Evaluation of Miscanthus × giganteus Tolerance to Trace Element Stress: Field Experiment with Soils Possessing Gradient Cd, Pb, and Zn Concentrations
Abstract
:1. Introduction
2. Results
2.1. Soil Physicochemical and T.E. Concentrations
2.2. Trace Element (T.E.) Leaf Concentrations
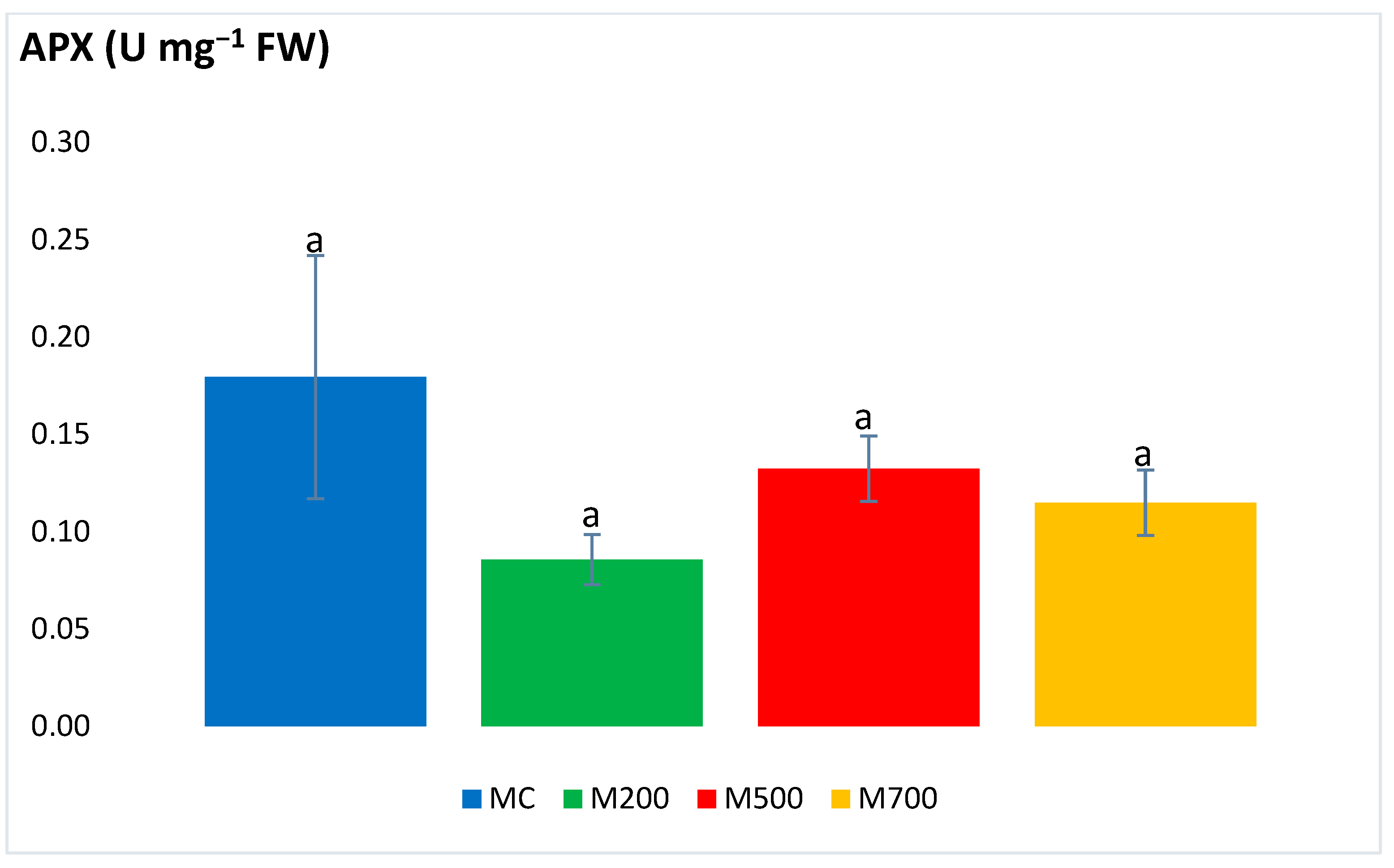
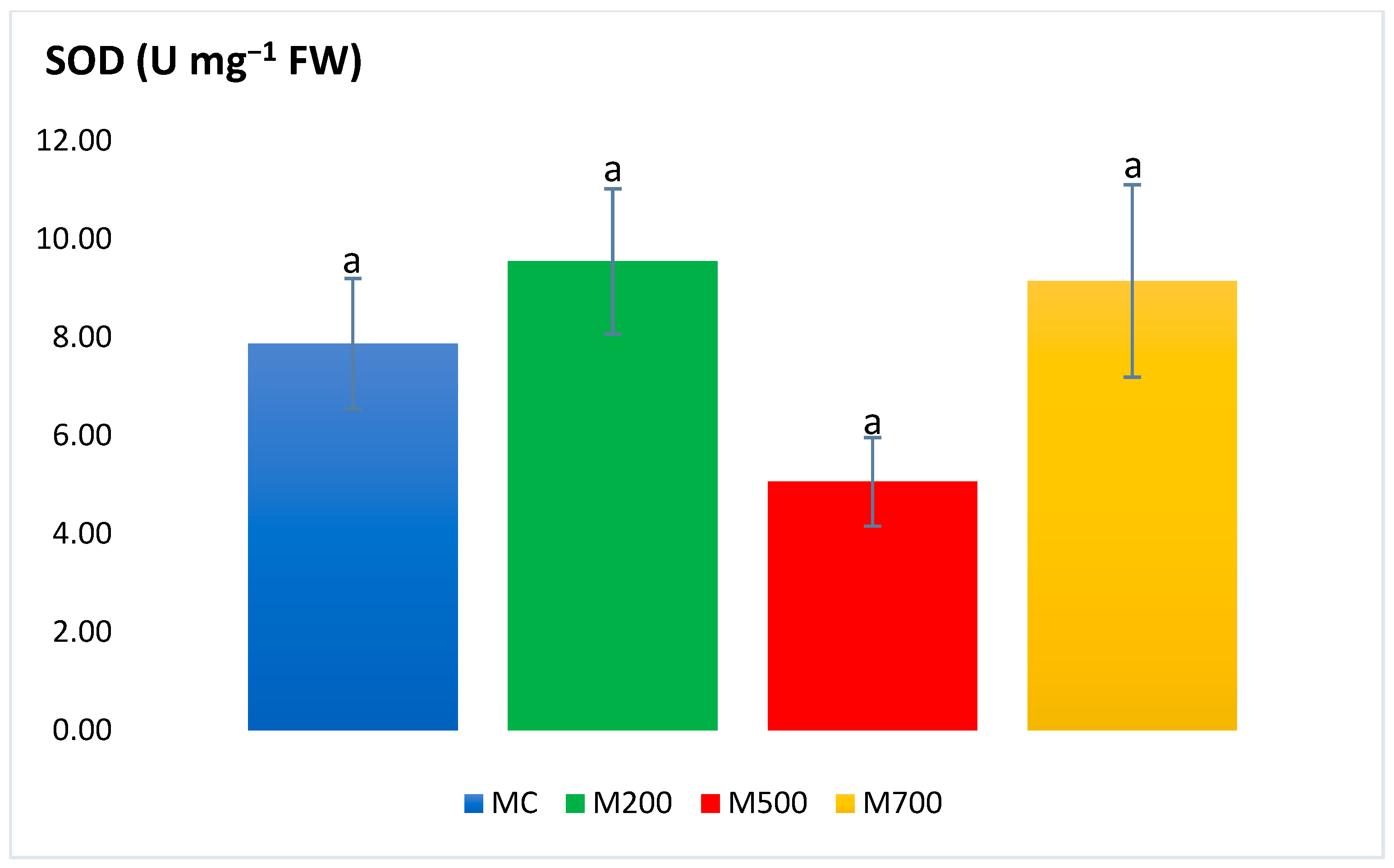
2.3. Antioxidant Enzymatic Activities
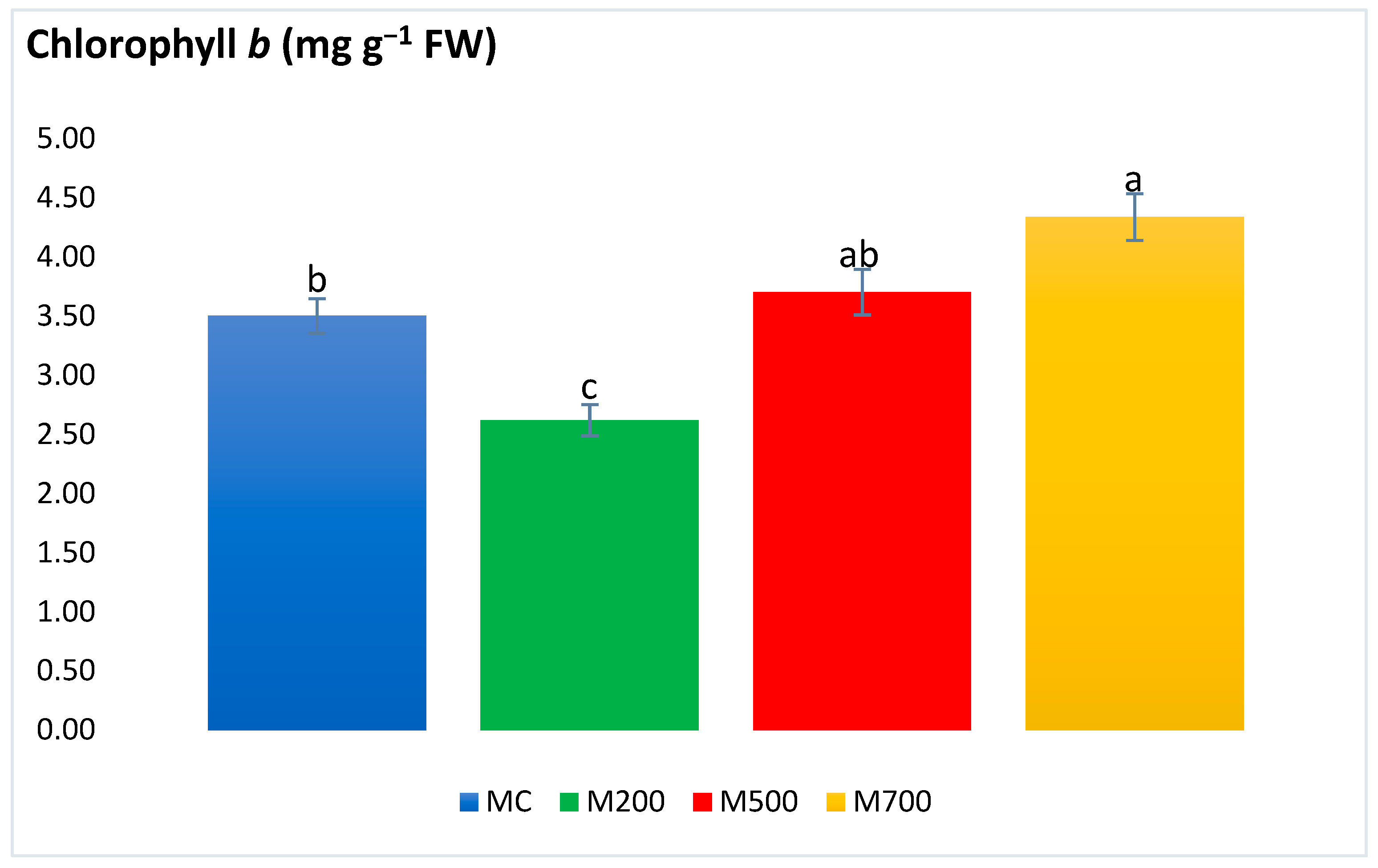
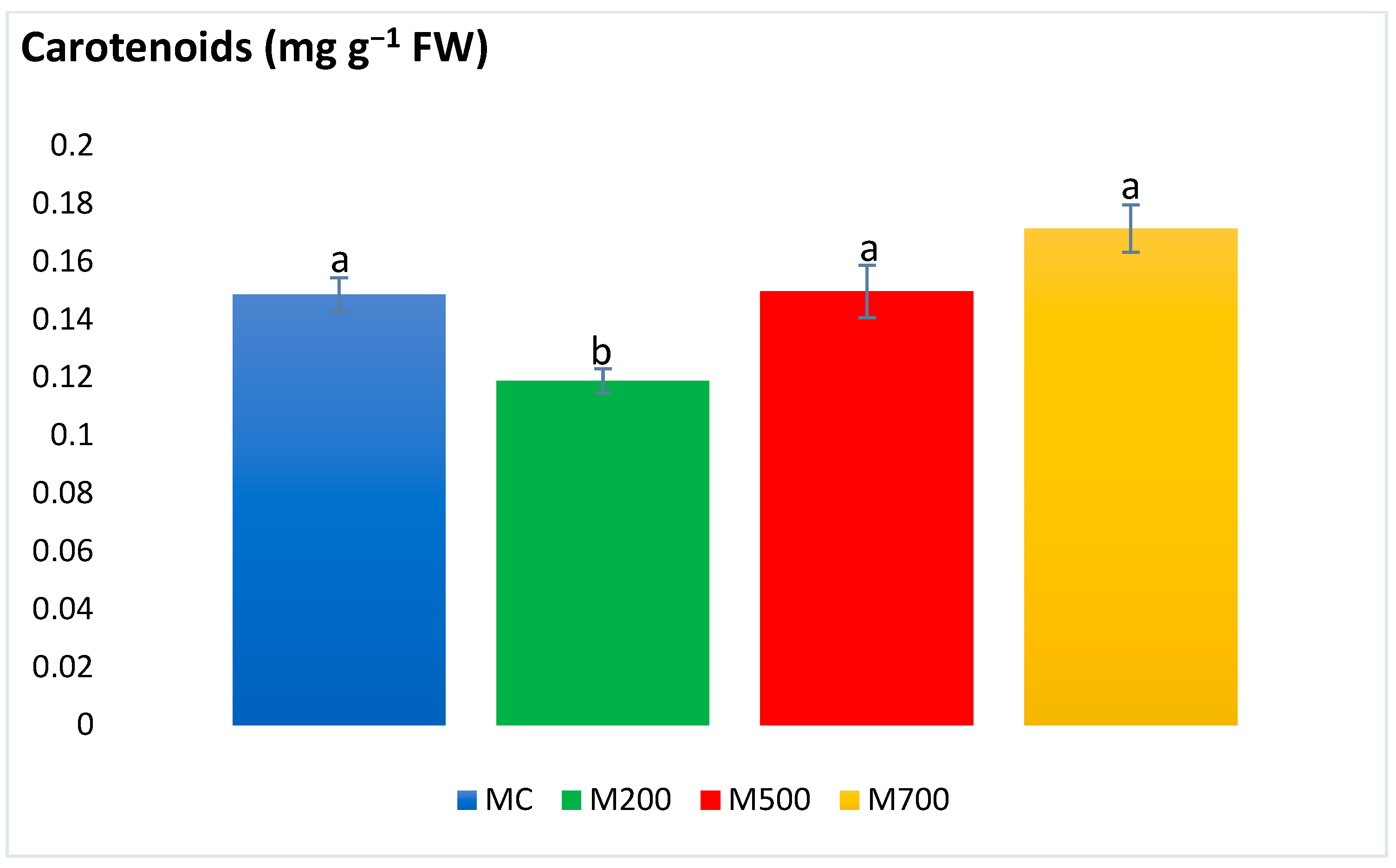
2.4. Photosynthetic Pigments and Secondary Metabolites
3. Discussion
4. Materials and Methods
4.1. Experimental Plots Characterization
4.2. Plant Sampling and Preparation
4.3. Trace Elements Concentration Determination in Plant Leaves
4.4. Stress Biomarker Measurement (Miscanthus Health Parameters)
4.4.1. Antioxidant Enzymatic Activities
4.4.2. Photosynthetic Pigments and Secondary Metabolites
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Su, C.; Jiang, L.; Zhang, W. A Review on Heavy Metal Contamination in the Soil Worldwide: Situation, Impact and Remediation Techniques. Environ. Skeptics Crit. 2014, 3, 24–38. [Google Scholar]
- Al Souki, K.S.; Burdová, H.; Mamirova, A.; Kuráň, P.; Kříženecká, S.; Oravová, L.; Tolaszová, J.; Nebeská, D.; Popelka, J.; Ust’ak, S.; et al. Evaluation of the Miscanthus × giganteus Short Term Impacts on Enhancing the Quality of Agricultural Soils Affected by Single and/or Multiple Contaminants. Environ. Technol. Innov. 2021, 24, 101890. [Google Scholar] [CrossRef]
