Next-Generation Sequencing (NGS) Identified Species-Specific SSR and SNP Markers, Allow the Unequivocal Identification of Strawberry Tree (Arbutus unedo L.) Germplasm Accessions and Contribute to Assess Their Genetic Relationships
Abstract
1. Introduction
2. Results
2.1. Establishment of the First Genome Assembly (Scafold) of Arbutus unedo L. by Next-Generation Sequencing (NGS)
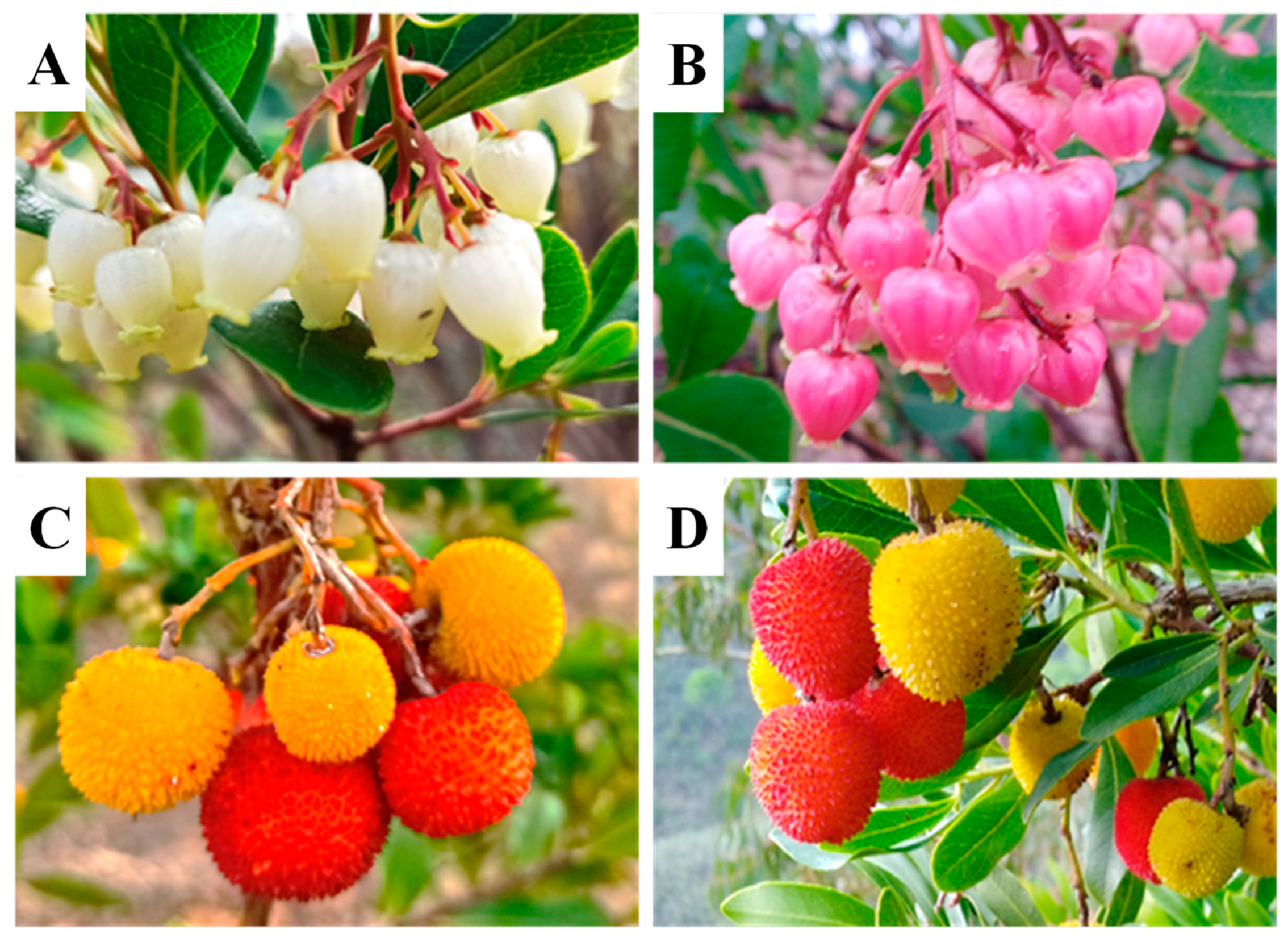
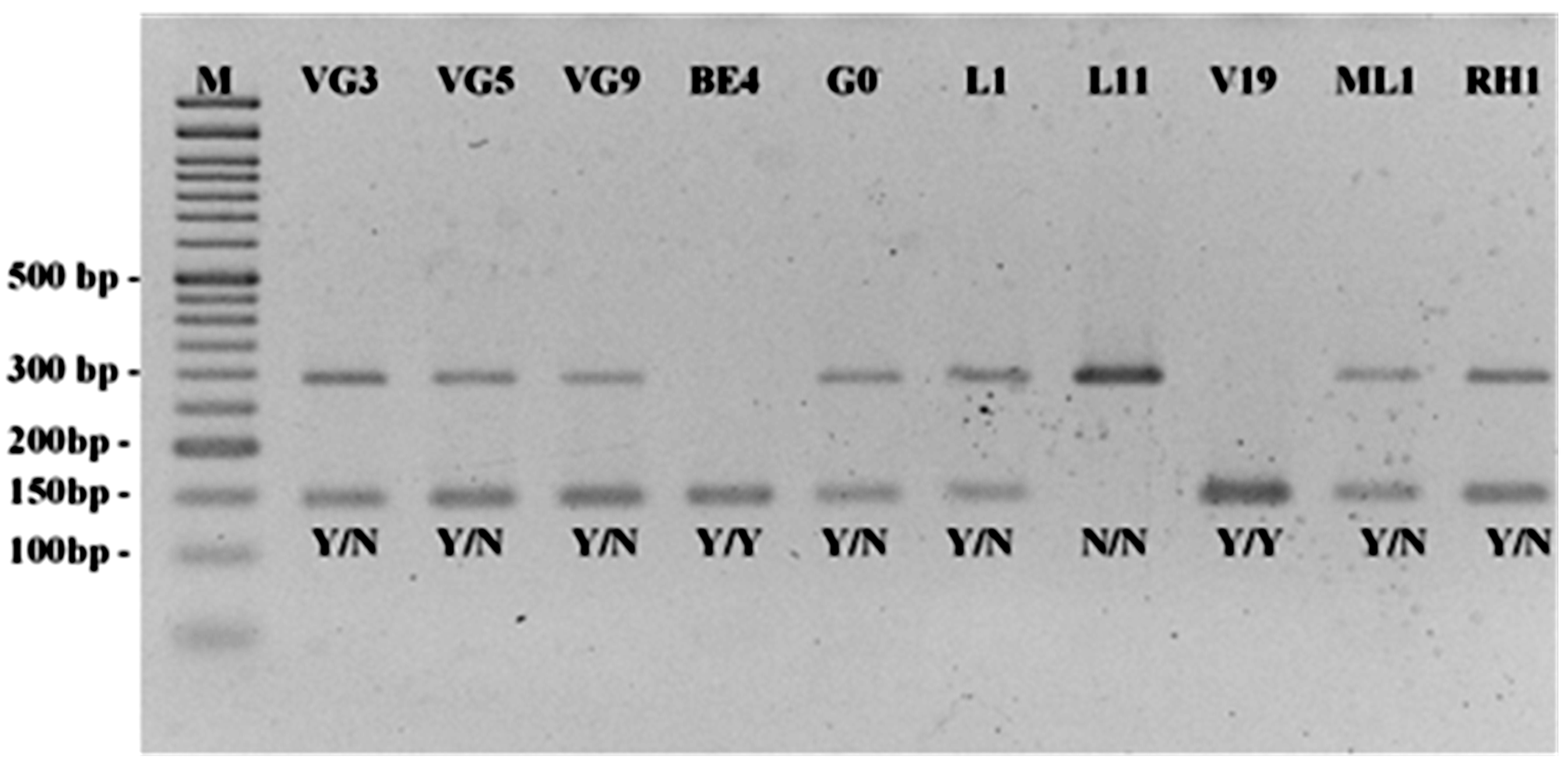
2.2. Unequivocal Identification of a Selected Set of 50 Germplasm Accessions of Arbutus unedo L. by Species-Specific SSR and SNP-CAPS Markers
