Monitoring Drought Stress in Common Bean Using Chlorophyll Fluorescence and Multispectral Imaging
Abstract
:1. Introduction
2. Results
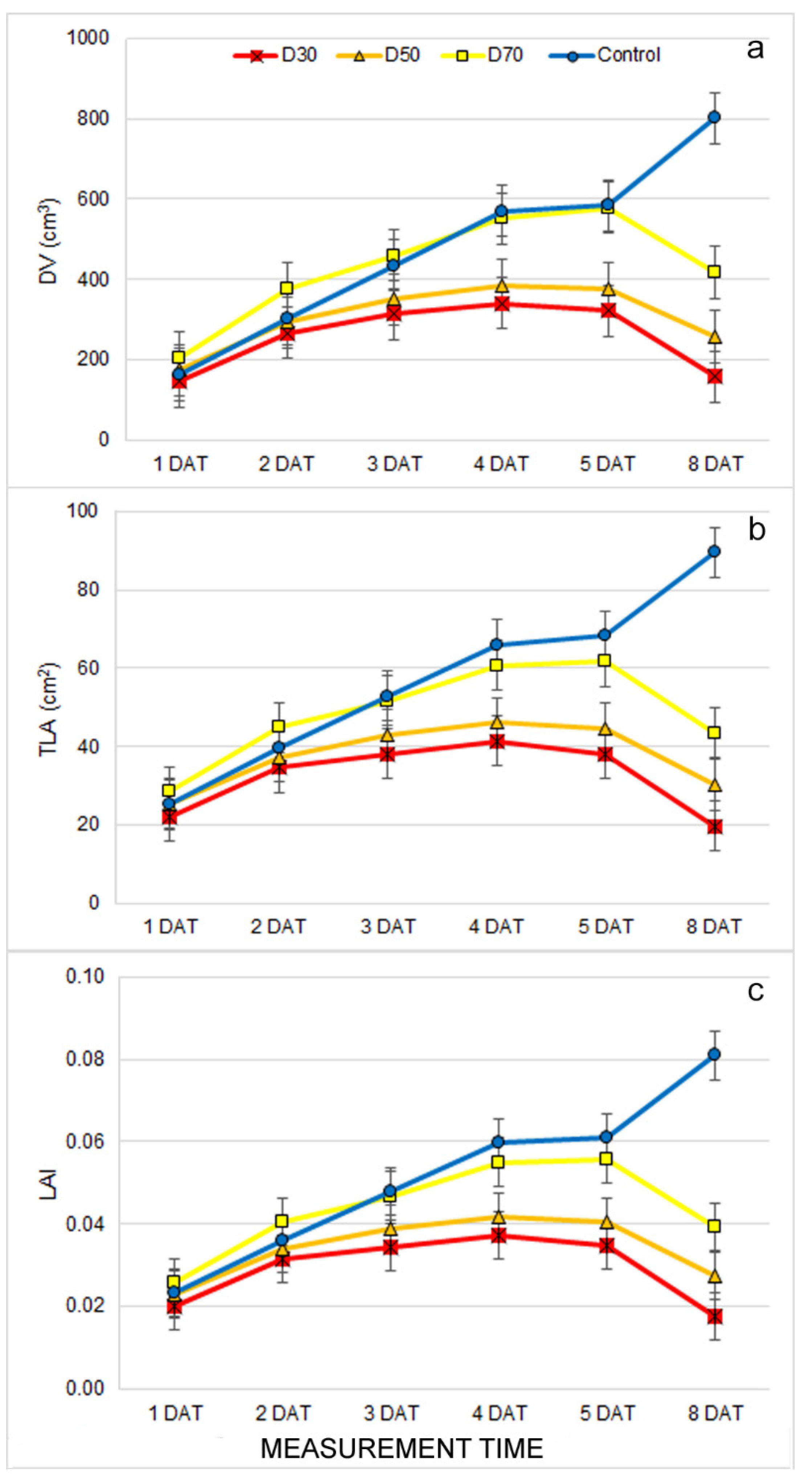
2.1. Effect of Drought Stress on Morphological Traits
2.2. Effect of Drought Stress on Multispectral traits
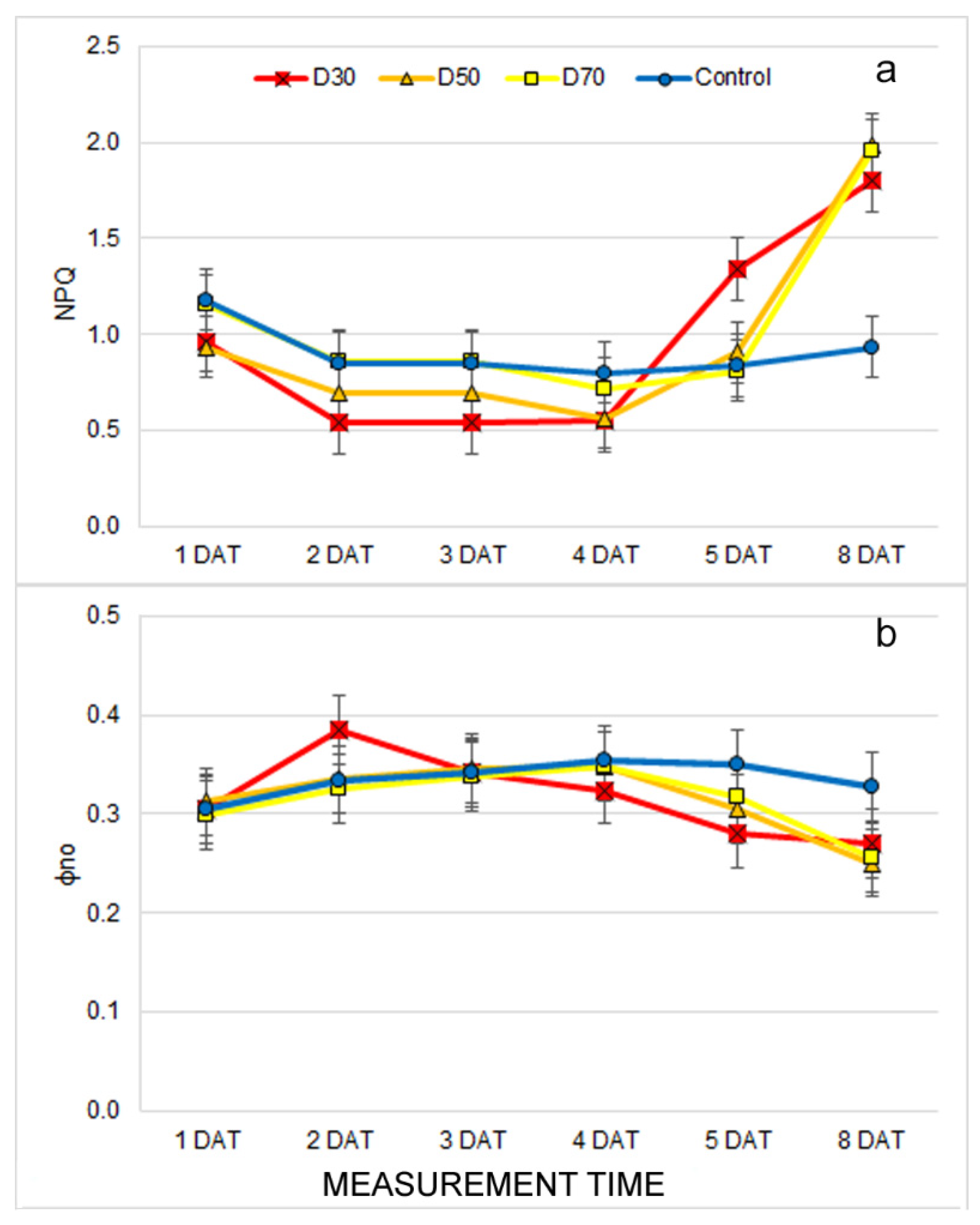
2.3. Effect of Drought Stress on Chlorophyll Fluorescence Traits
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Experimental Setup and Treatments
4.3. HTP Measurements
4.3.1. Chlorophyll Fluorescence Imaging
4.3.2. Multispectral Imaging
4.3.3. Multispectral 3D Scanning
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilhite, D.A. Drought Assessment, Management, and Planning: Theory and Case Studies, 2nd ed.; Springer: New York, NY, USA, 2012; pp. 1–289. [Google Scholar]
- Reyes, J.J.; Elias, E. Spatio-Temporal Variation of Crop Loss in the United States from 2001 to 2016. Environ. Res. Lett. 2019, 14, 074017. [Google Scholar] [CrossRef]
- Karthikeyan, L.; Chawla, I.; Mishra, A.K. A Review of Remote Sensing Applications in Agriculture for Food Security: Crop Growth and Yield, Irrigation, and Crop Losses. J. Hydrol. 2020, 586, 124905. [Google Scholar] [CrossRef]
- Nadeem, M.; Li, J.; Yahya, M.; Sher, A.; Ma, C.; Wang, X.; Qiu, L. Research Progress and Perspective on Drought Stress in Legumes: A Review. Int. J. Mol. Sci. 2019, 20, 2541. [Google Scholar] [CrossRef] [Green Version]
- Daryanto, S.; Wang, L.; Jacinthe, P.-A. Global Synthesis of Drought Effects on Cereal, Legume, Tuber and Root Crops Production: A Review. Agric. Water Manag. 2017, 179, 18–33. [Google Scholar] [CrossRef] [Green Version]
- Shao, H.-B.; Chu, L.-Y.; Jaleel, C.A.; Manivannan, P.; Panneerselvam, R.; Shao, M.-A. Understanding Water Deficit Stress-Induced Changes in the Basic Metabolism of Higher Plants—Biotechnologically and Sustainably Improving Agriculture and the Ecoenvironment in Arid Regions of the Globe. Crit. Rev. Biotechnol. 2009, 29, 131–151. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant Adaptation to Drought Stress. F1000Research 2016, 5, 1554. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant Drought Stress: Effects, Mechanisms and Management. Sustain. Agric. 2009, 29, 185–212. [Google Scholar]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M.; Al-Juburi, H.J.; Somasundaram, R.; Panneerselvam, R. Drought Stress in Plants: A Review on Morphological Characteristics and Pigments Composition. Int. J. Agric. Biol 2009, 11, 100–105. [Google Scholar]
- Anjum, S.A.; Xie, X.; Wang, L.; Saleem, M.F.; Man, C.; Lei, W. Morphological, Physiological and Biochemical Responses of Plants to Drought Stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar]
