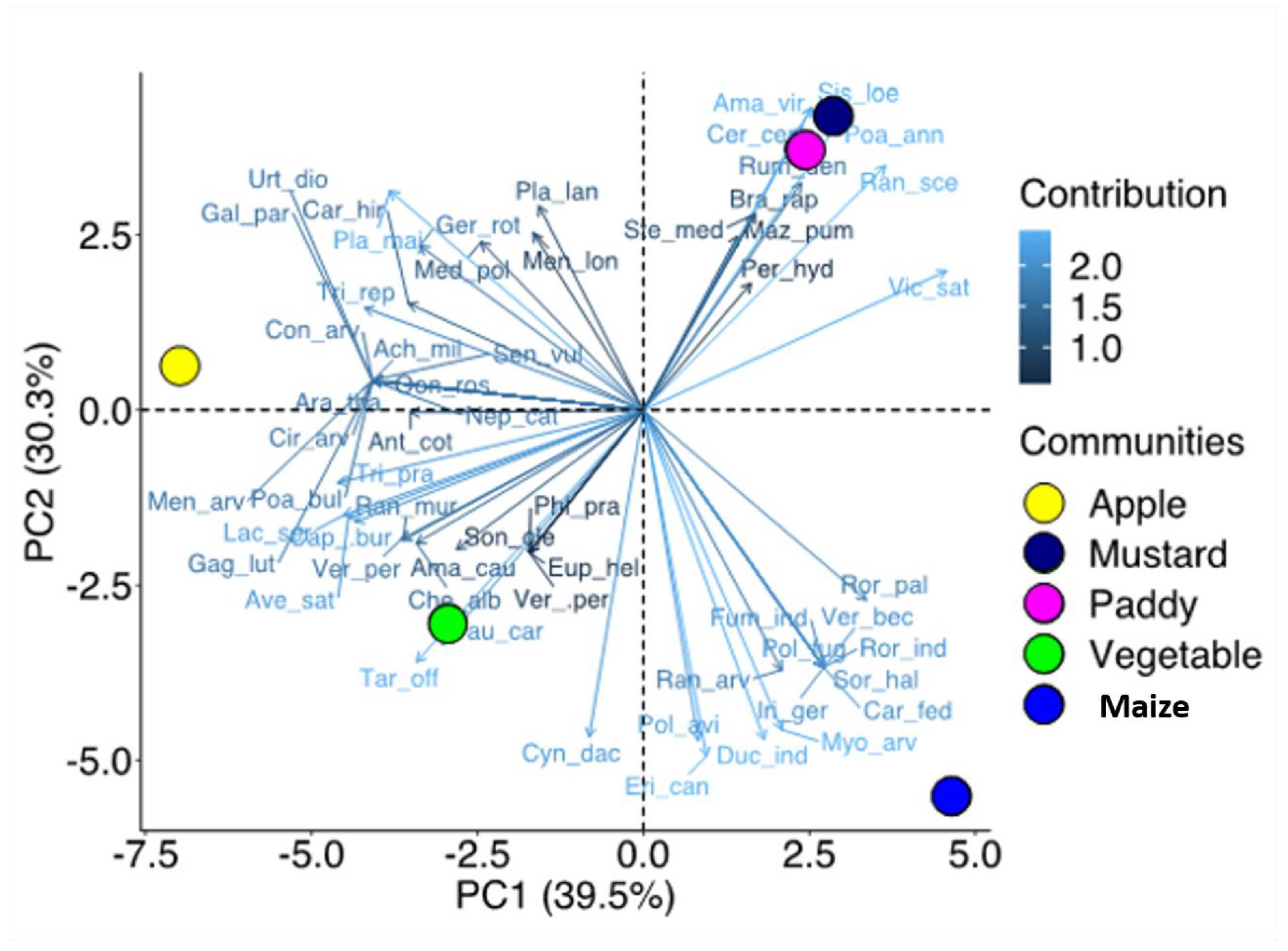
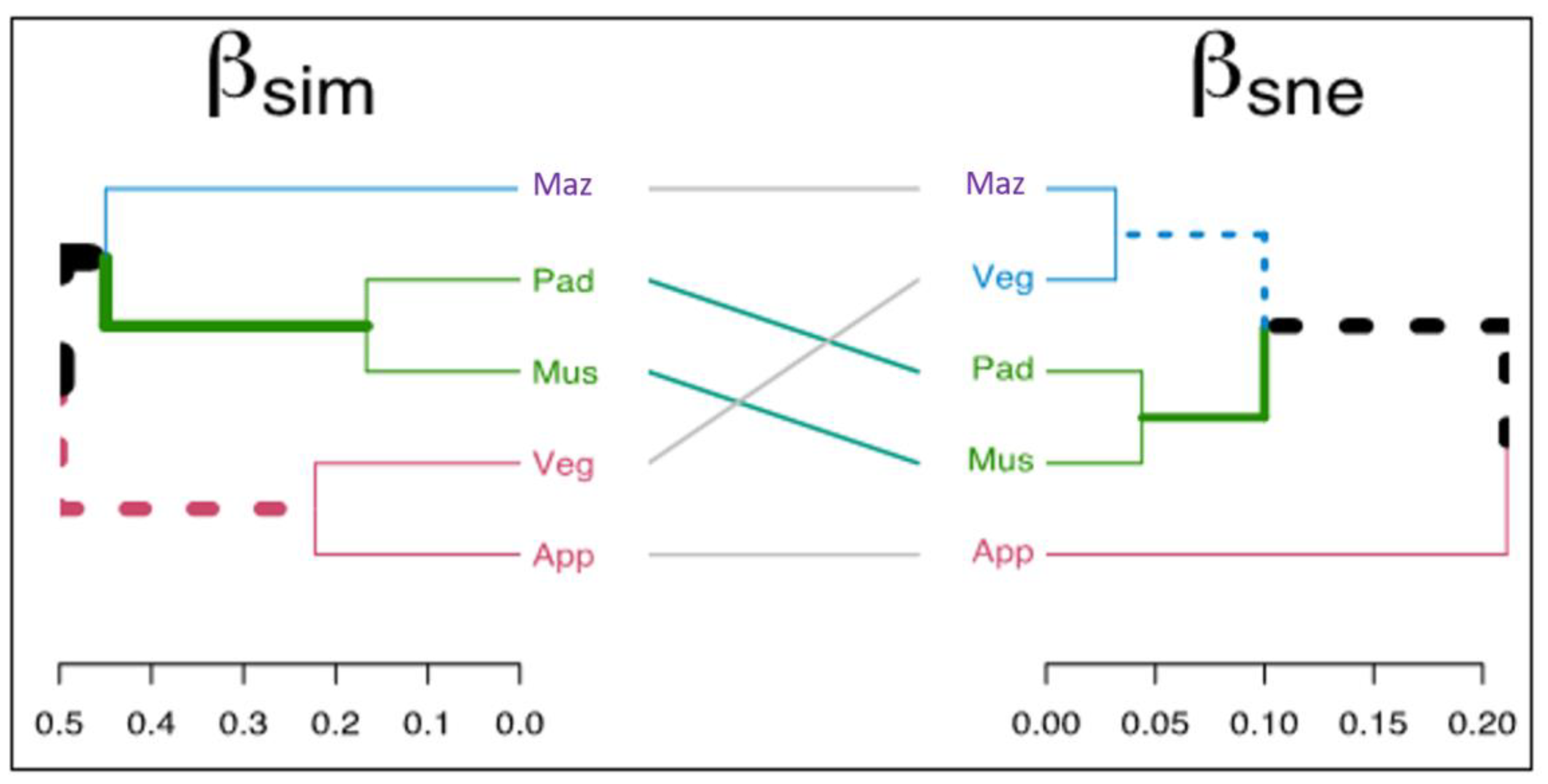


| Scientific Name | Family | Life Span | Agriculture | Horticulture | Origin | Flowering | Raunkiaer | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Paddy | Maize | Mustard | Apple | Vegetable | Phenology | Lifeform | ||||
| Achillea millefolium Linn. | Asteraceae | P | 0 | 0 | 0 | 2.74 | 0 | Native | July–Sept. | Hemicryptophyte |
| Amaranthus caudatus L. | Amaranthaceae | P | 0 | 0 | 0 | 2.74 | 11.20 | Exotic | June–Aug. | Therophyte |
| Amaranthus viridis L. | Amaranthaceae | A | 7.45 | 0 | 7.85 | 0 | 0 | Exotic | June–Aug. | Therophyte |
| Anthemis cotula L. | Asteraceae | A | 8.35 | 0 | 0 | 8.32 | 12.13 | Exotic | May–June | Therophyte |
| Arabidopsis thaliana (L.) Heynh. | Brassicaceae | A | 0 | 0 | 0 | 7.23 | 0 | Exotic | April–June | Therophyte |
| Avena sativa L. | Poaceae | A | 0 | 0 | 0 | 11.13 | 14.24 | Exotic | June–Aug. | Therophyte |
| Brassica rapa L. | Brassicaceae | A | 0 | 0 | 13.26 | 0 | 0 | Exotic | April–June | Hemicryptophyte |
| Capsella bursa-pastoris (L.) Medik. | Brassicaceae | A | 0 | 0 | 0 | 6.78 | 9.34 | Native | March–July | Therophyte |
| Cardamine hirsuta L. | Brassicaceae | B | 11.01 | 0 | 0 | 13.02 | 6.05 | Native | March–May | Therophyte |
| Carex fedia Nees | Cyperaceae | P | 0 | 20.63 | 0 | 0 | 0 | Native | March–June | Hemicryptophyte |
| Cerastium cerastoides (L.) | Caryophyllaceae | A | 3.76 | 0 | 7.19 | 0 | 0 | Native | May–Aug. | Therophyte |
| Chenopodium album L. | Chenopodiaceae | A | 0 | 0 | 0 | 5.05 | 12.61 | Exotic | July–Sept. | Therophyte |
| Cirsium arvense (L.) Scop. | Asteraceae | P | 0 | 0 | 0 | 2.08 | 0 | Exotic | May–Aug. | Hemicryptophyte |
