LED Lighting to Produce High-Quality Ornamental Plants
Abstract
1. Introduction
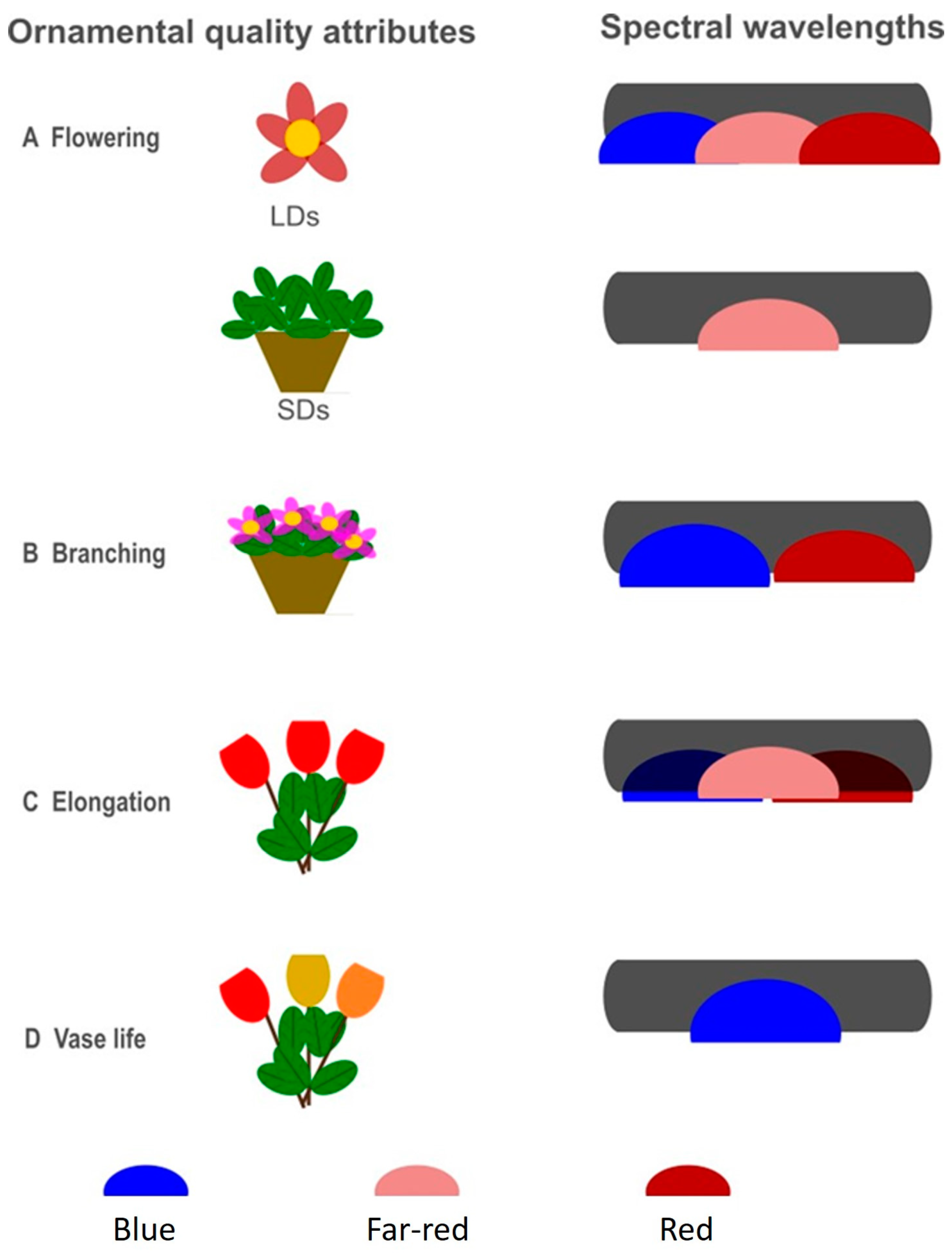
2. Flowering Regulation
3. Plant Architecture
4. Postharvest/Postproduction Longevity
5. Flower and Leaf Color
6. Pathogens and Disease Control
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Flower and Ornamental Plants Industry Research Report 2023: Competitive Landscape, Market Size, Regional Status and Prospect; Market Reports World, Absolute Reports; Market Reports World: Pune, India, 2023; p. 107.
