The Bacterial Volatile Organic Compound N,N-Dimethylhexadecylamine Induces Long-Lasting Developmental and Immune Responses throughout the Life Cycle of Arabidopsis thaliana
Abstract
:1. Introduction
2. Results
2.1. DMHDA Represses Growth of Arabidopsis Plants In Vitro
2.2. DMHDA Protects Arabidopsis Leaves from B. cinerea Infection
2.3. Contrasting Phenotypes in The Loss-of-Function of COI1 and EIN2 Genes for the Growth Response to DMHDA
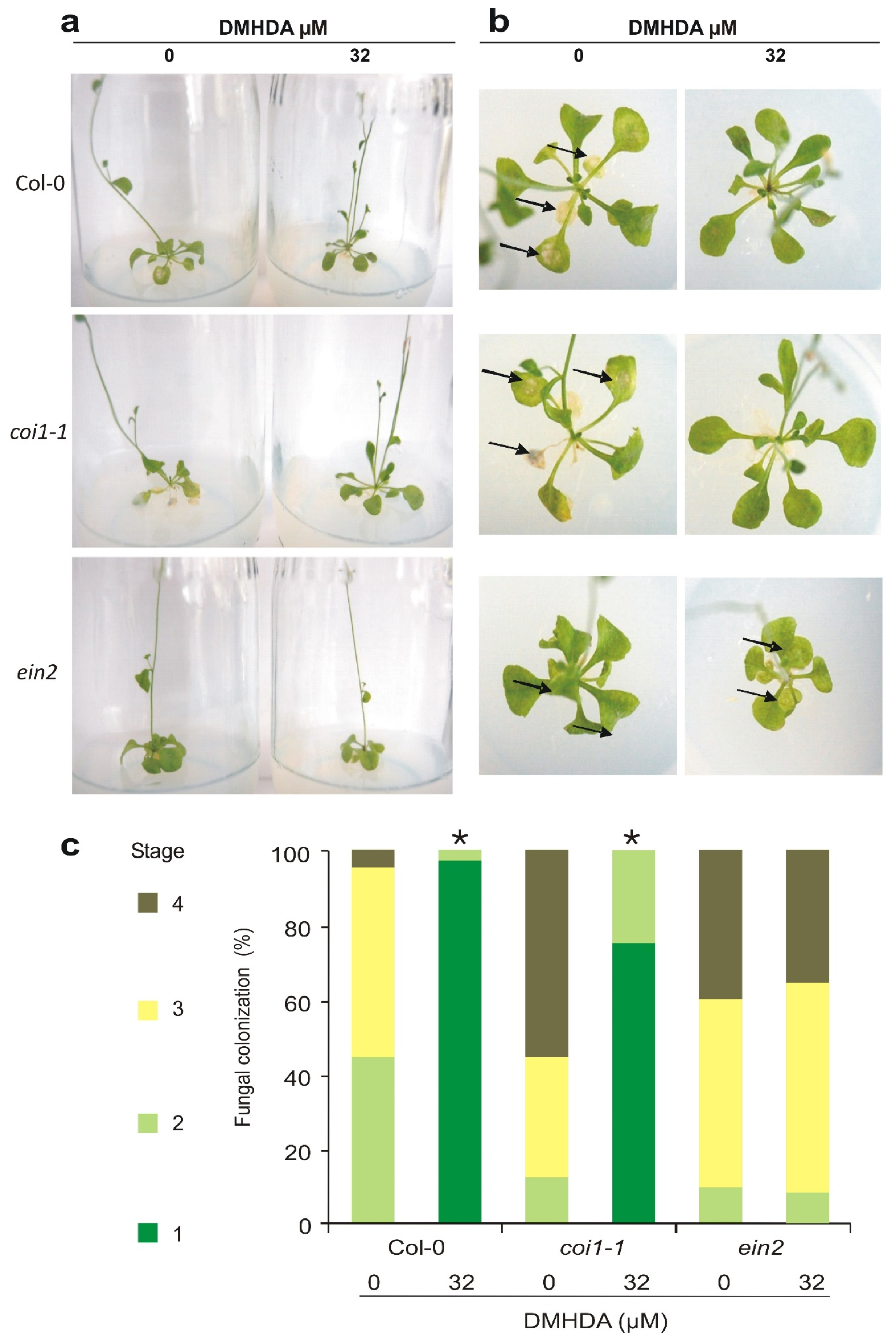
2.4. EIN2 Plays an Important Role in DMHDA-Elicited Plant Resistance to B. cinerea
2.5. Temporal Application of DMHDA Promotes Growth of A. thaliana
2.6. DMHDA Induces Long-Lasting Effects on The Immune Response of Arabidopsis Plants
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Propagation of Botrytis cinerea
4.3. Chemicals
4.4. Analysis of Plant Growth
4.5. Quantification of B. cinerea Colonization in Arabidopsis Leaves
4.6. Effect of DMHDA on Growth and Immune Response of Arabidopsis
4.7. DMHDA Long-Lasting Effect on The Growth of A. thaliana
4.8. DMHDA Long-Lasting Effect on The Immune Response of A. thaliana Plants
4.9. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kapoor, B.; Kumar, P.; Sharma, R.; Kumar, A. Regulatory interactions in phytohormone stress signaling implying plants resistance and resilience mechanisms. J. Plant Biochem. Biotechnol. 2021, 30, 813–828. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Soni, R.; Kumar, V.; Suyal, D.C.; Jain, L.; Goel, R. Metagenomics of plant rhizosphere microbiome. In Understanding Host-Microbiome Interactions—An Omics Approach; Singh, R., Kothari, R., Koringa, P., Singh, S., Eds.; Springer: Singapore, 2017; pp. 193–205. [Google Scholar] [CrossRef]
- Kumar, A.; Dubey, A. Rhizosphere microbiome: Engineering bacterial competitiveness for enhancing crop production. J. Adv. Res. 2020, 24, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Raaijmakers, J.M. Cross-kingdom similarities in microbiome functions. ISME 2015, 9, 1905–1907. [Google Scholar] [CrossRef] [Green Version]
- Li, J.T.; Wang, C.Y.; Liang, W.X.; Liu, S.H. Rhizosphere microbiome: The emerging barrier in plant-pathogen interactions. Front. Microbiol. 2021, 12, 772420. [Google Scholar] [CrossRef]
- Arnault, G.; Mony, C.; Vandenkoornhuyse, P. Plant microbiota dysbiosis and the Anna Karenina Principle. Trends Plant Sci. 2023, 28, 18–30. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Han, X.; Feng, D.; Yuan, D.; Huang, L.J. Signaling crosstalk between salicylic acid and ethylene/jasmonate in plant defense: Do we understand what they are whispering? Int. J. Mol. Sci. 2019, 20, 671. [Google Scholar] [CrossRef] [Green Version]
- Pieterse, C.M.; Leon-Reyes, A.; Van der Ent, S.; Van Wees, S. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 2009, 5, 308–316. [Google Scholar] [CrossRef] [Green Version]
- Van Loon, L.C.; Rep, M.; Pieterse, C.M.J. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef] [Green Version]
- Bürger, M.; Chory, J. Stressed out about hormones: How plants orchestrate immunity. Cell Host Microbe 2019, 26, 163–172. [Google Scholar] [CrossRef]
