Abstract
Rosehips of various Rosa spp. are well known for having human health-promoting compounds like mineral nutrients, vitamins, fatty acids, and phenolic compounds. However, little is known about rosehip characteristics which describe the fruit quality and may indicate appropriate harvest times. Our study evaluated the pomological (width, length, and weight of fruits, flesh weight, and seed weight), texture, and CIE colour parameters (L*, a*, and b*), chroma (C), and hue angle (h°) of rosehip fruits of Rosa canina, Rosa rugosa, and genotypes of Rosa rugosa ‘Rubra’ and ‘Alba’, harvested at five ripening stages (I–V). The main results revealed that genotype and ripening stage significantly affected parameters. The significantly longest (R. canina) and widest fruits (R. Rugosa) were measured at ripening stage V. Genotypes of R. rugosa ‘Rubra’ and ‘Alba’ had significantly higher fruit and flesh weights at ripening stage V. Rosehips of all investigated genotypes expressed darkness (lower L*) during ripening, and had the highest hue angle h° values at ripening stage I while the lowest was at stage V. The significantly lowest skin elasticity of rosehips was found at stage V. However, R. canina was distinguished by the highest fruit skin elasticity and strength. As our results show, the desired pomological, colour, and texture features of various species and cultivars rosehips can be optimised according to the harvest time.
1. Introduction
With a growing global population and diminishing natural resources, food supply and demand disparity have become more pronounced [1]. Diverse species employed in agricultural systems are crucial for human nutrition and sustainable food systems. Food diversity gained from or near agricultural fields and natural environments such as forests is an additional source of food system resilience [2]. The hunt for nutrient-dense food sources is a matter of great concern in today’s food-scarce, multibillion-person world. An unexploited functional food source is the Rosa species cultivated for the ornamental flowers where the fleshy fruits (hypanthium/haw), situated under the perianth, known as rosehips, are removed [3].
As interest in rosehips’ nutritional worth has increased, many studies on their morphological traits, phytochemical components, antioxidant capacity, and volatile chemicals have been conducted [1]. In addition, in natural growing conditions, different species and varieties show diversity in most morphological traits [4]. These plants are widely utilised in food; however, the interest in using them in cosmetics and pharmaceutical industries as effective materials for enhancing the quality of final goods with antioxidant activity features is prevailing [5,6,7]. Rosehips have antioxidant and anti-inflammatory, antibacterial, anti-mutagenic probiotic, anti-ulcerogenic, antinociceptive, and anti-carcinogenic properties [8,9]. In a recent study, over 500 s metabolites rosehips from five species were determined, most of which were flavonoids and phenolic acids [1]. In addition, rosehips are a rich source of mineral nutrients [10] and vitamins (particularly, vitamin C) and are abundant in carotenoids, tocopherols, tannins, organic acids, amino acids, and pectin [11,12]. In their seeds prevail polyunsaturated fatty acids, followed by monounsaturated and saturated fatty acids [13]. Linoleic, -linolenic, and oleic fatty acids were the most abundant unsaturated fatty acids in rosehip seed oil [14]. Due to these reasons, rosehips are used in developing new products in the food and pharmacy industries [4,15,16,17]. For example, the use of rosehip meal in eggs enriched with polyunsaturated fatty acids had a positive effect on the amino acid and fatty acid content, as well as the antioxidant capacity, and was very effective in extending the shelf-life of eggs [16]. The supplementation of Rosa canina rosehips may be recommended as a natural colourant in poultry diets in conventional or organic egg production [17].
Wild fruits exhibit diverse morphology, fruit quality, yield, and phytochemicals compared to cultivated ones, and all those traits can be influenced by ecotypes [4]. Rosa spp. are native to cold and highland areas but demonstrate high environmental adaptability, as they are widespread plants in different climate regions such as Turkey [4,18,19,20], Slovakia [21], Serbia [14,22,23], Croatia [24,25], Romania [26], or Lithuania [10,12,13].
Several studies showed that organic plants indicate a higher nutritional value and higher content of biologically active compounds in various crops from organic compared to conventional farming [27,28]. For example, organically cultivated rosehips had significantly higher phenolic compounds and antioxidant capacity values than conventional fruits [29,30]. However, genotype seems to be the more influential factor than cultivation techniques since the content of bioactive compounds like carotenoids was higher in wild rosehips than those cultivated in the organic or conventional systems [30].
Characterising the best harvesting time is also important in improving the quality and nutritional value of the processed product. Several studies reported that the qualitative and quantitative composition of the biologically active compounds in rosehips, such as fatty acids [13], phenolic compounds, or vitamin C [12,31,32] varies during the stages of maturity. These results may lead to the assumption that the accumulation of phytochemicals may correspond to pomological or organoleptic traits, such as colour characteristics. For example, darker rosehips showed higher phytochemical concentrations and antioxidant activity [1]. Additionally, in numerous fruit crops, colour is a significant determinant of the fruit’s appearance and maturity. Typically, the harvesting period is defined by colour changes of the rosehip’s skin, which turns from green to light orange or pink into red-orange or deeper red. In the study by Uggla et al. [33], the association between colour as assessed by CIE (L*, a*, b*) coordinates and °Brix values and qualitative factors during the ripening period in Rosa dumalis and R. rubiginosa have been published. However, compared to the phytochemical composition, pomological and colour parameters have been poorly presented for other Rosa species, and the studies on those characteristics associated with ripening are very limited. In addition, these data are relevant for the evaluation of the harvest time of rosehips and can be used to choose cultivars for future production in Lithuanian climatic conditions.
This study was aimed at evaluating the pomological, texture, and colour parameters of the fruits of different Rosa species/cultivars (R. canina, R. rugosa, R. rugosa ‘Rubra’, R. rugosa ‘Alba’) harvested at five ripening stages.
2. Results and Discussion
2.1. Pomological Parameters of Rosehip Fruits
Horticulture is primarily concerned with the growth of plant material for human consumption, medicinal usage, or functional and aesthetic objectives [34]. In this context, Rosa spp. are important plants for traditional pharmacological practices and landscape studies; the fruit quality is assessed by many pomological parameters, which can differ with climate conditions, geographical location, or geographic ecological origin. According to a two-way ANOVA, the examined pomological characteristics of rosehip fruits were affected by species and/or cultivars, ripening stage, and their interaction (Table 1).

Table 1.
Pomological parameters of rosehip fruits of different species/cultivars during five ripening stages (n = 60).
Our results showed that the significantly longest fruits (24.93 mm) were those of Rosa canina, while the widest fruits (31.88 mm) corresponded to Rosa rugosa at ripening stage V. Rosa rugosa ‘Rubra’ and Rosa rugosa ‘Alba’ had significantly higher fruit weights (12.53 and 13.79 g, respectively) and flesh weights (11.33 and 12.65 mm, respectively) at ripening stage V than the other genotypes (Table 1).
