Introgression of Heterotic Genomic Segments from Brassica carinata into Brassica juncea for Enhancing Productivity
Abstract
:1. Introduction
2. Results
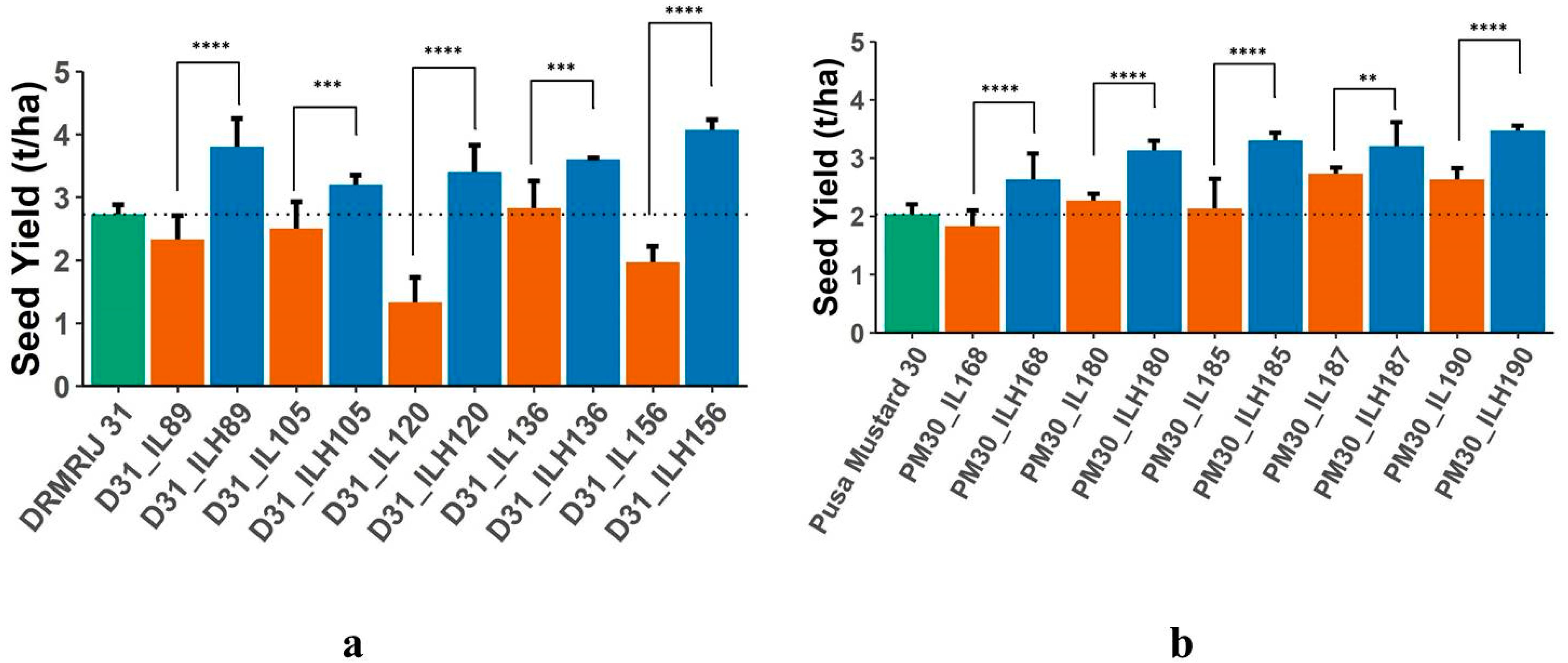
2.1. Mean Performance of Introgression Lines and Hybrids
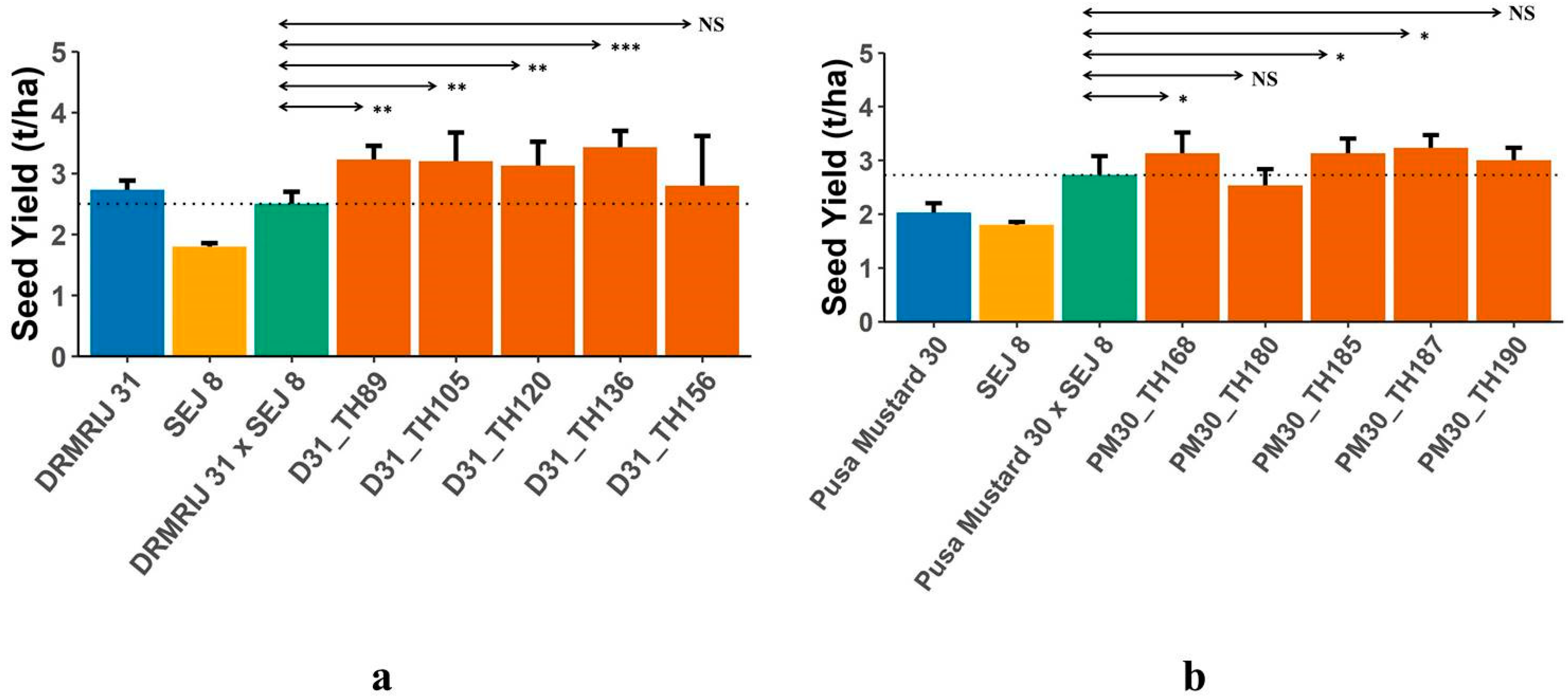
2.2. Heterosis Expressed in Introgression Line Hybrids and Test Hybrids
2.3. Confirmation of Heterosis Arising due to Introgressed Segments
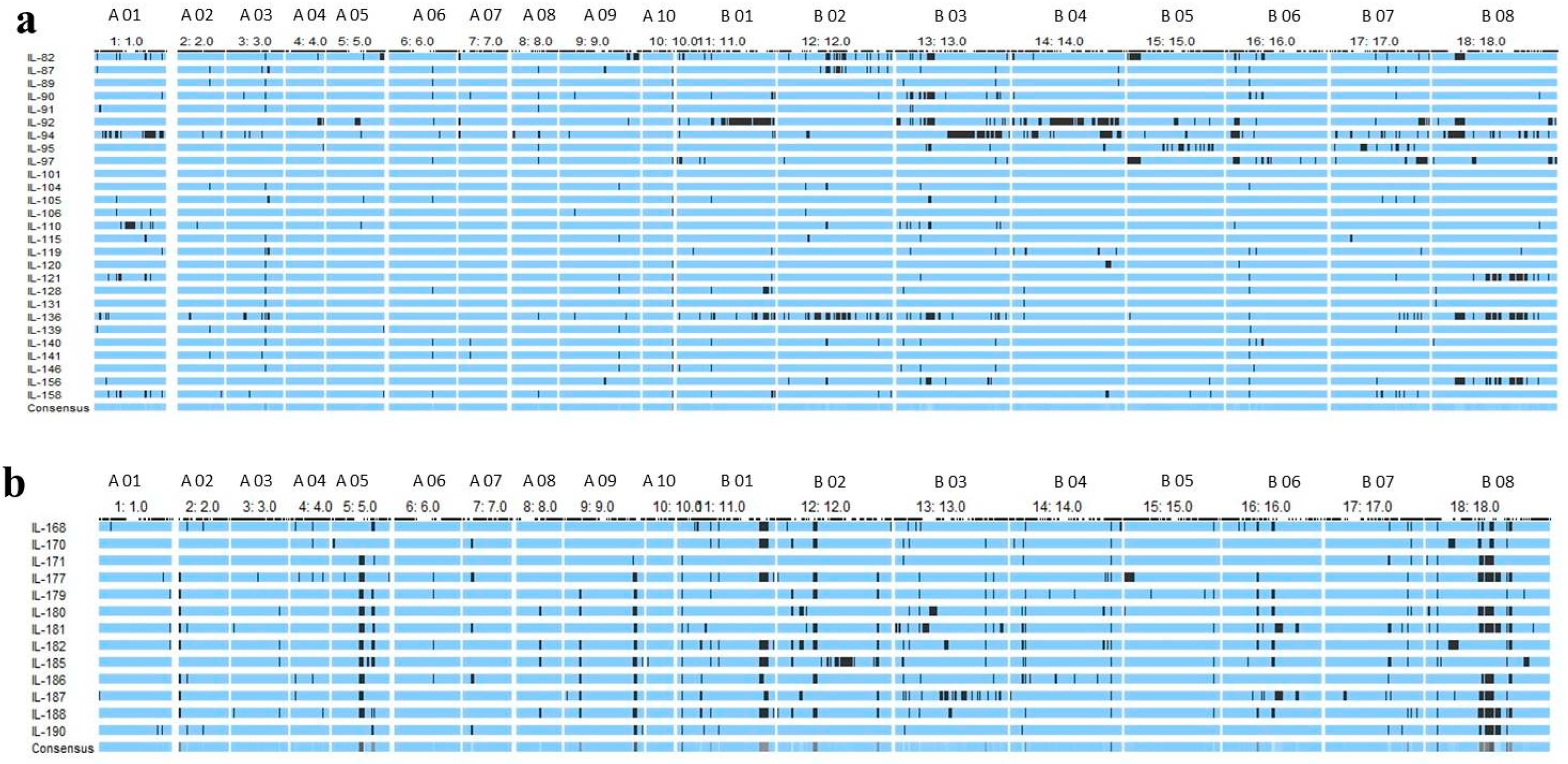
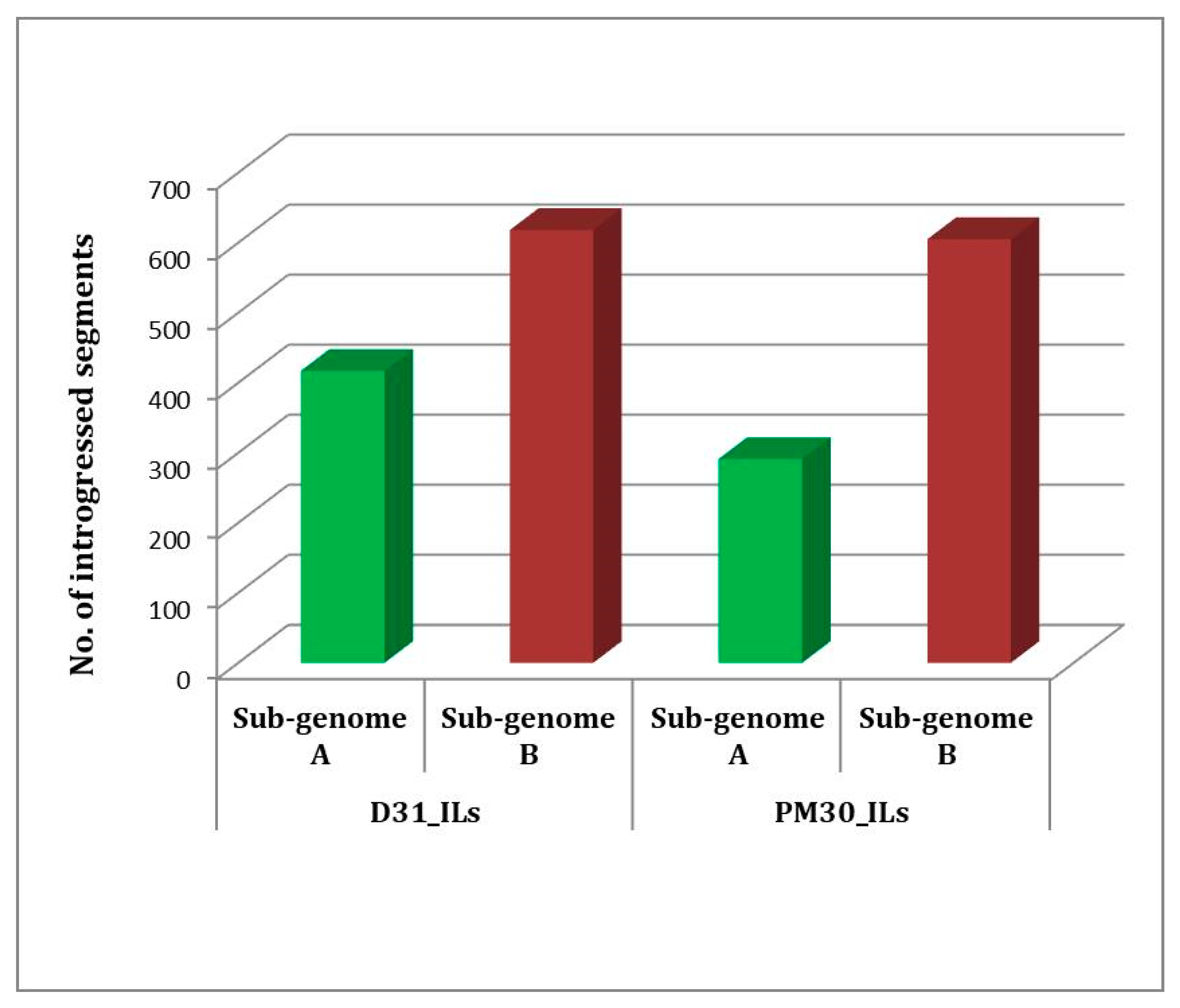
2.4. Marker Analysis and Detection of Introgressed B. carinata Genomic Segments
2.5. Identification of Heterotic Genomic Segments in ILs and Associated Candidate Genes
3. Discussion
3.1. Dissection of Heterotic Genomic Segments
3.2. Candidate Genes Associated with Heterotic Genomic Segments
4. Materials and Methods
4.1. Plant Materials
4.2. Phenotypic Evaluation
4.3. Genotyping by Sequencing
4.4. Identification of Heterotic Genomic Segments and Candidate Gene Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Nagaharu, U. Genome analysis in Brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilization. Jpn. J. Bot. 1935, 7, 389–452. [Google Scholar]
- USDA. Foreign Agricultural Service, Global Agricultural Information Network Circular Series, WAP 1–23. Available online: https://www.fas.usda.gov/data/world-agricultural-production (accessed on 13 January 2022).
