Seed Priming with Fullerol Improves Seed Germination, Seedling Growth and Antioxidant Enzyme System of Two Winter Wheat Cultivars under Drought Stress
Abstract
:1. Introduction
2. Results
2.1. Trial 1: Effect of Fullerol on Seed Germination in Two Winter Wheat Cultivars
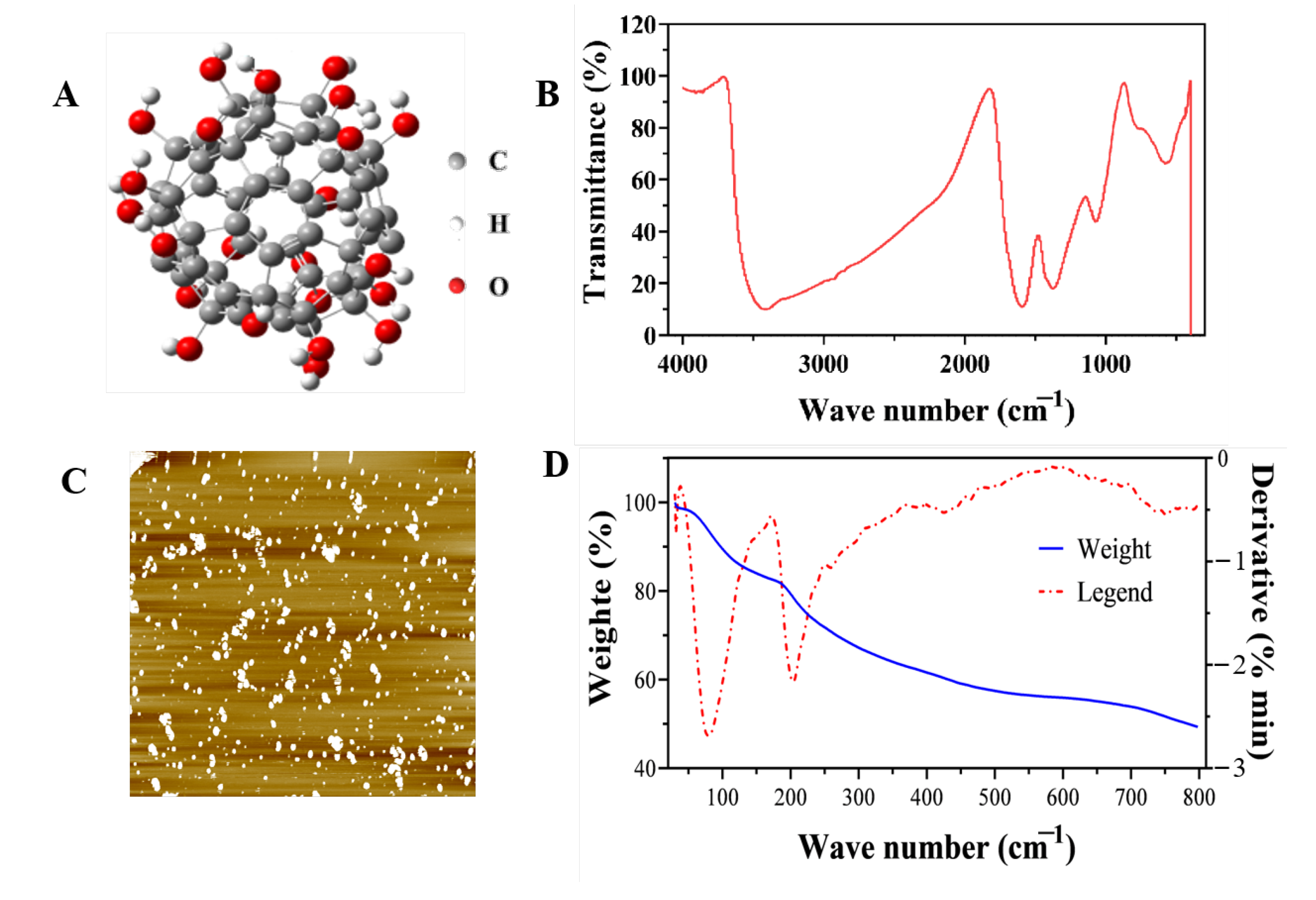
2.1.1. Characterization of Fullerol Nanoparticles
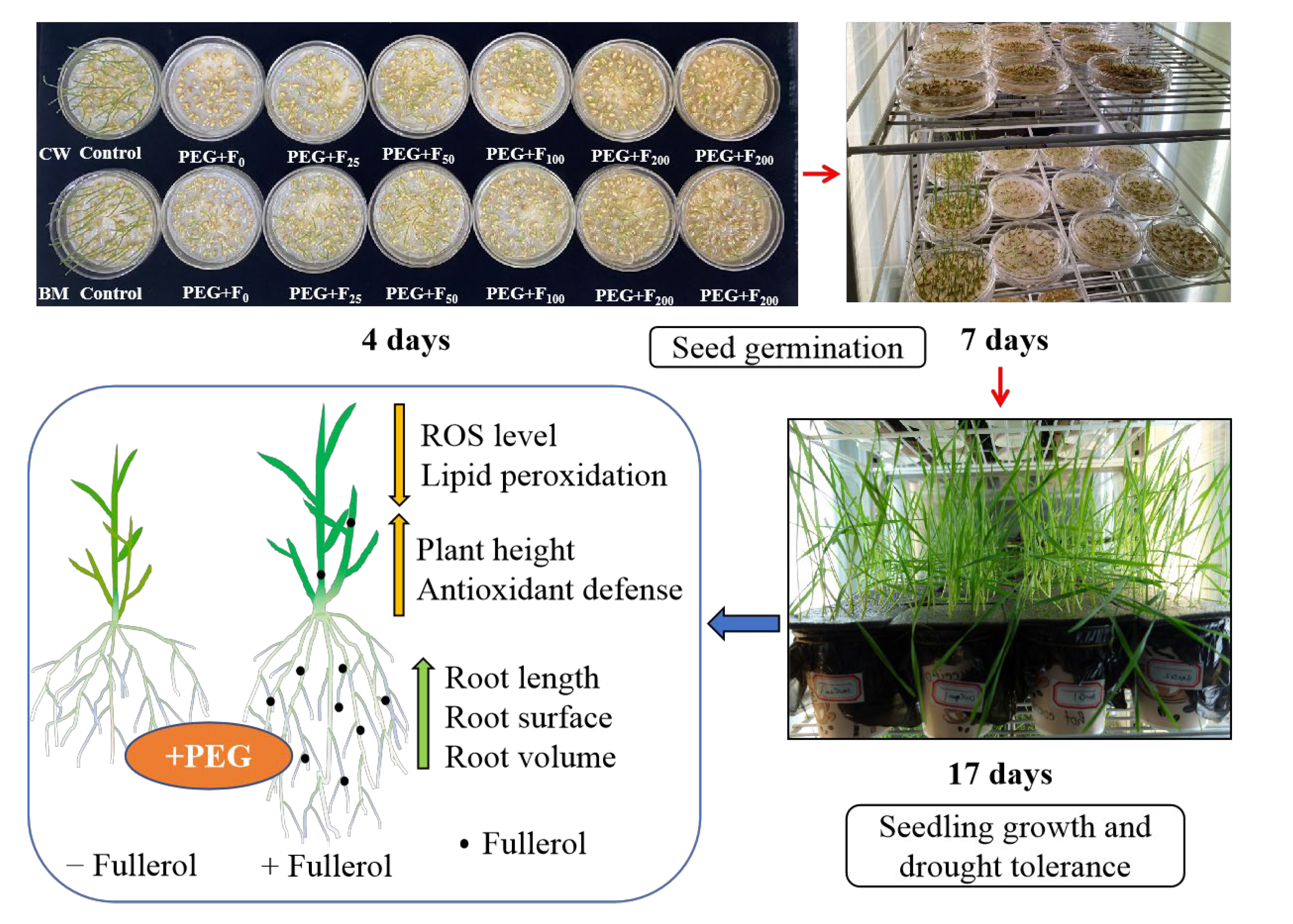
2.1.2. Seed Germination Parameters
2.2. Trial 2: Effect of Fullerol on Drought Tolerance in Two Winter Wheat Cultivars
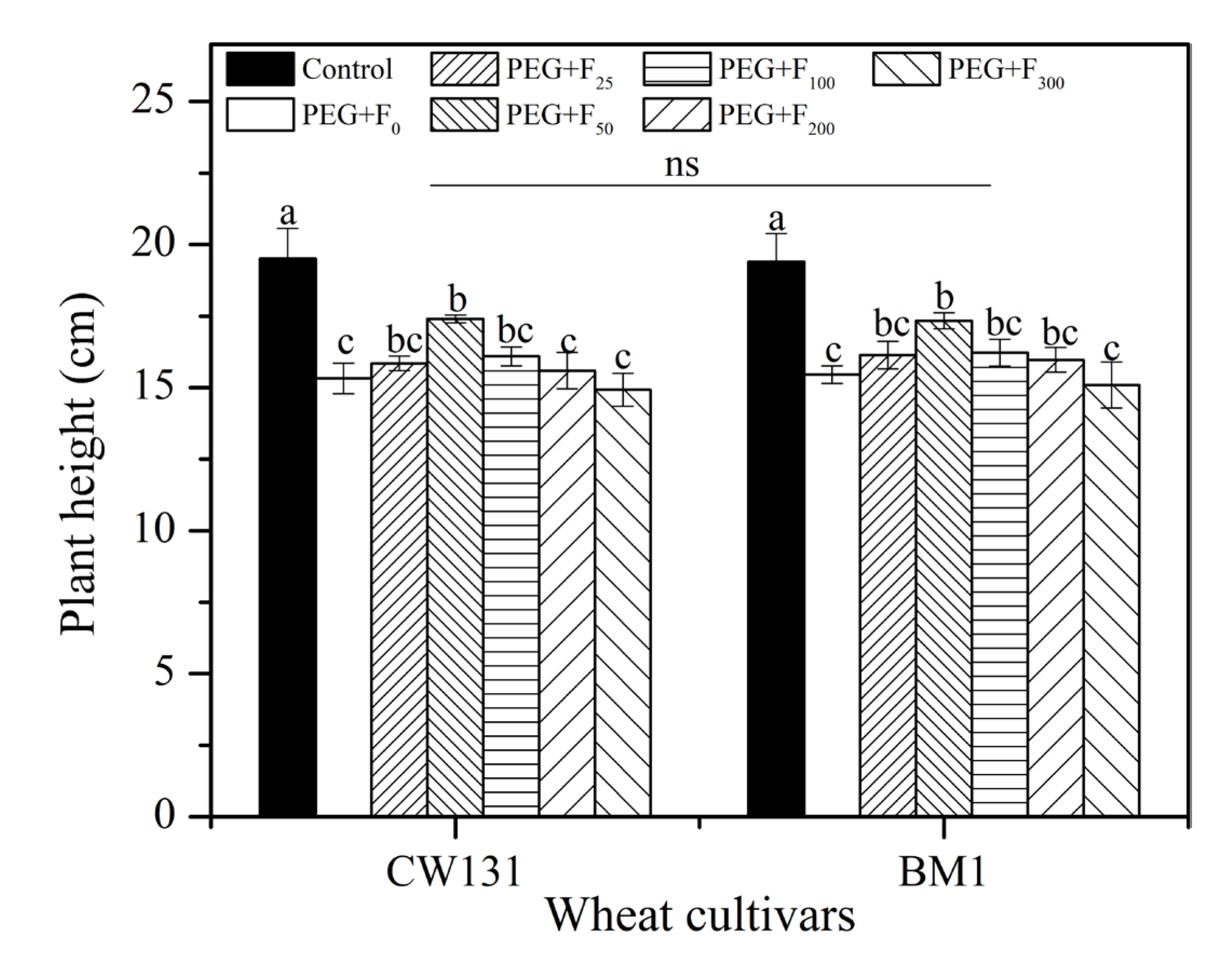
2.2.1. Plant Height and Root Growth Parameters
2.2.2. ROS Level and Membrane Lipid Peroxidation (MDA)
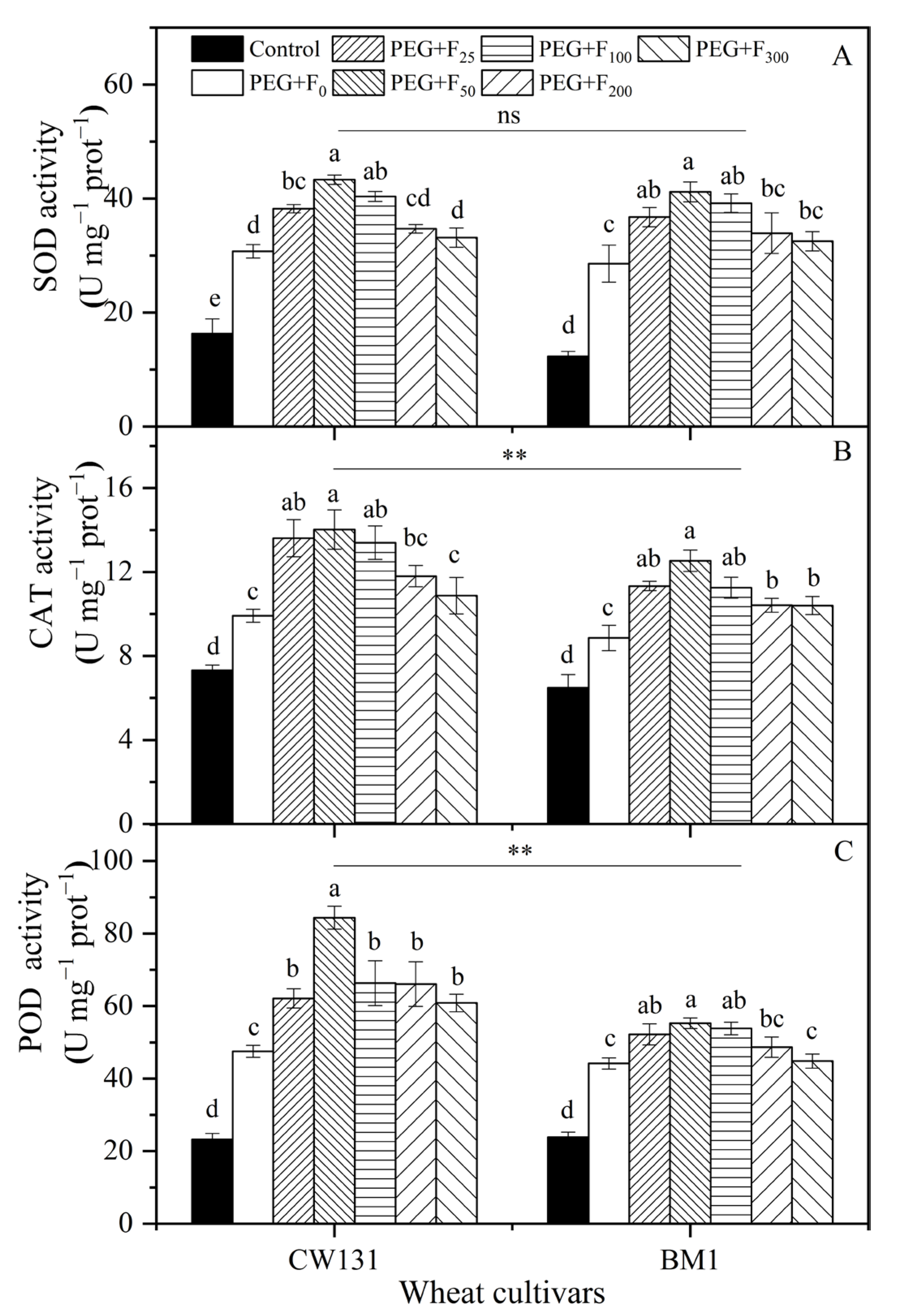
2.2.3. Antioxidant Enzyme Activities
3. Discussion
4. Materials and Methods
4.1. Trial 1: Effect of Fullerol on Seed Germination in Two Winter Wheat Cultivars
4.1.1. Characterization of Fullerol Nanoparticles
4.1.2. Drought Stress and Fullerol Treatment, and Seed Germination Measurements
4.2. Trial 2: Effect of Fullerol on Drought Tolerance in Two Winter Wheat Cultivars
4.2.1. Plant Materials and Growth Conditions
4.2.2. Measurement of Plant Height and Root Growth Parameters
4.2.3. Measurement of ROS Level, Malondialdehyde, and Antioxidant Enzyme Activities
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Long, S.P.; Ort, D.R. More than taking the heat: Crops and global change. Curr. Opin. Plant Biol. 2010, 13, 241–248. [Google Scholar] [CrossRef]
- Hughes, J.; Hepworth, C.; Dutton, C.; Dunn, J.A.; Hunt, L.; Stephens, J.; Waugh, R.; Cameron, D.D.; Gray, J.E. Reducing stomatal density in barley improves drought tolerance without impacting on yield. Plant Physiol. 2017, 174, 776–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. PPB 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Iwaniuk, P.; Borusiewicz, A.; Lozowicka, B. Fluazinam and its mixtures induce diversified changes of crucial biochemical and antioxidant profile in leafy vegetable. Sci. Hortic. 2022, 298, 110988. [Google Scholar] [CrossRef]