- Bulgari, R.; Petrini, A.; Cocetta, G.; Nicoletto, C.; Ertani, A.; Sambo, P.; Ferrante, A.; Nicola, S. The impact of COVID-19 on horticulture: Critical issues and opportunities derived from an unexpected occurrence. Horticulturae 2021, 7, 124. [Google Scholar] [CrossRef]
- Ferrante, A.; Trivellini, A.; Scuderi, D.; Romano, D.; Vernieri, P. Post-production physiology and handling of ornamental potted plants. Postharvest Biol. Technol. 2015, 100, 99–108. [Google Scholar] [CrossRef]
- Hill, J.; Von Maltitz, G.; Sommer, S.; Reynolds, J.; Hutchinson, C.; Cherlet, M. World Atlas of Desertification; Publications Office of the European Union: Luxembourg, 2018. [Google Scholar]
- Van Delden, S.H.; SharathKumar, M.; Butturini, M.; Graamans, L.J.A.; Heuvelink, E.; Kacira, M.; Kaiser, E.; Klamer, R.S.; Klerkx, L.; Kootstra, G.; et al. Current status and future challenges in implementing and upscaling vertical farming systems. Nat. Food 2021, 2, 944–956. [Google Scholar] [CrossRef]
- Xu, Y. Nature and source of light for plant factory. In Plant Factory Using Artificial Light; Anpo, M., Fukuda, H., Wada, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 47–69. [Google Scholar]
- Marondedze, C.; Liu, X.; Huang, S.; Wong, C.; Zhou, X.; Pan, X.; An, H.; Xu, N.; Tian, X.; Wong, A. Towards a tailored indoor horticulture: A functional genomics guided phenotypic approach. Hortic. Res. 2018, 5, 68. [Google Scholar] [CrossRef]
- Paucek, I.; Appolloni, E.; Pennisi, G.; Quaini, S.; Gianquinto, G.; Orsini, F. LED Lighting systems for horticulture: Business growth and global distribution. Sustainability 2020, 12, 7516. [Google Scholar] [CrossRef]
- Massa, G.D.; Kim, H.H.; Wheeler, R.M.; Mitchell, C.A.; Cary, A. Plant productivity in response to LED lighting. HortScience 2008, 43, 1951–1956. [Google Scholar] [CrossRef]
- Zheng, L.; He, H.; Song, W. Application of light-emitting diodes and the effect of light quality on horticultural crops: A review. HortScience 2019, 54, 1656–1661. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: The state of the art and the opportunities of modern LED systems. J. Plant Growth Regul. 2021, 41, 742–780. [Google Scholar] [CrossRef]
- Islam, M.A.; Kuwar, G.; Clarke, J.L.; Blystad, D.-R.; Gislerød, H.R.; Olsen, J.E.; Torre, S. Artificial light from light emitting diodes (LEDs) with a high portion of blue light results in shorter poinsettias compared to high pressure sodium (HPS) lamps. Sci. Hortic. 2012, 147, 136–143. [Google Scholar] [CrossRef]
- Singh, D.; Basu, C.; Meinhardt-Wollweber, M.; Roth, B. LEDs for energy efficient greenhouse lighting. Renew. Sustain. Energy Rev. 2015, 49, 139–147. [Google Scholar] [CrossRef]
- Bantis, F.; Smirnakou, S.; Ouzounis, T.; Koukounaras, A.; Ntagkas, N.; Radoglou, K. Current status and recent achievements in the field of horticulture with the use of light-emitting diodes (LEDs). Sci. Hortic. 2018, 235, 437–451. [Google Scholar] [CrossRef]
- Bula, R.J.; Morrow, R.C.; Tibbitts, T.W.; Barta, D.J.; Ignatius, R.W.; Martin, T.S. Light-emitting diodes as a radiation source for plants. HortScience 1991, 26, 203–205. [Google Scholar] [CrossRef]
- Morrow, R.C. LED lighting in horticulture. HortScience 2008, 43, 1947–1950. [Google Scholar] [CrossRef]
- Karabourniotis, G.; Liakopoulos, G.; Bresta, P.; Nikolopoulos, D. The optical properties of leaf structural elements and their contribution to photosynthetic performance and photoprotection. Plants 2021, 10, 1455. [Google Scholar] [CrossRef]
- Aliniaeifard, S.; Falahi, Z.; Dianati Daylami, S.; Li, T.; Woltering, E. Postharvest spectral light composition affects chilling injury in anthurium cut flowers. Front. Plant Sci. 2020, 11, 846. [Google Scholar] [CrossRef]
- Hao, Y.; Zeng, Z.; Zhang, X.; Xie, D.; Li, X.; Ma, L.; Liu, M.; Liu, H. Green means go: Green light promotes hypocotyl elongation via brassinosteroid signaling. Plant Cell 2023, koad022. [Google Scholar] [CrossRef]
- Christiaens, A.; Van Labeke, M.C.; Gobin, B.; Van Huylenbroeck, J. Rooting of ornamental cuttings affected by spectral light quality. Acta Hortic. 2015, 1104, 219–224. [Google Scholar] [CrossRef]
- Jeong, S.W.; Park, S.; Jin, J.S. Influences of four different light-emitting diode lights on flowering and polyphenol variations in the leaves of Chrysanthemum (Chrysanthemum morifolium). J. Agric. Food Chem. 2012, 60, 9793–9800. [Google Scholar] [CrossRef]
- Christiaens, A.; Gobin, B.; Van Huylenbroeck, J.; Van Labeke, M.C. Adventitious rooting of Chrysanthemum is stimulated by a low red: Far-red ratio. J. Plant Physiol. 2019, 236, 117–123. [Google Scholar] [CrossRef]
- Zheng, L.; Van Labeke, M.C. Long-term effects of red- and blue-light emitting diodes on leaf anatomy and photosynthetic efficiency of three ornamental pot plants. Front. Plant Sci. 2017, 8, 917. [Google Scholar] [CrossRef]
- Orlando, M.; Trivellini, A.; Puccinelli, M.; Ferrante, A.; Incrocci, L.; Mensuali, A. Increasing the functional quality of Crocus sativus L. by-product (tepals) by controlling spectral composition. Hortic. Environ. Biotechnol. 2022, 63, 363–373. [Google Scholar] [CrossRef]
- Heo, J.W.; Lee, C.W.; Murthy, H.N.; Paek, K.Y. Influence of light quality and photoperiod on flowering of Cyclamen persicum Mill. cv. ‘Dixie White’. Plant Growth Regul. 2003, 40, 7–10. [Google Scholar] [CrossRef]
