Effects of OsRCA Overexpression on Rubisco Activation State and Photosynthesis in Maize
Abstract
1. Introduction
2. Results
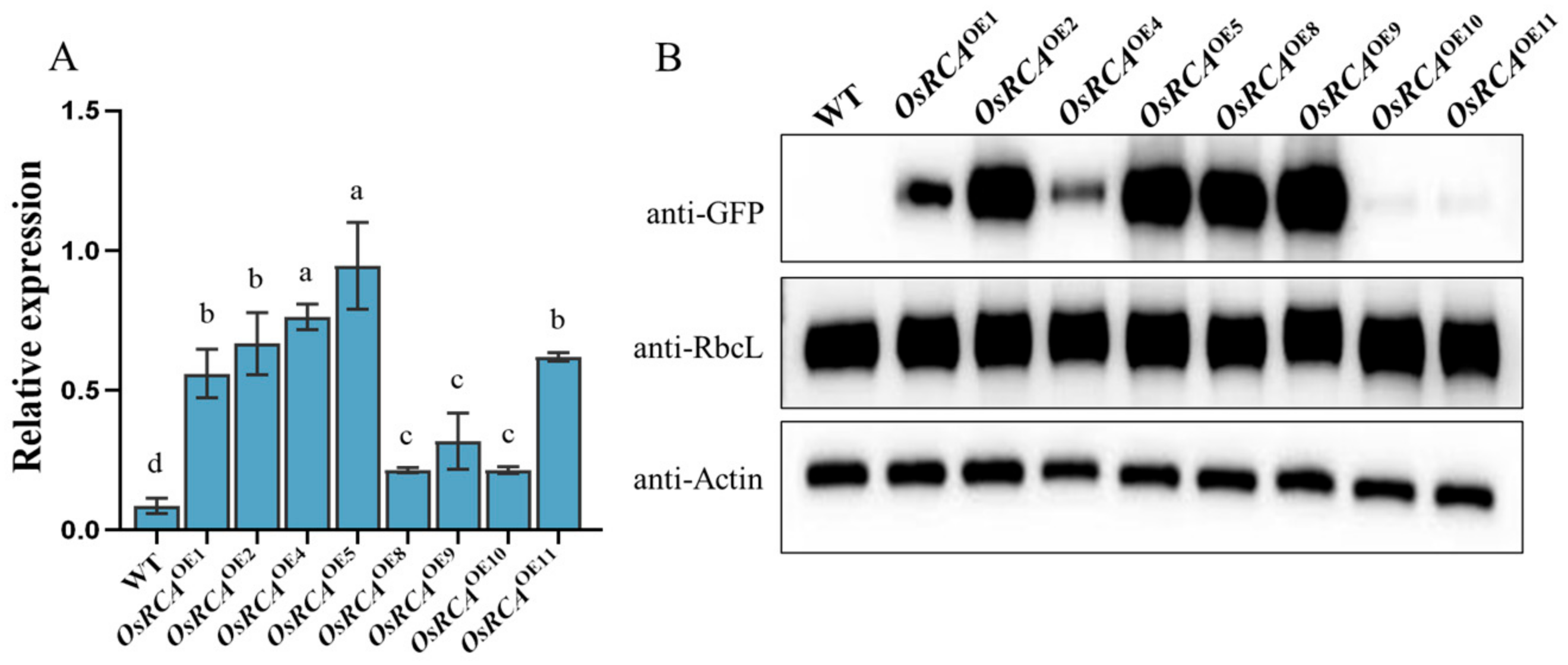
2.1. Molecular Characterization of Transgenic Plants
2.2. Rubisco Activity, Content, and Activation State in Transgenic Lines
2.3. Gas Exchange Parameters of Transgenic Lines
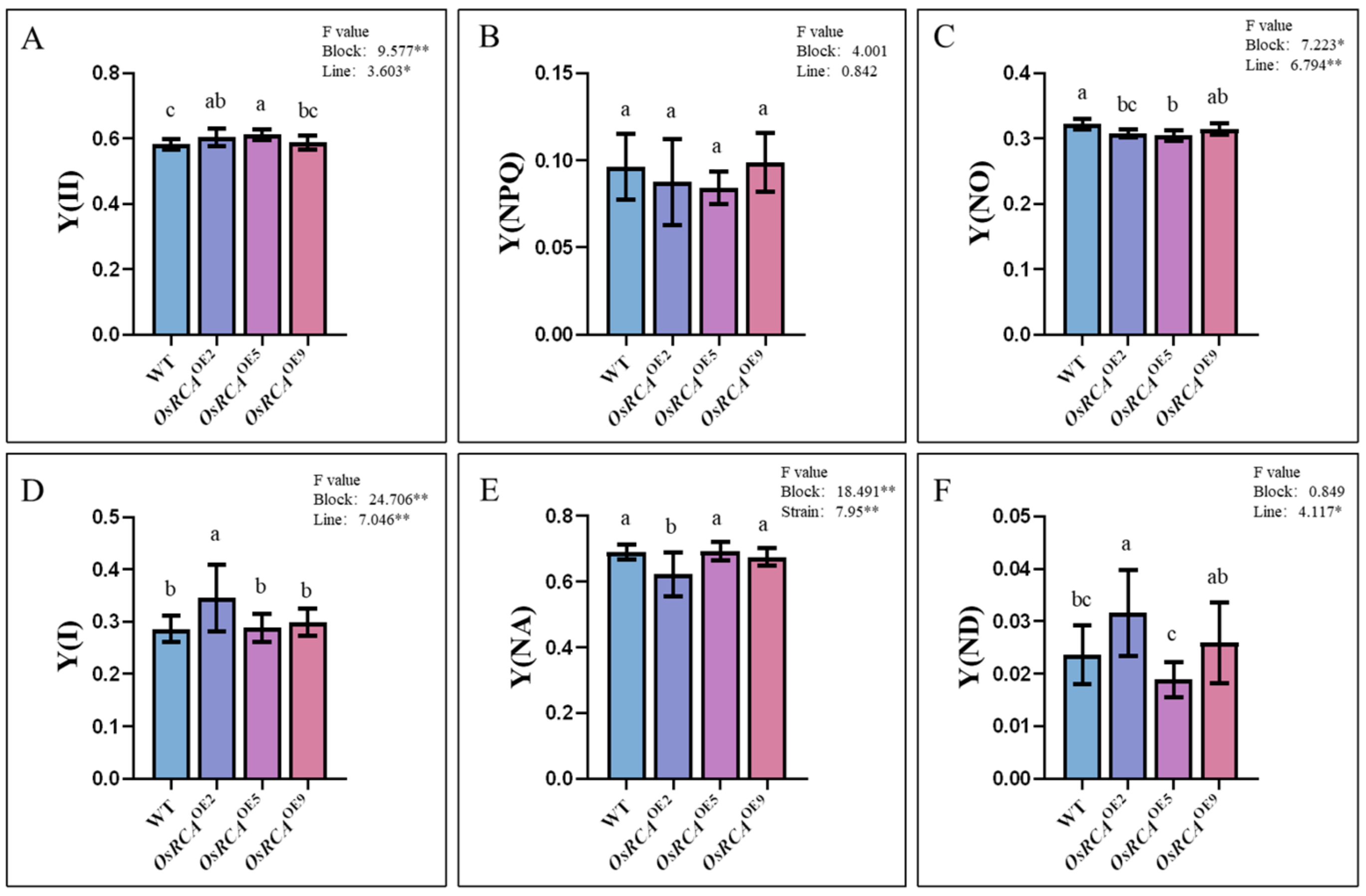
2.4. PSII and PSI Energy Conversion–Related Parameters in Transgenic Lines
3. Discussion
4. Materials and Methods
4.1. Cloning and Transformation of Rice RCA
4.2. Plant Materials and Growth Conditions
4.3. Real–Time PCR Assay
4.4. Western–Blot Analysis of OsRCA
4.5. Rubisco Protein Quantification by ELISA
4.6. Measurement of Gas Exchange
4.7. Rubisco Activity and Activation State in Leaf Extracts
4.8. Measurement of PSII and PSI Energy Conversion–Related Parameters
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nelson, N.; Ben-Shem, A. The complex architecture of oxygenic photosynthesis. Nat. Rev. Mol. Cell Biol. 2004, 5, 971–982. [Google Scholar] [CrossRef] [PubMed]
- von Caemmerer, S.; Evans, J.R. Enhancing C3 photosynthesis. Plant Physiol. 2010, 154, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, Y.; Lyu, M.A.; Zhu, X. Two major metabolic factors for an efficient NADP–malic enzyme type C4 photosynthesis. Plant Physiol. 2022, 189, 84–98. [Google Scholar] [CrossRef] [PubMed]
- P’yankov, V.; Voznesenskaya, E.; Kondratschuk, A.; Black, C. A comparative anatomical and biochemical analysis in salsola (Chenopodiaceae) species with and without a Kranz type leaf anatomy: A possible reversion of C4 to C3 photosynthesis. Am. J. Bot. 1997, 84, 597. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Bräutigam, A.; Weber, A.P.; Zhu, X.G. Systems analysis of cis–regulatory motifs in C4 photosynthesis genes using maize and rice leaf transcriptomic data during a process of de–etiolation. J. Exp. Bot. 2016, 67, 5105–5117. [Google Scholar] [CrossRef]
- Salesse-Smith, C.E.; Sharwood, R.E.; Busch, F.A.; Kromdijk, J.; Bardal, V.; Stern, D.B. Overexpression of Rubisco subunits with RAF1 increases Rubisco content in maize. Nat. Plants 2018, 4, 802–810. [Google Scholar] [CrossRef]