- Pidlisnyuk, V.; Erickson, L.; Stefanovska, T.; Popelka, J.; Hettiarachchi, G.; Davis, L.; Trögl, J. Potential Phytomanagement of Military Polluted Sites and Biomass Production Using Biofuel Crop Miscanthus × giganteus. Environ. Pollut. 2019, 249, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhao, X.; Fang, J.; Xiao, Y. Physiological Responses and Metal Uptake of Miscanthus under Cadmium/Arsenic Stress. Environ. Sci. Pollut. Res. 2018, 25, 28275–28284. [Google Scholar] [CrossRef]
- Sterckeman, T.; Douay, F.; Proix, N.; Fourrier, H.; Perdrix, E. Assessment of the Contamination of Cultivated Soils by Eighteen Trace Elements around Smelters in the North of France. Water Air Soil Pollut. 2002, 135, 173–194. [Google Scholar] [CrossRef]
- Douay, F.; Pruvot, C.; Waterlot, C.; Fritsch, C.; Fourrier, H.; Loriette, A.; Bidar, G.; Grand, C.; de Vaufleury, A.; Scheifler, R. Contamination of Woody Habitat Soils around a Former Lead Smelter in the North of France. Sci. Total Environ. 2009, 407, 5564–5577. [Google Scholar] [CrossRef] [PubMed]
- Nsanganwimana, F.; Pourrut, B.; Waterlot, C.; Louvel, B.; Bidar, G.; Labidi, S.; Fontaine, J.; Muchembled, J.; Sahraoui, A.L.-H.; Fourrier, H.; et al. Metal Accumulation and Shoot Yield of Miscanthus × giganteus Growing in Contaminated Agricultural Soils: Insights into Agronomic Practices. Agric. Ecosyst. Environ. 2015, 213, 61–71. [Google Scholar] [CrossRef]
- Nsanganwimana, F.; Waterlot, C.; Louvel, B.; Pourrut, B.; Douay, F. Metal, Nutrient and Biomass Accumulation during the Growing Cycle of Miscanthus Established on Metal-Contaminated Soils. J. Plant Nutr. Soil Sci. 2016, 179, 257–269. [Google Scholar] [CrossRef]