For each species and cultivar of Rosa spp., the weight of seeds varied from 0.39 to 1.42 g, depending on the ripening stage. Rosa canina had significantly greater seed weights at ripening stage V, while R. rugosa had significantly lower seed weights at ripening stage I. Our results agree with those of previously published studies that referred to pomological characteristics, such as fruit length, width, weight, flesh weight, seed weight, and the fruit–flesh ratio [26,35,36].
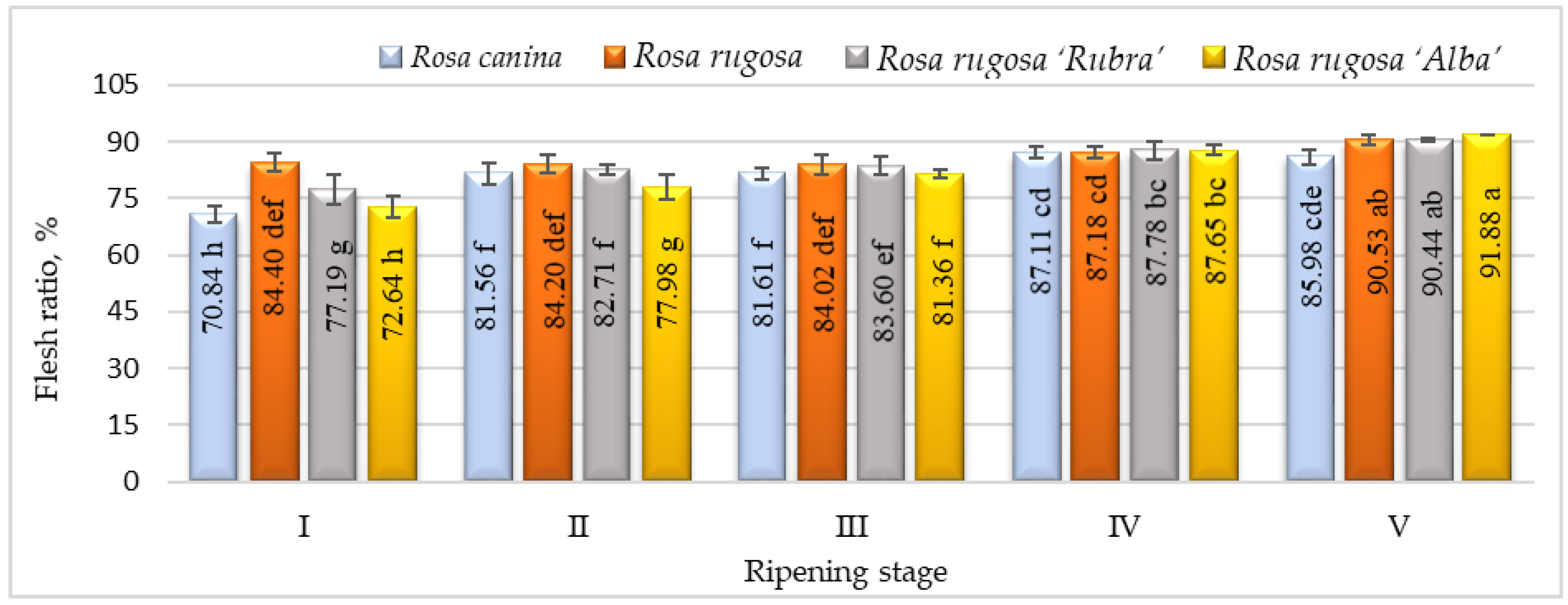
The flesh ratio is one of the most important criteria regarding rosehip fruit quality. In developing rosehip cultivars suitable for the industry, cultivars with large-sized fruits and a high flesh ratio are desired [19]. Our results indicated that the flesh ratio was quite variable, ranging from 70.84 % (ripening stage I, R. canina) to 91.88 % (ripening stage V, R. rugosa ‘Alba’) (Figure 1). This ratio was highest in R. rugosa, R. rugosa ‘Rubra’, and R. rugosa ‘Alba’ in ripening stage V. Previous research has also found a significant level of flesh ratio variability within Rosa L. species. Our results were comparable with the other studies [18,19,36,37], showing flesh ratios of 46.8–100% among rosehip genotypes.
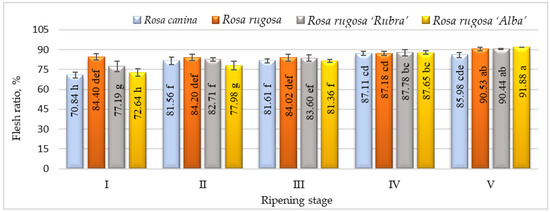
Figure 1.
Flesh ratio of rosehip of different species/cultivars at five ripening stages (n = 60). Data are given as the arithmetic mean ± standard deviation. Averages followed by different letters are significantly different at the 5% level of probability (p < 0.05). The effects of species/cultivars, ripening stage, and their interaction are significant at p < 0.0001.
2.2. Colour Parameters of Rosehips
CIE colour parameters were determined and are shown in Table 2: L* for the value for lightness, a* value for red/green colour, b* value for yellow/blue colour, hue angle (h°), and C* for the brightness of different genotypes of rosehip fruits at each ripening stage. A two-way ANOVA showed that rosehip species/cultivar, ripening stage, and their interaction significantly influenced the CIE colour coordinates L*, a*, b*, C, h° values. According to Kazankaya et al. [37], rosehip fruits had a base colour of red, dark red, light red, and orange. Our results showed that the value of L* varied differently in all species and/or cultivars during the ripening period (Table 2). Erogul and Oguz [38] researchers established the L* value of the investigated rosehip fruits from 32.35 to 37.62 among all genotypes. According to these authors, the differences can be caused by environmental conditions and depend on genotype. Our data showed that the fruits of R. canina, R. rugosa, R. rugosa ‘Rubra’, and R. rugosa ‘Alba’ expressed darkness during ripening, as demonstrated by the decreasing values of coordinate L*. Significantly higher rosehip lightness values L* were found for R. rugosa at ripening stages I, II, and IV (38.44, 39.24, and 38.07, respectively) and for R. rugosa cv. ‘Alba’ at ripening stages I, II, and III (39.31, 38.56, and 38.17, respectively). A significantly lowest coordinate L* (26.60) was found in the fruits of R. canina at ripening stage V. A study by Ercisli [20] showed higher colour L* (48.06), a* (41.70), and b* (39.39) values of R. canina rosehip fruits. Shades of red colours were found to be predominant among the investigated genotypes. Other studies showed that the L* value varied from 30.5 to 45.2 in the rosehips harvested from four Sicilian plants of Rosa canina [7].

Table 2.
Colour parameters of rosehip fruits of different species/cultivars during five ripening stages (n = 60).