- Chauhan, J.S.; Singh, K.H.; Singh, V.V.; Kumar, S. Hundred Years of Rapeseed-Mustard Breeding in India: Accomplishments and Future Strategies. Indian J. Agric. Sci. 2011, 81, 1093–1109. [Google Scholar]
- Jat, R.S.; Singh, V.V.; Sharma, P.; Rai, P.K. Oilseed Brassica in India: Demand, Supply, Policy Perspective and Future Potential. OCL 2019, 26, 8. [Google Scholar] [CrossRef]
- Akhatar, J.; Kumar, H.; Kaur, H. Recent Progress in Brassica Hybrid Breeding. In Plant Male Sterility Systems for Accelerating Crop Improvement; Springer: Berlin, Germany, 2022; pp. 195–219. [Google Scholar] [CrossRef]
- Gaikwad, K.B.; Singh, N.; Bhatia, D.; Kaur, R.; Bains, N.S.; Bharaj, T.S.; Singh, K. Reinventing Heterosis Phenomenon through Deployment of Alien Introgression Lines in Rice (Oryza sativa L.). Plant Breed. 2019, 138, 277–289. [Google Scholar] [CrossRef]
- Singh, N.; Yadava, D.K.; Vasudev, S.; Singh, R.; Giri, S.C.; Dass, B.; Barun, S.; Prabhu, K.V. Combining Ability and Heterobeltiosis for Yield and Yield Contributing Traits in High Quality Oil Indian Mustard (Brassica juncea) Genotypes. Indian J. Agric. Sci. 2015, 85, 498–503. [Google Scholar]
- East, E.M. HETEzROSIS. Genetics 1936, 21, 375–397. [Google Scholar] [CrossRef]
- Gaikwad, K.B.; Singh, N.; Bhatia, D.; Kaur, R.; Bains, N.S.; Bharaj, T.S.; Singh, K. Yield-Enhancing Heterotic QTL Transferred from Wild Species to Cultivated Rice Oryza sativa L. PLoS ONE 2014, 9, e96939. [Google Scholar] [CrossRef]
- Katche, E.; Quezada-Martinez, D.; Katche, E.I.; Vasquez-Teuber, P.; Mason, A.S. Interspecific Hybridization for Brassica Crop Improvement. Crop Breed. Genet. Genom. 2019, 1, e190007. [Google Scholar]
- Yu, F.; Lydiate, D.J.; Gugel, R.K.; Sharpe, A.G.; Rimmer, S.R. Introgression of Brassica rapa Subsp. Sylvestris Blackleg Resistance into B. napus. Mol. Breed. 2012, 30, 1495–1506. [Google Scholar] [CrossRef]
- Udall, J.A.; Quijada, P.A.; Polewicz, H.; Vogelzang, R.; Osborn, T.C. Phenotypic Effects of Introgressing Chinese Winter and Resynthesized Brassica napus L. Germplasm into Hybrid Spring Canola. Crop Sci. 2004, 44, 1990–1996. [Google Scholar] [CrossRef]
- Qian, W.; Liu, R.; Meng, J. Genetic Effects on Biomass Yield in Interspecific Hybrids between Brassica napus and B. rapa. Euphytica 2003, 134, 9–15. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, M.; Chang, S.; Wu, C.; Liu, P.; Meng, J.; Zou, J. Introgressing Subgenome Components from Brassica rapa and B. carinata to B. juncea for Broadening Its Genetic Base and Exploring Intersubgenomic Heterosis. Front. Plant Sci. 2016, 7, 1677. [Google Scholar] [CrossRef]
- Thakur, A.K.; Singh, K.H.; Sharma, D.; Parmar, N.; Nanjundan, J. Breeding and Genomics Interventions in Ethiopian Mustard (Brassica carinata A. Braun) Improvement—A Mini Review. S. Afr. J. Bot. 2019, 125, 457–465. [Google Scholar] [CrossRef]
- Choudhary, B.R.; Joshi, P.; Ramarao, S. Interspecific Hybridization between Brassica carinata and Brassica rapa. Plant Breed. 2000, 119, 417–420. [Google Scholar] [CrossRef]
- Jiang, Y.; Tian, E.; Li, R.; Chen, L.; Meng, J. Genetic Diversity of Brassica carinata with Emphasis on the Interspecific Crossability with B. rapa. Plant Breed. 2007, 126, 487–491. [Google Scholar] [CrossRef]
- Navabi, Z.K.; Stead, K.E.; Pires, J.C.; Xiong, Z.; Sharpe, A.G.; Parkin, I.A.P.; Rahman, M.H.; Good, A.G. Analysis of B-Genome Chromosome Introgression in Interspecific Hybrids of Brassica napus × B. carinata. Genetics 2011, 187, 659–673. [Google Scholar] [CrossRef]
- Chatterjee, D.; Banga, S.; Gupta, M.; Bharti, S.; Salisbury, P.A.; Banga, S.S. Resynthesis of Brassica napus through Hybridization between B. juncea and B. carinata. Theor. Appl. Genet. 2016, 129, 977–990. [Google Scholar] [CrossRef]
- Paritosh, K.; Rajarammohan, S.; Yadava, S.K.; Sharma, S.; Verma, R.; Mathur, S.; Mukhopadhyay, A.; Gupta, V.; Pradhan, A.K.; Kaur, J. A Chromosome-Scale Assembly of Brassica carinata (BBCC) Accession HC20 Containing Resistance to Multiple Pathogens and an Early Generation Assessment of Introgressions into B. juncea (AABB). bioRxiv, 2022; preprint. [Google Scholar]
