Biodiversity Evaluation and Preservation of Italian Stone Fruit Germplasm (Peach and Apricot) in Southern Italy
Abstract
1. Introduction
2. Results and Discussion
2.1. Cross-Transferable SSR Amplification and Genetic Variability Analysis
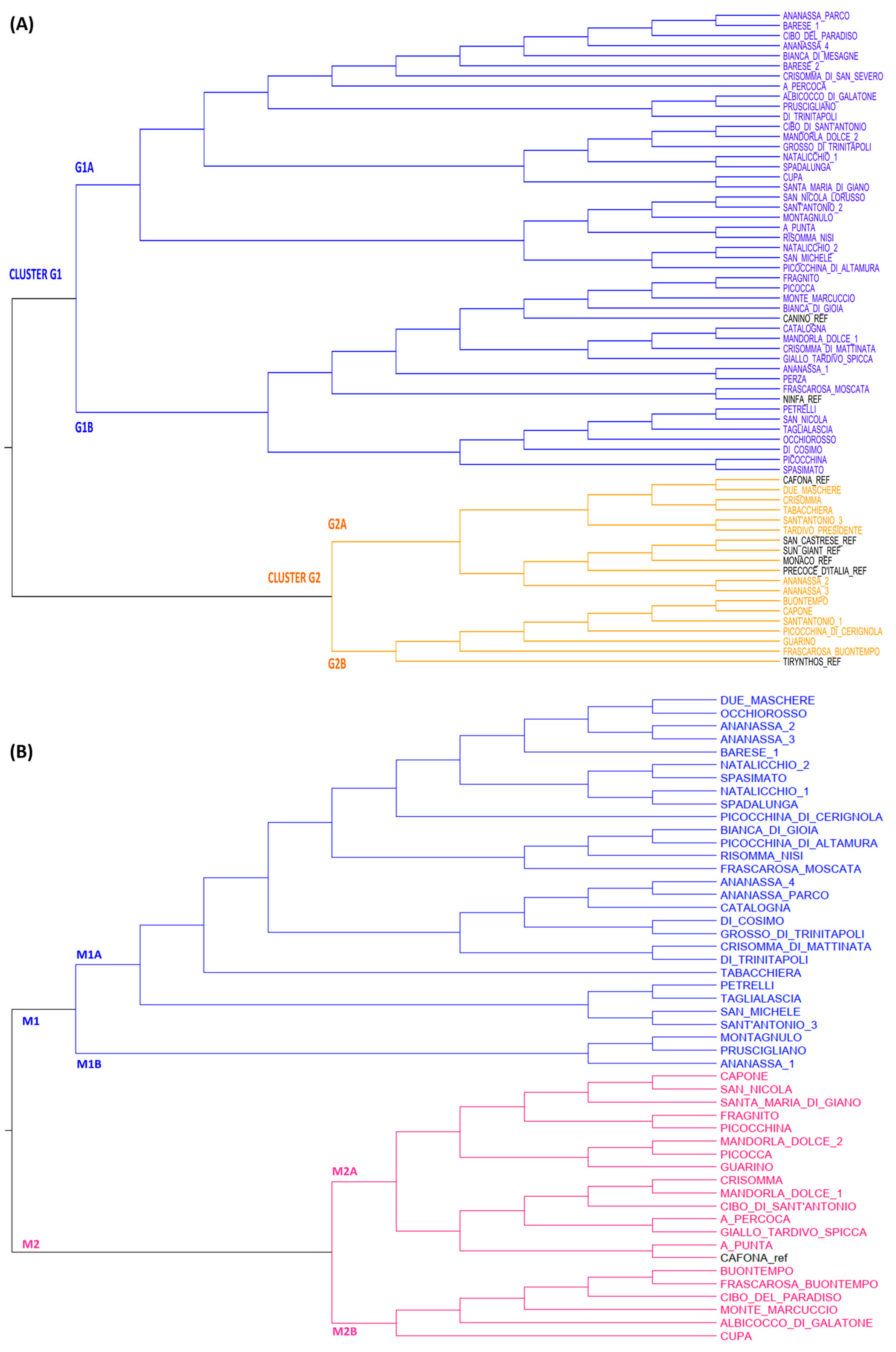
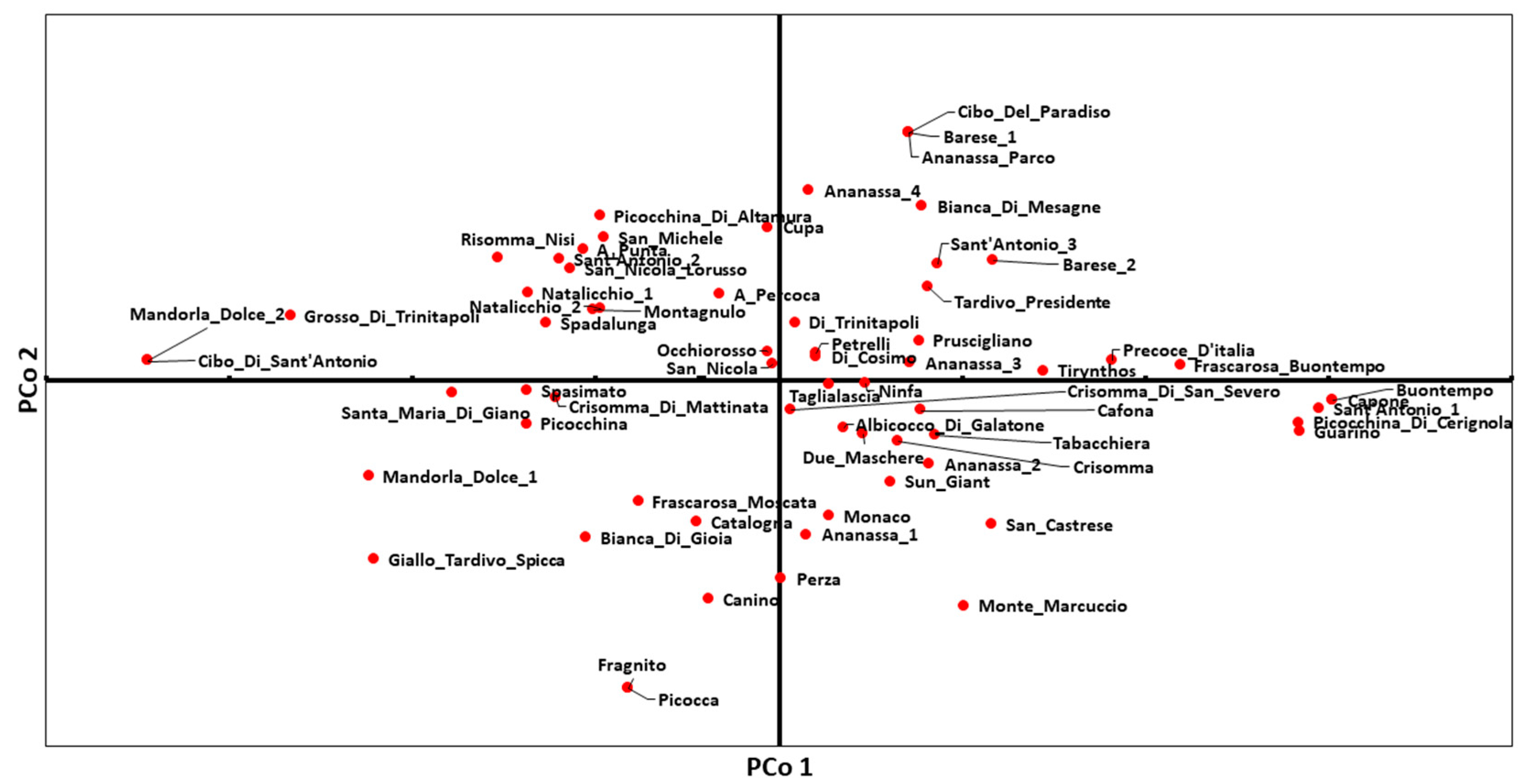
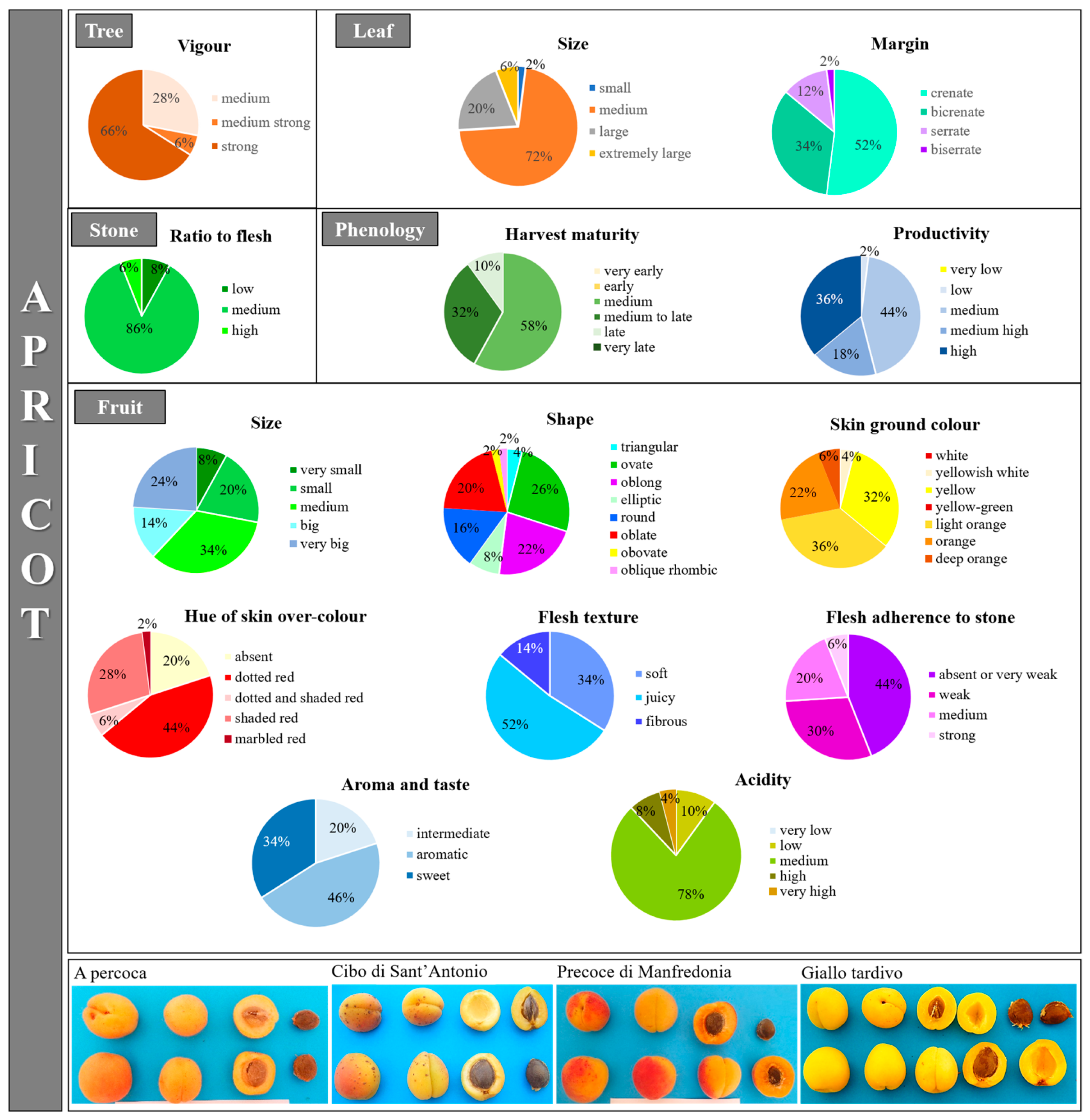
2.2. Genetic and Phenotypic Characterization of Apricot Collection
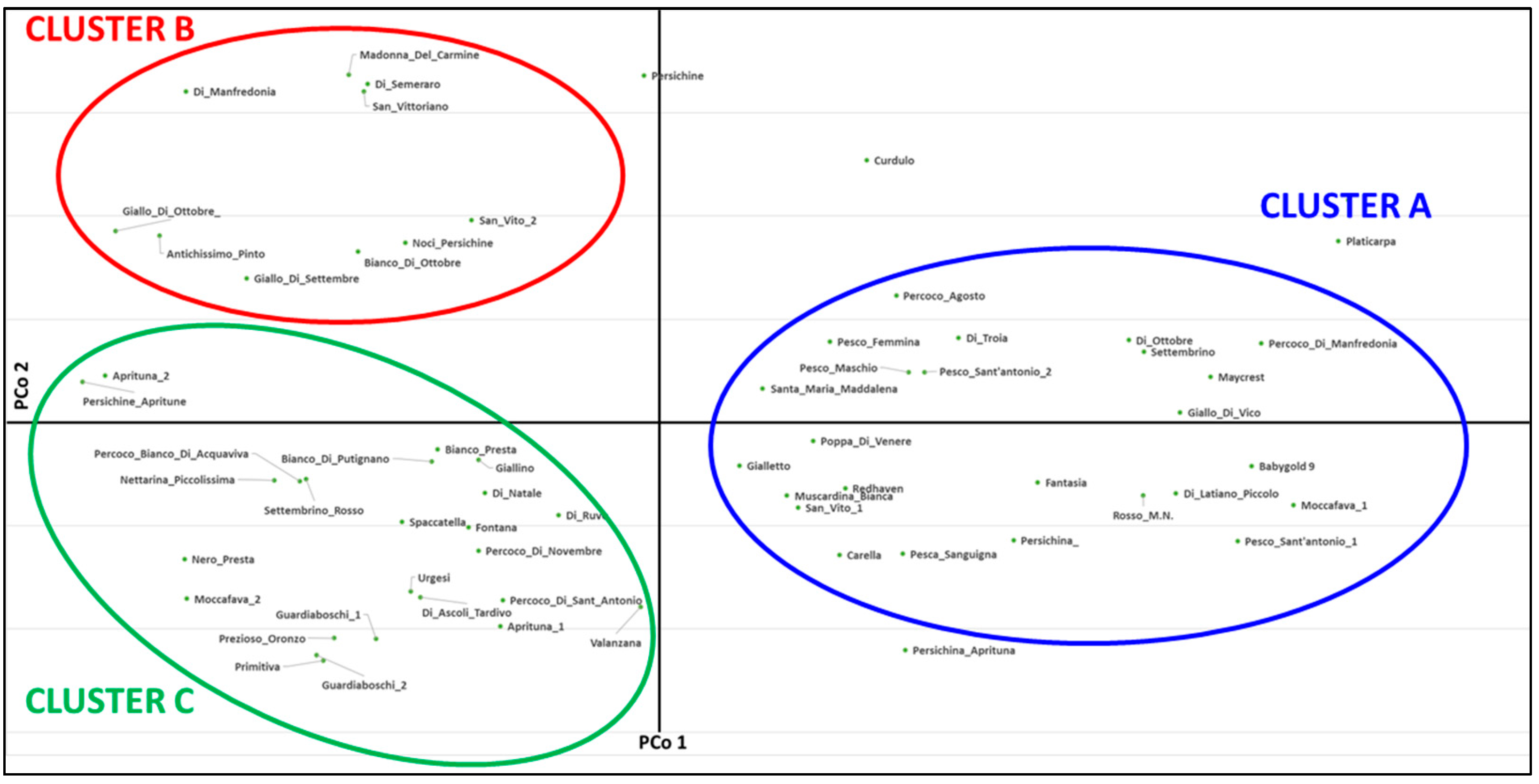
2.3. Genetic and Phenotypic Characterization of Peach Collection
3. Materials and Methods
3.1. Plant Material
3.2. DNA Extraction and SSR Analysis
3.3. Genetic Diversity