- Carmo-Silva, E.; Scales, J.C.; Madgwick, P.J.; Parry, M.A. Optimizing Rubisco and its regulation for greater resource use efficiency. Plant Cell Environ. 2015, 38, 1817–1832. [Google Scholar] [CrossRef]
- Seemann, J.R.; Kirschbaum, M.U.; Sharkey, T.D.; Pearcy, R.W. Regulation of Ribulose–1,5–bisphosphate carboxylase activity in Alocasia macrorrhiza in response to step changes in irradiance. Plant Physiol. 1988, 88, 148–152. [Google Scholar] [CrossRef]
- Chao, M.; Yin, Z.; Hao, D.; Zhang, J.; Song, H.; Ning, A.; Xu, X.; Yu, D. Variation in Rubisco activase (RCAβ) gene promoters and expression in soybean [Glycine max (L.) Merr]. J. Exp. Bot. 2014, 65, 47–59. [Google Scholar] [CrossRef]
- Martínez-Barajas, E.; Molina-Galán, J.D.; Jiménez, E.S. Regulation of Rubisco activity during grain–fill in maize: Possible role of Rubisco activase. J. Agric. Sci. 1997, 128, 155–161. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, Z.; Deng, D.; Chao, M.; Gao, Q.; Wang, Y.; Yang, Z.; Bian, Y.; Hao, D.; Xu, C. Characterization of Rubisco activase genes in maize: An α–isoform gene functions alongside a β–isoform gene. Plant Physiol. 2014, 164, 2096–2106. [Google Scholar] [CrossRef]
- Wang, Y.; Chan, K.X.; Long, S.P. Towards a dynamic photosynthesis model to guide yield improvement in C4 crops. Plant J. 2021, 107, 343–359. [Google Scholar] [CrossRef]
- von Caemmerer, S.; Hendrickson, L.; Quinn, V.; Vella, N.; Millgate, A.G.; Furbank, R.T. Reductions of Rubisco activase by antisense RNA in the C4 plant Flaveria bidentis reduces Rubisco carbamylation and leaf photosynthesis. Plant Physiol. 2005, 137, 747–755. [Google Scholar] [CrossRef]
- To, K.Y.; Suen, D.F.; Chen, S.C. Molecular characterization of ribulose–1,5–bisphosphate carboxylase/oxygenase activase in rice leaves. Planta 1999, 209, 66–76. [Google Scholar] [CrossRef]
- Fukayama, H.; Ueguchi, C.; Nishikawa, K.; Katoh, N.; Ishikawa, C.; Masumoto, C.; Hatanaka, T.; Misoo, S. Overexpression of rubisco activase decreases the photosynthetic CO2 assimilation rate by reducing rubisco content in rice leaves. Plant Cell Physiol. 2012, 53, 976–986. [Google Scholar] [CrossRef]