- Dutilloy, E.; Oni, F.E.; Esmaeel, Q.; Clément, C.; Barka, E.A. Plant beneficial bacteria as bioprotectants against wheat and barley diseases. J. Fungi 2022, 8, 632. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Trotel-Aziz, P.; Villaume, S.; Rabenoelina, F.; Clément, C.; Baillieul, F.; Aziz, A. Priming of camalexin accumulation in induced systemic resistance by beneficial bacteria against Botrytis cinerea and Pseudomonas syringae pv. tomato DC3000. J. Exp. Bot. 2022, 73, 3743–3757. [Google Scholar] [CrossRef]
- Huot, B.; Yao, J.; Montgomery, B.L.; He, S.Y. Growth–defense tradeoffs in plants: A balancing act to optimize fitness. Mol. Plant 2014, 7, 1267–1287. [Google Scholar] [CrossRef] [Green Version]
- van Hulten, M.; Pelser, M.; Van Loon, L.C.; Pieterse, C.M.; Ton, J. Costs and benefits of priming for defense in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 5602–5607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janse van Rensburg, H.C.; Van den Ende, W. Priming with γ-aminobutyric acid against Botrytis cinerea reshuffles metabolism and reactive oxygen species: Dissecting signalling and metabolism. Antioxidants 2020, 9, 1174. [Google Scholar] [CrossRef]
- Balmer, A.; Pastor, V.; Glauser, G.; Mauch-Mani, B. Tricarboxylates induce defense priming against bacteria in Arabidopsis thaliana. Front. Plant Sci. 2018, 9, 1221. [Google Scholar] [CrossRef]
- Mhlongo, M.I.; Piater, L.A.; Madala, N.E.; Labuschagne, N.; Dubery, I.A. The chemistry of plant–microbe interactions in the rhizosphere and the potential for metabolomics to reveal signaling related to defense priming and induced systemic resistance. Front. Plant Sci. 2018, 9, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortíz-Castro, R.; Martínez-Trujillo, M.; López-Bucio, J. N-acyl-L-homoserine lactones: A class of bacterial quorum-sensing signals alter post-embryonic root development in Arabidopsis thaliana. Plant Cell Environ. 2008, 31, 1497–1509. [Google Scholar] [CrossRef]
- Moshynets, O.V.; Babenko, L.M.; Rogalsky, S.P.; Lungin, O.S.; Foster, J.; Kosakivska, I.V.; Potters, G.; Spiers, A.J. Priming winter wheat seeds with the bacterial quorum sensing signal N-hexanoyl-L-homoserine lactone (C6-HSL) shows potential to improve plant growth and seed yield. PLoS ONE 2019, 14, e0209460. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, A. Quorum sensing N-acyl-homoserine lactone signal molecules of plant beneficial Gram-negative rhizobacteria support plant growth and resistance to pathogens. Rhizosphere 2020, 16, 100258. [Google Scholar] [CrossRef]
- Cordovez, V.; Schop, S.; Hordijk, K.; Dupré de Boulois, H.; Coppens, F.; Hanssen, I.; Raaijmakers, J.M.; Carrión, V.J. Priming of plant growth promotion by volatiles of root-associated Microbacterium spp. Appl. Environ. Microbiol. 2018, 84, e01865-18. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Calderón, E.; Aviles-Garcia, M.A.; Castulo-Rubio, D.Y.; Macías-Rodríguez, L.; Montejano-Ramírez, V.; Santoyo, G.; López-Bucio, J.; Valencia-Cantero, E. Volatile compounds from beneficial or pathogenic bacteria differentially regulate root exudation, transcription of iron transporters, and defense signaling pathways in Sorghum bicolor. Plant Mol. Biol. 2018, 96, 291–304. [Google Scholar] [CrossRef]
