Analysis of the Relative Importance of Stand Structure and Site Conditions for the Productivity, Species Diversity, and Carbon Sequestration of Cunninghamia lanceolata and Phoebe bournei Mixed Forest
Abstract
1. Introduction
2. Results
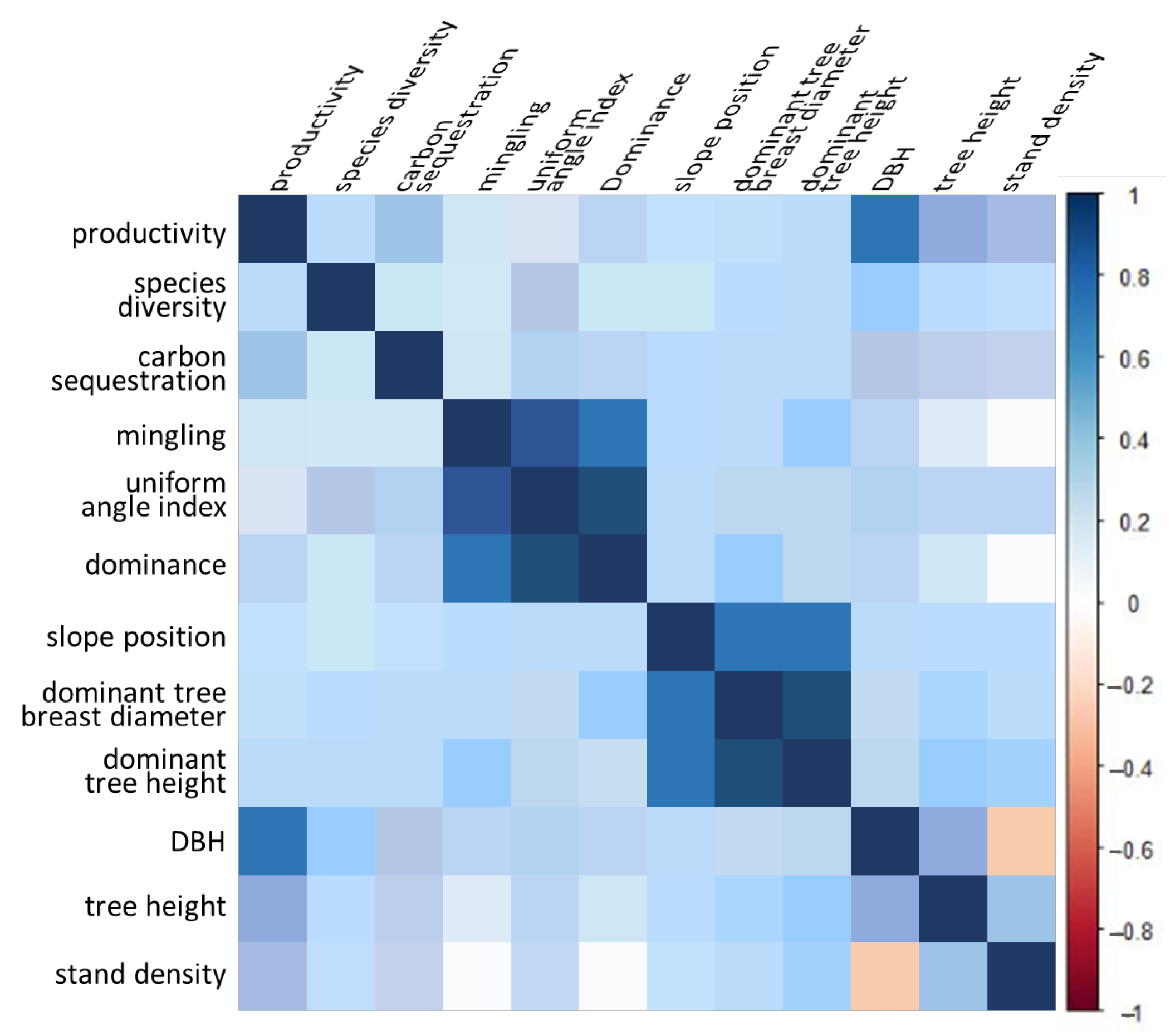
2.1. Response between Observed Variables
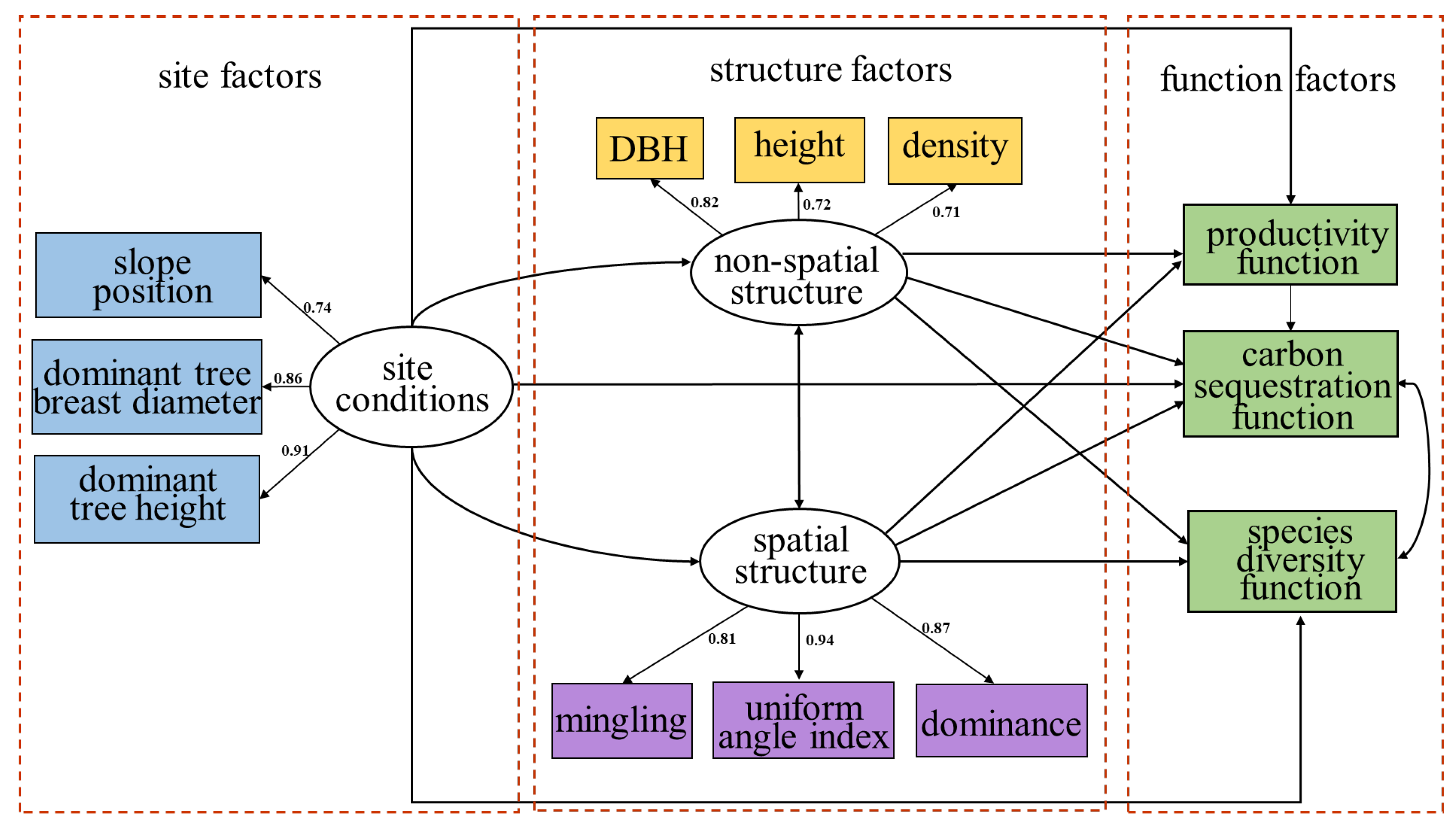
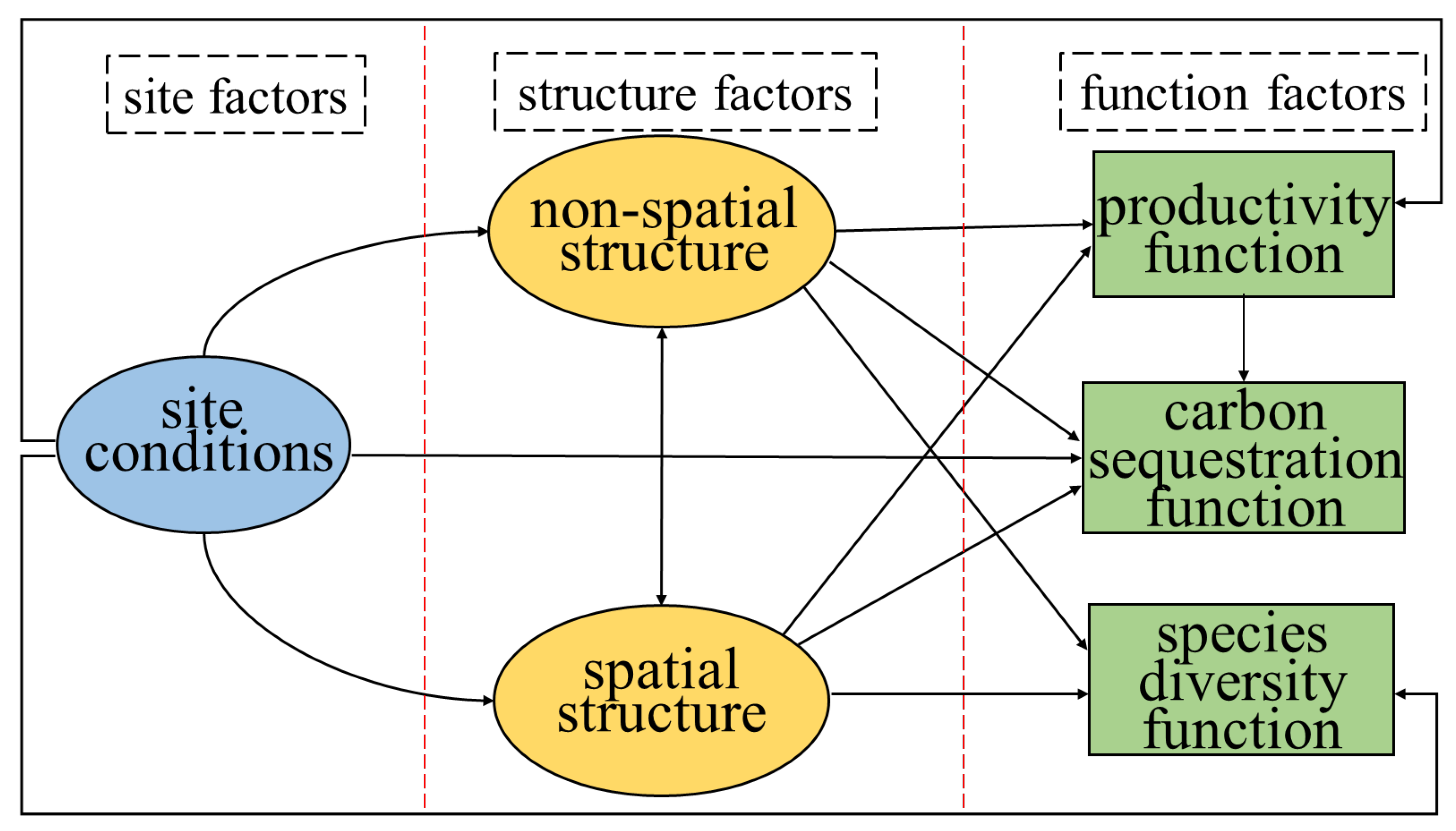
2.2. Path Diagram and Standardized Coefficients in the SEM Analysis
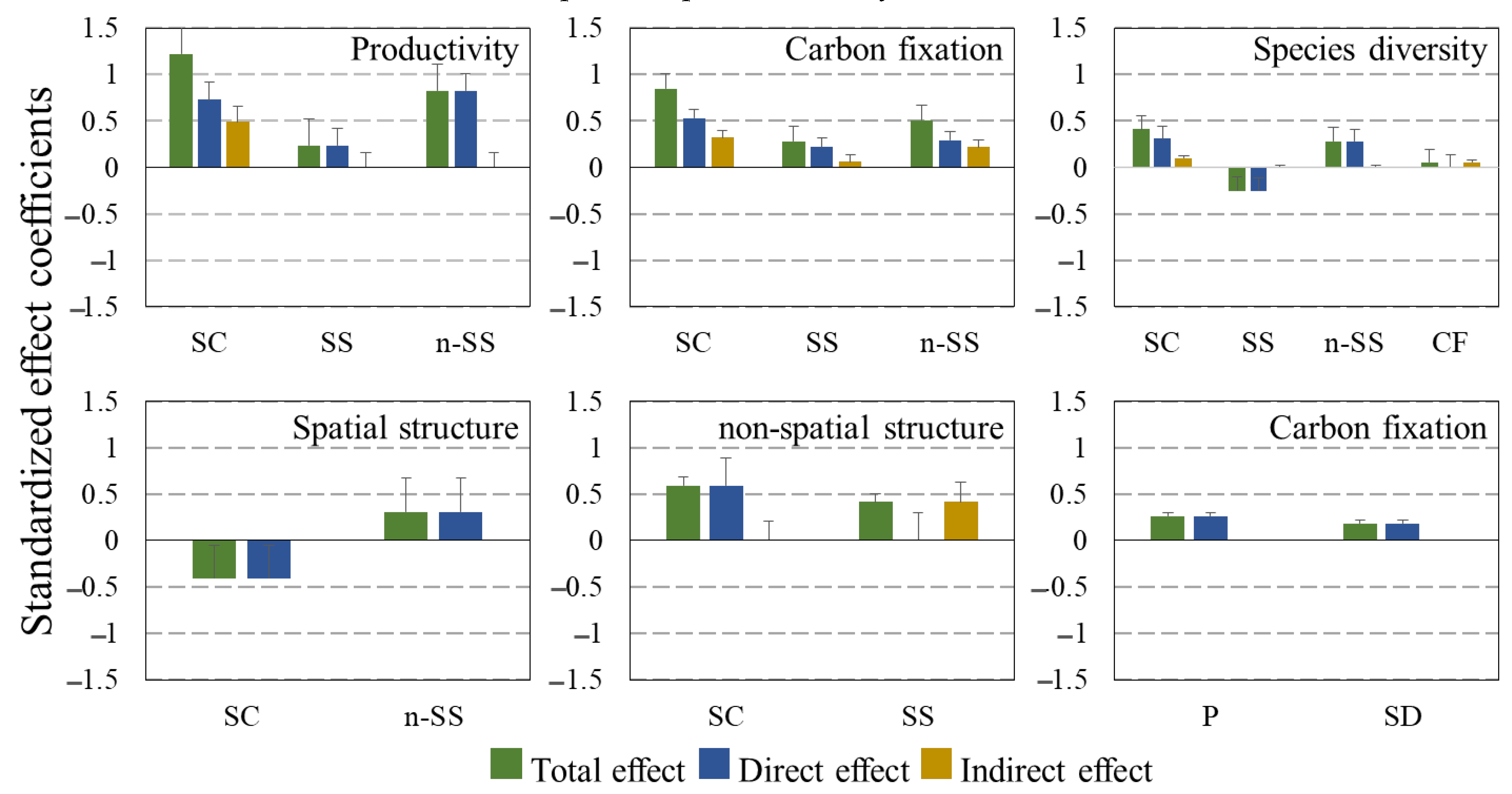
2.3. Multi-Factors Analysis of Stand Structures, Site Conditions and Forest Functions
3. Discussion
4. Materials and Methods
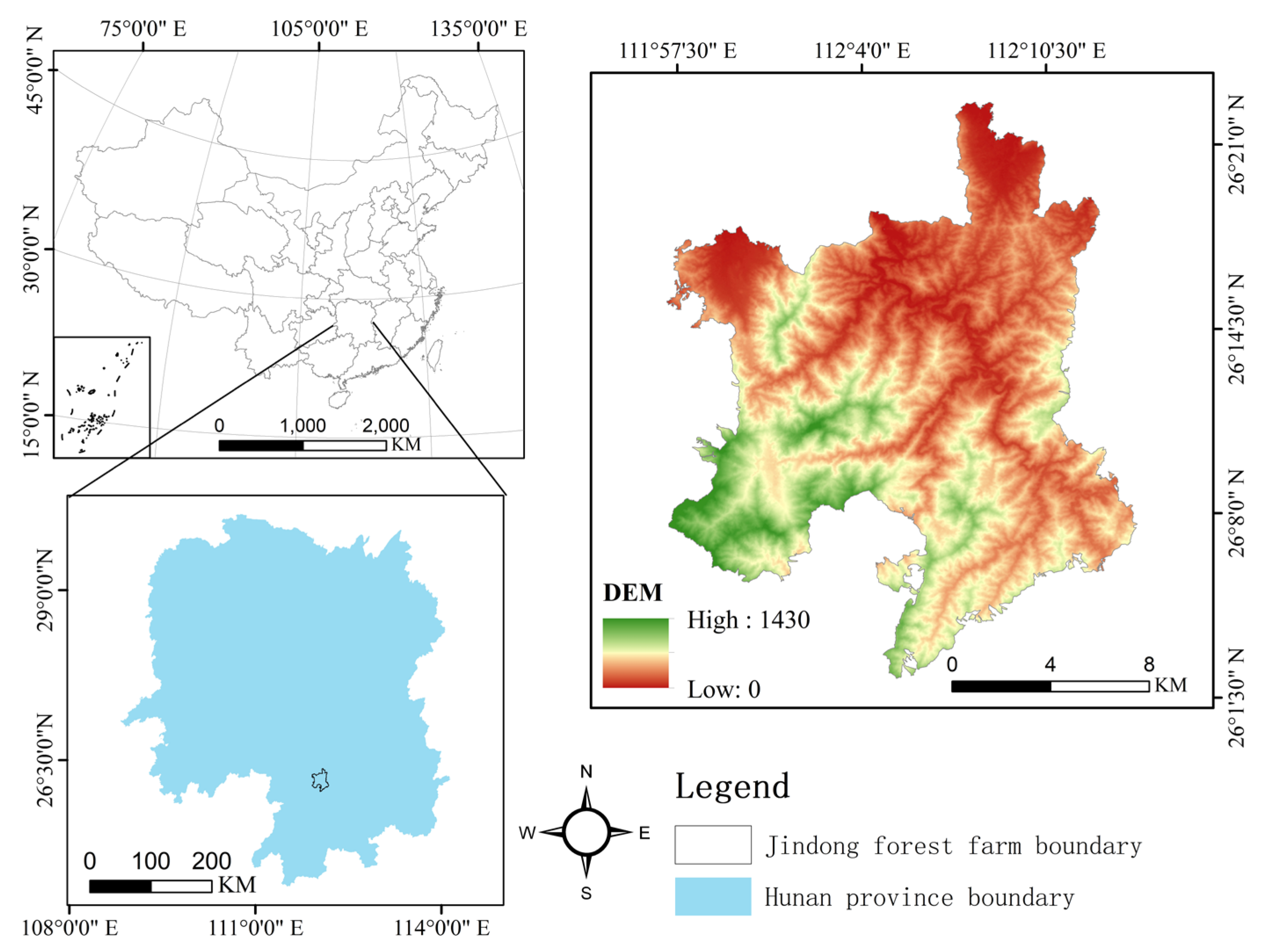
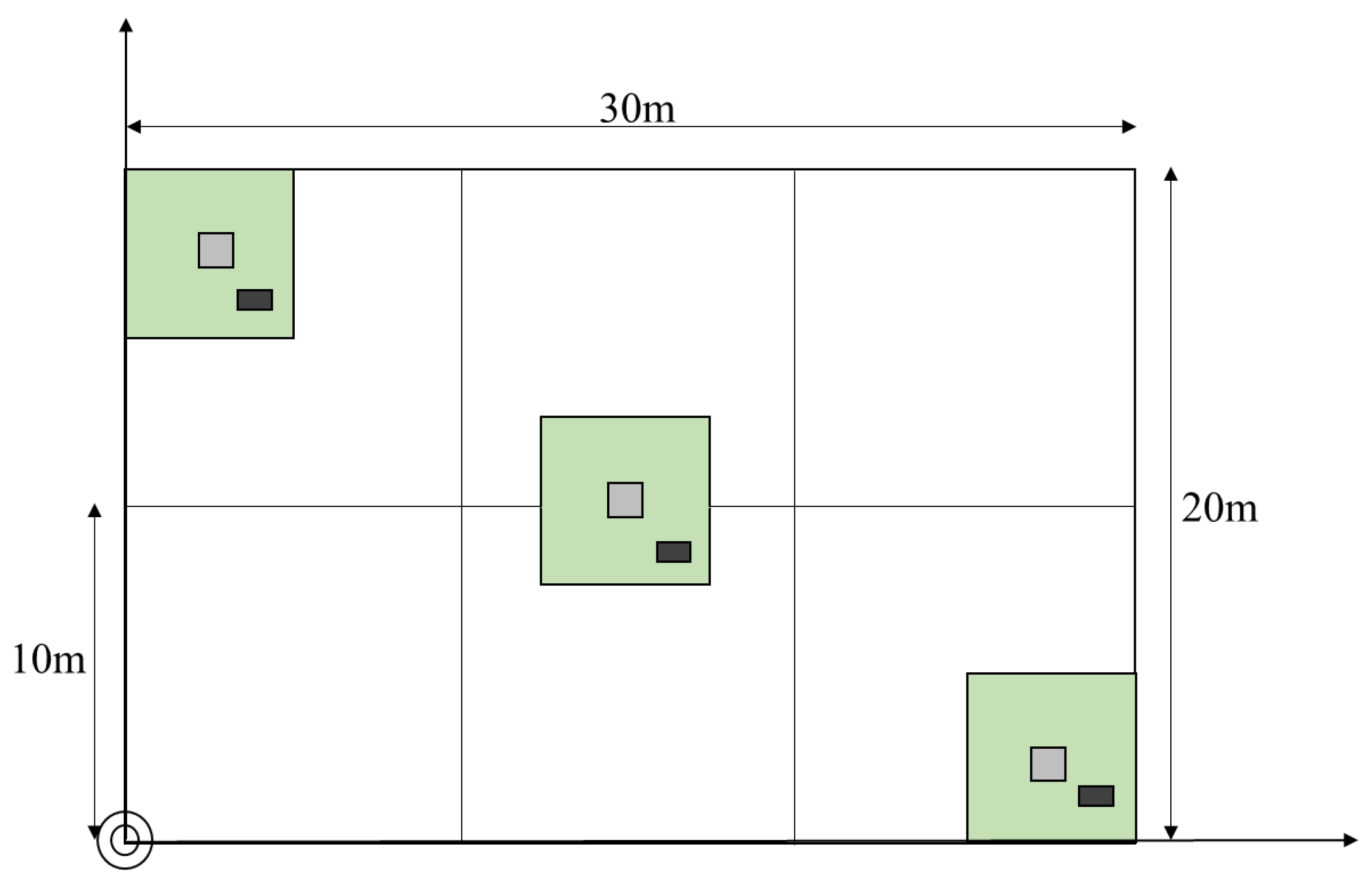
4.1. Study Site
4.2. Data Collection
4.3. Data Processing
4.3.1. Productivity Measurement
4.3.2. Carbon Sequestration of Vegetation and Soil
4.3.3. Species Diversity
4.4. Structural Equation Model
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aubinet, M.; Chermanne, B.; Vandenhaute, M.; Longdoz, B.; Yernaux, M.; Laitat, E. Long term carbon dioxide exchange above a mixed forest in the Belgian Ardennes. Agric. For. Meteorol. 2001, 108, 293–315. [Google Scholar] [CrossRef]
- Lagergren, F.; Eklundh, L.; Grelle, A.; Lundblad, M.; Molder, M.; Lankreijer, H.; Lindroth, A. Net primary production and light use efficiency in a mixed coniferous forest in Sweden. Plant Cell Environ. 2005, 28, 412–423. [Google Scholar] [CrossRef]
- Lu, Y. From Normal Forest to Close-to-nature Forest: Multi-functional Forestry and Its Practice at National, Regional and Forest Management Unit Levels in Germany. World For. Res. 2010, 23, 1–11. [Google Scholar] [CrossRef]
- Song, G.T. Comparison of species diversity between Larix gmelini pure forest and Larix gmelini-betula platyphylla mixed forest in Daxing’an Mountains. J. For. Res. 2001, 12, 136–138. [Google Scholar] [CrossRef]
- Ghareghiye, Z.N.; Etemed, V. Estimating the economic value of stored carbon in growing stocks of mixed and pure forest stands. Int. J. Agrisci. 2013, 3, 543–549. [Google Scholar]
- Möller, A. Der Dauerwaldgedanke—Sein Sinn und Seine Bedeutung; Springer: Berlin/Heidelberg, Germany, 1922; 136p. [Google Scholar]