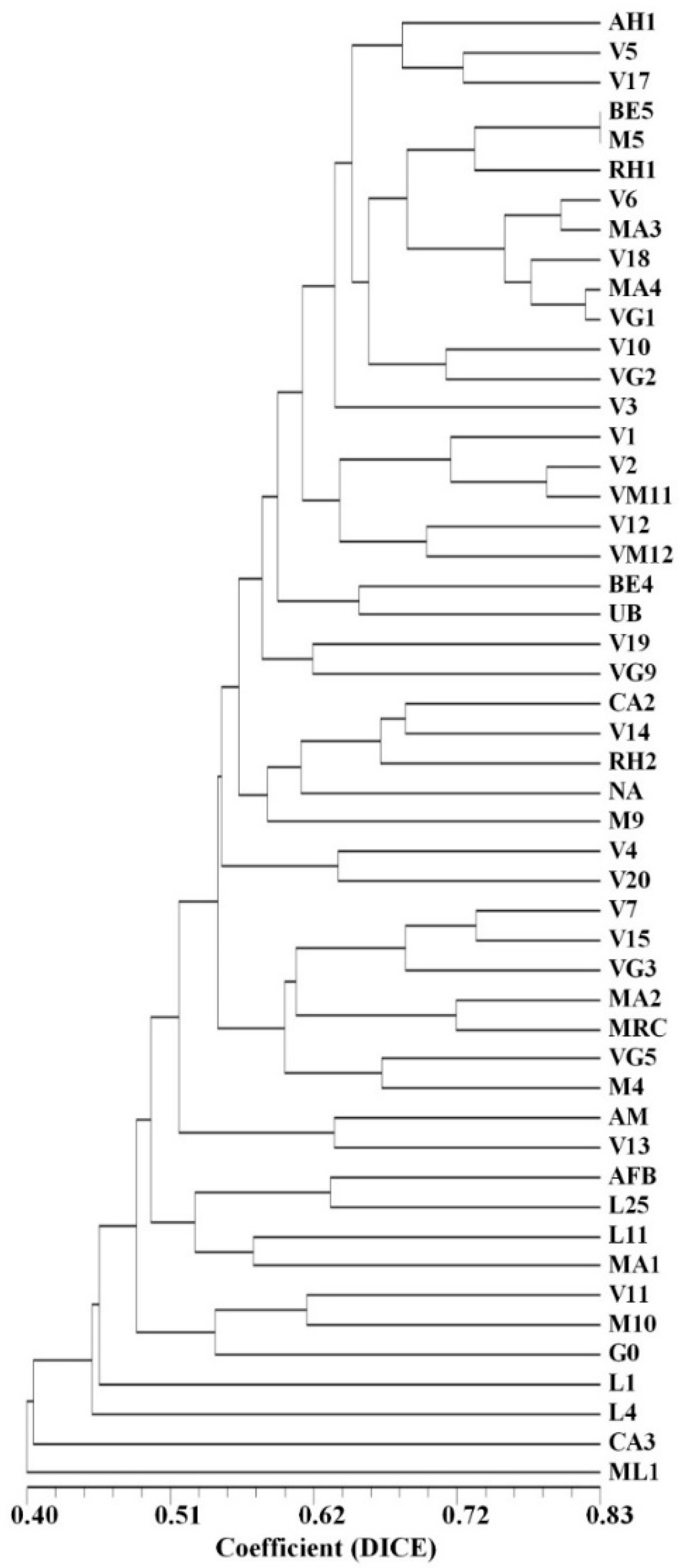
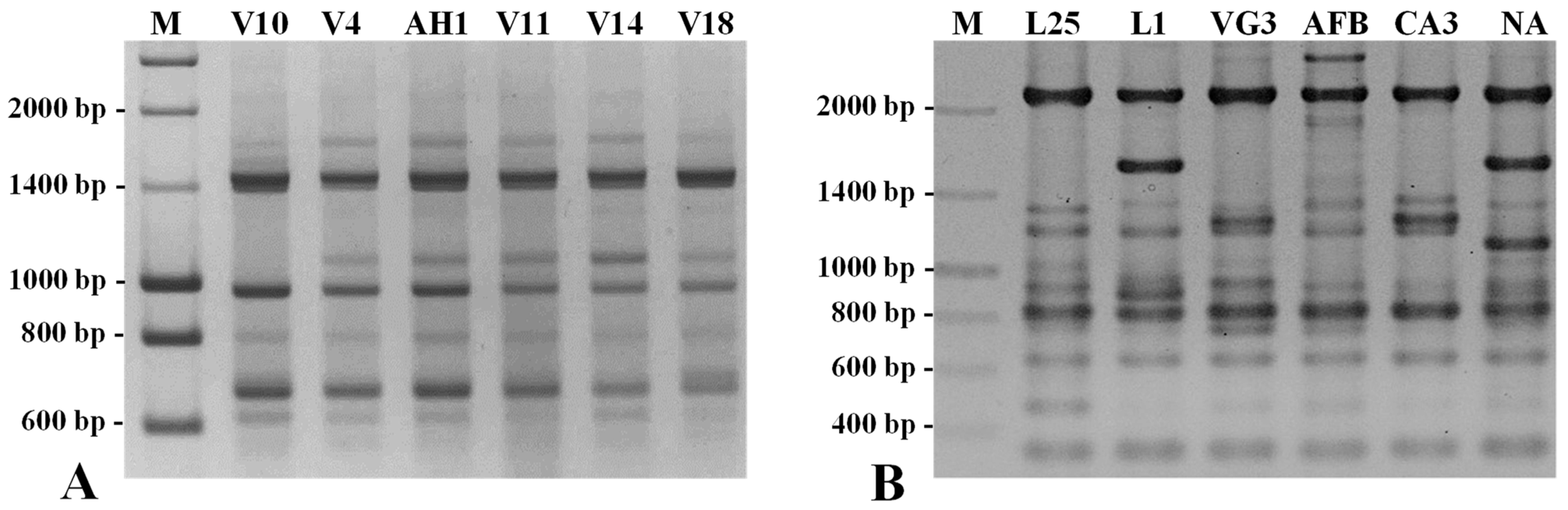
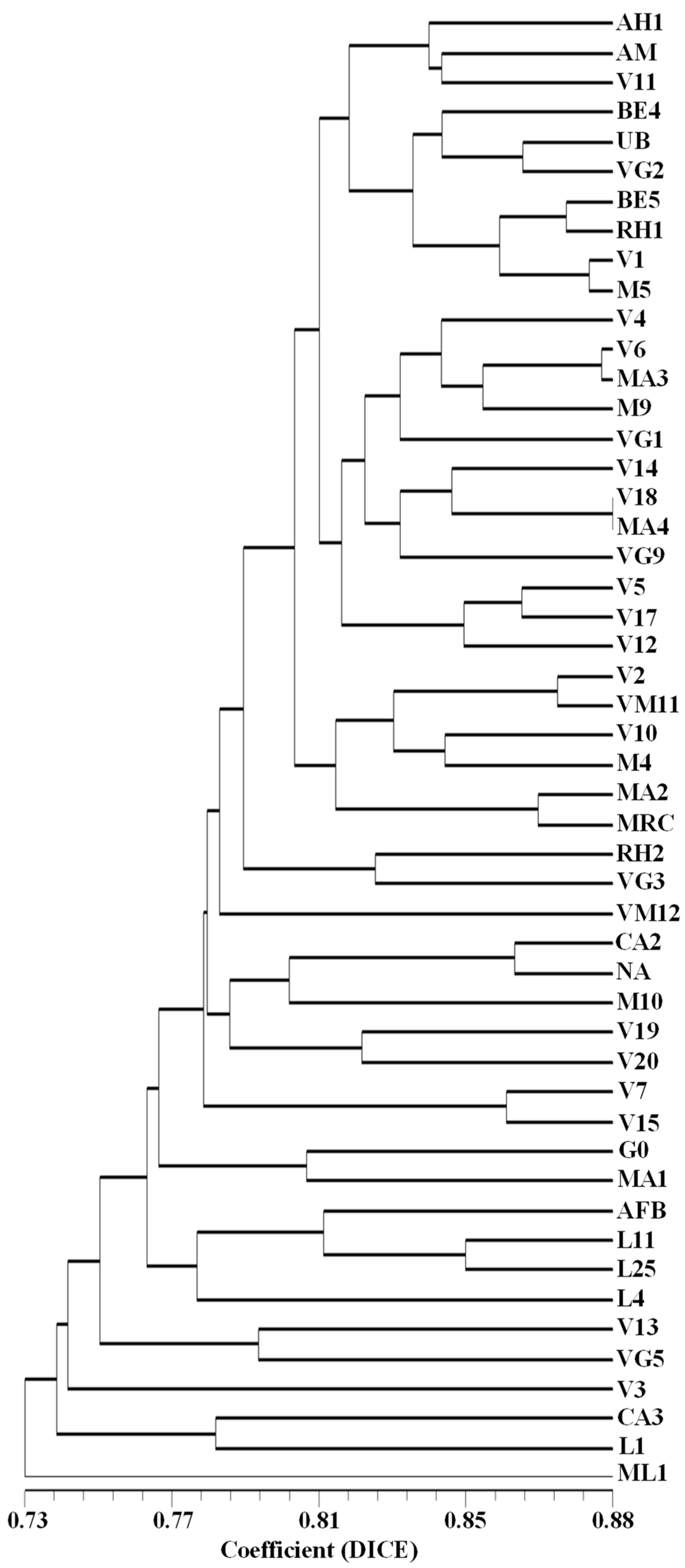
2.3. Assessment of the Genetic Diversity and Genetic Relationships among 50 Arbutus unedo L. Germplasm Accessions by SSR, SNP-CAPS, RAPD, and ISSR Markers
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. DNA Extraction
4.3. DNA Extraction for Next-Generation Sequencing (NGS)
4.4. Quality Evaluation and Quantification of the Extracted DNA
4.5. NGS Sequencing
4.6. Primer Design and Synthesis
4.7. Single-Sequence Repeats (SSR) Markers Analysis
4.8. Single Nucleotide Polymorphisms (SNP) Markers Analysis
4.9. Random Amplified Polymorphic DNA (RAPD) and Inter-Single Sequence Repeated (ISSR) Markers Analyses
4.10. Additional Data Analysis
5. Conclusions
- (a)
- Three major contributions for further genomic studies by the strawberry tree (Arbutus unedo L.) research community: (i) the first genome assembly (scaffold) for this fruit tree species; (ii) a set of 500 additional SSR loci; and (iii) a set of 500 SNP loci.
