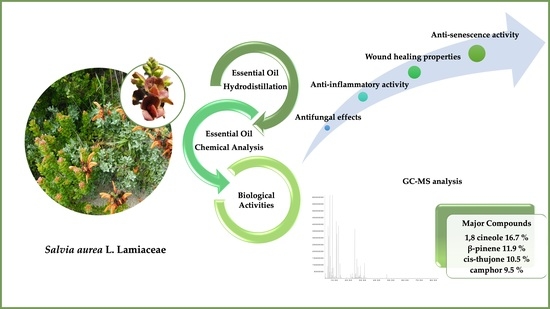
Advances in the Phytochemical Characterisation and Bioactivities of Salvia aurea L. Essential Oil
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition of S. aurea Essential Oil
2.2. Antifungal Effect of Salvia aurea
2.3. Anti-Inflammatory Potential of S. aurea
2.4. Wound Healing Properties of S. aurea Essential Oil
2.5. Anti-Senescence Potential of S. aurea Essential Oil
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Essential Oil Analysis
4.3. Antifungal Activity
4.4. Anti-Inflammatory Activity
4.4.1. Cell Culture
4.4.2. Nitric Oxide Production
4.4.3. Expression of Pro-Inflammatory Proteins, iNOS and COX-2
4.5. Cell Migration
4.5.1. Cell Culture
4.5.2. Cell Migration Assay
4.6. Cell Viability
4.7. Etoposide-Induced Senescence
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaur, N.; Ahmed, T. Bioactive secondary metabolites of medicinal and aromatic plants and their disease-fighting properties. In Medicinal and Aromatic Plants; Springer Nature: Basingstoke, UK, 2021; pp. 113–142. ISBN 978-3-030-58974-5. [Google Scholar]
- Pinto, E.; Pina-Vaz, C.; Salgueiro, L.; Gonçalves, M.J.; Costa-De-Oliveira, S.; Cavaleiro, C.; Palmeira, A.; Rodrigues, A.; Martinez-De-Oliveira, J. Antifungal activity of the essential oil of Thymus pulegioides on Candida, Aspergillus and dermatophyte species. J. Med. Microbiol. 2006, 55, 1367–1373. [Google Scholar] [CrossRef] [Green Version]
- Santos, E.L.; Freitas, P.R.; Araújo, A.C.J.; Almeida, R.S.; Tintino, S.R.; Paulo, C.L.R.; Silva, A.C.A.; Silva, L.E.; do Amaral, W.; Deschamps, C.; et al. Enhanced antibacterial effect of antibiotics by the essential oil of Aloysia gratissima (Gillies & Hook.) Tronc. and its major constituent beta-caryophyllene. Phytomedicine Plus 2021, 1, 100100. [Google Scholar] [CrossRef]
- Edris, A.E. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: A review. Phytother. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef]
- Piras, A.; Maccioni, A.; Falconieri, D.; Porcedda, S.; Gonçalves, M.J.; Alves-Silva, J.M.; Silva, A.; Cruz, M.T.; Salgueiro, L.; Maxia, A. Chemical composition and biological activity of essential oil of Teucrium scordium L. subsp. scordioides (Schreb.) Arcang. (Lamiaceae) from Sardinia Island (Italy). Nat. Prod. Res. 2022, 36, 5828–5835. [Google Scholar] [CrossRef]
- Maccioni, A.; Falconieri, D.; Porcedda, S.; Piras, A.; Gonçalves, M.J.; Alves-Silva, J.M.; Salgueiro, L.; Maxia, A. Antifungal activity and chemical composition of the essential oil from the aerial parts of two new Teucrium capitatum L. chemotypes from Sardinia Island, Italy. Nat. Prod. Res. 2020, 35, 6007–6013. [Google Scholar] [CrossRef]
- Piras, A.; Porcedda, S.; Falconieri, D.; Maxia, A.; Gonçalves, M.; Cavaleiro, C.; Gonc¸alves, M.J.; Salgueiro, L. Antifungal activity of essential oil from Mentha spicata L. and Mentha pulegium L. growing wild in Sardinia island (Italy). Nat. Prod. Res. 2021, 35, 993–999. [Google Scholar] [CrossRef]
- Walker, J.B.; Sytsma, K.J. Staminal evolution in the genus Salvia (Lamiaceae): Molecular phylogenetic evidence for multiple origins of the staminal lever. Ann. Bot. 2007, 100, 375–391. [Google Scholar] [CrossRef]
- Waller, S.B.; Cleff, M.B.; Serra, E.F.; Silva, A.L.; dos Reis Gomes, A.; de Mello, J.R.B.; de Faria, R.O.; Meireles, M.C.A. Plants from Lamiaceae family as source of antifungal molecules in humane and veterinary medicine. Microb. Pathog. 2017, 104, 232–237. [Google Scholar] [CrossRef]