- Ryu, C.M.; Farag, M.A.; Hu, C.H.; Reddy, M.S.; Wei, H.X.; Paré, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in, Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez-Luna, F.M.; López-Bucio, J.; Altamirano-Hernández, J.; Valencia-Cantero, E.; Reyes de la Cruz, H.; Macías-Rodríguez, L. Plant growth-promoting rhizobacteria modulate root-system architecture in Arabidopsis thaliana through volatile organic compound emission. Symbiosis 2010, 51, 75–83. [Google Scholar] [CrossRef]
- Cofer, T.; Seidl-Adams, I.; Tumlinson, J.H. From acetoin to (Z)-3-hexen-1-ol: The diversity of volatile organic compounds that Induce Plant Responses. J. Agric. Food Chem. 2018, 66, 11197–11208. [Google Scholar] [CrossRef]
- Pérez-Flores, P.; Valencia-Cantero, E.; Altamirano-Hernandez, J.; Pelagio-Flores, R.; López-Bucio, J.; García-Juárez, P.; Macías-Rdodríguez, L. Bacillus methylotrophicus M4-96 isolated from maize (Zea mays) rhizoplane increases growth and auxin content in Arabidopsis thaliana via emission of volatiles. Protoplasma 2017, 254, 2201–2213. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Mosqueda, M.C.; Velázquez-Becerra, C.; Macías-Rodríguez, L.I.; Santoyo, G.; Flores-Cortez, I.; Alfaro-Cuevas, R.; Valencia-Cantero, E. Arthrobacter agilis UMCV2 induces iron acquisition in Medicago truncatula (Strategy I plant) in vitro via dimethylhexadecylamine emission. Plant Soil 2013, 362, 51–66. [Google Scholar] [CrossRef]
- Farag, M.A.; Zhang, H.; Ryu, C.M. Dynamic chemical communication between plants and bacteria through airborne signals: Induced resistance by bacterial volatiles. J. Chem. Ecol. 2013, 39, 1007–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, K.; Liang, Y.; Mengiste, T.; Sharon, A. Killing softly: A roadmap of Botrytis cinerea pathogenicity. Trends Plant Sci. 2022, 28, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Choquer, M.; Rascle, C.; Gonçalves, I.R.; Vallée, A.; Ribot, C.; Loisel, E.; Smilevski, P.; Ferria, J.; Savadogo, M.; Souibgui, E.; et al. The infection cushion of Botrytis cinerea: A fungal “weapon” of plant-biomass destruction. Environ. Microbiol. 2021, 23, 2293–2314. [Google Scholar] [CrossRef] [PubMed]
- Hernández-León, R.; Rojas-Solís, D.; Contreras-Pérez, M.; Orozco-Mosqueda, M.C.; Macías-Rodríguez, L.I.; Reyes-de la Cruz, H.; Valencia-Cantero, E.; Santoyo, G. Characterization of the antifungal and plant-growth promoting effects of diffusible and volatile organic compounds produced by Pseudomonas fluorescens strains. Biol. Control 2015, 81, 83–92. [Google Scholar] [CrossRef]
- Liu, W.; Wei, M.; Bingyu, Z.; Feng, L. Antifungal activities and components of VOCs produced by Bacillus subtilis G8. Microbiol. Curr. Res. 2008, 1, 28–34. [Google Scholar] [CrossRef] [Green Version]