- Sheikh, F.A.; Banga, S.; Banga, S.S. Broadening the Genetic Base of Abyssinian Mustard (Brassica carinata A. Braun) through Introgression of Genes from Related Allotetraploid Species. Span. J. Agric. Res. 2014, 12, 742. [Google Scholar] [CrossRef]
- Nikzad, A.; Kebede, B.; Pinzon, J.; Bhavikkumar, J.; Wang, X.; Yang, R.-C.; Rahman, H. Potential of the C Genome of the Different Variants of Brassica oleracea for Heterosis in Spring B. napus Canola. Front. Plant Sci. 2020, 10, 1691. [Google Scholar] [CrossRef]
- Limbalkar, O.M.; Singh, R.; Kumar, P.; Nanjundan, J.; Parihar, C.M.; Vasisth, P.; Yadava, D.K.; Chinnusamy, V.; Singh, N. Deployment of Brassica carinata A. Braun Derived Brassica juncea (L.) Czern. Lines for Improving Heterosis and Water Use Efficiency Under Water Deficit Stress Conditions. Front. Plant Sci. 2021, 12, 765645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, X.; Chang, L.; Wang, T.; Liang, J.; Lin, R.; Wu, J.; Wang, X. Expanding the Genetic Variation of Brassica juncea by Introgression of the Brassica rapa Genome. Hortic. Res. 2022, 9, uhab054. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Mason, A.S.; Batley, J.; Bharti, S.; Banga, S.; Banga, S.S. Molecular-Cytogenetic Characterization of C-Genome Chromosome Substitution Lines in Brassica juncea (L.) Czern and Coss. Theor. Appl. Genet. 2016, 129, 1153–1166. [Google Scholar] [CrossRef] [PubMed]
- Schelfhout, C.J.; Wroth, J.M.; Yan, G.; Cowling, W.A. Enhancement of Genetic Diversity in Canola-Quality Brassica napus and B. juncea by Interspecific Hybridisation. Aust. J. Agric. Res. 2008, 59, 918. [Google Scholar] [CrossRef]
- Gaikwad, K.B.; Singh, N.; Bhatia, D.; Sharma, N.; Bains, N.S.; Bharaj, T.S.; Singh, K. Heterotic Response of Genomic Regions Derived from Oryza rufipogon and O. nivara in Improving Grain Morphology and Quality of Indica Rice (Oryza sativa L.). Indian J. Genet. Plant. Breed. 2018, 78, 155. [Google Scholar] [CrossRef]
- Liu, J.; Li, M.; Zhang, Q.; Wei, X.; Huang, X. Exploring the Molecular Basis of Heterosis for Plant Breeding. J. Integr. Plant Biol. 2020, 62, 287–298. [Google Scholar] [CrossRef]
- Huang, X.; Yang, S.; Gong, J.; Zhao, Q.; Feng, Q.; Zhan, Q.; Zhao, Y.; Li, W.; Cheng, B.; Xia, J.; et al. Genomic Architecture of Heterosis for Yield Traits in Rice. Nature 2016, 537, 629–633. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Y.; Liu, X.; Chen, B.; Tu, J.; Tingdong, F. Detection of QTL for Six Yield-Related Traits in Oilseed Rape (Brassica napus) Using DH and Immortalized F2 Populations. Theor. Appl. Genet. 2007, 115, 849–858. [Google Scholar] [CrossRef]
- Li, D.; Huang, Z.; Song, S.; Xin, Y.; Mao, D.; Lv, Q.; Zhou, M.; Tian, D.; Tang, M.; Wu, Q.; et al. Integrated Analysis of Phenome, Genome, and Transcriptome of Hybrid Rice Uncovered Multiple Heterosis-Related Loci for Yield Increase. Proc. Natl. Acad. Sci. USA 2016, 113, E6026–E6035. [Google Scholar] [CrossRef]
- Nassirou, T.Y.; He, W.; Chen, C.; Nevame, A.Y.M.; Nsabiyumva, A.; Dong, X.; Yin, Y.; Rao, Q.; Zhou, W.; Shi, H.; et al. Identification of Interspecific Heterotic Loci Associated with Agronomic Traits in Rice Introgression Lines Carrying Genomic Fragments of Oryza glaberrima. Euphytica 2017, 213, 176. [Google Scholar] [CrossRef]