- Aalifar, M.; Aliniaeifard, S.; Arab, M.; Zare Mehrjerdi, M.; Dianati Daylami, S.; Serek, M.; Woltering, E.; Li, T. Blue light improves vase life of carnation cut flowers through its effect on the antioxidant defense system. Front. Plant Sci. 2020, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- Aalifar, M.; Aliniaeifard, S.; Arab, M.; Mehrjerdi, M.; Serek, M. Blue light postpones senescence of carnation flowers through regulation of ethylene and abscisic acid pathway-related genes. Plant Physiol. Biochem. 2020, 151, 103–112. [Google Scholar] [CrossRef]
- De Keyser, E.; Dhooghe, E.; Christiaens, A.; Van Labeke, M.-C.; Van Huylenbroeck, J. LED light quality intensifies leaf pigmentation in ornamental pot plants. Sci. Hortic. 2019, 253, 270–275. [Google Scholar] [CrossRef]
- Kobori, M.M.R.G.; Mello, S.D.C.; Freitas, I.S.D.; Silveira, F.F.; Alves, M.C.; Azevedo, R.A. Supplemental light with different blue and red ratios in the physiology, yield and quality of Impatiens. Sci. Hortic. 2022, 306, 111424. [Google Scholar] [CrossRef]
- Wollaeger, H.M.; Runkle, E.S. Growth of impatiens, petunia, salvia, and tomato seedlings under blue, green, and red light-emitting diodes. HortScience 2014, 49, 734–740. [Google Scholar] [CrossRef]
- Wojciechowska, R.; Hanus-Fajerska, E.; Kamińska, I.; Koźmińska, A.; Długosz-Grochowska, O.; Kapczyńska, A. High ratio of red-to-blue LED light improves the quality of Lachenalia ‘Rupert’ inflorescence. Folia Hortic. 2019, 31, 93–100. [Google Scholar] [CrossRef]
- Meng, Q.; Runkle, E.S. Regulation of flowering by green light depends on its photon flux density and involves cryptochromes. Phys. Plant. 2019, 166, 762–771. [Google Scholar] [CrossRef]
- Flores-Perez, S.; Castillo-Gonzalez, A.M.; Valdez-Aguilar, L.A.; Garcia-Avita, E. Use of different proportions of red and blue LEDs to improve the growth of Lilium spp. Rev. Mex. Cienc. Agric. 2021, 12, 835–847. [Google Scholar] [CrossRef]
- Park, Y.; Runkle, E.S. Far-red radiation and photosynthetic photon flux density independently regulate seedling growth but interactively regulate flowering. Environ. Exp. Bot. 2018, 155, 206–216. [Google Scholar] [CrossRef]
- Kong, Y.; Schiestel, K.; Zheng, Y. Blue light associated with low phytochrome activity can promote flowering: A comparison with red light in four bedding plant species. Acta Hortic. 2020, 1296, 433–440. [Google Scholar] [CrossRef]
- Horibe, T.; Horie, K.; Kawai, M.; Kurachi, Y.; Watanabe, Y.; Makita, M. Effect of light environment on flower opening and water balance in cut rose. Environ. Control Biol. 2020, 58, 15–20. [Google Scholar] [CrossRef]
- Matysiak, B. The effect of supplementary LED lighting on the morphological and physiological traits of Miniature Rosa × hybrida ‘Aga’ and the development of Powdery Mildew (Podosphaera pannosa) under greenhouse conditions. Plants 2021, 10, 417. [Google Scholar] [CrossRef]
- Suthaparan, A.; Torre, S.; Stensvand, A.; Herrero, M.L.; Pettersen, R.I.; Gadoury, D.M.; Gislerod, H.R. Specific light-emitting diodes can suppress sporulation of Podosphaera pannosa on greenhouse roses. Plant Dis. 2010, 94, 1105–1110. [Google Scholar] [CrossRef]
- Yamori, N.; Matsushima, Y.; Yamori, W. Upward LED lighting from the base suppresses senescence of lower leaves and promotes flowering in indoor rose management. HortScience 2021, 56, 716–721. [Google Scholar] [CrossRef]
- Owen, W.G.; Lopez, R.G. Comparison of sole-source and supplemental lighting on callus formation and initial rhizogenesis of Gaura and Salvia cuttings. HortScience 2019, 54, 684–691. [Google Scholar] [CrossRef]
- Orlando, M.; Trivellini, A.; Incrocci, L.; Ferrante, A.; Mensuali, A. The inclusion of green light in a red and blue light background impact the growth and functional quality of vegetable and flower microgreen species. Horticulturae 2022, 8, 217. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Martínez-Ramírez, G.; Almansa, E.M.; Javier Barbero, F.; Chica, R.M.; Teresa Lao, M. Growth, photosynthesis, and physiological responses of ornamental plants to complementation with monochromic or mixed red-blue LEDs for use in indoor environments. Agronomy 2020, 10, 284. [Google Scholar] [CrossRef]
- Srikanth, A.; Schmid, M. Regulation of flowering time: All roads lead to Rome. Cell. Mol. Life Sci. 2011, 68, 2013–2037. [Google Scholar] [CrossRef]
- Cho, L.H.; Yoon, J.; An, G. The control of flowering time by environmental factors. Plant J. 2017, 90, 708–719. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.; Vince-Prue, D. Daylength Perception in Short-Day Plants, 2nd ed.; Academic Press: London, UK, 1996; pp. 1–427. [Google Scholar]
- Sung, S.; Amasino, R.M. Remembering winter: Toward a molecular understanding of vernalization. Annu. Rev. Plant Biol. 2005, 56, 491–508. [Google Scholar] [CrossRef] [PubMed]
- Lang, A. The effect of gibberellin upon flower formation. Proc. Natl. Acad. Sci. USA 1957, 43, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Simpson, G.C. The autonomous pathway: Epigenetic and post-transcriptional gene regulation in the control of Arabidopsis flowering time. Curr. Opin. Plant Biol. 2004, 7, 570–574. [Google Scholar] [CrossRef]