- Batool, A.; Akram, N.A.; Cheng, Z.G.; Lv, G.C.; Ashraf, M.; Afzal, M.; Xiong, J.L.; Wang, J.Y.; Xiong, Y.C. Physiological and biochemical responses of two spring wheat genotypes to non-hydraulic root-to-shoot signalling of partial and full root-zone drought stress. Plant Physiol. Biochem. PPB 2019, 139, 11–20. [Google Scholar] [CrossRef]
- Vithanage, M.; Seneviratne, M.; Ahmad, M.; Sarkar, B.; Ok, Y.S. Contrasting effects of engineered carbon nanotubes on plants: A review. Environ. Geochem. Health 2017, 39, 1421–1439. [Google Scholar] [CrossRef]
- Khan, M.N.; Mobin, M.; Abbas, Z.K.; AlMutairi, K.A.; Siddiqui, Z.H. Role of nanomaterials in plants under challenging environments. Plant Physiol. Biochem. PPB 2017, 110, 194–209. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.A.; Hashim, A.F.; Alghuthaymi, M.A.; Abd-Elsalam, K.A. Nano-carbon: Plant growth promotion and protection. In Nanobiotechnology Applications in Plant Protection; Springer: Berlin/Heidelberg, Germany, 2018; pp. 155–188. [Google Scholar]
- Kole, C.; Kole, P.; Randunu, K.M.; Choudhary, P.; Podila, R.; Pu, C.K.; Rao, A.M.; Marcus, R.K. Nanobiotechnology can boost crop production and quality: First evidence from increased plant biomass, fruit yield and phytomedicine content in bitter melon (Momordica Charantia). BMC Biotechnol. 2013, 13, 37. [Google Scholar] [CrossRef] [Green Version]
- Xiong, J.L.; Li, J.; Wang, H.C.; Zhang, C.L.; Naeem, M.S. Fullerol improves seed germination, biomass accumulation, photosynthesis and antioxidant system in brassica napus l. Under water stress. Plant Physiol. Biochem. PPB 2018, 129, 130–140. [Google Scholar] [CrossRef]
- Shafiq, F.; Iqbal, M.; Ali, M.; Ashraf, M.A. Seed pre-treatment with polyhydroxy fullerene nanoparticles confer salt tolerance in wheat through upregulation of h2o2 neutralizing enzymes and phosphorus uptake. J. Soil Sci. Plant Nutr. 2019, 19, 734–742. [Google Scholar] [CrossRef]
- Panova, G.G.; Ktitorova, I.N.; Skobeleva, O.V.; Sinjavina, N.G.; Charykov, N.A.; Semenov, K.N. Impact of polyhydroxy fullerene (fullerol or fullerenol) on growth and biophysical characteristics of barley seedlings in favourable and stressful conditions. Plant Growth Regul. 2016, 79, 309–317. [Google Scholar] [CrossRef]