- Tian, S.; Xu, X.; Zhu, X.; Wang, F.; Song, X.; Zhang, T. Overdominance Is the Major Genetic Basis of Lint Yield Heterosis in Interspecific Hybrids between G. hirsutum and G. barbadense. Heredity 2019, 123, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Semel, Y.; Nissenbaum, J.; Menda, N.; Zinder, M.; Krieger, U.; Issman, N.; Pleban, T.; Lippman, Z.; Gur, A.; Zamir, D. Overdominant Quantitative Trait Loci for Yield and Fitness in Tomato. Proc. Natl. Acad. Sci. USA 2006, 103, 12981–12986. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Zhou, C.; Chen, G.; Yu, J.; Wu, W.; Ge, Y.; Liu, X.; Li, J.; Jiang, X.; Tang, W.; et al. Heterosis-Associated Genes Confer High Yield in Super Hybrid Rice. Theor. Appl. Genet. 2020, 133, 3287–3297. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Yang, H.; Liu, X.; Li, H.; Yuan, L.; Li, W.; Fu, Z.; Tang, J.; Kang, D. Identification of Heterotic Loci Associated with Grain Yield and Its Components Using Two CSSL Test Populations in Maize. Sci. Rep. 2016, 6, 38205. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, D.; Surapaneni, M.; Mesapogu, S.; Neelamraju, S. Development and Use of Chromosome Segment Substitution Lines as a Genetic Resource for Crop Improvement. Theor. Appl. Genet. 2019, 132, 1–25. [Google Scholar] [CrossRef]
- Zhang, B.; Ma, L.; Wu, B.; Xing, Y.; Qiu, X. Introgression Lines: Valuable Resources for Functional Genomics Research and Breeding in Rice (Oryza sativa L.). Front. Plant Sci. 2022, 13, 863789. [Google Scholar] [CrossRef]
- Quezada-Martinez, D.; Addo Nyarko, C.P.; Schiessl, S.V.; Mason, A.S. Using Wild Relatives and Related Species to Build Climate Resilience in Brassica Crops. Theor. Appl. Genet. 2021, 134, 1711–1728. [Google Scholar] [CrossRef]
- Mason, A.S.; Huteau, V.; Eber, F.; Coriton, O.; Yan, G.; Nelson, M.N.; Cowling, W.A.; Chèvre, A.-M. Genome Structure Affects the Rate of Autosyndesis and Allosyndesis in AABC, BBAC and CCAB Brassica Interspecific Hybrids. Chromosome Res. 2010, 18, 655–666. [Google Scholar] [CrossRef]
- Katche, E.; Gaebelein, R.; Idris, Z.; Vasquez-Teuber, P.; Lo, Y.; Nugent, D.; Batley, J.; Mason, A.S. Stable, Fertile Lines Produced by Hybridization between Allotetraploids Brassica juncea (AABB) and Brassica carinata (BBCC) Have Merged the A and C Genomes. New Phytol. 2021, 230, 1242–1257. [Google Scholar] [CrossRef]
- Yadava, S.K.; Arumugam, N.; Mukhopadhyay, A.; Sodhi, Y.S.; Gupta, V.; Pental, D.; Pradhan, A.K. QTL Mapping of Yield-Associated Traits in Brassica juncea: Meta-Analysis and Epistatic Interactions Using Two Different Crosses between East European and Indian Gene Pool Lines. Theor. Appl. Genet. 2012, 125, 1553–1564. [Google Scholar] [CrossRef]
- Aakanksha; Yadava, S.K.; Yadav, B.G.; Gupta, V.; Mukhopadhyay, A.; Pental, D.; Pradhan, A.K. Genetic Analysis of Heterosis for Yield Influencing Traits in Brassica juncea Using a Doubled Haploid Population and Its Backcross Progenies. Front. Plant Sci. 2021, 12, 721631. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Yang, Q.; Yang, Q.; Zhao, Z.; Chen, H.; Wu, J.; Fan, C.; Zhou, Y. Identification of Candidate Genes of QTLs for Seed Weight in Brassica napus through Comparative Mapping among Arabidopsis and Brassica species. BMC Genet. 2012, 13, 105. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zaman, Q.U.; Huang, W.; Mei, D.; Liu, J.; Wang, W.; Ding, B.; Hao, M.; Fu, L.; Cheng, H.; et al. QTL and Candidate Gene Identification for Silique Length Based on High-Dense Genetic Map in Brassica napus L. Front. Plant Sci. 2019, 10, 1579. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xu, R.; Li, Y. Molecular networks of seed size control in plants. Annu. Rev. Plant Biol. 2019, 70, 435–463. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.B.; Huang, H.Y.; Hu, Y.W.; Zhu, S.W.; Wang, Z.Y.; Lin, W.H. Brassinosteroid Regulates Seed Size and Shape in Arabidopsis. Plant Physiol. 2013, 162, 1965–1977. [Google Scholar] [CrossRef]
- Liu, J.; Hua, W.; Hu, Z.; Yang, H.; Zhang, L.; Li, R.; Deng, L.; Sun, X.; Wang, X.; Wang, H. Natural Variation in ARF18 Gene Simultaneously Affects Seed Weight and Silique Length in Polyploid Rapeseed. Proc. Natl. Acad. Sci. USA 2015, 112, E5123–E5132. [Google Scholar] [CrossRef]
- Martín-Trillo, M.; Cubas, P. TCP Genes: A Family Snapshot Ten Years Later. Trends Plant Sci. 2010, 15, 31–39. [Google Scholar] [CrossRef]