3.4. Morphological Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, T.H.; Li, Y.X.; Li, Z.C.; Zhang, H.L.; Qi, Y.W.; Wang, T. Simple sequence repeat analysis of genetic diversity in primary core collection of peach (Prunus persica). J. Integr. Plant Biol. 2008, 50, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, D.; Yu, K.; Ji, J.; Liu, N.; Zhang, Y.; Xu, M.; Zhang, Y.; Ma, X.; Liu, S.; et al. Frequent germplasm exchanges drive the high genetic diversity of Chinese-cultivated common apricot germplasm. Hortic. Res. 2021, 8, 215. [Google Scholar] [CrossRef]
- Bunyard, E.A. The history and cultivation of the peach and nectarine. J. R. Hort. Sci. 1938, 63, 114–121. [Google Scholar]
- Zhang, Q.; Liu, W. Advances of the apricot resources collection, evaluation and germplasm enhancement. Acta Hortic. Sin. 2018, 45, 1642–1660. (In Chinese) [Google Scholar]
- Faust, M.; Timon, B. Origin and dissemination of peach. Hortic. Rev. 1995, 17, 331–379. [Google Scholar]
- Aranzana, M.J.; Abbassi, E.K.; Howad, W.; Arús, P. Genetic variation, population structure and linkage disequilibrium in peach commercial varieties. BMC Genet. 2010, 11, 69. [Google Scholar] [CrossRef]
- Yu, Y.; Fu, J.; Xu, Y.; Zhang, J.; Ren, F.; Zhao, H.; Tian, S.; Guo, W.; Tu, X.; Zhao, J.; et al. Genome re-sequencing reveals the evolutionary history of peach fruit edibility. Nat. Commun. 2018, 9, 5404. [Google Scholar] [CrossRef]
- I.Stat. Available online: http://dati.istat.it (accessed on 12 December 2021).