- Wang, J.W. Regulation of flowering time by the miR156-mediated age pathway. J. Exp. Bot. 2014, 65, 4723–4730. [Google Scholar] [CrossRef]
- Erwin, J. Factors affecting flowering in ornamental plants. In Flower Breeding and Genetics; Anderson, N.O., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 7–48. [Google Scholar]
- Weller, J.L.; Kendrik, R.E. Photomorphogenesis and photoperiodism in plants. In Photobiology: The Science of Light and Life; Bjorn, L.O., Ed.; Springer: New York, NY, USA, 2015; pp. 299–321. [Google Scholar]
- Proietti, S.; Scariot, V.; De Pascale, S.; Paradiso, R. Flowering mechanisms and environmental stimuli for flower transition: Bases for production scheduling in greenhouse floriculture. Plants 2022, 11, 432. [Google Scholar] [CrossRef]
- Higuchi, Y. Florigen and anti-florigen: Flowering regulation in horticultural crops. Breed. Sci. 2018, 68, 109–118. [Google Scholar] [CrossRef]
- Dixon, L.E.; Karsai, I.; Kiss, T.; Adamski, N.M.; Liu, Z.; Ding, Y.; Allard, V.; Boden, S.A.; Griffiths, S. VERNALIZATION1 controls developmental responses of winter wheat under high ambient temperatures. Development 2019, 146, dev172684. [Google Scholar] [CrossRef]
- Meng, Q.; Runkle, E.S. Moderate-intensity blue radiation can regulate flowering, but not extension growth, of several photoperiodic ornamental crops. Environ. Exp. Bot. 2017, 134, 12–20. [Google Scholar] [CrossRef]
- Park, Y.G.; Jeong, B.R. Both the quality and positioning of the night interruption light are important for flowering and plant extension growth. J. Plant Growth Regul. 2020, 39, 583–593. [Google Scholar] [CrossRef]
- Higuchi, Y.; Sumitomo, K.; Oda, A.; Shimizu, H.; Hisamatsu, T. Day light quality affects the night-break response in the short-day plant chrysanthemum, suggesting differential phytochrome-mediated regulation of flowering. J. Plant Physiol. 2012, 169, 1789–1796. [Google Scholar] [CrossRef]
- Cerdán, P.D.; Chory, J. Regulation of flowering time by light quality. Nature 2003, 423, 881–885. [Google Scholar] [CrossRef]
- Casal, J.J. Phytochromes, cryptochromes, phototropin: Photoreceptor interactions in plants. Photochem. Photobiol. 2000, 71, 1–11. [Google Scholar] [CrossRef]
- Meng, Q.; Runkle, E.S. Controlling flowering of photoperiodic ornamental crops with light-emitting diode lamps: A coordinated grower trial. HortTechnology 2014, 1, 702–711. [Google Scholar] [CrossRef]
- Garrett-Owen, W.; Meng, Q.; Lopez, R.G. Promotion of flowering from far-red radiation depends on the photosynthetic daily light integral. HortScience 2018, 53, 465–471. [Google Scholar] [CrossRef]
- Van Haeringen, C.J.; West, J.S.; Davis, F.J.; Gilbert, A.; Hadley, P.; Pearson, S.; Wheldon, A.E.; Henbest, R.G.C. The development of solid spectral filters for the regulation of plant growth. Photochem. Photobiol. 1998, 67, 407–413. [Google Scholar] [CrossRef]
- Runkle, E.S.; Heins, R.D. Specific functions of red, far red, and blue light in flowering and stem extension of long-day plants. J. Am. Soc. Hortic. Sci. 2001, 126, 275–282. [Google Scholar] [CrossRef]
- Kurilčik, A.; Miklušytė-Čanova, R.; Dapkūnienė, S.; Žilinskaitė, S.; Kurilčik, G.; Tamulaitis, G.; Duchovskis, P.; Žukauskas, A. In vitro culture of Chrysanthemum plantlets using light-emitting diodes. Open Life Sci. 2008, 3, 161–167. [Google Scholar] [CrossRef]
- Zhang, M.; Runkle, E.S. Regulating flowering and extension growth of poinsettia using red and far-red light-emitting diodes for end-of-day lighting. HortScience 2019, 54, 323–327. [Google Scholar] [CrossRef]
- Hisamatsu, T.; Sumitomo, K.; Shimizu, H. End-of-day far-red treatment enhances responsiveness to gibberellins and promotes stem extension in chrysanthemum. J. Hortic. Sci. Biotechnol. 2008, 83, 695–700. [Google Scholar] [CrossRef]
- SharathKumar, M.; Heuvelink, E.; Marcelis, L.F.M.; van Ieperen, W. Floral induction in the short-day plant chrysanthemum under blue and red extended long-days. Front. Plant Sci. 2021, 11, 610041. [Google Scholar] [CrossRef] [PubMed]
- Craig, D.S.; Runkle, E.S. A moderate to high red to far-red light ratio from light-emitting diodes controls flowering of short-day plants. J. Am. Soc. Hortic. Sci. 2013, 138, 167–172. [Google Scholar] [CrossRef]
- Park, Y.; Runkle, E.S. Blue radiation attenuates the effects of the red to far-red ratio on extension growth but not on flowering. Environ. Exp. Bot. 2019, 168, 103871. [Google Scholar] [CrossRef]
- Hamamoto, H.; Shimaji, H.; Higashide, T. Budding and bolting responses of horticultural plants to night-break treatments with LEDs of various colors. J. Agric. Meterol. 2003, 59, 103–110. [Google Scholar] [CrossRef]
- Yang, J.; Song, J.; Jeong, B.R. Low-intensity blue light supplemented during photoperiod in controlled environment induces flowering and antioxidant production in kalanchoe. Antioxidants 2022, 11, 811. [Google Scholar] [CrossRef]
- Hamamoto, H.; Yamazaki, K. Reproductive response of okra and native rosella to long-day treatment with red, blue, and green light-emitting diode lights. HortScience 2009, 5, 1494–1497. [Google Scholar] [CrossRef]
- Sampson, B.J.; Cane, J.H. Impact of enhanced ultraviolet-B radiation on flower, pollen, and nectar production. Am. J. Bot. 1999, 86, 108–114. [Google Scholar] [CrossRef]