For all investigated Rosa spp., colour changes during ripening were characterised by a significant increase of a* (redness) (Table 2). All investigated samples exhibited negative values of a* ranging from −1.89 to −4.52 at ripening stages I and II.
All tested rosehip samples were significantly greener at ripening stage I than those harvested at other ripening stages. However, from ripening stages III to V, there was a significant increase in the a* value, changing from negative to positive values (6.96–37.83) in all rosehips. According to the literature, colour variation in fruits is due to changes in the accumulation of pigments like carotenoids and chlorophyll degradation [39]. At ripening stage V, comparing the species and/or cultivars, the redness of rosehip fruits differed. Rosa rugosa showed a significantly higher a* value (37.83), followed by R. rugosa ’Rubra’ (35.59), R. rugosa (35.00), and R. rugosa ‘Alba’ (32.25). Nine Rosa spp. genotypes were investigated by Bilgin et al. [40] and they found a* values from 19.31 to 34.09, which express the red colour, while the L* value was from 21.71 to 36.01 and the b* from 9.75 to 22.57.
The value of coordinate b* showed a high level of variation (Table 2). The colour b* value of fruits was always positive (yellow h°) for all species/cultivars at all ripening stages. Significantly, the highest value of coordinate b* (41.51) was found for R. rugosa fruits at ripening stage I and the lowest (22.53) for R. canina at stage V, indicating that they became less yellow. In Palermo, Fascella et al. [7] investigated colour parameters for different Sicilian rose species with intense red colour fruits. The colour b* value varied from 14.1 to 26.0. According to these researchers, the rosehip colour may be a valuable predictor of the optimal harvesting time for different Rosa spp. genotypes [7].
Ripening stage and genotype significantly affected the C* values of the rosehips (Table 2). Our data showed that, as the fruit ripened, the C* values of all species and (or) cultivars increased significantly. For example, in the fruits of R. rugosa, the value of C* was significantly higher at ripening stages IV and V (49.87 and 48.28, respectively). According to Erogul and Oguz [39], the range of the C* value varied from 34.53 to 42.25, which is similar to ours.
In all investigated rosehip fruits, the hue angle (h°) of all ripening stages was significantly different (Table 2). All tested rosehip samples had the highest h° values at ripening stage I and the lowest at stage V. R. canina fruits had the significantly highest h° value of 98.39 (green) and the lowest h° value of 32.77 (red). Chae et al. [31] showed that the h° of R. rugosa rosehips also depended on the ripeness stage. h° decreased during ripening due to carotenoid accumulation, which is associated with changes in yellow and red colours [33,41].
2.3. Texture Properties of Rosehip Fruits
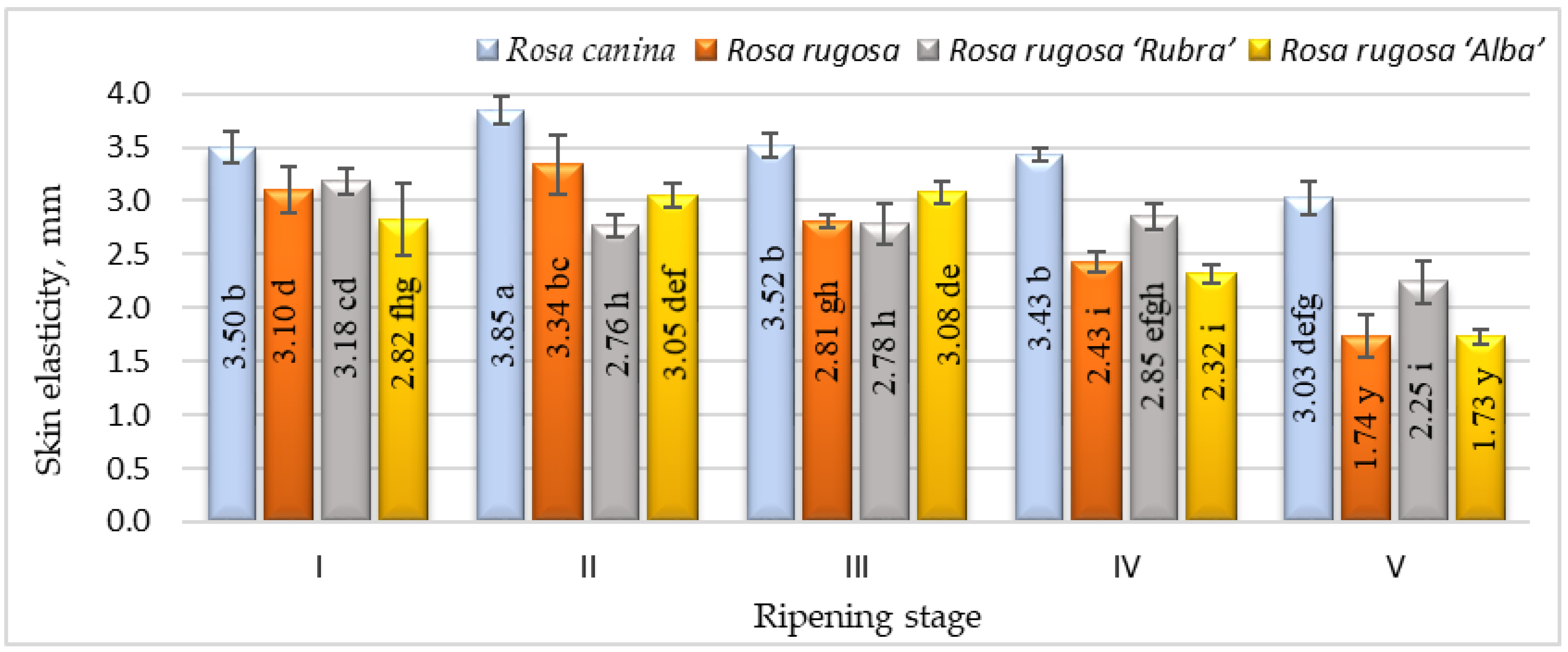
Ripening is the process by which fruits acquire desirable flavour, quality, colour, palatability, and other textural attributes. However, the textural aspects of the skin of rosehips during ripening have received little attention in the literature. The changes in elasticity during rosehip ripening for different cultivars and/or species are presented in Figure 2.
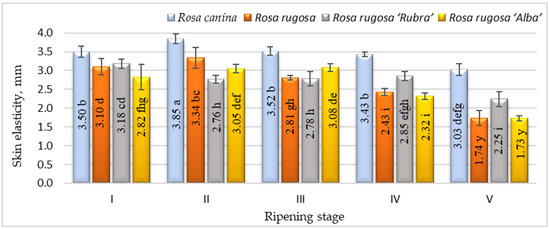
Figure 2.
Elasticity of different species/cultivars at five ripening stages (n = 60). Data are given as the arithmetic mean ± standard deviation. Averages followed by the different letters are significantly different at the 5% level of probability (p < 0.05). The effects of species/cultivars, ripening stage, and their interaction are significant at p < 0.0001.