- (b)
- The unequivocal molecular (SSR and SNP-CAPS markers) identification of a set of 50 (A. unedo) germplasm accessions selected for a plant breeding program.
- (c)
- The assessment of the genetic variability and genetic relationships among the same selected set of germplasm accessions using SSR, SNP-CAPS, RAPD, and ISSR markers.
- (d)
- The development of a fast, easy to perform and affordable protocol, based on SSR and SNP-CAPS markers, that will be used for identification of all accessions registered in the Corte Velada germplasm collection and is available for plant identification or other purposes by the strawberry tree research community.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sealy, J.R.; Webb, D.A. Arbutus unedo L. J. Ecol. 1950, 38, 223–236. [Google Scholar] [CrossRef]
- Soufleros, E.; Mygdalia, A.S.; Natskou, P. Production process and characterization of the traditional Greek fruit distillate “Koumaro” by aromatic and mineral composition. J. Food Compos. Anal. 2005, 18, 699–716. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Kucukislamoglu, M.; Reunanen, M. Sugar, Non-volatile and phenolic acids composition of strawberry tree (Arbutus unedo L. var. ellipsoidea) fruits. J. Food Compos. Anal. 2000, 13, 171–177. [Google Scholar] [CrossRef]
- Scarano, P.; Guida, R.; Zuzolo, D.; Tartaglia, M.; Prigioniero, A.; Postiglione, A.; Pinto, G.; Illiano, A.; Amoresano, A.; Schicchi, R.; et al. An Endemic Plant of the Mediterranean Area: Phytochemical Characterization of Strawberry Tree (Arbutus unedo L.) Fruits Extracts at Different Ripening Stages. Front. Nutr. 2022, 9, 915994. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, D.F.; Salvador, A.C.; Santos, S.A.; Vilela, C.; Freire, C.S.; Silvestre, A.J.; Rocha, S.M. Bioactive phytochemicals from wild Arbutus unedo L. berries from different locations in Portugal: Quantification of lipophilic components. Int. J. Mol. Sci. 2015, 16, 14194–14209. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.; Batista, T.; Pinto, G.; Canhoto, J. Seasonal variation of phenolic compounds in Strawberry tree (Arbutus unedo L.) leaves and inhibitory potential on Phytophthora cinnamomi. Trees 2021, 35, 1571–1586. [Google Scholar] [CrossRef]
- Ait Lhaj, Z.; Bchitou, R.; Gaboun, F.; Abdelwahd, R.; Benabdelouahab, T.; Kabbour, M.R.; Pare, P.; Diria, G.; Bakhy, K. Moroccan strawberry tree (Arbutus unedo L.) fruits: Nutritional value and mineral composition. Foods 2021, 10, 2263. [Google Scholar] [CrossRef]
- Mrabti, H.N.; Bouyahya, A.; Ed-Dra, A.; Kachmar, M.R.; Mrabti, N.N.; Benali, T.; Shariati, M.A.; Ouahbi, A.; Doudach, L.; Faouzi, M.E. Polyphenolic profile and biological properties of Arbutus unedo root extracts. Eur. J. Integr. Med. 2021, 42, 101266. [Google Scholar] [CrossRef]
- Caldeira, I.; Gomes, F.; Mira, H.; Botelho, G. Distillates composition obtained of fermented Arbutus unedo L. fruits from different seedlings and clonal plants. Ann. Agric. Sci. 2019, 64, 21–28. [Google Scholar] [CrossRef]
- Morgado, S.; Morgado, M.; Plácido, A.I.; Roque, F.; Duarte, A.P. Arbutus unedo L.: From traditional medicine to potential uses in modern pharmacotherapy. J. Ethnopharmacol. 2018, 225, 90–102. [Google Scholar] [CrossRef]
- Markovinović, A.B.; Karačonji, I.B.; Jurica, K.; Lasić, D.; Babojelić, M.S.; Duralija, B.; Žlabur, J.S.; Putnik, P.; Kovačević, D.B. Strawberry tree fruits and leaves (Arbutus unedo L.) as raw material for sustainable functional food processing: A review. Hortic. 2022, 8, 881. [Google Scholar] [CrossRef]
- Martins, J.; Pétriacq, P.; Flandin, A.; Gómez-Cadenas, A.; Monteiro, P.; Pinto, G.; Canhoto, J. Genotype determines Arbutus unedo L. physiological and metabolomic responses to drought and recovery. Front. Plant Sci. 2022, 13, 1011542. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.; Pardo-De-Santayana, M.; Aceituno, L.; Morales, R.; Tardío, J. Fruit production of strawberry tree (Arbutus unedo L.) in two Spanish forests. Forestry 2011, 84, 419–429. [Google Scholar] [CrossRef]
- Sutton, J. International Dendrology Society. Arbutus unedo L. Available online: https://treesandshrubsonline.org/articles/arbutus/arbutus-unedo/ (accessed on 22 December 2022).