- Gayer, K. Der Gemischte Wald—Seine Begründung und Pflege, Insbesondere Durch Horst-und Gruppenwirtschaft; Paul Parey Verlag: Berlin/Heidelberg, Germany, 1886. [Google Scholar]
- Schütz, J.P. Der Plenterwald und Weitere Formen strukturierter und Gemischter Wälder; Parey: Berlin/Heidelberg, Germany, 2001; 207p. [Google Scholar]
- Zhang, Y.T.; Ding, K.; Yrjala, K.; Liu, H.Y.; Tong, Z.K.; Zhang, J.H. Introduction of broadleaf species into monospecific Cunninghamia lanceolata plantations changed the soil Acidobacteria subgroups composition and nitrogen-cycling gene abundances. Plant Soil 2021, 467, 29–46. [Google Scholar] [CrossRef]
- Ding, K.; Zhang, Y.T.; Yrjala, K.; Tong, Z.K.; Zhang, J.H. The introduction of Phoebe bournei into Cunninghamia lanceolata monoculture plantations increased mi-crobial network complexity and shifted keystone taxa. For. Ecol. Manag. 2022, 509, 120072. [Google Scholar] [CrossRef]
- Brassard, B.W.; Chen, H.Y.H. Stand Structural Dynamics of North American Boreal Forests. Crit. Rev. Plant Sci. 2006, 25, 115–137. [Google Scholar] [CrossRef]
- Staudhammer, C.L.; Lemay, V.M. Introduction and evaluation of possible indices of stand structural diversity. Can. J. For. Res. 2001, 31, 1105–1115. [Google Scholar] [CrossRef]
- Ishii, H.T.; Tanabe, S.; Hiura, T. Exploring the Relationships Among Canopy Structure, Stand Productivity, and Bio-diversity of Temperate Forest Ecosystems. For. Sci. 2004, 50, 342–355. [Google Scholar] [CrossRef]
- Yin, G.F.; Li, J.; Liu, Q.H.; Fan, W.L.; Xu, B.D.; Zeng, Y.L.; Zhao, J. Regional Leaf Area Index Retrieval Based on Remote Sensing: The Role of Radiative Transfer Model Selection. Remote Sens. 2015, 7, 4604–4625. [Google Scholar] [CrossRef]
- Zhou, H.M.; He, B.T.; Peng, H.; Shen, B.; Wu, K.L.; Lin, F.; Liu, C.H. Research on Spatial Structure of Cunninghamia lanceolata Coppice Forest. For. Res. 2015, 28, 686–690. [Google Scholar] [CrossRef]
- Yin, Z.S.; Sun, C.Z.; Zhao, M.Y. Study on canopy interception model and its parameter characteristics of Pinus tabu-laeformis artificial plantation in the loess plateau. For. Res. 2015, 28, 261–264. [Google Scholar]
- Shi, B.K.; Gao, W.F.; Cai, H.Y.; Jin, G.Z. Spatial variation of soil respiration is linked to the forest structure and soil parameters in an old-growth mixed broadleaved-Korean pine forest in northeastern China. Plant Soil 2016, 400, 263–274. [Google Scholar] [CrossRef]