- Bridgen, M.P. Using ultraviolet-C (UV-C) irradiation on greenhouse ornamental plants for growth regulation. Acta Hortic. 2016, 1134, 49–56. [Google Scholar] [CrossRef]
- Darras, A.I.; Vlachodimitropoulou, A.; Dimitriadis, C. Regulation of corm sprouting, growth and flowering of pot Freesia hybrida L. plants by cold and UV-C irradiation forcing. Sci. Hortic. 2019, 252, 110–112. [Google Scholar] [CrossRef]
- Li, Y.; Xin, G.F.; Liu, C.; Shi, Q.H.; Yang, F.J.; Wei, M. Effects of red and blue light on leaf anatomy, CO2 assimilation and the photosynthetic electron transport capacity of sweet pepper (Capsicum annuum L.) seedlings. BMC Plant Biol. 2020, 20, 318. [Google Scholar] [CrossRef]
- Randall, W.C.; Lopez, R.G. Comparisons of bedding plant seedlings grown under sole source light-emitting diodes (LEDs) and greenhouse supplemental lighting from LEDs and high-pressure sodium lamps. HortScience 2015, 50, 705–713. [Google Scholar] [CrossRef]
- Mao, J.; Zhang, Y.; Sang, Y.; Li, Q.; Yang, H. From the cover: A role for Arabidopsis cryptochromes and COP1 in the regulation of stomatal opening. Proc. Natl. Acad. Sci. USA 2005, 102, 12270–12275. [Google Scholar] [CrossRef]
- Yang, J.; Li, C.; Kong, D.; Guo, F.; Wei, H. Light-mediated signaling and metabolic changes coordinate stomatal opening and closure. Front. Plant Sci. 2020, 11, 601478. [Google Scholar] [CrossRef]
- Llewellyn, D.; Schiestel, K.; Zheng, Y. Light-emitting diodes can replace high-pressure sodium lighting for cut gerbera production. HortScience 2019, 54, 95–99. [Google Scholar] [CrossRef]
- Ruberti, I.; Sessa, G.; Ciolfi, A.; Possenti, M.; Carabelli, M.; Morelli, G. Plant adaptation to dynamically changing environment: The shade avoidance response. Biotechnol. Adv. 2012, 30, 1047–1058. [Google Scholar] [CrossRef]
- Dierck, R.; Dhooghe, E.; Van Huylenbroeck, J.; Van Der Straeten, D.; De Keyser, E. Light quality regulates plant architecture in different genotypes of Chrysanthemum morifolium Ramat. Sci. Hortic. 2017, 218, 177–186. [Google Scholar] [CrossRef]
- Park, I.S.; Cho, K.J.; Kim, J.; Cho, J.Y.; Lim, T.J.; Oh, W. Growth and flowering responses of petunia to various artificial light sources with different light qualities. Korean J. Hortic. Sci. Technol. 2016, 34, 55–66. [Google Scholar] [CrossRef]
- Klein, R.M. Effects of green light on biological systems. Biol. Rev. 1992, 67, 199–284. [Google Scholar] [CrossRef]
- Cao, B.; Lv, X.; Chen, Z.; Xu, K. Supplementing green light under strong sunlight improves growth and functional ingredients of ginger (Zingiber officinale Rosc.) in summer. Ind. Crop Prod. 2021, 167, 113527. [Google Scholar] [CrossRef]
- Scariot, V.; Paradiso, R.; Rogers, H.; De Pascale, S. Ethylene control in cut flowers: Classical and innovative approaches. Postharvest Biol. Technol. 2014, 97, 83–92. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M. Ethylene role in plant growth, development and senescence: Interaction with other phytohormones. Front. Plant Sci. 2017, 8, 475. [Google Scholar] [CrossRef] [PubMed]
- Horibe, T. Use of light stimuli as a postharvest technology for cut flowers. Front. Plant Sci. 2020, 11, 573490. [Google Scholar] [CrossRef] [PubMed]
- Noichinda, S.; Bodhipadma, K.; Mahamontri, C.; Narongruk, T.; Ketsa, S. Light during storage prevents loss of ascorbic acid, and increases glucose and fructose levels in Chinese kale (Brassica oleracea var. alboglabra). Postharvest Biol. Technol. 2007, 44, 312–315. [Google Scholar] [CrossRef]
- Elibox, W.; Umaharan, P. Cultivar differences in the deterioration of vase-life in cut-flowers of Anthurium andraeanum is determined by mechanisms that regulate water uptake. Sci. Hortic. 2010, 124, 102–108. [Google Scholar] [CrossRef]
- Lü, P.; Cao, J.; He, S.; Liu, J.; Li, H.; Cheng, G.; Ding, Y.; Joyce, D.C. Nano-silver pulse treatments improve water relations of cut rose cv. Movie Star flowers. Postharvest Biol. Technol. 2010, 57, 196–202. [Google Scholar] [CrossRef]
- Hunter, D.A.; Ferrante, A.; Vernieri, P.; Reid, M.S. Role of abscisic acid in perianth senescence of daffodil (Narcissus pseudonarcissus ‘Dutch Master’). Physiol. Plant. 2004, 121, 313–321. [Google Scholar] [CrossRef]
- Hunter, D.A.; Yi, M.F.; Xu, X.; Reid, M.S. Role of ethylene in perianth senescence of daffodil (Narcissus pseudonarcissus L. ‘Dutch Master’). Postharvest Biol. Technol. 2004, 32, 269–280. [Google Scholar] [CrossRef]
- Trivellini, A.; Ferrante, A.; Vernieri, P.; Serra, G. Effects of abscisic acid on ethylene biosynthesis and perception in Hibiscus rosa-sinensis L. flower development. J. Exp. Bot. 2011, 62, 5437–5452. [Google Scholar] [CrossRef]
- Trivellini, A.; Ferrante, A.; Vernieri, P.; Serra, G. Effects of promoters and inhibitors of ABA and ethylene on flower senescence of Hibiscus rosa-sinensis L. J. Plant Growth Regul. 2011, 30, 175–184. [Google Scholar] [CrossRef]
- Zhao, D.; Tao, J. Recent advances on the development and regulation of flower color in ornamental plants. Front. Plant. Sci. 2015, 6, 1–13. [Google Scholar] [CrossRef]
- Lee, K.M.; Jeong, T.Y.; Song, J.Y.; Jeong, B.R. Flower color change of Boronia heterophylla as affected by light intensity and preservative chemicals. Korean J. Hortic. Sci. Technol. 2007, 25, 458–462. [Google Scholar]
