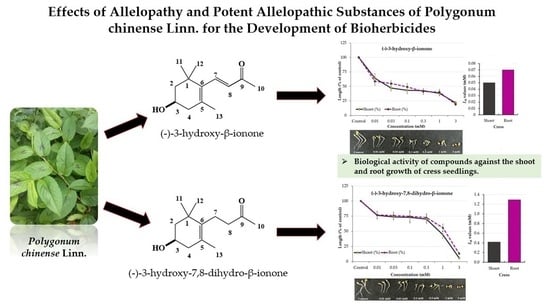
Isolation and Identification of Plant-Growth Inhibitory Constituents from Polygonum chinense Linn and Evaluation of Their Bioherbicidal Potential
Abstract
:1. Introduction
2. Results
2.1. Growth Inhibitory of Polygonum chinense Plant Extracts
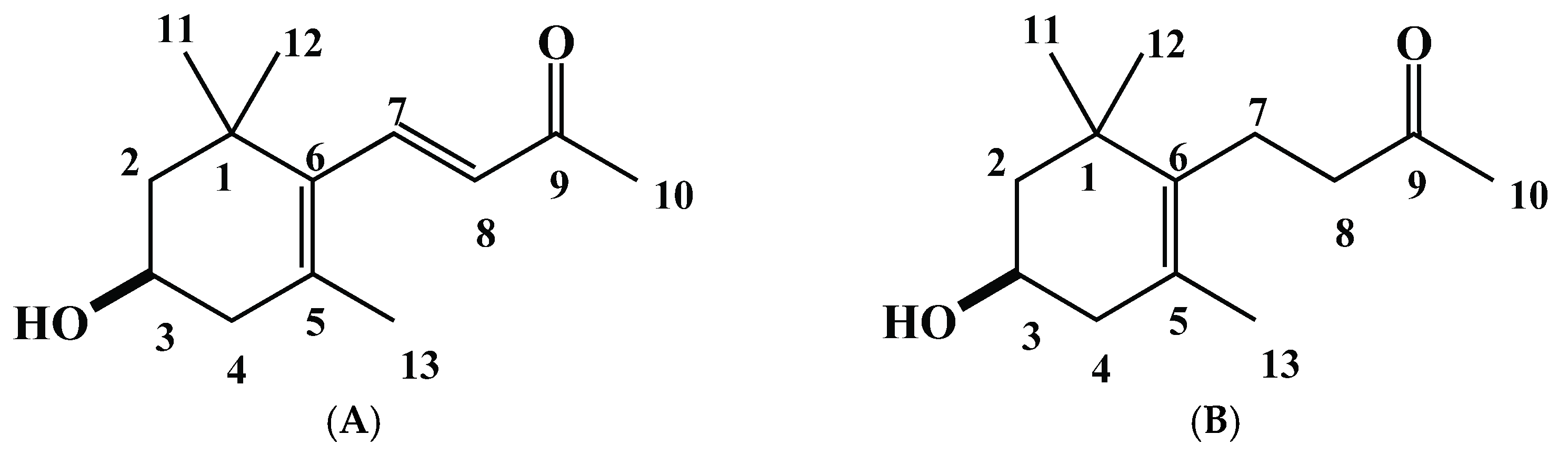
2.2. Isolation and Characterization of the Allelopathic Substances from the Polygonum chinense Extracts
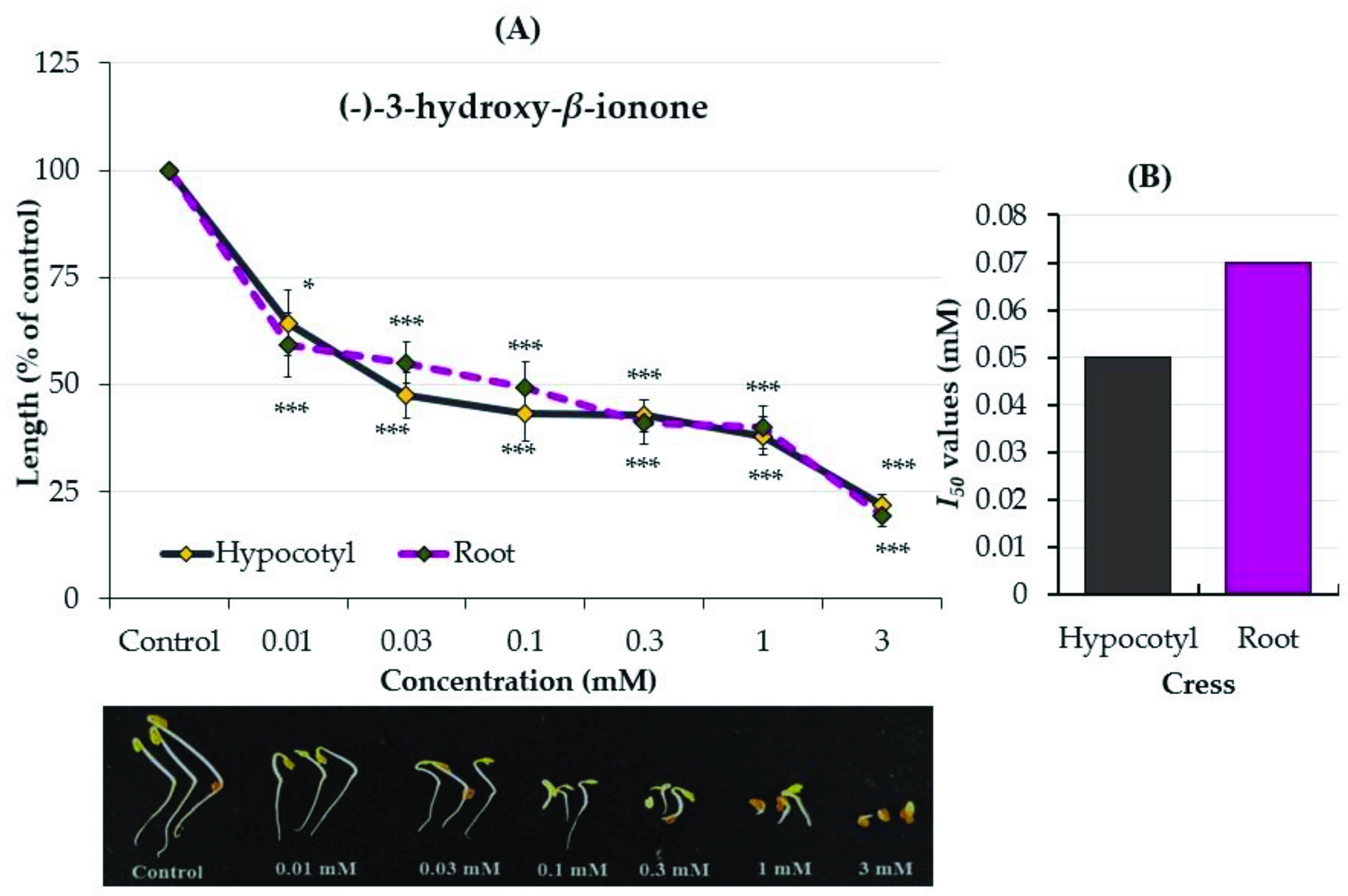
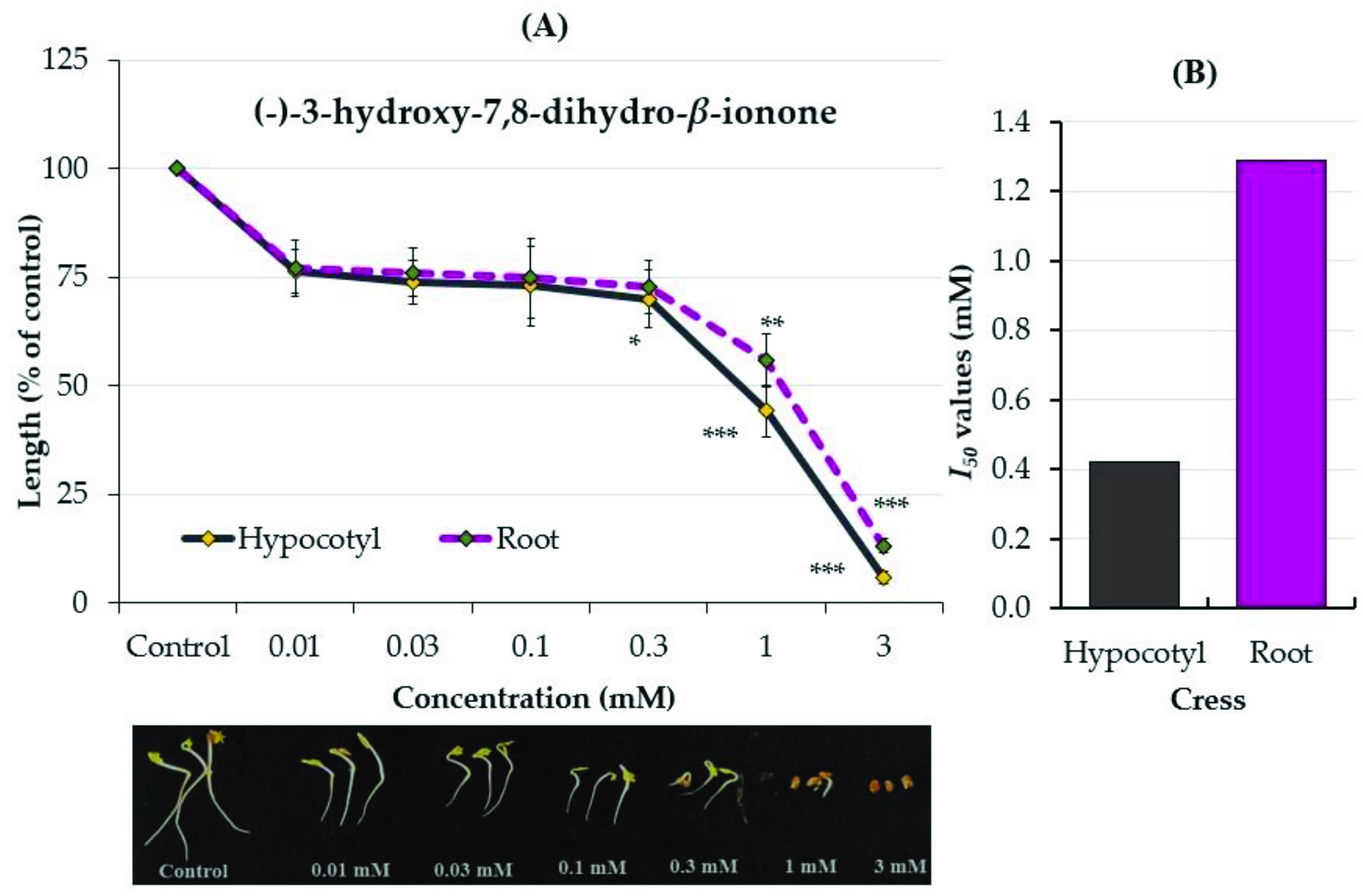
2.3. Growth Inhibitory Effects of the Two Compounds
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Extraction and Growth Inhibition Assay of P. chinense
4.3. Separation and Isolation of Active Substances from the Polygonum chinense Extracts
4.4. Germination Test for the Compounds
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kropff, M.J.; Walter, H. EWRS and the challenges for weed research at the start of a new millennium. Weed Res. 2000, 40, 7–10. [Google Scholar] [CrossRef]
- Awan, T.H.; Cruz, P.C.S.; Chauhan, B.S. Agronomic indices, growth, yield-contributing traits, and yield of dry-seeded rice under varying herbicides. Field Crop. Res. 2015, 177, 5–25. [Google Scholar] [CrossRef]
- Khamare, Y.; Chen, J.; Marble, S.C. Allelopathy, and its application as a weed management tool: A review. Front. Plant Sci. 2022, 13, 1034649. [Google Scholar] [CrossRef] [PubMed]
- Molisch, H. Der Einfluss Einer Pflanze auf die Andere-Allelopathie; Gustav Fischer Verlag: Jena, Germany, 1937; p. 136. [Google Scholar]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984. [Google Scholar]
- Cheng, F.; Cheng, Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Weston, P.A.; Gu, L.; Zhang, B.Y.; Li, M.J.; Wang, F.Q. Identification of phytotoxic metabolites released from Rehmannia glutinosa suggest their importance in the formation of its replant problem. Plant Soil. 2019, 441, 439–454. [Google Scholar] [CrossRef]