- Trenberth, K.E.; Dai, A.; Van Der Schrier, G.; Jones, P.D.; Barichivich, J.; Briffa, K.R.; Sheffield, J. Global Warming and Changes in Drought. Nat. Clim. Chang. 2014, 4, 17–22. [Google Scholar] [CrossRef]
- Zhao, T.; Dai, A. The Magnitude and Causes of Global Drought Changes in the Twenty-First Century under a Low–Moderate Emissions Scenario. J. Clim. 2015, 28, 4490–4512. [Google Scholar] [CrossRef]
- Wang, X.; Cai, X.; Xu, C.; Wang, Q.; Dai, S. Drought-Responsive Mechanisms in Plant Leaves Revealed by Proteomics. Int. J. Mol. Sci. 2016, 17, 1706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kar, R.K. Plant Responses to Water Stress: Role of Reactive Oxygen Species. Plant Signal. Behav. 2011, 6, 1741–1745. [Google Scholar] [CrossRef] [Green Version]
- Long, S.P.; Zhu, X.-G.; Naidu, S.L.; Ort, D.R. Can Improvement in Photosynthesis Increase Crop Yields? Plant Cell Environ. 2006, 29, 315–330. [Google Scholar] [CrossRef]
- White, J.W.; Ochoa, R.M.; Ibarra, F.P.; Singh, S.P. Inheritance of Seed Yield, Maturity and Seed Weight of Common Bean (Phaseolus vulgaris) under Semi-Arid Rainfed Conditions. J. Agric. Sci. 1994, 122, 265–273. [Google Scholar] [CrossRef]
- Broughton, W.J.; Hernández, G.; Blair, M.; Beebe, S.; Gepts, P.; Vanderleyden, J. Beans (Phaseolus Spp.)—Model Food Legumes. Plant Soil 2003, 252, 55–128. [Google Scholar] [CrossRef] [Green Version]
- Acosta-Gallegos, J.A.; Adams, M.W. Plant Traits and Yield Stability of Dry Bean (Phaseolus vulgaris) Cultivars under Drought Stress. J. Agric. Sci. 1991, 117, 213–219. [Google Scholar] [CrossRef]
- Hein, N.T.; Ciampitti, I.A.; Jagadish, S.V.K. Bottlenecks and Opportunities in Field-Based High-Throughput Phenotyping for Heat and Drought Stress. J. Exp. Bot. 2021, 72, 5102–5116. [Google Scholar] [CrossRef]
- Turner, N.C. Imposing and Maintaining Soil Water Deficits in Drought Studies in Pots. Plant Soil 2019, 439, 45–55. [Google Scholar] [CrossRef]
- Garg, A.; Bordoloi, S.; Ganesan, S.P.; Sekharan, S.; Sahoo, L. A Relook into Plant Wilting: Observational Evidence Based on Unsaturated Soil–Plant-Photosynthesis Interaction. Sci. Rep. 2020, 10, 22064. [Google Scholar] [CrossRef]
- Leonova, T.; Shumilina, J.; Kim, A.; Frolova, N.; Wessjohann, L.; Bilova, T.; Frolov, A. Agar-Based Polyethylene Glycol (PEG) Infusion Model for Pea (Pisum sativum L.)—Perspectives of Translation to Legume Crop Plants. Biol. Commun. 2022, 67, 3. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, H.; Chen, H.; Yang, X.; Yu, T.; Wang, Y.; Wang, Y.; Jiang, K.; Wang, Y.; Chen, Z.; et al. Proteomic Investigation of Molecular Mechanisms in Response to PEG-Induced Drought Stress in Soybean Roots. Plants 2022, 11, 1173. [Google Scholar] [CrossRef] [PubMed]