- Velázquez-Becerra, C.; Macías-Rodríguez, L.I.; López-Bucio, J.; Altamirano-Hernández, J.; Flores-Cortez, I.; Valencia-Cantero, E. A volatile organic compound analysis from Arthrobacter agilis identifies dimethylhexadecylamine, an amino-containing lipid modulating bacterial growth and Medicago sativa morphogenesis in vitro. Plant Soil 2011, 339, 329–340. [Google Scholar] [CrossRef]
- Martínez-Cámara, R.; Montejano-Ramírez, V.; Moreno-Hagelsieb, G.; Santoyo, G.; Valencia-Cantero, E. The volatile organic compound dimethylhexadecylamine affects bacterial growth and swarming motility of bacteria. Folia Microbiol. 2020, 65, 523–532. [Google Scholar] [CrossRef]
- Chávez-Moctezuma, M.P.; Martínez-Cámara, R.; Hernández-Salmerón, J.; Moreno-Hagelsieb, G.; Santoyo, G.; Valencia-Cantero, E. Comparative genomic and functional analysis of Arthrobacter sp. UMCV2 reveals the presence of luxR-related genes inducible by the biocompound N, N-dimethylhexadecilamine. Front. Microbiol. 2022, 13, 1040932. [Google Scholar] [CrossRef]
- Real-Sosa, K.M.; Hernández-Calderón, E.; Flores-Cortez, I.; Valencia-Cantero, E. Bacteria-derived N,N-dimethylhexadecylamine modulates the endophytic microbiome of Medicago truncatula in vitro. Rhizosphere 2022, 21, 100470. [Google Scholar] [CrossRef]
- Montejano-Ramírez, V.; García-Pineda, E.; Valencia-Cantero, E. Bacterial compound N, N-dimethylhexadecylamine modulates expression of iron deficiency and defense response genes in Medicago truncatula independently of the jasmonic acid pathway. Plants 2020, 9, 624. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Soberano, C.; Valencia-Cantero, E. Dimethylhexadecylamine, a bacterial volatile compound, regulates achene germination, in vitro growth, and defense priming in Fragaria × ananassa. Acta Physiol. Plant 2021, 43, 20. [Google Scholar] [CrossRef]
- Vázquez-Chimalhua, E.; Ruíz-Herrera, L.F.; Barrera-Ortiz, S.; Valencia-Cantero, E.; López-Bucio, J. The bacterial volatile dimethylhexadecilamine reveals an antagonistic interaction between jasmonic acid and cytokinin in controlling primary root growth of Arabidopsis seedlings. Protoplasma 2019, 256, 643–654. [Google Scholar] [CrossRef]
- Vázquez-Chimalhua, E.; Barrera-Ortiz, S.; Valencia-Cantero, E.; López-Bucio, J.; Ruiz-Herrera, L.F. The bacterial volatile N,N-dimethylhexadecylamine promotes Arabidopsis primary root elongation through cytokinin signaling and the AHK2 receptor. Plant Signal. Behav. 2021, 16, 1879542. [Google Scholar] [CrossRef]
- Vázquez-Chimalhua, E.; Valencia-Cantero, E.; López-Bucio, J.; Ruiz-Herrera, L.F. N, N, dimethyl-hexadecylamine modulates Arabidopsis root growth through modifying the balance between stem cell niche and jasmonic acid-dependent gene expression. Gene Expr. Patterns 2021, 41, 119201. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, C.; Gu, M.; Bai, Z.; Zhang, W.; Qi, T.; Cheng, Z.; Peng, W.; Luo, H.; Nan, F.; et al. The Arabidopsis CORONATINE INSENSITIVE1 protein is a jasmonate receptor. Plant Cell 2009, 21, 2220–2236. [Google Scholar] [CrossRef] [Green Version]