- Favaretto, A.; Chini, S.O.; Basso, S.M.S.; Sobottka, A.M.; Bertol, C.D.; Perez, N.B. Pattern of allelochemical distribution in leaves and roots of tough lovegrass (Eragrostis plana Nees.). Aust. J. Crop Sci. 2015, 9, 1119–1125. [Google Scholar]
- Tuyen, P.T.; Xuan, T.D.; Anh, T.T.T.; Van, T.M.; Ahmad, A.; Elzaawely, A.A.; Khanh, T.D. Weed Suppressing Potential and Isolation of Potent Plant Growth Inhibitors from Castanea crenata Sieb. et Zucc. Molecules 2018, 23, 345. [Google Scholar] [CrossRef] [Green Version]
- Selvi, E.K.; Turumatay, H.; Demir, A.; Turumatay, E.A. Phytochemical profiling and evaluation of the hepatoprotective effect of Cuscuta campestris by high-performance liquid chromatography with diode array detection. Anal. Lett. 2018, 51, 1464–1478. [Google Scholar] [CrossRef]
- Shaikh, A.C.; Gupta, A.; Chaphalkar, S.R. Identification of structurally unique molecules, phytochemical and immunological activity of medicinal plants. Int. J. Med. Pharm. Res. 2016, 4, 223–230. [Google Scholar]
- Zaïdi, M.A.; Huda, A.; Crow, S.A. Biological activity and elemental composition of Arceuthobium oxyedri (Dwarf Mistletoe) of juniper forest of Pakistan. Acta Bot. Hung. 2008, 50, 223–230. [Google Scholar] [CrossRef]
- Latif, S.G.; Chiapusio, G.; Weston, L.A. Chapter two-Allelopathy and the role of allelochmicals in plant defence. Adv. Bot. Res. 2017, 82, 19–54. [Google Scholar]
- Weston, L.A.; Duke, S.O. Weed and crop allelopathy. Crit. Rev. Plant Sci. 2003, 22, 367–389. [Google Scholar] [CrossRef]
- Han, C.; Zhou, S.; Mei, Y.; Cao, Q.; Shi, K.; Shao, H. Phytotoxic, insecticidal, and antimicrobial activities of Ajania tibetica essential oil. Front. Plant Sci. 2022, 13, 1028252. [Google Scholar] [CrossRef]