- Borisev, M.; Borisev, I.; Zupunski, M.; Arsenov, D.; Pajevic, S.; Curcic, Z.; Vasin, J.; Djordjevic, A. Drought impact is alleviated in sugar beets (Beta vulgaris L.) by foliar application of fullerenol nanoparticles. PLoS ONE 2016, 11, e0166248. [Google Scholar] [CrossRef]
- Shafiq, F.; Iqbal, M.; Ashraf, M.A.; Ali, M. Foliar applied fullerol differentially improves salt tolerance in wheat through ion compartmentalization, osmotic adjustments and regulation of enzymatic antioxidants. Physiol. Mol. Biol. Plants Int. J. Funct. Plant Biol. 2020, 26, 475–487. [Google Scholar] [CrossRef]
- Bityutskii, N.P.; Yakkonen, K.L.; Lukina, K.A.; Semenov, K.N.; Panova, G.G. Fullerenol can ameliorate iron deficiency in cucumber grown hydroponically. J. Plant Growth Regul. 2021, 40, 1017–1031. [Google Scholar] [CrossRef]
- Rokitskaya, T.I.; Antonenko, Y.N. Fullerenol c60(oh)24 increases ion permeability of lipid membranes in a ph-dependent manner. Biochim. Biophys. Acta 2016, 1858, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, S.; Ling, T.; Xu, L.; Shen, W. Heme oxygenase/carbon monoxide system participates in regulating wheat seed germination under osmotic stress involving the nitric oxide pathway. J. Plant Physiol. 2010, 167, 1371–1379. [Google Scholar] [CrossRef]
- Liu, F.; Xiong, F.; Fan, Y.; Li, J.; Wang, H.; Xing, G.; Yan, F.; Tai, F.; He, R. Facile and scalable fabrication engineering of fullerenol nanoparticles by improved alkaline-oxidation approach and its antioxidant potential in maize. J. Nanopart. Res. 2016, 18, 338. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, H.; Ruan, L.; Chen, L.; Li, H.; Chang, X.-L.; Zhang, X.; Yang, S.-T. Bioaccumulation of 13c-fullerenol nanomaterials in wheat. Environ. Sci. Nano 2016, 3, 799–805. [Google Scholar] [CrossRef]
- Lin, S.; Reppert, J.; Hu, Q.; Hudson, J.S.; Reid, M.L.; Ratnikova, T.A.; Rao, A.M.; Luo, H.; Ke, P.C. Uptake, translocation, and transmission of carbon nanomaterials in rice plants. Small 2009, 5, 1128–1132. [Google Scholar] [CrossRef]