- Cocco, E.; Maccioni, D.; Sanjust, E.; Falconieri, D.; Farris, E.; Maxia, A. Ethnopharmacobotany and diversity of Mediterranean endemic plants in Marmilla subregion, Sardinia, Italy. Plants 2022, 11, 3165. [Google Scholar] [CrossRef]
- Afonso, A.F.; Pereira, O.R.; Fernandes, Â.; Calhelha, R.C.; Silva, A.M.S.; Ferreira, R.C.F.; Cardoso, S.M. Phytochemical composition and bioactive effects of Salvia africana, Salvia officinalis “Icterina” and Salvia mexicana aqueous extracts. Molecules 2019, 24, 4327. [Google Scholar] [CrossRef] [Green Version]
- Alves-Silva, J.M.; Cocco, E.; Piras, A.; Gonçalves, M.J.; Silva, A.; Falconieri, D.; Porcedda, S.; Cruz, M.T.; Maxia, A.; Salgueiro, L. Unveiling the chemical composition and biological properties of Salvia cacaliifolia Benth. essential oil. Plants 2023, 12, 359. [Google Scholar] [CrossRef]
- Nkomo, M.M.; Katerere, D.D.; Vismer, H.H.; Cruz, T.T.; Balayssac, S.S.; Malet-Martino, M.M.; Makunga, N.N. Fusarium inhibition by wild populations of the medicinal plant Salvia africana-lutea L. linked to metabolomic profiling. BMC Complement. Altern. Med. 2014, 14, 99. [Google Scholar] [CrossRef] [Green Version]
- Codd, L.E. Flora of Southern Africa: Part. 4 Lamiaceae; Botanical Research Institute: Pretoria, South Africa, 1985; Volume 28, ISBN 0621082686. [Google Scholar]
- Makunga, N.P.; Van Staden, J. An efficient system for the production of clonal plantlets of the medicinally important aromatic plant: Salvia africana-lutea L. Plant Cell Tissue Organ Cult. 2007, 92, 63–72. [Google Scholar] [CrossRef]
- Aston Philander, L. An ethnobotany of Western Cape Rasta bush medicine. J. Ethnopharmacol. 2011, 138, 578–594. [Google Scholar] [CrossRef]
- Watt, J.M.; Breyer-Brandwijk, M.G. The Medicinal and Poisonous Plants of Southern and Eastern Africa: Being an Account of Their Medicinal and Other Uses, Chemical Composition, Pharmacological Effects and Toxicology in Man and Animal; E. & S. Livingstone: Edinburgh, UK, 1962. [Google Scholar]
- Gupta, A.K.; Cooper, E.A. Update in antifungal therapy of dermatophytosis. Mycopathologia 2008, 166, 353–367. [Google Scholar] [CrossRef]
- Matiz, C.; Friedlander, S.F. Subcutaneous tissue infections and abscesses. In Principles and Practice of Pediatric Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2012; pp. 454–462.e2. [Google Scholar] [CrossRef]
- De Oliveira, C.B.; Vasconcellos, C.; Sakai-Valente, N.Y.; Sotto, M.N.; Luiz, F.G.; Belda Júnior, W.; Sousa, M. da G.T. de; Benard, G.; Criado, P.R. Toll-like receptors (TLR) 2 and 4 expression of keratinocytes from patients with localized and disseminated dermatophytosis. Rev. Inst. Med. Trop. Sao Paulo 2015, 57, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Celestrino, G.A.; Reis, A.P.C.; Criado, P.R.; Benard, G.; Sousa, M.G.T. Trichophyton rubrum elicits phagocytic and pro-inflammatory responses in human monocytes through Toll-Like Receptor 2. Front. Microbiol. 2019, 10, 2589. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.-C. The non-canonical NF-B pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Rao, K.M.K. Molecular mechanisms regulating inos expression in various cell types. J. Toxicol. Environ. Health Part B 2000, 3, 27–58. [Google Scholar] [CrossRef]