- Guzmán, P.; Ecker, J.R. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 1990, 2, 513–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, C.; Yoon, G.M.; Shemansky, J.M.; Lin, D.Y.; Ying, Z.I.; Chang, J.; Garrett, W.M.; Kessenbrock, M.; Growth, G.; Tucker, M.L.; et al. CTR1 phosphorylates the central regulator EIN2 to control ethylene hormone signaling from the ER membrane to the nucleus in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 19486–19491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montejano-Ramírez, V.; Valencia-Cantero, E. Cross-Talk between iron deficiency response and defense establishment in plants. Int. J. Mol. Sci. 2003, 24, 6236. [Google Scholar] [CrossRef]
- Shrestha, A.; Grimm, M.; Ojiro, I.; Krumwiede, J.; Schikora, A. Impact of quorum sensing molecules on plant growth and immune system. Front. Microbiol. 2020, 11, 1545. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Schikora, A. AHL-priming for enhanced resistance as a tool in sustainable agriculture. FEMS Microbiol. 2020, 96, fiaa226. [Google Scholar] [CrossRef]
- Velázquez-Becerra, C.; Macías-Rodríguez, L.I.; López-Bucio, J.; Flores-Cortez, I.; Santoyo, G.; Hernández-Soberano, C.; Valencia-Cantero, E. The rhizobacterium Arthrobacter agilis produces dimethylhexadecylamine, a compound that inhibits growth of phytopathogenic fungi in vitro. Protoplasma 2013, 250, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Raya-González, J.; Velázquez-Becerra, C.; Barrera-Ortiz, S.; López-Bucio, J.; Valencia-Cantero, E. N, N-dimethylhexadecylamine and related amines regulate root morphogenesis via jasmonic acid signalling in Arabidopsis thaliana. Protoplasma 2017, 254, 1399–1410. [Google Scholar] [CrossRef]
- Janse van Rensburg, H.C.; Takács, Z.; Freynschlag, F.; Toksoy Öner, E.; Jonak, C.; Van den Ende, W. Fructans prime ROS dynamics and Botrytis cinerea resistance in Arabidopsis. Antioxidants 2020, 9, 805. [Google Scholar] [CrossRef]
- Janse van Rensburg, H.C.; Limami, A.M.; Van den Ende, W. Spermine and spermidine priming against Botrytis cinerea modulates ROS dynamics and metabolism in Arabidopsis. Biomolecules 2021, 11, 223. [Google Scholar] [CrossRef]
- Singh, P.; Kuo, Y.C.; Mishra, S.; Tsai, C.H.; Chien, C.C.; Chen, C.W.; Desclos-Theveniau, M.; Chu, P.W.; Schulze, B.; Chinchilla, D.; et al. The lectin receptor kinase-VI. 2 is required for priming and positively regulates Arabidopsis pattern-triggered immunity. Plant Cell 2012, 24, 1256–1270. [Google Scholar] [CrossRef] [Green Version]
- Luna, E.; López, A.; Kooiman, J.; Ton, J. Role of NPR1 and KYP in long-lasting induced resistance by β-aminobutyric acid. Front. Plant Sci. 2014, 5, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuźnicki, D.; Meller, B.; Drozda, A. BABA-Induced DNA methylome adjustment to intergenerational defense priming in potato to Phytophthora infestans. Front. Plant Sci. 2019, 10, 650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arévalo-Marín, D.F.; Briceño-Robles, D.M.; Mosquera, T.; Melgarejo, L.M.; Sarmiento, F. Jasmonic acid priming of potato uses hypersensitive response-dependent defense and delays necrotrophic phase change against Phytophthora infestans. Physiol. Mol. Plant Pathol. 2021, 115, 101680. [Google Scholar] [CrossRef]