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- Byrne, D.H.; Noratto, G.; Cisneros-Zevallos, L.; Porter, W.; Vizzotto, M. Health benefits of peach, nectarine and plums. Acta Hortic. 2009, 841, 267–274. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J. Antioxidant Activity Modulated by Polyphenol Contents in Apple and Leaves during Fruit Development and Ripening. Antioxidants 2020, 9, 567. [Google Scholar] [CrossRef]
- Moustafa, K.; Cross, J. Produzione, proprietà pomologiche e nutraceutiche dell’albicocca. J. Food Sci. Technol. 2019, 56, 12–23. [Google Scholar] [CrossRef]
- Cantin, C.M.; Moreno, M.A.; Gogorcena, Y. Evaluation of the antioxidant capacity, phenolic compounds, and vitamin C content of different peach and nectarine [Prunus persica (L.) Batsch] breeding progenies. J. Agric. Food Chem. 2009, 57, 4586–4592. [Google Scholar] [CrossRef] [PubMed]
- Minas, I.S.; Tanou, G.; Molassiotis, A. Environmental and orchard bases of peach fruit quality. Sci. Hortic. 2018, 235, 307–322. [Google Scholar] [CrossRef]
- Crisosto, C. How do we increase peach consumption? Acta Hort. 2002, 592, 601–605. [Google Scholar] [CrossRef]
- Raguseo, C.; Gerin, D.; Pollastro, S.; Rotolo, C.; Rotondo, P.R.; Faretra, F.; De Miccolis Angelini, R.M. A Duplex-Droplet Digital PCR Assay for Simultaneous Quantitative Detection of Monilinia fructicola and Monilinia laxa on Stone Fruits. Front. Microbiol. 2021, 12, 747560. [Google Scholar] [CrossRef] [PubMed]
- Cirilli, M.; Geuna, F.; Babini, A.R.; Bozhkova, V.; Catalano, L.; Cavagna, B.; Dallot, S.; Decroocq, V.; Dondini, L.; Foschi, S.; et al. Fighting sharka in peach: Current limitations and future perspectives. Front. Plant Sci. 2016, 7, 1290. [Google Scholar] [CrossRef] [PubMed]
- Greco, D.; Aprile, A.; De Bellis, L.; Luvisi, A. Diseases Caused by Xylella fastidiosa in Prunus Genus: An Overview of the Research on an Increasingly Widespread Pathogen. Front. Plant Sci. 2021, 12, 712452. [Google Scholar] [CrossRef]
- Confagricoltura. Available online: https://www.confagricoltura.it/ita (accessed on 20 September 2022).
- Miazzi, M.M.; di Rienzo, V.; Mascio, I.; Montemurro, C.; Sion, S.; Sabetta, W.; Vivaldi, G.A.; Camposeo, S.; Caponio, F.; Squeo, G.; et al. Re.Ger.O.P.: An Integrated Project for the Recovery of Ancient and Rare Olive Germplasm. Front. Plant Sci. 2020, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Miazzi, M.M.; D’Agostino, N.; di Rienzo, V.; Venerito, P.; Savino, V.N.; Fucilli, V.; Ruffa, P.; Roseti, V.; Pirolo, C.; Notte, P.L.; et al. Marginal grapevine germplasm from Apulia (Southern Italy) represents an unexplored source of genetic diversity. Agronomy 2020, 10, 563. [Google Scholar] [CrossRef]
- Sabetta, W.; Mascio, I.; Squeo, G.; Gadaleta, S.; Flamminii, F.; Conte, P.; Di Mattia, C.D.; Piga, A.; Caponio, F.; Montemurro, C. Bioactive potential of minor Italian olive genotypes from Apulia, Sardinia and Abruzzo. Foods 2021, 10, 1371. [Google Scholar] [CrossRef]
- Baird, W.V.; Ballard, R.E.; Rajapkse, S.; Abbott, A.G. Progress in Prunus mapping and application of molecular markers to germplasm improvement. Hort. Sci. 1996, 31, 1099–1106. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Z.; Miao, X.; Zhou, J. Genetic diversity and population structure of Siberian apricot (Prunus sibirica L.) in China. Int. J. Mol. Sci. 2013, 15, 377–400. [Google Scholar] [CrossRef] [PubMed]
- Zargar, S.A.; Wani, A.A.; Saggoo, M.I.S. Analysis of phenotypic diversity of apricot (Prunus armeniaca L.) accessions from Jammu and Kashmir, India. Plant Genet. Resour. 2021, 19, 203–215. [Google Scholar] [CrossRef]
- Sheikh, Z.N.; Sharma, V.; Shah, R.A.; Sharma, N.; Summuna, B.; Al-Misned, F.A.; El-Serehy, H.A.; Mir, J.I. Genetic diversity analysis and population structure in apricot (Prunus armeniaca L.) grown under north-western himalayas using ISSR markers. Saudi J. Biol. Sci. 2021, 28, 5986–5992. [Google Scholar] [CrossRef] [PubMed]
- Herrera, S.; Hormaza, J.I.; Lora, J.; Ylla, G.; Rodrigo, J. Molecular Characterization of Genetic Diversity in Apricot Cultivars: Current Situation and Future Perspectives. Agronomy 2021, 11, 1714. [Google Scholar] [CrossRef]
- Li, X.; Meng, X.; Jia, H.; Yu, M.; Ma, R.; Wang, L.; Cao, K.; Shen, Z.; Niu, L.; Tian, J.; et al. Peach genetic resources: Diversity, population structure and linkage disequilibrium. BMC Genet. 2013, 14, 84. [Google Scholar] [CrossRef]
- Trifonova, A.A.; Boris, K.V.; Mesyats, N.V.; Tsiupka, V.A.; Smykov, A.V.; Mitrofanova, I.V. Genetic Diversity of Peach Cultivars from the Collection of the Nikita Botanical Garden Based on SSR Markers. Plants 2021, 10, 2609. [Google Scholar] [CrossRef] [PubMed]
- Bouhadida, M.; Moreno, M.A.; Gonzalo, M.J.; Alonso, J.M.; Gogorcena, Y. Genetic variability of introduced and local Spanish peach cultivars determined by SSR markers. Tree Genet. Genom. 2011, 7, 257–270. [Google Scholar] [CrossRef]
- Aranzana, M.; Carbó, J.; Arús, P. Microsatellite variability in peach [Prunus persica (L.) Batsch]: Cultivar identification, marker mutation, pedigree inferences, and population structure. Theor. Appl. Genet. 2003, 106, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Zhebentyayeva, T.N.; Reighard, G.L.; Gorina, V.M.; Abbott, A.G. Simple sequence repeat (SSR) analysis for assessment of genetic variability in apricot germplasm. Theor. Appl. Genet. 2003, 106, 435–444. [Google Scholar] [CrossRef]