- Aluru, M.R.; Stessman, D.J.; Spalding, M.H.; Rodermel, S.R. Alterations in photosynthesis in Arabidopsis lacking IMMUTANS, a chloroplast terminal oxidase. Photosyn. Res. 2007, 91, 11–23. [Google Scholar] [CrossRef]
- Zhou, R.; Kan, X.; Chen, J.; Hua, H.; Li, Y.; Ren, J.; Feng, K.; Liu, H.; Deng, D.; Yin, Z. Drought–induced changes in photosynthetic electron transport in maize probed by prompt fluorescence, delayed fluorescence, P700 and cyclic electron flow signals. Environ. Exp. Bot. 2019, 158, 51–62. [Google Scholar] [CrossRef]
- Singh, J.; Pandey, P.; James, D.; Chandrasekhar, K.; Achary, V.M.; Kaul, T.; Tripathy, B.C.; Reddy, M.K. Enhancing C3 photosynthesis: An outlook on feasible interventions for crop improvement. Plant Biotechnol. J. 2014, 12, 1217–1230. [Google Scholar] [CrossRef]
- Suganami, M.; Suzuki, Y.; Kondo, E.; Nishida, S.; Konno, S.; Makino, A. Effects of overproduction of Rubisco activase on Rubisco content in transgenic rice grown at different N levels. Int. J. Mol. Sci. 2020, 21, 1626. [Google Scholar] [CrossRef]
- Nagarajan, R.; Gill, K.S. Evolution of Rubisco activase gene in plants. Plant Mol. Biol. 2018, 96, 69–87. [Google Scholar] [CrossRef]
- Salvucci, M.E. Association of Rubisco activase with chaperonin–60β: A possible mechanism for protecting photosynthesis during heat stress. J. Exp. Bot. 2007, 59, 1923–1933. [Google Scholar] [CrossRef] [PubMed]
- Degen, G.E.; Worrall, D.; Carmo-Silva, E. An isoleucine residue acts as a thermal and regulatory switch in wheat Rubisco activase. Plant J. 2020, 103, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Fukayama, H.; Mizumoto, A.; Ueguchi, C.; Katsunuma, J.; Morita, R.; Sasayama, D.; Hatanaka, T.; Azuma, T. Expression level of Rubisco activase negatively correlates with Rubisco content in transgenic rice. Photosyn. Res. 2018, 137, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, N.A.; Snyder, G.W.; Portis, A.R.; Orgen, W.L., Jr. Growth and photosynthesis under high and low irradiance of Arabidopsis thaliana antisense mutants with reduced ribulose–1,5–bisphosphate carboxylase/oxygenase activase content. Plant Physiol. 1997, 113, 575–586. [Google Scholar] [CrossRef]