- Huang, K.L.; Miyajima, I.; Okubo, H. Effects of temperature and shade treatment on flower colors and characteristics in newly established reddish-purple tuberose (Polianthes). J. Fac. Agric. Kyushu Univ. 2000, 45, 57–63. [Google Scholar] [CrossRef]
- Zhao, D.Q.; Hao, Z.J.; Tao, J. Effects of shade on plant growth and flower quality in herbaceous peony (Paeonia lactiflora Pall.). Plant Physiol. Biochem. 2012, 61, 187–196. [Google Scholar] [CrossRef]
- Young, H.K.; Hwan, C.J.; Sun, K.K.; Yang, H.K. Effects of light quality on growth and flowering of Hibiscus syriacus L. J. Korean Soc. Hortic. Sci. 1997, 38, 272–277. [Google Scholar]
- Thoma, F.; Somborn-Schulz, A.; Schlehuber, D.; Keuter, V.; Deerberg, G. Effects of light on secondary metabolites in selected leafy greens: A review. Front. Plant Sci. 2020, 11, 497. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant secondary metabolites as defenses, regulators, and primary metabolites: The blurred functional trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kanto, T.; Fujikawa, T.; Yamada, M.; Ishiwata, M.; Satou, M.; Hisamatsu, T. Supplemental UV radiation controls rose powdery mildew disease under greenhouse conditions. Environ. Control Biol. 2013, 51, 157–163. [Google Scholar] [CrossRef]
- Meyer, P.; Van de Poel, B.; De Coninck, B. UV-B light and its application potential to reduce disease and pest incidence in crops. Hortic. Res. 2021, 8, 194. [Google Scholar] [CrossRef]
- Yoon, J.B.; Nomura, M.; Ishikura, S. Analysis of the flight activity of the cotton bollworm Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) under yellow LED lighting. Jpn. J. Appl. Entomol. Zool. 2012, 56, 103–110. [Google Scholar] [CrossRef]
- Dielemana, J.A.; Kruidhof, H.M.; Weerheim, K. LED lighting strategies in cut flowers: Balancing plant physiology and biological control of pests. Acta Hortic. 2020, 1296, 591–604. [Google Scholar] [CrossRef]



| Species | Light Typologies | Effect of LEDs on Plants | References |
|---|---|---|---|
| Anthurium andraeanum Linden ‘Calore’, ‘Angel’ | Darkness (D); different light spectra (R, B, RB (70:30%), and W) at 125 µmol.m−2 s−1. | B and W increased electrolyte leakage (EL); R decreased EL; B increased water loss; D and R decreased water loss. Negative correlation for both cultivars between EL and vase life and anthocyanin concentration and EL, and a positive correlation between anthocyanin concentration and vase life, were found. Higher percentage of B spectra determined higher EL and a shorter vase life under a cold storage condition. | [18] |
| Arabidopsis thaliana (L.) Heynh. | Blue (B), red (R), far-red (F:R), UV-B light, and green (G) light sources. | G promoted hypocotyl elongation, and the brassinosteroid (BR) signaling pathway is involved in this process. G promoted the DNA binding activity of BRI1-EMS-SUPPRESSOR 1 (BES1), thus regulating gene transcription to promote hypocotyl elongation. | [19] |
| Chrysanthemum ×morifolium (Ramat.) Hemsl., Lavandula angustifolia Mill., and Rhododendron simsii Planch. hybrids | R:B 100:0, 90:10, 80:20, 50:50, 10:90 and 0:100 at a light intensity of 60 µmol m−2 s−1 for Chrysanthemum ×morifolium and Lavandula angustifolia and 30 µmol m−2 s−1 for Rhododendron simsii hybrids. | R 100% increased root formation. 10:90 R:B inhibited rooting in Chrysanthemum ×morifolium, while under 50:50 R:B was inhibited rooting in Rhododendron simsii. | [20] |
| Chrysanthemum ×morifolium (Ramat.) Hemsl. ‘Gaya yellow’ | Plants were grown under supplemental B (463 nm), G (518 nm), R (632 nm), and W LEDs. | W increased the weights of leaves and stems. G increased polyphenols (luteolin-7-O-glucoside, luteolin-7-O-glucuronide, quercetagetin-trimethyl ether); R increased dicaffeoylquinic acid isomer, dicaffeoylquinic acid isomer, naringenin, and apigenin-7-O-glucuronide. | [21] |
| Chrysanthemum morifolium ‘Orlando’ | Blue, red, far-red; daily light integral: 4.1 mol m−2 d−1 in interaction with auxin treatments. | Lowering the R:FR ratio improved rooting significantly. In contrast, adding blue light to solely red light decreased rooting. Phytochrome plays a role in adventitious root formation through the action of auxin, but the blue light receptors interact in this process. | [22] |
| Cordyline australis (G. Forst.) Endl., Ficus benjamina L., Sinningia speciosa Hiern | B (100% blue, 460 nm), R (100% red, 660 nm), and W (white, 7% blue (400–500 nm), 16% green (500–600 nm), 75% red (600–700 nm), and 2% far-red (700–800 nm)) and RB (75% R and 25% B, peaks at 460 and 660 nm). | B and RB increased Fv/Fm and ΦPSII; R decreased biomass. B increased stomatal conductance, leaf thickness, and palisade parenchyma in F. benjamina. B and RB increased palisade parenchyma in S. speciosa. | [23] |
| Crocus sativus L. | (i) R ʎ = 660 nm (62%) and B ʎ = 450 nm (38%) (RB); and (ii) R ʎ = 660 nm (50%), G ʎ = 500–600 nm (12%), and B ʎ = 450 nm (38%) (RGB) and a photosynthetic photon flux density of 120 µmol m−2 s−1. | The two LED treatments increased the antioxidant compounds. RGB enhanced the total flavonoid content and declined corolla fresh weight. RB and RGB increased DPPH. | [24] |