- Shukla, P.K.; Misra, P.; Kole, C. Uptake, Translocation, Accumulation, Transformation, and Generational Transmission of Nanoparticles in Plants in Plant Nanotechnol; Springer: Berlin/Heidelberg, Germany, 2016; pp. 183–218. [Google Scholar]
- Liang, C.; Xiao, H.; Hu, Z.; Zhang, X.; Hu, J. Uptake, transportation, and accumulation of c60 fullerene and heavy metal ions (cd, cu, and pb) in rice plants grown in an agricultural soil. Environ. Pollut. 2018, 235, 330–338. [Google Scholar] [CrossRef]
- Bityutskii, N.P.; Yakkonen, K.L.; Lukina, K.A.; Semenov, K.N. Fullerenol increases effectiveness of foliar iron fertilization in iron-deficient cucumber. PLoS ONE 2020, 15, e0232765. [Google Scholar] [CrossRef]
- Kong, H.; Palta, J.A.; Siddique, K.H.M.; Stefanova, K.; Xiong, Y.C.; Turner, N.C. Photosynthesis is reduced, and seeds fail to set and fill at similar soil water contents in grass pea (Lathyrus sativus L.) subjected to terminal drought. J. Agron. Crop Sci. 2015, 201, 241–252. [Google Scholar] [CrossRef]
- Shafiq, F.; Iqbal, M.; Ali, M.; Ashraf, M.A. Fullerenol regulates oxidative stress and tissue ionic homeostasis in spring wheat to improve net-primary productivity under salt-stress. Ecotoxicol. Environ. Saf. 2021, 211, 111901. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Folta, K.M.; Krishna, V.; Bai, W.; Indeglia, P.; Georgieva, A.; Nakamura, H.; Koopman, B.; Moudgil, B. Polyhydroxy fullerenes (fullerols or fullerenols): Beneficial effects on growth and lifespan in diverse biological models. PLoS ONE 2011, 6, e19976. [Google Scholar] [CrossRef] [Green Version]
- Miralles, P.; Johnson, E.; Church, T.L.; Harris, A.T. Multiwalled carbon nanotubes in alfalfa and wheat: Toxicology and uptake. J. R. Soc. Interface 2012, 9, 3514–3527. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; White, J.C.; Xing, B. Interactions between engineered nanomaterials and agricultural crops: Implications for food safety. J. Zhejiang Univ. Sci. A 2014, 15, 552–572. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.; Zhao, L.; Li, H.; Zhang, Q.; Tan, J.; Huang, M.; He, S.; Li, L. Single-walled carbon nanotubes selectively influence maize root tissue development accompanied by the change in the related gene expression. J. Hazard. Mater. 2013, 246–247, 110–118. [Google Scholar] [CrossRef]