- Kocoń, A.; Jurga, B. The Evaluation of Growth and Phytoextraction Potential of Miscanthus × giganteus and Sida Hermaphrodita on Soil Contaminated Simultaneously with Cd, Cu, Ni, Pb, and Zn. Environ. Sci. Pollut. Res. 2017, 24, 4990–5000. [Google Scholar] [CrossRef] [Green Version]
- Andrejić, G.; Šinžar-Sekulić, J.; Prica, M.; Dželetović, Ž.; Rakić, T. Phytoremediation Potential and Physiological Response of Miscanthus × giganteus Cultivated on Fertilized and Non-Fertilized Flotation Tailings. Environ. Sci. Pollut. Res. 2019, 26, 34658–34669. [Google Scholar] [CrossRef]
- Zgorelec, Z.; Bilandzija, N.; Knez, K.; Galic, M.; Zuzul, S. Cadmium and Mercury Phytostabilization from Soil Using Miscanthus × giganteus. Sci. Rep. 2020, 10, 6685. [Google Scholar] [CrossRef] [Green Version]
- Nurzhanova, A.; Pidlisnyuk, V.; Abit, K.; Nurzhanov, C.; Kenessov, B.; Stefanovska, T.; Erickson, L. Comparative Assessment of Using Miscanthus × giganteus for Remediation of Soils Contaminated by Heavy Metals: A Case of Military and Mining Sites. Environ. Sci. Pollut. Res. 2019, 26, 13320–13333. [Google Scholar] [CrossRef] [PubMed]
- Korzeniowska, J.; Stanislawska-Glubiak, E. Phytoremediation Potential of Miscanthus × giganteus and Spartina Pectinata in Soil Contaminated with Heavy Metals. Environ. Sci. Pollut. Res. 2015, 22, 11648–11657. [Google Scholar] [CrossRef]
- Pogrzeba, M.; Krzyżak, J.; Sas-Nowosielska, A. Environmental Hazards Related to Miscanthus × giganteus Cultivation on Heavy Metal Contaminated Soil. E3S Web Conf. 2013, 1, 29006. [Google Scholar] [CrossRef] [Green Version]