- Bhadoria, P. Allelopathy: A natural way towards weed management. Am. J. Exp. Agric. 2010, 1, 7–20. [Google Scholar] [CrossRef]
- Farnsworth, N.R. The role of ethnopharmacology in drug development. In Bioactive Compounds from Plants; Chadwick, D.J., Marsh, J., Eds.; Ciba Foundation Symposium 154; Wiley: Chichester, UK, 1990; pp. 2–11. [Google Scholar]
- Batish, D.R.; Arora, K.; Singh, H.P.; Kohli, R.K. Potential utilization of dried powder of Tagetes minuta as a natural herbicide for managing rice weeds. Crop Prot. 2006, 10, 1016. [Google Scholar] [CrossRef]
- Hong, N.H.; Xuan, T.D.; Tsuzuki, E.; Terao, H.; Mitsuhiro, M.; Khanhc, T.D. Weed control of four higher plant species in paddy rice fields in Southeast Asia. J. Agron. Crop Sci 2004, 190, 59–64. [Google Scholar] [CrossRef]
- Xuan, T.D.; Tawata, S.; Khanh, T.D.; Chung, I.M. Biological control of weeds and plant pathogens in paddy rice by exploiting plant allelopathy. Crop Prot. 2005, 24, 197–206. [Google Scholar] [CrossRef]
- Lin, D.; Tsuzuki, E.; Sugimoto, Y.; Dong, Y.; Matsuo, M.; Terao, H. Elementary identification of phenolic allelochemicals from dwarf lilyturf (Ophiopogon japonicus K.) against two weeds of paddy rice field. Plant Prod. Sci. 2004, 7, 260–265. [Google Scholar] [CrossRef]
- Tongma, S.; Kobayashi, K.; Usui, K. Allelopathic activity of Mexican sunflower [Tithonia diversifolia (Hemsl.) A. Gray] in soil under natural field conditions and different moisture conditions. Weed Biol. Manag. 2001, 1, 115–119. [Google Scholar] [CrossRef]
- Lin, W.X.; He, H.B.; Xiong, J.; Shen, L.H.; Wu, M.H.; Lin, R.Y.; He, H.Q.; Liang, Y.Y.; Li, Z.W.; Chen, T. Advances in the investigation of rice allelopathy and its molecular ecology. Acta Ecol. Sin. 2006, 26, 2687–2694. [Google Scholar]