- Martinez-Ballesta, M.C.; Zapata, L.; Chalbi, N.; Carvajal, M. Multiwalled carbon nanotubes enter broccoli cells enhancing growth and water uptake of plants exposed to salinity. J. Nanobiotechnol. 2016, 14, 42. [Google Scholar] [CrossRef] [Green Version]
- Cruz de Carvalho, M.H. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Gupta, A.K.; Kaur, N. Differential responses of antioxidative defence system to long-term field drought in wheat (Triticum aestivum L.) genotypes differing in drought tolerance. J. Agron. Crop Sci. 2012, 198, 185–195. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Li, F.-M.; Xiong, Y.-C.; Xu, B.-C. Soil-water threshold range of chemical signals and drought tolerance was mediated by ros homeostasis in winter wheat during progressive soil drying. J. Plant Growth Regul. 2008, 27, 309–319. [Google Scholar] [CrossRef]
- Du, Y.L.; Wang, Z.Y.; Fan, J.W.; Turner, N.C.; Wang, T.; Li, F.M. Β-aminobutyric acid increases abscisic acid accumulation and desiccation tolerance and decreases water use but fails to improve grain yield in two spring wheat cultivars under soil drying. J. Exp. Bot. 2012, 63, 4849–4860. [Google Scholar] [CrossRef]
- Husen, A.; Siddiqi, K.S. Carbon and fullerene nanomaterials in plant system. J. Nanobiotechnol. 2014, 12, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djordjevic, A.; Srdjenovic, B.; Seke, M.; Petrovic, D.; Injac, R.; Mrdjanovic, J. Review of synthesis and antioxidant potential of fullerenol nanoparticles. J. Nanomater. 2015, 2015, 567073. [Google Scholar] [CrossRef] [Green Version]
- Injac, R.; Prijatelj, M.; Strukelj, B. Fullerenol nanoparticles: Toxicity and antioxidant activity. Methods Mol. Biol. 2013, 1028, 75–100. [Google Scholar]