- Minghetti, L. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J. Neuropathol. Exp. Neurol. 2004, 63, 901–910. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Gupta, S. Protective manifestation of herbonanoceuticals as antifungals: A possible drug candidate for dermatophytic infection. Health Sci. Rep. 2022, 5, e775. [Google Scholar] [CrossRef]
- Guo, S.; DiPietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Zuzarte, M.; Gonçalves, M.J.; Cavaleiro, C.; Canhoto, J.; Vale-Silva, L.; Silva, M.J.; Pinto, E.; Salgueiro, L. Chemical composition and antifungal activity of the essential oils of Lavandula viridis LHér. J. Med. Microbiol. 2011, 60, 612–618. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Rossi, N.M.; Bitencourt, T.A.; Peres, N.T.A.; Lang, E.A.S.; Gomes, E.V.; Quaresemin, N.R.; Martins, M.P.; Lopes, L.; Rossi, A. Dermatophyte resistance to antifungal drugs: Mechanisms and prospectus. Front. Microbiol. 2018, 9, 1108. [Google Scholar] [CrossRef] [Green Version]
- Mourad, A.; Perfect, J.R. The war on cryptococcosis: A review of the antifungal arsenal. Mem. Inst. Oswaldo Cruz 2018, 113, 7. [Google Scholar] [CrossRef]
- Vonkeman, H.E.; van de Laar, M.A.F.J. Nonsteroidal anti-inflammatory drugs: Adverse effects and their prevention. Semin. Arthritis Rheum. 2010, 39, 294–312. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; van Zyl, R.L.; van Vuuren, S.F.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Viljoen, A.M. Seasonal variation in essential oil composition, oil toxicity and the biological activity of solvent extracts of three South African Salvia species. S. Afr. J. Bot. 2008, 74, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Kamatou, G.P.P.; van Zyl, R.L.; van Vuuren, S.F.; Viljoen, A.M.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Tilney, P.M.; Barroso, J.G. Chemical composition, leaf trichome types and biological activities of the essential oils of four related Salvia species indigenous to Southern Africa. J. Essent. Oil Res. 2006, 18, 72–79. [Google Scholar] [CrossRef]
- Najar, B.; Mecacci, G.; Nardi, V.; Cervelli, C.; Nardoni, S.; Mancianti, F.; Ebani, V.V.; Giannecchini, S.; Pistelli, L. Volatiles and antifungal-antibacterial-antiviral activity of South African Salvia spp. essential oils cultivated in uniform conditions. Molecules 2021, 26, 2826. [Google Scholar] [CrossRef]
- Cowling, R.M.; Rundel, P.W.; Lamont, B.B.; Arroyo, M.K.; Arianoutsou, M. Plant diversity in Mediterranean-climate regions. Trends Ecol. Evol. 1996, 11, 362–366. [Google Scholar] [CrossRef]
- Médail, F. Ecosystems: Mediterranean. In Encyclopedia of Ecology, Volume 3; Elservier Inc.: Oxford, UK, 2008; Volume 5, pp. 2296–2308. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- National Institute of Standards and Technology Mass Spectral Library (NIST/EPA/NIH). Available online: https://chemdata.nist.gov/ (accessed on 11 January 2023).
- Guijarro-Muñoz, I.; Compte, M.; Álvarez-Cienfuegos, A.; Álvarez-Vallina, L.; Sanz, L. Lipopolysaccharide activates Toll-like Receptor 4 (TLR4)-mediated NF-κB signaling pathway and proinflammatory response in human pericytes. J. Biol. Chem. 2014, 289, 2457–2468. [Google Scholar] [CrossRef] [Green Version]
- Manning, J.; Goldblatt, P. Plants of the Greater Cape Floristic Region. 1: The Core Cape Flora; South African National Biodiversity Institute: Pretoria, South Africa, 2012; ISBN 1919976744. [Google Scholar]
- Lim Ah Tock, M.J.; Kamatou, G.P.P.; Combrinck, S.; Sandasi, M.; Viljoen, A.M. A chemometric assessment of essential oil variation of three Salvia species indigenous to South Africa. Phytochemistry 2020, 172, 112249. [Google Scholar] [CrossRef]