- Ogushi, Y.; Terao, H.; Tsuzuki, E. Studies on the allelopathy in Kava (Piper methysticum). Jpn. J. Crop Sci. 2000, 69, 190–191. [Google Scholar]
- Fujii, Y. Allelopathy of hairy vetch and mucuna: Their application for sustainable agriculture. In Biodiversity and Allelopathy from Organisms to Ecosystems in the Pacific; Chou, C.H., Waller, G.R., Reinhardt, C., Eds.; Academia Sinica: Taipei, Taiwan, 1999; pp. 289–300. [Google Scholar]
- Fujii, Y.; Parvez, S.S.; Parvez, M.M.; Ohmae, S.; Iida, O. Screening of 239 medicinal plant species for allelopathic activity using the sandwich method. Weed Biol. Manag. 2003, 3, 233–241. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Hamada, Y.; Kojima, M.; Kumagai, S.; Iwasaki, A.; Suenaga, K. Allelopathic Substances of Osmanthus spp. For Developing Sustainable Agriculture. Plants 2023, 12, 376. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Salam, M.A.; Ohno, O.; Suenaga, K. Nimbolide B and nimbic acid B, phytotoxic substances in neem leaves with allelopathic activity. Molecules 2014, 19, 6929–6940. [Google Scholar] [CrossRef] [Green Version]
- Kyaw, E.H.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Assessment of the Phytotoxic Potential of Dregea volubilis (L.f.) Benth. ex Hook. f. and Identification of its Phytotoxic Substances for Weed Control. Agriculture 2022, 12, 1826. [Google Scholar] [CrossRef]
- Hossen, K.; Das, K.R.; Asato, Y.; Teruya, T.; Kato-Noguchi, H. Allelopathic activity and characterization of allelopathic substances from Elaeocarpus floribundus Blume leaves for the development of bioherbicides. Agronomy 2022, 12, 57. [Google Scholar] [CrossRef]