- Mathur, S.; Paritosh, K.; Tandon, R.; Pental, D.; Pradhan, A.K. Comparative Analysis of Seed Transcriptome and Coexpression Analysis Reveal Candidate Genes for Enhancing Seed Size/Weight in Brassica juncea. Front. Genet. 2022, 13, 814486. [Google Scholar] [CrossRef]
- Li, J.; Yu, M.; Geng, L.-L.; Zhao, J. The Fasciclin-like Arabinogalactan Protein Gene, FLA3, Is Involved in Microspore Development of Arabidopsis: Arabidopsis FLA3 Is Involved in Microspore Development. Plant J. 2010, 64, 482–497. [Google Scholar] [CrossRef]
- Kumar, R.; Saini, D.K.; Kumar, M.; Priyanka, V.; Akhatar, J.; Kaushik, D.; Sharma, A.; Dhanda, P.S.; Kaushik, P. Revealing the Genetic Architecture of Yield-Related and Quality Traits in Indian Mustard [Brassica juncea (L.) Czern. and Coss.] Using Meta-QTL Analysis. Agronomy 2022, 12, 2442. [Google Scholar] [CrossRef]
- Dhaka, N.; Jain, R.; Yadav, A.; Yadav, P.; Kumar, N.; Sharma, M.K.; Sharma, R. Transcriptome Analysis Reveals Cell Cycle-Related Transcripts as Key Determinants of Varietal Differences in Seed Size of Brassica juncea. Sci. Rep. 2022, 12, 11713. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.; Wells, R.; McKenzie, N.; Trick, M.; Ball, J.; Fatihi, A.; Dubreucq, B.; Chardot, T.; Lepiniec, L.; Bevan, M.W. Variation in Expression of the HECT E3 Ligase UPL3 Modulates LEC2 Levels, Seed Size, and Crop Yields in Brassica napus. Plant Cell 2019, 31, 2370–2385. [Google Scholar] [CrossRef] [PubMed]
- Panse, V.G.; Sukhatme, P.V. Statistical methods for agricultural workers. In Statistical Methods for Agricultural Workers; Indian Council of Agricultural Research: New Delhi, India, 1954. [Google Scholar]
- Coffman, F.A. Heterosis: Specific Not General in Nature. Science 1933, 77, 114–115. [Google Scholar] [CrossRef] [PubMed]
- Saghai-Maroof, M.A.; Soliman, K.M.; Jorgensen, R.A.; Allard, R.W. Ribosomal DNA Spacer-Length Polymorphisms in Barley: Mendelian Inheritance, Chromosomal Location, and Population Dynamics. Proc. Natl. Acad. Sci. USA 1984, 81, 8014–8018. [Google Scholar] [CrossRef] [PubMed]
- Elshire, R.J.; Glaubitz, J.C.; Sun, Q.; Poland, J.A.; Kawamoto, K.; Buckler, E.S.; Mitchell, S.E. A Robust, Simple Genotyping-by-Sequencing (GBS) Approach for High Diversity Species. PLoS ONE 2011, 6, e19379. [Google Scholar] [CrossRef]
- Paritosh, K.; Yadava, S.K.; Singh, P.; Bhayana, L.; Mukhopadhyay, A.; Gupta, V.; Bisht, N.C.; Zhang, J.; Kudrna, D.A.; Copetti, D.; et al. A Chromosome-scale Assembly of Allotetraploid Brassica juncea (AABB) Elucidates Comparative Architecture of the A and B Genomes. Plant Biotechnol. J. 2021, 19, 602–614. [Google Scholar] [CrossRef]
- Glaubitz, J.C.; Casstevens, T.M.; Lu, F.; Harriman, J.; Elshire, R.J.; Sun, Q.; Buckler, E.S. TASSEL-GBS: A High Capacity Genotyping by Sequencing Analysis Pipeline. PLoS ONE 2014, 9, e90346. [Google Scholar] [CrossRef]
- Van Berloo, R. GGT 2.0: Versatile Software for Visualization and Analysis of Genetic Data. J. Hered. 2008, 99, 232–236. [Google Scholar] [CrossRef]
- Young, N.D.; Tanksley, S.D. Restriction Fragment Length Polymorphism Maps and the Concept of Graphical Genotypes. Theor. Appl. Genet. 1989, 77, 95–101. [Google Scholar] [CrossRef]
- Guo, X.; Guo, Y.; Ma, J.; Wang, F.; Sun, M.; Gui, L.; Zhou, J.; Song, X.; Sun, X.; Zhang, T. Mapping Heterotic Loci for Yield and Agronomic Traits Using Chromosome Segment Introgression Lines in Cotton: HL Mapping for Yield Traits Using CSIL in Cotton. J. Integr. Plant Biol. 2013, 55, 759–774. [Google Scholar] [CrossRef]
- Hua, J.; Xing, Y.; Wu, W.; Xu, C.; Sun, X.; Yu, S.; Zhang, Q. Single-Locus Heterotic Effects and Dominance by Dominance Interactions Can Adequately Explain the Genetic Basis of Heterosis in an Elite Rice Hybrid. Proc. Natl. Acad. Sci. USA 2003, 100, 2574–2579. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.Y.; Wang, W.X.; Yang, J.S.; Luo, X.J. Genetic Analysis of Heterotic Loci Detected in a Cross between Indica and Japonica Rice (Oryza Sativa L.). Breed. Sci. 2011, 61, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Dhaka, N.; Rout, K.; Yadava, S.K.; Sodhi, Y.S.; Gupta, V.; Pental, D.; Pradhan, A.K. Genetic Dissection of Seed Weight by QTL Analysis and Detection of Allelic Variation in Indian and East European Gene Pool Lines of Brassica Juncea. Theor. Appl. Genet. 2017, 130, 293–307. [Google Scholar] [CrossRef] [PubMed]