- Martínez-Alberola, F.; del Campo, E.M.; Lázaro-Gimeno, D.; Mezquita-Claramonte, S.; Molins, A.; Mateu-Andrés, I.; Pedrola-Monfort, J.; Casano, L.M.; Barreno, E. Balanced gene losses, duplications and intensive rearrangements led to an unusual regularly sized genome in Arbutus unedo chloroplasts. PLoS ONE 2013, 8, e79685. [Google Scholar] [CrossRef] [PubMed]
- Fazenda, P.; Pereira, R.; Fonseca, M.; Carlier, J.; Leitão, J. Identification and validation of microsatellite markers in strawberry tree (Arbutus unedo L.). Turk. J. Agric. For. 2019, 43, 430–436. [Google Scholar] [CrossRef]
- Takrouni, M.M.; Boussaid, M. Genetic diversity and population’s structure in Tunisian strawberry tree (Arbutus unedo L.). Sci. Hort. 2010, 126, 330–337. [Google Scholar] [CrossRef]
- Lopes, L.; Sá, O.; Pereira, J.A.; Baptista, P. Genetic diversity of Portuguese Arbutus unedo L. populations using leaf traits and molecular markers: An approach for conservation purposes. Sci. Hort. 2012, 142, 57–67. [Google Scholar] [CrossRef]
- Gomes, F.; Costa, R.; Ribeiro, M.M.; Figueiredo, E.; Canhoto, J.M. Analysis of genetic relationship among Arbutus unedo L. genotypes using RAPD and SSR markers. J. For. Res. 2013, 24, 227–236. [Google Scholar] [CrossRef]
- Santiso, X.; Lopez, L.; Retuerto, R.; Barreiro, R. Population structure of a widespread species under balancing selection: The case of Arbutus unedo L. Front. Plant Sci. 2016, 6, 1264. [Google Scholar] [CrossRef]
- Bertsouklis, K.F.; Papafotiou, M. Morphometric and molecular analysis of the three Arbutus species of Greece. Not. Bot. Horti. Agrobo. 2016, 44, 423–430. [Google Scholar] [CrossRef]
- Ribeiro, M.M.; Piotti, A.; Ricardo, A.; Gaspar, D.; Costa, R.; Parducci, L.; Vendramin, G.G. Genetic diversity and divergence at the Arbutus unedo L. (Ericaceae) westernmost distribution limit. PLoS ONE 2017, 12, e0175239. [Google Scholar] [CrossRef]
- Kowalczyk, M.; Zawadzka, E.; Szewczuk, D.; Gryzińska, M.; Jakubczak, A. Molecular markers used in forensic genetics. Med. Sci. Law 2018, 58, 201–209. [Google Scholar] [CrossRef]
- Hakim, I.R.; Kammoun, N.G.; Makhloufi, E.; Rebaï, A. Discovery and potential of SNP markers in characterization of Tunisian olive germplasm. Diversity 2010, 2, 17–27. [Google Scholar] [CrossRef]
- Sengupta, S.; Das, B.; Prasad, M.; Acharyya, P.; Ghose, T.K. A comparative survey of genetic diversity among a set of Caricaceae accessions using microsatellite markers. Springerplus 2013, 2, 345. [Google Scholar] [CrossRef]
- Park, J.-S.; Kang, M.-Y.; Shim, E.-J.; Oh, J.H.; Seo, K.-I.; Kim, K.S.; Sim, S.-C.; Chung, S.-M.; Park, Y.; Lee, G.P.; et al. Genome-wide core sets of SNP markers and Fluidigm assays for rapid and effective genotypic identification of Korean cultivars of lettuce (Lactuca sativa L.). Hortic. Res. 2022, 9, uhac119. [Google Scholar] [CrossRef]
- Olejnik, A.; Parkitna, K.; Kozak, B.; Florczak, S.; Matkowski, J.; Nowosad, K. Asessment of the genetic diversity of Chrysanthemum cultivars using SSR markers. Agronomy 2021, 11, 2318. [Google Scholar] [CrossRef]
- Coelho, P.S.; Reis, J.M.; Pereira, A.L.; Vairinhos, A.; Lopes, V.; Leitão, J.M. Downy mildew resistance and genetic variability in a wild rocket germplasm. Agron. J. 2022, 114, 3083–3095. [Google Scholar] [CrossRef]
- Silva, D.; Duarte, J.M.; Miguel, M.G.; Leitão, J.M. AFLP assessment of the genetic relationships among 12 Thymus taxa occurring in Portugal. Plant Genet. Resour. 2017, 15, 89–92. [Google Scholar] [CrossRef]
- Rodrigues, R.; Veiga, I.; Marreiros, A.; Rocha, F.; Leitão, J. Correction of the misclassification of species in the Portuguese collection of Cucurbita pepo L. using DNA markers. Plant Genet. Resour. 2014, 12, 160–163. [Google Scholar] [CrossRef]
- Svetleva, D.; Pereira, G.; Carlier, J.; Cabrita, L.; Leitão, J.; Genchev, D. Molecular characterization of Phaseolus vulgaris L. genotypes included in Bulgarian collection by ISSR and AFLP analysis. Sci. Hortic. 2006, 109, 198–206. [Google Scholar] [CrossRef]
- Goulão, L.; Cabrita, L.; Oliveira, C.; Leitão, J. Comparing RAPD and AFLPTM analysis in discrimination and estimation of genetic similarities among apple (Malus domestica Borkh.) cultivars. Euphytica 2001, 119, 259–270. [Google Scholar] [CrossRef]
- Cabrita, L.; Aksoy, U.; Hepaksoy, S.; Leitão, J.M. Suitability of isozyme, RAPD and AFLP markers to assess genetic differences and relatedness among fig (Ficus carica L.) clones. Sci. Hortic. 2001, 87, 261–273. [Google Scholar] [CrossRef]
- CLC Genomics Workbench 12.0.3, Qiagen (Venlo, The Netherlands). Available online: https://digitalinsights.qiagen.com (accessed on 29 March 2020).
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. “QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef]
- Seppey, M.; Manni, M.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness. Methods Mol. Biol. 2019, 1962, 227–245. [Google Scholar] [CrossRef]
- Compeau, P.E.C.; Pevzner, P.A.; Tesler, G. How to apply de Bruijn graphs to genome assembly. Nat. Biotechnol. 2011, 29, 987–991. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010, Babraham Bioinformatics (Cambridge, UK). Available online: https://www.bioinformatics.babrham.ac.uk/projects/fastqc/ (accessed on 29 March 2020).