- Fokou, J.B.H.; Dongmo, P.M.J.; Boyom, F.F.; Fokou, J.B.H.; Dongmo, P.M.J.; Boyom, F.F. Essential oil’s chemical composition and pharmacological properties. In Essential Oils—Oils of Nature; El-Shemy, H., Ed.; IntechOpen: London, UK, 2020; pp. 13–36. ISBN 978-1-78984-641-6. [Google Scholar]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour. Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- van Vuuren, S.; Ramburrun, S.; Kamatou, G.; Viljoen, A. Indigenous South African essential oils as potential antimicrobials to treat foot odour (bromodosis). S. Afr. J. Bot. 2019, 126, 354–361. [Google Scholar] [CrossRef]
- Scott, G.; Springfield, E.P.; Coldrey, N. A pharmacognostical study of 26 South African plant species used as traditional medicines. Pharm Biol. 2004, 42, 186–213. [Google Scholar] [CrossRef]
- Oosthuizen, C.B.; Gasa, N.; Hamilton, C.J.; Lall, N. Inhibition of mycothione disulphide reductase and mycobacterial biofilm by selected South African plants. S. Afr. J. Bot. 2019, 120, 291–297. [Google Scholar] [CrossRef] [Green Version]
- Alves, M.; Gonçalves, M.J.; Zuzarte, M.; Alves-Silva, J.M.; Cavaleiro, C.; Cruz, M.T.; Salgueiro, L. Unveiling the antifungal potential of two Iberian thyme essential oils: Effect on C. albicans germ tube and preformed biofilms. Front. Pharmacol. 2019, 10, 446. [Google Scholar] [CrossRef]
- Shukla, R.; Singh, P.; Prakash, B.; Dubey, N.K. Antifungal, aflatoxin inhibition and antioxidant activity of Callistemon lanceolatus (Sm.) Sweet essential oil and its major component 1,8-cineole against fungal isolates from chickpea seeds. Food Control 2012, 25, 27–33. [Google Scholar] [CrossRef]
- Yu, D.; Wang, J.; Shao, X.; Xu, F.; Wang, H. Antifungal modes of action of tea tree oil and its two characteristic components against Botrytis cinerea. J. Appl. Microbiol. 2015, 119, 1253–1262. [Google Scholar] [CrossRef] [Green Version]
- Morcia, C.; Malnati, M.; Terzi, V. In vitro antifungal activity of terpinen-4-ol, eugenol, carvone, 1,8-cineole (eucalyptol) and thymol against mycotoxigenic plant pathogens. Food Addit. Contam. Part A 2011, 29, 415–422. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-M.; Kwon, H.; Kim, K.; Lee, S.-E. Antifungal and antiaflatoxigenic activities of 1,8-cineole and t-cinnamaldehyde on Aspergillus flavus. Appl. Sci. 2018, 8, 1655. [Google Scholar] [CrossRef] [Green Version]
- da Silva, A.C.R.; Lopes, P.M.; de Azevedo, M.M.B.; Costa, D.C.M.; Alviano, C.S.; Alviano, D.S. Biological activities of a-pinene and -pinene enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.-K.; Lee, S.-Y.; Kim, S.-H.; Hong, C.-Y.; Park, M.-J.; Choi, I.-G. Antifungal activities of essential oils from six conifers against Aspergillus fumigatus. J. Korean Wood Sci.Technol. 2012, 40, 133–140. [Google Scholar] [CrossRef] [Green Version]
- de Macêdo Andrade, A.C.; Rosalen, P.L.; Freires, I.A.; Scotti, L.; Scotti, M.T.; Aquino, S.G.; de Castro, R.D. Antifungal activity, mode of action, docking prediction and anti-biofilm effects of (+)-β-pinene enantiomers against Candida spp. Curr. Top. Med. Chem. 2018, 18, 2481–2490. [Google Scholar] [CrossRef]
- Shin, S. Antifungal activities of essential oils from Glehnia littoralis alone and in combination with ketoconazole. Nat. Prod. Sci. 2005, 11, 92–96. [Google Scholar]