- Di Gaspero, G.; Peterlunger, E.; Testolin, R.; Edwards, K.J.; Cipriani, G. Conservation of microsatellite loci within the genus Vitis. Theor. Appl. Genet. 2000, 101, 301–308. [Google Scholar] [CrossRef]
- Plieske, J.; Struss, D. Microsatellite markers for genome analysis in Brassica. Development in Brassica napus and abundance in Brassicaceae species. Theor. Appl. Genet. 2001, 102, 680–694. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kimura, T.; Sawamura, Y.; Kotobuki, K.; Ban, Y.; Hayashi, T.; Matsuta, N. SSRs isolated from apple can identify polymorphism and genetic diversity in pear. Theor. Appl. Genet. 2001, 102, 865–870. [Google Scholar] [CrossRef]
- Rigoldi, M.P.; Rapposelli, E.; De Giorgio, D.; Resta, P.; Porceddu, A. Genetic diversity in two Italian almond collections. Electron. J. Biotechnol. 2015, 18, 40–45. [Google Scholar] [CrossRef]
- Halász, J.; Kodad, O.; Galiba, G.M.; Skola, I.; Ercisli, S.; Ledbetter, C.A.; Hegedűs, A. Genetic variability is preserved among strongly differentiated and geographically diverse almond germplasm: An assessment by simple sequence repeat markers. Tree Genet. Genomes 2019, 15, 12. [Google Scholar] [CrossRef]
- Savoia, M.A.; Del Faro, L.; Venerito, P.; Gaeta, L.; Palasciano, M.; Montemurro, C.; Sabetta, W. The Relevance of Discovering and Recovering the Biodiversity of Apulian Almond Germplasm by Means of Molecular and Phenotypic Markers. Plants 2022, 11, 574. [Google Scholar] [CrossRef] [PubMed]
- Dirlewanger, E.; Graziano, E.; Joobeur, T.; Garriga-Calderé, F.; Cosson, P.; Howad, W.; Arús, P. Comparative mapping and marker-assisted selection in Rosaceae fruit crops. Proc. Natl. Acad. Sci. USA 2004, 101, 9891–9896. [Google Scholar] [CrossRef]
- Wünsch, A. Cross-Transferable Polymorphic SSR loci in Prunus Species. Sci. Hortic. 2009, 120, 348–352. [Google Scholar] [CrossRef]
- Mnejja, M.; Garcia-Mas, J.; Audergon, J.M.; Arús, P. Prunus microsatellite marker transferability across rosaceous crops. Tree Genet. Genomes 2010, 6, 689–700. [Google Scholar] [CrossRef]
- Tian-Ming, H.; Xue-Sen, C.; Zheng, X.; Jiang-Sheng, G.; Pei-Jun, L.; Wen, L.; Qing, L.; Yan, W. Using SSR markers to determine the population genetic structure of wild apricot (Prunus armeniaca L.) in the Ily Valley of West China. Genet. Resour. Crop. Evol. 2007, 54, 563–572. [Google Scholar] [CrossRef]
- Decroocq, S.; Cornille, A.; Tricon, D.; Babayeva, S.; Chague, A.; Eyquard, P.; Karychev, R.; Dolgikh, S.; Kostritsyna, T.; Liu, S.; et al. New insights into the history of domesticated and wild apricots and its contribution to plum pox virus resistance. Mol. Ecol. 2016, 25, 2712–4729. [Google Scholar] [CrossRef]
- Zhang, Q.P.; Liu, D.C.; Liu, S.; Liu, N.; Wei, X.; Zhang, A.M.; Liu, W.S. Genetic diversity and relationships of common apricot (Prunus armeniaca L.) in China based on simple sequence repeat (SSR) markers. Genet. Resour. Crop. Evol. 2014, 61, 357–368. [Google Scholar] [CrossRef]
- Ferrer, M.M.; Eguiarte, L.E.; Montana, C. Genetic structure and outcrossing rates in Flourensia cernua (Asteraceae) growing at different densities in the Southwestern Chihuahuan Desert. Ann. Bot. 2004, 94, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Corrado, G.; Forlani, M.; Rao, R.; Basile, B. Diversity and Relationships among Neglected Apricot (Prunus armeniaca L.) Landraces Using Morphological Traits and SSR Markers: Implications for Agro-Biodiversity Conservation. Plants 2021, 10, 1341. [Google Scholar] [CrossRef]
- Rallo, P.; Rocio Jiménez, M.R.; Casanova, L.; Morales-Sillero, A.; Paz Suárez, M. Genetic diversity of stone fruit cultivars preserved on-farm in southern Spain. JAST 2019, 21, 943–955. [Google Scholar]
- Vilanova, S.; Soriano, J.M.; Lalli, D.A.; Romero, C.; Abbott, A.G.; Llacer, G.; Badenes, M.L. Development of SSR markers located in the G1 linkage group of apricot (Prunus armeniaca L.) using a bacterial artificial chromosome library. Mol. Ecol. Notes 2006, 3, 789–791. [Google Scholar] [CrossRef]
- Raji, R.; Jannatizadeh, A.; Fattahi, R.; Esfahlani, M.A. Investigation of variability of apricot (Prunus armeniaca L.) using morphological traits and microsatellite markers. Sci. Hortic. 2014, 176, 225–231. [Google Scholar] [CrossRef]
- Bourguiba, H.; Scotti, I.; Sauvage, C.; Zhebentyayeva, T.; Ledbetter, C.; Krska, B.; Remay, A.; Donofrio, C.; Iketani, H.; Christen, D.; et al. Genetic structure of a worldwide germplasm collection of Prunus armeniaca L. reveals three major diffusion routes for varieties coming from the species’ center of origin. Front. Plant Sci. 2020, 25, 638. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.J.; Ma, R.J.; Cai, Z.X.; Yu, M.L.; Zhang, Z. Diversity, population structure, and evolution of local peach cultivars in China identified by simple sequence repeats. Genet. Mol. Res. 2015, 14, 101–117. [Google Scholar] [CrossRef]
- Cao, K.; Wang, L.; Zhu, G.; Fang, W.; Chen, C.; Luo, J. Genetic diversity, linkage disequilibrium, and association mapping analyses of peach (Prunus persica) landraces in China. Tree Genet. Genom. 2012, 8, 975–990. [Google Scholar] [CrossRef]