| Genotype(s) | Parameter | Traits | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SL | SPS | SMS | TS | OC | TSW | HI | SY | ||
| ILs in genetic background of DRMRIJ 31 (D31_ILs) | Mean ± SD | 3.67 ± 0.51 | 12.7 ± 1.45 | 48.16 ± 4.36 | 307.20 ± 44.09 | 35.35 ± 2.39 | 4.52 ± 0.56 | 21.76 ± 2.66 | 2.05 ± 0.51 |
| Range | 2.83–5.20 | 9.83–16.53 | 39.40–56.33 | 218–376.60 | 31.37–39.73 | 3.60–5.90 | 16.27–26.87 | 1.07–2.93 | |
| DRMRIJ 31 | Mean | 3.87 | 15.63 | 56.27 | 291.47 | 37.13 | 4.07 | 25.60 | 2.73 |
| ILHs in genetic background of DRMRIJ 31 (D31_ILs × DRMRIJ 31) | Mean ± SD | 4.02 ± 0.33 | 14.18 ± 0.86 | 51.03 ± 3.39 | 313.25 ± 40.27 | 36.37 ± 1.72 | 4.88 ± 0.33 | 24.8 ± 1.84 | 3.07 ± 0.39 |
| Range | 3.27–4.73 | 12.73–16.23 | 44.73–56.67 | 237.93–387.67 | 32.10–39.50 | 4.30–5.63 | 20.03–28.60 | 2.40–4.07 | |
| Critical difference (p = 0.05) | 0.401 | 1.4034 | 6.4279 | 68.7943 | 1.8523 | 0.6118 | 4.4766 | 0.4941 | |
| ILs in genetic background of Pusa Mustard 30 (PM30_ILs) | Mean ± SD | 3.73 ± 0.46 | 13.47 ± 1.14 | 51.78 ± 6.42 | 357.12 ± 46.55 | 35.11 ± 1.77 | 4.81 ± 0.76 | 23.09 ± 2.37 | 2.38 ± 0.35 |
| Range | 3.10–4.50 | 11.50–14.80 | 40.53–62.87 | 294.87–452.60 | 32.60–38.47 | 3.93–6.40 | 19.83–27.03 | 1.77–2.83 | |
| Pusa Mustard 30 | Mean | 3.90 | 11.17 | 43.87 | 233.80 | 31.77 | 5.97 | 21.30 | 2.03 |
| ILHs in genetic background of Pusa Mustard 30 (PM30_ILs × Pusa Mustard 30) | Mean ± SD | 4.21 ± 0.32 | 13.25 ± 0.70 | 49.53 ± 3.77 | 333.34 ± 51.52 | 35.31 ± 0.61 | 5.52 ± 0.49 | 24.54 ± 0.97 | 2.95 ± 0.31 |
| Range | 3.80–4.70 | 12.07–14.47 | 44.20–55.07 | 267.80–434.20 | 34.07–36.17 | 5.07–7.00 | 22.27–26.10 | 2.47–3.47 | |
| Critical difference (p = 0.05) | 0.4921 | 1.234 | 5.4801 | 68.6253 | 1.5683 | 0.6887 | 3.7609 | 0.4907 | |
| Traits | ILHs in Genetic Background of DRMRIJ 31 (D31_ILs × DRMRIJ 31) | ILHs in Genetic Background of Pusa Mustard 30 (PM30_ILs × Pusa Mustard 30) | ||
|---|---|---|---|---|
| Mean | Range | Mean | Range | |
| Siliqua length (cm) | 7.79 ± 6.85 | −6.33–19.40 | 10.56 ± 8.96 | −4.84–25.47 |
| Seeds/Siliqua | 0.20 ± 5.69 | −11.81–16.85 | 7.73 ± 6.20 | −3.23–20.72 |
| Total siliquae on main shoot | −2.73 ± 5.43 | −11.67–8.67 | 3.81 ± 8.23 | −8.23–16.98 |
| Total number of siliquae/plant | 5.16 ± 15.53 | −17.67–33.95 | 14.01 ± 22.94 | −16.92–51.34 |
| Oil content (%) | 0.00 ± 3.79 | −11.93–6.57 | 5.65 ± 2.28 | 1.00–8.54 |
| 1000 seed weight (g) | 13.48 ± 6.02 | 4.65–24.68 | 2.73 ± 9.23 | −13.75–18.31 |
| Harvest index (%) | 5.00 ± 9.21 | −21.41–24.12 | 10.82 ± 6.76 | −1.10–22.92 |
| Seed yield (t/ha) | 31.36 ± 19.41 | −7.69–76.81 | 34.19 ± 15.85 | 11.76–58.40 |
| Traits | Hybrids between ILs of DRMRIJ 31 and SEJ 8 (D31_ILs × SEJ 8) # | Hybrids between ILs of Pusa Mustard 30 and SEJ 8 (PM30_ILs × SEJ 8) $ | ||
|---|---|---|---|---|
| Mean | Range | Mean | Range | |
| Siliqua length (cm) | −1.75 ± 8.40 | −19.86–15.07 | −0.47 ± 5.86 | −9.09–11.36 |
| Seeds/Siliqua | −2.05 ± 6.61 | −13.97–12.23 | 9.02 ± 4.28 | 1.70–14.84 |
| Total siliquae on main shoot | 27.44 ± 6.79 | 10.6–41.47 | 9.25 ± 7.53 | −2.68–22.43 |
| Total number of siliquae/plant | 33.96 ± 16.66 | 9.13–69.39 | 9.68 ± 11.07 | −14.61–24.41 |
| Oil content (%) | −2.28 ± 4.07 | −11.13–4.07 | −0.84 ± 2.77 | −5.53–3.20 |
| 1000 seed weight (g) | −6.36 ± 7.03 | −18.62–7.59 | −14.2 ± 6.87 | −26.03–−3.55 |
| Harvest index (%) | 6.09 ± 9.14 | −8.55–25.91 | 11.64 ± 6.20 | 0.86–19.23 |
| Seed yield (t/ha) | 22.77 ± 13.11 | −8.00–42.67 | 2.63 ± 11.97 | −17.07–18.29 |