- Iraji, A.; Yazdanpanah, S.; Alizadeh, F.; Mirzamohammadi, S.; Ghasemi, Y.; Pakshir, K.; Yang, Y.; Zomorodian, K. Screening the antifungal activities of monoterpenes and their isomers against Candida species. J. Appl. Microbiol. 2020, 129, 1541–1551. [Google Scholar] [CrossRef]
- Jaafar, M.; Mitri, S.; Na’was, T. Inhibition of gram negative bacterial growth and biofilm formation by alpha thujone. IOSR J. Pharm. Biol. Sci. 2018, 13, 2. [Google Scholar] [CrossRef]
- Teker, T.; Sefer, Ö.; Gazdağlı, A.; Yörük, E.; Varol, G.İ.; Albayrak, G. α-thujone exhibits an antifungal activity against F. graminearum by inducing oxidative stress, apoptosis, epigenetics alterations and reduced toxin synthesis. Eur. J. Plant Pathol. 2021, 160, 611–622. [Google Scholar] [CrossRef]
- Huo, H.; Gu, Y.; Cao, Y.; Liu, N.; Jia, P.; Kong, W. Antifungal activity of camphor against four phytopathogens of Fusarium. S. Afr. J. Bot. 2022, 148, 437–445. [Google Scholar] [CrossRef]
- Wu, K.; Lin, Y.; Chai, X.; Duan, X.; Zhao, X.; Chun, C. Mechanisms of vapor-phase antibacterial action of essential oil from Cinnamomum camphora var. linaloofera Fujita against Escherichia coli. Food Sci. Nutr. 2019, 7, 2546–2555. [Google Scholar] [CrossRef] [Green Version]
- Magiatis, P.; Skaltsounis, A.-L.; Chinou, I.; Haroutounian, S.A. Chemical Composition and In-Vitro Antimicrobial Activity of the Essential Oils of Three Greek Achillea Species. Z. Für Nat. C 2002, 57, 287–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahham, S.; Tabana, Y.; Iqbal, M.; Ahamed, M.; Ezzat, M.; Majid, A.; Majid, A. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Pieri, F.A.; de Castro Souza, M.C.; Vermelho, L.L.R.; Vermelho, M.L.R.; Perciano, P.G.; Vargas, F.S.; Borges, A.P.B.; da Veiga-Junior, V.F.; Moreira, M.A.S. Use of β-caryophyllene to combat bacterial dental plaque formation in dogs. BMC Vet. Res. 2016, 12, 216. [Google Scholar] [CrossRef] [Green Version]
- Goren, A.C.; Piozzi, F.; Akcicek, E.; Kılıç, T.; Çarıkçı, S.; Mozioğlu, E.; Setzer, W.N. Essential oil composition of twenty-two Stachys species (mountain tea) and their biological activities. Phytochem. Lett. 2011, 4, 448–453. [Google Scholar] [CrossRef]
- Yadav, N.; Chandra, H. Suppression of inflammatory and infection responses in lung macrophages by eucalyptus oil and its constituent 1,8-cineole: Role of pattern recognition receptors TREM-1 and NLRP3, the MAP kinase regulator MKP-1, and NFκB. PLoS ONE 2017, 12, e0188232. [Google Scholar] [CrossRef] [Green Version]
- Beer, A.M.; Zagorchev, P.; Filipova, D.M.; Lukanov, J. Effects of 1,8-cineole on the activity of cyclooxygenase and cyclooxygenase 1 and cyclooxygenase 2 isoforms. Nat. Prod. Chem. Res. 2017, 5, 1000253. [Google Scholar] [CrossRef]
- Bastos, V.P.D.; Gomes, A.S.; Lima, F.J.B.; Brito, T.S.; Soares, P.M.G.; Pinho, J.P.M.; Silva, C.S.; Santos, A.A.; Souza, M.H.L.P.; Magalhães, P.J.C. inhaled 1,8-cineole reduces inflammatory parameters in airways of ovalbumin-challenged guinea pigs. Basic. Clin. Pharmacol. Toxicol. 2011, 108, 34–39. [Google Scholar] [CrossRef]
- Juergens, L.J.; Racké, K.; Tuleta, I.; Stoeber, M.; Juergens, U.R. Anti-inflammatory effects of 1,8-cineole (eucalyptol) improve glucocorticoid effects in vitro: A novel approach of steroid-sparing add-on therapy for COPD and asthma? Synergy 2017, 5, 1–8. [Google Scholar] [CrossRef]