- Hu, Y.J.; Li, J.P.; Cao, X.Y.; Chen, J. Dynamic changes and their relationship of spatial structure and soil’s water conservation function in Cunninghamia lanceolata forest after stand improvement. J. Cent. South Univ. For. Technol. 2018, 38, 103–109. [Google Scholar] [CrossRef]
- Yasuhiro, K. Effects of disturbance and size structure on the regeneration process in a sub-boreal coniferous forest, northern Japan. Ecol. Res. 1995, 10, 135–142. [Google Scholar] [CrossRef]
- Ryan, M.G.; Stape, J.L.; Binkley, D.; Fonseca, S.; Loos, R.A.; Takahashi, E.N.; Silva, C.R.; Silva, S.R.; Hakamada, R.E.; Ferreira, J.M.; et al. Factors controlling Eucalyptus productivity: How water availability and stand structure alter production and carbon allocation. For. Ecol. Manag. 2010, 259, 1695–1703. [Google Scholar] [CrossRef]
- Pretzsch, H.; Biber, P.; Uhl, E.; Dauber, E. Long-term stand dynamics of managed spruce–fir–beech mountain forests in Central Europe: Structure, productivity and regeneration success. Forestry 2015, 88, 407–428. [Google Scholar] [CrossRef]
- Saha, S.; Rajwar, G.S.; Kumar, M. Forest structure, diversity and regeneration potential along altitudinal gradient in Dhanaulti of Garhwal Himalaya. For. Syst. 2016, 25, e058. [Google Scholar] [CrossRef]
- Zhao, Y.; Duan, X.; Shu, S.H. Relationship between space structure characteristics and site environment of Pinus Yunnanensis secondary forests on Mopan Mountain in the middle of Yunnan, Southern China. Bulg. Chem. Commun. 2017, 49, 83–88. [Google Scholar]
- Wang, D.M.; Li, J.P.; Tang, T. Determining the Optimal Density of Phoebe bournei Plantations Based on Dynamic Programming under Close-to-Nature Management Measures. Sustainability 2022, 14, 1. [Google Scholar] [CrossRef]
- Tang, Q.; Li, J.P.; Tang, T.; Liao, P.C.; Wang, D.M. Construction of a Forest Ecological Network Based on the Forest Ecological Suitability Index and the Morphological Spatial Pattern Method: A Case Study of Jindong Forest Farm in Hunan Province. Sustainability 2022, 14, 3082. [Google Scholar] [CrossRef]
- Grime, J.P. Benefits of plant diversity to ecosystems: Immediate, filter and founder effects. J. Ecol. 1998, 86, 902–910. [Google Scholar] [CrossRef]