- Al Souki, K.S.; Liné, C.; Moravec, J.; Douay, F.; Pourrut, B. Response of Three Miscanthus × giganteus Cultivars to Toxic Elements Stress: Part 2, Comparison between Two Growing Seasons. Plants 2022, 11, 945. [Google Scholar] [CrossRef]
- Nsanganwimana, F.; Al Souki, K.S.; Waterlot, C.; Douay, F.; Pelfrêne, A.; Ridošková, A.; Louvel, B.; Pourrut, B. Potentials of Miscanthus × giganteus for Phytostabilization of Trace Element-Contaminated Soils: Ex Situ Experiment. Ecotoxicol. Environ. Saf. 2021, 214, 112125. [Google Scholar] [CrossRef]
- Al Souki, K.S.; Louvel, B.; Douay, F.; Pourrut, B. Assessment of Miscanthus × giganteus Capacity to Restore the Functionality of Metal-Contaminated Soils: Ex Situ Experiment. Appl. Soil Ecol. 2017, 115, 44–52. [Google Scholar] [CrossRef]
- Al Souki, K.S.; Liné, C.; Louvel, B.; Waterlot, C.; Douay, F.; Pourrut, B. Miscanthus × giganteus Culture on Soils Highly Contaminated by Metals: Modelling Leaf Decomposition Impact on Metal Mobility and Bioavailability in the Soil–Plant System. Ecotoxicol. Environ. Saf. 2020, 199, 110654. [Google Scholar] [CrossRef]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy Metal Tolerance in Plants: Role of Transcriptomics, Proteomics, Metabolomics and Ionomics. Front. Plant Sci. 2016, 6, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Ullah, F.; Zhou, D.