- Santos, F.A.; Rao, V.S.N. Antiinflammatory and antinociceptive effects of 1,8-cineole a terpenoid oxide present in many plant essential oils. Phytother. Res. 2000, 14, 240–244. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Mohammed, S.A.A.; Khan, O.; Ali, H.M. Topical eucalyptol ointment accelerates wound healing and exerts antioxidant and anti-inflammatory effects in rats’ skin burn model. J. Oleo Sci. 2022, 71, ess22214. [Google Scholar] [CrossRef]
- Juergens, U.R.; Dethlefsen, U.; Steinkamp, G.; Gillissen, A.; Repges, R.; Vetter, H. Anti-inflammatory activity of 1.8-cineol (eucalyptol) in bronchial asthma: A double-blind placebo-controlled trial. Respir. Med. 2003, 97, 250–256. [Google Scholar] [CrossRef] [Green Version]
- Lima, P.R.; de Melo, T.S.; Carvalho, K.M.M.B.; de Oliveira, Í.B.; Arruda, B.R.; de Castro Brito, G.A.; Rao, V.S.; Santos, F.A. 1,8-cineole (eucalyptol) ameliorates cerulein-induced acute pancreatitis via modulation of cytokines, oxidative stress and NF-κB activity in mice. Life Sci. 2013, 92, 1195–1201. [Google Scholar] [CrossRef] [Green Version]
- Coté, H.; Boucher, M.-A.; Pichette, A.; Legault, J. Anti-inflammatory, antioxidant, antibiotic, and cytotoxic activities of tanacetum vulgare l. essential oil and its constituents. Medicines 2017, 4, 34. [Google Scholar] [CrossRef]
- Rufino, A.T.; Ribeiro, M.; Judas, F.; Salgueiro, L.; Lopes, M.C.; Cavaleiro, C.; Mendes, A.F. Anti-inflammatory and chondroprotective activity of (+)-α-pinene: Structural and enantiomeric selectivity. J. Nat. Prod. 2014, 77, 264–269. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Kushnarenko, S.V.; Özek, G.; Kirpotina, L.N.; Utegenova, G.A.; Kotukhov, Y.A.; Danilova, A.N.; Özek, T.; Başer, K.H.C.; Quinn, M.T. Inhibition of human neutrophil responses by the essential oil of Artemisia kotuchovii and its constituents. J. Agric. Food Chem. 2015, 63, 4999–5007. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, E.; Leitão, M.M.; Aguero Ito, C.N.; Silva-Filho, S.E.; Arena, A.C.; de Souza Silva-Comar, F.M.; Nakamura Cuman, R.K.; Oliveira, R.J.; Nazari Formagio, A.S.; Leite Kassuya, C.A. Analgesic and anti-inflammatory articular effects of essential oil and camphor isolated from Ocimum kilimandscharicum Gürke leaves. J. Ethnopharmacol. 2021, 269, 113697. [Google Scholar] [CrossRef]
- Adhikari, A.; Bhandari, S.; Pandey, D.P. Anti-inflammatory compounds camphor and methylsalicylate from traditionally used pain curing plant Equisetum arvense L. J. Nepal Chem. Soc. 2019, 40, 1–4. [Google Scholar] [CrossRef]
- Silva-Filho, S.; de Souza Silva-Comar, F.; Wiirzler, L.; do Pinho, R.; Grespan, R.; Bersani-Amado, C.; Cuman, R. Effect of camphor on the behavior of leukocytes in vitro and in vivo in acute inflammatory response. Trop. J. Pharm. Res. 2015, 13, 2031. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.Y.; Chang, H.-J.; Lee, S.-K.; Kim, H.-J.; Hwang, J.-K.; Chun, H.S. Amelioration of dextran sulfate sodium-induced colitis in mice by oral administration of β-caryophyllene, a sesquiterpene. Life Sci. 2007, 80, 932–939. [Google Scholar] [CrossRef]
- Gushiken, L.F.S.; Beserra, F.P.; Hussni, M.F.; Gonzaga, M.T.; Ribeiro, V.P.; de Souza, P.F.; Campos, J.C.L.; Massaro, T.N.C.; Hussni, C.A.; Takahira, R.K.; et al. Beta-caryophyllene as an antioxidant, anti-inflammatory and re-epithelialization activities in a rat skin wound excision model. Oxid. Med. Cell Longev. 2022, 2022, 1–21. [Google Scholar] [CrossRef]