- Milne, I.; Stephen, G.; Bayer, M.; Cock, P.J.A.; Pritchard, L.; Cardle, L.; Shaw, P.D.; Marshall, D. Using Tablet for visual exploration of second-generation sequencing data. Brief. Bioinform. 2013, 14, 193–202. [Google Scholar] [CrossRef]
- Kalendar, R.; Khassenov, B.; Ramankulov, Y.; Samuilova, O.; Ivanov, K.I. FastPCR: An in silico tool for fast primer and probe design and advanced sequence analysis. Genomics 2017, 109, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Vincze, T.; Posfai, J.; Roberts, R.J. NEBcutter: A program to cleave DNA with restriction enzymes. Nucleic Acids Res. 2003, 31, 3688–3691. [Google Scholar] [CrossRef] [PubMed]
- Farinhó, M.; Coelho, P.; Carlier, J.; Svetleva, D.; Monteiro, A.; Leitão, J. Mapping of a locus for adult plant resistance to downy mildew in broccoli (Brassica oleracea convar. italica). Theor. Appl. Genet. 2004, 109, 1392–1398. [Google Scholar] [CrossRef]
- Rohlf, F.J. NTSYS-pc: Numerical Taxonomy and Multivariate Analysis System; Version 2.2; Applied Biostatistics Inc.: New York, NY, USA; State University of New York Stony Brook: New York, NY, USA, 2009; ISBN 0-925031-31-3. [Google Scholar]
- Nei, M.; Li, W.H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc. Natl. Acad. Sci. USA 1979, 76, 5269–5273. [Google Scholar] [CrossRef]
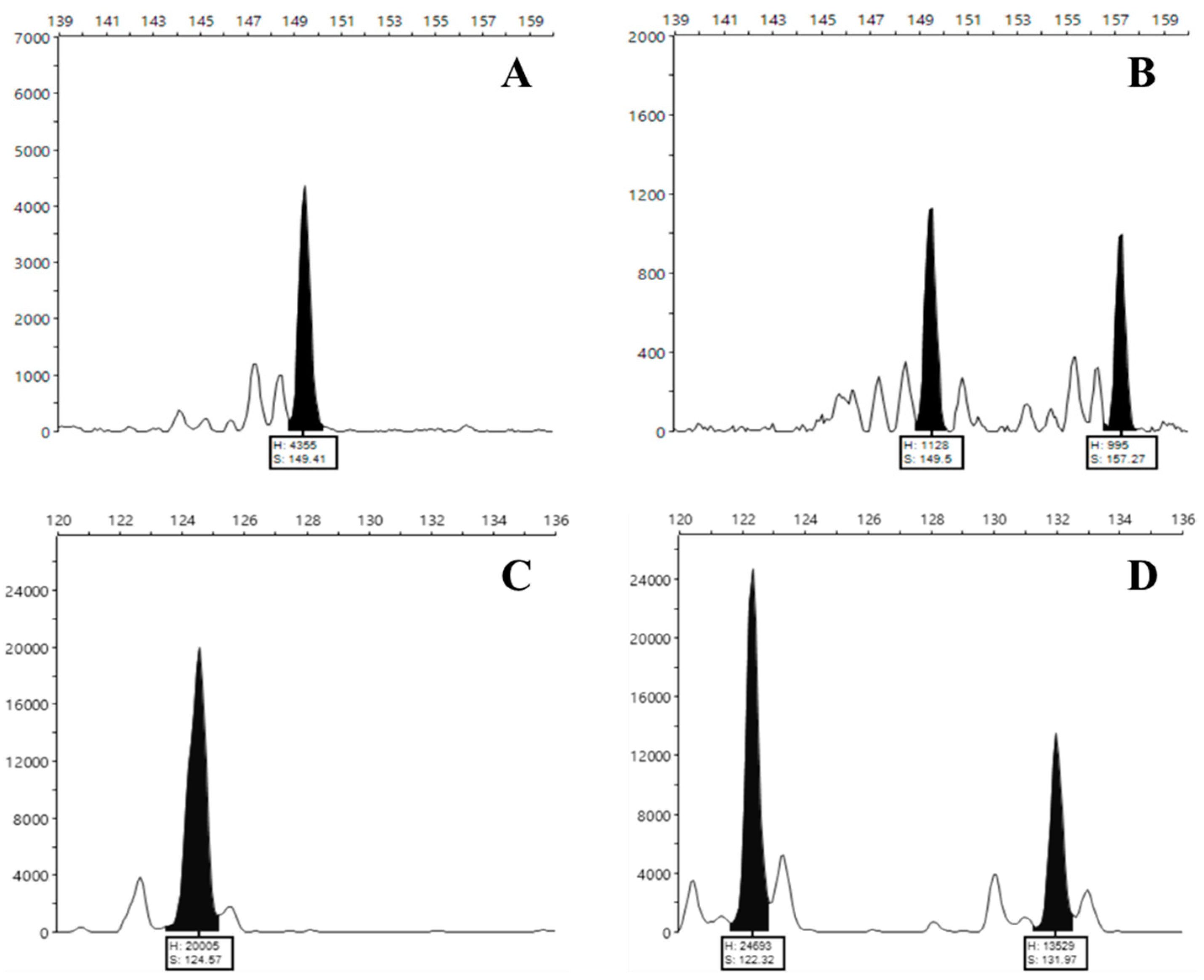
| Loci (GenBank) | Primers | 5′ Fluorophore Modification |
|---|---|---|
| MT327202 | Fw_CACCGCAACTTCCTAA * | Atto 550 |
| Rv_CTCAACTTTCTAAACGTCAC | ||
| MT327224 | Fw_ACCACTCTTTGTCTCC * | Hex |
| Rv_TTGGCAAATGTATTACGG | ||
| MT327513 | Fw_TCTAGTTCGAGACTCTAAGC * | 6-FAM |
| Rv_ACGAATCGAATCAGATTGAC | ||
| MT327557 | Fw_AACTTAGATTTGGCATGAAG * | Atto 565 |
| Rv_ACATTGGACTGTTTAGATCA |
| Accessions | MT327224 | MT327202 | MT327557 | MT327513 | OM145552 | OM145840 | OM145884 | OM145971 | OM145551 | OM145712 | OM145708 | OM145595 | OM145977 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AH1 | 166;174 | 170;182 | 149;157 | 124;124 | Y/Y | Y/Y | Y/Y | Y/Y | N/N | Y/Y | Y/Y | N/N | Y/Y |
| AFB | 166;180 | 162;162 | 147;147 | 122;124 | Y/Y | Y/N | N/N | Y/Y | N/N | Y/Y | N/N | Y/N | Y/Y |
| AM | 164;174 | 164;174 | 137;157 | 120;120 | Y/N | Y/Y | Y/N | Y/Y | N/N | Y/Y | N/N | Y/N | Y/Y |
| BE4 | 172;174 | 164;168 | 149;149 | 126;132 | Y/N | Y/Y | Y/Y | Y/Y | Y/Y | Y/Y | N/N | Y/Y | N/N |
| BE5 | 170;174 | 172;176 | 149;149 | 124;124 | Y/Y | Y/N | Y/Y | Y/Y | N/N | Y/Y | N/N | Y/Y | Y/Y |
| CA2 | 164;170 | 166;180 | 159;159 | 120;120 | Y/Y | Y/N | N/N | Y/Y | N/N | Y/Y | N/N | Y/N | Y/N |
| CA3 | 176;176 | 168;172 | 139;159 | 122;130 | N/N | Y/Y | Y/Y | Y/Y | Y/N | N/N | N/N | N/N | Y/N |
| G0 | 164;172 | 164;180 | 159;159 | 132;132 | Y/N | Y/N | Y/N | Y/N | Y/N | Y/N | Y/N | Y/N | Y/N |
| L1 | 168;170 | 168;178 | 159;163 | 134;134 | N/N | Y/Y | Y/N | N/N | Y/N | Y/Y | N/N | Y/N | Y/N |
| L4 | 164;166 | 170;170 | 137;149 | 122;124 | N/N | Y/Y | N/N | N/N | Y/N | Y/Y | N/N | Y/Y | Y/N |
| L11 | 160;168 | 172;180 | 159;159 | 124;124 | Y/Y | Y/N | Y/N | N/N | N/N | Y/Y | N/N | Y/N | Y/N |
| L25 | 164;166 | 176;192 | 147;147 | 120;124 | Y/Y | Y/Y | Y/N | N/N | N/N | Y/Y | N/N | Y/N | Y/N |