- Zhao, L.; Zhang, H.; Wang, J.; Tian, L.; Li, F.; Liu, S.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L.; White, J.C.; Huang, Y.; et al. C60 fullerols enhance copper toxicity and alter the leaf metabolite and protein profile in cucumber. Environ. Sci. Technol. 2019, 53, 2171–2180. [Google Scholar] [CrossRef]
- Du, Y.-L.; Wang, Z.-Y.; Fan, J.-W.; Turner, N.C.; He, J.; Wang, T.; Li, F.-M. Exogenous abscisic acid reduces water loss and improves antioxidant defence, desiccation tolerance and transpiration efficiency in two spring wheat cultivars subjected to a soil water deficit. Funct. Plant Biol. 2013, 40, 494–506. [Google Scholar] [CrossRef]
- Kong, H.Y.; Zhang, Z.; Qin, J.; Akram, N.A. Interactive effects of abscisic acid (aba) and drought stress of the physiological responses of winter wheat (Triticum aestivum L.). Pak. J. Bot. 2021, 53, 1545–1551. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Cavalcanti, F.R.; Lima, J.P.; Ferreira-Silva, S.L.; Viegas, R.A.; Silveira, J.A. Roots and leaves display contrasting oxidative response during salt stress and recovery in cowpea. J. Plant Physiol. 2007, 164, 591–600. [Google Scholar] [CrossRef]
- Khodakovskaya, M.V.; Silvaa, a.d.; Nedosekin, D.A.; Dervishi, E.; Biris, A.S.; Galanzha, E.I.; Zharov, V.P. Complex genetic, photothermal, and photoacoustic analysis of nanoparticle-plant interactions. Proc. Natl. Acad. Sci. USA 2011, 108, 1028–1033. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, A.; Majumdar, S.; Servin, A.D.; Pagano, L.; Dhankher, O.P.; White, J.C. Carbon nanomaterials in agriculture: A critical review. Front. Plant Sci. 2016, 7, 172. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, A.; Rao, D.P.P. Enhancement of seed germination and plant growth of wheat, maize, peanut and garlic using multiwalled carbon nanotubes. Eur. Chem. Bull. 2014, 3, 502–504. [Google Scholar]
- Xi, D.M.; Liu, W.S.; Yang, G.D.; Wu, C.A.; Zheng, C.C. Seed-specific overexpression of antioxidant genes in arabidopsis enhances oxidative stress tolerance during germination and early seedling growth. Plant Biotechnol. J. 2010, 8, 796–806. [Google Scholar] [CrossRef]