- Wang, S.Y.; Mi, F.; Pan, W.J.; Zhang, C.H. The Research on the Influential Factors of Forest Biomass Energy Industry Chain. In Proceedings of the International Conference On New Energy and Renewable Resources, Guangzhou, China, 2 June 2015; pp. 181–189. [Google Scholar]
- Yamamoto, T.; Ikeda, S. Relationship between changes of the forests and runoff property in soil and water conservation function reinforcement synthesis model basin: Direction of forest management for improving of water conservation func-tion. Bull. Hiroshima Prefect. For. Res. Cent. 2005, 37, 15–33. [Google Scholar]
- Sherk, J.T.; Fu, W.Y.; Neal, J.C. Site Conditions, Maintenance Costs, and Plant Performance of 10 Extensive Green Roofs in the Research Triangle Area of Central North Carolina. HortTechnology 2020, 30, 761–769. [Google Scholar] [CrossRef]
- Elliott, K.J.; Miniat, C.F.; Pederson, N.; Laseter, S.H. Forest tree growth response to hydroclimate variability in the southern Appalachians. Glob. Chang. Biol. 2015, 21, 4627–4641. [Google Scholar] [CrossRef]
- Yang, X.C.; Liu, D.P.; Fu, Q.; Li, T.X.; Hou, R.J.; Li, Q.L.; Li, M.; Meng, F.X. Characteristics of greenhouse gas emissions from farmland soils based on a structural equation model: Regulation mechanism of biochar. Environ. Res. 2022, 206, 112303. [Google Scholar] [CrossRef]
- Singhai, S.; Singh, R.; Sardana, H.K.; Madhukar, A. Analysis of Factors Influencing Technology Transfer: A Structural Equation Modeling Based Approach. Sustainability 2021, 13, 5600. [Google Scholar] [CrossRef]
- Howard, J.L.; Gagne, M.; Morin, A.J.S.; Forest, J. Using Bifactor Exploratory Structural Equation Modeling to Test for a Continuum Structure of Motivation. J. Manag. 2016, 44, 2638–2664. [Google Scholar] [CrossRef]
- Guo, J.S.; Marsh, H.W.; Parker, P.D.; Dicke, T.; Lüdtke, O.; Diallo, T.M.O. A Systematic Evaluation and Comparison Between Exploratory Structural Equation Modeling and Bayesian Structural Equation Modeling. Struct. Equ. Model. A Multidiscip. J. 2019, 26, 529–556. [Google Scholar] [CrossRef]
- Fan, X.; Thompson, B.; Wang, L. Effects of sample size, estimation methods, and model specification on structural equation modeling fit indexes. Struct. Equ. Model. A Multidiscip. J. 1999, 6, 56–83. [Google Scholar] [CrossRef]