- Murchie, E.H.; Lawson, T. Chlorophyll Fluorescence Analysis: A Guide to Good Practice and Understanding Some New Applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bukhov, N.G.; Carpentier, R. Effects of Water Stress on the Photosynthetic Efficiency of Plants. In Chlorophyll a Fluorescence, 1st ed.; Govindjee, G.C.P., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 623–635. [Google Scholar]
- Kim, J.; Kim, K.-S.; Kim, Y.; Chung, Y.S. A Short Review: Comparisons of High-Throughput Phenotyping Methods for Detecting Drought Tolerance. Sci. Agric. 2020, 78. [Google Scholar] [CrossRef]
- Brestic, M.; Zivcak, M. PSII Fluorescence Techniques for Measurement of Drought and High Temperature Stress Signal in Crop Plants: Protocols and Applications. In Molecular Stress Physiology of Plants; Rout, G.R., Das, A.B., Eds.; Springer: Delhi, India, 2013; pp. 87–131. [Google Scholar]
- Bresson, J.; Vasseur, F.; Dauzat, M.; Koch, G.; Granier, C.; Vile, D. Quantifying Spatial Heterogeneity of Chlorophyll Fluorescence during Plant Growth and in Response to Water Stress. Plant Methods 2015, 11, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Humplík, J.F.; Lazár, D.; Husičková, A.; Spíchal, L. Automated Phenotyping of Plant Shoots Using Imaging Methods for Analysis of Plant Stress Responses–a Review. Plant Methods 2015, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Sun, D.; Cen, H.; Xu, H.; Weng, H.; Yuan, F.; He, Y. Phenotyping of Arabidopsis Drought Stress Response Using Kinetic Chlorophyll Fluorescence and Multicolor Fluorescence Imaging. Front. Plant Sci. 2018, 9, 603. [Google Scholar] [CrossRef]
- Huang, W.; Li, J.; Wang, Q.; Chen, L. Development of a Multispectral Imaging System for Online Detection of Bruises on Apples. J. Food Eng. 2015, 146, 62–71. [Google Scholar] [CrossRef]
- Wang, H.; Qian, X.; Zhang, L.; Xu, S.; Li, H.; Xia, X.; Dai, L.; Xu, L.; Yu, J.; Liu, X. A Method of High Throughput Monitoring Crop Physiology Using Chlorophyll Fluorescence and Multispectral Imaging. Front. Plant Sci. 2018, 9, 407. [Google Scholar] [CrossRef] [Green Version]
- Vadez, V.; Kholová, J.; Hummel, G.; Zhokhavets, U.; Gupta, S.K.; Hash, C.T. LeasyScan: A Novel Concept Combining 3D Imaging and Lysimetry for High-Throughput Phenotyping of Traits Controlling Plant Water Budget. J. Exp. Bot. 2015, 66, 5581–5593. [Google Scholar] [CrossRef] [Green Version]