- Brito, L.F.; Oliveira, H.B.M.; Neves Selis, N.; Souza, C.L.S.; Júnior, M.N.S.; Souza, E.P.; da Silva, L.S.C.; Souza Nascimento, F.; Amorim, A.T.; Campos, G.B.; et al. Anti-inflammatory activity of β -caryophyllene combined with docosahexaenoic acid in a model of sepsis induced by Staphylococcus aureus in mice. J. Sci. Food Agric. 2019, 99, 5870–5880. [Google Scholar] [CrossRef]
- Sousa, L.F.B.; Oliveira, H.B.M.; das Neves Selis, N.; Morbeck, L.L.B.; Santos, T.C.; da Silva, L.S.C.; Viana, J.C.S.; Reis, M.M.; Sampaio, B.A.; Campos, G.B.; et al. β-caryophyllene and docosahexaenoic acid, isolated or associated, have potential antinociceptive and anti-inflammatory effects in vitro and in vivo. Sci. Rep. 2022, 12, 19199. [Google Scholar] [CrossRef]
- Scandiffio, R.; Geddo, F.; Cottone, E.; Querio, G.; Antoniotti, S.; Gallo, M.P.; Maffei, M.E.; Bovolin, P. Protective effects of (e)-β-caryophyllene (bcp) in chronic inflammation. Nutrients 2020, 12, 3273. [Google Scholar] [CrossRef]
- Salas-Oropeza, J.; Jimenez-Estrada, M.; Perez-Torres, A.; Castell-Rodriguez, A.E.; Becerril-Millan, R.; Rodriguez-Monroy, M.A.; Jarquin-Yañez, K.; Canales-Martinez, M.M. Wound healing activity of α-pinene and α-phellandrene. Molecules 2021, 26, 2488. [Google Scholar] [CrossRef]
- Rocha Caldas, G.F.; da Silva Oliveira, A.R.; Araújo, A.V.; Lafayette, S.S.L.; Albuquerque, G.S.; da Costa Silva-Neto, J.; Costa-Silva, J.H.; Ferreira, F.; da Costa, J.G.M.; Wanderley, A.G. Gastroprotective mechanisms of the monoterpene 1,8-cineole (eucalyptol). PLoS ONE 2015, 10, e0134558. [Google Scholar] [CrossRef] [Green Version]
- Chabane, S.; Boudjelal, A.; Napoli, E.; Benkhaled, A.; Ruberto, G. Phytochemical composition, antioxidant and wound healing activities of Teucrium polium subsp. capitatum (L.) Briq. essential oil. J. Essent. Oil Res. 2021, 33, 143–151. [Google Scholar] [CrossRef]
- Tran, T.A.; Ho, M.T.; Song, Y.W.; Cho, M.; Cho, S.K. Camphor induces proliferative and anti-senescence activities in human primary dermal fibroblasts and inhibits uv-induced wrinkle formation in mouse skin. Phytother. Res. 2015, 29, 1917–1925. [Google Scholar] [CrossRef]
- Rodenak-Kladniew, B.; Castro, A.; Stärkel, P.; Galle, M.; Crespo, R. 1,8-Cineole promotes G0/G1 cell cycle arrest and oxidative stress-induced senescence in HepG2 cells and sensitizes cells to anti-senescence drugs. Life Sci. 2020, 243, 117271. [Google Scholar] [CrossRef]
- Directorate for the Quality of Medicines & HealthCare of the Council of Europe. European Pharmacopoeia; EDQM: Strasbourg, France, 2010; ISBN 978-92-871-6700-2. [Google Scholar]
- Van den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- CLSI Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. CLSI Document M38-A2, Approved Standard, 2nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008; Volume 28, ISBN 1-56238-668-9. [Google Scholar]
- Zuzarte, M.; Alves-Silva, J.M.; Alves, M.; Cavaleiro, C.; Salgueiro, L.; Cruz, M.T. New insights on the anti-inflammatory potential and safety profile of Thymus carnosus and Thymus camphoratus essential oils and their main compounds. J. Ethnopharmacol. 2018, 225, 10–17. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Martinotti, S.; Ranzato, E. Scratch wound healing assay. In Epidermal Cells: Methods in Molecular Biology; Turksen, K., Ed.; Humana: New York, NY, USA, 2019; Volume 2109, pp. 225–229. [Google Scholar] [CrossRef]