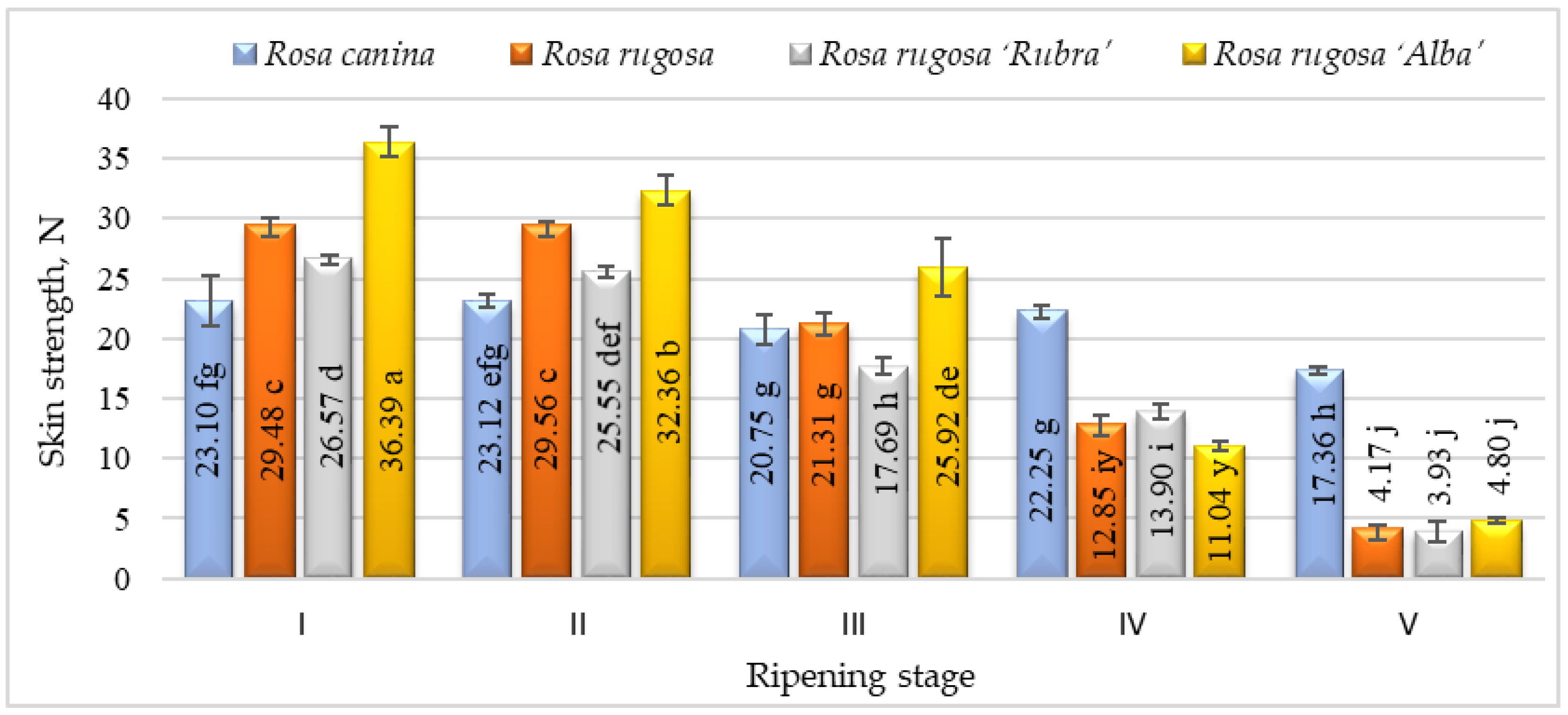
The elasticity of the rosehip skin had a tendency to decrease during the ripening period and the significantly lowest elasticity was found at stage V. According to the results, Rosa canina presented the highest skin elasticity during ripening. We found significant differences in strength among the cultivars and/or species studied and ripening stages (Figure 3). Fruits harvested at stages I and II have shown higher skin strength than at other ripening stages. During ripening, the fruit of all investigated cultivars/species had a decrease in skin strength. The strength parameters of all species and/or cultivars also changed throughout ripening. At ripening stage V, Rosa canina fruit had the strongest skin compared with other genotypes at the same stage. A decrease in the strength of the fruit skin during ripening can be associated with the metabolic pathways that are responsible for textural changes in fruits, which are believed to involve loss in turgor pressure, degradation, and other physiological changes in the composition of membranes, degradation of starch, and modifications in the cell wall structure and dynamics [42].
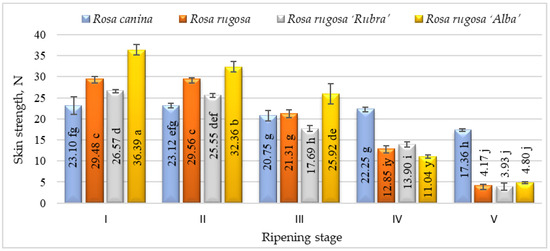
Figure 3.
Strength of different species/cultivars at ripening stages (n = 60). Averages in the column followed by different letters are significantly different at the 5% probability level (p < 0.05). The effects of species/cultivars, ripening stage, and their interaction are significant at p < 0.0001.
During fruit ripening, various biochemical changes occur, including seed maturation, colour change, texture softening, volatile taste generation, wax formation on the skin, tissue permeability, and changes in the content of carbohydrates, organic acids, and proteins. These ripening-related changes account for fruit skin elasticity and strength variations during fruit maturity 28]. Unfortunately, as far as we know, there is no research on rosehip fruit skins.
2.4. Correlation among Investigated Traits
The correlation matrix between the pomological, colour, and textural properties of rose hip species/cultivars is shown in Table 3. Strong positive correlations were found between fruit weight and flesh weight, seed weight, and fruit length (r = 0.999, r = 0.778, and r = 0.856, respectively). According to other researchers, the highest correlation was that between fruit weight and fruit width (r = 0.898) [40].

Table 3.
Correlation matrix between pomological, colour, and texture properties.
Our study showed that fruit length had a negative correlation with L ∗, b*, and h° values (r = −0.751, r = −0.641, and r = −0.933, respectively) and a high positive correlation (r = 0.910) with the a* value. In comparison, values of h° were strongly negative for a* and C (r = −0.933 and r = −0.823, respectively). Bilgin et al. [40] discovered highly negative associations between fruit weight, stone weight, and width and colour L*, a*, b*, and C* values. In our study, skin elasticity negatively correlated with fruit weight, flesh weight, and fruit width (r = −0.737, r = −0.741, and r = −0.876, respectively). As well, fruit skin strength correlated negatively with fruit weight, flesh weight, fruit length, and a * (r = −0.878, r = −0.880, r = −0.800, and r = −0.855) while it positively correlated with the h° value (r = 0.839). No correlation was found between skin elasticity, C, and rosehip L* and b* values.