- Paulus, S. Measuring Crops in 3D: Using Geometry for Plant Phenotyping. Plant Methods 2019, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ludovisi, R.; Tauro, F.; Salvati, R.; Khoury, S.; Mugnozza Scarascia, G.; Harfouche, A. UAV-Based Thermal Imaging for High-Throughput Field Phenotyping of Black Poplar Response to Drought. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Subhash, C.; Yan, J.; Song, C.; Zhao, J.; Li, J. Maize Leaf Temperature Responses to Drought: Thermal Imaging and Quantitative Trait Loci (QTL) Mapping. Environ. Exp. Bot. 2011, 71, 158–165. [Google Scholar] [CrossRef]
- Xiao, Q.; Bai, X.; Zhang, C.; He, Y. Advanced High-Throughput Plant Phenotyping Techniques for Genome-Wide Association Studies: A Review. J. Adv. Res. 2022, 35, 215–230. [Google Scholar] [CrossRef]
- Trapp, J.J.; Urrea, C.A.; Zhou, J.; Khot, L.R.; Sankaran, S.; Miklas, P.N. Selective Phenotyping Traits Related to Multiple Stress and Drought Response in Dry Bean. Crop Sci. 2016, 56, 1460–1472. [Google Scholar] [CrossRef] [Green Version]
- Saglam, A.; Saruhan, N.; Terzi, R.; Kadioglu, A. The Relations between Antioxidant Enzymes and Chlorophyll Fluorescence Parameters in Common Bean Cultivars Differing in Sensitivity to Drought Stress. Russ. J. Plant Physiol. 2011, 58, 60–68. [Google Scholar] [CrossRef]
- Dastneshan, S.; Bihamta, M.R.; Abbasi, A.; Sabokdast, M. The Effect of Different Levels of Drought Stress on Some Physiological Traits and Chlorophyll Fluorescence of Bean Genotypes (Phaseolus vulgaris L.). J. Crop Breed. 2019, 11, 92–104. [Google Scholar] [CrossRef]
- Sankaran, S.; Zhou, J.; Khot, L.R.; Trapp, J.J.; Mndolwa, E.; Miklas, P.N. High-Throughput Field Phenotyping in Dry Bean Using Small Unmanned Aerial Vehicle Based Multispectral Imagery. Comput. Electron. Agric. 2018, 151, 84–92. [Google Scholar] [CrossRef]
- Zhou, J.; Khot, L.R.; Boydston, R.A.; Miklas, P.N.; Porter, L. Low Altitude Remote Sensing Technologies for Crop Stress Monitoring: A Case Study on Spatial and Temporal Monitoring of Irrigated Pinto Bean. Precis. Agric. 2018, 19, 555–569. [Google Scholar] [CrossRef]
- Hong, M.; Bremer, D.J.; van der Merwe, D. Using Small Unmanned Aircraft Systems for Early Detection of Drought Stress in Turfgrass. Crop Sci. 2019, 59, 2829–2844. [Google Scholar] [CrossRef]