- Moreira, P.; Sousa, F.J.; Matos, P.; Brites, G.S.; Gonçalves, M.J.; Cavaleiro, C.; Figueirinha, A.; Salgueiro, L.; Batista, M.T.; Branco, P.C.; et al. Chemical composition and effect against skin alterations of bioactive extracts obtained by the hydrodistillation of Eucalyptus globulus leaves. Pharmaceutics 2022, 14, 561. [Google Scholar] [CrossRef]




| RI | RI (Litt) | Compound | Area, % |
|---|---|---|---|
| 925 | 921 | tricyclene | 0.1 |
| 927 | 924 | α-thujene | 1.0 |
| 934 | 932 | α-pinene | 4.7 |
| 951 | 946 | camphene | 3.9 |
| 973 | 969 | sabinene | 0.7 |
| 980 | 974 | β-pinene | 11.9 |
| 989 | 988 | myrcene | 2.1 |
| 1017 | 1014 | α-terpinene | 0.7 |
| 1024 | 1022 | ortho-cymene | 0.4 |
| 1029 | 1024 | limonene | 1.5 |
| 1033 | 1026 | 1,8-cineole | 16.7 |
| 1044 | 1044 | (E)-β-ocimene | 0.3 |
| 1056 | 1054 | γ-terpinene | 1.2 |
| 1068 | 1065 | cis-sabinene hydrate | 0.2 |
| 1084 | 1086 | terpinolene | 0.3 |
| 1100 | 1095 | linalool | 0.3 |
| 1107 | 1101 | cis-thujone | 10.5 |
| 1117 | 1112 | trans-thujone | 6.9 |
| 1146 | 1141 | camphor | 9.5 |
| 1169 | 1165 | borneol | 2.7 |
| 1178 | 1174 | terpinen-4-ol | 0.4 |
| 1192 | 1186 | α-terpineol | 0.2 |
| 1343 | 1345 | α-cubebene | 0.5 |
| 1364 | 1373 | α-ylangene | 0.3 |
| 1371 | 1374 | α-copaene | 0.7 |
| 1415 | 1417 | (E)-caryophyllene | 9.3 |
| 1424 | 1430 | β-copaene | 0.6 |
| 1432 | 1439 | aromadendrene | 0.8 |
| 1449 | 1452 | α-humulene | 3.0 |
| 1469 | 1478 | γ-muurolene | 1.6 |
| 1486 | 1495 | γ-amorphene | 0.3 |
| 1493 | 1500 | α-muurolene | 0.3 |
| 1507 | 1513 | γ-cadinene | 0.5 |
| 1513 | 1522 | δ-cadinene | 1.8 |
| 1574 | 1582 | caryophyllene oxide | 0.4 |
| 1587 | 1592 | viridiflorol | 2.4 |
| Total identified | 98.8 | ||
| Hydrocarbon monoterpenes | 28.8 | ||
| Oxygenated monoterpenes | 47.4 | ||
| Hydrocarbon sesquiterpenes | 19.8 | ||
| Oxygenated sesquiterpenes | 2.8 | ||
| Strains | S. aurea | Fluconazole | ||
|---|---|---|---|---|
| MIC a | MLC | MIC b | MLC | |
| Trichophyton mentagrophytes FF7 | 1.25 | 1.25 | 16–32 | 32–64 |
| T. rubrum CECT 2794 | 1.25 | 2.5 | 16 | 64 |
| T. mentagrophytes var. interdigitale CECT 2958 | 5 | >5 | 128 | ≥128 |
| T. verrucosum CECT 2992 | 5 | >5 | >128 | >128 |
| Microsporum canis FF1 | 5–2.5 | >5 | 128 | 128 |
| M. gypseum CECT 2908 | 5 | >5 | 128 | >128 |
| Epidermophyton floccosum FF9 | 1.25 | 1.25 | 16 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves-Silva, J.M.; Maccioni, D.; Cocco, E.; Gonçalves, M.J.; Porcedda, S.; Piras, A.; Cruz, M.T.; Salgueiro, L.; Maxia, A. Advances in the Phytochemical Characterisation and Bioactivities of Salvia aurea L. Essential Oil. Plants 2023, 12, 1247. https://doi.org/10.3390/plants12061247
Alves-Silva JM, Maccioni D, Cocco E, Gonçalves MJ, Porcedda S, Piras A, Cruz MT, Salgueiro L, Maxia A. Advances in the Phytochemical Characterisation and Bioactivities of Salvia aurea L. Essential Oil. Plants. 2023; 12(6):1247. https://doi.org/10.3390/plants12061247
Chicago/Turabian StyleAlves-Silva, Jorge Miguel, Delia Maccioni, Emma Cocco, Maria José Gonçalves, Silvia Porcedda, Alessandra Piras, Maria Teresa Cruz, Lígia Salgueiro, and Andrea Maxia. 2023. "Advances in the Phytochemical Characterisation and Bioactivities of Salvia aurea L. Essential Oil" Plants 12, no. 6: 1247. https://doi.org/10.3390/plants12061247
APA StyleAlves-Silva, J. M., Maccioni, D., Cocco, E., Gonçalves, M. J., Porcedda, S., Piras, A., Cruz, M. T., Salgueiro, L., & Maxia, A. (2023). Advances in the Phytochemical Characterisation and Bioactivities of Salvia aurea L. Essential Oil. Plants, 12(6), 1247. https://doi.org/10.3390/plants12061247