2.5. Principal Component Analysis
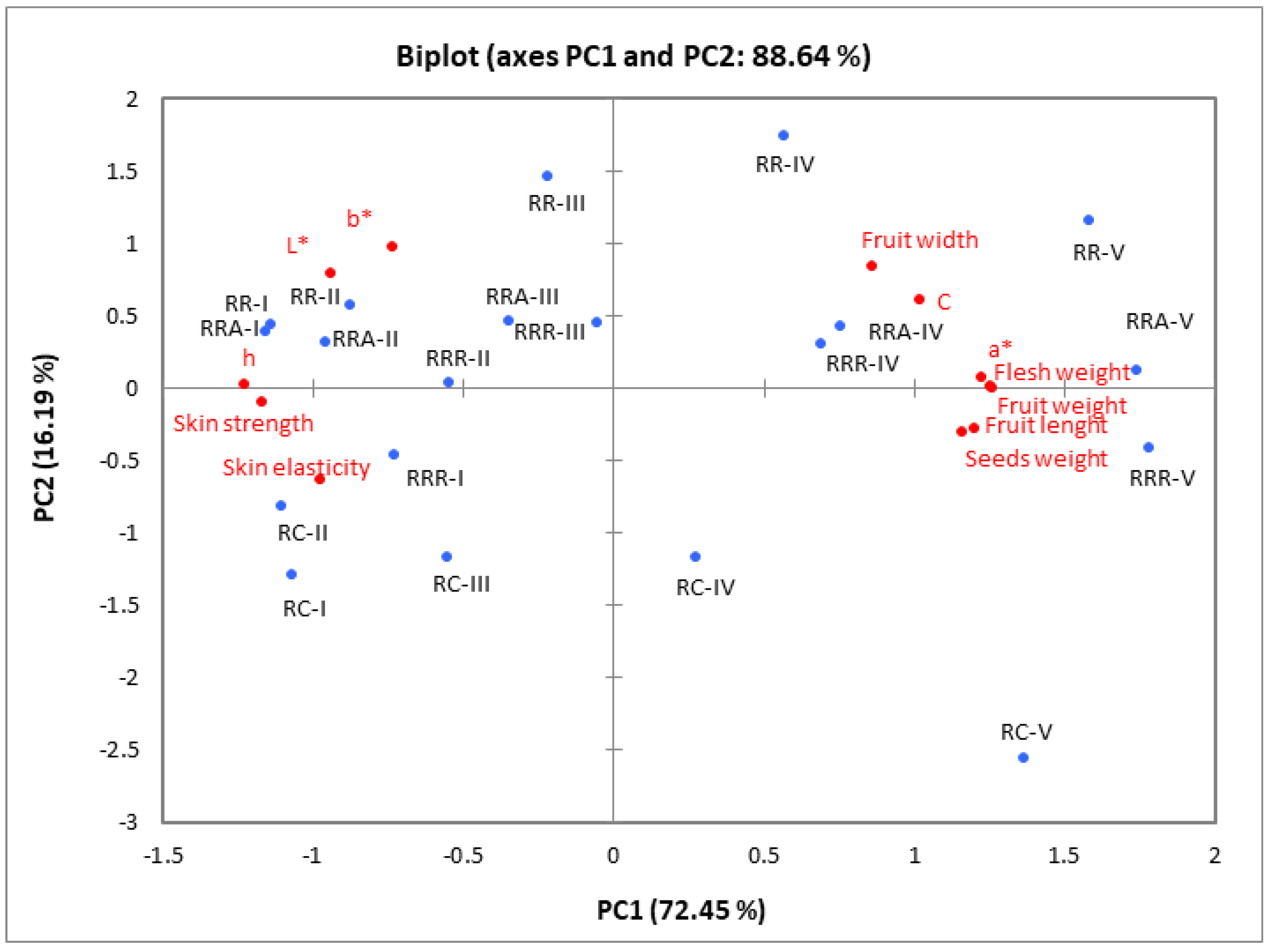
The relationships between rosehip samples of different species/cultivars harvested at five ripening stages and the pomological (Table 1), colour (Table 2), and textural (Figure 2 and Figure 3) properties were examined using a principal component analysis (PCA). The PCA findings showed that the first two axes (PC1: 72.45% and PC2: 16.19%) explained 88.64% of the total variance (Figure 4). In addition, the first principal component’s (PC1) and second principal component’s (PC2) eigenvalues were both greater than one (8.69 and 1.94, respectively).
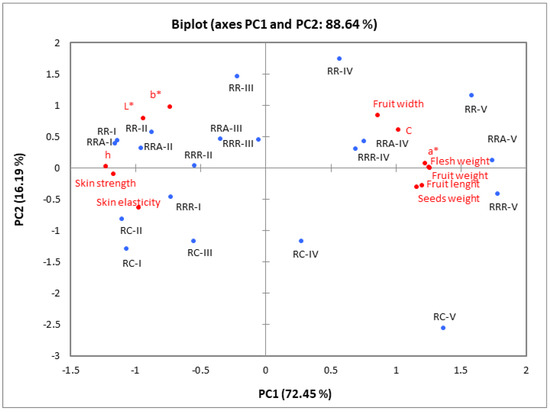
Figure 4.
PCA for pomological (fruit weight, flesh weight, seeds weight, fruit width, fruit length), colour (L*, a*, b*, C* and h◦), and texture (skin elasticity, skin strength) parameters of the rosehip of different species and (or) cultivars. RC—Rosa canina, RR—Rosa rugosa, RRR—Rosa rugosa ‘Rubra’, RRA—Rosa rugosa ‘Alba’ harvested at five ripening stages (I, II, III, IV, and V).
All pomological characteristics and colour characteristics a* and C were strongly and positively associated with PC1. In contrast, the skin’s colour parameters L*, h◦, and textural properties (strength and elasticity) were negatively associated with PC1. PC2 was positively associated with colour coordinate b*. As illustrated in Figure 4, PC1 separated the rosehip samples harvested at ripening stages I, II, and III from the rosehip samples harvested at ripening stages IV and V. The fruits harvested at ripening stages IV and V are defined by higher width, length and weight, and also, greater values of colour characteristics a* and C. However, these samples contained lower values of colour parameters L*, h°, skin strength, and elasticity compared to fruits harvested at ripening stages I, II, and III.