- Berger, B.; Parent, B.; Tester, M. High-Throughput Shoot Imaging to Study Drought Responses. J. Exp. Bot. 2010, 61, 3519–3528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salekdeh, G.H.; Reynolds, M.; Bennett, J.; Boyer, J. Conceptual Framework for Drought Phenotyping during Molecular Breeding. Trends Plant Sci. 2009, 14, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Lazarević, B.; Šatović, Z.; Nimac, A.; Vidak, M.; Gunjača, J.; Politeo, O.; Carović-Stanko, K. Application of Phenotyping Methods in Detection of Drought and Salinity Stress in Basil (Ocimum basilicum L.). Front. Plant Sci. 2021, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lipovac, A.; Bezdan, A.; Moravčević, D.; Djurović, N.; Ćosić, M.; Benka, P.; Stričević, R. Correlation between Ground Measurements and UAV Sensed Vegetation Indices for Yield Prediction of Common Bean Grown under Different Irrigation Treatments and Sowing Periods. Water 2022, 14, 3786. [Google Scholar] [CrossRef]
- McClung, C.R. Plant Circadian Rhythms. Plant Cell 2006, 18, 792–803. [Google Scholar] [CrossRef] [Green Version]
- Alves, D.S.; Nitsche, P.R.; Cirino, V.M.; dos Santos Scholz, M.B.; dos Santos Neto, J.; Caramori, P.H. Remote Sensing Allows to Estimate Water Stress and Yield losses of Bean Cultivars. IRRIGA 2021, 26, 1–12. [Google Scholar] [CrossRef]
- Bielczynski, L.W.; Łącki, M.K.; Hoefnagels, I.; Gambin, A.; Croce, R. Leaf and Plant Age Affects Photosynthetic Performance and Photoprotective Capacity. Plant Physiol. 2017, 175, 1634–1648. [Google Scholar] [CrossRef]
- Shin, Y.K.; Bhandari, S.R.; Jo, J.S.; Song, J.W.; Lee, J.G. Effect of Drought Stress on Chlorophyll Fluorescence Parameters, Phytochemical Contents, and Antioxidant Activities in Lettuce Seedlings. Horticulturae 2021, 7, 238. [Google Scholar] [CrossRef]
- Singh, G.M.; Goldberg, S.; Schaefer, D.; Zhang, F.; Sharma, S.; Mishra, V.K.; Xu, J. Biochemical, Gas Exchange, and Chlorophyll Fluorescence Analysis of Maize Genotypes under Drought Stress Reveals Important Insights into Their Interaction and Homeostasis. Photosynthetica 2022, 60, 376–388. [Google Scholar] [CrossRef]
- Vijayaraghavareddy, P.; Lekshmy, S.V.; Struik, P.C.; Makarla, U.; Yin, X.; Sreeman, S. Production and Scavenging of Reactive Oxygen Species Confer to Differential Sensitivity of Rice and Wheat to Drought Stress. Crop Environ. 2022, 1, 15–23. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The Relationship between the Quantum Yield of Photosynthetic Electron Transport and Quenching of Chlorophyll Fluorescence. Biochim. Biophys. Acta-Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Bilger, W.; Björkman, O. Role of the Xanthophyll Cycle in Photoprotection Elucidated by Measurements of Light-Induced Absorbance Changes, Fluorescence and Photosynthesis in Leaves of Hedera Canariensis. Photosynth. Res. 1990, 25, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, U.; Schliwa, U.; Bilger, W. Continuous Recording of Photochemical and Non-Photochemical Chlorophyll Fluorescence Quenching with a New Type of Modulation Fluorometer. Photosynth. Res. 1986, 10, 51–62. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New Fluorescence Parameters for the Determination of Q A Redox State and Excitation Energy Fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef]