| Traits | Parameter | Introgression Line Hybrids (ILHs) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D31_ILH89 | D31_ILH105 | D31_ILH120 | D31_ILH136 | D31_ILH156 | PM30_ILH168 | PM30_ILH180 | PM30_ILH185 | PM30_ILH187 | PM30_ILH190 | ||
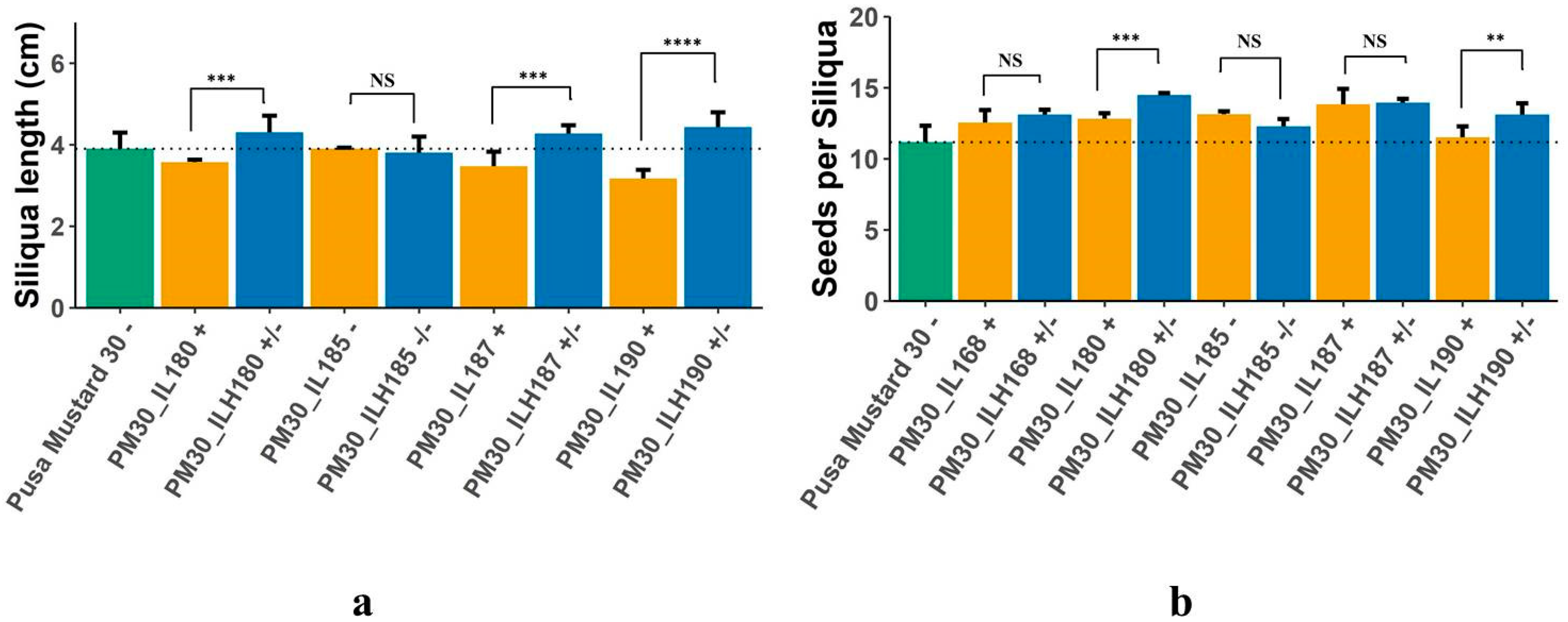
| SL | MPH | 4.00 | 1.12 | 8.86 | 8.82 | 7.04 | −1.29 | 15.18 * | −2.56 | 15.84 * | 25.47 * |
| Heterosis over IL | 5.41 | −11.76 * | 4.88 | 23.33 * | 15.15 * | −0.86 | 20.56 * | −2.56 | 23.08 * | 40.00 * | |
| Heterosis over GB | 2.63 | 18.42 * | 13.16 * | −2.63 | 0 | −1.71 | 10.26 | −2.56 | 9.40 | 13.67 * | |
| SPS | MPH | 1.12 | −3.43 | 3.45 | −1.12 | −4.32 | 10.55 * | 20.72 * | 0.96 | 11.47 * | 15.59 * |
| Heterosis over IL | 21.62 * | −6.06 | 11.94 * | 18.92 * | 9.02 | 4.52 | 13.02 * | −6.60 | 0.72 | 13.91 * | |
| Heterosis over GB | −13.46 * | −0.64 | −3.85 | −15.38 * | −14.74 * | 17.31* | 29.55 * | 9.85 | 24.78 * | 17.31 * | |
| SMS | MPH | −9.68 | −3.36 | 3.33 | 0.54 | −2.08 | −4.81 | 11.78 * | 15.08* | −8.23 | −6.93 |
| Heterosis over IL | 0.65 | 5.22 | 24.87 * | 2.78 | −0.18 | −9.80 | 1.23 | 10.49 | −18.51 * | −21.00 * | |
| Heterosis over GB | −18.09 * | −10.64 | −11.88 * | −1.60 | −3.90 | 0.76 | 24.77 * | 20.06* | 5.02 | 13.22 * | |
| TS | MPH | 4.27 | −10.60 | 32.56 * | 4.27 | −9.17 | 12.95 | 30.70 * | 0.13 | −16.92 | 15.58 |
| Heterosis over IL | 0.77 | −16.41 | 35.36 * | −7.51 | −14.05 | −0.90 | 12.68 | −20.08 * | −34.82 * | −0.47 | |
| Heterosis over GB | 8.03 | −3.91 | 29.88 * | 19.49 | −3.70 | 31.31 * | 55.57 * | 34.02 * | 14.54 | 37.81 * | |
| OC | MPH | 2.53 | 1.02 | 1.30 | 2.36 | 2.25 | 7.00 * | 6.22 * | 6.12 * | 6.71 * | 5.75 * |
| Heterosis over IL | 1.58 | 10.54 * | −2.01 | 5.73 * | 7.40 * | 1.51 | −0.46 | 3.17 | 2.01 | 1.85 | |
| Heterosis over GB | 3.49 | −6.99 * | 4.84 * | −0.81 | −2.42 | 13.12 * | 13.85 * | 9.23 * | 11.86 * | 9.97 * | |
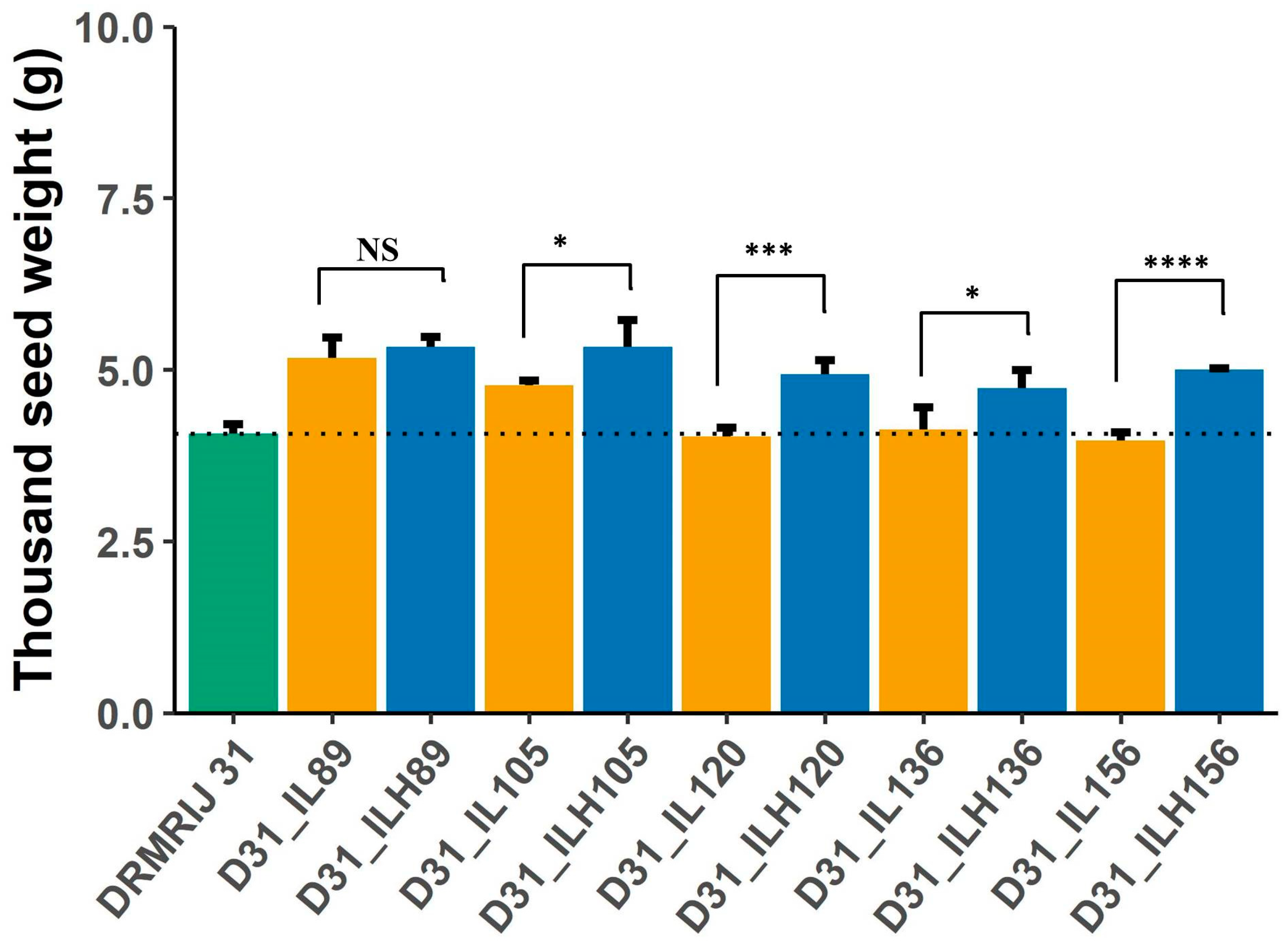
| TSW | MPH | 16.13 * | 20.45 * | 20.99 * | 15.66 * | 23.46 * | 7.79 | 4.60 | −6.87 | 11.11 | 6.42 |
| Heterosis over IL | 3.85 | 12.77 | 22.50 * | 14.29 | 25.00 * | 28.68 * | 27.20 * | 0 | 39.83 * | 17.57 * | |
| Heterosis over GB | 31.71 * | 29.27 * | 19.51 * | 17.07 * | 21.95 * | −7.26 | −11.17 | −12.85 * | −7.82 | −2.79 | |
| HI | MPH | 11.30 | 6.21 | 17.42 | 1.08 | −4.00 | 15.55 | 9.79 | 22.92 * | −1.10 | 3.89 |
| Heterosis over IL | 19.82 | 9.05 | 50.92 * | 12.44 | −6.32 | 19.10 * | 1.05 | 23.31 * | −11.59 | 3.25 | |
| Heterosis over GB | 3.91 | 3.52 | −3.91 | −8.20 | −1.56 | 12.21 | 20.19 * | 22.54 * | 12.21 | 4.54 | |
| SY | MPH | 49.33 * | 23.08 * | 70.00 * | 30.12 * | 76.81 * | 36.21 * | 45.73 * | 58.40 * | 34.27 * | 48.57 * |
| Heterosis over IL | 60.00 * | 26.32 * | 155.00 * | 25.58 * | 110.34 * | 43.64 * | 38.23 * | 54.69 * | 17.07 * | 31.65 * | |
| Heterosis over GB | 40.00 * | 20.00 * | 27.50 * | 35.00 * | 52.50 * | 29.51 * | 54.10 * | 62.30 * | 57.38 * | 70.50 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasisth, P.; Singh, N.; Limbalkar, O.M.; Sharma, M.; Dhanasekaran, G.; Meena, M.L.; Jain, P.; Jaiswal, S.; Iquebal, M.A.; Watts, A.; et al. Introgression of Heterotic Genomic Segments from Brassica carinata into Brassica juncea for Enhancing Productivity. Plants 2023, 12, 1677. https://doi.org/10.3390/plants12081677
Vasisth P, Singh N, Limbalkar OM, Sharma M, Dhanasekaran G, Meena ML, Jain P, Jaiswal S, Iquebal MA, Watts A, et al. Introgression of Heterotic Genomic Segments from Brassica carinata into Brassica juncea for Enhancing Productivity. Plants. 2023; 12(8):1677. https://doi.org/10.3390/plants12081677
Chicago/Turabian StyleVasisth, Prashant, Naveen Singh, Omkar Maharudra Limbalkar, Mohit Sharma, Gokulan Dhanasekaran, Mohan Lal Meena, Priyanka Jain, Sarika Jaiswal, Mir Asif Iquebal, Anshul Watts, and et al. 2023. "Introgression of Heterotic Genomic Segments from Brassica carinata into Brassica juncea for Enhancing Productivity" Plants 12, no. 8: 1677. https://doi.org/10.3390/plants12081677