- Santangelo, G.M.; Drubin, D.G. Article-level assessment of influence and translation in biomedical research. Mol. Biol. Cell 2017, 28, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Kirkman, L.K.; Mitchell, R.J.; Helton, R.C.; Drew, M.B. Productivity and species richness across an environmental gradient in a fire-dependent ecosystem. Am. J. Bot. 2001, 88, 2119–2128. [Google Scholar] [CrossRef] [PubMed]
- Kahmen, A.; Perner, J.; Audorff, V.; Weisser, W.; Buchmann, N. Effects of plant diversity, community composition and environmental parameters on productivity in montane European grasslands. Ecosyst. Ecol. 2005, 142, 606–615. [Google Scholar] [CrossRef]
- Georges, M.; Anne, F.; Cyril, F.; Bodil, B.; Claire, M.; Stine, H.K.; Ola, M.; Florence, P. 11q13 Alterations in two cases of hibernoma: Large heterozygous deletions and rearrangement breakpoints near GARP in 11q13.5. Aust. J. Bot. 2003, 37, 389–395. [Google Scholar] [CrossRef]
- Caspersen, J.P.; Pacala, S.W. Successional diversity and forest ecosystem function. Ecol. Res. 2001, 16, 895–903. [Google Scholar] [CrossRef]
- Palik, B.J.; Montgomery, R.A.; Reich, P.B.; Boyden, S.B. Biomass growth response to spatial pattern of variable-retention harvesting in a northern Minnesota pine ecosystem. Ecol. Appl. 2014, 24, 2078–2088. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, D.; Xun, B.; Sun, Y.; Hao, R. The potential effects of climate change on the distribution and productivity of Cunninghamia lanceolatain China. Environ. Monit. Assess. 2014, 186, 135–149. [Google Scholar] [CrossRef]
- Bustamante, R.; Badano, E.; Pickett, S. Impacts of land use change on seed removal patterns of native and exotic species in a forest landscape. Community Ecol. 2012, 13, 171–177. [Google Scholar] [CrossRef]
- Geir, S.V.; Colin, F.; Loubère, M. Influence of Progeny and Initial Stand Density on the Relationship between Diameter at Breast Height and Knot Diameter of Picea abies. Scand. J. For. Res. 1999, 14, 470–480. [Google Scholar] [CrossRef]