- Rao, R.; Bencivenni, M.; Mura, L.; Araujo-Burgos, T.; Corrado, G. Molecular characterisation of Vesuvian apricot cultivars: Implications for the certification and authentication of protected plant material. J. Hortic. Sci. Biotechnol. 2010, 85, 42–47. [Google Scholar] [CrossRef]
- Corrado, G.; La Mura, M.; Ambrosino, O.; Pugliano, G.; Varricchio, P.; Rao, R. Relationships of Campanian olive cultivars: Comparative analysis of molecular and phenotypic data. Genome 2009, 52, 692–700. [Google Scholar] [CrossRef]
- Manco, R.; Basile, B.; Capuozzo, C.; Scognamiglio, P.; Forlani, M.; Rao, R.; Corrado, G. Molecular and phenotypic diversity of traditional european plum (Prunus domestica L.) germplasm of southern Italy. Sustainability 2019, 11, 4112. [Google Scholar] [CrossRef]
- UPOV. Available online: http://www.upov.int (accessed on 10 October 2022).
- Rete Rurale Nazionale 2007–2013. Guidelines for the Conservation and Characterization of Plant, Animal, and Microbial Biodiversity of Interest to Agriculture, Published by the Ministry of Agricultural Food and Forestry Policies. Available online: http://www.reterurale.it/flex/cm/pages/ServeBLOB.php/L/IT/IDPagina/9580 (accessed on 20 September 2022).
- Marchese, A.; Tobutt, K.R.; Caruso, T. Molecular characterization of Sicilian Prunus persica cultivars using microsatellites. J. Hortic. Sci. Biotechnol. 2005, 80, 121–129. [Google Scholar] [CrossRef]
- Chaar, J.E. Characterization of peach (Prunus persica (L.) Batsch.) cultivars by frost resistance. Acta Agronómica 2015, 64, 246–253. [Google Scholar] [CrossRef]
- Qadri, R.; Ubaid, Z.; Saleem, A.; Iqbal, A.; Nisar, N.; Khan, M.M.; Haq, I.U.; Hanif, A.; Khan, I.; Feroz, A.; et al. Phenotypic and biochemical diversity among peach cultivars grown under environmental conditions of Pothohar (salt range) Pakistan. PeerJ Prepr. 2017, 5, 2833. [Google Scholar] [CrossRef]
- Spadoni, A.; Sion, S.; Gadaleta, S.; Savoia, M.A.; Piarulli, L.; Fanelli, V.; Di Rienzo, V.; Taranto, F.; Miazzi, M.M.; Montemurro, C.; et al. A simple and rapid method for genomic DNA extraction and microsatellite analysis in tree plants. J. Agric. Sci. Techol. 2019, 21, 1215–1226. [Google Scholar]
- Peakall, R.; Smouse, P.E. GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. A mathematical theory of communication. Bell Syst. Technol. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Wright, S. The genetical structure of populations. Ann. Eugen. 1951, 15, 323–354. [Google Scholar] [CrossRef]
- Lynch, M.; Ritland, K. Estimation of pairwise relatedness with molecular markers. Genetics 1999, 152, 1753–1766. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighborjoining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Gower, J.C. Wiley StatsRef: Statistics Reference Online; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; 70p. [Google Scholar]
- Dirlewanger, E.; Cosson, P.; Tavaud, M.; Aranzana, M.J.; Poizat, C.; Zanetto, A.; Arus, P.; Laigret, F. Development of microsatellite markers in peach [Prunus persica (L.) Batsch.] and their use in genetic diversity analysis in peach and sweet cherry (Prunus avium L.). Theor. Appl. Genet. 2002, 105, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Mnejja, M.; GarciaMas, M.; Howad, W.; Badenes, M.L.; Arús, P. Simple sequence repeat (SSR) markers of Japanese plum (Prunus salicina Lindl.) are highly polymorphic and transferable to peach and almond. Mol. Ecol. 2004, 4, 163–166. [Google Scholar] [CrossRef]
- Mnejja, M.; GarciaMas, J.; Howad, W.; Arús, P. Development and transportability across Prunus species of 42 polymorphic almond microsatellites. Mol. Ecol. 2005, 5, 531–535. [Google Scholar] [CrossRef]
- Aranzana, M.J.; GarciaMas, J.; Carbó, J.; Arús, P. Development and variability analysis of microsatellite markers in peach. Plant Breed. 2002, 121, 87–92. [Google Scholar] [CrossRef]
- Howad, W.; Yamamoto, T.; Dirlewanger, E.; Testolin, R.; Cosson, P.; Cipriani, G.; Monforte, A.J.; Georgi, L.; Abbott, A.G.; Arús, P. Mapping with a few plants: Using selective mapping for microsatellite saturation of the Prunus reference map. Genetics 2005, 171, 1305–1309. [Google Scholar] [CrossRef]
- Sosinski, B.; Gannavarapu, M.; Hager, L.; Beck, L.; King, G.; Ryder, C.D.; Rajapakse, S.; Baird, W.; Ballard, R.; Abbott, A.G. Characterization of microsatellite markers in peach [Prunus persica (L.) Batsch]. Theor. Appl. Genet. 2000, 101, 421–428. [Google Scholar] [CrossRef]
- Cipriani, G.; Lot, G.; Huang, W.G.; Marrazzo, M.T.; Peterlunger, E.; Testolin, R. AG/GT and AG/CT microsatellite repeats in peach [Prunus persica (L.) Batsch]: Isolation, characterization and cross-species amplification in Prunus. Theor. Appl. Genet. 1999, 99, 65–72. [Google Scholar] [CrossRef]
- Testolin, R.; Marrazzo, T.; Cipriani, G.; Quarta, R.; Verde, I.; Dettori, M.T.; Sansavini, S. Microsatellite DNA in peach (Prunus persica L. Batsch) and its use in fingerprinting and testing the genetic origin of cultivars. Genome 2000, 43, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Genome Database of Rosaceae (GDR). Available online: https://www.rosaceae.org/ (accessed on 30 September 2022).