- Moh, S.M.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Allelopathic activity of a novel compound, 5,6-dihydrogen-11-O-acetyl-12-O-tigloyl-17-marsdenin, and a known steroidal glycoside from the leaves of Marsdenia tenacissima (Roxb.) Moon. Agronomy 2022, 12, 1536. [Google Scholar] [CrossRef]
- Lun, T.L.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Two allelopathic substances from Plumbago rosea stem extracts and their allelopathic effects. Agronomy 2022, 12, 2020. [Google Scholar] [CrossRef]
- Rob, M.M.; Iwasaki, A.; Suzuki, R.; Suenaga, K.; Kato-Noguchi, H. Garcienone, a Novel Compound Involved in Allelopathic Activity of Garcinia xanthochymus Hook. Plants 2019, 8, 301. [Google Scholar] [CrossRef] [Green Version]
- Fan, P.; Hostettmann, K.; Lou, H. Allelochemicals of the invasive neophyte Polygonum cuspidatum Sieb. & Zucc. (Polygonaceae). Chemoecology 2010, 20, 223–227. [Google Scholar] [CrossRef]
- Sanchez, A.; Tanja, M.; Schuster, J.M.; Kron, B.K.A. Taxonomy of Polygonoideae (Polygonaceae): A new tribal classification. Taxon 2011, 60, 151–160. [Google Scholar] [CrossRef]
- DeFilipps, R.A.; Krupnick, G.A. The medicinal plants of Myanmar. PhytoKeys 2018, 102, 1–341. [Google Scholar] [CrossRef] [Green Version]
- China Pharmacopoeia Committee (CPC). Pharmacopoeia of the People’s Republic of China; China Chemical Industry Press: Beijing, China, 1999; p. 167.
- Maharajan, M.; Rajendran, A. Taxonomic studies on selected species of the genus Polygonum L. (Polygonaceae) in South India. J. Sci. 2014, 4, 144–148. Available online: http://www.journalofscience.net/File_Folder/144-148 (accessed on 3 March 2023).
- Swingle, W.T. The Botany of Citrus and Its Wild Relatives in the Citrus Industry; University of California Press: Berkeley, CA, USA, 1967; pp. 190–430. Available online: http://lib.ucr.edu/agnic/webber/Vol1/Chapter3.html (accessed on 3 March 2023).