- Zhang, J.; Bruelheide, H.; Chen, X.; Eichenberg, D.; Kröber, W.; Xu, X.; Xu, L.; Schuldt, A. Tree diversity promotes generalist herbivore community patterns in a young subtropical forest experiment. Oecologia 2007, 183, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.Q.; Liu, Q.J.; Xu, W.J.; Li, X.R.; Liu, Y.C. Carbon Storage of Artificial Forest in Qianyanzhou, Jiangxi Province. Sci. Silvae Sin. 2007, 43, 1–7. [Google Scholar] [CrossRef]
- Dai, W.; Fu, W.J.; Jiang, P.K.; Zhao, K.L.; Li, Y.H.; Tao, J.X. Spatial pattern of carbon stocks in forest ecosystems of a typical subtropical region of southeastern China. For. Ecol. Manag. 2018, 409, 288–297. [Google Scholar] [CrossRef]
- Wang, Z.C.; Du, H.; Song, T.Q.; Peng, W.X.; Zhang, H. Allometric models of major tree species and forest biomass in Guangxi. Acta Ecol. Sin. 2015, 35, 4462–4472. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, X.; Wang, X.; Fei, L. Biomass and its allocation of Chinese forest ecosystems. Ecology 2016, 95, 2026. [Google Scholar] [CrossRef]
- Lu, J.; Feng, J.K.; Zhu, Y. Estimation of Forest Biomass and Carbon Storage in China Based on Forest Resources Inventory Data. Forests 2019, 10, 650. [Google Scholar] [CrossRef]
- Laughlin, D.C.; Abella, S.R.; Covington, W.W.; Grace, J.B. Species richness and soil properties in Pinus ponderosa forests: A structural equation modeling analysis. J. Veg. Sci. 2007, 18, 231–242. [Google Scholar] [CrossRef]
- Schmidt, T.S.; Van, M.P.C.; Carlisle, M.M.; Carlisle, D.M. Linking the Agricultural Landscape of the Midwest to Stream Health with Structural Equation Modeling. Environ. Sci. Technol. 2019, 53, 452–462. [Google Scholar] [CrossRef]
- Feng, M.I.; Pan, W.; Chen, K. Study on Forest Biomass Energy Industry Chain External Driving Force Based on Structural Equation Modeling. Sci. Technol. Manag. Res. 2015, 35, 128–132. [Google Scholar] [CrossRef]
- Zhu, Y.W.; Bathelt, H.; Zeng, G. Learning in Context: A Structural Equation Modeling Approach to Analyze Knowledge Acquisition at Trade Fairs. Z. Für Wirtsch. 2020, 64, 165–179. [Google Scholar] [CrossRef]
- Goodboy, A.K.; Bolkan, S.; Brisini, K.; Solomon, D.H. Relational Uncertainty Within Relational Turbulence Theory: The Bifactor Exploratory Structural Equation Model. J. Commun. 2021, 71, 403–430. [Google Scholar] [CrossRef]