| M4 | 160;168 | 174;178 | 157;157 | 122;138 | Y/Y | Y/Y | N/N | Y/Y | Y/N | Y/Y | N/N | Y/Y | Y/N |
| M5 | 170;174 | 172;178 | 149;159 | 124;124 | Y/Y | Y/N | Y/N | Y/Y | Y/N | Y/Y | N/N | Y/Y | Y/N |
| M9 | 166;170 | 168;168 | 159;159 | 132;132 | Y/N | Y/Y | Y/Y | Y/Y | N/N | Y/Y | N/N | N/N | Y/Y |
| M10 | 166;166 | 176;188 | 137;159 | 122;132 | Y/N | Y/N | Y/N | Y/Y | N/N | Y/Y | Y/N | Y/N | Y/N |
| MA1 | 168;184 | 164;168 | 147;159 | 124;132 | Y/Y | Y/Y | N/N | N/N | N/N | Y/Y | Y/N | Y/Y | Y/Y |
| MA2 | 168;170 | 172;182 | 147;157 | 124;134 | Y/Y | Y/Y | N/N | N/N | Y/N | Y/Y | N/N | Y/N | Y/N |
| MA3 | 162;164 | 166;172 | 137;159 | 122;132 | Y/Y | Y/Y | Y/Y | Y/Y | N/N | Y/Y | Y/N | Y/N | Y/Y |
| MA4 | 174;184 | 172;180 | 149;159 | 122;136 | Y/Y | Y/Y | Y/Y | Y/Y | N/N | Y/Y | N/N | Y/Y | Y/Y |
| MRC | 168;170 | 164;172 | 151;157 | 122;134 | Y/Y | Y/Y | N/N | Y/Y | N/N | Y/Y | N/N | Y/N | Y/Y |
| ML1 | 168;182 | 154;162 | 147;157 | 122;131 | N/N | Y/N | N/N | Y/Y | Y/N | Y/N | N/N | Y/N | Y/N |
| NA | 168;170 | 154;166 | 149;159 | 121;137 | Y/Y | Y/N | Y/N | Y/Y | N/N | Y/Y | N/N | N/N | Y/N |
| RH1 | 160;174 | 172;172 | 141;149 | 124;132 | Y/Y | Y/Y | Y/N | Y/Y | Y/N | Y/Y | Y/N | Y/Y | Y/Y |
| RH2 | 170;174 | 168;178 | 137;159 | 120;132 | Y/Y | Y/Y | N/N | Y/Y | N/N | Y/Y | Y/N | Y/Y | Y/N |
| UB | 164;170 | 164;178 | 149;149 | 122;122 | N/N | Y/Y | Y/Y | Y/Y | N/N | Y/Y | N/N | Y/Y | Y/N |
| V1 | 170;174 | 178;188 | 149;149 | 122;132 | Y/Y | Y/Y | N/N | Y/Y | Y/N | Y/Y | N/N | Y/Y | Y/N |
| V2 | 168;170 | 166;178 | 149;157 | 132;132 | Y/Y | Y/Y | N/N | Y/N | Y/N | Y/Y | N/N | Y/Y | Y/N |
| V3 | 168;172 | 172;176 | 139;139 | 124;132 | Y/Y | Y/Y | Y/Y | Y/N | Y/N | Y/Y | N/N | Y/Y | Y/N |
| V4 | 166;170 | 172;172 | 145;159 | 122;132 | Y/Y | N/N | Y/Y | Y/Y | Y/Y | Y/Y | N/N | Y/Y | N/N |
| V5 | 164;166 | 168;180 | 149;153 | 124;132 | Y/Y | Y/Y | Y/Y | Y/Y | Y/N | Y/Y | Y/N | Y/N | Y/N |
| V6 | 164;170 | 176;180 | 149;159 | 122;132 | Y/Y | Y/Y | Y/Y | Y/Y | N/N | Y/Y | Y/N | Y/Y | Y/Y |
| V7 | 168;176 | 164;184 | 149;159 | 134;136 | Y/Y | Y/Y | N/N | Y/Y | Y/N | Y/Y | N/N | Y/N | Y/N |
| V10 | 170;176 | 172;174 | 137;177 | 124;136 | Y/Y | Y/Y | Y/Y | Y/Y | Y/N | Y/Y | N/N | Y/N | Y/N |
| V11 | 166;174 | 164;168 | 145;145 | 122;130 | Y/N | Y/N | Y/N | Y/Y | Y/N | Y/Y | Y/N | N/N | Y/N |
| V12 | 172;184 | 176;182 | 149;157 | 132;136 | Y/Y | Y/Y | Y/N | Y/Y | N/N | Y/Y | N/N | Y/N | Y/Y |
| V13 | 164;168 | 164;164 | 151;151 | 121;121 | Y/N | Y/Y | Y/Y | Y/Y | N/N | Y/Y | N/N | N/N | Y/Y |
| V14 | 168;170 | 172;178 | 143;159 | 120;120 | Y/Y | Y/Y | Y/Y | Y/Y | N/N | Y/Y | Y/N | Y/N | Y/N |
| V15 | 166;168 | 172;172 | 149;159 | 134;134 | Y/Y | Y/Y | N/N | Y/Y | Y/Y | Y/Y | N/N | Y/N | N/N |
| V17 | 172;174 | 164;180 | 137;161 | 124;132 | Y/Y | Y/Y | Y/Y | Y/Y | N/N | Y/Y | Y/N | Y/N | Y/Y |
| V18 | 168;184 | 172;172 | 149;149 | 122;132 | Y/Y | Y/Y | Y/Y | Y/Y | Y/N | Y/Y | N/N | Y/Y | Y/Y |
| V19 | 164;168 | 172;178 | 149;157 | 120;122 | Y/N | Y/Y | Y/N | Y/Y | Y/Y | Y/Y | N/N | Y/N | N/N |
| V20 | 160;168 | 168;172 | 137;159 | 130;134 | Y/Y | Y/N | Y/N | Y/Y | Y/Y | Y/Y | Y/N | Y/Y | N/N |