-X.; Zhao, Y. Mechanisms of ROS Regulation of Plant Development and Stress Responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Pourrut, B.; Shahid, M.; Dumat, C.; Winterton, P.; Pinelli, E. Lead Uptake, Toxicity, and Detoxification in Plants. Rev. Environ. Contam. Toxicol. 2011, 213, 113–136. [Google Scholar]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and Redox Signalling in the Response of Plants to Abiotic Stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, B.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, S.; Zulfiqar, F.; Alam, M.; Fujita, M. Regulation of ROS Metabolism in Plants under Environmental Stress: A Review of Recent Experimental Evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, S.; Huang, Y.; Zhou, S. The Tolerance and Accumulation of Miscanthus sacchariflorus (Maxim.) Benth., an Energy Plant Species, to Cadmium. Int. J. Phytoremediat. 2015, 17, 538–545. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [Green Version]
- Das, S.K.; Patra, J.K.; Thatoi, H. Antioxidative Response to Abiotic and Biotic Stresses in Mangrove Plants: A Review. Int. Rev. Hydrobiol. 2016, 101, 3–19. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as Important Molecules of Plant Interactions with the Environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baek, S.-A.; Han, T.; Ahn, S.-K.; Kang, H.; Cho, M.R.; Lee, S.-C.; Im, K.-H. Effects of Heavy Metals on Plant Growths and Pigment Contents in Arabidopsis Thaliana. Plant Pathol. J. 2012, 28, 446–452. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Hong, C.; Chen, X.; Xu, Y.; Liu, Y.; Jiang, D.; Zheng, B. Different Growth and Physiological Responses to Cadmium of the Three Miscanthus Species. PLoS ONE 2016, 11, e0153475. [Google Scholar] [CrossRef] [Green Version]