3. Materials and Methods
3.1. Field Experiments
This research was conducted in 2018–2020 at the organic farm in Pakruojis District, Lithuania (field coordinates 56°10′29.0″ N 23°49′02.6″ E). Two species, Rosa rugosa and Rosa canina and two cultivars, Rosa rugosa cv. ‘Rubra’ and Rosa rugosa cv. ‘Alba’ were planted in 2011. The distance between the rows was 4 m, and the distance between rosehip shrubs was 2 m. The interrow was loosened, and weeds were manually removed. The soil pH at the experimental site was from 6.8 to 7.0 mg kg−1 plant-available potassium from 97.7 to 181.0 mg kg−1, available phosphorus from 120.6 to 153.3 mg kg−1, and total nitrogen from 2.5%. The experimental plots were arranged in the randomised block with four replicates per treatment. Each replication consisted of four plants of each species/cultivar. Fruits were randomly collected for analysis from July to September, depending on the ripening stage. For the analyses, 1 kg of fruits was randomly harvested from each block of each treatment.
The rose fruits were harvested at five ripening stages (I-V; Figure 5): (I) was reached when fruit colour changed slightly from green to yellow, pink, or red in no less than 10% of its surface; (II) fruit colour changed from green to tarnish—yellow, pink, or red in no less than 30% of the surface; (III) fruit colour changed from green to light orange or red or a combination thereof in no less than 60% of the surface; (IV) fruit became pinkish or orange depending on the species; (V) fruit surface was red.

Figure 5.
Fruits at ripening stages (I–V) for: (A)—Rosa canina, (B)—Rosa rugosa, (C)—Rosa rugosa ‘Rubra’, (D)—Rosa rugosa ‘Alba’ (photos by B. Medveckienė).
Compared to standard climate normal (SCN), in 2018, 2019, and 2020, the temperature was higher by 2.5, 1.4, and 0.7 ℃. During the rosehip vegetation period of 2018, 2019, and 2020, the average precipitation was 86, 106.6, and 72.5 mm less than in the SCN. In addition, in 2018, 2019, and 2020, the sunshine was 195, 118, and 8 h longer than SCN (Table 4).

Table 4.
Weather conditions during the rosehip vegetation period in 2018, 2019, and 2020 (Šiauliai meteorological station, Lithuania).
3.2. Soil Agrochemical Analyses
The soil agrochemical characteristic was evaluated in the Vytautas Magnus University Agriculture Academy Laboratory of Food Raw materials, Agronomical and Zoo-technical Investigations. From the soil samples, the small stones, roots, and other organic plant parts were removed, then air-dried in open plastic boxes and crushed. Homogenised soil was sieved through a 1 mm mesh size sieve. Soil samples were analysed for pH in potassium chloride (KCl) solution (pHKCl), plant-available phosphorus, potassium, and total nitrogen. Soil pHKCl was established by the potentiometric method in 1N KCl extract [43]. Plant-available phosphorus and potassium were extracted with ammonium lactate according to the Egner–Riehm–Domingo method [44]. Total nitrogen concentration (mg kg−1) was determined by the Kjeldahl method using Kjeldatherm (Gerhardt, Königswinter, Germany) [45].
3.3. Pomological Parameters
Fruit length and width in mm were measured with a digital calliper. The fresh fruit biomass (g), flesh and seed weights (g) were measured using a technical balance (Radwag, WPS 510/C/1, Radom, Poland). The results are displayed in grams with a precision of 0.01 g. The fruit-to-flesh ratio was calculated by dividing flesh weight by fruit weight and multiplying by 100. The pomological fruit parameters were calculated as the mean of 60 (3 replicates × 20 fruits) fruits.
3.4. Colour Parameter Analysis
The CIE colour parameters L* (lightness), a* (positive—red, negative—green), and b* (positive—yellow, negative—blue) of rosehips were measured using a spectrophotometer ColorFlex (Hunter Associates Laboratory, Inc., Reston, VA, USA) in NBS units.
Chroma presents the quality and intensity of fruit colour. Calculations were performed on colour attributes chroma (C), using the following formula:
Hue angle (h°) was calculated using the following formula:
The colour parameters were calculated as the mean of 60 (3 replicates × 20 fruits) fruits.
3.5. Fruit Skin Texture Analysis
The skin elasticity and strength of rosehips were measured using a texture analyser, TA.XTPlus, with a P/2 probe (Stable Micro Systems, Godalming, UK).
The probe descended into the sample at a speed of 1 mm s−1 and reached a depth of 5 mm. Skin strength is the force (N) required to puncture the fruit skin, whereas skin elasticity is the distance (mm) to which the skin deflects before probe penetration.
The texture parameters were calculated as the mean of 60 (3 replicates × 20 fruits) fruits.
3.6. Statistical Analysis
Data were analysed using Microsoft®Excel®2016 MSO and confirmed using the STATISTICA 10 (StatSoft, Inc., Tulsa, OK, USA, 2010). All analyses were conducted in triplicate, with the mean and standard deviation presented. Since the analysis of variance did not show year interaction, the data are presented as two-year averages. The samples were defined by two qualitative factors (species/cultivars and ripening stage), and therefore, the reliability of the results was evaluated by a two-way analysis of variance (ANOVA) method. Tukey’s test was applied to assess significant differences between the means at p < 0.05. The nature and strength of the correlations between the variables were determined using correlation analysis. Finally, the relationships between rosehip samples of different species/cultivars harvested at five ripening stages and the pomological, colour, and texture properties were evaluated with the XLSTAT 2018 (New York, NY, USA) using principal components analysis (PCA).
4. Conclusions
The results of this experimental investigation revealed that the genotype and ripening stage significantly affected the fruit’s pomological, colour, and textural parameters. According to our results, appropriate for transportation was Rosa canina having the strongest fruit skin elasticity (3.03 mm) and strength (17.36 N). R. rugosa, R. rugosa ‘Rubra’, and R. rugosa ‘Alba’ are suitable for the industry because these species/cultivars had the largest-sized fruits and the highest flesh ratio at the ripening stage V. Comparing the species and (or) cultivars, Rosa rugosa fruits were the reddest, at ripening stage V. The pomological, colour, and texture features of rosehips can be optimised and used for many purposes with the appropriate harvesting time and species/cultivar selection.