- Srividya, A.R.; Shalom, A.; Chandrasekhar, R.; Vijayan, P.; Vishnuvarthtan, V.J. Cytotoxic, Antioxidant and Antimicrobial Activity of Polygonum chinensis Linn. Int. J. Pharm. Sci. Nanotechnol. IJPSN 2012, 4, 1569–1574. [Google Scholar] [CrossRef]
- Lai, S.M.; Sudhahar, D.; Anandarajagopal, K. Evaluation of Antibacterial and Antifungal activities of Persicaria chinensis Leaves. Int. J. Pharm. Sci. Res. 2012, 3, 2825–2830. [Google Scholar]
- Hossen, M.J.; Kim, S.C.; Son, Y.J.; Baek, K.S.; Kim, E.; Yang, W.S.; Jeong, D.; Park, J.G.; Kim, H.G.; Chung, W.J.; et al. AP-1-Targeting Anti-Inflammatory Activity of the Methanolic Extract of Persicaria chinensis. Hindawi Publ. Corp. Evid. Based Complement. Altern. Med. 2015, 2015, 608126. [Google Scholar] [CrossRef] [Green Version]
- Hossen, M.J.; Baek, K.S.; Kim, E.; Yang, W.S.; Jeong, D.; Kim, J.H.; Kweon, D.H.; Yoon, D.H.; Kim, T.W.; Kim, J.H.; et al. In vivo and in vitro anti-inflammatory activities of Persicaria chinensis methanolic extract targeting Src/Syk/NF-κB. J. Ethnopharmacol. 2015, 159, 9–16. [Google Scholar] [CrossRef]
- Xiao, H.T.; Tsang, S.W.; Qin, H.Y.; Choi, F.F.K.; Yang, Z.J.; Han, Q.B.; Chen, H.B.; Xu, H.X.; Shen, H.; Lu, A.P.; et al. A bioactivity-guided study on the anti-diarrheal activity of Polygonum chinense Linn. J. Ethnopharmacol. 2013, 149, 499–505. [Google Scholar] [CrossRef]
- Ismail, I.F.; Golbabapour, S.; Hassandarvish, P.; Hajrezaie, M.; Majid, N.A.; Kadir, F.A.; Al-Bayaty, F.; Awang, K.; Hazni, H. and Abdulla, M.A. Gastroprotective Activity of Polygonum chinense Aqueous Leaf Extract on Ethanol-Induced Hemorrhagic Mucosal Lesions in Rats. Evid. Based Complement. Altern. Med. 2012, 9, 404012. [Google Scholar]
- Manasa, K.S.; Kuppast, I.J.; Kishan, K.M.A.; Akshara, K. A review on Polygonum chinensis. Res. J. Pharmacol. Pharmacodyn. 2016, 8, 185–188. [Google Scholar] [CrossRef]
- Fujimori, T.; Kasuga, R.; Noguchi, M.; Kaneko, H. Isolation of R-(−)-3-hydroxy-β-ionone from burley tobacco. Agric Biol Chem. 1974, 38, 891–892. [Google Scholar]
- Perez, C.; Trujillo, J.; Almonacid, L.N.; Trujillo, J.; Navarro, E.; Alonso, S.J. Absolute structures of two new Cl3-norisoprenoids from Apollonias barbujana. J. Nat. Prod. 1996, 59, 69–72. [Google Scholar] [CrossRef]
- Macías, F.A.; Lacret, R.; Varela, R.M.; Nogueiras, C.; Molinillo, J.M.G. Bioactive apocarotenoids from Tectona grandis. Phytochemistry 2008, 69, 2708–2715. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-C.; Fang, J.-M.; Cheng, Y.-S. Terpenes and lignans from leaves of Chamaecyparis formosensis. Phytochemistry 1999, 51, 793–801. [Google Scholar] [CrossRef]
- Lun, T.L.; Kato-Noguchi, H. Assessment of the allelopathic potential of Leucas cephalotes (Roth) Spreng. extracts on the seedling growth of six test plants. Plant Omics J. 2021, 14, 72–77. [Google Scholar]