- Genty, B.; Harbinson, J.; Cailly, A.L.; Rizza, F. Fate of Excitation at PS II in Leaves: The Non-Photochemical Side. In Third BBSRC Robert Hill Symposium on Photosynthesis; University of Sheffield, Department of Molecular Biology and Biotechnology: Sheffield, UK, 1996. [Google Scholar]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between Leaf Chlorophyll Content and Spectral Reflectance and Algorithms for Non-Destructive Chlorophyll Assessment in Higher Plant Leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Chivkunova, O.B. Optical Properties and Nondestructive Estimation of Anthocyanin Content in Plant Leaves. Photochem. Photobiol. 2001, 74, 38. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with ERTS; Goddard Space Flight Center 3d ERTS-1 Symp; NASA: Washington, DC, USA, 1973; Volume 1, Section A; pp. 309–317. [Google Scholar]
- Merzlyak, M.N.; Gitelson, A.A.; Chivkunova, O.B.; Rakitin, V.Y. Non-Destructive Optical Detection of Pigment Changes during Leaf Senescence and Fruit Ripening. Physiol. Plant. 1999, 106, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Littell, R.C.; Pendergast, J.; Natarajan, R. Modelling Covariance Structure in the Analysis of Repeated Measures Data. Stat. Med. 2000, 19, 1793–1819. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javornik, T.; Carović-Stanko, K.; Gunjača, J.; Vidak, M.; Lazarević, B. Monitoring Drought Stress in Common Bean Using Chlorophyll Fluorescence and Multispectral Imaging. Plants 2023, 12, 1386. https://doi.org/10.3390/plants12061386
Javornik T, Carović-Stanko K, Gunjača J, Vidak M, Lazarević B. Monitoring Drought Stress in Common Bean Using Chlorophyll Fluorescence and Multispectral Imaging. Plants. 2023; 12(6):1386. https://doi.org/10.3390/plants12061386
Chicago/Turabian StyleJavornik, Tomislav, Klaudija Carović-Stanko, Jerko Gunjača, Monika Vidak, and Boris Lazarević. 2023. "Monitoring Drought Stress in Common Bean Using Chlorophyll Fluorescence and Multispectral Imaging" Plants 12, no. 6: 1386. https://doi.org/10.3390/plants12061386
APA StyleJavornik, T., Carović-Stanko, K., Gunjača, J., Vidak, M., & Lazarević, B. (2023). Monitoring Drought Stress in Common Bean Using Chlorophyll Fluorescence and Multispectral Imaging. Plants, 12(6), 1386. https://doi.org/10.3390/plants12061386