Author Contributions
Conceptualisation, J.K., B.M., D.L., N.V. and V.V.-K.; methodology, J.K. and B.M.; software, B.M. and N.V.; formal analysis, B.M. and N.V.; investigation, J.K. and B.M.; data curation, B.M. and N.V.; writing—original draft preparation, J.K., B.M., D.L., N.V. and V.V.-K.; writing—review and editing, D.L and V.V.-K.; visualisation, J.K., B.M., D.L., N.V. and V.V.-K.; supervision, J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sun, Y.; Zhou, M.; Luo, L.; Pan, H.; Zhang, Q.; Yu, C. Metabolic Profiles, Bioactive Compounds and Antioxidant Activity of Rosehips from Xinjiang, China. LWT 2023, 174, 114451. [Google Scholar] [CrossRef]
- Lachat, C.; Raneri, J.E.; Smith, K.W.; Kolsteren, P.; van Damme, P.; Verzelen, K.; Penafiel, D.; Vanhove, W.; Kennedy, G.; Hunter, D.; et al. Dietary Species Richness as a Measure of Food Biodiversity and Nutritional Quality of Diets. Proc. Natl. Acad. Sci. USA 2018, 115, 127–132. [Google Scholar] [CrossRef]
- Patel, S. Rose Hip as an Underutilized Functional Food: Evidence-Based Review. Trends Food Sci. Technol. 2017, 63, 29–38. [Google Scholar] [CrossRef]
- Bozhuyuk, M.R.; Ercisli, S.; Karatas, N.; Ekiert, H.; Elansary, H.O.; Szopa, A. Morphological and Biochemical Diversity in Fruits of Unsprayed Rosa canina and Rosa Dumalis Ecotypes Found in Different Agroecological Conditions. Sustainability 2021, 13, 8060. [Google Scholar] [CrossRef]
- Butkevičiūtė, A.; Urbštaitė, R.; Liaudanskas, M.; Obelevičius, K.; Janulis, V. Phenolic Content and Antioxidant Activity in Fruit of the Genus rosa L. Antioxidants 2022, 11, 912. [Google Scholar] [CrossRef]
- Cosmulescu, S.; Trandafir, I.; Nour, V. Phenolic Acids and Flavonoids Profiles of Extracts from Edible Wild Fruits and Their Antioxidant Properties. Int. J. Food Prop. 2017, 20, 3124–3134. [Google Scholar] [CrossRef]
- Fascella, G.; D’Angiolillo, F.; Mammano, M.M.; Amenta, M.; Romeo, F.V.; Rapisarda, P.; Ballistreri, G. Bioactive Compounds and Antioxidant Activity of Four Rose Hip Species from Spontaneous Sicilian Flora. Food Chem. 2019, 289, 56–64. [Google Scholar] [CrossRef]
- Gruenwald, J.; Uebelhack, R.; Moré, M.I. Rosa canina—Rose Hip Pharmacological Ingredients and Molecular Mechanics Counteracting Osteoarthritis—A Systematic Review. Phytomedicine 2019, 60, 152958. [Google Scholar] [CrossRef]
- Gulbagca, F.; Ozdemir, S.; Gulcan, M.; Sen, F. Synthesis and Characterization of Rosa canina-Mediated Biogenic Silver Nanoparticles for Anti-Oxidant, Antibacterial, Antifungal, and DNA Cleavage Activities. Heliyon 2019, 5, e02980. [Google Scholar] [CrossRef]
- Medveckienė, B.; Kulaitienė, J.; Vaitkevičienė, N.; Levickienė, D.; Bunevičienė, K. Effect of Harvesting in Different Ripening Stages on the Content of the Mineral Elements of Rosehip (Rosa Spp.) Fruit Flesh. Horticulturae 2022, 8, 467. [Google Scholar] [CrossRef]
- Demir, F.; Özcan, M. Chemical and Technological Properties of Rose (Rosa canina L.) Fruits Grown Wild in Turkey. J. Food Eng. 2001, 47, 333–336. [Google Scholar] [CrossRef]
- Medveckienė, B.; Kulaitienė, J.; Levickienė, D.; Hallmann, E. The Effect of Ripening Stages on the Accumulation of Carotenoids, Polyphenols and Vitamin C in Rosehip Species/Cultivars. Appl. Sci. 2021, 11, 6761. [Google Scholar] [CrossRef]
- Kulaitienė, J.; Medveckienė, B.; Levickienė, D.; Vaitkevičienė, N.; Makarevičienė, V.; Jarienė, E. Changes in Fatty Acids Content in Organic Rosehip (Rosa spp.) Seeds during Ripening. Plants 2020, 9, 1793. [Google Scholar] [CrossRef] [PubMed]
- Popović-Djordjević, J.; Špirović-Trifunović, B.; Pećinar, I.; Fernando Cappa de Oliveira, L.; Krstić, Đ.; Mihajlović, D.; Akšić, M.F.; Simal-Gandara, J. Fatty Acids in Seed Oil of Wild and Cultivated Rosehip (Rosa canina L.) from Different Locations in Serbia. Ind. Crops Prod. 2023, 191, 115797. [Google Scholar] [CrossRef]
- Mármol, I.; Sánchez-De-Diego, C.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C.; Rodríguez-Yoldi, M. Therapeutic Applications of Rose Hips from Different Rosa Species. Int. J. Mol. Sci. 2017, 18, 1137. [Google Scholar] [CrossRef]
- Vlaicu, P.A.; Untea, A.E.; Turcu, R.P.; Panaite, T.D.; Saracila, M. Rosehip (Rosa canina L.) Meal as a Natural Antioxidant on Lipid and Protein Quality and Shelf-Life of Polyunsaturated Fatty Acids Enriched Eggs. Antioxidants 2022, 11, 1948. [Google Scholar] [CrossRef]
- Konca, Y.; Kaliber, M.; Uzkulekci, H.H.; Cimen, B.; Yalcin, H. The Effect of Rosehip (Rosa canina L.) Supplementation to Diet on the Performance, Egg and Meat Quality, Antioxidant Activity in Laying Quail. Sains Malays. 2021, 50, 3617–3629. [Google Scholar] [CrossRef]
- Ersoy, N.; Salman Özen, M. Some Physico-Chemical Characteristics in Fruits of Rose Hip (Rosa Spp.) Genotypes from Bolu Province in Western Part of Turkey. Agro-knowledge 2017, 17, 191. [Google Scholar] [CrossRef]
- Celik, F.; Kazankaya, A.; Ercisli, S. Fruit characteristics of some selected promising rose hip (Rosa spp.) genotypes from Van region of Turkey. Afr. J. Agric. Res. 2009, 4, 236–240. [Google Scholar]
- Ercisli, S. Chemical Composition of Fruits in Some Rose (Rosa spp.) Species. Food Chem. 2007, 104, 1379–1384. [Google Scholar] [CrossRef]
- Rovná, K.; Ivanišová, E.; Žiarovská, J.; Ferus, P.; Terentjeva, M.; Kowalczewski, P.Ł.; Kačániová, M. Characterization of Rosa canina Fruits Collected in Urban Areas of Slovakia. Genome Size, IPBS Profiles and Antioxidant and Antimicrobial Activities. Molecules 2020, 25, 1888. [Google Scholar] [CrossRef] [PubMed]