- Nowack, B.; Koehler, S.; Schulin, R. Use of Diffusive Gradients in Thin Films (DGT) in Undisturbed Field Soils. Environ. Sci. Technol. 2004, 38, 1133–1138. [Google Scholar] [CrossRef]
- Wang, Z.; Shan, X.-Q.; Zhang, S. Comparison between Fractionation and Bioavailability of Trace Elements in Rhizosphere and Bulk Soils. Chemosphere 2002, 46, 1163–1171. [Google Scholar] [CrossRef]
- Conesa, H.M.; Robinson, B.H.; Schulin, R.; Nowack, B. Growth of Lygeum Spartum in Acid Mine Tailings: Response of Plants Developed from Seedlings, Rhizomes and at Field Conditions. Environ. Pollut. 2007, 145, 700–707. [Google Scholar] [CrossRef]
- Cheng, S. Effects of Heavy Metals on Plants and Resistance Mechanisms. Environ. Sci. Pollut. Res. 2003, 10, 256–264. [Google Scholar] [CrossRef]
- Robinson, B.H.; Leblanc, M.; Petit, D.; Brooks, R.R.; Kirkman, J.H.; Gregg, P.E.H. The Potential of Thlaspi Caerulescens for Phytoremediation of Contaminated Soils. Plant Soil 1998, 203, 47–56. [Google Scholar] [CrossRef]
- Hu, Y.; Nan, Z.; Su, J.; Wang, N. Heavy Metal Accumulation by Poplar in Calcareous Soil with Various Degrees of Multi-Metal Contamination: Implications for Phytoextraction and Phytostabilization. Environ. Sci. Pollut. Res. 2013, 20, 7194–7203. [Google Scholar] [CrossRef]
- Sterckeman, T.; Douay, F.; Proix, N.; Fourrier, H. Vertical Distribution of Cd, Pb and Zn in Soils near Smelters in the North of France. Environ. Pollut. 2000, 107, 377–389. [Google Scholar] [CrossRef]