- Jung, S.; Lee, T.; Cheng, C.H.; Buble, K.; Zheng, P.; Yu, J.; Humann, J.; Ficklin, S.P.; Gasic, K.; Scott, K.; et al. 15 years of GDR: New data and functionality in the Genome Database for Rosaceae. Nucleic Acids Res. 2019, 47, D1137–D1145. [Google Scholar] [CrossRef] [PubMed]


| SSR Locus | N | Na | Ho | He | PIC |
|---|---|---|---|---|---|
| BPPCT001 | 124 | 13 | 0.419 | 0.831 | 0.812 |
| BPPCT007 | 123 | 10 | 0.528 | 0.824 | 0.802 |
| BPPCT025 | 128 | 14 | 0.406 | 0.790 | 0.763 |
| CPDCT045 | 123 | 9 | 0.325 | 0.711 | 0.667 |
| CPPCT006 | 126 | 10 | 0.540 | 0.786 | 0.759 |
| CPPCT033 | 115 | 8 | 0.148 | 0.649 | 0.585 |
| UDP98409 | 129 | 13 | 0.457 | 0.773 | 0.749 |
| UDP98412 | 127 | 12 | 0.441 | 0.863 | 0.848 |
| Total | 129 | 89 | - | - | - |
| Mean | - | 11 | 0.408 | 0.778 | 0.748 |
| Crop Name | SSR Locus | Allele Size | Na | Ne | I | Ho | He | F | PIC |
|---|---|---|---|---|---|---|---|---|---|
| Apricot | BPPCT001 | 111–117 | 2 | 2 | 0.662 | 0.350 | 0.469 | 0.25 | 0.36 |
| BPPCT007 | 135–167 | 5 | 3 | 1.088 | 0.594 | 0.609 | 0.03 | 0.54 | |
| BPPCT010 | 114–120 | 2 | 2 | 0.615 | 0.391 | 0.424 | 0.08 | 0.33 | |
| BPPCT014 | 184–186 | 2 | 1 | 0.148 | 0.068 | 0.065 | −0.04 | 0.06 | |
| BPPCT025 | 145–157 | 4 | 2 | 0.810 | 0.563 | 0.478 | −0.18 | 0.41 | |
| CPDCT025 | 196–202 | 9 | 2 | 1.154 | 0.477 | 0.501 | 0.05 | 0.48 | |
| CPDCT045 | 128–134 | 3 | 1 | 0.377 | 0.153 | 0.198 | 0.23 | 0.18 | |
| CPPCT006 | 184–202 | 9 | 4 | 1.654 | 0.774 | 0.747 | −0.04 | 0.72 | |
| CPPCT033 | 137–141 | 3 | 1 | 0.459 | 0.151 | 0.235 | 0.36 | 0.22 | |
| CPSCT012 | 142–174 | 4 | 1 | 0.547 | 0.246 | 0.283 | 0.13 | 0.26 | |
| CPSCT018 | 139–147 | 4 | 2 | 1.066 | 0.590 | 0.582 | −0.01 | 0.53 | |
| Pchgms1 | 156–172 | 5 | 3 | 1.222 | 0.651 | 0.665 | 0.02 | 0.61 | |
| UDP96003 | 91–109 | 4 | 2 | 0.946 | 0.443 | 0.555 | 0.20 | 0.48 | |
| UDP98409 | 134–164 | 9 | 4 | 1.732 | 0.662 | 0.765 | 0.14 | 0.73 | |
| UDP98412 | 82–114 | 5 | 3 | 1.357 | 0.635 | 0.702 | 0.10 | 0.65 | |
| Mean | - | 5 | 2 | 0.922 | 0.450 | 0.485 | 0.09 | 0.44 | |
| Peach | BPPCT001 | 131–161 | 11 | 6 | 1.963 | 0.492 | 0.829 | 0.41 | 0.81 |
| BPPCT007 | 127–149 | 7 | 3 | 1.488 | 0.458 | 0.710 | 0.36 | 0.68 | |
| BPPCT015 | 150–236 | 18 | 6 | 2.196 | 0.508 | 0.830 | 0.39 | 0.82 | |
| BPPCT017 | 132–178 | 11 | 2 | 1.097 | 0.385 | 0.474 | 0.19 | 0.45 | |
| BPPCT025 | 173–195 | 10 | 3 | 1.593 | 0.262 | 0.690 | 0.62 | 0.66 | |
| BPPCT038 | 124–154 | 8 | 2 | 1.209 | 0.484 | 0.542 | 0.11 | 0.52 | |
| CPDCT045 | 138–154 | 6 | 3 | 1.133 | 0.492 | 0.617 | 0.20 | 0.55 | |
| CPPCT006 | 174–192 | 7 | 3 | 1.334 | 0.323 | 0.675 | 0.52 | 0.63 | |
| CPPCT022 | 248–294 | 7 | 4 | 1.570 | 0.415 | 0.753 | 0.45 | 0.72 | |
| CPPCT033 | 143–157 | 5 | 2 | 0.705 | 0.143 | 0.348 | 0.59 | 0.32 | |
| CPPCT044 | 149–191 | 10 | 4 | 1.742 | 0.323 | 0.758 | 0.57 | 0.73 | |
| CPSCT012 | 154–166 | 7 | 2 | 0.999 | 0.406 | 0.484 | 0.16 | 0.45 | |
| EPPCU5176 | 118–128 | 5 | 2 | 0.866 | 0.354 | 0.452 | 0.22 | 0.41 | |
| UDP96005 | 152–172 | 8 | 4 | 1.561 | 0.354 | 0.748 | 0.53 | 0.71 | |
| UDP96008 | 134–164 | 6 | 2 | 1.119 | 0.492 | 0.546 | 0.10 | 0.51 | |
| UDP98022 | 124–136 | 5 | 4 | 1.427 | 0.308 | 0.743 | 0.59 | 0.70 | |