- Wu, H.R. Photosynthetic Characterizations of Transgenic Rice with Rca Gene Encoding the Large Isoformof Rice Rubisco Activase. Ph.D. Thesis, The Institute of Botany of the Chinese Academy of Sciences, Beijing, China, 2004. [Google Scholar]
- Bi, H.; Liu, P.; Jiang, Z.; Ai, X. Overexpression of the rubisco activase gene improves growth and low temperature and weak light tolerance in Cucumis sativus. Physiol. Plant 2017, 161, 224–234. [Google Scholar] [CrossRef]
- Chen, G.Y.; Chen, J.; Xu, D.Q. Thinking about the relationship between net photosynthetic rate and intercellular CO2 concentration. Plant Physiol. J. 2010, 46, 64–66. [Google Scholar]
- Zhang, D.; Wang, X.; Chen, Y.; Xu, D. Determinant of photosynthetic capacity in rice leaves under ambient air conditions. Photosynthetica 2005, 43, 273–276. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Govindjee, Eds.; Kluwer Academic Publishers Press: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Stirbet, A.; Govindjee. On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and Photosystem II: Basics and applications of the OJIP fluorescence transient. J. Photochem. Photobiol. B 2011, 104, 236–257. [Google Scholar] [CrossRef]
- Suganami, M.; Konno, S.; Maruhashi, R.; Takagi, D.; Tazoe, Y.; Wada, S.; Yamamoto, H.; Shikanai, T.; Ishida, H.; Suzuki, Y.; et al. Expression of flavodiiron protein rescues defects in electron transport around PSI resulting from overproduction of Rubisco activase in rice. J. Exp. Bot. 2022, 73, 2589–2600. [Google Scholar] [CrossRef]
- Niyogi, K.K.; Grossman, A.R.; Björkman, O. Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 1998, 10, 1121–1134. [Google Scholar] [CrossRef]
- Chen, W.; Jia, B.; Chen, J.; Feng, Y.; Li, Y.; Chen, M.; Liu, H.; Yin, Z. Effects of different planting densities on photosynthesis in maize determined via prompt fluorescence, delayed Fluorescence and P700 signals. Plants 2021, 10, 276. [Google Scholar] [CrossRef]
- Schreiber, U. Pulse–Amplitude–Modulation (PAM) fluorometry and saturation pulse method: An overview. In Chlorophyll a Fluorescence a Signature of Photosynthesis; Springer: Dordrecht, The Netherlands, 2004; pp. 279–319. [Google Scholar]