| Convolvulus arvensis L. | Convolvulaceae | P | 0 | 0 | 0 | 3.37 | 0 | Exotic | April–Aug. | Therophyte |
| Cynodon dactylon (L.) Pers. | Poaceae | P | 0 | 21.21 | 0 | 13.32 | 11.47 | Native | May–Aug. | Hemicryptophyte |
| Daucus carota L. | Apiaceae | B | 0 | 2.97 | 0 | 4.18 | 8.41 | Exotic | June–Sept. | Hemicryptophyte |
| Duchesnea indica (Andrews) Teschem. | Rosaceae | P | 0 | 14.51 | 0 | 0 | 7.41 | Native | March–Oct. | Hemicryptophyte |
| Erigeron canadensis L. | Asteraceae | A-B | 5.17 | 16.73 | 5.62 | 7.28 | 12.61 | Exotic | April–Sept. | Therophyte |
| Euphorbia helioscopia L. | Euphorbiaceae | A | 0 | 0 | 0 | 0 | 3.29 | Exotic | April–July | Therophyte |
| Fumaria indica (Hausskn.) Pugsley | Papaveraceae | A | 0 | 5.64 | 0 | 0 | 0 | Native | April-June | Therophyte |
| Gagea lutea (L.) Ker Gawl. | Liliaceae | P | 0 | 0 | 0 | 3.37 | 0 | Native | April–June | Geophyte |
| Galinsoga parviflora Cav. | Asteraceae | A | 0 | 0 | 0 | 3.75 | 0 | Exotic | June–Aug. | Therophyte |
| Geranium rotundifolium L. | Geraniaceae | P | 3.29 | 0 | 3.56 | 10.51 | 0 | Native | June–July | Therophyte |
| Iris kashmiriana Baker | Iridaceae | P | 0 | 6.39 | 0 | 0 | 0 | Native | April–Jun | Geophyte |
| Lactuca serriola L. | Asteraceae | A | 0 | 0 | 0 | 4.62 | 5.63 | Native | July–Sept. | Therophyte |
| Mazus pumilus (Burm.f.) Steenis | Mazaceae | A | 6.67 | 0 | 0 | 0 | 0 | Native | April–May | Therophyte |
| Medicago polymorpha L. | Fabaceae | A | 0 | 0 | 7.92 | 9.81 | 0 | Exotic | April–June | Therophyte |
| Mentha arvensis L. | Lamiaceae | P | 0 | 0 | 0 | 5.35 | 0 | Native | July–Oct. | Hemicryptophyte |
| Mentha longifolia (L.) Huds. | Lamiaceae | P | 7.47 | 0 | 0 | 5.92 | 0 | Exotic | July–Sept. | Hemicryptophyte |