| UDP98409 | 120–152 | 6 | 1 | 0.676 | 0.262 | 0.328 | 0.20 | 0.30 | |
| UDP98412 | 106–130 | 7 | 4 | 1.502 | 0.262 | 0.748 | 0.65 | 0.71 | |
| Mean | - | 8 | 3 | 1.343 | 0.373 | 0.626 | 0.38 | 0.59 |
| Crop Name | Genotypes with LRM = 0.50 | |
|---|---|---|
| Apricot | Ananassa_2 | Ananassa_3 |
| Ananassa_4 | Ananassa_Parco | |
| Ananassa_4 | Barese_1 | |
| Ananassa_4 | Cibo_Del_Paradiso | |
| Buontempo | Capone | |
| Buontempo | Guarino | |
| Buontempo | Picocchina_Di_Cerignola | |
| Cibo_Di_Sant’Antonio | Grosso_Di_Trinitapoli | |
| Cibo_Di_Sant’Antonio | Mandorla_Dolce_2 | |
| Cafona_ref | Due_Maschere | |
| Fragnito | Picocca | |
| Natalicchio_1 | Spadalunga | |
| Natalicchio_2 | San_Michele | |
| Occhiorosso | San_Nicola | |
| Petrelli | Taglialascia | |
| Picocchina | Spasimato | |
| Montagnulo | San_Nicola_Lorusso | |
| Montagnulo | Sant’Antonio_2 | |
| Genotypes with 0.40 < LRM < 0.50 | ||
| A_Punta | Sant’Antonio_2 | |
| A_Punta | Risomma_Nisi | |
| A_Punta | San_Nicola_Lorusso | |
| Buontempo | Sant’Antonio_1 | |
| Buontempo | Frascarosa_Buontempo | |
| Capone | Sant’Antonio_1 | |
| Capone | Frascarosa_Buontempo | |
| Frascarosa_Buontempo | Guarino | |
| Frascarosa_Buontempo | Picocchina_Di_Cerignola | |
| Guarino | Sant’Antonio_1 | |
| Montagnulo | San_Michele | |
| Montagnulo | Natalicchio_2 | |
| Natalicchio_2 | Sant’Antonio_2 | |
| Natalicchio_2 | San_Nicola_Lorusso | |
| Natalicchio_2 | Risomma_Nisi | |
| Occhiorosso | Petrelli | |
| Occhiorosso | Taglialascia | |
| Petrelli | San_Nicola | |
| Picocchina_Di_Cerignola | Sant’Antonio_1 | |
| Risomma_Nisi | San_Michele | |
| San_Michele | Sant’Antonio_2 | |
| San_Michele | San_Nicola_Lorusso | |
| San_Nicola | Taglialascia | |
| Sant’Antonio_3 | Tardivo_Presidente | |
| Peach | Genotypes with LRM = 0.50 | |
| Persichine_Apritune | Aprituna_2 | |
| Genotypes with 0.40 < LRM < 0.50 | ||
| Moccafava_2 | Nero_Presta | |
| Giallo_Di_Ottobre_ | Antichissimo_Pinto | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savoia, M.A.; Del Faro, L.; Turco, A.; Fanelli, V.; Venerito, P.; Montemurro, C.; Sabetta, W. Biodiversity Evaluation and Preservation of Italian Stone Fruit Germplasm (Peach and Apricot) in Southern Italy. Plants 2023, 12, 1279. https://doi.org/10.3390/plants12061279
Savoia MA, Del Faro L, Turco A, Fanelli V, Venerito P, Montemurro C, Sabetta W. Biodiversity Evaluation and Preservation of Italian Stone Fruit Germplasm (Peach and Apricot) in Southern Italy. Plants. 2023; 12(6):1279. https://doi.org/10.3390/plants12061279
Chicago/Turabian StyleSavoia, Michele Antonio, Loredana Del Faro, Andrea Turco, Valentina Fanelli, Pasquale Venerito, Cinzia Montemurro, and Wilma Sabetta. 2023. "Biodiversity Evaluation and Preservation of Italian Stone Fruit Germplasm (Peach and Apricot) in Southern Italy" Plants 12, no. 6: 1279. https://doi.org/10.3390/plants12061279
APA StyleSavoia, M. A., Del Faro, L., Turco, A., Fanelli, V., Venerito, P., Montemurro, C., & Sabetta, W. (2023). Biodiversity Evaluation and Preservation of Italian Stone Fruit Germplasm (Peach and Apricot) in Southern Italy. Plants, 12(6), 1279. https://doi.org/10.3390/plants12061279