- Yamori, W.; Masumoto, C.; Fukayama, H.; Makino, A. Rubisco activase is a key regulator of non–steady–state photosynthesis at any leaf temperature and, to a lesser extent, of steady–state photosynthesis at high temperature. Plant J. Cell Mol. Biol. 2012, 71, 871–880. [Google Scholar] [CrossRef]

| Lines | Net Photosynthetic Rate (μmol·m−2·s−1) | Intercellular CO2 Concentration | Stomatal Conductance (mol·m−2·s−1) | Transportation Rate (m mol·m−2·s−1) |
|---|---|---|---|---|
| WT | 29.694 ± 4.490c | 47.500 ± 11.776a | 163.125 ± 27.217a | 5.124 ± 0.580a |
| OsRCAOE2 | 33.610 ± 4.962a | 30.700 ±1 4.407bc | 175.600 ± 26.222a | 5.0520 ± 0.491a |
| OsRCAOE5 | 32.700 ± 3.945ab | 26.308 ± 16.373c | 167.846 ± 19.196a | 4.861 ± 0.333a |
| OsRCAOE9 | 30.120 ± 4.380bc | 37.933 ± 13.172ab | 160.333 ± 27.168a | 4.937 ± 0.448a |
| F value | ||||
| Block | 34.926 ** | 1.448 | 28.217 ** | 0.840 |
| Line | 5.258 ** | 6.453 ** | 2.220 | 0.925 |
| Fluorescence parameter | Description |
|---|---|
| Y(II) = (Fm’ − Fs’)/Fm’ | PSII photochemical energy conversion yield |
| Y(NPQ) = Fs’/Fm’ − Fs’/Fm | Quantum yield of regulatory energy dissipation at PSII |
| Y(NO) = Fs’/Fm | Quantum production of non–regulatory energy dissipation at PSII |
| Y(ND) = 1 − P700red | Non–photochemical quantum yield of the PSI donor side |
| Y(NA) = (Pm − Pm’)/Pm | Non–photochemical quantum yield of the PSI acceptor side |
| Y(I) = 1 − Y(ND) − Y(NA) | Photochemical efficiency of PSI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Wu, H.; Liu, H.; He, Y.; Yin, Z. Effects of OsRCA Overexpression on Rubisco Activation State and Photosynthesis in Maize. Plants 2023, 12, 1614. https://doi.org/10.3390/plants12081614
Feng Y, Wu H, Liu H, He Y, Yin Z. Effects of OsRCA Overexpression on Rubisco Activation State and Photosynthesis in Maize. Plants. 2023; 12(8):1614. https://doi.org/10.3390/plants12081614
Chicago/Turabian StyleFeng, Yujiao, Hao Wu, Huanhuan Liu, Yonghui He, and Zhitong Yin. 2023. "Effects of OsRCA Overexpression on Rubisco Activation State and Photosynthesis in Maize" Plants 12, no. 8: 1614. https://doi.org/10.3390/plants12081614
APA StyleFeng, Y., Wu, H., Liu, H., He, Y., & Yin, Z. (2023). Effects of OsRCA Overexpression on Rubisco Activation State and Photosynthesis in Maize. Plants, 12(8), 1614. https://doi.org/10.3390/plants12081614