| Myosotis arvensis (L.) Hill | Boraginaceae | P | 0 | 18.93 | 0 | 0 | 7.38 | Exotic | May–June | Therophyte |
| Nepeta cataria L. | Lamiaceae | P | 0 | 0 | 0 | 3.37 | 0 | Native | June–Aug. | Hemicryptophyte |
| Oenothera rosea L’Hér. ex Aiton | Onagraceae | P | 0 | 0 | 0 | 6.35 | 0 | Native | April–Aug. | Therophyte |
| Persicaria hydropiper Delarbre | Polygonaceae | A | 56.71 | 14.92 | 3.91 | 9.36 | 6.05 | Native | July–Sept. | Therophyte |
| Phleum pratense L. | Poaceae | P | 0 | 0 | 0 | 0 | 25.15 | Native | July–Aug. | Hemicryptophyte |
| Plantago lanceolata L. | Plantaginaceae | A-B-P | 7.47 | 0 | 7.92 | 5.05 | 9.38 | Native | May–Aug. | Therophyte |
| Plantago major L. | Plantaginaceae | P | 6.31 | 5.07 | 6.92 | 7.61 | 6.51 | Native | May–Sept. | Hemicryptophyte |
| Poa annua L. | Poaceae | A | 95.64 | 42.03 | 99.22 | 34.75 | 21.26 | Native | March–Aug. | Therophyte |
| Poa bulbosa L. | Poaceae | P | 0 | 0 | 0 | 10.54 | 0 | Native | March–July | Hemicryptophyte |
| Polygonum aviculare L. | Polygonaceae | A | 4.96 | 18.55 | 5.28 | 9.39 | 10.27 | Native | June–Aug. | Therophyte |
| Polypogon fugax Nees ex Steud. | Poaceae | P | 0 | 19.19 | 0 | 0 | 0 | Native | May–June | Therophyte |
| Ranunculus arvensis L. | Ranunculaceae | P | 0 | 16.21 | 0 | 2.77 | 0 | Native | March–April | Hemicryptophyte |
| Ranunculus muricatus L. | Ranunculaceae | A-B-P | 0 | 0 | 0 | 4.62 | 10.14 | Exotic | April–June | Therophyte |
| Ranunculus sceleratus L. | Ranunculaceae | P | 15.53 | 5.91 | 16.13 | 0 | 0 | Exotic | May–July | Therophyte |
| Rorippa palustris (L.) Besser | Brassicaceae | A-B | 8.64 | 21.17 | 0 | 0 | 0 | Native | June–Aug. | Geophyte |