- Singh, P.; Dave, A.; Vaistij, F.E.; Worrall, D.; Holroyd, G.H.; Wells, J.G.; Kaminski, F.; Graham, I.A.; Roberts, M.R. Jasmonic acid-dependent regulation of seed dormancy following maternal herbivory in Arabidopsis. New Phytol. 2017, 214, 1702–1711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baccelli, I.; Mauch-Mani, B. Beta-aminobutyric acid priming of plant defense: The role of ABA and other hormones. Plant Mol. Biol. 2016, 91, 703–711. [Google Scholar] [CrossRef]
- Thevenet, D.; Pastor, V.; Baccelli, I.; Balmer, A.; Vallat, A.; Neier, R.; Glauser, G.; Mauch-Mani, B. The priming molecule β-aminobutyric acid is naturally present in plants and is induced by stress. New Phytol. 2017, 213, 552–559. [Google Scholar] [CrossRef]
- Cai, J.; Aharoni, A. Amino acids and their derivatives mediating defense priming and growth tradeoff. Curr. Opin. Plant Biol. 2022, 69, 102288. [Google Scholar] [CrossRef] [PubMed]
- Feys, B.J.F.; Benedetti, C.E.; Penfold, C.N.; Turner, J.G. Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male-sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell 1994, 6, 751–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigotti, S.; Gindro, K.; Richter, H.; Viret, O. Characterization of molecular markers for specific and sensitive detection of Botrytis cinerea Pers.: Fr. in strawberry (Fragaria × ananassa Duch.) using PCR. FEMS Microbiol. Lett. 2002, 209, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–160. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Soberano, C.; López-Bucio, J.; Valencia-Cantero, E. The Bacterial Volatile Organic Compound N,N-Dimethylhexadecylamine Induces Long-Lasting Developmental and Immune Responses throughout the Life Cycle of Arabidopsis thaliana. Plants 2023, 12, 1540. https://doi.org/10.3390/plants12071540
Hernández-Soberano C, López-Bucio J, Valencia-Cantero E. The Bacterial Volatile Organic Compound N,N-Dimethylhexadecylamine Induces Long-Lasting Developmental and Immune Responses throughout the Life Cycle of Arabidopsis thaliana. Plants. 2023; 12(7):1540. https://doi.org/10.3390/plants12071540
Chicago/Turabian StyleHernández-Soberano, Christian, José López-Bucio, and Eduardo Valencia-Cantero. 2023. "The Bacterial Volatile Organic Compound N,N-Dimethylhexadecylamine Induces Long-Lasting Developmental and Immune Responses throughout the Life Cycle of Arabidopsis thaliana" Plants 12, no. 7: 1540. https://doi.org/10.3390/plants12071540
APA StyleHernández-Soberano, C., López-Bucio, J., & Valencia-Cantero, E. (2023). The Bacterial Volatile Organic Compound N,N-Dimethylhexadecylamine Induces Long-Lasting Developmental and Immune Responses throughout the Life Cycle of Arabidopsis thaliana. Plants, 12(7), 1540. https://doi.org/10.3390/plants12071540