- Piepho, H.P.; Edmondson, R.N. A tutorial on the statistical analysis of factorial experiments with qualitative and quantitative treatment factor levels. J. Agron. Crop Sci. 2018, 204, 429–455. [Google Scholar] [CrossRef] [Green Version]

| Cultivar | Treatment | Final Germination Percentage (%) | Germination Index | Radical Plus Plumule Length (cm) | Seed Vigor Index |
|---|---|---|---|---|---|
| CW131 | Control | 98.7 ± 0.7 a | 44.0 ± 1.4 a | 15.7 ± 0.1 a | 1545.1 ± 13.0 a |
| PEG + F0 | 87.3 ± 3.7 c | 20.3 ± 1.5 d | 5.3 ± 0.1 d | 460.5 ± 19.9 e | |
| PEG + F25 | 94.7 ± 1.3 ab | 25.1 ± 0.6 bc | 6.3 ± 0.1 c | 599.4 ± 9.0 c | |
| PEG + F50 | 99.3 ± 0.7 a | 27.7 ± 1.8 b | 7.1 ± 0.1 b | 710.0 ± 9.9 b | |
| PEG + F100 | 96.0 ± 0.0 ab | 23.3 ± 0.1 c | 6.2 ± 0.2 c | 595.2 ± 15.5 c | |
| PEG + F200 | 96.7 ± 0.7 a | 23.4 ± 0.2 c | 5.5 ± 0.2 d | 536.3 ± 18.3 d | |
| PEG + F300 | 91.3 ± 0.7 bc | 21.3 ± 0.3 d | 5.5 ± 0.3 d | 506.9 ± 26.7 de | |
| BM1 | Control | 99.3 ± 0.7 a | 40.7 ± 0.8 a | 13.2 ± 0.1 a | 1313.1 ± 6.1 a |
| PEG + F0 | 82.7 ± 3.5 c | 19.3 ± 1.4 d | 3.6 ± 0.2 d | 301.4 ± 25.0 d | |
| PEG + F25 | 89.3 ± 1.8 b | 21.6 ± 0.3 bc | 4.8 ± 0.5 c | 427.0 ± 7.4 c | |
| PEG + F50 | 90.7 ± 2.9 b | 23.3 ± 0.5 b | 6.8 ± 0.3 b | 620.5 ± 46.3 b | |
| PEG + F100 | 90.0 ± 2.0 b | 21.3 ± 0.6 bc | 5.2 ± 0.4 c | 468.1 ± 37.1 c | |
| PEG + F200 | 87.3 ± 1.3 bc | 20.5 ± 0.3 cd | 5.1 ± 0.2 c | 447.7 ± 15.2 c | |
| PEG + F300 | 87.0 ± 0.6 bc | 20.1 ± 0.4 cd | 3.9 ± 0.3 d | 339.0 ± 24.1 d | |
| Cultivar (C) | ** | ** | ** | ** | |
| Fullerol (F) | ** | ** | ** | ** | |
| C × F | ns | ns | * | ns |
| Cultivar | Treatment | Root Length (cm) | Root Surface Area (cm2) | Root Volume (cm3) | Root Mean Diameter (mm) |
|---|---|---|---|---|---|
| CW131 | Control | 53.9 ± 2.3 a | 6.4 ± 0.1 a | 0.055 ± 0.001 a | 0.37 ± 0.02 ab |
| PEG + F0 | 40.4 ± 1.3 cd | 4.1 ± 0.1 d | 0.036 ± 0.001 c | 0.37 ± 0.01 ab | |
| PEG + F25 | 44.8 ± 1.7 bc | 4.6 ± 0.1 cd | 0.036 ± 0.002 c | 0.37 ± 0.02 ab | |
| PEG + F50 | 47.8 ± 0.1 b | 5.2 ± 0.2 b | 0.046 ± 0.001 b | 0.39 ± 0.01 a | |