- Kannan, E.; Palayian, L. Allelopathic potential of Annona muricata (L.) on physiological and biochemical changes of Vigna radiata (L.) and Eleusine coracana (L.) Gaertn. J. Appl. Biol. Biotechnol. 2022, 10, 145–153. [Google Scholar] [CrossRef]
- Krumsri, R.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Assessment of allelopathic potential of Senna garrettiana leaves and identification of potent phytotoxic substances. Agronomy 2022, 12, 139. [Google Scholar] [CrossRef]
- Khan, M.S.I.; Kato-Noguchi, H. Assessment of allelopathic potential of Couroupita guianensis Aubl. POJ 2016, 9, 115–120. [Google Scholar] [CrossRef]
- Krumsri, R.; Boonmee, S.; Kato-Noguchi, H. Evaluation of the allelopathic potential of leaf extracts from Dischidia imbricata (Blume) Steud. on the seedling growth of six test plants. Not. Bot. Horti Agrobot. 2019, 47, 1019–1024. [Google Scholar] [CrossRef] [Green Version]
- Nishida, N.; Tamotsu, S.; Nagata, N.; Saito, C.; Sakai, A. Allelopathic effects of volatile monoterpenoids produced by Salvia leucophylla: Inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. J. Chem. Ecol. 2005, 31, 1187–1203. [Google Scholar] [CrossRef]
- Rial, C.; Novaes, P.; Varela, R.M.G.; Molinillo, J.M.; Macias, F.A. Phytotoxicity of cardoon (Cynara cardunculus) allelochemicals on standard target species and weeds. J. Agric. Food Chem. 2014, 62, 6699–6706. [Google Scholar] [CrossRef]
- Yoshimura, H.; Sawa, Y.; Tamotsu, S.; Sakai, A. 1,8-cineole inhibits both proliferation and elongation of by-2 cultured tobacco cells. J. Chem. Ecol. 2011, 37, 320–328. [Google Scholar] [CrossRef] [Green Version]
- Enzell, C. Biodegradation of carotenoids-an important route to aroma components. Pure Appl. Chem. 1985, 57, 693–700. [Google Scholar] [CrossRef]
- Schwab, W.; Schreier, P. Simultaneous enzyme catalysis extraction: A versatile technique for the study of flavor precursors. J. Agric. Food Chem. 1988, 36, 1238–1242. [Google Scholar] [CrossRef]
- Strauss, C.R.; Gooley, P.R.; Wilson, B.; Williams, P.J. Application of droplet countercurrent chromatography for the analysis of conjugated forms of terpenoids, phenols, and other constituents of grape juice. J. Agric. Food Chem. 1987, 35, 519–524. [Google Scholar] [CrossRef]
- Schwab, W.; Mahr, C.; Schreier, P. Studies on the enzymic hydrolysis of bound aroma components from Carica papaya fruit. J. Agric. Food Chem. 1989, 37, 1009–1012. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. An endogenous growth inhibitor, 3-hydroxy-β-ionone. I. Its role in light-induced growth inhibition of hypocotyls of Phaseolus vulgaris. Physiol. Plant. 1992, 86, 583–586. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Yamamoto, M.; Tamura, K.; Teruya, T.; Suenaga, K.; Fujii, Y. Isolation and identification of potent allelopathic substances in rattail fescue. Plant Grow. Regul. 2010, 60, 127–131. [Google Scholar] [CrossRef]