| VG1 | 170;184 | 172;182 | 149;159 | 122;124 | Y/Y | Y/Y | Y/Y | Y/Y | N/N | Y/Y | Y/N | Y/N | Y/Y |
| VG2 | 168;170 | 168;178 | 137;149 | 122;136 | Y/Y | Y/N | Y/Y | Y/Y | Y/N | Y/Y | N/N | Y/Y | Y/N |
| VG3 | 172;184 | 164;178 | 149;159 | 120;120 | Y/Y | Y/Y | N/N | Y/Y | Y/N | Y/Y | N/N | Y/N | Y/N |
| VG5 | 160;168 | 164;184 | 149;157 | 124;138 | Y/Y | Y/Y | N/N | Y/Y | Y/N | N/N | N/N | N/N | Y/N |
| VG9 | 166;184 | 172;178 | 149;149 | 120;136 | Y/Y | Y/Y | Y/Y | Y/Y | Y/N | N/N | Y/N | Y/N | Y/N |
| VM11 | 160;170 | 166;166 | 149;157 | 132;132 | Y/N | Y/Y | N/N | Y/Y | Y/N | Y/Y | Y/N | Y/N | Y/N |
| VM12 | 174;184 | 178;182 | 149;157 | 132;132 | Y/Y | Y/Y | Y/Y | N/N | Y/N | Y/Y | N/N | Y/Y | Y/N |
| Loci (GenBank) | Primers | T (°C) * | Product Length (bp) (Restriction Fragments) |
|---|---|---|---|
| OM145551 | FW: AGAAAGAGCTGAACACG | 57 | 292 (147/145) |
| RV: AGTTATTTCCTAGCCGAATC | |||
| OM145552 | FW: AAATATCACCACATCGGG | 60 | 218 (135/83) |
| RV: GATCAACCCTTTTGTACAC | |||
| OM145595 | FW: GTTGGATTTGTGTAGATCATG | 57 | 278 (139/139) |
| RV: TTGGTCTCTGGAGTTCTA | |||
| OM145708 | FW: GAAGATGATTCAGCATGTTAG | 58 | 258 (182/76) |
| RV: TGAAATAAGCAACGGTACA | |||
| OM145712 | FW: CAGATATTTGTCCTAACATGAAG | 59 | 273 (143/130) |
| RV: GATATGAATAGAACAACGCG | |||
| OM145840 | FW: TTCCAGTATAAGTTCTTGGTG | 61 | 291 (154/137) |
| RV: CAGGAACCATAAGAATAGTGA | |||
| OM145884 | FW: TCTATTGCTGCCAAGTAC | 59 | 272 (154/118) |
| RV: TCAAAGGTATAACTGAGGC | |||
| OM145971 | FW: ATACTAGGAACTTGGAAGTG | 60 | 296 (155/141) |
| RV: AAGTCAAATGGAGTTATTTCC | |||
| OM145977 | FW: TTGTAGGAGTACATGGTCT | 59 | 286 (145/141) |
| RV: TATTGTGAGCATGGTGATAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, R.; Anjos, I.; Reis, J.; Dias, C.; Leitão, J. Next-Generation Sequencing (NGS) Identified Species-Specific SSR and SNP Markers, Allow the Unequivocal Identification of Strawberry Tree (Arbutus unedo L.) Germplasm Accessions and Contribute to Assess Their Genetic Relationships. Plants 2023, 12, 1517. https://doi.org/10.3390/plants12071517
Pereira R, Anjos I, Reis J, Dias C, Leitão J. Next-Generation Sequencing (NGS) Identified Species-Specific SSR and SNP Markers, Allow the Unequivocal Identification of Strawberry Tree (Arbutus unedo L.) Germplasm Accessions and Contribute to Assess Their Genetic Relationships. Plants. 2023; 12(7):1517. https://doi.org/10.3390/plants12071517
Chicago/Turabian StylePereira, Ricardo, Isabela Anjos, João Reis, Carolina Dias, and José Leitão. 2023. "Next-Generation Sequencing (NGS) Identified Species-Specific SSR and SNP Markers, Allow the Unequivocal Identification of Strawberry Tree (Arbutus unedo L.) Germplasm Accessions and Contribute to Assess Their Genetic Relationships" Plants 12, no. 7: 1517. https://doi.org/10.3390/plants12071517
APA StylePereira, R., Anjos, I., Reis, J., Dias, C., & Leitão, J. (2023). Next-Generation Sequencing (NGS) Identified Species-Specific SSR and SNP Markers, Allow the Unequivocal Identification of Strawberry Tree (Arbutus unedo L.) Germplasm Accessions and Contribute to Assess Their Genetic Relationships. Plants, 12(7), 1517. https://doi.org/10.3390/plants12071517