- Zhang, J.H.; Tong, H.; Jiao, G.; Zhao, L. Research on soil organic carbon spatial distribution in Qilian Mountain based on geographic infor-mation system and spatial analysis technology. In Proceedings of the SPIE—The International Society for Optical Engineering, San Diego, CA, USA, 12–16 August 2012; Volume 8334, p. 95. [Google Scholar] [CrossRef]
- Tian, X.; Zheng, J.; Wang, D.; Huang, X.R.; Zhang, Z.D. Structural equation modeling analysis of the response of herbaceous species richness to landscape factors in a forest-steppe zone. Acta Ecol. Sin. 2018, 38, 4649–4656. [Google Scholar] [CrossRef]
- Wang, S.L.; Han, Y.H.C. Diversity of northern plantations peaks at intermediate management intensity. For. Ecol. Manag. 2010, 259, 360–366. [Google Scholar] [CrossRef]
- Torresan, C.; del Río, M.; Hilmers, T.; Notarangelo, M.; Bielak, K.; Binder, F.; Boncina, A.; Bosela, M.; Forrester, D.I.; Hobi, M.L.; et al. Importance of tree species size dominance and heterogeneity on the productivity of spruce-fir-beech mountain forest stands in Europe. For. Ecol. Manag. 2020, 457, 117716. [Google Scholar] [CrossRef]
- Wolf, E.J.; Harrington, K.M.; Clark, S.L.; Miller, M.W. Sample Size Requirements for Structural Equation Models: An Evaluation of Power, Bias, and Solution Propriety. Educ. Psychol. Meas. 2013, 73, 913–934. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Liu, S.; Sun, Y.; An, Y.; Zhao, S.; Liu, Y.; Li, M. Ecological network construction of the heterogeneous agro-pastoral areas in the upper Yellow River basin. Agric. Ecosyst. Environ. 2020, 302, 107069. [Google Scholar] [CrossRef]

| Statistics | Fitting Index | Evaluation Standard | A Priori Model | Optimal Model |
|---|---|---|---|---|
| Absolute fit statistics | Between 1–3 means the model fits well | 1.889 | 1.889 | |
| GFI | >0.90 | 0.949 | 0.935 | |
| RMSEA | <0.05 | 0.055 | 0.038 | |
| NCP | The smaller the better | 39.995 | 28.367 | |
| Value-added adaptation statistics | NFI | Between 0–1, the closer to 1, the better the model fit | 0.948 | 0.954 |
| RFI | 0.931 | 0.933 | ||
| IFI | 0.975 | 0.978 | ||
| TLI | 0.962 | 0.967 | ||
| CFI | 0.974 | 0.978 | ||
| Minimal adaptation statistics | PGFI | >0.5, The higher the value, the better | 0.548 | 0.550 |
| PNFI | >0.5, The higher the value, the better | 0.649 | 0.651 |
| Species | Formula | a | b | c |
|---|---|---|---|---|