| Rorippa indica (L.) Hiern. | Brassicaceae | A-B | 0 | 5.76 | 0 | 0 | 0 | Native | April–June | Geophyte |
| Rumex dentatus L. | Polygonaceae | P | 13.87 | 4.71 | 15.12 | 2.75 | 10.28 | Native | June–July | Hemicryptophyte |
| Senecio vulgaris L. | Asteraceae | A-B | 0 | 0 | 0 | 2.08 | 0 | Exotic | April–Sept. | Therophyte |
| Sisymbrium loeselii L. | Brassicaceae | A | 11.01 | 0 | 11.84 | 0 | 0 | Exotic | June–Aug. | Therophyte |
| Sonchus oleraceus L. | Asteraceae | A | 0 | 0 | 0 | 0 | 5.62 | Exotic | May–July | Therophyte |
| Sorghum halepense (L.) Pers. | Poaceae | P | 0 | 8.61 | 0 | 0 | 0 | Exotic | June–Sept. | Geophyte |
| Stellaria media (L.) Vill. | Poaceae | A | 0 | 0 | 58.93 | 0 | 0 | Native | March–Oct. | Therophyte |
| Taraxacum officinale Weber | Asteraceae | P | 0 | 3.31 | 0 | 5.73 | 7.41 | Native | March–Aug. | Hemicryptophyte |
| Trifolium pratense L. | Fabaceae | P | 9.49 | 8.41 | 10.07 | 19.63 | 21.28 | Native | May–Aug. | Hemicryptophyte |
| Trifolium repens L. | Fabaceae | P | 11.21 | 5.86 | 12.57 | 33.19 | 10.14 | Native | May–Sept. | Hemicryptophyte |
| Urtica dioica L. | Urticaceae | P | 0 | 0 | 0 | 4.96 | 0 | Exotic | June–Aug. | Therophyte |
| Veronica anagallis-aquatica L. | Plantaginaceae | P | 0 | 8.12 | 0 | 0 | 0 | Native | June–Sept. | Hemicryptophyte |
| Veronica peregrina L. | Plantaginaceae | A | 0 | 0 | 0 | 0 | 13.47 | Exotic | May–Oct. | Therophyte |
| Veronica persica Poir. | Plantaginaceae | A | 0 | 0 | 0 | 6.64 | 14.24 | Native | March–July | Therophyte |
| Vicia sativa L. | Fabaceae | A | 5.21 | 4.71 | 6.31 | 0 | 0 | Exotic | May–June | Therophyte |
| Indices | Agriculture | Horticulture | |||
|---|---|---|---|---|---|
| Paddy Field | Maize Field | Mustard Field | Apple Garden | Vegetable Garden | |