| Cyclamen persicum Mill. ‘Dixie White’ | B light treatment; R light treatment; mixing of B and R (BR) light treatments (1:1 photon flux density). Photoperiod of 10 or 12 h per day. | BR improved flower induction, with number of flower buds and open flowers being highest in the plants grown under RB (10 h per day). B and R alone reduced the flowering response. Peduncle length and blooming period of flowers were also influenced by light qualities and photoperiod treatments. Red length increased peduncle length. R increased the blooming period. | [25] |
| Dianthus caryophyllus L. ‘Moon light’ | W (400–730 nm), B (460 nm), and R (660 nm). | B maintained a higher membrane stability index; higher activities of SOD, POD, CAT, and APX; a decline in petal carotenoid; a higher Fv/Fm percentage of open stomata; and a higher sugar content. | [26] |
| Dianthus caryophyllus L. | W (400–730 nm), R (660 nm), and B (460 nm). | B determined the lowest relative membrane permeability (RMP) in flowers, and longest vase life. The R and W lights accelerated flower senescence and increased expression of DcACS and DcACO. B inhibited the expression of ethylene biosynthetic genes. | [27] |
| (i) Hypoestes phyllostachya Baker ‘Decor Pink’ and ‘Decor Red’, Guzmania lingulata Mez. ‘Theresa’; (ii) Cryptanthus carnosus Mez. ‘Tricolor’ | (i) 100R0B, 80R20B, 50R50B, 20R80B, and 0R100B (ii) 100R, 100R + FR, and 83R17B and 86R14B. | R and B are needed to preserve plant quality. In Hypoestes, the R LEDs determined curly leaves and plants that were not sufficiently compact. Without B light in Guzmania, bracts turn entirely yellow and Cryptanthus leaves are much paler. The B light improves the anthocyanin synthesis and qualitative pigmentation. | [28] |
| Impatiens hybrida hort (‘Sunpatiens Compact Royal Magenta’ = Magenta and ‘Sunpatiens Compact White’ = White) | 83%R:17%B; 75%R:25%B; 67%R:33%B; and 50%R:25%R:25%B. | 75R:25B and 83R:17B increased the cutting number in both cultivars. White cv. produced a higher number of cuttings compared to magenta, but only at 83 DAT in the 67R:33B treatment. At 167 days, 83R:17B produced a higher number of cuttings than 67R:33B. At 202 days, 83R:17B improved the number of cuttings compared to control. 67R:33B and 83R:17B increased leaf trichome numbers compared to the control. | [29] |
| Impatiens walleriana Hook.f., Salvia splendens Sellow ex Nees, Petunia hybrida E. Vilm. | B100, B50 + G50, B50 + R50, B25 + G25 + R50, G50 + R50, and R100, with a photosynthetic photon flux of 160 µmol·m−2·s−1 for 18 h·d−1. | For all species, plants grown under 25% or greater B light were shorter than those under R light. For all species, the plants under R light increased leaf area and fresh shoot weight more than plants grown under treatments with 25% or greater B light. B increased in Impatiens walleriana the flower bud. | [30] |
| Lachenalia spp. | Three light treatments: red (660 nm) and blue (440 nm) lights in different ratios: 100% R (100/0), 90% R + 10% B (90/10), and 80% + 20% B (80/20). The PPFD at the plant leaf level was approx. 150 μmol m−2 s−1. | The 90/10 spectrum induced the longest inflorescences with the highest stem diameter and number of florets. B light increased the anthocyanin content in the corolla (+~35%) compared to plants exposed to 100% R light and nonirradiated ones (control plants). | [31] |
| LDPs (long-day plants): two petunia cultivars, ageratum, snapdragons, and Arabidopsis; and SDPs (short-day plants): three chrysanthemum cultivars and marigold | Greenhouse undertruncated 9 h short days with or without 7 h day-extension lighting from G (peak = 521 nm) at 0, 2, 13, or 25 μmol m−2 s−1 or R + W + FR light at 2 μmol m−2 s−1. | Increasing the G photon flux density from 0 to 25 μmol m−2 s−1 accelerated flowering of all LDPs and delayed flowering of all SDPs. Petunias flowered similarly fast under R + W + FR light and moderate G light; under G, petunia plants were shorter and developed more branches. To be as effective as the R + W + FR light, saturation of G photon flux densities were 2 μmol m−2 s−1 for ageratum and marigold and 13 μmol m−2 s−1 for petunias. Snapdragons were the least sensitive to G. In Arabidopsis, cryptochrome 2 mediated the promotion of flowering under moderate G, whereas both phytochrome B and cryptochrome 2 mediated that under R + W + FR light. | [32] |
| Lilium spp. ‘Corvara’ | 20:80 (R4B); 40:60 (2R3B); 60:40 (3R2B); 80:20 (4RB); and control (W) (100% white light). | 2R3B reduced the number of days to harvest maturity and flower height. Control increases were achieved in the following variables: R4B = leaf area, tepal color; 3R2B = vase life; and 4RB = plant height, flower diameter, and number of days to maturity. | [33] |
| Petunia hybrida E. Vilm., Geranium (Pelargonium ×hortorum L.H. Bailey), and Coleus (Solenostemon scutellariodes (L.) Codd) | R:FR (1:0, 2:1, and 1:1) at two PPFDs (96 and 288 μmol m−2 s−1), all with a B photon flux density of 32 μmol m−2 s−1. | As R:FR decreased, stem length in all species increased. Decreasing R:FR increased the leaf area in petunias, and increased shoot dry weight in petunias and coleus. Decreasing R:FR promoted in petunias subsequent flowering at both PPFDs. In geraniums, the addition of FR had no effect on flowering, irrespective of PPFD. | [34] |