| Cunninghamia lanceolata | V = a × Db × Hc | 0.000058777042 | 1.969983 | 0.896462 |
| Other conifer | V = a × Db × Hc | 0.000062341803 | 1.855150 | 0.956825 |
| One type of hardwood broad-leaf | V = a × Db × Hc | 0.000068563400 | 1.933221 | 0.867885 |
| Second type of hardwood broad-leaf | V = a × Db × Hc | 0.000050479055 | 1.88452 | 0.990765 |
| Soft broad-leaf | V = a × Db × Hc | 0.000041028005 | 1.80063 | 1.130599 |
| Species | Biomass Equation | R2 |
|---|---|---|
| Cunninghamia lanceolata | Wtrunk = 37.9323D2.598 | 0.975 |
| Wbranch = 1.6255D2.0074 | 0.764 | |
| Wleaf = 5.2619D2.1515 | 0.788 | |
| lgWroot = −1.995 + 2.4541lgD | 0.962 | |
| Hard broad-leaf class | Wtrunk = 0.065D2.548 | 0.972 |
| Wbranch = 0.025D2.390 | 0.91 | |
| Wleaf = 0.036D1.818 | 0.876 | |
| Wroot = 0.027D2.394 | 0.922 | |
| Soft broad-leaf class | Wtrunk = 0.080D2.348 | 0.995 |
| Wbranch = 0.027D1.762 | 0.975 | |
| Wleaf = 0.027D1.371 | 0.954 | |
| Wroot = 0.027D2.165 | 0.873 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, Z.; Tang, T.; Li, J. Analysis of the Relative Importance of Stand Structure and Site Conditions for the Productivity, Species Diversity, and Carbon Sequestration of Cunninghamia lanceolata and Phoebe bournei Mixed Forest. Plants 2023, 12, 1633. https://doi.org/10.3390/plants12081633
Wang Y, Liu Z, Tang T, Li J. Analysis of the Relative Importance of Stand Structure and Site Conditions for the Productivity, Species Diversity, and Carbon Sequestration of Cunninghamia lanceolata and Phoebe bournei Mixed Forest. Plants. 2023; 12(8):1633. https://doi.org/10.3390/plants12081633
Chicago/Turabian StyleWang, Yiru, Zhaohua Liu, Tao Tang, and Jiping Li. 2023. "Analysis of the Relative Importance of Stand Structure and Site Conditions for the Productivity, Species Diversity, and Carbon Sequestration of Cunninghamia lanceolata and Phoebe bournei Mixed Forest" Plants 12, no. 8: 1633. https://doi.org/10.3390/plants12081633
APA StyleWang, Y., Liu, Z., Tang, T., & Li, J. (2023). Analysis of the Relative Importance of Stand Structure and Site Conditions for the Productivity, Species Diversity, and Carbon Sequestration of Cunninghamia lanceolata and Phoebe bournei Mixed Forest. Plants, 12(8), 1633. https://doi.org/10.3390/plants12081633