- Joret, F.; Hébert, J. Contribution à La Détermination Du Besoin Des Sols En Acide Phosphorique. Ann. Agron. Ser. 1955, 6, 233–299. [Google Scholar]
- Waterlot, C.; Bidar, G.; Pelfrêne, A.; Roussel, H.; Fourrier, H.; Douay, F. Contamination, Fractionation and Availability of Metals in Urban Soils in the Vicinity of Former Lead and Zinc Smelters, France. Pedosphere 2013, 23, 143–159. [Google Scholar] [CrossRef]
- Al Souki, K.S.; Liné, C.; Douay, F.; Pourrut, B. Response of Three Miscanthus × giganteus Cultivars to Toxic Elements Stress: Part 1, Plant Defence Mechanisms. Plants 2021, 10, 2035. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases: I. Occurrence in Higher Plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]





| MC | M200 | M500 | M700 | |
|---|---|---|---|---|
| Clay (%) | 18.9 ± 1.1 | 21.3 ± 4.7 | 31.1 ± 1.9 | 19.5 ± 2.0 |
| Silt (%) | 69.8 ± 1.4 | 49.8 ± 9.0 | 52.2 ± 3.9 | 53.0 ± 2.5 |
| Sand (%) | 11.3 ± 0.9 | 28.9 ± 10.6 | 16.7 ± 5.2 | 27.4 ± 1.3 |
| pH | 6.2 ± 0.1 | 7.5 ± 0.5 | 7.7 ± 0.1 | 7.5 ± 0.4 |
| SOC (g kg−1) | 18.3 ± 3.7 | 16.4 ± 1.8 | 35.7 ± 1.6 | 18.2 ± 0.4 |
| TN (g kg−1) | 1.6 ± 0.1 | 1.2 ± 0.2 | 2.2 ± 0.3 | 1.1 ± 0.1 |
| P2O5 (g kg−1) | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 |
| CEC (cmol+ kg−1) | 11.2 ± 1.2 | 16.3 ± 1.5 | 32.8 ± 2.1 | 14.9 ± 1.6 |
| CaCO3 (g kg−1) | 1.1 ± 0.2 | 7.7 ± 0.9 | 22.7 ± 2.8 | 10.2 ± 1.7 |
| Cd (mg kg−1) | 0.5 ± 0.0 | 5.1 ± 0.3 | 10.0 ± 0.8 | 15.0 ± 1.2 |