| Petunia hybrida E. Vilm. ’Duvet Red’, Calibrachoa ×hybrida ‘Kabloom Deep Blue’, Pelargonium ×hortorum L.H. Bailey ‘Pinto Premium Salmon’, and Tagetes erecta L. ‘Antigua Orange’ | R (660 nm); B (455 nm); BRF0; BRF2; BRF4; and BRF6. Unpure B light was created by mixing B with low-level (6%) R, and further adding far-red light of 0, 2, 4, and 6 μmol m−2 s−1, respectively. | B and BRF6 promoted flowering compared to R or BRF0. The promotion effect of unpure B light increased, following the order of BRF0, BRF2, BRF4, and BRF6, which varied in sensitivity among plant species. The calculated phytochrome photostationary state was higher for R and decreased gradually for unpure blue light treatments: BRF0, BRF2, BRF4, and BRF6. | [35] |
| Rosa ‘Red Star’ | R (660 nm), B (440 nm), W (white); and darkness (dark). | W increased water uptake and evaporation rates; water uptake and evaporation did not modify the quality of cut roses subjected to red light treatment. | [36] |
| Rosa ×hybrida ‘Aga’ | R, B, W, RBW + FR (far-red) (high R:FR), and RBW + FR (low R:FR). | Both RBW + FR lights increased plant growth and total shoot length. Light treatments increased Fv/Fm. R and RBW + FR at high R:FR stimulated flower bud formation. R increased the resistant to Podosphaera pannosa. B increased the flavonol index. | [37] |
| Rosa ×hybrida ‘Mistral’ | To study the effects of light quality and light intensity on conidial productivity, chambers for conidia (Podosphaera pannosa) production were equipped with LEDs of B (465 nm), R (675 nm), F-R (755 nm), or W (full spectrum) in confront of mercury lamps (white light source). | The number of conidia trapped under F-R LEDs was approximately 4.7 times higher than in W light, and 13.3 times higher than under R. When mildewed plants were exposed to cycles of 18 h of W light followed by 6 h of B, R, or F-R light, or darkness, R reduced the number of conidia trapped by ~88% compared with darkness or F-R. Interrupting the dark period with 1 h of R light reduced the number of conidia trapped, while 1 h of F-R following the 1 h of light from R nullified the suppressive effect of R. | [38] |
| Rosa ×hybrida L. | Control (no supplemental lighting); downward lighting at 150 μmol⋅m−2⋅s−1; and upward lighting at 150 μmol⋅m−2⋅s−1. | Control decreased flower number and lower-leaf senescence. Downward LED lighting promoted blooming and lower-leaf senescence. Upward LED lighting promoted blooming and maintained the photosynthetic abilities of the leaves, including the lower leaves. | [39] |
| Salvia nemorosa L. ‘Lyrical Blues’, Gaura lindheimeri Engelm. and Gray ‘Siskiyou Pink’ | [R (660 nm)]:[B (460 nm)] light ratios (%) of 100:0 (R100:B0), 75:25 (R75:B25), 50:50 (R50:B50), or 0:100 (R0:B100). | All light-quality treatments did not change callus diameter and rooting percentage. R75:B25 or R50:B50 increased relative leaf chlorophyll content. R50:B50 decreased stem lengths of both species’ cuttings, and increased the root biomass compared to SL. | [40] |
| Tagetes tenuifolia Cav., Celosia argentea L. | RB: 65% R, 35% B; RGB: 47% R, 19% G, 34% B; and different photosynthetic photon flux densities (110, 220, and 340 µmol m−2 s−1). | Lowest level of photosynthetically active photon flux (110 µmol m−2 s−1) reduced growth and decreased the phenolic contents in all species. Total carotenoid content and antioxidant capacity were enhanced by the middle intensity (220 µmol m−2 s−1), regardless of spectral combination. The inclusion of green light at 340 µmol m−2 s−1 in the RB increased the growth (dry weight biomass) and the accumulation of bioactive phytochemicals. | [41] |
| Tradescantia zebrina Bosse Chlorophytum comosum (Thunb.) Jacques | Different light treatments: TO Tube luminescent Dunn (TLD) lamps or control, TB (TLD lamps + blue light-emitting diodes (LEDs)), TR (TLD lamps + red LEDs), and TBR (TLD lamps + blue and red LEDs). | Both species had increased root, shoot, and total dry weights under blue LED conditions. The chlorophyll concentration showed a specific response in each species under monochromic or mixed red–blue LEDs. The highest photosynthetic rate was measured under the addition of mixed red–blue LEDs with TLD lamps. The addition of blue LEDs increased the production of ornamental foliage species. | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trivellini, A.; Toscano, S.; Romano, D.; Ferrante, A. LED Lighting to Produce High-Quality Ornamental Plants. Plants 2023, 12, 1667. https://doi.org/10.3390/plants12081667
Trivellini A, Toscano S, Romano D, Ferrante A. LED Lighting to Produce High-Quality Ornamental Plants. Plants. 2023; 12(8):1667. https://doi.org/10.3390/plants12081667
Chicago/Turabian StyleTrivellini, Alice, Stefania Toscano, Daniela Romano, and Antonio Ferrante. 2023. "LED Lighting to Produce High-Quality Ornamental Plants" Plants 12, no. 8: 1667. https://doi.org/10.3390/plants12081667
APA StyleTrivellini, A., Toscano, S., Romano, D., & Ferrante, A. (2023). LED Lighting to Produce High-Quality Ornamental Plants. Plants, 12(8), 1667. https://doi.org/10.3390/plants12081667