| PEG + F100 | 44.4 ± 1.8 bc | 4.8 ± 0.4 bc | 0.044 ± 0.004 b | 0.37 ± 0.01 ab | |
| PEG + F200 | 42.3 ± 1.4 cd | 4.6 ± 0.1 cd | 0.041 ± 0.005 bc | 0.36 ± 0.01 ab | |
| PEG + F300 | 39.6 ± 0.5 d | 4.4 ± 0.3 cd | 0.034 ± 0.002 c | 0.35 ± 0.05 b | |
| BM1 | Control | 50.8 ± 0.6 a | 6.1 ± 0.4 a | 0.052 ± 0.005 a | 0.34 ± 0.03 a |
| PEG + F0 | 38.5 ± 0.8 c | 4.0 ± 0.1 cd | 0.034 ± 0.006 c | 0.33 ± 0.01 a | |
| PEG + F25 | 39.3 ± 1.3 c | 4.5 ± 0.2 bc | 0.038 ± 0.006 bc | 0.36 ± 0.02 a | |
| PEG + F50 | 44.9 ± 1.0 b | 4.9 ± 0.2 b | 0.044 ± 0.001 ab | 0.37 ± 0.01 a | |
| PEG + F100 | 40.7 ± 0.6 c | 4.7 ± 0.1 bc | 0.036 ± 0.002 bc | 0.35 ± 0.02 a | |
| PEG + F200 | 38.2 ± 1.7 c | 4.2 ± 0.3 bcd | 0.033 ± 0.003 c | 0.34 ± 0.02 a | |
| PEG + F300 | 37.5 ± 1.5 c | 3.8 ± 0.3 d | 0.031 ± 0.002 c | 0.33 ± 0.02 a | |
| Cultivar (C) | ** | * | * | * | |
| Fullerol (F) | ** | ** | ** | ns | |
| C × F | ns | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, H.; Meng, X.; Akram, N.A.; Zhu, F.; Hu, J.; Zhang, Z. Seed Priming with Fullerol Improves Seed Germination, Seedling Growth and Antioxidant Enzyme System of Two Winter Wheat Cultivars under Drought Stress. Plants 2023, 12, 1417. https://doi.org/10.3390/plants12061417
Kong H, Meng X, Akram NA, Zhu F, Hu J, Zhang Z. Seed Priming with Fullerol Improves Seed Germination, Seedling Growth and Antioxidant Enzyme System of Two Winter Wheat Cultivars under Drought Stress. Plants. 2023; 12(6):1417. https://doi.org/10.3390/plants12061417
Chicago/Turabian StyleKong, Haiyan, Xiangzhan Meng, Nudrat Aisha Akram, Fengru Zhu, Jiaxing Hu, and Zhen Zhang. 2023. "Seed Priming with Fullerol Improves Seed Germination, Seedling Growth and Antioxidant Enzyme System of Two Winter Wheat Cultivars under Drought Stress" Plants 12, no. 6: 1417. https://doi.org/10.3390/plants12061417
APA StyleKong, H., Meng, X., Akram, N. A., Zhu, F., Hu, J., & Zhang, Z. (2023). Seed Priming with Fullerol Improves Seed Germination, Seedling Growth and Antioxidant Enzyme System of Two Winter Wheat Cultivars under Drought Stress. Plants, 12(6), 1417. https://doi.org/10.3390/plants12061417