| Pb (mg kg−1) | 10.9 ± 0.8 | 203.5 ± 7.8 | 496.9 ± 12.7 | 703.8 ± 21.9 |
| Zn (mg kg−1) | 49.8 ± 3.9 | 329.0 ± 19.2 | 549.1 ± 14.2 | 1018.8 ± 27.3 |
| Cd (mg kg−1) | Pb (mg kg−1) | Zn (mg kg−1) | |
|---|---|---|---|
| MC | 0.42 ± 0.02 a | 1.1 ± 0.1 a | 31.41 ± 2.29 a |
| M200 | 0.43 ± 0.03 a | 9.64 ± 2.29 b | 44.73 ± 2.62 b |
| M500 | 0.46 ± 0.04 a | 11.87 ± 1.48 b | 55.80 ± 5.13 c |
| M700 | 0.69 ± 0.04 b | 12.85 ± 2.16 b | 64.60 ± 5.69 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastia, G.; Al Souki, K.S.; Pourrut, B. Evaluation of Miscanthus × giganteus Tolerance to Trace Element Stress: Field Experiment with Soils Possessing Gradient Cd, Pb, and Zn Concentrations. Plants 2023, 12, 1560. https://doi.org/10.3390/plants12071560
Bastia G, Al Souki KS, Pourrut B. Evaluation of Miscanthus × giganteus Tolerance to Trace Element Stress: Field Experiment with Soils Possessing Gradient Cd, Pb, and Zn Concentrations. Plants. 2023; 12(7):1560. https://doi.org/10.3390/plants12071560
Chicago/Turabian StyleBastia, Giulia, Karim Suhail Al Souki, and Bertrand Pourrut. 2023. "Evaluation of Miscanthus × giganteus Tolerance to Trace Element Stress: Field Experiment with Soils Possessing Gradient Cd, Pb, and Zn Concentrations" Plants 12, no. 7: 1560. https://doi.org/10.3390/plants12071560
APA StyleBastia, G., Al Souki, K. S., & Pourrut, B. (2023). Evaluation of Miscanthus × giganteus Tolerance to Trace Element Stress: Field Experiment with Soils Possessing Gradient Cd, Pb, and Zn Concentrations. Plants, 12(7), 1560. https://doi.org/10.3390/plants12071560