| Species number | 20 | 24 | 18 | 37 | 27 |
| Dominance | 0.153 | 0.062 | 0.164 | 0.048 | 0.045 |
| Shannon | 2.412 | 2.955 | 2.307 | 3.325 | 3.197 |
| Simpson | 0.847 | 0.937 | 0.835 | 0.951 | 0.955 |
| Evenness | 0.557 | 0.801 | 0.558 | 0.751 | 0.905 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haq, S.M.; Lone, F.A.; Kumar, M.; Calixto, E.S.; Waheed, M.; Casini, R.; Mahmoud, E.A.; Elansary, H.O. Phenology and Diversity of Weeds in the Agriculture and Horticulture Cropping Systems of Indian Western Himalayas: Understanding Implications for Agro-Ecosystems. Plants 2023, 12, 1222. https://doi.org/10.3390/plants12061222
Haq SM, Lone FA, Kumar M, Calixto ES, Waheed M, Casini R, Mahmoud EA, Elansary HO. Phenology and Diversity of Weeds in the Agriculture and Horticulture Cropping Systems of Indian Western Himalayas: Understanding Implications for Agro-Ecosystems. Plants. 2023; 12(6):1222. https://doi.org/10.3390/plants12061222
Chicago/Turabian StyleHaq, Shiekh Marifatul, Fayaz A. Lone, Manoj Kumar, Eduardo Soares Calixto, Muhammad Waheed, Ryan Casini, Eman A. Mahmoud, and Hosam O. Elansary. 2023. "Phenology and Diversity of Weeds in the Agriculture and Horticulture Cropping Systems of Indian Western Himalayas: Understanding Implications for Agro-Ecosystems" Plants 12, no. 6: 1222. https://doi.org/10.3390/plants12061222
APA StyleHaq, S. M., Lone, F. A., Kumar, M., Calixto, E. S., Waheed, M., Casini, R., Mahmoud, E. A., & Elansary, H. O. (2023). Phenology and Diversity of Weeds in the Agriculture and Horticulture Cropping Systems of Indian Western Himalayas: Understanding Implications for Agro-Ecosystems. Plants, 12(6), 1222. https://doi.org/10.3390/plants12061222