- Pećinar, I.; Krstić, D.; Caruso, G.; Popović-Djordjević, J.B. Rapid Characterization of Hypanthium and Seed in Wild and Cultivated Rosehip: Application of Raman Microscopy Combined with Multivariate Analysis. R. Soc. Open Sci. 2021, 8, 202064. [Google Scholar] [CrossRef] [PubMed]
- Popović-Djordjević, J.; Paunović, D.; Milić, A.; Krstić, Đ.; Siavash Moghaddam, S.; Roje, V. Multi-Elemental Analysis, Pattern Recognition Techniques of Wild and Cultivated Rosehips from Serbia, and Nutritional Aspect. Biol. Trace Elem. Res. 2021, 199, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Grdiša, M.; Šic Žlabur, J.; Varga, F.; Bosilj, P.; Skendrović-Babojelić, M.; Klepo, T.; Šatović, Z. Phytochemical Diversity of Rosa canina L. Populations in Croatia. Maced. Pharm. Bull. 2022, 68, 57–58. [Google Scholar] [CrossRef]
- Benković-Lačić, T.; Japundžić-Palenkić, B.; Mirosavljević, K.; Rakić, M.; Obradović, V.; Japundžić, M.; Benković, R. Morphological, Pomological, and Nutritional Value of Wild and Cultivated Rosehip (Rosa canina L.) Genotypes in Slavonia, Croatia. Acta Agrobot. 2022, 75, 1–19. [Google Scholar] [CrossRef]
- Rosu, C.M.; Manzu, C.; Olteanu, Z.; Oprica, L.; Oprea, A.; Ciornea, E.; Zamfirache, M.M. Several Fruit Characteristics of Rosa sp. Genotypes from the Northeastern Region of Romania. Not. Bot. Horti Agrobot. Cluj-Napoca 2011, 39, 203–208. [Google Scholar] [CrossRef]
- Hallmann, E. The Influence of Organic and Conventional Cultivation Systems on the Nutritional Value and Content of Bioactive Compounds in Selected Tomato Types. J. Sci. Food Agric. 2012, 92, 2840–2848. [Google Scholar] [CrossRef]
- Vaitkeviciene, N.; Kulaitiene, J.; Jariene, E.; Levickiene, D.; Danillcenko, H.; Srednicka-Tober, D.; Rembiałkowska, E.; Hallmann, E. Characterization of Bioactive Compounds in Colored Potato (Solanum tuberosum L.) Cultivars Grown with Conventional, Organic, and Biodynamic Methods. Sustainability 2020, 12, 2701. [Google Scholar] [CrossRef]
- Voća, S.; Žlabur, J.Š.; Dobričević, N.; Pliestić, S.; Galić, A.; Stanić, L.; Babojelić, M.S. The influence of cultivation on rosehip chemical fruit composition. In Proceedings of the 54th Croatian and 14th International Agronomy Symposium, Vodice, Croatia, 17–22 February 2019; pp. 567–570. [Google Scholar]
- Žlabur, J.Š.; Voća, S.; Dobričević, N.; Pliestić, S.; Galić, A.; Stanić, L.; Babojelić, M.S. Specialized metabolites of rosehip fruits (Rosa canina L.). In Proceedings of the 54th Croatian and 14th International Agronomy Symposium, Vodice, Croatia, 17–22 February 2019; pp. 558–561. [Google Scholar]
- Chae, S.H.; Lee, Y.S.; Kim, J.H.; Han, T.H.; Ku, K.M. Metabolite and Elastase Activity Changes in Beach Rose (Rosa rugosa) Fruit and Seeds at Various Stages of Ripeness. Plants 2021, 10, 1283. [Google Scholar] [CrossRef]
- Elmastaş, M.; Demir, A.; Genç, N.; Dölek, Ü.; Güneş, M. Changes in Flavonoid and Phenolic Acid Contents in Some Rosa Species during Ripening. Food Chem. 2017, 235, 154–159. [Google Scholar] [CrossRef]
- Uggla, M.; Gustavsson, K.E.; Olsson, M.E.; Nybom, H. Changes in Colour and Sugar Content in Rose Hips (Rosa dumalis L. and R. rubiginosa L.) during Ripening. J. Hortic. Sci. Biotechnol. 2005, 80, 204–208. [Google Scholar] [CrossRef]
- Adams, C.R. Principles of Horticulture; Routledge: New York, NY, USA, 2012. [Google Scholar] [CrossRef]
- Dogan, A.; Kazmkaya, A. Fruit Properties of Rose Hip Species Grown in Lake Van Basin (Eastern Anatolia Region). Asian J. Plant Sci. 2006, 5, 120–122. [Google Scholar]
- Stoenescu, A.M.; Cosmulescu, S. Variability of morphological characteristics in hawthorn (Crataegus monogyna L.) Fruit genotypes. South West. J. 2010, 11, 15–26. [Google Scholar]
- Kazankaya, A.; Yilmaz, H.; Yilmaz, M. Adilcevaz yöresinde doğal olarak yetişen kuşburnuların (Rosa spp.) seleksiyonu. Yuz. Yıl Univ. J. Agric. Sci. 2001, 11, 29–34. [Google Scholar]
- Eroğul, D.; Oğuz, H.İ. Determining the Physico-Chemical Characterstics of the Rosehip Genotypes Grown Naturally in Adiyaman Province. Erwerbs-Obstbau 2018, 60, 195–201. [Google Scholar] [CrossRef]
- Ammawath, W.; bin Che Man, Y.; Yusof, S.; b Abdul Rahman, R. Effects of Variety and Stage of Fruit Ripeness on the Physicochemical and Sensory Characteristics of Deep-fat-fried Banana Chips. J. Sci. Food Agric. 2001, 81, 1166–1171. [Google Scholar] [CrossRef]
- Bilgin, N.A.; Mısırlı, A.; Şen, F.; Türk, B.; Yağmur, B. Fruit Pomological, Phytochemical Characteristic and Mineral Content of Rosehip Genotypes. ETP Int. J. Food Eng. 2020, 6, 18–23. [Google Scholar] [CrossRef]
- Zhong, L.; Gustavsson, K.E.; Oredsson, S.; Głąb, B.; Yilmaz, J.L.; Olsson, M.E. Determination of Free and Esterified Carotenoid Composition in Rose Hip Fruit by HPLC-DAD-APCI+-MS. Food Chem. 2016, 210, 541–550. [Google Scholar] [CrossRef]
- Goulao, L.F.; Oliveira, C.M. Cell Wall Modifications during Fruit Ripening: When a Fruit Is Not the Fruit. Trends Food Sci. Technol. 2008, 19, 4–25. [Google Scholar] [CrossRef]
- ISO 10390:2005; Second Edition Soil Quality-Determination of PH. International Organization for Standardization: Geneva, Switzerland, 2005.
- Oreshkin, N.G. Extraction of mobile forms of phosphorus and potassium by the Egner-Riehm-Domingo method [Determination of available for plants phosphates in the soil]. Agrokhimiia 1980, 8, 135–138. [Google Scholar]
- ISO 11261:1995; Soil Quality—Determination of Total Nitrogen—Modified Kjeldahl Method. International Organization for Standardization: Geneva, Switzerland, 1995.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).