- Masum, S.M.; Hossain, M.A.; Akamine, H.; Sakagami, J.I.; Ishii, T.; Gima, S.; Kensaku, T.; Bhowmik, P.C. Isolation and characterization of allelopathic compounds from the indigenous rice variety ‘Boterswar’ and their biological activity against Echinochloa crus-galli L. Allelopath. J. 2018, 43, 31–42. [Google Scholar] [CrossRef]
- Ida, N.; Iwasaki, A.; Teruya, T.; Suenaga, K.; Kato-Noguchi, H. Tree fern Cyathea lepifera may survive by its phytotoxic property. Plants 2020, 9, 46. [Google Scholar] [CrossRef] [Green Version]
- Aloum, L.; Alefishat, E.; Adem, A.; Petroianu, G. Ionone is more than a violet’s fragrance: A review. Molecules 2020, 25, 5822. [Google Scholar] [CrossRef] [PubMed]
- Miyase, T.; Ueno, A.; Takizawa, N.; Kobayashi, H.; Oguchi, H. Studies on the glycosides of Epimedium grandiflorum var thunbergianum miq. Nakai. Chem. Pharm. Bull. 1988, 36, 2475–2484. [Google Scholar] [CrossRef] [Green Version]
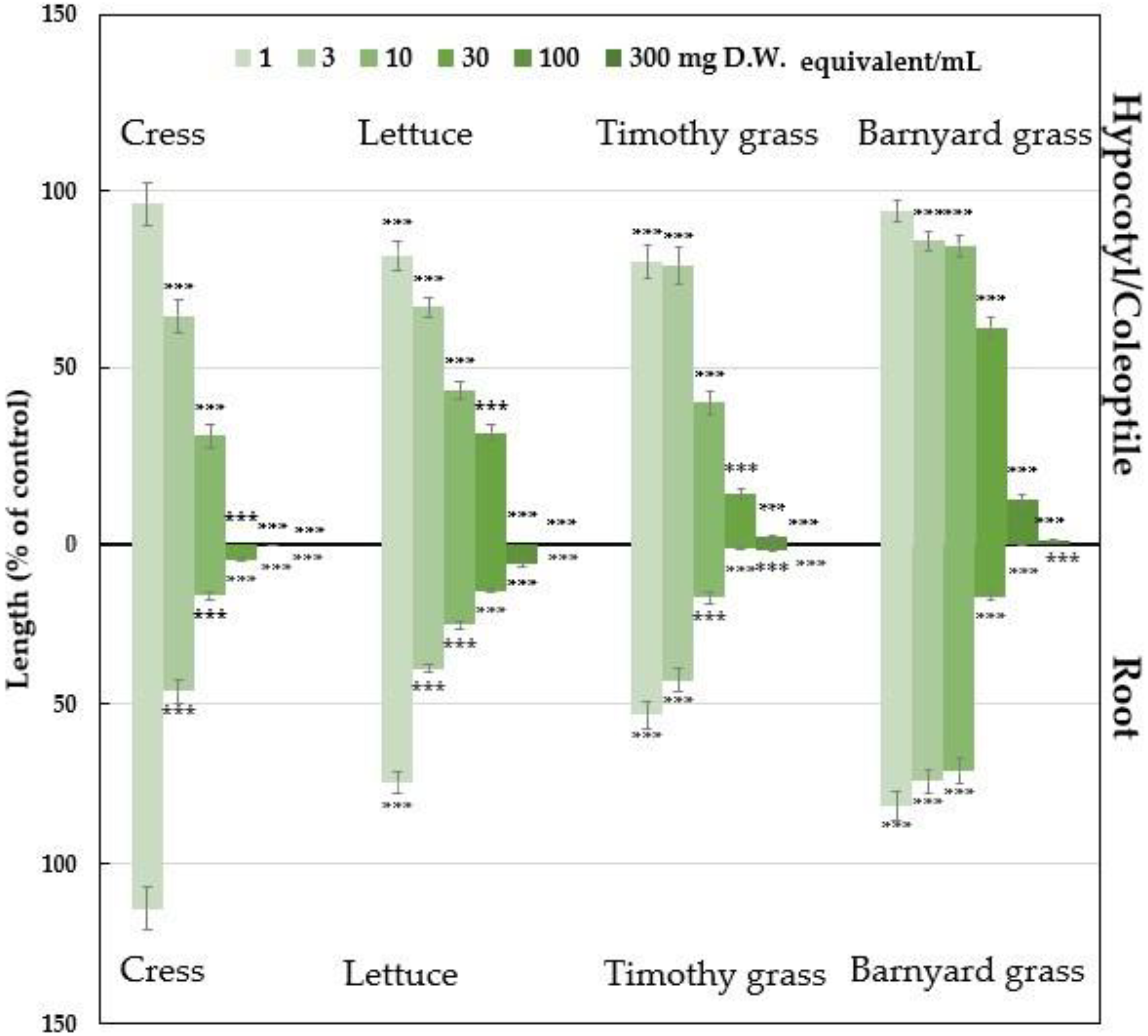

| Test Plants | I50 Value (mg D.W. Equivalent Extract/mL) | Correlation Coefficient (R) | ||
|---|---|---|---|---|
| Hypocotyl/ Coleoptile | Root | Hypocotyl/ Coleoptile | Root | |
| Cress | 5.01 | 2.97 | −0.789 ** | −0.744 ** |
| Lettuce | 7.25 | 2.54 | −0.852 ** | −0.864 ** |
| Timothy grass | 7.00 | 1.46 | −0.803 ** | −0.796 ** |
| Barnyard grass | 35.09 | 11.70 | −0.832 ** | −0.805 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lun, T.L.; Iwasaki, A.; Suenaga, K.; Kato-Noguchi, H. Isolation and Identification of Plant-Growth Inhibitory Constituents from Polygonum chinense Linn and Evaluation of Their Bioherbicidal Potential. Plants 2023, 12, 1577. https://doi.org/10.3390/plants12071577
Lun TL, Iwasaki A, Suenaga K, Kato-Noguchi H. Isolation and Identification of Plant-Growth Inhibitory Constituents from Polygonum chinense Linn and Evaluation of Their Bioherbicidal Potential. Plants. 2023; 12(7):1577. https://doi.org/10.3390/plants12071577
Chicago/Turabian StyleLun, Thang Lam, Arihiro Iwasaki, Kiyotake Suenaga, and Hisashi Kato-Noguchi. 2023. "Isolation and Identification of Plant-Growth Inhibitory Constituents from Polygonum chinense Linn and Evaluation of Their Bioherbicidal Potential" Plants 12, no. 7: 1577. https://doi.org/10.3390/plants12071577
APA StyleLun, T. L., Iwasaki, A., Suenaga, K., & Kato-Noguchi, H. (2023). Isolation and Identification of Plant-Growth Inhibitory Constituents from Polygonum chinense Linn and Evaluation of Their Bioherbicidal Potential. Plants, 12(7), 1577. https://doi.org/10.3390/plants12071577